Abstract
Microbial contamination of cell culture is a major problem encountered both in academic labs and in the biotechnology/pharmaceutical industries. A broad spectrum of microbes including mycoplasma, bacteria, fungi, and viruses are the causative agents of cell culture contamination. Unfortunately, the existing disinfection techniques lack selectivity and/or lead to the development of drug-resistance, and more importantly there is no universal method to address all microbes. Here, we report a novel, chemical-free visible ultrashort pulsed laser method for cell culture disinfection. The ultrashort pulsed laser technology inactivates pathogens with mechanical means, a paradigm shift from the traditional pharmaceutical and chemical approaches. We demonstrate that ultrashort pulsed laser treatment can efficiently inactivate mycoplasma, bacteria, yeast, and viruses with good preservation of mammalian cell viability. Our results indicate that this ultrashort pulsed laser technology has the potential to serve as a universal method for the disinfection of cell culture.
Index Terms: Photonic disinfection, ultrashort pulsed laser, cell cultures, pathogens
I. INTRODUCTION
Microbial contamination of cell culture affects both academia and the biotechnology/pharmaceutical industries, and can have disastrous consequences including the loss of entire experiments or products. The primary causative agents of cell culture contamination include mycoplasma, bacteria, fungi (including yeasts), and viruses [1,2]. Certain contaminants such as bacteria and fungi are more easily detected since they cause cytotoxicity and an increase in the turbidity of culture media; others, such as mycoplasma and viruses, often require special assays for detection.
There are a few papers in the literature addressing disinfection of cell cultures or tissues with light through photodynamic and/or photocatalytic processes that requires addition of materials such as semiconductor nanoparticles or photosensitizers. [3–12].
Antibiotics are often used to inhibit bacterial and fungal growth in cell culture. However, routine antibiotic use leads to the development of resistant microorganisms [2]. In addition, prolonged exposure to chemicals such as antibiotics often results in adverse effects such as altered cell metabolism or behavior. Furthermore, antibiotics are ineffective against mycoplasma and viral contamination. On the other hand, physical techniques such as ultraviolet (UV) light, gamma rays, heat, and microwave radiation all cause indiscriminate destruction of both the cultured cells and microbes. Thus, there is a need for a universal disinfection method that is capable of selectively inactivating these microorganisms without causing toxicity to the cultured cells.
Ultrashort pulsed (USP) laser technology has recently emerged as a novel chemical-free method for pathogen inactivation [13]. Previous studies indicate that visible USP lasers inactivate pathogens by vibrational excitation of protein structures via impulsive stimulated Raman scattering [14–16]. This inactivation mechanism provides several advantages over existing disinfection techniques. Unlike UV light, visible light shows negligible intrinsic absorption by proteins and nucleic acids, and thus USP laser treatment does not heat up the sample. Relative to gamma rays and UV light, the photons in visible USP lasers do not have sufficient energy to disrupt covalent structures in biological macromolecules, and therefore have less concern of adverse effects on desirable entities such as mammalian cells. Finally, the USP laser method does not involve adding any chemical or biological agents. USP laser treatment was previously shown to efficiently inactivate a wide variety of enveloped/nonenveloped, DNA/RNA viruses including human immunodeficiency virus (HIV) [17], human papillomavirus (HPV) [18], murine cytomegalovirus (MCMV) [19], murine norovirus (MNV) [16], encephalomyocarditis virus (EMCV) [20], and M13 bacteriophage, as well as bacteria including E- coli [21] and Salmonella typhimurium [20].
We have recently demonstrated the selectivity of this visible USP laser technology. For instance, irradiation of a visible USP laser can (1) significantly reduce pathogen infectivity, while retaining ≥ 70% of most of the coagulation factors in human plasma [22]; (2) inactivate enveloped viruses through laser-driven aggregation of viral capsid proteins without altering the structure of surface proteins -- hemagglutinin, leading to the very efficient generation of USP laser-inactivated whole influenza virus vaccines [23,24]. In this report, we show that, in addition to bacteria and viruses, visible USP laser treatment can efficiently inactivate mycoplasma and yeast, which are major cell culture contaminants, with good preservation of the viability of mammalian cell lines. Our findings suggest that this visible USP laser technology has the potential to serve as a universal method for the disinfection of cell culture.
II. MATERIALS AND METHODS
Mycoplasma
M. Orale (Arizona State University, Tempe, AZ), which had a titer of about 1.0 × 108 cfu/ml, was diluted in yeast extract and horse serum solution for laser treatment. Viability assays were performed on agar plates and was expressed in cfu. A. laidlawii (Arizona State University, Tempe, AZ), which had a titer of about 1.2 × 108 cfu/ml, was diluted in BSA, glucose monohydrate and polypeptone solution for laser treatment. Viability assays were performed on agar plates and results were expressed in cfu.
Yeast
Saccharomyces cerevisiae yeast cells (Washington University, St. Louis, MO) were used. Yeast cells were grown to exponential phase and diluted to a concentration of 4 × 104 cfu/mL in water for laser treatment. Viability assays were performed on standard rich media (1% yeast extract, 2% peptone, 2% dextrose(YPD)) plates with incubation at 30°C for 3 days. Results were expressed in cfu.
Bacteria
We chose E-coli and Salmonella typhimurium as representatives for gram-negative bacteria and listeria for gram-positive bacteria in our study.
E. coli, Salmonella typhimurium and Listeria samples (Arizona State University, Tempe, AZ), which had titers of about 1.5 × 108 cfu/ml, 2.0 × 108 cfu/ml, and 1.0 × 108 cfu/ml, respectively, were diluted in phosphate buffered saline (PBS) solution for laser treatment. Viability assays were performed on agar plates and results were expressed in colony forming units (cfu).
Viruses
We picked M13 bacteriophage as a representative for non-enveloped viruses and MCMV for enveloped viruses in the study.
Murine cytomegalovirus (MCMV) samples (Washington University, St Louis, MO) and infectivity assay have been previously described [19]. The titer used was 5 ×107/ml. Tissue culture infective dose (TCID50) assay was used as viability assay, and results were expressed in plaque-forming units (pfu).
M13 bacteriophage samples (Stratagene, La Jolla, CA) were diluted in PBS buffer solution for laser treatment. The typical titer used in the experiments was 5 × 107/cm3. Viability assays were performed on cultured bacteria grown on agar plates, and results were expressed in pfu.
Mammalian cell lines
A3.01 cells (human T cell line) were obtained from Dr. Thomas Folks of the NIH AIDS Reagent Program, Division of AIDS, NIAID, NIH. A3.01 cells were cultured in RPMI supplemented with 10 mM HEPES, 2 mM L-glutamine, and 10% heat inactivated fetal bovine serum. Density of cells per vial was 1 × 106 and viability of cells was measured according to trypan blue exclusion.
IC-29 (murine macrophage) and HEK293 (human embryonic kidney) cell lines were obtained from American Type Culture Collection (Manassas, VA). U937 (human monocyte) cells were previously described [25]. These cell lines were cultured in DMEM/F12 medium supplemented with 10% FBS, 0.1 mg/ml streptomycin, and 0.1 unit/ml penicillin.
Cells were diluted in complete medium to a final concentration of 1×106/ml. 120μl of cells were treated by laser irradiation for 90 min at ambient temperature under stirring conditions. Trypan Blue solution (Sigma-Aldrich Co. St. Louis, MO) was used for testing of cell viability.
Femtosecond laser irradiation
Fig. 1 shows the experimental setup for selective photonic disinfection employed in this work. It is a diode-pumped cw mode-locked Ti-sapphire laser system, which includes A 5Ws-cw diode-pumped laser operated at a wavelength of 532 nm (Coherent Inc. Santa Clara, CA) and a Ti-sapphire laser (Del Mar Photonics Inc., San Diego, CA) [13–24]. The laser produced a continuous train of 60-fs pulses at a repetition rate of 80 MHz. The output of the second harmonic generation system (Coherent Inc. Santa Clara, CA) of the mode-locked Ti-sapphire laser was used to irradiate the sample. The excitation laser was chosen to operate at a wavelength of λ= 415 ± 5 nm and with an average power of approximately 80 mW. It has a pulse width of full-width at half maximum (FWHM) = 100 fs. An achromatic lens with a focus length of 5 cm (Melles Griot Inc. Rochester, NY) was used to focus the laser beam so that the tightest laser region was a cylinder with about 50 μm in diameter and about 2.5 mm in height within the sample. Samples of bacteria, yeast, mycoplasma, virus, or mammalian cells in buffered solutions were loaded inside a Pyrex vial. The samples had a volume of about 120 μl and were irradiated for 1.5 hours. The Pyrex vial is a capped cylinder with a diameter of about 1.0 cm and a height of about 3.0 cm (VWR International Inc. Radnor, PA). In order to facilitate the interaction of laser with the microorganisms/cells, a magnetic stirring system (Corning Inc. Corning, NY) was used and operated at a stirring rate of about 0.5 Hz so that the microorganisms/cells would enter the laser-focused cylindrical volume as described above and interact with the photons. Controls were similarly stirred but without laser irradiation. Irradiation was carried out at 22°C and with the single laser beam excitation configuration. All experiments were carried out in triplicate.
Fig. 1.
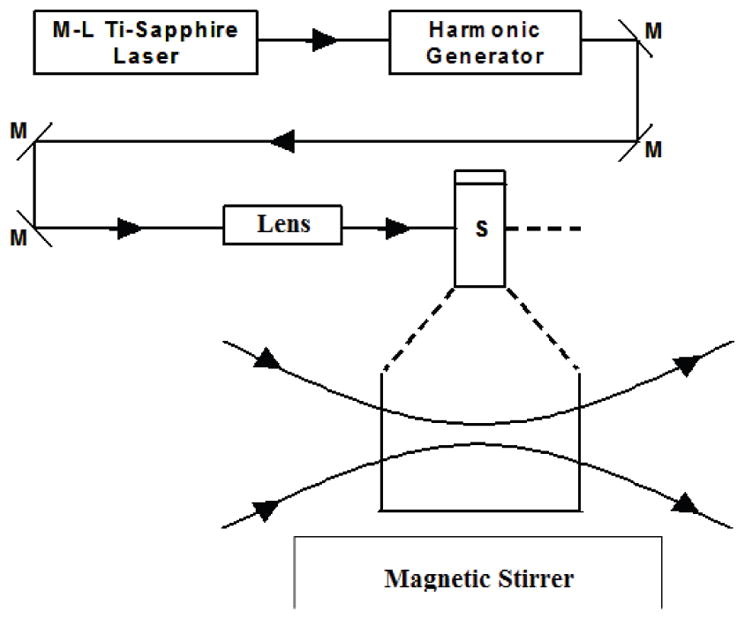
Experimental set up for the selective disinfection of pathogens with a visible USP laser. Lens: focusing lens; M: mirror; S: vial containing pathogens in buffer solutions.
Statistics
Differences between means of control and laser-treated microorganisms/cells were analyzed by Student’s t-test. p<0.05 was used as a threshold for statistical significance. The experimental results were expressed as means ± standard deviation.
III. EXPERIMENTAL RESULTS
Inactivation of mycoplasma by USP laser treatment
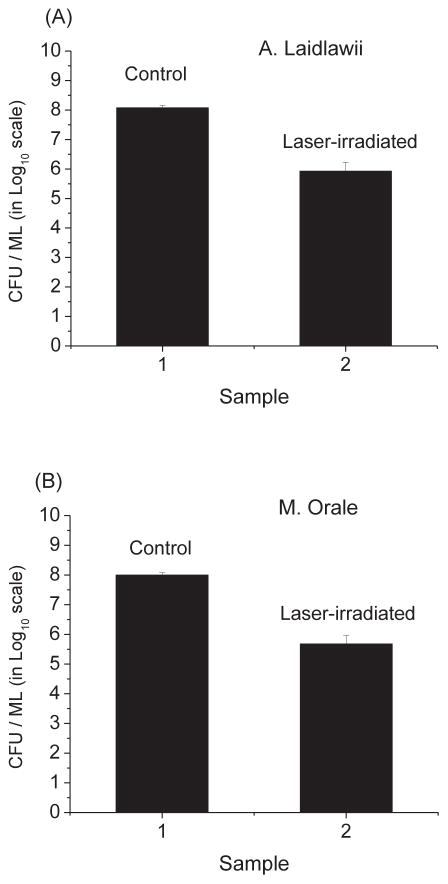
As shown in Fig. 2, we have found that the load reduction values for A. laidlawii and M. Orale were (1.4 ± 0.1) × 102 and (2.1 ± 0.2) × 102, respectively. The load reduction value is defined to be the ratio of the cfu/ml in the control to that in the laser-irradiated sample. Therefore, the USP laser under our experimental conditions can effectively inactivate mycoplasma.
Fig. 2.
The cfu/ml (in log10 scale) of control and laser-treated samples for (A) A. laidlawii and (B) M. orale mycoplasma. Irradiation of the visible USP laser results in load reduction values of (1.4 ± .1) × 102 and (2.1± 0.2) × 102 for A. laidlawii and M. Orale, respectively.
Inactivation of yeast cells by USP laser treatment
To determine whether USP laser treatment could inactivate yeast, we irradiated samples of Saccharomyces cerevisiae yeast cells using the USP laser. Fig. 3 shows a load reduction value of (2.1 ± 0.2) × 102 for the laser-treated yeast. These data indicate that USP laser treatment can effectively inactivate yeast cells.
Fig. 3.
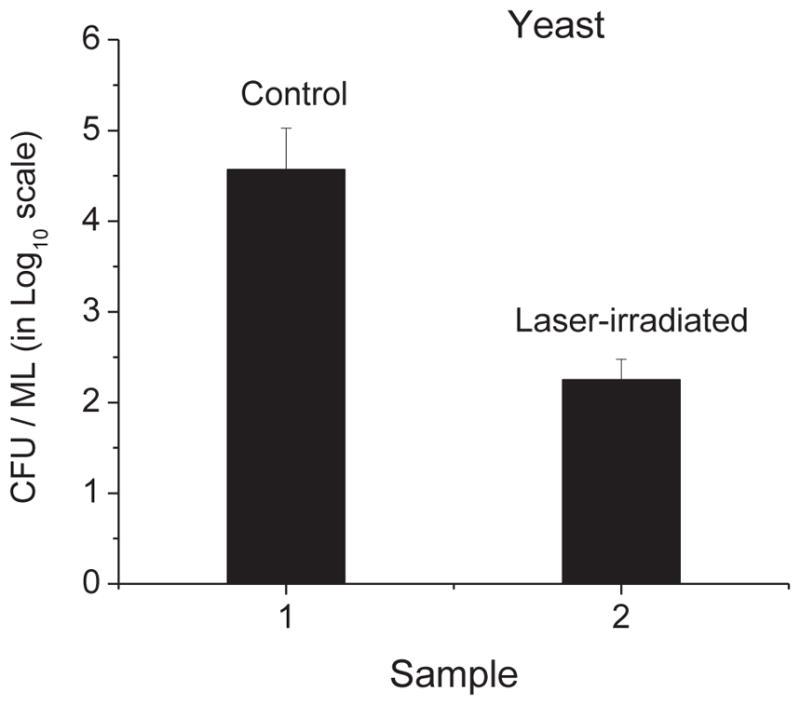
The cfu/ml (in log10 scale) of control and laser-treated samples of Saccharomyces cerevisiae yeast. Irradiation of the visible USP laser causes a load reduction value of (2.1 ± 0.2) × 102 for yeast cells.
Inactivation of bacteria by USP laser treatment
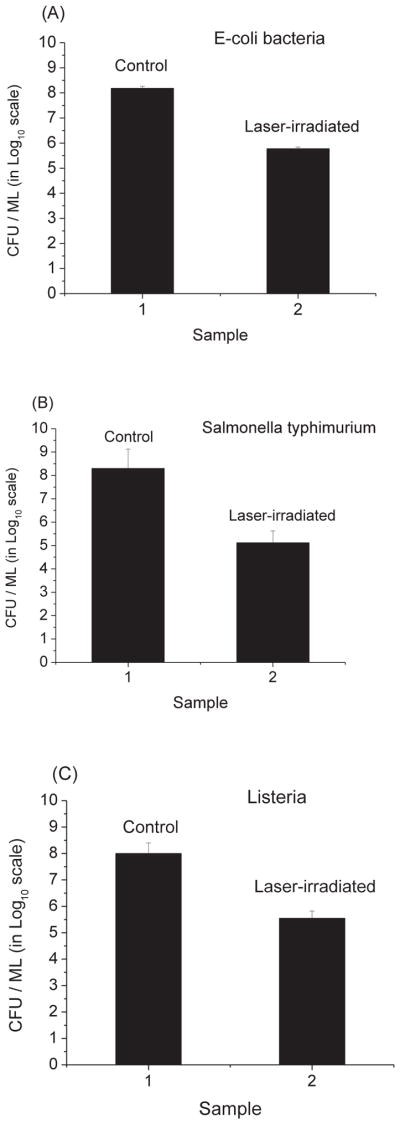
Fig. 4 shows that the load reduction values are (2.5 ± 0.1) × 102, (1.5 ± 0.1) × 103, and (2.8 × 102, for E. coli, Salmonella typhimurium, and Listeria, respectively. These data indicate that the USP laser treatment is able to effectively inactivate these bacteria.
Fig. 4.
The cfu/ml (in log10 scale) of control and laser-treated samples of (A) E- coli, (B) Salmonella typhimurium, and (C) Listeria bacteria. Irradiation of the visible USP laser leads to load reduction values of (2.5 ± 0.1) × 102, (1.5 ± 0.1) × 103, (2.8 ± 0.2) × 102 for E- coli, Salmonella typhimurium, and Listeria, respectively.
Inactivation of viruses by USP laser treatment
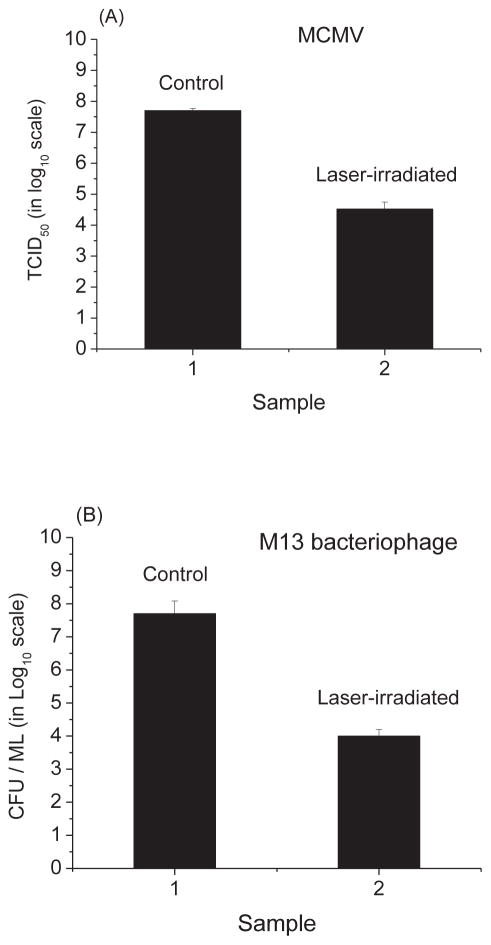
Fig. 5 shows that the load reduction values for MCMV and M13 bacteriophage are (1.5 ± 0.1) × 103 and (4.1 ± 0.4) × 103, respectively. Therefore, the USP laser treatment under our experimental conditions can very effectively inactivate viruses.
Fig. 5.
(A) The tissue culture infective dose (TCID50) (in log10 scale) of control and laser-treated samples of MCMV; (B) the cfu/ml (in log10 scale) of control and laser-treated samples of M13 bacteriophage. Irradiation of the visible USP laser leads to load reduction values of (1.5 ± 0.1) × 103 and (4.1 ± 0.4) × 103 for MCMV and M13 bacteriophage, respectively.
Effect of USP laser treatment on mammalian cell lines
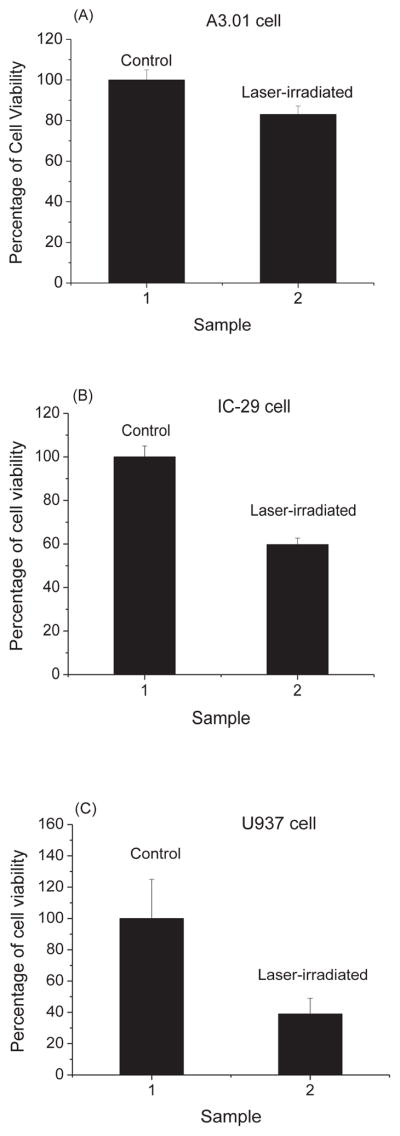
As shown in Fig. 6, we have found that the percentage of cell viability for A3.01 cells, IC-29 cells, U937 cells, and HEK293 cells are (81 ± 8)%, (60 ± 2)%, (39 ± 10)%, and (41 ± 4)%, respectively. The percentage of cell viability is defined to be the ratio of the number of live cells in the laser-irradiated sample to that in the control multiplied by 100%. Consequently, after USP laser treatment under our experimental conditions, the viability of mammalian cells was well preserved.
Fig. 6.
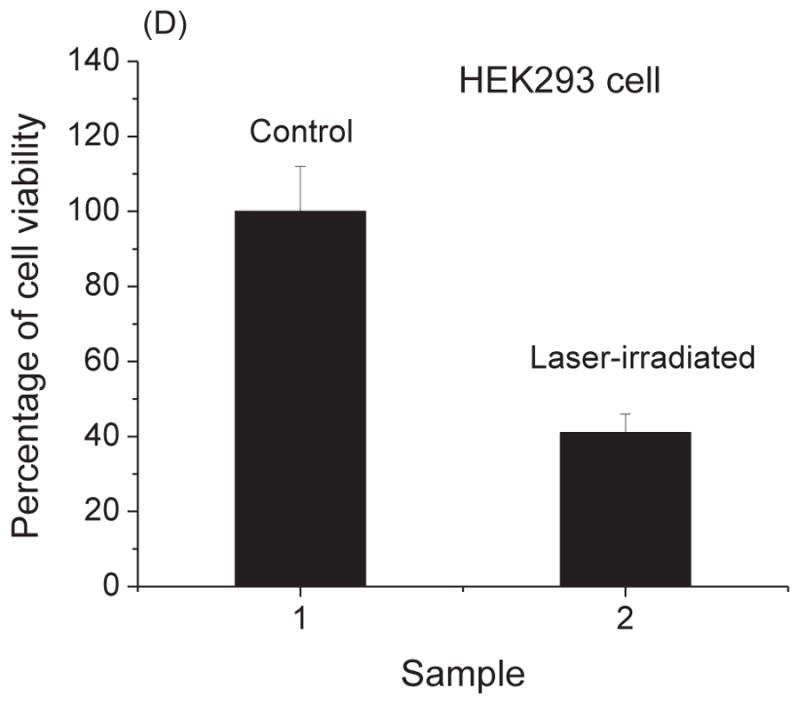
The percentage of cell viability of control and laser-treated samples for (A) A3.01 cells, (B) IC-29 cells, (C) U937 cells, and (D) HEK293 cells as determined by trypan blue exclusion assay. Irradiation of the visible USP laser retains about 81%, 60%, 39%, and 41% viability of A3.01 cells, IC-29 cells, U937 cells, and HEK293 cells, respectively.
For the purpose of comparison, the experimental results for the percentage of cell viability for the mammalian cells are summarized in Table 1, and the load reduction values for virus, yeast, bacteria and mycoplasma are summarized in Table 2.
TABLE 1.
SURVIVAL RATE/PERCENTAGE OF CELL VIABILITY AFTER THE IRRADIATION OF A VISIBLE USP LASER FOR A VARIETY OF CELL LINES
| Cell type | Survival rate/percentage of cell viability |
|---|---|
| A3.01 | 81 ± 8 |
| IC29 | 60 ± 2 |
| U937 | 39 ± 10 |
| HEK293 | 41 ± 4 |
TABLE 2.
LOAD REDUCTION VALUE OF A VARIETY OF MICROOGANISMS BY THE IRRADIATION OF A VISIBLE USP LASER (THE CONCENTRATION OF EACH MICROORGANISM, WHICH WAS IN ITS BUFFER SOLUTION WAS INDICATED IN THE MATERIALS AND METHODS SECTION)
| Microorganism | Load reduction value |
|---|---|
| M13 bacteriophage | (4.1 ± 0.4) × 103 |
| MCMV | (1.5± 0.1) × 103 |
| E. coli | (2.5 ± 0.1) × 102 |
| Salmonella typhimurium | (1.5 ± 0.1) × 103 |
| Listeria | (2.8 ± 0.2) × 102 |
| Saccharomyces cerevisiae | (2.1 ± 0.2) × 102 |
| A. laidlawii | (1.4 ± 0.1) × 102 |
| M. orale | (2.1 ± 0.2) × 102 |
IV. DISCUSSION
We now address the question – why does the visible USP laser irradiation efficiently inactivate viruses, bacteria, mycoplasma and yeast, whereas leaving to a great extent (about 50%) the mammalian cells unharmed?
It has been established that [16, 19, 20] viruses can be efficiently inactivated by the visible USP laser irradiation through the impulsive stimulated Raman scattering (ISRS) process. For example, it has been demonstrated that enveloped viruses such as MCMV are inactivated by a visible USP laser through density-dependent protein aggregation within the virion via the ISRS process [19]; non-enveloped viruses such as M13 bacteriophage [20], murine norovirus (MNV) [16] are inactivated by a visible USP laser through the ISRS process as well, in which the USP laser excites Raman-active global acoustic/low frequency vibrational modes on the capsid of the non-enveloped virus. This vibrational excitation causes the capsid to disintegrate into subunits in space, leading to its inactivation.
Maclean et al. [26] found that MRSA – a gram-positive bacterium could be efficiently inactivated by irradiation of a lamp with monochromatic wavelength at 405 nm. They attributed the inactivation to the presence of porphyrin molecules inside the bacterium. The porphyrin molecules produced reactive oxygen species (ROS) upon the 405nm irradiation which damaged nucleic acids of the bacterium, causing the inactivation of MRSA. Lu et al.[27] observed that E-coli bacteria could be efficiently inactivated by the USP laser irradiation at a wavelength of 425 nm. They suggested that the density-dependent protein aggregation induced by the visible USP laser irradiation inactivated the E-coli bacteria. Since the wavelength of the visible USP laser used in our experiments is 415 ± 5 nm, which is very close to either 405nm or 425 nm, in particular after considering the inevitable spectral spread (full-width at half-maximum is about 5 nm) of the ultrashort pulsed laser, we believe that these two mechanisms can explain the efficient inactivation of E-coli, Salmonella typhimurium and listeria observed in our experiments. Moreover, because mycoplasma and yeast have similar cellular structures to bacteria at the global level, it is reasonable to expect that they can be inactivated by the USP laser through these two mechanisms as well. As a result these two inactivation mechanisms can readily account for the efficient inactivation of yeast and mycoplasma by the visible USP laser irradiation.
Mammalian cells, mycoplasma, bacteria, and yeast have similar cellular structure. As a matter of fact they are usually referred to as “cells”. In principle, the two inactivation mechanisms by the visible USP laser irradiation applicable to the bacteria stated above are expected to be operative in mammalian cells as well. Nevertheless, it is very intriguing to note that four very different mammalian cells tested in our USP laser experiments survive to a great extent (around 50%) after the visible USP laser irradiation.
If mammalian cells contain porphyrin molecule which absorbs visible light at a wavelength of 415 nm, leading to the production of ROS, then mammalian cells will be efficiently inactivated by the visible USP laser irradiation. Therefore, our experimental results that mammalian cells survive in large percentage (about 50%) under the USP laser irradiation suggest that there is no porphyrin molecule inside the mammalian cells which absorbs photon at a wavelength of 415 nm used in our experiments
It is well-known that protein density is approximately the same in the mammalian cells as in the bacteria (about 0.17 g/ml); this raises an interesting question: why do mammalian cells escape the damage caused by density-dependent protein aggregation through ISRS process, while the bacteria do not? It has been shown that proteins have varied propensities to aggregation depending on their structure [28]. For example, at a protein density of 5.0 mg/cm3, bovine serum albumin (BSA) proteins suffer almost no aggregation; while monoclonal antibodies such as mAb(04) have around 20% aggregation under the visible USP laser irradiation [19]. It should also be noted that the protein content in mammalian cells and bacteria is quite different. If the proteins in the mammalian cells exhibit lesser tendency for aggregation upon USP laser irradiation than those in bacteria, then this density-dependent protein aggregation mechanism will have lesser effect on mammalian cells, leading to their much higher viability after the laser treatment. Consequently, the relatively high survival of mammalian cells under the visible USP laser irradiation observed in our visible USP laser experiments is simply a manifestation of the difference in the protein structures between the mammalian cells and bacteria.
We have also performed experiments in which the concentration of each microorganism in its buffer solution as described in the METHODS and MATERIALS section was kept at 1.0 × 106/ml. The results are summarized in Table 3. These results, when compared with those of Table 2, indicate that within our experimental uncertainties different density does not cause any difference to the laser-microorganism interaction.
TABLE 3.
LOAD REDUCTION VALUE OF A VARIETY OF MICROOGANISMS BY THE IRRADIATION OF A VISIBLE USP LASER (THE CONCENTRATION OF EACH MICROORGANISM, WHICH WAS IN ITS BUFFER SOLUTION AS DESCRIBED IN THE MATERIALS AND METHODS SECTION, WAS KEPT AT 1.0 × 106/ml)
| Microorganism | Load reduction value |
|---|---|
| M13 bacteriophage | (4.4 ± 0.4) × 103 |
| MCMV | (1.5± 0.1) × 103 |
| E. coli | (2.7 ± 0.1) × 102 |
| Salmonella typhimurium | (1.5 ± 0.1) × 103 |
| Listeria | (2.9± 0.2) × 102 |
| Saccharomyces cerevisiae | (2.2 ±0.2) × 102 |
| A. laidlawii | (1.6 ± 0.1) × 102 |
| M. orale | (2.3 ± 0.2) × 102 |
In order to obtain a direct view of how effective the USP laser for cell culture disinfection, we have carried out similar laser experiments in which the microorganism was mixed/spiked in IC-29 cell solution. The buffer solution of IC-29 cells was described in METHODS and MATERIALS section. In other words, we only varied the microorganism while all the variables in the experiment were kept the same. The concentration of either the microorganism or IC-29 cells was kept at 1.0 × 106/ml. The results are summarized in Table 4. These results, when compared with those of Table 3, suggest that under our experimental conditions the different buffer solution does not cause significant difference to the effectiveness of the USP laser for cell culture disinfection.
TABLE 4.
LOAD REDUCTION VALUE OF A VARIETY OF MICROOGANISMS BY THE IRRADIATION OF A VISIBLE USP LASER (THE MICROORGANISM WAS MIXED/SPIKED IN IC-29 CELL SOLUTION. THE BUFFER SOLUTION OF IC-29 WAS DESCRIBED IN THE MATERIALS AND METHODS SECTION. THE CONCENTRATION OF EITHER THE MICROORGANISM OR IC-29 CELLS WAS KEPT AT 1.0 × 106/ml)
| Microorganism | Load reduction value |
|---|---|
| M13 bacteriophage | (4.0 ±0.4) × 103 |
| MCMV | (1.7± 0.1) × 103 |
| E. coli | (2.4 ±0.1) × 102 |
| Salmonella typhimurium | (1.6 ±0.1) × 103 |
| Listeria | (2.7± 0.2) × 102 |
| Saccharomyces cerevisiae | (2.1 ± 0.2) × 102 |
| A. laidlawii | (1.5 ± 0.1) × 102 |
| M. orale | (2.0 ± 0.2) × 102 |
It is generally accepted by the medical community that a disinfection method with threshold load reduction value equal to or larger than 100 is a useful disinfection technique of microbes in medical applications. From the experimental results summarized in Tables 1 and 2, we can see that with the visible USP laser treatment a therapeutic window/a set of laser parameters exists in which it is possible to effectively inactivate mycoplasma, bacteria, yeast, and viruses, with good preservation of mammalian cell viability. Therefore, our work represents a step forward in the development of a novel, universal chemical-free disinfection method for cell cultures.
V. CONCLUSION
In this report, we demonstrate a novel, chemical-free visible USP laser treatment method for the disinfection of cell culture. We show that the visible USP laser treatment can efficiently inactivate cell culture contaminants such as mycoplasma, bacteria, yeast, and viruses with good preservation of mammalian cell viability. Our results indicate that this visible USP laser technology has the potential to serve as a universal method for chemical-free disinfection of cell culture.
Acknowledgments
This work was supported in part by NHLBI Ruth L. Kirschstein NRSA F30 grant HL116183-01 (Shaw-Wei Tsen).
Biographies
Kong-Thon Tsen is a professor of Physics and center for Biophysics at Arizona State University, Tempe, AZ. His research interest focuses on the interaction of light with solid state and biological systems; in particular, on the use of ultrafast laser sources to elucidate novel electron transport phenomena, dynamical properties of lattice vibrations in low-dimensional and nanostructure semiconductors as well as microscopic mechanisms in biological systems such as viruses, bacteria and cells.
Shaw-Wei D. Tsen is a candidate of MD/Ph. D. student at the Washington University school of Medicine in St Louis, MO. His interest is in the general area of pathology and particularly in the interaction of pathogens with ultrashort pulsed lasers.
Samuel Achilefu, PhD is a professor of Radiology, Biomedical Engineering, and Biochemistry & Molecular Biophysics at Washington University in St. Louis, MO. His research interests are in the development of molecular imaging probes and therapeutic molecules, new methods, and devices for imaging and treatment of cancer and other biological applications. He has received several honors and awards for his innovative work, including the Technical Innovation Award (1995), Extraordinary Performance Award (1998), Achiever Award (2008), Medical Innovator Award (2014), St. Louis Award (2014), Best Global Impact Award (2015), and Outstanding Scientist Award (2015).
Bert Jacobs is a professor at the School of Life Sciences and Biodesign institute’s Center for Infectious Diseases and Vaccinology at Arizona State University Tempe, AZ. He is one of the world’s foremost experts on a poxvirus called vaccinia, a cousin of the smallpox virus. Bert Jacobs has been named Academia Winner at 2006 Governor’s Celebration of Innovation Awards.
Karen Kibler is currently an Assistant Research Professor in the Center for Infectious Diseases and Vaccinology. Dr. Kibler received her Bachelor of Science Degree from the University of Iowa in 1977 and completed her PhD in Microbiology at ASU in 1997. After holding postdoctoral positions at both NIAID at NIH in Bethesda, Maryland and Mayo Clinic in Scottsdale, Arizona, she returned to ASU. Most of her work in the past eight years has been within large consortia funded by the Bill and Melinda Gates Foundation in an effort to develop an HIV vaccine. IACUC.
Justin Fay is an associate professor in the Genetics department at Washington University’s School of Medicine in St Louis, MO. He is interested in the genetic basis of evolutionary change and specifically interested in the role of changes in gene regulation.
Tatiana Ugarova is a professor at the School of Life Sciences at Arizona State University, Tempe, AZ. She is a cell biologist studying how cells in our bodies communicate with the surrounding extracellular matrix, and with other cells in normal physiology and disease. She investigates integrins, the receptors on the cell surface, which mediate cell adhesion and migration by sensing and transducing mechanical forces into intracellular signaling. She focuses on adhesive interactions of platelets and leukocytes, the blood cells involved in normal hemostasis and protective inflammatory responses.
N.P. Podolnikova is a Research Assistant professor at School of Life Sciences at Arizonan State University, Tempe, AZ. She is a cell biologist. She studies how cells in our bodies communicate with the surrounding extracellular matrix, and with other cells in normal physiology and disease.
Contributor Information
Shaw-Wei D. Tsen, Email: tsens@wusm.wustl.edu, Department of Radiology, Washington University School of Medicine, St Louis, MO 63110
Karen Kibler, Email: karen.kibler@asu.edu, Biodesign Institute, Arizona State University, Tempe, AZ 85287.
Bert Jacobs, Email: bert.jacobs@asu.edu, Biodesign Institute, Arizona State University, Tempe, AZ 85287.
Justin C. Fay, Email: jfay@genetics.wustl.edu, Department of Genetics, Washington University School of Medicine, St Louis, MO 63110
NP Podolnikova, Email: Nataly.Podolnikova@asu.edu, ASU/Mayo Center for Metabolic and Vascular Biology, Arizona State University Tempe, AZ 85287.
TP Ugarova, Email: Tatiana.Ugarova@asu.edu, ASU/Mayo Center for Metabolic and Vascular Biology, Arizona State University Tempe, AZ 85287.
Samuel Achilefu, Email: achilefus@mir.wustl.edu, Department of Radiology, Washington University School of Medicine, St Louis, MO 63110.
Kong-Thon Tsen, Email: tsen@asu.edu, Department of Physics and Center for Biophysics, Arizona State University, Tempe, AZ 85287-1504.
References
- 1.Langdon SP. Cell culture contamination: an overview. Methods in molecular medicine. 2004;88:309–317. doi: 10.1385/1-59259-406-9:309. [DOI] [PubMed] [Google Scholar]
- 2.Mirjalili A, Parmoor E, Moradi Bidhendi S, Sarkari B. Microbial contamination of cell cultures: a 2 years study. Biologicals : journal of the International Association of Biological Standardization. 2005;33:81–85. doi: 10.1016/j.biologicals.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 3.Blake Daniel M, Maness Pin-Ching, Huang Zheng, Wolfrum Edward J, Huang Jie, Jacoby William A. Application of the Photocatalytic Chemistry of Titanium Dioxide to Disinfection and the Killing of Cancer Cells. Separation & Purification Reviews. 1999;28(1):1–50. [Google Scholar]
- 4.Xu Y, Young MJ, Battaglino RA, Morse LR, Fontana CR, Pagonis TC, Kent R, Soukos NS. Endodontic Antimicrobial Photodynamic Therapy: Safety Assessment in Mammalian Cell Cultures. Journal of Endodontics. 2009;35(11):1567–1572. doi: 10.1016/j.joen.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tseng SP, Teng LJ, Chen CT, Lo TH, Hung WC, Chen HJ, Hsueh PR, Tsai JC. Toluidine blue O photodynamic inactivation on multidrug-resistant Pseudomonas aeruginosa. Laser Surg Med. 2009;41:391–397. doi: 10.1002/lsm.20765. [DOI] [PubMed] [Google Scholar]
- 6.Komerik N, Nakanishi H, MacRobert AJ, Henderson B, Speight P, Wilson M. In vivo killing of Porphyromonas gingivalis by toluidine blue-mediated photosensitization in an animal model. Antimicrob Agents Chemother. 2003;47:932–940. doi: 10.1128/AAC.47.3.932-940.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Seguier S, Souza SL, Sverzut AC, Simioni AR, Primo FL, Bodineau A, Correa VM, Coulomb B, Tedesco AC. Impact of photodynamic therapy on inflammatory cells during human chronic periodontitis. J Photochem Photobiol B. 2010;101:348–354. doi: 10.1016/j.jphotobiol.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 8.Giroldo LM, Felipe MP, de Oliveira MA, Munin E, Alves LP, Costa MS. Photodynamic antimicrobial chemotherapy (PACT) with methylene blue increases membrane permeability in Candida albicans. Lasers Med Sci. 2009;24:109–112. doi: 10.1007/s10103-007-0530-2. [DOI] [PubMed] [Google Scholar]
- 9.Gonzales FP, da Silva SH, Roberts DW, Braga GU. Photodynamic inactivation of conidia of the fungi Metarhizium anisopliae and Aspergillus nidulans with methylene blue and toluidine blue. Photochem Photobiol. 2010;86:653–661. doi: 10.1111/j.1751-1097.2009.00689.x. [DOI] [PubMed] [Google Scholar]
- 10.Smijs TG, van der Haas RN, Lugtenburg J, Liu Y, de Jong RL, Schuitmaker HJ. Photodynamic treatment of the dermatophyte Trichophyton rubrum and its microconidia with porphyrin photosensitizers. Photochem Photobiol. 2004;80:197–202. doi: 10.1562/2004-04-22-RA-146. [DOI] [PubMed] [Google Scholar]
- 11.Lin HY, Chen CT, Huang CT. Use of merocyanine 540 for photodynamic inactivation of Staphylococcus aureus planktonic and biofilm cells. Appl Environ Microbiol. 2004;70:6453–6460. doi: 10.1128/AEM.70.11.6453-6458.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Smijs TG, Schuitmaker HJ. Photodynamic inactivation of the dermatophyte Trichophyton rubrum. Photochem Photobiol. 2003;77:556–560. doi: 10.1562/0031-8655(2003)077<0556:PIOTDT>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 13.Tsen SWD, Wu TC, Kiang JG, Tsen KT. Prospects for a novel ultrashort pulsed laser technology for pathogen inactivation. Journal of biomedical science. 2012;19:62. doi: 10.1186/1423-0127-19-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tsen KT, et al. Inactivation of viruses with a very low power visible femtosecond laser. Journal of Physics: Condensed Matter. 2007;19:322102. doi: 10.1186/1743-422X-4-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tsen KT, et al. Inactivation of viruses by laser-driven coherent excitations via impulsive stimulated Raman scattering process. Journal of biomedical optics. 2007;12:064030. doi: 10.1117/1.2821713. [DOI] [PubMed] [Google Scholar]
- 16.Tsen SWD, et al. Studies of inactivation mechanism of non-enveloped icosahedral virus by a visible ultrashort pulsed laser. Virology journal. 2014;11:20. doi: 10.1186/1743-422X-11-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tsen KT, Tsen SWD, Hung CF, Wu TC, Kiang JG. Selective inactivation of human immunodeficiency virus with subpicosecond near-infrared laser pulses. Journal of Physics: Condensed Matter. 2008;20:252205. [Google Scholar]
- 18.Tsen KT, et al. Photonic approach to the selective inactivation of viruses with a near-infrared subpicosecond fiber laser. Journal of biomedical optics. 2009;14:064042. doi: 10.1117/1.3275477. [DOI] [PubMed] [Google Scholar]
- 19.Tsen SWD, et al. Inactivation of enveloped virus by laser-driven protein aggregation. Journal of biomedical optics. 2012;17:128002. doi: 10.1117/1.JBO.17.12.128002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tsen KT, et al. Studies of inactivation of encephalomyocarditis virus, M13 bacteriophage, and Salmonella typhimurium by using a visible femtosecond laser: insight into the possible inactivation imechanisms. Journal of biomedical optics. 2011;16:078003. doi: 10.1117/1.3600771. [DOI] [PubMed] [Google Scholar]
- 21.Tsen KT, Tsen SWD, Sankey OF, Kiang JG. Selective inactivation of micro-organisms with near-infrared femtosecond laser pulses. Journal of Physics: Condensed Matter. 2007;19:472201. [Google Scholar]
- 22.Tsen SWD, Kingsley DH, Kibler K, Jacobs B, Sizemore S, Vaiana SM, Anderson J, Tsen KT, Achilefu S. Pathogen Reduction in Human Plasma using an Ultrashort Pulsed Laser. PLOS one. 2014;9(11):e111673. doi: 10.1371/journal.pone.0111673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsen SWD, Chapa T, Beatty W, Xu B, Tsen KT, Achilefu S. Ultrashort pulsed laser treatment inactivates viruses by inhibiting viral replication and transcription in the host nucleus. Antiviral Research. 2014;110:70–76. doi: 10.1016/j.antiviral.2014.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tsen SWD, Donti N, La V, Hsieh WH, Li YD, Knoff J, Chen A, Wu TC, Hung CF, Achilefu S, Tsen KT. Chemical-free inactivated whole influenza virus vaccine prepared by ultrashort pulsed laser treatment. J Biomedical Optics. 2015;20(5):051008. doi: 10.1117/1.JBO.20.5.051008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lishko VK, Novokhatny VV, Yakubenko VP, Skomorovska-Prokvolit HV, Ugarova TP. Characterization of plasminogen as an adhesive ligand for integrins alphaMbeta2 (Mac-1) and alpha5beta1 (VLA-5) Blood. 2004;104(3):719–26. doi: 10.1182/blood-2003-09-3016. [DOI] [PubMed] [Google Scholar]
- 26.Maclean M, Macgregor SJ, Anderson JG, Woolsey G. High-intensity narrow- spectrum light inactivation and wavelength sensisitivity of staphylococcus aureus. FEMS microbiol Lett. 2008;285:227–232. doi: 10.1111/j.1574-6968.2008.01233.x. [DOI] [PubMed] [Google Scholar]
- 27.Lu CH, Lin KH, Hsu YY, Tsen KT, Kuan YS. Inhibition of Escherichia Coli respiratory enzymes by short visible femtosecond laser irradiation. Journal of Physics D: Applied Physics. 2014;47:315402. [Google Scholar]
- 28.Chiti F. Protein Misfolding, Aggregation, and Conformational Diseases. Springer US; 2006. Relative importance of hydrophobicity, net charge, and secondary structure propensities in protein aggregation; pp. 43–59. [Google Scholar]