Abstract
Climate change pressures will influence marine planktonic systems globally, and it is conceivable that harmful algal blooms may increase in frequency and severity. These pressures will be manifest as alterations in temperature, stratification, light, ocean acidification, precipitation-induced nutrient inputs, and grazing, but absence of fundamental knowledge of the mechanisms driving harmful algal blooms frustrates most hope of forecasting their future prevalence. Summarized here is the consensus of a recent workshop held to address what currently is known and not known about the environmental conditions that favor initiation and maintenance of harmful algal blooms. There is expectation that harmful algal bloom (HAB) geographical domains should expand in some cases, as will seasonal windows of opportunity for harmful algal blooms at higher latitudes. Nonetheless there is only basic information to speculate upon which regions or habitats HAB species may be the most resilient or susceptible. Moreover, current research strategies are not well suited to inform these fundamental linkages. There is a critical absence of tenable hypotheses for how climate pressures mechanistically affect HAB species, and the lack of uniform experimental protocols limits the quantitative cross-investigation comparisons essential to advancement. A HAB “best practices” manual would help foster more uniform research strategies and protocols, and selection of a small target list of model HAB species or isolates for study would greatly promote the accumulation of knowledge. Despite the need to focus on keystone species, more studies need to address strain variability within species, their responses under multifactorial conditions, and the retrospective analyses of long-term plankton and cyst core data; research topics that are departures from the norm. Examples of some fundamental unknowns include how larger and more frequent extreme weather events may break down natural biogeographic barriers, how stratification may enhance or diminish HAB events, how trace nutrients (metals, vitamins) influence cell toxicity, and how grazing pressures may leverage, or mitigate HAB development. There is an absence of high quality time-series data in most regions currently experiencing HAB outbreaks, and little if any data from regions expected to develop HAB events in the future. A subset of observer sites is recommended to help develop stronger linkages among global, national, and regional climate change and HAB observation programs, providing fundamental datasets for investigating global changes in the prevalence of harmful algal blooms. Forecasting changes in HAB patterns over the next few decades will depend critically upon considering harmful algal blooms within the competitive context of plankton communities, and linking these insights to ecosystem, oceanographic and climate models. From a broader perspective, the nexus of HAB science and the social sciences of harmful algal blooms is inadequate and prevents quantitative assessment of impacts of future HAB changes on human well-being. These and other fundamental changes in HAB research will be necessary if HAB science is to obtain compelling evidence that climate change has caused alterations in HAB distributions, prevalence or character, and to develop the theoretical, experimental, and empirical evidence explaining the mechanisms underpinning these ecological shifts.
Keywords: Harmful algal blooms, HAB, Climate change
1. Introduction
The warming of the global system is unequivocal and has resulted in unprecedented changes in climate, meaning decadal or longer time scale shifts in overall weather characteristics (Stocker et al., 2013). The proximal changes, manifest primarily in terms of temperature, precipitation, and wind, work interactively with surface water acidification stemming from increased CO2 emissions to alter mean surface water conditions, and perhaps more importantly their extremes. There is increasing concern that this shifting milieu will cause changes in phytoplankton community structure and composition, including a greater prevalence and geographical spread of harmful algal blooms. But the anticipated linkages between climate change and harmful algal blooms are founded on limited and often conflicting experimental and observational data. Scientists are left mostly to “informed” speculation about whether future climate may enhance or diminish the frequency, intensity, and distribution of HAB outbreaks. A fundamental challenge to HAB scientists is to identify key indicators and demonstrated relationships that reveal solid evidence of climate-induced changes in harmful algal blooms.
The current state of knowledge stems from a rich literature on the taxonomy, growth characteristics, and ecophysiology of freshwater and marine phytoplankton collectively grouped as “harmful algae”. This societally defined category includes toxic species that express toxicity to higher trophic levels, largely fish, shellfish, marine mammals, or humans, and include members of the cyanobacteria, dinoflagellates, raphidophytes, haptophytes, and diatoms. Included also under the HAB umbrella are largely human-caused high-biomass events that, while often comprising non-toxic phytoplankton species, still critically alter ecosystems through hypoxia/anoxia, altered food web efficiencies, stimulation of pathogenic bacteria, or other ecological consequences.
Current spatial and temporal ranges of HAB species will most certainly change under future climate scenarios. Spatially, one can expect that the geographic domains of species may expand, contract, or just shift latitudinally. Temporally, the seasonal windows for growth will also contract and expand. Successful “invasions” of new HAB species will depend fundamentally on the species “getting there”, through spatial transport, “being there” as indigenous species (hidden flora) that potentially can grow in abundance within the phytoplankton community, and “staying there” by persistence through unfavorable conditions (e.g., high temperature, nutrient depletion, overwintering).
The HAB research community is largely unprepared to address these questions. The central challenge is to achieve consensus about the way forward from both research and management perspectives. This focused community synergy will be critical if the knowledge base is to advance faster than the influence of climate-related changes on harmful algal blooms, and if statistically credible evidence of this change can be provided soon enough to contribute to the societal debate over climate change impacts. These preparations will be particularly critical for high latitude regions where climate change impacts are liable to be most rapid and substantial (Stocker et al., 2013). The foundation of HAB knowledge has accumulated mainly through isolated investigations, as with most environmental sciences, but this piecemeal process does not readily foster as powerful a knowledge structure as can be achieved through synergistic, collective, and collaborative approaches. That is, a collective vision is needed that can identify the “known knowns” and rank the levels of the “known unknowns” if the community is to presage climate change-HAB linkages before they develop.
In working to achieve a higher level of cooperation among HAB and climate scientists, there is some guidance to be gleaned from the ocean acidification field, which used broad collaboration to create the infrastructure and standard methods needed to generate scientific awareness and funding streams that critically address the environmental and biological questions of greatest importance. Moving the understanding of HAB-climate change interactions beyond informed speculation will require rigorous, testable hypotheses to guide scientists, managers and the public on what changes are happening or are projected, estimation of the confidence limits on those projected changes, and establishing the infrastructure and studies needed to capture these necessary data.
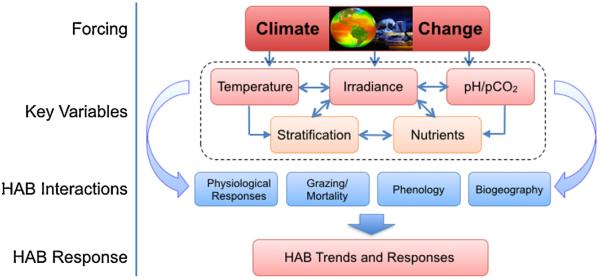
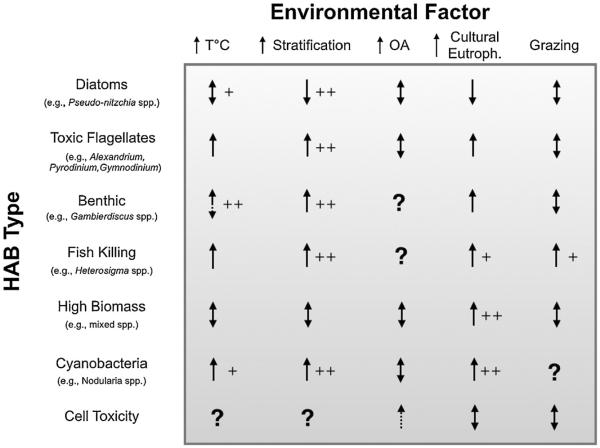
As a beginning, there is a strong need to outline clearly what currently is known (and not known) about the environmental conditions that favor initiation and maintenance of different types of HAB events, and how sensitive those key parameters are to changes in the climate system (Fig. 1). As a first step in that process, a four-day workshop was held in Spring 2013 at Friday Harbor Laboratories, University of Washington, comprised of 11 HAB researchers with diverse expertise spanning the ecophysiology and nutrient acquisition of HAB species, their nutritional quality and implications for food web structure and ecosystem health, and observational platforms, time series analyses and prediction. This paper summarizes the discussion at that workshop, illustrated by an overview assessment of how environmental change may affect different HAB types (Fig. 2). This document is not intended to be a comprehensive description of potential linkages between climate change and harmful algal blooms, but rather to: (1) give a sense of near-term research that may hold the greatest promise for knowledge advancement and impact, (2) provide funding agencies, managers, and interested stakeholders an overview assessment of current knowledge and key gaps in this knowledge, (3) assist in leveraging the use of current ocean observing systems to obtain important, HAB-related parameters, and (4) perhaps most importantly, attract the interest of non-HAB researchers who are developing relevant new tools or approaches (molecular, cellular, modeling, sensor) that hold promise for HAB research. A focus on this broad picture will be necessary if there is to be improvement in the understanding and tools needed to identify and forecast the links of harmful algal blooms and climate, and to ascertain the earliest signals of this change.
Fig. 1.
The progression of climate change pressure on key variables and related HAB interactions that will drive HAB responses in the future ocean.
Fig. 2.
A general overview of the current understanding from the literature of how different HAB types will be affected by climate change stressors. Arrows indicate changes that either increase, decrease, or can occur in both directions. Symbols suggest the level of confidence: + (reasonably likely), ++ (more likely).
2. Anticipated linkages between harmful algal blooms and climate change
2.1. Direct temperature effects on harmful algal blooms
Temperature is one of the main environmental factors affecting physiological processes in phytoplankton, acting at many different stages of growth and bloom development. It not only is one of the most important environmental drivers expected to change with climate, but also is the least contentious, since there already has been measurable warming of the surface mixed layer (Beardall et al., 2009) and the upper several hundred meters of the oceans (Roemmich et al., 2012). But this warming is not globally uniform (Roemmich et al., 2012; Stocker et al., 2013). Regional assessments and downscaling of global models are needed to understand and predict temperature changes in specific coastal regions. Some regions clearly will be affected more by global warming than others, but high latitudes will experience the greatest absolute and relative warming (Locarnini et al., 2006; Stocker et al., 2013).
Increases in atmospheric and surface water temperature will be accompanied by altered seasonal patterns, with longer duration of summertime conditions and corresponding shifts in the timing of spring and fall transitions. These changes lengthen the “windows of opportunity” for growth (Moore et al., 2009) and germination (Itakura and Yamaguchi, 2005), affecting both species selection and phytoplankton population dynamics in temperate and boreal habitats. At the same time, increased temperatures may narrow “growth windows” of HAB species in sub-tropical/tropical waters if temperature optima are exceeded.
2.1.1. What is known about temperature effects on the relative success of HAB species?
The link between increasing water temperatures and phytoplankton growth rates has long been recognized (Bissenger et al., 2008; Eppley, 1972), and it is expected that increased potential growth rates will accompany warming. Temperature influences motility (Kamykowski and McCollum, 1986), germination (Montresor and Lewis, 2006; Yamochi and Joh, 1986), nutrient uptake, photosynthesis, and other physiological processes (Beardall and Raven, 2004; Raven and Geider, 1988). Optimal and inhibitory threshold temperatures differ among metabolic processes. The optimal temperature for photosynthesis is generally greater than the optimum for growth (Li, 1980). Indeed, the biogeography of phytoplankton species boundaries is determined in large part by temperature regimes (Longhurst, 1998; Okolodkov, 1999, 2005), as is almost certainly is true for HAB species. But phytoplankton, including HAB species, can show strong intraspecific differences in temperature tolerance and responses (de Boer, 2005).
All species have a temperature niche described by optimal, lethal and tolerable water temperatures at which cells do not grow well, but can survive (de Boer, 2005; Fehling et al., 2004; Magaña and Villareal, 2006; Rhodes and O'Kelly, 1994). Even so, there usually is a mismatch between optimal growth rate temperatures for species in culture and those at which these species occur in natural systems, with many species dropping out of the successional sequence prior to appearance of their optimum isotherms (Karentz and Smayda, 1984, 1998). While methodological artifacts explain some of the discrepancy, the mismatch largely reflects the multifactorial control of in situ populations that is absent in the ungrazed, non-dispersed, monospecific, and nutritionally optimal culture experiments. Since the optimal temperature and tolerance of a species are genetically, not environmentally determined, current insights may stem disproportionately from an inherent selection of the best growers, and often the experimental focus on non-HAB species. There also are genetic differences among isolates that may reflect their adaptive strategies to different regions (Ruiz et al., 2005; Scholin et al., 1995).
Relative to diatoms and raphidophytes, dinoflagellates generally have low growth rates (Honjo, 1987; Honjo and Tabata, 1985; Tang, 1996), but species capable of rapid growth in culture, including HAB species, are known (Smayda, 1996). Some non-toxic species differ from toxic species within the same genus in their temperature–growth relationship (Rhodes and O'Kelly, 1994), but in most cases, specific information about differences between HAB and non-HAB species is lacking. Various cyanobacterial species respond differently than other groups to temperature changes (Fu et al., 2007), but it seems unlikely that there are differences in temperature tolerance between toxic and non-toxic cyanobacteria within the same species (Huisman et al., 2005). Gambierdiscus toxicus is known to generally favor warmer conditions, and increased ciguatera fish poisoning has been observed with elevated sea surface temperatures related to El Niño Southern Oscillation (ENSO) and the Pacific Decadal Oscillation (PDO) (Rongo and van Woesik, 2011). But this linkage is correlative, rather than determined, and temperature optima differ substantially among different Gambierdiscus species or phylotypes (Yoshimatsu et al., 2014). The cell-size dependent population response to warming also differs among phytoplankton groups. Specifically, picophytoplankton biomass appears to increase with temperature, unlike non-cyanobacterial communities which tend to respond in the opposite (Karlberg and Wulff, 2013; Morán et al., 2010). Despite this, unusual blooms of both may be linked to climatic events (Gómez and Souissi, 2007).
Temperature, along with light, influences the germination of dinoflagellate cysts (Anderson et al., 2005; Bravo and Anderson, 1994; although exceptions are known—Perez et al., 1998). Earlier spring warming trends might result in HAB seed populations appearing sooner in surface coastal waters, reflecting earlier onset of permissive temperatures for germination (Kremp and Anderson, 2000; Pfiester and Anderson, 1987) and increased germination rates at higher temperatures (Anderson et al., 2005). An important caveat is that both low and high temperatures can be inhibitory, thereby maintaining cyst quiescence (e.g. Anderson and Rengefors, 2006; Hallegraeff et al., 1998; Itakura and Yamaguchi, 2005). This temperature “window” for germination figures prominently in a species' response to a changing climate.
The chemical composition of a species' (e.g., lipids, fatty acids, and toxicity) also is a function of temperature (Guerrini et al., 2007; Jahnke, 1989). While higher toxicity (i.e., toxin accumulation) of some species can occur with slowing growth, temperature and toxin production appear to be directly linked in some species (Ogata et al., 1989) but not others (e.g., Lewis et al., 1993). Much of the basic information needed to generate a preliminary forecast of which regions or habitats (poles vs. tropics, estuaries vs. coasts) HAB species will be the most resilient or susceptible to temperature change likely is available. As a start, the temperature niche approach versus the temperature at which HAB species isolates were collected can be utilized (Boyd et al., 2013). Cells isolated from areas where temperature variations are small likely are not as resilient to temperature change as those from areas experiencing large seasonal temperature fluctuations. It may be, however, that resilience is less a cellular trait than a population trait wherein the molecular diversity (strain composition) of the population, not individual cells, provides the resilience (Burkholder and Glibert, 2009).
2.1.2. What are important “known unknowns” about direct temperature effects on harmful algal blooms?
At the present time, knowledge of in situ population dynamics of representative HAB species on appropriate temporal and spatial time scales, and environmental regulation of their selection, blooms, toxicity and trophic impacts is inadequate. This knowledge gap is a major impediment. It compromises extrapolation of laboratory-based results to natural populations and identification of thresholds, and because of imperfect insight into the indirect effects of temperature, it restricts understanding of the regulation of population growth and succession. Another constraint is the difficulty in downscaling global climate model predictions to forecast the extent and rate of temperature changes in specific regional habitats. One approach to deal with this uncertainty is to evaluate a range of possible temperature windows, thereby generating an ensemble of forecasts that may reveal thresholds for HAB species growth, distribution, and physiology; thresholds that model output of downscaled regional temperature changes can be compared against. But even then, not all species will respond equally, and there is little information about how HAB species may adapt to temperature increases or decreases relative to non-HAB species. For example, does the net progressive northward migration of warmer temperatures exceed the ability of HAB species, relative to other phytoplankton, to adapt and optimize their growth? It would be useful to rank HAB species in terms of the most temperature tolerant to the least tolerant using the large body of laboratory-based experimental data available, but the degree of genetic heterogeneity among strains of key HAB species with respect to temperature tolerances needs to be determined. Understanding strain variability within co-occurring non-HAB species is equally important, as it will influence their ability to adaptively compete.
Temperature effects will cascade through the ecosystem, affecting bacteria, HAB species, competing phytoplankton grazers, parasites, and other organisms (Alheit et al., 2005; Edwards and Richardson, 2004; Hansen, 1991). How will these indirect temperature effects affect the success of HAB species? Are there temperature-sensitive processes or stages in the bloom progression of some HAB species? For example, the effects of temperature on the quiescence, timing of germination, or survival of HAB resting cysts and spores is not understood (Lewis et al., 1999; Pfiester and Anderson, 1987), nor is the effect of warming in lengthening the duration of the seeding process. Will increasing temperatures affect the long-term persistence of a species in a given region, and will the establishment of range extensions become easier as temperatures increase? What temperature changes are expected for benthic environments (e.g. reefs)? These “known unknowns” all are relevant issues, but there are central stumbling blocks that hamper understanding temperature:HAB interactions where significant advances in research are feasible over the next decade.
2.1.3. Pressing questions about direct temperature effects on harmful algal blooms
Most studies of temperature effects on HAB species utilize single strains—how representative are these responses to that of the more genetically diverse natural populations? While the available limited evidence suggests that increasing temperatures will lead to expansion or contraction of a problem species (e.g., Gambierdiscus spp., Heterosigma spp.), the real situation is almost certainly more complex as a consequence of strain variability. It is critical that there be quantitative assessment of the strain variability in temperature tolerances and optima of key HAB species. This will require studies involving many strains of individual species—a dramatic departure from current practices (Burkholder and Glibert, 2009; Martinez et al., 2010). A good place to start are culture collections that contain numerous isolates of the species of interest, but even these may not be ideally representative of natural population variability. A high priority should also then be given to isolating and characterizing strains associated with different temperature regimes.
What is the potential for multiplicative or additive effects of temperature with other environmental parameters in affecting the relative success of HAB species? There are good examples where temperature strongly modulates the effects of other stressors (e.g., elevated CO2—Feng et al., 2009), which argues for a shift to multifactorial experiments as the norm (e.g., Kremp et al., 2012). But the resulting large matrices create practical challenges for traditional laboratory culture studies. One way around this constraint may be to take advantage of natural weather perturbations to simulate future scenarios with complete ecosystems (e.g., see Moore et al., 2011), although identifying the driving parameters for HAB species behavior will be challenging. Long-term records of temperature are robust, and will make it easier to identify anomalies for study.
Can range expansions or contractions due to warm temperatures be identified? This is a fundamental question that is remarkably hard to answer, much the same as for phytoplankton species introductions via ship's ballast water. Range extensions and species shifts are being reported for other phytoplankton, but with few exceptions (e.g., Hajdu et al., 2000) there are no firm conclusions about HAB species, through either monitoring, cyst distributions, toxicity events, or retrospective analysis of long-term data sets and time series (e.g., the Continuous Plankton Recorder). More common are reports of significant temporal and spatial changes in the abundance of indigenous dinoflagellate species in response to climate change, as in the North Sea where decreasing dinoflagellate abundance appears to be the collective result of increased summer temperature combined with stronger and more frequent winds (Hinder et al., 2012).
From an ecological perspective, will any significant shifts in HAB distributions stem more from temperature-linked physiological effects, such as growth rates, or simply to longer periods of adequate growth windows, leading to a greater probability that HAB events may occur in a given year? Answering this question will depend upon acquisition of better mechanistic understanding of HAB initiation, maintenance, and demise as well as physical characterizations of ocean temperature effects on circulation, which will affect the spatial redistribution of species. There also is the challenge to distinguish between blooms of introduced species from those of indigenous, cryptic species that emerge (i.e., the “hidden flora”) in response to climate-induced changes in temperature and anthropogenic disruption, such as eutrophication. An observational network of sensors and quantitative field studies would facilitate in situ validation of model simulations or other forecast efforts. Regional or local model simulations (physical/chemical/biological) based on HAB versus non-HAB species are lacking in the literature, but would serve as an intellectual foundation for better identifying (with sensitivity analysis) the direct effects of temperature on these and other HAB climate-change issues.
2.1.4. Summary of direct temperature effects on harmful algal blooms
Temperature is a keystone parameter differentially affecting a range of metabolic processes, and thus is anticipated to have a strong influence on phytoplankton community compositions and trajectories. While increasing annual temperatures should broaden the windows of some HAB activity it will not affect others or even lessen other harmful algal blooms. Ecosystem interactions, strain variability within HAB and non-HAB species, and concurrent hydrographic or oceanographic changes all complicate even this seemingly straightforward expectation. It is logical that HAB habitats should expand to higher latitudes, but there is little clear evidence that this has happened and the time series observation systems needed to verify this change currently are lacking. There is little evidence to date that changing temperatures directly affect toxin production in HAB species although these findings are by no means comprehensive. It is unlikely that temperature alone will drive competitive selection of HAB species over non-HAB species, but it is probable that temperature effects on metabolic rates will magnify or lessen the influence of other climatic pressures on HAB prevalence.
2.2. Direct effects of stratification on harmful algal blooms
The surface ocean is anticipated to become generally more stratified with increasing global temperature, glacial and snow-pack meltwater, and precipitation runoff (Stocker et al., 2013). In addition to increasing strength and depth of stratification, variations in its seasonal timing will alter mid-latitude spring and fall transitional periods with corresponding changes to phytoplankton phenology and community succession. Based future global biomass and productivity projections (e.g., Behrenfeld et al., 2006), it is likely that mid- and high-latitude regions will be most strongly impacted by changes in stratification, while changes low-latitude tropical systems will be relatively minor. But species respond to local and regional changes, not global patterns, and the manifested effects will incorporate changes in wind stress (leading to changes in turbulence), local hydrography, depth and intensity of the pycnocline, relative contribution of upwelled nutrients versus runoff as well as changes in nutrient ratios, and indirect changes in subsurface irradiance (e.g., Kahru et al., 2015).
Perhaps the most obvious effect of increased stratification on phytoplankton, and therefore HAB organisms, will be the changing patterns of nutrient availability (e.g., Marinov et al., 2010). At a global scale, changes in thermal stratification already have been linked to oligotrophication at low latitudes (decreased mixing and nutrient inputs) and increases in phytoplankton biomass at higher latitude transitional zones where greater stratification provides enhanced stability and shoaling of the mixed layer (Behrenfeld et al., 2006). These altered seasonal patterns may lengthen the “window of opportunity” for some HAB species (Moore et al., 2009), particularly chain-forming dinoflagellates that take advantage of strong thermal stratification with higher nutrient concentrations at relatively shallow depths (Figueras et al., 2006; Smayda and Trainer, 2010).
2.2.1. What is known about stratification effects on the relative success of HAB species?
As a generalization, prolonged stratification favors organisms with a smaller surface area to volume ratio, such as nano- and picoplankton, which facilitates nutrient uptake (e.g., Hein et al., 1995) and, where vertical migration distances are reasonable, stratification also favors swimmers (Peacock and Kudela, 2014). Moderate turbulence can be expected to help chain-forming swimmers (Acker and Leptoukh, 2007), such as Alexandrium, Cochlodinium, and Gymnodinium spp., to thrive (Fraga et al., 1989).
Stratification also promotes intensification of the pycnocline, which potentially leads to formation of thin layers. These can serve as biological “hotspots” accounting for 50–75% of the total water column biomass, and affect a multitude of biological processes including growth, reproduction, grazing, and toxin production (c.f. Berdalet et al., 2012). There are numerous examples of HAB organisms associated with thin layers, defined here as being a few cm to several meters thick (see review by Sullivan et al., 2010). These include Karenia mikimotoi and Dinophysis spp. (e.g., Farrell et al., 2012; Raine, 2014), Chattonella antiqua and Pseudochattonella spp. (e.g., Aoki et al., 2014; Pettersson and Pozdnyakov, 2013), and Heterosigma akashiwo (e.g., Strom et al., 2013). The diatom Pseudo-nitzschia also is commonly found in layers (McManus et al., 2008; Rines et al., 2002; Ryan et al., 2010), despite the generalization that diatoms are not favored with increased stratification.
There are several recent dramatic examples of emerging HAB issues associated with changes in stratification. Decadal changes in temperature and stratification in the California Current system have led to prolonged periods of increased dinoflagellate dominance (Jester et al., 2009). This shift is closely related to increasing intensity and decreasing depth of the pycnocline (Kudela et al., 2010), and is associated with an increased frequency of visual “red tide” events (Ryan et al., 2008, 2010, 2014). Bird mortalities have been associated with these stratification events through foam production with intense Akashiwo sanguinea events in Monterey Bay, CA in 2007 (Jessup et al., 2009) and the Pacific Northwest in 2010 (Berdalet et al., 2014), as well as elsewhere (e.g., off eastern Africa in 2013—Wim Mullié, pers. comm.). Sub-lethal but disruptive effects of harmful algal blooms in stratified conditions on seabird ecology also may include reduced feeding, lowered fecundity, and loss of motor coordination (Shumway et al., 2003). Another example of an apparent linkage of harmful algal blooms and increased stratification is the recent emergence of Dinophysis spp. in several regions where it has not historically caused problems, including several coastal areas of the United States, the west coast of Canada, and coastal South Africa (Campbell et al., 2010; Hattenrath-Lehmann et al., 2013; Hubbart et al., 2012; Pitcher et al., 1993; Taylor et al., 2013; Trainer et al., 2013). But all the correlative associations of harmful algal blooms and stratification lack the mechanistic insights to be considered causative, and more often than not increased stratification does not result in harmful algal blooms.
2.2.2. What are important “known unknowns” about direct effects of stratification on harmful algal blooms?
Stratification and associated changes in temperature, salinity, mixed layer depth, subsurface irradiance, and nutrient concentrations and ratios will affect HAB (and all phytoplankton) ecophysiology. A key question is do changes in stratification (duration, position, persistence) alter the frequency or intensity of harmful algal blooms? In particular, climate change likely will affect large-scale circulation, thermal stratification, runoff (including the dynamics of flow) and both wind intensity and timing. The selection of turbulent-tolerant organisms versus stratification-tolerant organisms will be strongly influenced by the balance between thermal heating, freshwater inputs, and increased wind stress. Not only are downscaled climate and regional ocean predictions needed to address these unknowns, but also fundamental knowledge about how HAB species (and strains of those species) respond to changes in temperature, nutrients, irradiance, and turbulence.
An important task will be to identify those areas that are more susceptible to changes in stratification. For example, it is broadly assumed that low-latitude systems will be relatively unaffected since they already exhibit strong annual stratification, but this assumption does not take into account the destabilizing effect of increased storm/typhoon/hurricane activity, or shifts in the global wind fields (Stocker et al., 2013). For regions strongly influenced by terrestrial runoff, how will climate change affect the timing and pulsed magnitudes of freshwater inputs, and how will these changes influence the frequency and intensity of stratification events driven by temperature changes? How will these projected changes in either the strength or seasonal duration of stratification affect the windows of opportunity for HAB species?
Although often considered to enhance harmful algal blooms, stratification is a double-edged sword because harmful algal blooms also can be restrained under intensely stratified conditions. For example, the Gulf of Maine is subject to large recurrent annual blooms of Alexandrium fundyense, with the dramatic exception of 2010 (McGillicuddy et al., 2011). An influx of an abnormally fresh and warm water mass that year led to unusually intense stratification, resulting in an early and large spring diatom bloom that crashed rapidly leaving highly nutrient-deplete surface waters. Alexandrium spp. cyst germination, which is controlled by an internal clock and thus is annually invariant (Binder and Anderson, 1987), introduced vegetative cells into those waters, and growth was constrained by the unfavorable conditions. This example emphasizes the difficulties that some HAB species may have in dealing with stratification changes that exceed the levels of interannual variability to which they have adapted.
2.2.3. Pressing questions about direct effects of stratification on harmful algal blooms
How will changes in stratification work in conjunction with other environmental parameters to influence the relative success of HAB species? Given these potentially complex interactions, there are two clear ways forward. First, there are benefits from recent technological advances, emergence of new sampling methods, and a better understanding of the complex biological interactions and life cycles that govern harmful algal blooms in stratified systems. To better ascertain the effects of stratification on HAB species success, these methods must be consistently applied to build high-quality time-series of HAB observations in stratified systems. Coupling these observations with coincident datasets of physical and ecological drivers, including stratification intensity and depth, wind stress and turbulence, nutrient concentrations and ratios, runoff, and upwelling intensity will provide the mechanistic underpinning for the pattern of HAB eruptions. Second, these combined observations should lead to advances in both conceptual and numerical modeling that already are beginning to improve our understanding of harmful algal blooms in stratified systems (GEOHAB, 2013; Berdalet et al., 2014).
How important will swimming behavior, the response to turbulence, and pycnoclines and thin layers as biological refugia be in contributing to HAB species success relative to other phytoplankton? These remain key questions that are readily amenable to direct testing in laboratory, in situ imaging systems, and mesocosm studies (Greer et al., 2013; Smayda, 2002). There also are some natural experiments, such as the changes in HAB frequency and intensity associated with climate patterns of ENSO, PDO, and the North Pacific Gyre Oscillation (NPGO) or changes in coastal communities in response to historically wet and dry years, which provide opportunities to assess measured changes in stratification relative to HAB frequency, intensity, and duration. While these complex systems cannot be used to provide clear evidence that increased stratification enhances (or disrupts) HAB events, it should be possible to ascertain what stratification characteristics (e.g., strength, duration) may correlate consistently with harmful algal blooms. Regardless of the methods chosen, regions that globally exhibit a larger signal-to-noise relationship for HAB impacts, including coastal enclosed and semi-enclosed water bodies, brackish estuaries and fjords, and coastal upwelling systems, offer good opportunities to specifically investigate stratification effects on HAB development.
2.2.4. Summary of direct effects of stratification on harmful algal blooms
Climate change will expand and intensify vertical stratification, particularly at high latitudes. It is clear that water column stratification alone does not cause harmful algal blooms; vertical stability of the water column underpins all global primary production while HAB represent sporadic shifts in an otherwise “non-harmful” planktonic system. But multiple HAB species can flourish under well-stratified conditions (Berdalet et al., 2014), and the emergence of new HAB threats, and expansion of existing HAB organisms, appear to be coincident with global changes in stratification, suggesting that this physical process is a significant contributor. The challenge will be to ascertain whether and how these climate-induced changes will affect the prevalence of harmful algal blooms.
2.3. Altered light field effects on harmful algal blooms
Climate change projections for atmospheric conditions are beginning to be more consistent in global models. Most show a net global increase in clouds near the tropopause and a general trend of reduced cloud fraction (i.e., the percentage of each gridbox in a climate model that is covered with clouds) between 50°N and 50°S, except near the equator (Stocker et al., 2013). Increasing cloud fractions are projected for higher latitudes (Stocker et al., 2013). The in situ light fields also are expected to change in association with the introduction of particulate material from runoff or ice melting (Häder et al., 2010), and the trends towards “brownification” of coastal waters due to increased inputs of humic matter (Hansson et al., 2013; Monteith et al., 2007). In contrast, the expectation of shallower mixed layers due to increased stratification will cause phytoplankton to be exposed to higher intensities/doses of photosynthetically active radiation (PAR: 400–700 nm) and ultraviolet radiation (UVR: 315–400 nm). Any effects from UVR, particularly UVB radiation; 280–315 nm) may be magnified because increased levels of greenhouse gases are expected to delay recovery of the stratospheric ozone layer (McKenzie et al., 2010). While there remains considerable uncertainty over these projections (Stocker et al., 2013), there seems little doubt that the light field in surface waters will be shifting.
2.3.1. What currently is known about light effects on harmful algal blooms?
There are no unique PAR requirements or tolerances of HAB species relative to non-HAB species, but surface irradiance may influence toxin production, particularly at high light intensities. Phytoplankton species have different strategies to adjust to radiation conditions by altering their photosynthetic and photo-protective pigments, including the production of UV-absorbing compounds such as mycosporine-like amino acids (MAA) (Jeffrey et al., 1999). Many of the MAA-containing species are toxic and form dense surface blooms, so enhanced UVR might facilitate some harmful algal blooms. For example, surface-dwelling harmful cyanobacteria contain photoprotective carotenoids and UV-absorbing compounds (e.g. MAA), enabling high growth rates under intense radiation conditions (Carreto and Carignan, 2011; García-Pichel and Castenholz, 1993; Mohlin et al., 2012; Mohlin and Wulff, 2009). Several cyanobacteria also produce the sheath pigment scytonemin with maximum absorbance around 380 nm (Castenholz and Garcia-Pichel, 2005), complementing the UVR absorption of MAA (310–360 nm). Like carotenoids and other photoprotective pigments, some MAA also are proposed to act as antioxidants providing protection against photodamage at high PAR (Castenholz and Garcia-Pichel, 2005).
High amounts of MAA have been found in the raphidophyte Chattonella spp. (Jeffrey et al., 1999) responsible for fish-killing blooms in Asian and European waters. Raphidophytes, such as Heterosigma akashiwo and Chattonella subsalsa, show no sign of photoinhibition at PAR intensities >600 μmol photons m−2 s−1 (Zhang et al., 2006). Some toxic dinoflagellates also have been reported to tolerate high radiation intensities, including Alexandrium catenella (Carignan et al., 2002; Laabir et al., 2011; Smayda and Borkman, 2008), Ceratium furca and Ceratium fusus (Baek et al., 2008), Karenia brevis (Evens et al., 2001), among others. The polar water HAB species, Phaeocystis pouchetii, also produces high concentrations of MAA (Marchant et al., 1991). But not all HAB species tolerate high radiation intensities, with Aureococcus spp. and K. brevis preferring low radiation conditions (MacIntyre et al., 2004; Magaña and Villareal, 2006).
The significance of the high-light tolerance of some HAB species has less to do with their survival than with their ability to thrive throughout a wider portion of the photic zone to maximize photosynthetic potential. Some HAB species can limit photodamage through vertical migration or regulation of buoyancy through gas vesicles (Huisman et al., 2005; Walsby et al., 1995), but non-HAB species also have this capability so it does not necessary give HAB species a competitive advantage.
There is evidence that high penetrating radiation intensities (including UVR) may influence toxin production in some HAB species, but the findings remain equivocal. The toxic filamentous cyanobacterium Nodularia spumigena shows the highest intra- and extracellular nodularin concentrations under the combined conditions of high ambient radiation and low nitrogen availability, when shielded from UVR (Pattanaik et al., 2010). The toxicity of two isolates of Alexandrium fundyense generally increased with increasing PAR intensities up to 425 μmol photons m−2 s−1 (Etheridge and Roesler, 2005); however, there is no apparent overall trend in light dependent toxicity for Alexandrium spp. (Fu et al., 2012). In Microcystis aeruginosa, microcystin synthesis increased with increasing radiation (Kaebernik et al., 2000) and Van de Waal et al. (2011) concluded that growth of the toxic M. aeruginosa strain was greater than the non-toxic variety in high light. Light intensity also positively correlated with the ichthyotoxicity of Chattonella marina (Ishimatsu et al., 1996; Marshall et al., 2001). Based on in situ observations, Mengelt and Prézelin (2005) concluded that the toxic diatom Pseudo-nitzschia sp. was well adapted to utilizing UVA (320–400 nm) to enhance carbon fixation, contributing to its capacity to produce large surface blooms. Field observations by MacIntyre et al. (2011) support the findings that toxin concentration and abundance of Pseudo-nitzschia are strongly correlated with high radiation. Interestingly, a toxic variety of Pseudo-nitzschia pungens/multiseries was shown to be more tolerant to UVB than its non-toxic variety (Hargraves et al., 1993). While none of these relationships are characterized well enough to generalize, there clearly is significant reason to expect that major changes in the global light field of surface waters potentially may influence the competitive success and toxicity of HAB species.
2.3.2. What are important “known unknowns” about altered light field effects on harmful algal blooms?
The projected changes in insolation at both lower and higher latitudes are likely to affect phytoplankton community compositions, but without comparative studies among HAB and non-HAB species there is little basis to conclude that HAB species will be better adapted. Similarly, there are few data to assess the light-induced production of UV-absorbing compounds and toxins in HAB species. Although some toxins absorb UVR, it is yet to be determined whether these compounds serve a physiological role as UV-absorbing compounds (i.e., provide a competitive metabolic advantage). Nevertheless the frequent co-occurrence of MAA and toxins in cyanobacteria, dinoflagellates, and at least a few diatom species (see Klisch and Hader, 2008), argues for the need of more studies.
Increased light intensity with depth in tropical and sub-tropical regions may benefit benthic HAB communities and their macrophytic hosts, with implications for more widespread ciguatera effects. This would be particularly important if ciguatoxin-producing species were more UVR tolerant than non-toxic benthic microalgae. Ecosystems where cysts are vital life stages that experience increased benthic radiant flux may also experience selective effects, though light is rapidly attenuated in sediments, so the effects could only be important at the thin veneer of the sediment surface (Stock et al., 2005). Turbulent systems may also experience increased light penetration with deeper mixing and potentially increased production at depth. In contrast, climate projections for increased runoff and prevalence of low, dense clouds at high latitudes will reduce light availability. These changes will increase the selective pressures for low-light adapted species, and changes in UVR may diminish metal (e.g., Fe) availability (e.g., Roy and Wells, 2011), potentially decreasing the toxicity of Pseudo-nitzschia spp. (Maldonado et al., 2002; Wells et al., 2005).
2.3.3. What are the pressing questions regarding altered light field effects on harmful algal blooms?
Do HAB species possess unique light harvesting and protection capabilities that increase their competitiveness, and do changes in the spectrum and intensity of surface irradiance contribute to altered toxin production? For the first, it will be important to determine whether HAB species are better adapted than non-HAB species to respond to increased, or decreased PAR and UVR; essential knowledge to enable first order assessment of the likelihood of irradiance-derived changes in HAB outbreaks. Second, one of our key unknowns is the extent that UVR influences the putative relationship between MAA and other toxin synthesis. A better understanding of the postulated role of some toxins as photoprotective compounds (Ha et al., 2014), and the competitive advantages provided, would help to forecast the community responses to elevated UVR. While these fundamentals can be tested mechanistically with well-controlled laboratory studies, investigation under ambient radiation conditions will be needed to tease out the community-scale responses.
Will increasing PAR and UV light fields have particular relevance to benthic HAB species in lower latitude regions? Ciguatera Fish Poisoning arguably is the single greatest global HAB-human health problem today. Determining the light factors that may exacerbate the toxicity or niche dominance of these HAB species in reef environments should be of high priority.
2.3.4. Summary of altered light field effects on harmful algal blooms
The modeled trends in cloud feedback mechanisms suggest that climate-driven changes in the light fields will occur at both low and high latitudes, and these changes will impact phytoplankton metabolism and community composition. While currently there are no compelling insights that changing light fields will cause competitive selection of HAB species, there are reasons that they might. Increased light penetration could magnify high biomass harmful algal blooms, and some toxins may enhance UVR protection. If climate driven changes in light fields do contribute to increased harmful algal blooms, these effects would manifest at the extremes, either at high (inhibiting) irradiance or by extending (or diminishing) the depth of the light field.
2.4. Ocean acidification effects on harmful algal blooms
Increasing atmospheric CO2 is being modulated partly by its dissolution into surface seawater, leading to ocean acidification (OA) (Stocker et al., 2013). Although OA is a consequence rather than proximal effect of climate change, its influence is distributed across all ocean surface waters and so its potential effects on harmful algal blooms are considered here. While uncertain, projections suggest that surface water pH may decrease by 0.3–0.4 units by the end of the century (Feely et al., 2004), with upwelling regions being sites of extreme acidification (Feely et al., 2008). Although the majority of early concern about ocean acidification has focused on calcium carbonate precipitating marine organisms (e.g., Bednarsek et al., 2014; Orr et al., 2005), the pH dependent changes in the marine carbonate system also have broad implications for carbon acquisition for both marine phytoplankton (e.g., Beardall et al., 2009; Hansen, 2002; Hinga, 2002) and microbes (e.g., Hutchins et al., 2009). The opposite trend of surface water basification, where biological production drives increased pH during high biomass blooms, is not considered here.
2.4.1. What is known about the effects of ocean acidification on harmful algal blooms?
The complexity of the myriad effects stemming from decreased pH and increased pCO2 on phytoplankton community composition is daunting. Although marine phytoplankton appear to be well adapted to grow at low pH levels (by oceanic standards, Berge et al., 2010) understanding the subtleties of individual species responses is far more difficult. CO2 enrichment is expected to relieve the energy requirements of carbon concentrating mechanisms (CCM) and particularly benefit those species having Form II Rubisco (ribulose-1,5-bis-phosphate carboxylase-oxygenase), which has a low affinity for CO2 (Beardall et al., 2009; Giordano et al., 2005; Raven and Beardall, 2014; Rost et al., 2003; Tortell, 2000). Many bloom-forming dinoflagellates fall into this category, and in particular some toxic dinoflagellates are known to have very limited CCM abilities (Dason and Colman, 2004) while some raphidophytes appear not to have CCM systems at all (Nimer et al., 1997). Even so, the effects of increasing CO2 on dinoflagellates are not uniform. Kremp et al. (2012) found that increasing CO2 stimulated only two of eight Alexandrium ostenfeldii clones, while increasing temperatures universally stimulated growth. Similarly, elevated pCO2 in culture has been observed to increase (Sun et al., 2011; Tatters et al., 2012), decrease (Lundholm et al., 2004), or have no effect on growth of Pseudo-nitzschia (Cho et al., 2001), depending on the species and experimental conditions. So while there is concern that blooms may reach high biomass faster, and that these blooms may have increasing abundances of harmful species, the jury remains out on the likely effects of OA on harmful algal blooms.
The study of OA effects on phytoplankton has focused mainly on carbon acquisition, but decreasing pH also will directly affect cellular membrane potential, enzyme activity, and energy partitioning (Beardall and Raven, 2004; Giordano et al., 2005), all of which influence cell metabolism. Altered transmembrane potential affects a wide range of cellular processes that depend upon proton pumps, such as nutrient uptake. Lowered pH also may affect nutrient acquisition by altering the chemical speciation of dissolved nutrients, particularly trace metals, which can affect interspecies competition (Shi et al., 2010) and HAB toxicity (Maldonado et al., 2002; Wells et al., 2005). Transmembrane proton gradients also are essential for flagellar motion (Manson et al., 1977), so changes in extracellular pH can affect cell mobility and thus perhaps behaviors that enhance nutrient uptake (Hallegraeff et al., 2012a). For example exposure of the harmful algal bloom species Heterosigma akashiwo to lower pH increased its photosynthetic growth rates (Fu et al., 2008; Nimer et al., 1997) and substantially increased its swimming speed (30%) and downward velocities (46%), implying that changing pCO2 is a signaling factor for this organism to alter its behavior (Kim et al., 2013). In contrast, increasing pCO2 diminished the presence of toxic haptophyte Chrysochromulina spp. in a mesocosm study (Hama et al., 2012). On the other hand, the filamentous toxic cyanobacteria Nodularia spumigena and Aphanizomenon sp. were not affected by increased levels of pCO2 (Karlberg and Wulff, 2013). It is no exaggeration to say that current knowledge of the growth or competitive responses of phytoplankton to OA, let alone those of HAB species, is extraordinarily limited.
There also is very little information regarding the potential impact of OA on the cellular toxicity of HAB species, and findings that do appear in the literature are conflicting. Kremp et al. (2012) found an overall trend of increasing cell toxicity with increasing pCO2 (and temperature) for eight strains of Alexandrium ostenfeldii. In culture experiments, strains of Alexandrium fundyense isolated from Northport Bay, New York, and the Bay of Fundy, Canada, grew significantly faster when exposed to elevated levels of pCO2 compared to lower levels (Hattenrath-Lehmann et al., 2015), and high levels of pCO2 significantly increased cellular toxicity in the Northport Bay strain but had no effect in the Bay of Fundy strain. In another study, acidification led to a doubling of saxitoxin concentrations in nutrient replete Alexandrium catenella, but toxicity increased by an order of magnitude under P limitation (Tatters et al., 2013a), indicating the OA effect was minor relative to nutrient status. Even then, the toxicity increases with OA were strongly suppressed by elevated temperatures, illustrating how difficult it is to interpret and compare OA effects even in simple laboratory experiments without fully considering all factors that contribute to the response (see Boyd and Hutchins, 2012). Similarly, Van de Waal et al. (2011) found using different culturing methods that elevated pCO2 led to decreasing cellular toxicity in Alexandrium tamarense.
Current certainty about the effects of pH on toxicity of the diatom genus Pseudo-nitzschia is not much better. Some experiments show 2–3 fold increases in cellular domoic acid concentrations under elevated pCO2 conditions (reduced pH) when either P-(Sun et al., 2011) or Si-limited (Tatters et al., 2012), and these authors suggest that increased availability of inorganic carbon may be mechanistically responsible. But other studies, using different techniques and Pseudo-nitzschia species, found that cellular toxicity increased up to 70-fold at elevated pH rather than more acidic conditions (Lundholm et al., 2004; Trimborn et al., 2008). Although these differences are not yet explained, the implication is that ocean acidification may impact diatom toxicity even if the sign and magnitude of this change so far is unpredictable.
On perhaps a brighter note, early evidence suggests that “medium” term (1–2 yr) evolutionary shifts in phytoplankton adaptation to decreased pH are not sufficient to substantially alter interspecific competitive success. Tatters et al. (2013c) found that the recombination of cell lines acclimated for one year to different pCO2 conditions yielded the same general competitive responses observed in the two-week natural diatom community experiment from which they were isolated. Similar “artificial” communities comprising clonal isolates of dinoflagellates conditioned to altered pCO2 conditions for one year generally matched the outcome of the short-term (2 week) experiment with the natural dinoflagellate community (Tatters et al., 2013b). Even so, it may be that the successful evolutionary adaptations of competitive non-HAB species (e.g., Collins et al., 2014) will be more important in determining the frequency of future harmful algal blooms.
2.4.2. What are important “known unknowns” about ocean acidification effects on harmful algal blooms?
The key issues surrounding high biomass HAB events and OA (vs. other aspects of climate change) are still not well defined. On the one hand, there is ample evidence that the potential growth rates of phytoplankton could increase as the need for CCM diminish, and so increased CO2 availability may intensify blooms and their impacts, particularly where impact zones are confined (e.g., embayments, restricted coastlines). There currently is little understanding about how decreased pH affects the rates of zooplankton grazing (Caron and Hutchins, 2013) or mixotrophy (e.g., Kim et al., 2013) (see below). Changes in grazing pressure in high biomass harmful algal blooms may exacerbate (less feeding on harmful species) or help modulate bloom intensity and disperse carbon export over larger spatial zones, thereby potentially diminishing the potential for hypoxia. To date there is no clarity regarding the balance of these drivers in response to OA.
On the other hand, there is evidence that increasing CO2 concentrations will affect competition among major phytoplankton groups (Feng et al., 2009; Fu et al., 2007; Hare et al., 2007; Tortell et al., 2002), and toxic algal groups (Fu et al., 2008; Ratti et al., 2007; Rost et al., 2006), although very few species have been studied. It is, however, unclear whether these changes in carbon acquisition potential will affect harmful species disproportionately, contributing to either increases or decreases in the frequency of harmful algal blooms. That is, the important issue is not whether ocean acidification will increase the predominance of dinoflagellates, as anticipated, but whether it will select for more frequent flourishing of toxic or harmful dinoflagellate species. More comparative studies along the lines of Eberlein et al. (2014) are needed. In this vein, multiple stressor effects very likely will alter how OA influences HAB species.
The question of whether OA increases the toxicity of HAB species remains open, with conflicting findings in the literature sowing more confusion than consensus. Phytoplankton toxins account for a very small proportion of total cellular carbon (e.g., Fu et al., 2010) and there is no evidence to suggest that cellular toxicity is directly related to intra- or extracellular pH. Even subtle shifts in metabolic processes could affect toxin production or retention by the cell. So slight differences in culture conditions, even those independent of pCO2, might affect the trends in toxin production. Identifying the indirect drivers of ocean acidification on cell toxicity will be challenging.
The effects of OA on the grazing pressure may significantly alter top-down controls of HAB prevalence. Decreasing calcite saturation levels projected for future oceans lower the recruitment success of benthic grazers (Waldbusser et al., 2015), as already observed in upwelling regions (Ekstrom et al., 2015), potentially leading to higher toxin loadings in surviving bivalves. Effects of OA on mesozooplankton are less well understood, but there are indications that it may be more important than previously recognized (Cripps et al., 2014).
2.4.3. What are the pressing questions regarding ocean acidification effects on harmful algal blooms?
What effect does increased CO2 availability have on the competitive success of HAB species, and does it alter bloom dynamics sufficiently to intensify their impacts? There is a rich literature describing the physiology, growth characteristics, and toxicity of HAB species in laboratory and field conditions but there is very little quantitative insight to how competitive these organisms are relative to their “non-HAB” counterparts, let alone how OA may alter their competitive success. In the case of high biomass blooms, if rates or magnitude of bloom formation increase significantly there is a potential to spatially compress the zones of impact, leading to greater hypoxia or other environmental consequences.
Are our current methods adequate for gaining consensus about how OA may affect HAB toxicity? First, there is little definitive knowledge of the role toxins have in cell physiology or ecological interactions, so there are no clear, testable hypotheses for why OA might affect cell toxicity. Second, culture conditions vary among studies (light, temperature, nutrient addition, the basal seawater used for media amendments). Given current uncertainty over the metabolic controls of toxin production, it will be critical to seek uniformity in experimental methods among studies, and this is particularly true in the methodology of pH alteration and maintenance (bubbling, acid/base additions). A best practices manual for ocean acidification studies exists (Riebesell et al., 2010) and should be expanded to serve as a benchmark for HAB investigations. Without unifying methods, confusion will persist over whether disparate results among studies stem from true variability among organisms, or from methodological quirks. The third, and perhaps main challenge ahead is defining how best to go about isolating and evaluating the integrative effects of environmental and biochemical processes altered by OA on HAB species success and toxicity.
2.4.4. Summary of ocean acidification effects on harmful algal blooms
Progressively increasing OA has the potential to alter many aspects of phytoplankton physiology. The two proximal effects of OA on cell physiology will result from increased pCO2 (reducing the value and costs for CCM) and potentially altered transmembrane proton gradients (with impacts on ion transport and ion-channel activities). More distal effects include altered micronutrient chemistries with likely impacts on their bioavailability. Both will exert selective pressure on phytoplankton community composition, but how these might alter the competitive success of HAB species is unknown. More than any other climate-associated stress factor discussed here, small methodological differences among OA studies have the potential to alter experimental findings dramatically, so it is critical that well-defined and uniform methods be adopted.
2.5. Nutrients and harmful algal blooms
Species causing HAB events are primarily photoautrotrophs with generally simple macronutrient needs. The majority of high-biomass harmful algal blooms, in many cases resulting in hypoxia, are linked unequivocally to cultural eutrophication (Paerl et al., 2014; Rabalais et al., 2010), but as for “natural” blooms, there is little understanding of the proximate reasons for species selection within these phytoplankton blooms. The most common approach to study this core ecological principle is to quantify the presence or flux of dissolved forms of nitrogen, phosphorus, and silicon, or trace nutrients such as iron and vitamins, and to then seek correlations with the competitive outcomes among species (e.g., nutrient preferences, nutrient affinities). The most common metrics for measuring HAB species success are biomass accumulation (i.e., assimilation of a larger portion of the nutrient pool), or changes in the levels of ecologically- or socially-important toxins (e.g., allelochemicals, food chain toxins). The challenge is to stretch this thin knowledge across the projected alterations in nutrient fluxes to the photic zone through warming-induced decreases in vertical mixing, changes in upwelling intensities, and change in the spatial and temporal patterns of freshwater inputs.
2.5.1. What is known about nutrient effects on HAB species?
There is little experimental or theoretical information to indicate that changes in macronutrient supply and form will lead directly to a switch towards HAB species and bloom events in most marine environments, in contrast to the impact of increased phosphorus and nitrogen inputs to brackish and freshwater environments (e.g., Thornton et al., 2013). Futurist visions of climate-driven increases in harmful algal blooms often emphasize “Global Change” issues, which include population-driven anthropogenic alterations of the earth system, and there is little dispute that future population increases are likely to alter regional nitrogen or phosphorus cycles (e.g., Glibert et al., 2006). Here the emphasis instead is to describe how climate change pressures may affect the prevalence of harmful algal blooms by alteration of surface water nutrient fields through physical or precipitation-related processes, without assuming added changes due to localized human pressures.
While there remain significant uncertainties in global climate models, projections for the climate system suggest that there will be net increases in rainfall over the next century but that these changes will not be uniform among regions, with southern Europe, the Middle East, and southwestern USA experiencing decreases in precipitation while increases are projected for Southeast Asia, tropical East Africa and high northern latitudes (Boberg et al., 2010; Gutowski et al., 2007; Sun et al., 2007). Moreover, there are expectations for a shift to more intense precipitation events (Seneviratne et al., 2012), increasing the episodic flux of macro- and micro-nutrients into coastal waters.
These “great floods” may serve to supply coastal plankton communities in a massive, and temporally restricted manner—as a horizontal “upwelling” event. When presented to possibly warmer, predictably more stable surface waters, the runoff provides a nutrient source to increase existing biomass or to possibly modify community structure through enhanced competitiveness of certain species. The end result of a more nutrient-rich, warm, stable regime could be the selection of certain HAB species (Smayda, 1998). This type of precipitation pulse, when combined with possibly increased rates of in situ nutrient recycling (Doney et al., 2012), may result in the elevated growth of HAB species that are not held `in check' by grazing and do not rely on recycled nutrients (Sunda and Shertzer, 2012). Thus, there would be a tendency for “late-bloom species”, such as ecosystem destructive algal blooms (EDAB), to dominate more frequently providing that the stable temperature/salinity environment is maintained.
With increased frequency of climate-change precipitation events, higher runoff and unused (excess) fertilizer inputs can be anticipated, leading to the overall enhancement of phytoplankton growth and biomass accumulation. Depending on the fertilizer type and method of application, this input can be highly concentrated in ammonium, urea, or nitrate, and each may have differential effects on phytoplankton and HAB community composition. Much attention has been directed to the increased use of urea fertilizers and the correlation with regions of increased cyanobacteria and dinoflagellate HAB events of the last few decades (Glibert et al., 2006). But urea has been shown to support the growth of both HAB and non-HAB species, and it has not been proven whether urea selects for HAB species, although one might expect dinoflagellates to dominate given their higher rates of urease activity per cell compared to other phytoplankton classes grown in culture (Glibert et al., 2008). A review of HAB species associated with upwelling regions by Kudela et al. (2010) summarizes the inexact relationship between the ecophysiology of cells, their toxin content, and the comparative nutrient supply. Under present nutrient regimes and ratios, cellular toxin levels are genus specific, and thus no global statement is applicable. The effect of N substrate (oxidized vs. reduced forms) on the toxicity of HAB species is complex, illustrated by the many laboratory studies of dinoflagellates. Here, exponential growth on nitrate generates greater overall cellular toxin content (=cell toxin quota) compared to growth on ammonium or urea for Alexandrium fundyense, whereas during N-depleted stationary growth, nitrate-grown cells are slightly less toxic than ammonium-grown cells, but more toxic than cells grown on urea (Dyhrman and Anderson, 2003). Another strain of Alexandrium fundyense, however, showed increased cellular toxicity on ammonium-grown cells compared to nitrate-grown cells during exponential phase, but similar or less during stationary phase depending on the degree of phosphorus stress (John and Flynn, 2000). Similarly enhanced toxicity for ammonium-grown cells compared to nitrate-grown cells has been reported during exponential growth for A. excavatum (Levasseur et al., 1995), during batch growth of Gymnodinium catenatum (Flynn et al., 1996), and for Alexandrium tamarense where ammonium induced the highest cellular toxin concentrations, followed by urea, and then nitrate (Leong et al., 2004). Increased intracellular toxin in ammonium-grown stationary cells compared to nitrate-grown cells of Japanese strains of Alexandrium tamarense is also suggested by Hamasaki et al. (2001), but direct experimental comparisons using the two N substrates were not conducted. The opposite result was found for Alexandrium catenella where growth on nitrate resulted in 5–6 fold increases in cellular toxin concentration compared to growth on urea during both the N-replete exponential and N-depleted stationary phases of growth (Dyhrman and Anderson, 2003), and for nitrate-grown cells of Karenia brevis (formerly G. breve), toxin content per cell was only marginally influenced by urea enrichment (Shimizu et al., 1995).
The influence of N substrate on cellular toxicity of diatoms of the genus Pseudo-nitzschia is also highly variable but unlike most dinoflagellates, the cellular toxin (domoic acid) concentration is generally much greater during the stationary phase of growth, rather than the nutrient-replete exponential growth phase. This generalization is based primarily on culture studies of Pseudo-nitzschia multiseries and Pseudo-nitzschia australis, where stationary growth is induced by P or Si depletion (cf. reviews by Bates and Trainer, 2006; Lelong et al., 2012; Trainer et al., 2012), rather than N limitation where enhancement of cellular toxicity is not observed during stationary growth (Auro and Cochlan, 2013). Absolute N concentrations, light availability, and cellular growth phase have all been shown to affect the differential toxicity of Pseudo-nitzschia species as a function of N substrate (Auro and Cochlan, 2013, and references therein); these and other abiotic factors associated with changing climate need to be considered when evaluating the influence of N substrate on the cellular toxicity of toxigenic diatoms. Based on current evidence, neither specific growth rate nor cellular domoic acid concentration can be predicted based on the N substrate supporting growth as both vary among species and strains of Pseudo-nitzschia (Auro and Cochlan, 2013; Thessen et al., 2009, and references therein). Arguments to suggest that a specific N source will result in greater growth or toxicity of HAB species in general (or even just toxic diatom blooms), and thus more frequent, or more toxic HAB events, are not supported by empirical data from culture studies.
The projected increase in the flux of dissolved organic matter (DOM), or brownification of coastal waters (Hansson et al., 2013; Monteith et al., 2007) could affect the nutrition of marine plankton communities. The role of elevated DOM containing dissolved organic nitrogen and phosphorus is not well studied, and the trace (nutrient) metal loading and complexation capacity of DOM (Laglera and Berg, 2009) also may facilitate the growth or toxicity of HAB species (see in Loureiro et al., 2011).
Climate-related changes in the intensity of storm events, the frequency of hurricanes, or great floods may break down the natural biogeographical barriers that restrict the expansion of HAB species. Any new, higher frequency exchange of waters due to storms may enhance the transport of invasive harmful algal blooms to areas presently outside their range. Even without the elevated flux of new nutrients, variations in storm events increase the likelihood of invasive, exploitive HAB species. As documented a decade ago by Anderson et al. (2002), there is more advanced understanding regarding the extent of climate-induced supply of nutrients than on the selective pressures that these changes have on the HAB community structure. The importance of nutrient supply-changes is, without question, critically important in community development, yet the complexity of response of individual species and their subsequent competitive or adversarial activities (allelopathy, mixotrophy) remains elusive.
A well-stratified surface water provides a common environment of enhanced nutrient regeneration, and comparative studies generally show that these conditions favor flagellate dominance (Smayda, 1998). Flagellates can flourish under long periods of low nutrient concentrations where reduced forms of nitrogen (e.g., ammonium) comprise a large proportion of the available N pool [e.g., Alexandrium spp. (Maguer et al., 2007); Prorocentrum minimum (Fan et al., 2003)]. This does not mean that all flagellates prefer reduced nitrogen forms—only that reduced forms tend to dominate when ambient N concentrations are low (Bonachela et al., 2011). Indeed some HAB species appear to favor greater ambient (i.e., non-reduced) nutrient conditions, due perhaps to higher physiological quota requirements; e.g., Alexandrium spp. (Collos et al., 2006), Heterosigma akashiwo (Herndon and Cochlan, 2007), Noctiluca spp. (Harrison et al., 2011), Prorocentrum spp. (Anderson et al., 2012); or perhaps it is a consequence of the higher nutrients establishing more robust cyst seed beds. For example, the dramatic drawdown of nutrients that occurs during high-density blooms leads to nutrient-depletion, followed by encystment of Alexandrium spp., creating a seedbed density sufficient for the reintroduction of the HAB population in subsequent years (Anderson et al., 2014).
The relative supply rates of nitrogen and phosphorus to surface waters can significantly impact the physiology and dynamics of the phytoplankton community, as well as the community composition (Geider and La Roche, 2002; Sterner et al., 2008). But this ratio is of most use in interpreting succession and resultant community composition only when either the ambient nitrogen or phosphorus concentrations are low. When there is an abundance of both, or when nutrients are not limiting phytoplankton growth, there is no compelling evidence that N:P ratios provide a predictive value for the floristic composition of the phytoplankton community and the likelihood of HAB development (Davidson et al., 2012, and references therein).
2.5.2. What are important “known unknowns” about nutrients and harmful algal blooms?
As a driving force, nutrients control the production and influence the composition of phytoplankton communities. Nutrient concentrations, ratios, and speciation are known to directly affect the community and metabolites produced, such as toxins and allelopathic compounds, even though the specifics of these interactions remain elusive. Perhaps the biggest unknowns though are the extent and scale of change in nutrient influx via runoff or altered upwelling intensities that these communities will experience, balanced against other global factors such as eutrophication, demophoric (population × energy consumption) growth and land use. Ecological shifts due to climate-induced changes in nutrient fluxes or nutrient use might already be emerging, but they may not be evident or significant in the milieu of other coastal ocean processes.
There are large knowledge gaps in how nutrient quality and quantity effect changes in phytoplankton community physiology and ecology to select for HAB species. For example, how do the nutrient-use efficiencies of HAB species change relative to non-HAB species when presented with lower pH, increased temperature or salinity. This baseline understanding, related primarily through nutrient uptake kinetic studies, is essential to provide a theoretical and quantitative foundation to evaluate the potential role of nutrients under future climate conditions. While N tends to be the primary nutrient of interest (in its oxidized and reduced forms), equal efforts will have to be devoted to assessing under what conditions will shifting nutrient ratios contribute to shaping phytoplankton communities in the future ocean.
Paramount to forecasting potential nutrient effects in the future ocean is the need for clear insight to the range of conditions that will be faced. What changes in regional supply patterns of reduced or oxidized forms of N and other nutrients are anticipated (e.g., Hutchins et al., 2009) and will they vary in concert or disproportionately, resulting in altered nutrient ratios? It is ambitious to obtain these quantitative insights from current models, but consideration of the possible ranges would help to set the boundaries for experimental studies that then could be used to inform improved model development.
In almost all cases, “nutrient effects” on harmful algal blooms pertains to macronutrients such as N, P, or Si, yet there is almost no understanding of micronutrient effects on harmful algal blooms: i.e., trace elements and vitamins. Micronutrients have strong potential effects on phytoplankton community composition (Brand et al., 1983; Sunda, 1989), given their role in photosynthesis, enzymatic and other functions throughout cell metabolism. But little is known about micronutrient requirements (and tolerances in the case of metals) of HAB species compared to non-HAB species, or whether these differences are likely to shape phytoplankton communities. Given that experimental cultures traditionally have metal conditions radically different than natural conditions, most current data are inadequate to address this question. Moreover, the chemistry of trace metals and the HAB specific availabilities and quotients remain poorly understood, and thus it will be difficult to predict climate-driven changes in micronutrient supply may affect the prevalence of harmful algal blooms without concerted research in this direction.
2.5.3. Pressing questions about nutrients and harmful algal blooms?
How do nutrient uptake kinetics and preferences of HAB species compare quantitatively to non-HAB species? These critical data are missing for many HAB species, and in particular their relation to non-HAB species, and thus models lack appropriate parameterization. It is essential to know if HAB species in general, or certain specific HAB species, will thrive by outcompeting other species in the future ocean chemical matrix. Current foundation knowledge regarding the role of nutrients in establishing the competitive selection of HAB species needs to be challenged. Nutrient kinetic and nutrient use efficiency studies have been generated under controlled laboratory conditions using cells in seawater with a relative stable chemical matrix. Given the anticipated changes in P, iron, and other micronutrient inputs to surface waters, these nutrient competition studies must be revisited. Embedded within this research must be efforts to normalize experimental methods enough to enable better assessment of nutrient effects on cell toxicity. Similarly, HAB responses in hypereutrophic environments, where nutrients are available in considerable excess, need to be revisited because existing models employing rectangular hyperbolic (e.g., Michaelis–Menten) approaches to parameterize phytoplankton growth or uptake relative to external nutrient concentration are inadequate for assessing competitive success. New theoretical and experimental approaches need to be considered.
How can linkages be improved between observed nutrient fields, nutrient acquisition kinetics by HAB and non-HAB species, and physical, ecological, and climate change models to increase competence in forecasting harmful algal blooms in present day and future oceans? More stabilized surface waters, extended seasons, and intense storms all are predicted for the future ocean – factors that will influence the extent and timing of nutrient inputs into the photic zone. There are few biological models of individual HAB species that couple alterations in seasonal and pulsed nutrient supply to physical models for HAB forecasts under present day or future ocean scenarios. Moving forward it will be important that these modeling activities develop along with modified understanding of changes in cell physiology, species competition, and the physical environment of the HAB and non-HAB species.
How do changes in micronutrient and vitamin supply to phytoplankton communities affect success of HAB species relative to the broader phytoplankton community, and what effect may fluctuations in this supply affect cell toxicity? While it is known from culture studies that trace elements and vitamins (micronutrients) are critical in harmful algal blooms, there is uncertainty with regards to the natural supply levels of these trace nutrients. The chemistry and availability of trace metals remain poorly understood and characterized for HAB species and habitats, and thus it will be difficult to predict their effects on specific harmful algal blooms as a function of climate change.
2.5.4. Summary of nutrients and harmful algal blooms
Current understanding is insufficient to determine how absolute concentrations and speciation of nutrients challenge the metabolic “skills” of phytoplankton, so there is limited ability to predict how changing nutrient fields result in “winners” and “losers” within natural phytoplankton assemblages. Reduced N species, particularly from anthropogenic sources, often appear associated with some HAB and HAB species, yet non-HAB species also flourish in culture with these N sources, so the issues are more complicated. Climate change will affect nutrient flux to surface waters, through alteration in vertical mixing and runoff, and the challenge will be to incorporate quantitative measures of nutrient acquisition among HAB and non-HAB species, along with the effects of strain variability, to forecast likely outcomes. It will be particularly difficult to differentiate between climate change pressures and those stemming from societal changes (population/social/economic) in many regions.
2.6. Grazing effects on harmful algal blooms
Most efforts to understand and forecast harmful algal blooms have focused on bottom up controls of species success and biomass development. The ecological consequences of grazing pressures on bloom characteristics either are not effective, in the case of high-biomass blooms, or are not well understood in the case of toxic blooms. Indeed among the factors that govern harmful algal blooms, grazing arguably is the most difficult because it adds not only the complexity of top down effects upon the bottom-up controls introduced by temperature, stratification, acidification, nutrients, and other environmental stressors, but also these effects upon the grazer activity.
2.6.1. What is known about grazing effects on harmful algal blooms?
Five different tiers of grazers predate on HAB species, each characterized by unique modes of predation, prey preference, toxin effects, and ecologies. Arrayed along a template of increasing size, the primary grazers on HAB species are: microbial pathogens, micro-zooplankton, copepods and other mesozooplankton components, benthic invertebrates, and fish. Microbial pathogens include algicidal bacteria, infectious viruses, and parasites (Coats and Park, 2002; Salomon and Umai, 2006). The microzooplankton, known also as the protozooplankton (Smetacek, 1981), include heterotrophic dinoflagellates and ciliates < 200 μm in size, tintinnids and aloricate ciliates (Stoecker and Evans, 1985; Turner, 2006). Copepods and cladocerans are the major mesozooplankton (>200 μm) grazers (Turner, 2006), with copepods being particularly voracious, having prey clearance rates that exceed filter-feeding rates of cladocerans and meroplankton larvae by 10-fold (Hansen et al., 1997). Commercially important shellfish are prominent filter feeders on HAB species among the diverse benthic invertebrate grazers, and are important vectors of shellfish borne toxins that impact human health (Shumway, 1990). The larval and adult stages of many other invertebrate species are also copious feeders on phytoplankton. Farmed fish stocks, particularly salmonid species, are vulnerable to ichthyotoxic blooms (see Rensel et al., 2010), and the larval and adult stages of many species are sensitive to dinoflagellate toxins ingested directly or in other prey-vectoring toxins acquired through food web transfer (Bruslé, 1995; Smayda, 1992; White et al., 1989). But the first-feeding or adult stages of many naturally-occurring and commercially important fish species selectively graze dinoflagellate species (Lasker and Zweifel, 1978; Last, 1980; Samson et al., 2008), whose caloric value generally is twice that of diatoms of equivalent biovolume (Hitchcock, 1981). Some fish species have evolved spawning migrations to coincide with blooms on which their first-feeding stages consume (Gosselin et al., 1989). There is spawn cueing behavior, i.e., synchrony between invertebrate spawning and increases in phytoplankton biomass on which larvae graze (Fernandez et al., 2012; Himmelman, 1975; Starr et al., 1990). Trophic linkages between harmful algal blooms and predatory food web components are reviewed in Smayda (1992). All life cycle stages and bloom phases of HAB species are subject to multiple and changing grazer attacks. For example, Jeong et al. (2013) listed 33 species of heterotrophic dinoflagellates, microzooplankton, mesozooplankton and invertebrate larvae that graze on ichthyotoxic Heterosigma akashiwo, a predator–prey association they termed a “grazing hub”; a concept that also can be applied to some dinoflagellates (Jeong et al., 2010).
The prevailing conceptual view is that HAB species are grazed, their blooms develop when the population growth rate exceeds grazing rate, and phycotoxins allelochemically protect against grazers. But the ultimate predator–prey interactions occur at the organismal level, and the experimental results, primarily with mesozooplankton (copepod) species, indicate that those concepts are conditional. The physiological and ecological effects on copepods that graze on toxic species, and their growth and bloom responses vary greatly. Copepod species generally feed on a variety of HAB species, selecting prey based on their cell size and toxicity, with the latter selection mode indicative of highly sensitive and specific chemoreceptive abilities (Teegarden and Cembella, 1996). Ingestion of toxic prey can adversely affect predator grazing rate, fecundity, egg hatching success, growth, survival, and incapacitate or cause death of the grazer (Fiedler, 1982; Huntley et al., 1986; Koski et al., 1999, 2002; Schultz and Kjørboe, 2009; Smayda, 1992; Teegarden, 1999). Adverse effects can be reversed when the grazer is exposed to different diets, or the HAB species to different grazers (Koski et al., 2002; Lincoln et al., 2001; Teegarden et al., 2001). Some copepod species actively graze, grow and reproduce on HAB prey without deleterious effects; conversely, they also reject nontoxic species, and a species toxic to or avoided by one species is grazed by another species (Smayda, 1992; Turner, 2006; Uye and Takamatsu, 1990). The availability of alternative prey influences grazing rates on HAB species, with the intensity of grazing a function of the inherent toxicity of each prey species, its strain, and the magnitude of toxicity induced by nutrient-limitation. The presence of a toxic species can reduce feeding rates on all species (Schultz and Kjørboe, 2009). There is the potential that all of these interactions may shift if climate change pressures lead to changes in the toxicity or nutritional quality of the HAB prey (e.g., Fu et al., 2010; Leu et al., 2013).
The impacts of zooplankton community grazing on a HAB likewise vary. Grazing may retard initial development, terminate harmful algal blooms, or be too sluggish to prevent a bloom from developing (Schultz and Kjørboe, 2009). Trophic breakdowns in community grazing may sustain a bloom for years, as in a Texas brown tide (Buskey et al., 2001). The impact of grazing on HAB species phenology and their blooms is likely a function of the historical exposure of the predator–prey pairings, with both predator and prey species capable of counter-measure capture and avoidance adaptations, respectively. Some copepods routinely exposed to a toxic species and its specific toxin become less vulnerable physiologically to that toxin/HAB species than other meta-populations of the same copepod species not naturally exposed to that species/toxin (Colin and Dam, 2002). There is experimental evidence that waterborne cues emanating from copepods induce prey to develop morphological defense protectants (Jiang et al., 2010), or to synthesize chemical grazing deterrents, re-directing grazing pressure towards competing non-toxic species (Bergkvist et al., 2008; Guissande et al., 2002). While lethal effects of prey on grazers can be dramatic, this vulnerability is not equally distributed among grazer groups. Microzooplankton are considered the dominant grazers on HAB species, consuming 60 to 70% of the biomass (Turner, 2006), but most toxic prey within the microzooplankton are not known to kill their protistan grazers, relying, instead, on sub-lethal chemical defenses that impair grazer activity, or chemically signal noxiousness (Strom, 2002). Lethal effects of HAB species against mesozooplankton, invertebrate larvae, and larval fish appear to be more common.
2.6.2. What are important “known unknowns” about grazing effects on harmful algal blooms?
Extrapolation of experimental grazing data to in situ behavior is problematic. For many studies, it is difficult to determine whether the responses are ecologically representative because of the methodological use of high prey population densities, un-natural predator–prey pairings, strain differences, and experimental presentation of a single prey species rather than a mixed-diet. Trainer et al. (2002) found domoic acid concentrations were higher during natural blooms than expected from cellular production rates in laboratory experiments. Extrapolation of bivalve data to assess potential climate change effects on shellfish-borne phycotoxin uptake and transmission is likewise problematic.
Detailed understanding of saxitoxin kinetics in bivalves is largely restricted to a few commercially important species that are relatively resistant to toxins, e.g. the mussel Mytilus edulis and Atlantic surf clam Spisula solidissima (Bricelj and Shumway, 1998). Under-study of PSP and other shellfish-borne toxin kinetics greatly hampers the current understanding because bivalves show up to 100-fold interspecific differences in their accumulation of PSP toxins. In general, bivalves are avid suspension feeders (Jørgensen, 1966). Species with nerves less sensitive to saxitoxins (e.g. M. edulis, Bricelj et al., 2005; sea scallop Placopecten magellanicus, Cembella et al., 1994) readily feed on toxic cells and accumulate high toxin levels, whereas bivalves more sensitive to PSP toxins (e.g. oyster Crassostrea virginica) attain relatively low toxicities, although factors other than toxin sensitivity also influence bivalve toxicity (Bricelj and Shumway, 1998).
The HAB-grazing relationship and outcomes are not fixed, linear or invariant in any given predator–prey coupling. A more important impediment in forecasting climate-induced predator–prey alterations is that grazing reciprocally affects both prey and predator. That is, each element of the predator:prey interaction is both an ecological driver and a responder, unlike the unidirectional, non-reciprocal affects that changing temperature or pH have on HAB species growth rate, range extension and bloom phenology. The altered distribution, abundance and phenology of both grazers and their prey in response to climate change may intensify or diminish the prior predator–prey association, with the response dependent on biogeographical location, trophic structure and strength of benthic (i.e., filter feeding bivalve)—pelagic coupling. Adding further complexity, the influence of climate change likely also will influence the temporal convergence of “bottom-up” (i.e., nutrient regulated growth) and “top-down” grazing control during bloom and succession cycles.
Similarly, mixotrophy is a critical feature of many HAB species, particularly those that inhabit an ecological niche near the end of a bloom sequence. While generally relegated to secondary processes in productive waters, alteration in the balance of carbon fixation and growth in the future ocean may elevate the role of mixotrophy in ocean surface waters. Thus, mixotrophic HAB species may have a significant ecological space opened for their activities depending on whether future ocean conditions alter present patterns of balanced/unbalanced cell growth.
2.6.3. What are the pressing questions regarding grazing effects on harmful algal blooms?
What is the best approach to study grazing effects on the success or demise of harmful algal blooms? Forecasting climate-induced changes in the coupled grazing-HAB relationship differs fundamentally, and is considerably less tractable, than for other climate-linked impacts. Grazing is a process of transient seasonal, regional, and trophic predator–prey interactions, each governed by its own range of tolerance and vulnerability to the operative climate change variable, further modified by the particular prey and predator species pairings. Altered grazing pressures will affect both HAB and non-HAB species, so it is unlikely that climate-altered grazing effects will be singular, or easily tractable. Temperature effects will act as a community and trophic pacemaker, regulating grazer and prey metabolic rates, life history transitions, and community development. Acidification will pose major physiological threats in some species, e.g. through decreased skeletal calcification of larvae that cause secondary impacts on grazing potential (Byrne, 2011). The challenge will be to disentangle these intricate couplings, perhaps starting with prominent grazer-prey couplings, to ascertain net effects on HAB species from these climate-altered balances in predator–prey interactions.
Will the effects of climate change in some cases decouple existing grazer and prey life histories sufficiently to fundamentally alter their predator:prey relationships, or to create opportunities for new grazer coupling? There exist primary unknowns about the optimal ranges of environmental conditions for grazer life histories. For example, will range extension of grazer populations depend more upon their environmental requirements or that of their prey, so that potential ranges extensions of current predator–prey interactions will become decoupled. If so, conditions may be moving into novel and unpredictable future states in some coastal regions. Will metabolic shifts in prey species disproportionately affect their food “quality”, changing grazing selective pressures on either HAB or non-HAB species and the resultant trajectories of planktonic communities? In shallow coastal regions, how will the temperature dependency of collective benthic feeding rates affect seasonal feeding windows differently than HAB growth in overlying waters, and what effect will this have on HAB and non-HAB abundance? Longer warm seasonal windows will increase the relative impacts of gelatinous zooplankton with possible changes in the relative grazing pressure on HAB vs. non-HAB species.
How will grazer behavior and sensing capabilities be affected by OA, and will this alter grazing pressure? Given that chemical cues can enhance or diminish grazer:prey interactions, it is important to recognize the potentially unique effect of pH on grazing pressures. Recent evidence shows that OA depresses the sensory detection of chemical cues in fish (e.g., Lonnstedt et al., 2013), so there may be precedent for similar effects in planktonic and benthic grazers. The potential effects of OA on grazing of HAB and non-HAB species, as well as the relative degree of toxin accumulation in bivalves, remains unknown.
2.6.4. Summary of grazing effects on harmful algal blooms
Projecting how climate change may affect HAB prevalence by altering grazing pressure, and carrying out the requisite ecological experiments to test these hypotheses, will be extraordinarily difficult. These experiments and field investigations must deal with the enormous functional and ecological diversity of the grazers (mixotrophy through pelagic and benthic heterotrophs), the diversity of phycotoxins, and their differing inimical effects. No single predator–prey relationship describes the great diversity of prey ecology and predator feeding strategies found, so it is unlikely that any uniform, generally applicable predator–prey interaction model can be expected. Instead the focus will need to be on the interactions of specific grazer-prey couples – e.g., how prey characteristics influence grazing efficiencies, and how these in turn affect net growth – with hope that some general patterns of response emerge so that more adequate representation of grazing pressure can be incorporated into ecological and climate change models.
3. Multiple stressor effects on harmful algal blooms
An overwhelming proportion of laboratory studies on environmental effects on HAB organisms are single stressor or single factor experiments, yet it can be expected that parameter interactions will yield unpredicted outcomes and mechanisms driving harmful algal blooms (“unknown unknowns”). There is a very short list of known interactions, and in some cases they generate unexpected results. For example, unlike the diatoms tested, nutrient stress does not increase the sensitivity of the Baltic HAB cyanobacteria Nodularia spumigena to high ambient UVR (Mohlin et al., 2012; Mohlin and Wulff, 2009; Pattanaik et al., 2010), meaning the multistressor conditions do not necessarily lead to increasingly negative effects. Another example is the combined effects of stratification and nutrient limitation that intuitively should enhance the potential for toxic HAB formation (Raine, 2014) but may also prevent HAB formation (McGillicuddy et al., 2011). On the other hand, the interaction between stratification and grazing may lead to more concentrated blooms of Heterosigma akashiwo (Strom et al., 2013). In the broader context, the multiple stressor effects on non-HAB species may be highly relevant as well in that they affect the relative competitiveness and opportunities for HAB species to flourish or perhaps just survive better, which is all that is needed for increased HAB impacts of some toxic species. Far greater efforts are needed to assess interactive parameter outcomes using laboratory, mesocosm and field studies. Although quantitative predictive models for harmful algal blooms are in their infancy (Anderson et al., 2013), there is much that can be learned from these multifactorial data by using quantitative models and sensitivity analysis to evaluate our understanding of multiple stressor effects. Findings from multifactorial experiments at all scales should be incorporated step-wise into global models to evaluate whether ecologically realistic outcomes are generated, and to guide experimentalists to better refine their studies, evaluate other HAB strains, or to help identify key observer sites for validation of apparent knowledge advancement.
4. Local human-introduced pressures and global change
Climate related changes in the intensity of storm events, the frequency of hurricanes, or great floods may break down the natural biogeographical barriers that restrict the expansion of HAB species, but it is important to also consider societal-derived transport of invasive harmful algal blooms to areas presently outside their range. The human transfer of HAB and grazer species to new regions via ballast water, fouling of vessels, and aquaculture is well documented (e.g., Hallegraeff, 2007; Ruiz et al., 1997). This often cryptic seeding and spreading mechanism may lead to false positive signals of climate change effects on phytoplankton community composition, and the changing prevalence of harmful algal blooms. Similarly, global change effects stemming from increased future populations will lead to greater cultural eutrophication pressures in some coastal regions, a factor well recognized to affect HAB conditions (GEOHAB, 2006) as well as certain HAB species and predators (Smayda, 2007, 2008). These human-introduced “point source” effects will vary greatly among coastal regions according to local social and economic conditions, and may obscure underlying shifts in harmful algal blooms driven by climate change. The challenge will be to develop suitable metrics for disentangling the ecological outcomes from climate and global change pressures in those regions.
5. The critical need for HAB observer sites
Although culture and mesocosm studies can serve as foundations for hypothesis testing of mechanistic linkages between climate change stressors and the competitive success of HAB organisms, structured field observations will be essential not only to inform on shifting HAB responses within natural ecosystems, but also to reveal unexpected patterns that transcend our knowledge. Currently, very few time series of HAB data describing annual phytoplankton cycles are available in the literature. This trend needs to change if researchers are to have the information needed to proactively assess the potential changes in HAB impacts, rather than simply hindcasting the mechanisms underlying emerged patterns of new HAB eruptions. National HAB monitoring data are a useful starting point, but these datasets most often lack the requisite physical, chemical and biological data needed to critically test hypotheses explaining the observed changes in seafood safety. Automation of HAB sensing is an emerging science (Campbell et al., 2010; Preston et al., 2011) and the continuing development towards user friendly systems will greatly accelerate this critical data acquisition. Trend detection of harmful algal blooms in marine and freshwater systems could be greatly assisted by development of simplified tools for societal-based sampling [e.g., microscope ranking of relative abundance and simplified optical measurements of chlorophyll (Hydrocolor, iTunes Store)]. As was the case for climate change, greater accumulation of data across broad regions and environments will be critical for establishing long-term HAB trends.
Moving forward it is important that existing datasets on HAB occurrence and absence be integrated to the extent possible with environmental data, and this base then needs to be expanded to create a global-scale program of observations to facilitate investigations of HAB-climate interactions. To this end, it is proposed that a suite of Long Term Observer sites be chosen that combine climate, oceanography and HAB-specific observations to provide the essential test beds for these evaluations. Suggestions of potential sites for consideration are shown in Table 1, which is not intended to be comprehensive. This list of reference sites encompass key ecosystem types, and includes biogeographical transition zones (e.g. ~40–50°N), high latitude (Greenland, Russia, Canada, USA), and polar environments (Palmer LTR). These sites all represent existing or near-existing monitoring programs, so facilitating their use as HAB sentinels could be accomplished by only expanding their currently monitored parameters. Grouping data from these sentinel sites would enable for the first time a comprehensive review of the region-to-region patterns that necessarily underlie any critical assessment of global changes in harmful algal blooms.
Table 1.
General ecosystem types for climate and HAB studies: example reference sites and selected publications describing analyses of long-term data sets. See also GEOHAB references on HABs in upwelling systems (GEOHAB, 2005), fjords and embayments (GEOHAB, 2010), eutrophic systems (GEOHAB, 2006), stratified systems (GEOHAB, 2013) and benthic systems (GEOHAB, 2012).
| Ecosystem types | Example study areas | Example HAB genus | Selected references |
|---|---|---|---|
| Open coast | East China Sea | Cochlodinium | (Matsuoka et al., 2010) |
| East China Sea and Yellow Sea | Trichodesmium | (Tang et al., 2006) | |
| Prorocentrum | |||
| Arabian Sea | Dinophysis | (Singh et al., 2014) | |
| French coast of the English channel, Southern North Sea, Australian east coast | Pseudo-nitzschia | (Hernandez-Farinas et al., 2013; Singh et al., 2014) | |
| Pseudo-nitzschia | (Ajani et al., 2014) | ||
| Noctiluca | (Hallegraeff et al., 2012b) | ||
| Gambierdiscus | |||
| Coastal marine systems (semi-enclosed) | Gulf of Maine | Alexandrium | (McGillicuddy et al., 2005) |
| Monterey Bay | Pseudo-nitzschia | (Jessup et al., 2009; Jester et al., 2009; Kudela et al., 2008) | |
| Cochlodinium | |||
| Akashiwo sanguinea | |||
| Todos Santos Bay | Pseudo-nitzschia | (García-Mendoza et al., 2009) | |
| Paracas Bay | Akashiwo sanguinea | (Kahru et al., 2004) | |
| Peter the Great Bay | Amphidinium | (Selina et al., 2014) | |
| Ostreopsis | |||
| Prorocentrum | |||
| Kattegat-Skagerrak | Dinophysis, Prymnesium, Chrysochromulina, Pseudochattonella, Karenia | (Håkansson, 2002) | |
| Bohai Sea | Ceratium, Heterosigma, Mesodinium, Prorocentrum | (Wu et al., 2013) | |
| Seto Inland Sea | Alexandrium, Chattonella, Heterosigma, Eucampia | (Itakura et al., 2002; Nishikawa et al., 2011, 2014, 2009) | |
| Buzzards Bay | Cochlodinium, Alexandrium, Pseudo-nitzschia | (Turner et al., 2009) | |
| Florida coast | Karenia | (Brand and Compton, 2007) | |
| Korean coast | Cochlodinium | (Lee et al., 2013) | |
| Bay of Fundy | Alexandrium | (Kaczmarska et al., 2007; Martin et al., 2009) | |
| Pseudo-nitzschia | |||
| W. Scottish waters | Pseudo-nitzschia | (Fehling et al., 2006) | |
| Coastal marine system (enclosed) | Narragansett Bay | Heterosigma, Alexandrium, Cochlodinium, Fibrocapsa, Karlodinium, Pfiesteria, Prorocentrum, Pseudo-nitzschia | (Borkman et al., 2014, 2012; Li and Smayda, 2000; Smayda, 1998; Smayda and Borkman, 2008; Smayda et al., 2004) |
| Salish Sea | Alexandrium | (Moore et al., 2010; Trainer et al., 2007, 2003, 2013) | |
| Dinophysis | |||
| Pseudo-nitzschia | |||
| Manila Bay | Pyrodinium bahamense var. compressum | (Azanza and Taylor, 2001) | |
| Arabian Gulf | Chaetoceros, Karenia, Dinophysis, Gymnodinium, Gonyaulax, Noctiluca, Prorocentrum, Cochlodinium | (Al-Azri et al., 2012) | |
| Coastal brackish system (enclosed) | Baltic Sea | Cyanobacteria | (Suikkanen et al., 2007) |
| Alexandrium | |||
| Prymnesium, Chrysochromulina | |||
| Chesapeake Bay | Pseudo-nitzschia | (Anderson et al., 2010; Brownlee et al., 2005; Moncheva et al., 2001) | |
| P. minimum | |||
| Black Sea | Pseudo-nitzschia, Gymnodinium, Prorocentrum | (Moncheva et al., 2001) | |
| Fjords | Norwegian coastline | Dinophysis | (Naustvoll et al., 2012) |
| Gullmar fjord | Dinophysis | (Belgrano and Lindahl, 1999) | |
| Coastal upwelling system | Washington State | Pseudo-nitzschia | (Trainer et al., 2009; Trainer and Suddleson, 2005) |
| Benguela | Alexandrium, Dinophysis | (Pitcher and Calder, 2000) | |
| Iberian coast | Pseudo-nitzschia | (Pérez et al., 2010) | |
| Shallow estuarine systems | Thau Lagoon | Alexandrium | (Collos et al., 2009, 2014) |
| Nauset Estuary | Alexandrium, Dinophysis | (Ralston et al., 2014) | |
| Northport Harbor | Alexandrium, Dinophysis | (Hattenrath et al., 2010) | |
| (Hattenrath-Lehmann et al., 2013) | |||
| Freshwater systems | Great Lakes, reservoirs, lakes | Anabaena, Lyngbya, Aphanizomenon, Nodularia, Nostoc, Oscillatoria | (Lopez et al., 2008) |
| Microcystis | |||
| Lake Taihu | Cyanobacteria | (Chen et al., 2003) | |
| Microcystis | |||
| Benthic tropical | Reef systems, Mediterranean | Ostreopsis, Gambierdiscus | (GEOHAB, 2012) |
| Sea, St. Thomas, Virgin Islands | |||
| Caribbean Sea, West Indies | (Tester et al., 2010) | ||
| Open ocean | E1 Plymouth; Station Papa | Pseudo-nitzschia, Trichodesmium | (Edwards et al., 2013; Edwards and Johns, 2006; Kraberg et al., 2012; Widdicombe et al., 2010) |
| Northeast Atlantic | Dinophysis | (Edwards and Johns, 2006) | |
| Polar | Palmer Site | Pseudo-nitzschia | (Grigorov et al., 2014) |
It is to be expected that not all of these sites will be equally rich in data acquisition, and indeed even sites that regularly monitor a minor subset of parameters will be of high value. The observing network should comprise a combination of reference (climate sensitive) sites and a small subset of HAB Super Observer Sites (HAB SOS). In this case, reference sites would focus on collecting basic time series data while the HAB SOS sites instead would be geared toward the more intense data acquisition needed to facilitate process studies (Table 2). These Long Term Observer sites should correspond to satellite ground-truthing sites for the Global Coastal Ocean Observing System, thereby facilitating the future development of algorithms for remotely monitoring the HAB initiation and progression. All of these data should be made available over appropriate time frames through publicly accessible data centers. Together, these collective datasets should be coupled to downscaled physical ecosystem models to evaluate the drivers of HAB distributions.
Table 2.
A suggested list of parameters for routine measurement at HAB reference observer sites and super observer sites.
| Reference observer sites | Super observer sites |
|---|---|
| Single depth sampling in the photic zone | Full vertical resolution |
| 1. Physical parameters (T, S, chlorophyll, pH, light field (Secchi)) | 1. Physical parameters (T, S, chlorophyll a fluorescence, chlorophyll a, CO2, O2, pH, light field (radiometer)) |
| 2. Qualitative phytoplankton identification and enumeration (microscope) of HAB species (whole water bottle samples) | 2. Phytoplankton speciation–whole water, total abundance and biomass (e.g., light microscopy, Flow-CAM, electron microscopy, imaging flow cytometry, in-situ autonomous sensors, or molecular methods including metagenomic analyses for HAB and non-HAB species) |
| 3. Macronutrient (N, P, Si) concentrations | 3. Macronutrients concentrations of NO3, NH4, urea, P, Si |
| 4. HAB toxin concentrations or shellfish toxicity | 4. Zooplankton biomass and community composition (macro and microzooplankton) |
| 5. Toxin identification and quantification (shellfish if appropriate or whole water and solid phase adsorption toxin tracking bags) | |
| 6. Bio-optics for ocean color validation |
The goal for HAB Reference observer sites is the routine monitoring of HAB presence, character, and intensity in conjunction with time series data on a few or several oceanographic parameters. Even sites that can provide a consistent subset of these data will be highly valued. These combined long-term datasets should reveal any regional and intraregional correlations between environmental parameters and the spatial and temporal distribution of HABs. In contrast, a much more limited number of Super observer sites in the global HAB monitoring program would be targeted for more extensive measurements that would enable process-style assessments of the mechanisms underlying the patterns of change observed.
6. Linkage to other programs and the social sciences
There are many global, regional and national programs and initiatives that can provide substantial capabilities to better understand the linkages between climate change and HAB dynamics, distribution and the underlying causative mechanisms. These range from components of global observational systems to national resource monitoring programs. They provide a wide scope of capabilities that need to be assessed with regard to quantitative applicability, and fully capitalized upon through engagement and even optimization with regard to HAB observation and characterization.
6.1. Global observation systems: GEOSS and GOOS
The Global Earth Observation System of Systems (GEOSS) is the primary vehicle of the Group on Earth Observations (GEO), an international initiative promoting the use of integrated earth observations for sustainable development. The Global Ocean Observing System (GOOS) is considered to be the ocean implementation of GEOSS. The GEO Task SB-01 Oceans and Society: Blue Planet (Djavidnia et al., 2014) is the single largest initiative of relevance here and consists of components addressing global ocean information systems, forecasting networks, ecosystem monitoring, and fisheries/aquaculture management. HAB specific applications are a recognized task component (Bernard et al., 2014), namely
Integrated Earth observation-based systems can play a significant role in the detection, monitoring and analysis of harmful algal blooms in marine and freshwater ecosystems. The GEO Blue Planet HAB initiative seeks to consolidate and expand on existing capabilities, building a global community to develop and maximise the use and societal benefits of an integrated HAB observation and prediction system.
Freshwater and cyanobacterial blooms are considered in another GEO Task; WA-01-C4 Global Water Quality Products and Services. The GEO Work Plan also is seen as an implementation plan for programmatic funding in Europe and other countries, and so linkages with GEO also provide a means of policy integration and impact. The International Ocean Colour Co-ordinating Group (IOCCG) is of particular relevance to ongoing earth observation HAB capabilities, with outputs focusing on methods for phytoplankton functional type, harmful algal blooms and water quality from space. In addition the IOCCG provides a mechanism to provide space agencies with recommendations from the HAB community for optimising HAB applications for current and future sensors.
6.2. Regional and national programs
Regional or national initiatives can contribute to elucidating the link between climate and HAB phenomena in a number of ways. Operational programs such as NOAA CoastWatch, or the MyOcean-linked ASIMUTH, MarCoast and AquaMar projects can provide considerable regionally-optimized earth observation and historical in situ data. In addition, national water quality programs and monitoring frameworks provide policy impact vehicles and can potentially serve as sources of data for long time series of in situ data relevant to HAB geographical distribution. Examples of such programs include the European Water Framework Directive; the EPA and other agencies monitoring under the Clean Water Act in the USA; the Canadian Water Act; the South African National Eutrophication Monitoring Programme; and the Australian National Water Quality Management Strategy, among others.
6.3. Linkages to social science
“Harmful” algal bloom is a societal-based moniker that traditionally stems from the consequence of either their direct or indirect human health impacts, but these blooms also have socio-economic and cultural implications (e.g., Smayda, 1997; Trainer and Yoshida, 2014). There is emerging recognition of the complexities and synergies that exist among the fields of marine science, social science and economics (e.g., Moore et al., 2013), and that human well-being, the state characterized by health, happiness, and prosperity, should be the currency by which environmental perturbations are measured. Yet research on this broader perspective occurs largely independent of traditional HAB science, which focuses more on the ecological (e.g., species characterization, presence/absence), toxicity, or direct economic consequences of harmful algal blooms. The insular nature of HAB research needs to diminish if the field is to adequately respond to the shifting perspectives of human well-being overlain upon changes in the natural environment over the next century.
7. Summary and next steps
As a field of research, harmful algal science has progressed to the stage where numerous avenues by which climate change might alter HAB prevalence, distribution, and character can be anticipated. For example, there can be high confidence that, barring improved societal practices in agriculture and other land use policies, climate-driven increases in riverine inflows from human-modified water-sheds will increase the prevalence of HAB-induced hypoxia/anoxia. The case for other types of harmful algal blooms, however, remains equivocal because compelling evidence that climate change will enhance the growth of HAB species over the far larger pool of competing non-HAB species is lacking. This shortcoming is partly due to the complexity and multiplicity of phytoplankton community processes (e.g., interspecies competition, grazing) but it also stems in part from the “insular” nature of most HAB research, where a species is studied in far more detail than the community of organisms in which it occurs. Studies of the physiology of HAB species often occur within the limited perspective of clonal cultures, and the benefits of this simplicity falter when efforts are made to use these experimental insights to forecast a species' potential success in complex communities. Additionally, HAB scientists generally focus their research either on localized regions or locally important HAB species, and rarely conform to common experimental designs and methods. While there clearly are exceptions, this “boutique” style of science is poorly suited for acquiring the datasets needed to globally assess the current distributions of HAB frequency and character, let alone for estimating how climate change pressures may alter those patterns. Global datasets on HAB observations, such as IOC–ICES–PICES Harmful Algae Event Database (HAEDAT), are a good beginning but some “change” also is needed regarding how HAB science is done. Suggestions are offered on steps to advance more rapidly the understanding of how climate change may affect harmful algal blooms.
A best practices manual for HAB and climate change research: There is a strong need to develop some consensus with regard to the “best” practices for studying the effect of climate change pressures on harmful algal blooms. By example, the guide to best practices for ocean acidification research (Riebesell et al., 2010) provides a unifying methodological framework that enables direct intercomparison among independent studies from different global regions. To accomplish this within the HAB community, a small working group should be established, perhaps through the Intergovernmental Oceanographic Commission (IOC) Intergovernmental Panel on Harmful Algal Blooms (IPHAB), that would formulate draft guidelines. After review and revision by the broader community, the resultant manual should adopted by IPHAB and kept current with advancing knowledge.
Select representative model organisms of HAB types for intensified study: Biomedical research has been greatly accelerated by utilizing a small subset of model organisms (e.g., the thale cress Arabidopsis thaliana, the common fruit fly Drosophila melanogaster, the water flea Daphnia, and the zebrafish Danio rerio). Rather than directly representing the species of interest (Homo sapiens), these model systems are effective investigative platforms of the linkages among genomic, molecular, and metabolic processes. Selecting a small subset of HAB species/isolates for similar intensive study would generate greater advances than the equivalent investigative effort spread across many species/isolates. The quantitative genomic knowledge that emerged from these “model” systems would offer critical insight for deciphering the genetic and molecular architecture that regulates the growth and toxicity of these and other HAB species. This subset of model organisms also would serve as valuable reference species for inter-lab comparisons and for inclusion into experiments testing other experimental strains or organisms. A draft listing of proposed HAB genera or species for this purpose is shown in Table 3. This list is not meant to be inclusive or exhaustive, but rather a start for discussion among the HAB community on what species or genera should be included, and indeed, the relative values of such a prioritization process.
Inclusion of co-occurring non-HAB species in longer term HAB laboratory studies: Culture studies investigating the responses of HAB species to climate change stressors or factors should as a rule include at least one non-HAB phytoplankton species common to the coastal waters of interest. Better still, simulated or “artificial” community studies should become more prevalent, where simplified “communities” comprising HAB clonal isolates and a few to several commonly co-occurring non-HAB isolates are subjected to climate change pressures. A step beyond parallel clonal culture studies, artificial communities allow for direct interactions among phytoplankton and so provide a better integrative assessment of the relative success of HAB species. These laboratory culture experiments should range from short (days/weeks) to long (months) duration so as to better evaluate HAB (and non-HAB) physiological plasticity to the climate stress conditions. Long-term acclimation to experimental conditions should be part of many studies, as responses to applied stresses may vary significantly depending on the extent of prior pre-conditioning. In the case of artificial communities, a combination of cultures acclimated for extended periods provides an opportunity to test the stability of competitive responses (see Tatters et al., 2013c).
A greater emphasis on multifactorial laboratory experiments: There currently is insufficient understanding about the responses of HAB and non-HAB species under more realistic climate change scenarios, where simultaneous variation of multiple factors (e.g., temperature, salinity, OA) will regulate competitive outcomes. These data then can be fed into coupled HAB-IPCC climate models to generate ecologically realistic results that, in turn, can be used to design new multifactorial experiments and highlight key observer sites for exploring these ecological transitions.
Better global assessment of HAB species responses through “Common Garden” experiments: Greater emphasis should be placed on conducting the same investigations across multiple laboratories with identical experimental designs (temperature, light, nutrients, etc.) using widely distributed HAB and non-HAB species. An example would be determining the response of several HAB species, or multiple clones of a single species, from different geographical locations or habitats to the same environmental perturbations. These studies would provide critical insight into interspecies and interclonal phenotypic variability.
Retrospective analyses of long-term plankton and cyst core data sets: Looking to the past may help inform our forecasts for the future. Re-examination, and continued collection, of long-term plankton and core data sets in the context of climate change data likely will provide the first “canary in the mine” evidence of climate-driven changes in ecological conditions.
Rapid response strategies to investigate harmful algal blooms during major weather or other environmental fluctuations: One of the best ways to study climate change effects on harmful algal blooms may be to utilize major weather shifts that mimic projected future climate scenarios. These shifts for example, might be a major warming event, increased runoff (or drought), water mass intrusion, or other perturbations that have occurred recently in areas subject to harmful algal blooms (see McGillicuddy et al., 2011, the emerging warming/Pseudo-nitzschia bloom conditions in the western USA in summer 2015). Taking advantage of these situations to study entire eco- and hydrographic systems under a common forcing would help develop a more complete understanding.
A stronger linkage among global, national, and regional climate change and HAB observation programs: A greater focus on unifying global HAB observations is essential if the changes in HAB distribution, character, and intensity are to be understood, and in particular to support needed annual or biannual global assessments of the environmental and human impacts from harmful algal blooms. As suggested here, a network of sentinel ocean-observing reference sites should be established that includes both “basic” and HAB SOS (Table 2). This network should encompass existing regional and national monitoring programs and expand observations to regions of concern in the future (e.g., high latitude environments). Stronger linkages need be fostered with the Global Ocean Observing System (GOOS) to improve algorithms for the detection and monitoring of HAB outbreaks. On a practical level, HAB investigators need to: (a) utilize data from state or federal monitoring programs that provide continuity through time and broad spatial coverage, as well as information on the onset, termination, and intensity of outbreaks; (b) identify key locations within individual countries or regions where sufficient data can be collected to provide the necessary perspectives; (c) add HAB species (and techniques to identify them) to existing plankton monitoring programs; and (d) develop and utilize new sensors and instruments that can obtain long-term data in an automated and relatively inexpensive fashion. Installing HAB sensors on existing ocean observing moorings and linking HAB researchers to established Long Term Ecological Research (LTER) sites, which already are measuring and archiving ecologically relevant data, would greatly facilitate collection of the needed time-series data.
A greater emphasis on development of HAB modeling and forecasting platforms: As with other complex systems, there is a need to develop virtual assessments of the factors initiating, maintaining, and dispersing HAB outbreaks. There are few numerical oceanographic models currently optimized for HAB forecasting, but advances have made these tools important learning platforms for investigating interspecies competition and HAB impacts. Models are essential for any quantitative forecasting of climate change effects on harmful algal blooms in the future oceans, so a high priority should be given to the development of such capabilities for multiple harmful algal blooms in multiple regions. Many elements of the existing models can be transferred from one HAB to another or one region to another with appropriate guidance and precautions, so progress can be accelerated through a concerted community effort to share model formulations and to collaborate.
Explicit coupling of HAB modeling and forecasting platforms to complex ecosystem models: Regional physical-biological HAB models need to be better coupled to larger, ecosystem-level models. That is, creating model products that incorporate growth and success of HAB species within the context of complex ecosystem changes under different climate scenarios. Developing the modeling infrastructure needed for these efforts would provide tools for stepwise assessment of our understanding about the likely prevalence of harmful algal blooms under future conditions.
A stronger connection among HAB and climate change researchers: The research network investigating the physical science basis of climate change (e.g. temperature, precipitation, sea level change) has developed a strong foundation for forecasting the magnitude and character of change. Determining how these changes will differentially affect phytoplankton functional groups is more complex (e.g., Marinov et al., 2010), and extending this approach to studying intra-functional group changes (i.e., harmful algal blooms) is far more challenging. Success along this path will depend upon greater levels of organization and cooperation between HAB scientists and their counterparts in the broader climate change research community.
Expand studies on the social science of harmful algal blooms: Incorporation of social science into HAB climate change research should be a firm goal, enabling a more comprehensive assessment of HAB impacts on human well-being (e.g., cultural, economic, human health). “HAB” is a societal-defined term, so better understanding of how societal effects will change with climate stress is essential to attaining a comprehensive assessment of projected changes in future harmful algal blooms.
Retain a strong focus on the fundamental core research on HAB species and events: Although this paper focuses on questions related to climate change and the current inadequate understanding of how harmful algal blooms may be affected, it must be recognized that many important research questions persist in HAB ecology, physiology, genetics, and other aspects of HAB science. The current understanding of harmful algal blooms stems from this basic foundation, and it would be counterproductive if an overemphasis on climate change studies led to a decrease in these "core" research efforts.
Table 3.
Marine HAB species suggested as model organisms for deep study towards quantitative genomic capabilities to determine their physiological, genetic and molecular architecture regulating their responses to climate change factors. The quantitative insight on this subset of organisms would elevate the foundation for study of all other HAB organisms.
| HAB types | Species | Justification |
|---|---|---|
| 1. Paralytic shellfish poisoning | Alexandrium fundyense | Subject of extensive field and laboratory studies, northern expansion is expected |
| 2. Diatom (domoic acid poisoning) | Pseudo-nitzschia multiseries | Wide distribution, toxic, and the genome has been mapped, quantitative genomic studies are underway |
| 3. Fish killing | Heterosigma akashiwo (toxic Pacific strain) | Historical harmful impact, growth-temperature relationships known, northern expansion is expected |
| Cochlodinium polykrikoides (toxic Pacific strain) | Widespread impacts in Asian waters, long subject of field and laboratory studies | |
| Karenia brevis (Atlantic strain) | Large spatial scale blooms, broad field studies, direct human health impacts (aerosols) | |
| 4. Diarrhetic shellfish poisoning | Dinophysis spp. | Limited data makes a species selection difficult, but this genus serves a useful role as both toxic and mixotrophic model organism |
| 5. High biomass | Aureococcus anophageffens (Long Island, NY) | Large scale blooms with long lasting ecological and aesthetic consequences. Already subject to increasing genomic study |
| 6. Cyanobacteria | Nodularia spumigena (Baltic strain) | Persistent high biomass over large salinity ranges, stimulated by eutrophication |
| 7. Ciguatera fish poisoning | Gambierdiscus spp. | Large species complex with highly variable toxicity and temperature tolerances |
The response of harmful algal blooms to the multifaceted forcing of projected climate change is largely unknown and highly speculative. Worse, it appears unlikely that this understanding will improve quickly without broad changes in the general strategies of HAB research, which largely fail to address the complex ecological and multi-environmental stresses that shape phytoplankton communities. There are two central goals moving forward. First, obtain compelling evidence that climate change has caused alterations in HAB distribution, prevalence or character. This fundamental foundation currently is lacking. Second, develop the theoretical, experimental, and empirical evidence for how changes in environmental and ecological factors may influence the geospatial distribution, range expansion or contraction, and emergence of new patterns for harmful algal blooms. Fundamental changes in HAB research strategies will be necessary to meet these critical challenges.
Acknowledgements
Support for the workshop was provided by the North Pacific Marine Science Organization (PICES) and the U.S. National Office for Marine Biotoxins and Harmful Algal Blooms, Woods Hole Oceanographic Institution. Additional support for MLW, CGT, and WPC was from the National Science Foundation (NSF) Grants OCE-1131657 and OCE 1130748 and NOAA NCCOS grants NA10NOS4780161 and NA10NOS4780160, respectively. Support for DMA was provided through the Woods Hole Center for Oceans and Human Health, National Science Foundation (NSF) Grants OCE-1128041 and OCE-1314642; and National Institute of Environmental Health Sciences (NIEHS) Grant 1-P50-ES021923-01. Support for RMK was provided through California Sea Grant and California Ocean Protection Council award R/OPCCONT-12 A 10, National Aeronautics and Space Administration grant NNX13AL28G, and the NOAA award NA11NOS4780030. We express our sincere appreciation to G. Hallegraeff, S. Moore, and an anonymous reviewer for their contributions that improved this work. This is ECOHAB Contribution ECO829.
References
- Acker JG, Leptoukh G. Online analysis enhances use of NASA earth science data. EOS. 2007;88(2):14–17. [Google Scholar]
- Ajani PA, Allen AP, Ingleton T, Armand L. A decadal decline in relative abundance and a shift in microphytoplankton composition at a long-term coastal station off southeast Australia. Limnol. Oceanogr. 2014;59(2):519–531. [Google Scholar]
- Al-Azri A, Piontkovski S, Al-Hashmi K, Al-Gheilani H, Al-Habsi H, Al-Khusaibi S, Al-Azri N. The occurrence of algal blooms in Omani coastal waters. Aquat. Ecosyst. Health Manage. 2012;15(1):56–63. [Google Scholar]
- Alheit J, Mollmann C, Dutz J, Kornilovs G, Loewe P, Mohrholz V, Wasmund N. Synchronous ecological regime shifts in the central Baltic and the North Sea in the late 1980s. ICES J. Mar. Sci. 2005;62(7):1205–1215. [Google Scholar]
- Anderson DM, Alpermann TJ, Cembella AD, Collos Y, Masseret E, Montresor M. The globally distributed genus Alexandrium: multifaceted roles in marine ecosystems and impacts on human health. Harmful Algae. 2012;14:10–35. doi: 10.1016/j.hal.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Glibert PM, Burkholder JM. Harmful algal blooms and eutrophication: nutrient sources, composition, and consequences. Estuaries. 2002;25(4B):704–726. [Google Scholar]
- Anderson DM, Keafer BA, Kleindinst JL, McGillicuddy DJ, Martin JL, Norton K, Pilskaln CH, Smith JL, Sherwood CR, Butman B. Alexandrium fundyense cysts in the Gulf of Maine: long-term time series of abundance and distribution, and linkages to past and future blooms. Deep-Sea Res., II—Top. Stud. Oceanogr. 2014;103:6–26. doi: 10.1016/j.dsr2.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson DM, Keafer BA, McGillicuddy DJ, Solow AR, Kleindinst JL. Improving The Accuracy and Utility of Harmful Algal Bloom Forecasting Systems. Geological Soc Publishing House; Bath: 2013. [Google Scholar]
- Anderson DM, Rengefors K. Community assembly and seasonal succession of marine dinoflagellates in a temperate estuary: the importance of life cycle events. Limnol. Oceanogr. 2006;51(2):860–873. [Google Scholar]
- Anderson CR, Sapiano MRP, Prasad MBK, Long W, Tango PJ, Brown CW, Murtugudde R. Predicting potentially toxigenic Pseudo-nitzschia blooms in the Chesapeake Bay. J. Mar. Syst. 2010;83(3–4):127–140. [Google Scholar]
- Anderson DM, Stock CA, Keafer BA, Nelson AB, Thompson B, McGillicuddy DJ, Keller M, Matrai PA, Martin J. Alexandrium fundyense cyst dynamics in the Gulf of Maine. Deep-Sea Res., II—Top. Stud. Oceanogr. 2005;52(19–21):2522–2542. [Google Scholar]
- Aoki K, Onitsuka G, Shimizu M, Kuroda H, Matsuo H, Kitadai Y, Sakurada K, Ando H, Nishi H, Tahara Y. Variability of factors driving spatial and temporal dispersion in river plume and Chattonella antiqua bloom in the Yatsushiro Sea, Japan. Mar. Pollut. Bull. 2014;81(1):131–139. doi: 10.1016/j.marpolbul.2014.02.005. [DOI] [PubMed] [Google Scholar]
- Auro ME, Cochlan WP. Nitrogen utilization and toxin production by two diatoms of the Pseudo-nitzschia pseudodelicatissima complex: P. cuspidata and P. fryxelliana. J. Phycol. 2013;49(1):156–169. doi: 10.1111/jpy.12033. [DOI] [PubMed] [Google Scholar]
- Azanza R, Taylor FJR. Are Pyrodinium blooms in the southeast Asian region recurring and spreading? A view at the end of the millennium. AMBIO J. Hum. Environ. 2001;30(6):356–364. [PubMed] [Google Scholar]
- Baek SH, Shimode S, Kikuchi T. Growth of dinoflagellates, Ceratium furca and Ceratium fusus in Sagami Bay, Japan: the role of temperature, light intensity and photo-period. Harmful Algae. 2008;7:163–173. [Google Scholar]
- Bates SS, Trainer VL. The ecology of harmful diatoms. In: Graneli E, Turner JT, editors. Ecology of Harmful Algae. Springer-Verlag; Berlin, Germany: 2006. pp. 81–93. [Google Scholar]
- Beardall J, Raven JA. The potential effects of global climate change on microbial photosynthesis, growth and ecology. Phycologia. 2004;43:26–40. [Google Scholar]
- Beardall J, Stojkovic S, Larsen S. Living in a high CO2 world: impacts of global climate change on marine phytoplankton. Plant Ecol. Diversity. 2009;2(2):191–205. [Google Scholar]
- Bednarsek N, Tarling GA, Bakker DCE, Fielding S, Feely RA. Dissolution dominating calcification process in polar pteropods close to the point of aragonite undersaturation. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0109183. http://dx.doi.org/10.1371/journal.-pone.0109183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrenfeld MJ, O'Malley RT, Siegel DA, McClain CR, Sarmiento JL, Feldman GC, Milligan AJ, Falkowski PG, Letelier RM, Boss ES. Climate-driven trends in contemporary ocean productivity. Nature. 2006;444(7120):752–755. doi: 10.1038/nature05317. [DOI] [PubMed] [Google Scholar]
- Belgrano A, Lindahl OBH. North Atlantic Oscillation primary productivity and toxic phytoplankton in the Gullmar Fjord, Sweden (1986–1996) Proc. R. Soc. Lond. Ser. B. 1999;266:425–430. [Google Scholar]
- Berdalet E, McManus MA, Ross ON, Burchard H, Chavez FP, Jaffe JS, Jenkinson IR, Kudela R, Lips I, Lips U, Lucas A, Rivas D, Ruiz-de la Torre MC, Ryan J, Sullivan JM, Yamazaki H. Understanding harmful algae in stratified systems: review of progress and future directions. Deep-Sea Res., II—Top. Stud. Oceanogr. 2014;101:4–20. [Google Scholar]
- Berdalet E, Tester P, Zingone A. Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Benthic Systems. IOC of UNESCO and SCOR; Paris and Newark: 2012. p. 64. [Google Scholar]
- Berge T, Daugbjerg N, Andersen BB, Hansen PJ. Effect of lowered pH on marine phytoplankton growth rates. Mar. Ecol. Prog. Ser. 2010;416:79–91. [Google Scholar]
- Bergkvist J, Selander E, Pavia H. Induction of toxin production in dinoflagellates: the grazer makes a difference. Oecologia. 2008;156:147–154. doi: 10.1007/s00442-008-0981-6. [DOI] [PubMed] [Google Scholar]
- Bernard S, Kudela R, Velo-Suarez L. Developing global capabilities for the observation and prediction of harmful algal blooms. In: Djavidnia S, Cheung V, Ott M, Seeyave S, editors. Oceans and Society, Blue Planet. Cambridge Scholars Publishing; Newcastle upon Tyne: 2014. pp. 46–52. [Google Scholar]
- Binder BJ, Anderson DM. Physiological and environmental-control of germination in Scrippsiella-trochoidea (Dinophyceae) resting cysts. J. Phycol. 1987;23(1):99–107. [Google Scholar]
- Bissenger JE, Montagnes SJ, Atkinson D. Predicting marine phytoplankton maximum growth rates from temperature: improving on the Eppley curve using quantile regression. Limnol. Oceanogr. 2008;53:487–493. [Google Scholar]
- Boberg F, Berg P, Thejll P, Gutowski WJ, Christensen JH. Improved confidence in climate change projections of precipitation further evaluated using daily statistics from ENSEMBLES models. Clim. Dyn. 2010;35(7–8):1509–1520. [Google Scholar]
- Bonachela JA, Raghib M, Levin SA. Dynamic model of flexible phytoplankton nutrient uptake. Proc. Natl. Acad. Sci. U.S.A. 2011;108(51):20633–20638. doi: 10.1073/pnas.1118012108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borkman DG, Smayda TJ, Schwarz EN, Flewelling LJ, Tomas CR. Recurrent vernal presence of the toxic Alexandrium tamarense/Alexandrium fundyense (Dinoflagellata) species complex in Narragansett Bay, USA. Harmful Algae. 2014;32:73–80. [Google Scholar]
- Borkman DG, Smayda TJ, Tomas CR, York R, Strangman W, Wright JLC. Toxic Alexandrium peruvianum (Balech and de Mendiola) Balech and Tangen in Narragansett Bay, Rhode Island (USA) Harmful Algae. 2012;19:92–100. [Google Scholar]
- Boyd PW, Hutchins DA. Understanding the responses of ocean biota to a complex matrix of cumulative anthropogenic change. Mar. Ecol. Prog. Ser. 2012;470:125–135. [Google Scholar]
- Boyd PW, Rynearson TA, Armstrong EA, Fu FX, Hayashi K, Hu ZX, Hutchins DA, Kudela RM, Litchman E, Mulholland MR, Passow U, Strzepek RF, Whittaker KA, Yu E, Thomas MK. Marine phytoplankton temperature versus growth responses from polar to tropical waters—outcome of a scientific community-wide study. PLoS ONE. 2013;8(5) doi: 10.1371/journal.pone.0063091. http://dx.doi.org/10.1371/journal.-pone.0063091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand LE, Compton A. Long-term increase in Karenia brevis abundance along the Southwest Florida Coast. Harmful Algae. 2007;6(2):232–252. doi: 10.1016/j.hal.2006.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brand LE, Sunda WG, Guillard RRL. Limitation of marine phytoplankton reproductive rates by zinc, manganese, and iron. Limnol. Oceanogr. 1983;28(6):1182–1198. [Google Scholar]
- Bravo I, Anderson DM. The effects of temperature, growth medium and darkness on excystment and growth of the toxic dinoflagellate Gymnodinium catenatum from northwest Spain. J. Plankton Res. 1994;16:513–525. [Google Scholar]
- Bricelj VM, Connell L, Konoki K, MacQuarrie SP, Scheuer T, Catterall WA. Trainer, V.L., 2005. Sodium channel mutation leading to saxitoxin resistance in clams increases risk of PSP. Nature. 434(7034):763–767. doi: 10.1038/nature03415. [DOI] [PubMed] [Google Scholar]
- Bricelj VM, Shumway SE. Paralytic shellfish toxins in bivalve molluscs: occurrence, transfer kinetics and biotransformation. Rev. Fish. Sci. 1998;6:315–383. [Google Scholar]
- Brownlee EF, Sellner SG, Sellner KG. Prorocentrum minimum blooms: potential impacts on dissolved oxygen and Chesapeake Bay oyster settlement and growth. Harmful Algae. 2005;4(3):593–602. [Google Scholar]
- Bruslé J. IFREMER Repéres Océan No. 10. 1995. The impact of harmful algal blooms on finfish mortality, pathology and toxicology; pp. 1–75. [Google Scholar]
- Burkholder HM, Glibert PM. The importance of intraspecific variability in harmful algae—preface to a collection of topical papers. Harmful Algae. 2009;8:744–822. [Google Scholar]
- Buskey EJ, Liu H, Collumb C, Bersano JGF. The decline and recovery of a persistent Texas brown tide algal bloom in the Laguna Madre (Texas, USA) Estuaries. 2001;24:337–346. [Google Scholar]
- Byrne M. Impact of ocean warming and ocean acidification on marine invertebrate life history stages: vulnerabilities and potential for persistence in a changing ocean. Oceanogr. Mar. Biol. Annu. Rev. 2011;49:1–42. [Google Scholar]
- Campbell L, Olson RJ, Sosik HM, Abraham A, Henrichs DW, Hyatt CJ, Buskey EJ. First harmful Dinophysis (Dinophyceae, Dinophysiales) bloom in the US is revealed by automated imaging flow cytometry. J. Phycol. 2010;46(1):66–75. [Google Scholar]
- Carignan MO, Montoya NG, Carreto JI. Long-term effects of ultraviolet radiation on the composition of pigment and mycosporine-like amino acids (MAAs) composition in Alexandrium catenella. In: Arzul G, editor. Aquaculture, Environment and Marine Phytoplankton. Vol. 34. IFREMER, Actes Colloq; 2002. pp. 191–207. [Google Scholar]
- Caron DA, Hutchins DA. The effects of changing climate on microzooplankton grazing and community structure: drivers, predictions and knowledge gaps. J. Plankton Res. 2013;35(2):235–252. [Google Scholar]
- Carreto JI, Carignan MO. Mycosporine-like amino acids: relevant secondary metabolites. Chemical and ecological aspects. Mar. Drugs. 2011;9(3):387–446. doi: 10.3390/md9030387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castenholz RW, Garcia-Pichel F. Cyanobacterial responses to UV radiation. In: Whitton BA, Potts M, editors. The Ecology of Cyanobacteria. Kluwer Academic Publishers; Dordrecht: 2005. pp. 591–611. [Google Scholar]
- Cembella AD, Shumway SE, Larocque R. Sequestering and putative biotransformation of paralytic shellfish toxins by the sea scallop Placopecten magellanicus—seasonal and spatial scales in natural-populations. J. Exp. Mar. Biol. Ecol. 1994;180(1):1–22. [Google Scholar]
- Chen Y, Qin B, Teubner K, Dokulil MT. Long-term dynamics of phytoplankton assemblages: Microcystis-domination in Lake Taihu, a large shallow lake in China. J. Plankton Res. 2003;25(1):445–453. [Google Scholar]
- Cho ES, Kotaki Y, Park JG. The comparison between toxic Pseudo-nitzschia multiseries (Hasle) Hasle and non-toxic P. pungens (Grunow) Hasle isolated from Jinhae Bay, Korea. Algae. 2001;16:275–285. [Google Scholar]
- Coats DW, Park MG. Parasitism of photosynthetic dinoflagellates by three strains of Amoebophrya (Dinophyta): parasite survival, infectivity, generation time, and host specificity. J. Phycol. 2002;38:520–528. [Google Scholar]
- Colin SP, Dam H. Latitudinal differentiation in the effects of the toxic dinoflagellate Alexandrium spp. on the feeding and reproduction of populations of the copepod Acartia hudsonica. Harmful Algae. 2002;1:113–125. [Google Scholar]
- Collins S, Rost B, Rynearson TA. Evolutionary potential of marine phytoplankton under ocean acidification. Evol. Appl. 2014;7:140–155. doi: 10.1111/eva.12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collos Y, Bec B, Jauzein C, Abadie E, Laugier T, Lautier J, Pastoureaud A, Souchu P, Vaquer A. Oligotrophication and emergence of picocyano-bacteria and a toxic dinoflagellate in Thau lagoon, southern France. J. Sea Res. 2009;61(1–2):68–75. [Google Scholar]
- Collos Y, Jauzein C, Ratmaya W, Souchu P, Abadie E, Vaquer A. Comparing diatom and Alexandrium catenella/tamarense blooms in Thau lagoon: importance of dissolved organic nitrogen in seasonally N-limited systems. Harmful Algae. 2014;37:84–91. [Google Scholar]
- Collos Y, Lespilette M, Vaquer A, Laabir M, Pastoureaud A. Uptake and accumulation of ammonium by Alexandrium catenella during nutrient pulses. Afr. J. Mar. Sci. 2006;28(2):313–318. [Google Scholar]
- Cripps G, Lindeque P, Flynn KJ. Have we been underestimating the effects of ocean acidification in zooplankton? Global Change Biol. 2014;20(11):3377–3385. doi: 10.1111/gcb.12582. [DOI] [PubMed] [Google Scholar]
- Dason JS, Colman B. Inhibition of growth in two dinoflagellates by rapid changes in external pH. Can. J. Bot.—Revue Canadienne De Botanique. 2004;82(4):515–520. [Google Scholar]
- Davidson K, Gowen RJ, Tett P, Bresnan E, Harrison PJ, McKinney A, Milligan S, Mills DK, Silke J, Crooks AM. Harmful algal blooms: how strong is the evidence that nutrient ratios and forms influence their occurrence? Estuar. Coast. Shelf Sci. 2012;115:399–413. [Google Scholar]
- de Boer MK. Temperature responses of three Fibrocapsa japonica strains (Raphidophyceae) from different climate regions. J. Plankton Res. 2005;27(1):47–60. [Google Scholar]
- Djavidnia S, Cheung V, Ott M, Seeyave S. Oceans and Society, Blue Planet. Cambridge Scholars Publishing; Newcastle upon Tyne: 2014. [Google Scholar]
- Doney SC, Ruckelshaus M, Duffy JE, Barry JP, Chan F, English CA, Galindo HM, Grebmeier JM, Hollowed AB, Knowlton N, Polovina J, Rabalais NN, Sydeman WJ, Talley LD. Climate change impacts on marine ecosystems. Annu. Rev. Mar. Sci. 2012;4:11–37. doi: 10.1146/annurev-marine-041911-111611. [DOI] [PubMed] [Google Scholar]
- Dyhrman ST, Anderson DM. Urease activity in cultures and field populations of the toxic dinoflagellate Alexandrium. Limnol. Oceanogr. 2003;48(2):647–655. [Google Scholar]
- Eberlein T, de Waal DBV, Rost B. Differential effects of ocean acidification on carbon acquisition in two bloom-forming dinoflagellate species. Physiol. Plant. 2014;151(4):468–479. doi: 10.1111/ppl.12137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards KF, Litchman E, Klausmeier CA. Functional traits explain phytoplankton community structure and seasonal dynamics in a marine ecosystem. Ecol. Lett. 2013;16(1):56–63. doi: 10.1111/ele.12012. [DOI] [PubMed] [Google Scholar]
- Edwards M, Johns D. Regional climate change and harmful algal blooms in the northeast Atlantic. Limnol. Oceanogr. 2006;51:820–829. [Google Scholar]
- Edwards ME, Richardson AJ. Impact of climage change on marine pelagic phenology and trophic mismatch. Nature. 2004;430:881–883. doi: 10.1038/nature02808. [DOI] [PubMed] [Google Scholar]
- Ekstrom JA, Suatoni L, Cooley SR, Pendleton LH, Waldbusser GG, Cinner JE, Ritter J, Langdon C, van Hooidonk R, Gledhill D, Wellman K, Beck MW, Brander LM, Rittschof D, Doherty C, Edwards PET, Portela R. Vulnerability and adaptation of US shellfisheries to ocean acidification. Nat. Clim. Chang. 2015;5(3):207–214. [Google Scholar]
- Eppley RW. Temperature and phytoplankton growth in the sea. Fish. Bull. 1972;70:1063–1085. [Google Scholar]
- Etheridge SM, Roesler CS. Effects of temperature, irradiance, and salinity on photosynthesis, growth rates, total toxicity, and toxin composition for Alexandrium fundyense isolates from the Gulf of Maine. Deep-Sea Res., II—Top. Stud. Oceanogr. 2005;52:2491–2500. [Google Scholar]
- Evens TJ, Kirkpatrick GJ, Millie DF, Chapman DJ, Schofield OME. Photophysiological responses of the toxic red-tide dinoflagellate Gymnodinium breve (Dinophyceae) under natural sunlight. J. Plankton Res. 2001;23:1177–1193. [Google Scholar]
- Fan CL, Glibert PM, Burkholder JM. Characterization of the affinity for nitrogen, uptake kinetics, and environmental relationships for Prorocentrum minimum in natural blooms and laboratory cultures. Harmful Algae. 2003;2(4):283–299. [Google Scholar]
- Farrell H, Gentien P, Fernand L, Lunven M, Reguera B, González-Gil S, Raine R. Scales characterising a high density thin layer of Dinophysis acuta Ehrenberg and its transport within a coastal jet. Harmful Algae. 2012;15:36–46. [Google Scholar]
- Feely RA, Sabine CL, Lee K, Berelson W, Kleypas J, Fabry VJ, Millero FJ. Impact of anthropogenic CO2 on the CaCO3 system in the oceans. Science. 2004;305(5682):362–366. doi: 10.1126/science.1097329. [DOI] [PubMed] [Google Scholar]
- Feely RA, Sbaine CL, Hernandex-Ayon JM, Ianson D, Hales B. Evidence of upwelling of corrosive acidified water onto the continental shelf. Science. 2008;320:1490–1492. doi: 10.1126/science.1155676. [DOI] [PubMed] [Google Scholar]
- Fehling J, Davidson K, Bolch C, Tett P. Seasonality of Pseudo-nitzschia spp. (Bacillariophyceae) in western Scottish waters. Mar. Ecol. Prog. Ser. 2006;323:91–105. [Google Scholar]
- Fehling J, Green DH, Davidson K, Bolch CJ, Bates SS. Domoic acid production by Pseudo-nitzschia seriata (Bacillariophyceaae) in Scottish waters. J. Phycol. 2004;40:622–630. [Google Scholar]
- Feng YY, Hare CE, Leblanc K, Rose JM, Zhang YH, DiTullio GR, Lee PA, Wilhelm SW, Rowe JM, Sun J, Nemcek N, Gueguen C, Passow U, Benner I, Brown C, Hutchins DA. Effects of increased pCO(2) and temperature on the North Atlantic spring bloom. I. The phytoplankton community and biogeochemical response. Mar. Ecol. Prog. Ser. 2009;388:13–25. [Google Scholar]
- Fernandez L, Quintanila J, Monteiro-Ribas W, Gonzalez-Rodriguez E, Goutinho R. Seasonal and interannual coupling between sea surface temperature, phyyoplankton and meroplankton in the subtropical south-western Atlantic Ocean. J. Plankton Res. 2012;34:236–244. [Google Scholar]
- Fiedler P. Zooplankton avoidance and reduced grazing responses to Gymnodinium splendens (Dinophyceae) Limnol. Oceanogr. 1982;27:961–965. [Google Scholar]
- Figueras FG, Pitcher GC, Estrada M. Harmful algal bloom dynamics in relation to physical processes. In: Granéli E, Turner JT, editors. Ecology of Harmful Algae. Springer-Verlag; Berlin: 2006. pp. 127–138. [Google Scholar]
- Flynn KJ, Flynn K, John EH, Reguera B, Reyero MI, Franco JM. Changes in toxins, intracellular and dissolved free amino acids of the toxic dinoflagellate Gymnodinium catenatum in response to changes in inorganic nutrients and salinity. J. Plankton Res. 1996;18(11):2093–2111. [Google Scholar]
- Fraga S, Gallager SM, Anderson DM. Chain-forming dinoflagellates: an adaptation to red tides. In: Okaichi T, Anderson DM, Nemoto T, editors. Red Tides: Biology, Environmental and Science and Toxicology. Elsevier; New York, NY: 1989. pp. 281–284. [Google Scholar]
- Fu F-X, Warner ME, Zhang Y, Feng Y, Hutchins DA. Effects of increased temperature and CO2 on photosynthesis, growth, and elemental ratios in marine Synechococcus and Prochlorococcus (cyanobacteria) J. Phycol. 2007;43(3):485–496. [Google Scholar]
- Fu FX, Place AR, Garcia NS, Hutchins DA. CO2 and phosphate availability control the toxicity of the harmful bloom dinoflagellate Karlodinium veneficum. Aquat. Microbiol. Ecol. 2010;59(1):55–65. [Google Scholar]
- Fu FX, Tatters AO, Hutchins DA. Global change and the future of harmful algal blooms in the ocean. Mar. Ecol. Prog. Ser. 2012;470:207–233. [Google Scholar]
- Fu FX, Zhang YH, Warner ME, Feng YY, Sun J, Hutchins DA. A comparison of future increased CO2 and temperature effects on sympatric Heterosigma akashiwo and Prorocentrum minimum. Harmful Algae. 2008;7(1):76–90. [Google Scholar]
- García-Mendoza E, Rivas D, Olivos-Ortiz A, Almazán-Becerril A, Castañeda-Vega C, Peña-Manjarrez JL. A toxic Pseudo-nitzschia bloom in Todos Santos Bay, northwestern Baja California, Mexico. Harmful Algae. 2009;8(3):493–503. [Google Scholar]
- García-Pichel F, Castenholz RW. Occurrence of UV-absorbing, mycosporine-like compounds among cyanobacterial isolates and an estimate of their screening capacity. Appl. Environ. Microbiol. 1993;59(1):163–169. doi: 10.1128/aem.59.1.163-169.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geider RJ, La Roche J. Redfield revisited: variability of C:N:P in marine microalgae and its biochemical basis. Eur. J. Phycol. 2002;37(1):1–17. [Google Scholar]
- GEOHAB . Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Upwelling Systems. 2005. [Google Scholar]
- GEOHAB . Global Ecology and Oceanography of Harmful Algal Blooms, Harmful Algal Blooms in Eutrophic Systems. IOC and SCOR; Paris and Baltimore: 2006. [Google Scholar]
- GEOHAB . Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Fjords and Coastal Embayments A. IOC and SCOR; Paris, France and Newark, Delaware USA: 2010. [Google Scholar]
- GEOHAB . Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Benthic Systems. IOC of UNESCO and SCOR; Paris and Newark: 2012. [Google Scholar]
- GEOHAB . Workshop on “Advances and Challenges for Understanding Physical–Biological Interactions in HABs in Stratified Environments”. IOC and SCOR; Paris, France, and Newark, Delaware, USA: 2013. Global Ecology and Oceanography of Harmful Algal Blooms, GEOHAB Core Research Project: HABs in Stratified Systems. [Google Scholar]
- Giordano M, Beardall J, Raven JA. CO2 concentrating mechanisms in algae: mechanisms, environmental modulation, and evolution. Annu. Rev. Plant Biol. 2005;56:99–131. doi: 10.1146/annurev.arplant.56.032604.144052. [DOI] [PubMed] [Google Scholar]
- Glibert PM, Azanza R, Burford M, Furuya K, Abal E, Al-Azri A, Al-Yamani F, Andersen P, Anderson DM, Beardall J, Berg GM, Brand L, Bronk D, Brookes J, Burkholder JM, Cembella A, Cochlan WP, Collier JL, Collos Y, Diaz R, Doblin M, Drennen T, Dyhrman S, Fukuyo Y, Furnas M, Galloway J, Graneli E, Ha DV, Hallegraeff G, Harrison J, Harrison PJ, Heil CA, Heimann K, Howarth R, Jauzein C, Kana AA, Kana TM, Kim H, Kudela R, Legrand C, Mallin M, Mulholland M, Murray S, O'Neil J, Pitcher G, Qi YZ, Rabalais N, Raine R, Seitzinger S, Salomon PS, Solomon C, Stoecker DK, Usup G, Wilson J, Yin KD, Zhou MJ, Zhu MY. Ocean urea fertilization for carbon credits poses high ecological risks. Mar. Pollut. Bull. 2008;56(6):1049–1056. doi: 10.1016/j.marpolbul.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glibert PM, Harrison J, Heil C, Seitzinger S. Escalating worldwide use of urea—a global change contributing to coastal eutrophication. Biogeochemistry. 2006;77(3):441–463. [Google Scholar]
- Gómez F, Souissi S. Unusual diatoms linked to climatic events in the northeastern English Channel. J. Sea Res. 2007;58(4):283–290. [Google Scholar]
- Gosselin S, Fortier L, Gagné A. Vulnerability of marine fish larvae to the toxic dinoflagellate Protogonyaulax tamarensis. Mar. Ecol. Prog. Ser. 1989;57:1–10. [Google Scholar]
- Greer AT, Cowen RK, Guigand CM, McManus MA, Sevadjian JC, Timmerman AHV. Relationships between phytoplankton thin layers and the fine-scale vertical distributions of two trophic levels of zooplankton. J. Plankton Res. 2013;35(5):939–956. [Google Scholar]
- Grigorov I, Rigual-Hernandez AS, Honjo S, Kemp AES, Armand LK. Settling fluxes of diatoms to the interior of the Antarctic circumpolar current along 170° W. Deep Sea Res. Part I: Oceanogr. Res. Pap. 2014;93:1–13. [Google Scholar]
- Guerrini F, Ciminiello P, Dell'Aversano C, Tartaglione L, Fattorusso E, Boni L, Pistocchi R. Influence of temperature, salinity and nutrient limitation on yessotoxin production and release by the dinoflagellate Protoceratium reticulatum in batch-cultures. Harmful Algae. 2007;6(5):707–717. [Google Scholar]
- Guissande C, Frangopulos M, Maneiro I, Vergara AR, Riveiro I. Ecological advantages of toxin production by the dinoflagellate Alexandrium minutum under phosphorus limitation. Mar. Ecol. Prog. Ser. 2002;225:169–176. [Google Scholar]
- Gutowski WJ, Kozak KA, Arritt RW, Christensen JH, Patton JC, Takle ES. A possible constraint on regional precipitation intensity changes under global warming. J. Hydrometeorol. 2007;8(6):1382–1396. [Google Scholar]
- Ha SY, La HS, Min JO, Chung KH, Kang SH, Shin KH. Photoprotective function of mycosporine-like amino acids in a bipolar diatom (Porosira glacialis): evidence from ultraviolet radiation and stable isotope probing. Diatom Res. 2014;29(4):399–409. [Google Scholar]
- Häder DP, Helbling W, Williamson CE, Worrest RC. 2010 AssessmentUNEP. 2010. Effects of UV radiation on aquatic ecosystems and interactions with climate change, the environmental effects of ozone depletion and its interactions with climate change; pp. 113–150. [Google Scholar]
- Hajdu S, Edler L, Olenina I, Witek B. Spreading and establishment of the potentially toxic dinoflagellate Prorocentrum minimum in the Baltic Sea. Int. Rev. Gesamten Hydrobiol. 2000;85:561–575. [Google Scholar]
- Håkansson B, editor. Swedish National Report on Eutrophication Status in the Kattegat and Skagerrak. Swedish Meteorological and Hydrological Institute; Göteborg, Sweden: 2002. [Google Scholar]
- Hallegraeff G. Special issue: ballast water. Harmful Algae. 2007;6:461–622. [Google Scholar]
- Hallegraeff GM, Blackburn SI, Doblin MA, Bolch CJS. Global toxicology, ecophysiology and population relationships of the chainforming PST dinoflagellate Gymnodinium catenatum. Harmful Algae. 2012a;14:130–143. [Google Scholar]
- Hallegraeff G, Beardall J, Brett S, Doblin M, Thompson P. Phytoplankton. In: Poloczanska ES, Hobday AJ, Richardson AJ, editors. Marine Climage Change Impacts and Adaptation Report Card for Australia 2012. 2012b. p. 240. [Google Scholar]
- Hallegraeff GM, Marssahall JA, Valentine J, Hardiman S. Short cyst-dormancy period of an Australian isolate of the toxic dinoflagellate Alexandrium catenella. Mar. Freshwater Res. 1998;49:415–420. [Google Scholar]
- Hama T, Kawashima S, Shimotori K, Satoh Y, Omori Y, Wada S, Adachi T, Hasegawa S, Midorikawa T, Ishii M, Saito S, Sasano D, Endo H, Nakayama T, Inouye I. Effect of ocean acidification on coastal phytoplankton composition and accompanying organic nitrogen production. J. Oceanogr. 2012;68(1):183–194. [Google Scholar]
- Hamasaki K, Horie M, Tokimitsu S, Toda T, Taguchi S. Variability in toxicity of the dinoflagellate Alexandrium tamarense isolated from Hiroshima Bay, western Japan, as a reflection of changing environmental conditions. J. Plankton Res. 2001;23(3):271–278. [Google Scholar]
- Hansen PJ. Quantitative importance and trophic role of heterotrophic dinoflagellates in a coastal pelagial food web. Mar. Ecol. Prog. Ser. 1991;73:253–261. [Google Scholar]
- Hansen PJ. Effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquat. Microb. Ecol. 2002;28(3):279–288. [Google Scholar]
- Hansen PJ, Bjørnsen PK, Hansen BW. Zooplankton grazing and growth: scaling within the 2–2000-μm body size range. Limnol. Oceanogr. 1997;42:687–704. [Google Scholar]
- Hansson LA, Nicolle A, Graneli W, Hallgren P, Kritzberg E, Persson A, Bjork J, Nilsson PA, Bronmark C. Food-chain length alters community responses to global change in aquatic systems. Nat. Clim. Change. 2013;3(3):228–233. [Google Scholar]
- Hare CE, Leblanc K, DiTullio GR, Kudela RM, Zhang Y, Lee PA, Riseman S, Hutchins DA. Consequences of increased temperature and CO2 for phytoplankton community structure in the Bering Sea. Mar. Ecol. Prog. Ser. 2007;352:9–16. [Google Scholar]
- Hargraves PE, Zhang J, Wang R, Shimizu Y. Growth characteristics of the diatoms Pseudonitzschia pungens and P. fraudulenta exposed to ultraviolet radiation. Hydrobiologia. 1993;269:207–212. [Google Scholar]
- Harrison PJ, Furuya K, Glibert PM, Xu J, Liu HB, Yin K, Lee JHW, Anderson DM, Gowen R, Al-Azri AR, Ho AYT. Geographical distribution of red and green Noctiluca scintillans. Chin. J. Oceanol. Limnol. 2011;29(4):807–831. [Google Scholar]
- Hattenrath TK, Anderson DM, Gobler CJ. The influence of anthropogenic nitrogen loading and meteorological conditions on the dynamics and toxicity of Alexandrium fundyense blooms in a New York (USA) estuary. Harmful Algae. 2010;9(4):402–412. [Google Scholar]
- Hattenrath-Lehmann KT, Marcoval MA, Berry DL, Fire S, Wang Z, Morton SL, Gobler CJ. The emergence of Dinophysis acuminata blooms and DSP toxins in shellfish in New York waters. Harmful Algae. 2013;26:33–44. [Google Scholar]
- Hattenrath-Lehmann TK, Smith JL, Wallace RB, Merlo LR, Koch F, Mittelsdorf H, Goleski JA, Anderson DM, Gobler CJ. The effects of elevated CO2 on the growth and toxicity of field populations and cultures of the saxitoxin-producing dinoflagellate, Alexandrium fundyense. Limnol. Oceanogr. 2015;60(1):198–214. doi: 10.1002/lno.10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hein M, Pedersen MF, Sandjensen K. Size-dependent nitrogen uptake in micro- and macroalgae. Mar. Ecol. Prog. Ser. 1995;118(1–3):247–253. [Google Scholar]
- Hernandez-Farinas T, Soudant D, Barille L, Belin C, Lefebvre A, Bacher C. Temporal changes in the phytoplankton community along the French coast of the eastern English Channel and the southern Bight of the North Sea. ICES J. Mar. Sci. 2013;71(4):821–833. [Google Scholar]
- Herndon J, Cochlan WP. Nitrogen utilization by the raphidophyte Hetero-sigma akashiwo: growth and uptake kinetics in laboratory cultures. Harmful Algae. 2007;6(2):260–270. [Google Scholar]
- Himmelman JH. Phytoplankton as a stimulus for spawning in 3 marine invertebrates. J. Exp. Mar. Biol. Ecol. 1975;20:199–214. [Google Scholar]
- Hinder SL, Hays GC, Edwards M, Roberts EC, Walne AW, Gravenor MB. Changes in marine dinoflagellate and diatom abundance under climate change. Nat. Clim. Change. 2012;2(4):271–275. [Google Scholar]
- Hinga KR. Effects of pH on coastal marine phytoplankton. Mar. Ecol. Prog. Ser. 2002;238:281–300. [Google Scholar]
- Hitchcock GL. A comparative study of the size-dependent organic composition of marine diatoms and dinoflagellates. J. Plankton Res. 1981;4:363–377. [Google Scholar]
- Honjo T. Growth potential of Chattonella marina (Raphidophyceae) collected in Gokasho Bay, Japan. Bull. Plankton Soc. Japan. 1987;34:119–124. [Google Scholar]
- Honjo T, Tabata K. Growth dynamics of Olisthodiscus luteus in outdoor tanks with flowing coastal water and in small vessels. Limnol. Oceanogr. 1985;30:653–664. [Google Scholar]
- Hubbart B, Pitcher GC, Krock B, Cembella AD. Toxigenic phytoplankton and concomitant toxicity in the mussel Choromytilus meridionalis off the west coast of South Africa. Harmful Algae. 2012;20:30–41. [Google Scholar]
- Huisman JM, Matthijs HCP, Visser PM. Harmful Cyanobacteria. Springer; Dordrecht, The Netherlands: 2005. [Google Scholar]
- Huntley M, Sykes P, Rohan S, Martin V. Chemically mediated rejection of dinoflagellate prey by the copepods Calanus pacificus and Paracalanus parvus: mechanism, occurrence and significance. Mar. Ecol. Prog. Ser. 1986;28:105–128. [Google Scholar]
- Hutchins DA, Mulholland MR, Fu FX. Nutrient cycles and marine microbes in a CO2-enriched ocean. Oceanography. 2009;22(4):128–145. [Google Scholar]
- Ishimatsu A, Oda T, Yoshida M, Ozaki M. Oxygen radicals are probably involved in the mortality of yellowtail by Chattonella marina. Fish. Sci. 1996;62:836–837. [Google Scholar]
- Itakura S, Yamaguchi M. Morphological and physiological dfferences between the cysts of Alexandrium catenella and A. tamarense (Dinophyceae) in the Seto Inland Sea, Japan. Plankton Biol. Ecol. 2005;57:85–91. [Google Scholar]
- Itakura S, Yamaguchi M, Yoshida MYF. The seasonal occurrence of Alexandrium tamarense (Dinophyceae) vegetative cells in Hiroshima Bay, Japan. Fish. Sci. 2002;68:77–86. [Google Scholar]
- Jahnke J. The light and temperatuae dependence of growth rate and elemental composition of Phaeocystis globosa Scherffel and P. pouchtetii (Har.) Lager in batch cultures. Neth. J. Sea Res. 1989;23:15–21. [Google Scholar]
- Jeffrey SW, MacTavish HS, Dunlap WC, Vesk M, Groenewoud K. Occurrence of UVA- and UVB-absorbing compounds in 152 species (206 strains) of marine microalgae. Mar. Ecol. Prog. Ser. 1999;189:35–51. [Google Scholar]
- Jeong HJ, Yoo YD, Kim JS, Seong KA, Kang NS, Kim TH. Growth, feeding and ecological roles of the mixotrophic and heterotrophic dinoflagellates in marine planktonic food webs. Ocean Sc. J. 2010;45((2):65–91. [Google Scholar]
- Jeong HJ, Yoo YD, Lim AS, Kim T-W, Lee K, Kang CK. Raphidophyte red tides in Korean waters. Harmful Algae. 2013;30S:541–552. [Google Scholar]
- Jessup DA, Miller MA, Ryan JP, Nevins HM, Kerkering HA, Mekebri A, Crane DB, Johnson TA, Kudela RM. Mass stranding of marine birds caused by a surfactant-producing red tide. PLoS ONE. 2009;4(2):8. doi: 10.1371/journal.pone.0004550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jester R, Lefebvre K, Langlois G, Vigilant V, Baugh K, Silver MW. A shift in the dominant toxin-producing algal species in central California alters phycotoxins in food webs. Harmful Algae. 2009;8(2):291–298. [Google Scholar]
- Jiang X, Lonsdale D, Gobler CJ. Grazers and vitamins shape chain formation in a bloom-forming dinoflagellate, Cochlodinium polykrikoides. Oecologia. 2010;164:455–464. doi: 10.1007/s00442-010-1695-0. [DOI] [PubMed] [Google Scholar]
- John EH, Flynn KJ. Growth dynamics and toxicity of Alexandrium fundyense (Dinophyceae): the effect of changing N:P supply ratios on internal toxin and nutrient levels. Eur. J. Phycol. 2000;35(1):11–23. [Google Scholar]
- Jørgensen CB. Biology of Suspension Feeding. Pergamon, Oxford: 1966. [Google Scholar]
- Kaczmarska I, Martin JL, Ehrman JM, LeGresley MM. Pseudo-nitzschia species population dynamics in the Quoddy Region, Bay of Fundy. Harmful Algae. 2007;6(6):861–874. [Google Scholar]
- Kaebernik M, Brett AN, Börner T, Dittmann E. Light and the transcriptional response of the microcystin biosynthesis gene cluster. Appl. Environ. Microbiol. 2000;66:3387–3392. doi: 10.1128/aem.66.8.3387-3392.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahru M, Lee Z, Kudela R, Manzano-Sarabia M. Multi-satellite time series of inherent optical properties in the California Current. Deep-Sea Res., II—Top. Stud. Oceanogr. 2015;112:91–106. [Google Scholar]
- Kahru MB, Michell G, Diaz A, Miura M. MODIS detects a devastating algal bloom in Paracas Bay, Peru. Eos. Trans. Am. Geophys. Union. 2004;85(45):465–472. [Google Scholar]
- Kamykowski D, McCollum SA. The temperature acclimatized swimming speed of selected marine dinoflagellates. J. Plankton Res. 1986;8:275–287. [Google Scholar]
- Karentz D, Smayda TJ. Temperature and the seasonal occurrence pattern of 30 dominant phytoplankton species in Narragansett Bay over a 22-year period (1959–1980) Mar. Ecol. Prog. Ser. 1984;18:277–293. [Google Scholar]
- Karentz D, Smayda TJ. Temporal patterns and variations in phytoplankton community organization and abundance in Narragansett Bay during 1959–1980. J. Plankton Res. 1998;20:145–168. [Google Scholar]
- Karlberg M, Wulff A. Impact of temperature and species interaction on filamentous cyanobacteria may be more important than salinity and increased pCO(2) levels. Mar. Biol. 2013;160(8):2063–2072. [Google Scholar]
- Kim H, Spivack AJ, Menden-Deuer S. pH alters the swimming behaviors of the raphidophyte Heterosigma akashiwo: implications for bloom formation in an acidified ocean. Harmful Algae. 2013;26:1–11. [Google Scholar]
- Klisch M, Hader DP. Mycosporine-like amino acids and marine toxins–the common and the different. Mar. Drugs. 2008;6(2):147–163. doi: 10.3390/md20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koski M, Rosenberg M, Viitasalo M, Tanskanen S, Sjöland U. Is Prymnesium patelliferum toxic for copepods?—Grazing, egg production, and egestion of the calanoid copepod Eurytermora affinis in mixtures of “good” and “bad” food. ICES J. Mar. Sci. 1999;56:131–139. [Google Scholar]
- Koski M, Schmidt K, Engström-Ost J, Viitasalo M, Jónasddottir SH, Repka S, Sivonen K. Calanoid copepods feed and produce eggs in the presence of toxic cyanobacteria Nodularia spumigena. Limnol. Oceanogr. 2002;47:878–885. [Google Scholar]
- Kraberg A, Widdicombe C, Wiltshire K, Bresnan E, Soudant D, Tarran G, Smyth T. Phytoplankton and microbial plankton of the North Sea and English Channel. ICES Phytoplankton and Microbial Plankton Status Report 2009/2010. 2012;6:70–91. [Google Scholar]
- Kremp A, Anderson DM. Factors regulating germination of resting cysts of the spring bloom dinoflagellate Scrippsiella hangoei from the northern Baltic Sea. J. Plankton Res. 2000;22(7):1311–1327. [Google Scholar]
- Kremp A, Godhe A, Egardt J, Dupont S, Suikkanen S, Casabianca S, Penna A. Intraspecific variability in the response of bloom-forming marine microalgae to changed climate conditions. Ecol. Evol. 2012;2(6):1195–1207. doi: 10.1002/ece3.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kudela RM, Seeyave S, Cochlan WP. The role of nutrients in regulation and promotion of harmful algal blooms in upwelling systems. Prog. Oceanogr. 2010;85(1–2):122–135. [Google Scholar]
- Kudela RM, Ryan JP, Blakely MD, Lane JQ, Peterson TD. Linking the physiology and ecology of Cochlodinium to better understand harmful algal bloom events: a comparative approach. Harmful Algae. 2008;7(3):278–292. [Google Scholar]
- Laabir M, Jauzein C, Genovesi B, Masseret E, Grzebyk D, Cecchi P, Vaquer A, Perrin Y, Collos Y. Influence of temperature, salinity and irradiance on the growth and cell yield of the harmful red tide dinoflagellate Alexandrium catenella colonizing Mediterranean waters. J. Plankton Res. 2011;33(10):1550–1563. [Google Scholar]
- Laglera LM, Berg C.M.G.v.d. Evidence for geochemical control of iron by humic substances in seawater. Limnol. Oceanogr. 2009;54(2):610–619. [Google Scholar]
- Lasker R, Zweifel JR. Growth and survival of first-feeding northern anchovy larvae (Engraulis mordax) in patches containing different proportions of large and small prey. In: Steele JH, editor. Spatial Patterns in Plankton Communities. Plenum Press; New York, NY: 1978. pp. 329–354. [Google Scholar]
- Last JM. Fisheries Research Technical ReportMAFF Directorate Fishery Research. Lowestoft; 1980. The food of twenty species of fish larvae in the west-central North Sea; pp. 1–44. [Google Scholar]
- Lee C-K, Park T-G, Park Y-T, Lim W-A. Monitoring and trends in harmful algal blooms and red tides in Korean coastal waters, with emphasis on Cochlodinium polykrikoides. Harmful Algae. 2013;30:S3–S14. [Google Scholar]
- Lelong A, Hegaret H, Soudant P, Bates SS. Pseudo-nitzschia (Bacillariophyceae) species, domoic acid and amnesic shellfish poisoning: revisiting previous paradigms. Phycologia. 2012;51(2):168–216. [Google Scholar]
- Leong SCY, Murata A, Nagashima Y, Taguchi S. Variability in toxicity of the dinoflagellate Alexandrium tamarense in response to different nitrogen sources and concentrations. Toxicon. 2004;43(4):407–415. doi: 10.1016/j.toxicon.2004.01.015. [DOI] [PubMed] [Google Scholar]
- Leu E, Daase M, Schulz KG, Stuhr A, Riebesell U. Effect of ocean acidification on the fatty acid composition of a natural plankton community. Biogeosciences. 2013;10(2):1143–1153. [Google Scholar]
- Levasseur M, Gamache T, St.-Pierre I, Michaud S. Does the cost of NO3 reduction affect the production of harmful compounds by Alexandrium excavatum? In: Lassus P, Arzul G, Erard E, Gentien P, Marcaillou C, editors. Harmful Marine Algal Blooms. Technique et Documentation-Lavoisier, Intercept Ltd; Paris: 1995. pp. 463–468. [Google Scholar]
- Lewis J, Harris ASD, Jones KJ, Edmonds RL. Long-term survival of marine planktonic diatoms and dinoflagellates in stored sediment samples. J. Plankton Res. 1999;21:343–354. [Google Scholar]
- Lewis NI, Bates SS, McLachlan JL, Smith JC. Elsevier Science Publ B V. Amsterdam: 1993. Temperature Effects on Growth, Domoic Acid Production, and Morphology of the Diatom Nitzschiapungens f. multiseries. [Google Scholar]
- Li Y, Smayda TJ. Heterosigma akashiwo (Raphidophyceae): on prediction of the week of bloom initiation and maximum during the initial pulse of its bimodal bloom cycle in Narragansett Bay. Plankton Biol. Ecol. 2000;47(2):80–84. [Google Scholar]
- Li WKW. Temperature adaptation in phytoplankton; cellular and photosynthetic characteristics. In: Falkowski PG, editor. Primary Productivity in the Sea. Plenum Press; NY: 1980. [Google Scholar]
- Lincoln JA, Turner JT, Bates SS, Léger C, Gauthier DA. Feeding, egg production, and egg hatching success of the copepods Acartia tonsa and Temora longicornis on diets of the toxic diatom Pseudo-nitzschia multiseries and the non-toxic diatom Pseudo-nitzschia pungens. Hydrobioloiga. 2001;453/454:107–120. [Google Scholar]
- Locarnini RA, Mishonov V, Antonov JI, Boyer TP, Garcia HE. World Ocean Atlas 2005. Vol. 1. Temperature. U.S. Government Printing Office; Washington, D.C.: 2006. p. 182. [Google Scholar]
- Longhurst A. Ecological Geography of the Sea. Academic Press; San Diego: 1998. p. 398. [Google Scholar]
- Lonnstedt OM, Munday PL, McCormick MI, Ferrari MCO, Chivers DP. Ocean acidification and responses to predators: can sensory redundancy reduce the apparent impacts of elevated CO2 on fish? Ecol. Evol. 2013;3(10):3565–3575. doi: 10.1002/ece3.684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez CB, Jewett EB, Dortch QTWB, Hudnell HK. Hypoxia, and Human Health of the Joint Subcommittee on Ocean Science and Technology. 2008. Scientific Assessment of Freshwater Harmful Algal Blooms. [Google Scholar]
- Loureiro C, Castro BB, Pereira JL, Goncalves F. Performance of standard media in toxicological assessments with Daphnia magna: chelators and ionic composition versus metal toxicity. Ecotoxicology. 2011;20(1):139–148. doi: 10.1007/s10646-010-0565-1. [DOI] [PubMed] [Google Scholar]
- Lundholm N, Hansen PJ, Kotaki Y. Effect of pH on growth and domoic acid production by potentially toxic diatoms of the genera Pseudo-nitzschia and Nitzschia. Mar. Ecol. Prog. Ser. 2004;273:1–15. [Google Scholar]
- MacIntyre HL, Lomas MW, Cornwell J, Suggett DJ, Gobler CJ, Koch EW, Kana TM. Mediation of benthic pelagic coupling by microphytobenthos: an energy- and material-based model for initiation of blooms of Aureococcus anophagefferens. Harmful Algae. 2004;3:403–437. [Google Scholar]
- MacIntyre HL, Stutes AL, Smith WL, Dorsey CP, Abraham A, Dickey RW. Environmental correlates of community composition and toxicity during a bloom of Pseudo-nitzschia spp. in the northern Gulf of Mexico. J. Plankton Res. 2011;33:273–295. [Google Scholar]
- Magan~a HA, Villareal TA. The effect of environmental factors on the growth rate of Karenia brevis (Davis) G. Hansen and Moestrup. Harmful Algae. 2006;5(2):192–198. [Google Scholar]
- Maguer JF, L'Helguen S, Madec C, Labry C, Le Corre P. Nitrogen uptake and assimilation kinetics in Alexandrium minutum (Dynophyceae): effect of N-limited growth rate on nitrate and ammonium interactions. J. Phycol. 2007;43(2):295–303. [Google Scholar]
- Maldonado MT, Hughes M, Rue E, Wells ML. The effect of Fe and Cu on the growth and domoic acid production of Pseudo-nitzschia multiseries and Pseudo-nitzschia australis. Limnol. Oceanogr. 2002;47:515–526. [Google Scholar]
- Manson MD, Tedesco P, Berg HC, Harold FM, Vanderdrift C. Protonmotive force drives bacterial flagella. Proc. Natl. Acad. Sci. U.S.A. 1977;74(7):3060–3064. doi: 10.1073/pnas.74.7.3060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant HJ, Davidson AT, Kelly GJ. UV-B absorbing compounds in the marine alga Phaeocystis pouchetii from Antarctica. Mar. Biol. 1991;109:391–395. [Google Scholar]
- Marinov I, Doney SC, Lima ID. Response of ocean phytoplankton community structure to climate change over the 21st century: partitioning the effects of nutrients, temperature and light. Biogeosciences. 2010;7(12):3941–3959. [Google Scholar]
- Marshall JA, Munday B, Yoshizawa Y, Hallegraeff GM. Effect of irradiance on superoxide production by Chattonella marina (Raphidophyceae) from South Australia and Japan. In: Hallegraeff GM, Blackburn SI, Bolch CJ, Lewis RD, editors. Harmful Algal Blooms 2000. UNESCO; Paris: 2001. pp. 316–319. [Google Scholar]
- Martin JL, Hanke AR, LeGresley MM. Long term phytoplankton monitoring, including harmful algal blooms, in the Bay of Fundy, eastern Canada. J. Sea Res. 2009;61(1–2):76–83. [Google Scholar]
- Martinez R, Orive E, Laza-Martinez A, Seoane S. Growth response of six strains of Heterosigma akashiwo to varying temperature, salinity and irradiance conditions. J. Plankton Res. 2010;32(4):529–538. [Google Scholar]
- Matsuoka K, Mizuno A, Iwataki M, Takano Y, Yamatogi T, Yoon YH, Lee J-B. Seed populations of a harmful unarmored dinoflagellate Cochlodinium polykrikoides Margalef in the East China Sea. Harmful Algae. 2010;9(6):548–556. [Google Scholar]
- McGillicuddy DJ, Townsend DW, He R, Keafer BA, Kleindinst JL, Li Y, Manning JP, Mountain DG, Thomas MA, Anderson DM. Suppression of the 2010 Alexandrium fundyense bloom by changes in physical, biological, and chemical properties of the Gulf of Maine. Limnol. Oceanogr. 2011;56(6):2411–2426. doi: 10.4319/lo.2011.56.6.2411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGillicuddy DJ, Anderson DM, Solow AR, Townsend DW. Interannual variability of Alexandrium fundyense abundance and shellfish toxicity in the Gulf of Maine. Deep Sea Res. Part II: Top. Stud. Oceanogr. 2005;52(19–21):2843–2855. [Google Scholar]
- McKenzie RL, Aucamp PJ, Bais AF, Björn LO, Ilyas M, Madronich S. The environmental effects of ozone depletion and its interactions with climate changeIn. UNEP Assessment Panel Report for 2010; 2010. pp. 1–30. [Google Scholar]
- McManus MA, Kudela RM, Silver MW, Steward GF, Donaghay PL, Sullivan JM. Cryptic blooms: are thin layers the missing connection? Estuaries Coasts. 2008;31(2):396–401. [Google Scholar]
- Mengelt C, Prézelin BB. UVA enhancement of carbon fixation and resilience to UV inhibition in the genus Pseudo-nitzschia may provide a competitive advantage in high UV surface waters. Mar. Ecol. Prog. Ser. 2005;301:81–93. [Google Scholar]
- Mohlin M, Roleda MY, Pattanaik B, Tenne SJ, Wulff A. Interspecific resource competition-combined effects of radiation and nutrient limitation on two diazotrophic filamentous cyanobacteria. Microb. Ecol. 2012;63(4):736–750. doi: 10.1007/s00248-011-9964-y. [DOI] [PubMed] [Google Scholar]
- Mohlin M, Wulff A. Interaction effects of ambient UV radiation and nutrient limitation on the toxic cyanobacterium Nodularia spumigena. Microb. Ecol. 2009;57(4):675–686. doi: 10.1007/s00248-008-9427-2. [DOI] [PubMed] [Google Scholar]
- Moncheva S, Gotsis-Skretas O, Pagou K, Krastev A. Phytoplankton blooms in black sea and Mediterranean coastal ecosystems subjected to anthropogenic eutrophication: similarities and differences. Estuar. Coast. Shelf Sci. 2001;53(3):281–295. [Google Scholar]
- Monteith DT, Stoddard JL, Evans CD, de Wit HA, Forsius M, Hogasen T, Wilander A, Skjelkvale BL, Jeffries DS, Vuorenmaa J, Keller B, Kopacek J, Vesely J. Dissolved organic carbon trends resulting from changes in atmospheric deposition chemistry. Nature. 2007;450(7169):537–U539. doi: 10.1038/nature06316. [DOI] [PubMed] [Google Scholar]
- Montresor M, Lewis J. Phases, stages and shifts in the life cycles of marine phytoplankton. In: Subba-Rao DV, editor. Algal Cultures Analogues of Blooms and Applications. Science Publishers, Enfield; USA: 2006. pp. 91–129. [Google Scholar]
- Moore MN, Depledge MH, Fleming L, Hess P, Lees D, Leonard P, Madsen L, Owen R, Pirlet H, Seys J, Vasconcelos V, Viarengo A, Marine Board ESFWGO. Oceans and Human Health (OHH): a European Perspective from the Marine Board of the European Science Foundation (Marine Board-ESF) Microb. Ecol. 2013;65(4):889–900. doi: 10.1007/s00248-013-0204-5. [DOI] [PubMed] [Google Scholar]
- Moore SK, Mantua NJ, Hickey BM, Trainer VL. The relative influences of El Niño-Southern Oscillation and Pacific Decadal Oscillation on paralytic shell-fish toxin accumulation in northwest Pacific shellfish. Limnol. Oceanogr. 2010;55(6):2262–2274. [Google Scholar]
- Moore SK, Mantua NJ, Hickey BM, Trainer VL. Recent trends in paralytic shellfish toxins in Puget Sound, relationships to climate, and capacity for prediction of toxic events. Harmful Algae. 2009;8(3):463–477. [Google Scholar]
- Moore SK, Mantua NJ, Salathe EP. Past trends and future scenarios for environmental conditions favoring the accumulation of paralytic shellfish toxins in Puget Sound shellfish. Harmful Algae. 2011;10(5):521–529. [Google Scholar]
- Morán SAG, López-Urrutia A, Calvo-Díaz A, Li WKW. Increasing importance of small phytoplanktonin a warmer ocean. Global Change Biol. 2010;16:1137–1144. [Google Scholar]
- Naustvoll LJ, Gustad E, Dahl E. Monitoring of Dinophysis species and diarrhetic shellfish toxins in Flodevigen Bay, Norway: inter-annual variavility over a 25-year time-series. Food Addi. Contamin. A. 2012;29:1605–1615. doi: 10.1080/19440049.2012.714908. [DOI] [PubMed] [Google Scholar]
- Nimer NA, Iglesias-Rodriguez MD, Merrett MJ. Bicarbonate utilization by marine phytoplankton species. J. Phycol. 1997;33(4):625–631. [Google Scholar]
- Nishikawa T, Hori Y, Nagai S, Miyahara K, Hakamura Y, Harada K, Tada K, Imai I. Long time-series observations in population dynamics of the harmful diatom Eucampia zodiacus and environmental factors in Harima-Nada, eastern Seto Inland Sea, Japan during 1974–2008. Plankton Benthos Res. 2011;6:26–34. [Google Scholar]
- Nishikawa T, Hori Y, Nagai S, Miyahara K, Nakamura Y, Harada K, Tada K, Imai I. Long-term (36-year) observations on the dynamics of the fish-killing raphidophyte Chattonella in Harima-Nada, eastern Seto Inland Sea, Japan. J. Oceanogr. 2014;70(2):153–164. [Google Scholar]
- Nishikawa T, Hori Y, Nagai S, Miyahara K, Nakamura Y, Harada K, Tanda M, Manabe T, Tada K. Nutrient and phytoplankton dynamics in Harima-Nada, Eastern Seto Inland Sea, Japan during a 35-year period from 1973 to 2007. Estuaries Coasts. 2009;33(2):417–427. [Google Scholar]
- Ogata T, Kodama M, Ishimaru T. Effect of water temperature and light intensity on growth rate and toxin production of toxic dinoflagellates. In: Okaichi T, Anderson DM, Nemoto T, editors. Red Tides, Biology, Environmental Science and Toxicology. Elsevier, NY: 1989. pp. 423–426. [Google Scholar]
- Okolodkov YB. Species range types of recent marine dinoflagellates recorded from the Arctic. Grana. 1999;38(2–3):162–169. [Google Scholar]
- Okolodkov YB. The global distributional patterns of toxic, bloom dinoflagellates recorded from the Eurasian Arctic. Harmful Algae. 2005;4(2):351–369. [Google Scholar]
- Orr JC, Fabry VJ, Aumont O, Bopp L, Doney SC, Feely RA, Gnanadesikan A, Gruber N, Ishida A, Joos F, Key RM, Lindsay K, Maier-Reimer E, Matear R, Monfray P, Mouchet A, Najjar RG, Plattner GK, Rodgers KB, Sabine CL, Sarmiento JL, Schlitzer R, Slater RD, Totterdell IJ, Weirig MF, Yamanaka Y, Yool A. Anthropogenic ocean acidification over the twenty-first century and its impact on calcifying organisms. Nature. 2005;437(7059):681–686. doi: 10.1038/nature04095. [DOI] [PubMed] [Google Scholar]
- Paerl HW, Hall NS, Peierls BL, Rossignol KL. Evolving paradigms and challenges in estuarine and coastal eutrophication dynamics in a culturally and climatically stressed world. Estuaries Coasts. 2014;37(2):243–258. [Google Scholar]
- Pattanaik B, Wulff A, Roleda MY, Garde K, Mohlin M. Production of the cyanotoxin nodularin - a multifactorial approach. Harmful Algae. 2010;10:30–38. [Google Scholar]
- Peacock MB, Kudela RM. Evidence for active vertical migration by two dinoflagellates experiencing iron, nitrogen, and phosphorus limitation. Limnol. Oceanogr. 2014;59(3):660–673. [Google Scholar]
- Perez CC, Roy S, Levasseur M, Anderson DM. Control of germination of Alexandrium tamarense (Dinophyceae)_cysts from the lower St. Lawrence estuary (Canada) J. Phycol. 1998;34:242–249. [Google Scholar]
- Pérez F, Padin X, Pazos Y, Gilcoto M, Cabanas M, Pardo PC, Doval M, Farina-Busto L. Plankton response to weakening of the Iberian coastal upwelling. Glob. Change Biol. 2010;16:1258–1267. [Google Scholar]
- Pettersson LH, Pozdnyakov D. Biology and Ecology of Harmful Algal Species. Springer; Berlin Heidelberg: 2013. pp. 25–47. [Google Scholar]
- Pfiester LA, Anderson DM. Dinoflagellate reproduction. In: Taylor FJR, editor. The Biology of Dinoflagellates. Blackwell Scientific Publications Ltd; Boston: 1987. pp. 611–648. [Google Scholar]
- Pitcher GC, Calder D. Harmful algal blooms of the southern Benguela Current: a review and appraisal of monitoring from 1989 to 1997. South Afr. J. Mar. Sci. 2000;22:255–271. [Google Scholar]
- Pitcher GC, Horstman DA, Calder D, Debruyn JH, Post BJ. The first record of diarrhetic shellfish poisoning on the south-african coast. S. Afr. J. Sci. 1993;89(10):512–514. [Google Scholar]
- Preston CM, Harris A, Ryan JP, Roman B, Marin R, Jensen S, Everlove C, Birch J, Dzenitis JM, Pargett D, Adachi M, Turk K, Zehr JP, Scholin CA. Underwater application of quantitative PCR on an ocean mooring. PLoS ONE. 2011;6(8):12. doi: 10.1371/journal.pone.0022522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabalais NN, Diaz RJ, Levin LA, Turner RE, Gilbert D, Zhang J. Dynamics and distribution of natural and human-caused hypoxia. Biogeosciences. 2010;7(2):585–619. [Google Scholar]
- Raine R. A review of the biophysical interactions relevant to the promotion of HABs in stratified systems: the case study of Ireland. Deep-Sea Res., II—Top. Stud. Oceanogr. 2014;101:21–31. [Google Scholar]
- Ralston DK, Keafer BA, Brosnahan ML, Anderson DM. Temperature dependence of an estuarine harmful algal bloom: resolving interannual variability in bloom dynamics using a degree day approach. Limnol. Oceanogr. 2014;59(4):1112–1126. doi: 10.4319/lo.2014.59.4.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratti S, Giordano M, Morse D. CO2-concentrating mechanisms of the potentially toxic dinoflagellate Protoceratium reticulatum (Dinophyceae, Gonyaulacales) J. Phycol. 2007;43(4):693–701. [Google Scholar]
- Raven JA, Beardall J. CO2 concentrating mechanisms and environmental change. Aquat. Bot. 2014;118:24–37. [Google Scholar]
- Raven JA, Geider RJ. Temperature and algal growth. New Phytol. 1988;110:411–416. [Google Scholar]
- Rensel JEJ, Haigh N, Tynan TJ. Fraser river sockeye salmon marine survival decline and harmful blooms of Heterosigma akashiwo. Harmful Algae. 2010;10:98–115. [Google Scholar]
- Rhodes LL, O'Kelly HJA. Comparison of growth characteristics of New Zealand isolates of the prymnesiophytes Chrysochromulina quadrikonta and C. camella with those of the ichthyotoxic species C. polylepis. J. Plankton Res. 1994;16:69–82. [Google Scholar]
- Riebesell U, Fabry VJ, Hansson L, Gattuso J-P. Guide to Best Practices for Ocean Acidification Research and Data Reporting. Publications Office of the European Union; Luxembourg: 2010. p. p260. [Google Scholar]
- Rines JEB, Donaghay PL, Dekshenieks MM, Sullivan JM, Twardowski MS. Thin layers and camouflage: hidden Pseudo-nitzschia spp. (Bacillariophyceae) populations in a fjord in the San Juan Islands, Washington, USA. Mar. Ecol. Prog. Ser. 2002;225:123–137. [Google Scholar]
- Roemmich D, Gould WJ, Gilson J. 135 years of global ocean warming between the Challenger expedition and the Argo Programme. Nat. Clim. Change. 2012;2(6):425–428. [Google Scholar]
- Rongo T, van Woesik R. Ciguatera poisoning in Rarotonga, southern Cook Islands. Harmful Algae. 2011;10(4):345–355. [Google Scholar]
- Rost B, Richter KU, Riebesell U, Hansen PJ. Inorganic carbon acquisition in red tide dinoflagellates. Plant Cell Environ. 2006;29(5):810–822. doi: 10.1111/j.1365-3040.2005.01450.x. [DOI] [PubMed] [Google Scholar]
- Rost B, Riebesell U, Burkhardt S, Sultemeyer D. Carbon acquisition of bloom-forming marine phytoplankton. Limnol. Oceanogr. 2003;48(1):55–67. [Google Scholar]
- Roy E, Wells ML. Regulation of Fe(II) oxidation rates by organic complexing ligands in the eastern subarctic Pacific. Mar. Chem. 2011;127:115–122. [Google Scholar]
- Ruiz GM, Carlton JT, Grosholz ED, Hines AH. Global invasions of marine and estuarine habitats by non-indigenous species: mechanisms, extent, and consequences. Am. Zool. 1997;37:621–634. [Google Scholar]
- Ruiz S, Etheridge SM, Cook PA, O'Ryan C, Pitcher GC. Phylogenetic analysis of toxic Alexandrium (Dinophyceae) isolates from South Africa: implications for the global phylogeography of the Alexandrium tamarense species complex. Phycologia. 2005;44:49–60. [Google Scholar]
- Ryan JP, McManus MA, Kudela RM, Artigas ML, Bellingham JG, Chavez FP, Doucette G, Foley D, Godin M, Harvey JBJ, Marin R, Messie M, Mikulski C, Pennington T, Py F, Rajan K, Shulman I, Wang Z, Zhang Y. Boundary influences on HAB phytoplankton ecology in a stratification-enhanced upwelling shadow. Deep-Sea Res., II—Top. Stud. Oceanogr. 2014;101:63–79. [Google Scholar]
- Ryan JP, McManus MA, Paduan JD, Chavez FP. Phytoplankton thin layers caused by shear in frontal zones of a coastal upwelling system. Mar. Ecol. Prog. Ser. 2008;354:21–34. [Google Scholar]
- Ryan JP, McManus MA, Sullivan JM. Interacting physical, chemical and biological forcing of phytoplankton thin-layer variability in Monterey Bay, California. Cont. Shelf Res. 2010;30(1):7–16. [Google Scholar]
- Salomon PS, Umai I. Pathogens of harmful algae. In: Granéli E, Turner JT, editors. Ecology of Harmful Algae. Springer; Heidelberg: 2006. pp. 271–282. [Google Scholar]
- Samson JC, Shumway SE, Weis JS. Effects of the toxic dinoflagellate Alexandrium fundyense on three species of larval fish: a food-chain approach. J. Fish Biol. 2008;72:168188. [Google Scholar]
- Scholin CA, Hallegraeff GM, Anderson DM. Molecular evolution of the Alexandrium tamarense `species complex' (Dinophyceae): dispersal in North American and West Pacific regions. Phycologia. 1995;34:472–485. [Google Scholar]
- Schultz M, Kjørboe T. Active prey selection in two pelagic copepods feeding on potentially toxic and non-toxic dinoflagellates. J. Plankton Res. 2009;31:553–561. [Google Scholar]
- Selina MS, Morozova TV, Vyshkvartsev DI, Orlova TY. Seasonal dynamics and spatial distribution of epiphytic dinoflagellates in Peter the Great Bay (Sea of Japan) with special emphasis on Ostreopsis species. Harmful Algae. 2014;32:1–10. [Google Scholar]
- Seneviratne SI, Nichollsm N, Easterling D, Goodess CM, Kanae S, Kossin J, Luo Y, Marengo J, McInnes K, Rahimi M, Reichstein M, Sorteberg A, Vera C, Zhang X. Changes in climate extremes and their impacts on the natural physical environment. In: Field CB, Barros V, Stocker TF, Dahe Q, Dokken DJ, Ebi KL, Mastrandrea MD, Mach KJ, Plattner G-K, Allen SK, Tignor M, Midgley PM, editors. Managing the Risks of Extreme Events and Disasters to Advance Climate Change Adaptation. A Special Report of Working Groups I and II of the Intergovernmental Panel on Climate Change (IPCC) Cambridge University Press; Cambridge, UK, and New York, NY, USA: 2012. pp. 109–203. [Google Scholar]
- Shi DL, Xu Y, Hopkinson BM, Morel FMM. Effect of ocean acidification on iron availability to marine phytoplankton. Science. 2010;327(5966):676–679. doi: 10.1126/science.1183517. [DOI] [PubMed] [Google Scholar]
- Shimizu Y, Watanabe N, Wrensford G. Biosynthesis of brevetoxins and heterotrophic metabolism in Gymnodinium breve. In: Lassus P, Arzul G, Erard E, Gentien P, Marcaillou C, editors. Harmful Marine Algal Blooms. Technique et Documentation-Lavoisier, Intercept Ltd; Paris: 1995. pp. 351–357. [Google Scholar]
- Shumway SE. A review of the effects of algal blooms on shellfish and aquaculture. J. World Aquacult. Soc. 1990;21:65–104. [Google Scholar]
- Shumway SE, Allen SM, Dee Boersma P. Marine birds and harmful algal blooms: sporadic victims or under-reported events? Harmful Algae. 2003;2:1–17. [Google Scholar]
- Singh A, Hårding K, Reddy HRV, Godhe A. An assessment of Dinophysis blooms in the coastal Arabian Sea. Harmful Algae. 2014;34:29–35. [Google Scholar]
- Smayda TJ. Global epidemic of noxious phytoplankton blooms and food chain consequences in large ecosystems. In: Sherman K, Alexander L, Gold BD, editors. Food Chains, Yields, Models, and Management of Large Marine Ecosystems. Westview Press; San Francisco: 1992. pp. 275–307. [Google Scholar]
- Smayda TJ. Dinoflagellate bloom cycles: what is the role of cellular growth rate and bacteria? In: Yasumoto T, Oshima Y, Fukuyo Y, editors. Harmful and Toxic Algal Blooms. Intergovernmental Commission on Oceanography of UNESCO; Paris: 1996. pp. 331–334. [Google Scholar]
- Smayda TJ. What is a bloom? A commentary. Limnol. Oceanogr. 1997;42(5):1132–1136. [Google Scholar]
- Smayda TJ. Patterns of variability characterizing marine phytoplankton, with examples from Narragansett Bay. ICES J. Mar. Sci. 1998;55(4):562–573. [Google Scholar]
- Smayda TJ. Turbulence, watermass stratification and harmful algal blooms: an alternative view. Harmful Algae. 2002;1:95–112. [Google Scholar]
- Smayda TJ, Borkman D, Beaugrand G, Belgrano A. Ecological Effects of Climate Variation in the North Atlantic: Phytoplankton. Oxford University Press; 2004. [Google Scholar]
- Smayda TJ. Reflections on the ballast water dispersal-harmful algal bloom paradigm. Harmful Algae. 2007;6:601–622. [Google Scholar]
- Smayda TJ. Complexity in the eutrophication-harmful algal bloom relationship, with comment on the importance of grazing. Harmful Algae. 2008;8:140–151. [Google Scholar]
- Smayda TJ, Borkman D. Nutrient and phytoplankton gradients in Narragansett Bay. In: Desbonnet A, Costa-Pierce B, editors. Science for Ecosystem-Based Management: Narragansett Bay in the 21st Century. Springer; Berlin: 2008. pp. 423–476. [Google Scholar]
- Smayda TJ, Trainer VL. Dinoflagellate blooms in upwelling systems: seeding, variability, and contrasts with diatom bloom behaviour. Prog. Oceanogr. 2010;85(1–2):92–107. [Google Scholar]
- Smetacek V. The annual cycle of protozooplankton in the Kiel Bight. Mar. Biol. 1981;63:1–11. [Google Scholar]
- Starr M, Himmelman JH, Therriault JC. Direct coupling of marine invertebrate spawning with phytoplankton blooms. Science. 1990;247(4946):1071–1074. doi: 10.1126/science.247.4946.1071. [DOI] [PubMed] [Google Scholar]
- Sterner RW, Andersen T, Elser JJ, Hessen DO, Hood JM, McCauley E, Urabe J. 2008 Scale-dependent carbon: nitrogen: phosphorus seston stoichiometry in marine and freshwaters. Limnol. Oceanogr. 53(3):1169–1180. [Google Scholar]
- Stock CA, McGillicuddy DJ, Solow AR, Anderson DM. Evaluating hypotheses for the initiation and development of Alexandrium fundyense blooms in the western Gulf of Maine using a coupled physical-biological model. Deep-Sea Res., II—Top. Stud. Oceanogr. 2005;52(19–21):2715–2744. [Google Scholar]
- Stocker TF, Qin D, Plattner G-K, Tignor M, Allen SK, Boschung J, Nauels A, Xia Y, Bex V, Midgle PM. IPCC, 2013: climate change 2013: the physical science basis. Contribution of Working Group I to the Fifth Assessment Report of the Intergovern-mental Panel on Climate ChangeCambridge University Press; Cambridge, United Kingdom and New York, NY USA. 2013. p. 1535. [Google Scholar]
- Stoecker DK, Evans GT. Effects of protozoan herbivory and carnivory in a microplankton food web. Mar. Ecol. Prog. Ser. 1985;24:159–167. [Google Scholar]
- Strom SL, Harvey EL, Fredrickson KA, Menden-Deuer S. Broad salinity tolerance as a refuge from predation in the harmful raphidophyte alga Heterosigma akashiwo (Raphidophyceae) J. Phycol. 2013;49(1):20–31. doi: 10.1111/jpy.12013. [DOI] [PubMed] [Google Scholar]
- Strom Z. Novel interactions between phytoplankton and microzooplankton: their influence on the coupling between growth and grazing rates in the sea. Hydrobiologia. 2002;180:41–54. [Google Scholar]
- Suikkanen S, Laamanen M, Huttunen M. Long-term changes in summer phytoplankton communities of the open northern Baltic Sea. Estuar. Coast. Shelf Sci. 2007;71(3–4):580–592. [Google Scholar]
- Sullivan JM, Van Holliday D, McFarland M, McManus MA, Cheriton OM, Benoit-Bird KJ, Goodman L, Wang ZK, Ryan JP, Stacey M, Greenlaw C, Moline MA. Layered organization in the coastal ocean: an introduction to planktonic thin layers and the LOCO project. Cont. Shelf Res. 2010;30(1):1–6. [Google Scholar]
- Sun J, Hutchins DA, Feng YY, Seubert EL, Caron DA, Fu FX. Effects of changing pCO(2) and phosphate availability on domoic acid production and physiology of the marine harmful bloom diatom Pseudo-nitzschia multiseries. Limnol. Oceanogr. 2011;56(3):829–840. [Google Scholar]
- Sun Y, Solomon S, Dai A, Portmann RW. How often will it rain? J. Clim. 2007;20(19):4801–4818. [Google Scholar]
- Sunda WG. Trace metal interactions with marine phytoplankton. Biol. Oceanogr. 1989;6:411–442. [Google Scholar]
- Sunda WG, Shertzer KW. Modeling ecosystem disruptive algal blooms: positive feedback mechanisms. Mar. Ecol. Prog. Ser. 2012;447:31–U69. [Google Scholar]
- Tang EPY. Why do dinoflagellates have lower growth rates? J. Phycol. 1996;32(80–84) [Google Scholar]
- Tang DL, Di BP, Wei G, Ni I-H, Oh ISSFW. Spatial, seasonal and species variations of harmful algal blooms in the South Yellow Sea and East China Sea. Hydrobiologia. 2006;568:245–253. [Google Scholar]
- Tatters AO, Flewelling LJ, Fu FX, Granholm AA, Hutchins DA. High CO2 promotes the production of paralytic shellfish poisoning toxins by Alexandrium catenella from Southern California waters. Harmful Algae. 2013a;30:37–43. [Google Scholar]
- Tatters AO, Fu FX, Hutchins DA. High CO2 and silicate limitation synergistically increase the toxicity of Pseudo-nitzshia fraudulenta. PLoS ONE. 2012;7(2) doi: 10.1371/journal.pone.0032116. http://dx.doi.org/10.1371/journal.pone.0032116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatters AO, Roleda MY, Schnetzer A, Fu FX, Hurd CL, Boyd PW, Caron DA, Lie AAY, Hoffmann LJ, Hutchins DA. Short- and long-term conditioning of a temperate marine diatom community to acidification and warming. Philos. Trans. R. Soc., Ser. B—Biol. Sci. 2013b;368(1627) doi: 10.1098/rstb.2012.0437. http://dx.doi.org/10.1098/rstb.2012.0437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tatters AO, Schnetzer A, Fu FX, Lie AYA, Caron DA, Hutchins DA. Short- versus long-term responses to changing CO2 in a coastal dinoflagellate bloom: implications for interspecific competitive interactions and community structure. Evolution. 2013c;67(7):1879–1891. doi: 10.1111/evo.12029. [DOI] [PubMed] [Google Scholar]
- Taylor M, McIntyre L, Ritson M, Stone J, Bronson R, Bitzikos O, Rourke W, Galanis E. Outbreak of diarrhetic shellfish poisoning associated with mussels, British Columbia, Canada. Mar. Drugs. 2013;11(5):1669–1676. doi: 10.3390/md11051669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teegarden GJ. Copepod grazing selection and particle discrimination on the basis of PSP toxin content. Mar. Ecol. Prog. Ser. 1999;181:163–176. [Google Scholar]
- Teegarden GJ, Campbell RG, Durbin EG. Zooplankton feeding behavior and particle selection in natural plankton assemblages containing toxic Alexandrium spp. Mar. Ecol. Prog. Ser. 2001;218:213–226. [Google Scholar]
- Teegarden GJ, Cembella AD. Grazing of toxic dinoflagellates, Alexandrium spp., by adult copepods of coastal Maine: implications for the fate of paralytic shellfish toxins in marine food webs. J. Exp. Mar. Biol. Ecol. 1996;196:145–176. [Google Scholar]
- Tester PA, Feldman RL, Nau AW, Kibler SR, Wayne Litaker R. Ciguatera fish poisoning and sea surface temperatures in the Caribbean Sea and the West Indies. Toxicon. 2010;56(5):698–710. doi: 10.1016/j.toxicon.2010.02.026. [DOI] [PubMed] [Google Scholar]
- Thessen AE, Bowers HA, Stoecker DK. Intra- and interspecies differences in growth and toxicity of Pseudo-nitzschia while using different nitrogen sources. Harmful Algae. 2009;8(5):792–810. [Google Scholar]
- Thornton JA, Harding WR, Dent M, Hart RC, Lin H, Rast CL, Rast W, Ryding S-O, Slawski TM. Eutrophication as a `wicked' problem. Lakes Reserv. Res. Manage. 2013;18(4):298–316. [Google Scholar]
- Tortell PD. Evolutionary and ecological perspectives on carbon acquisition in phytoplankton. Limnol. Oceanogr. 2000;45(3):744–750. [Google Scholar]
- Tortell PD, DiTullio GR, Sigman DM, Morel FMM. CO2 effects on taxonomic composition and nutrient utilization in an Equatorial Pacific phytoplankton assemblage. Mar. Ecol. Prog. Ser. 2002;236:37–43. [Google Scholar]
- Trainer VL, Cochlan WP, Erickson A, Bill BD, Cox FH, Borchert JA, Lefebvre KA. Recent domoic acid closures of shellfish harvest areas in Washington State inland waterways. Harmful Algae. 2007;6(3):449–459. [Google Scholar]
- Trainer VL, Eberhart B-TL, Wekell JC, Adams NA, Hanson L, Cox F, Dowell J. Paralytic shellfish toxins in Puget Sound, Washington State. J. Shellfish Res. 2003;22(1):213–224. [Google Scholar]
- Trainer VL, Hickey BM, Lessard EJ, Cochlan WP, Trick CG, Wells ML, MacFadyen A, Moore SK. Variability of Pseudo-nitzschia and domoic acid in the Juan de Fuca eddy region and its adjacent shelves. Limnol. Oceanogr. 2009;54(1):289–308. [Google Scholar]
- Trainer VL, Moore L, Bill BD, Adams NG, Harrington N, Borchert J, da Silva DAM, Eberhart BTL. Diarrhetic shellfish toxins and other lipophilic toxins of human health concern in Washington State. Mar. Drugs. 2013;11(6):1815–1835. doi: 10.3390/md11061815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trainer VL, Suddleson M. Monitoring approaches for early warning of domoic acid events in Washington state. Oceanography. 2005;18(2):228–237. [Google Scholar]
- Trainer V, Yoshida M. Proceedings of the workshop on economic impacts of harmful algal blooms on fisheries and aquacultureIn. PICES Sci. Rep. 2014:85. [Google Scholar]
- Trainer VA, Hickey BM, Horner RA. Biological and physical dynamics of domoic acid production off the Washington coast. Limnol. Oceanogr. 2002;47:1438–1446. [Google Scholar]
- Trainer VL, Bates SS, Lundholm N, Thessen AE, Cochlan WP, Adams NG, Trick CG. Pseudo-nitzschia physiological ecology, phylogeny, toxicity, monitoring and impacts on ecosystem health. Harmful Algae. 2012;14:271–300. [Google Scholar]
- Trimborn S, Lundholm N, Thoms S, Richter KU, Krock B, Hansen PJ, Rost B. Inorganic carbon acquisition in potentially toxic and non-toxic diatoms: the effect of pH-induced changes in seawater carbonate chemistry. Physiol. Plant. 2008;133(1):92–105. doi: 10.1111/j.1399-3054.2007.01038.x. [DOI] [PubMed] [Google Scholar]
- Turner JT. Harmful algae interactions with marine planktonic grazers. In: Granéli E, Turner JT, editors. Ecology of Harmful Algae. Springer; Heidelberg: 2006. pp. 259–270. [Google Scholar]
- Turner JT, Borkman DG, Lincoln JA, Gauthier DA, Petitpas CM. Plankton studies in Buzzards Bay, Massachusetts, USA. VI. Phytoplankton and water quality, 1987 to 1998. Phytoplankton Water Qual. 2009;376:103–122. [Google Scholar]
- Uye SI, Takamatsu K. Feeding interactions between planktonic copepods and red tide flagellates from Japanese coastal waters. Mar. Ecol. Prog. Ser. 1990;49:97–107. [Google Scholar]
- Van de Waal DB, Verspagen JM, Finke JF, Vournazou V, Immers AK, Kardinaal WE, Tonk L, Becker S, Van Donk E, Visser PM, Huisman J. Reversal in competitive dominance of a toxic versus non-toxic cyanobacterium in response to rising CO2. ISME J. 2011;5(9):1438–1450. doi: 10.1038/ismej.2011.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldbusser GG, Hales B, Langdon CJ, Haley BA, Schrader P, Brunner EL, Gray MW, Miller CA, Gimenez I. Saturation-state sensitivity of marine bivalve larvae to ocean acidification. Nat. Clim. Change. 2015;5(3):273–280. doi: 10.1371/journal.pone.0128376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsby AE, Hayes PK, Boje R. The gas vesicles, buoyancy and vertical distribution of cyanobacteria in the Baltic Sea. Eur. J. Phycol. 1995;30(2):87–94. [Google Scholar]
- Wells ML, Trick CG, Cochlan WP, Hughes P, Trainer VL. Domoic acid: the synergy of iron, copper and the toxicity of diatoms. Limnol. Oceanogr. 2005;50(6):1908–1917. [Google Scholar]
- White AW, Fukuhara O, Anraku M. Mortality of fish larvae from eating toxic dinoflagellates or zooplankton containing dinoflagellate toxins. In: Okaichi T, Anderson DM, Nemoto T, editors. Red Tides: Biology, Environmental Science and Toxicology. Elsevier; Amsterdam: 1989. pp. 3–8. [Google Scholar]
- Widdicombe CE, Eloire D, Harbour D, Harris RP, Somerfield PJ. Long-term phytoplankton community dynamics in the Western English Channel. J. Plankton Res. 2010;32(5):643–655. [Google Scholar]
- Wu Z, Yu Z, Song X, Yuan Y, Cao X, Liang Y. The spatial and temporal characteristics of harmful algal blooms in the southwest Bohai Sea. Cont. Shelf Res. 2013;59(0):10–17. [Google Scholar]
- Yamochi S, Joh H. Effects of temperature on the vegetataive cell liberation of seven species of red-tide algae from the bottom mud in Osaka Bay, Japan. J. Oceanogr. Soc. Jpn. 1986;42:266–275. [Google Scholar]
- Yoshimatsu T, Yamaguchi H, Iwamoto H, Nishimura T, Adachi M. Effects of temperature, salinity and their interaction on growth of Japanese Gambierdiscus spp. (Dinophyceae) Harmful Algae. 2014;35:29–37. [Google Scholar]
- Zhang Y, Fu F-X, Whereat E, Coyne KJ, Hutchins DA. Bottom-up controls on a mixed-species HAB assemblage: a comparison of sympatric Chattonella subsalsa and Heterosigma akashiwo (Raphidophyceae) isolates from the Delaware Inland Bays, USA. Harmful Algae. 2006;5(3):310–320. [Google Scholar]