Abstract
Purpose
The present study aimed to investigate the value of apolipoproteins, including ApoA-1, ApoC-III, and ApoE, in patients with small cell lung cancer (SCLC) as potential biomarkers for diagnosis, prognosis, and cancer progression.
Materials and Methods
Lung samples were collected from 89 patients with SCLC. Nineteen lung samples from non-small cell lung cancer (NSCLC) patients and 12 normal lung tissues were used as controls. Expression profiles of ApoA-1, ApoC-III, and ApoE in different samples were examined using immunohistochemical methods, and the expression levels were correlated with cancer types, treatment, and outcomes using chi-square and Mann-Whitney tests.
Results
Expression of ApoA-1 and ApoC-III in SCLC was significantly different, compared with that in NSCLC and normal lung tissues, and was correlated with recurrence of SCLC. Patients undergoing neoadjuvant chemotherapy before surgery showed significantly reduced expression of ApoA-1 and increased expression of ApoC-III and ApoE. Nevertheless, the expression levels of ApoA-1, ApoC-III, and ApoE were not correlated with SCLC staging.
Conclusion
ApoA-1 and ApoC-III may be used as differentiating and predictive markers for SCLC. ApoA-1, ApoC-III, and ApoE may be used to monitor the efficacy of chemotherapy.
Keywords: Small cell lung cancer, apolipoprotein, biomarker, prognosis
INTRODUCTION
As one of the most common malignancies, lung cancer accounted for approximately 1.38 million deaths in 2008, and is expected to cause more deaths in the future, particularly in developing countries.1,2 The majority of lung cancers originates from epithelial cells, and can be categorized morphologically as non-small cell lung cancer (NSCLC) and small cell lung cancer (SCLC). Although constituting only 15–20% of all lung cancers, SCLC is considered a fatal disease with extremely low survival rates, due to early metastases and high sensitivity to cytotoxic chemotherapy.3 The past few decades have witnessed tremendous developments in drugs and biomarkers targeting SCLC, in large part to extensive research into the molecular mechanisms of SCLC.4,5 Nevertheless, the average outcomes of SCLC patients have not significantly improved, and 5-year survival rates have not been satisfactorily improved over the last 40 years.6 Approximately 60–70% of patients are not diagnosed until an extensive stage (ES), with a median survival of only 8–10 months.6 Since the therapeutic options for SCLC are still limited, diagnosing the disease as early as possible is critical.
Apolipoproteins are structural components of lipoproteins, and play a critical role in lipid transportation. Genetic mutations in apolipoproteins are widely associated with metabolic and cardiovascular diseases.7,8 Furthermore, accumulating evidence suggests that apolipoproteins may also be related to the development and/or outcomes of certain cancers, for instance ovarian cancer, renal cancer, bladder cancer, etc.9,10 We previously reported on the up-regulation of ApoE in the serum of SCLC patients, suggesting that apolipoproteins can serve as potential biomarkers for SCLC.11 Therefore, in order to analyze the possibility of utilizing apolipoproteins in SCLC diagnosis, we sought to systematically characterize the expression of different apolipoproteins in SCLC tissue samples from patients of various clinical histories. Our studies revealed that the expression of apolipoproteins may be useful for SCLC diagnosis and monitoring treatment.
MATERIALS AND METHODS
Subjects
Patients with lung cancers were enrolled at the Fourth Hospital of Hebei Medical University from January 2011 to December 2013. All lung cancer patients were histopathologically diagnosed, and all tissue samples were obtained by surgery. The inclusion criteria for SCLC consisted of the following: patients without clear diagnosis from endoscopy or lymph node biopsy, but histopathologically diagnosed after surgery; patients with a medical history of SCLC and who had undergone preoperative neoadjuvant chemotherapy before surgery. The exclusion criteria included mixed cancers and patients without clear postoperative pathological diagnosis. In total, 89 SCLC samples were selected for study. For controls, 12 adjacent normal lung tissues (collected from SCLC patients at least 10 cm away from the tumor) and 19 NSCLC samples (eight cases of squamous cell carcinoma and 11 cases of adenocarcinoma) were used. In addition, eight normal liver tissues obtained from volunteers were used as positive controls for immunohistochemical staining. All original tissue samples were approximately 0.3×1.5×2.0 cm3. Samples were formalin-fixed and paraffin-embedded, and sectioned to make biopsies of 4 µm thickness. All specimens were processed following the standard hematoxylin and eosin stain protocol and independently diagnosed by two physicians.
The protocol for this research project was approved by the Ethics Committee of the Fourth Hospital of Hebei Medical University and conformed to the provisions of the Declaration of Helsinki (as revised in Edinburgh 2000). All subjects in this research provided informed consent and underwent surgery at their own discretion.
Immunohistochemical staining
The 4-µm thick biopsy specimens were first deparaffinized in xylene I and II for 10 minutes each, rehydrated in ethanol I, ethanol II, 95% ethanol, and 80% ethanol for 5 minutes each, and rinsed with phosphate buffered saline (PBS, pH7.4) twice for 5 minutes each. Samples were then soaked in 3% hydrogen peroxide for 10 minutes, followed by a rinse with tap water. A SPlink Detection kit (ZSGC-BIO/ORIGENE, Shenzhen, China) was used for immunohistochemical staining following the manufacture's protocol. Briefly, samples were heated in 0.01 M sodium citrate (pH6.0) for 10 minutes at 95℃, followed by three rinses with PBS for 5 minutes each. Samples were incubated with primary antibodies (rabbit anti-ApoE, ApoC-III, and ApoA-1, diluted 100, 100, and 200 times, respectively; Abcam, Cambridge, United Kingdom) for 2–3 hours at room temperature, and then rinsed three times with PBS for 5 minutes each. Samples were incubated with secondary antibody (biotin-conjugated goat anti-rabbit IgG at a dilution of 1:400) for 20 minutes at room temperature, followed by three rinses with PBS for 5 minutes each. Samples were then stained with DAB kit (ZSGC-BIO/ORIGENE) following the manufacture's protocol and mounted with neutral resin on slides.
Assessment of immunohistochemical staining
Slides were observed and imaged with a DM-LB2 microscope (Leica, Wetzlar, Germany) and Olympus BX51 microscope (Olympus, Tokyo, Japan). Stains of normal liver tissues were used as a reference. Brown colored granules in the cytoplasm indicated the expression of apolipoproteins. Cells showing these stained granules were considered as expressing the targeted apolipoproteins. For each slide, the total degree of staining was scored for five randomly selected fields as negative (-), if only 10–30% of cells were stained; 1+, if 30–50% of cells were stained; 2+, if 50–70% of cells were stained; and 3+, if more than 70% of cells were stained. Overall reactivity was defined as negative if less than 30% of cells were stained, regardless of staining intensity, and positive if more than 30% of cells were stained.
Statistics
Statistical analyses were performed with SPSS software (version 13.0, SPSS Inc., Chicago, IL, USA). Chi-square test was used to compare the number of positive cells in different tissues, and Mann-Whitney test was performed to compare the expression levels of apolipoproteins in different samples. For all tests, two-tailed distribution was used, and a p value less than 0.05 (p<0.05) was considered to indicate a statistically significant difference between groups.
RESULTS
Demographic and baseline data
A total of 89 patients with SCLC were recruited to the present study. Their demographic and baseline clinical characteristics are shown in Table 1. The median age of the SCLC patients was 53 years (range: 36–75 y), and the male to female ratio was approximately 2:1. Tumor samples were obtained from all patients and their pathologic stages were determined. According to the updated Veteran's Administration Lung Study Group (VALSG) standard that assesses the location and metastases of tumors,12 87 were at a limited stage and two were at an ES. According to the tumor, node, and metastasis (TNM) standard [International Association for the Study of Lung Cancer (IASLC), 7th update13], 12, 19, 52, and 4 cases were in stages I, II, III, and IV, respectively. There were 13 patients with recurrent SCLC, and there were 20 who received neoadjuvant chemotherapy before surgery.
Table 1. Demographic Information and Baseline Clinical Data in SCLC Patients.
| Parameter | Value/cases |
|---|---|
| Age | |
| Median (yr) | 53 |
| Range (yr) | 36-75 |
| Male/female (n/n) | 60/29 |
| Anatomic stage (n) | |
| I | 12 |
| II | 19 |
| III | 52 |
| IV | 4 |
| VALSG stage (n) | |
| Limited | 87 |
| Extensive | 2 |
| Recurrence (n) | |
| Yes | 13 |
| No | 76 |
| Neoadjuvant chemotherapy (n) | |
| Yes | 20 |
| No | 69 |
SCLC, small cell lung cancer; VALSG, Veteran's Administration Lung Study Group.
The corresponding TNM classifications for each anatomic stage were as follows: stage I: T1aN0M0, T1bN0M0, and T2aN0M0, stage II: T1aN1M0, T1b-N1M0, T2aN1M0, T2bN0M0, T2bN1M0, and T3N0M0, stage III: T1N2M0, T2N2M0, T3N1M0, T3N2M0, T4N0M0, T4N1M0, T4N2M0, T1N3M0, T2N3M0, T3N3M0, and T4N3M0, stage IV: T any, N any, and M1a or 1b.
ApoA-1 and ApoC-III can be used as differentiating markers for SCLC
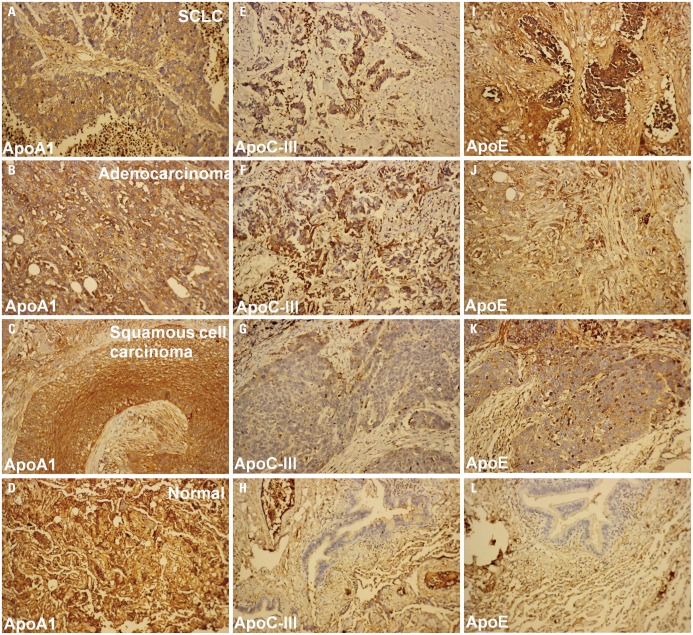
Immunohistochemical staining for the three apolipoproteins revealed brown granules in the cytoplasm, indicating the expression of apolipoproteins in SCLC samples (Fig. 1). Tissue samples with 30% of cells in the cytoplasm containing brown granules were considered as positively expressing apolipoproteins (Fig. 1, Table 2).
Fig. 1. Immunohistochemical analysis of apolipoproteins. The expression of ApoA-1 (A-D), ApoC-III (E-H), and ApoE (I-L) in different tissue samples, including SCLC (A, E, and I), adenocarcinoma (B, F, and J), squamous cell carcinoma (C, G, and K), and normal lung tissues (D, H, and L). Brown dots represent stained proteins. SCLC, small cell lung cancer.
Table 2. Expression of ApoA-1, ApoC-III, and ApoE in Different Tissues.
| Tissues | ApoA-1 | ApoC-III | ApoE | |||
|---|---|---|---|---|---|---|
| Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | |
| SCLC (89) | 27.0% (24/89) | 73.0% (65/89)* | 41.6% (37/89) | 58.4% (52/89)* | 22.5% (20/89) | 77.5% (69/89) |
| NSCLC (19) | 42.1% (8/19) | 57.9% (11/19) | 31.6% (6/19) | 68.4% (13/19) | 26.3% (5/19) | 73.7% (14/19) |
| Normal (12) | 50% (6/12) | 50% (6/12) | 25% (3/12) | 75% (9/12) | 25% (3/12) | 75% (9/12) |
| Squamous cell carcinoma (8) | 50% (4/8) | 50% (4/8) | 25% (2/8) | 75% (17/19) | 25% (2/8) | 75% (6/8) |
| Adenocarcinoma (11) | 36.4% (4/11) | 63.6% (7/11) | 36.4% (4/11) | 63.6% (7/11) | 27.4% (3/11) | 72.6% (8/11) |
SCLC, small cell lung cancer; NSCLC, non-small cell lung cancer.
*p<0.05 (compared with NSCLC or normal lung tissue).
To avoid possible gender bias, we first analyzed the expression of the three apolipoproteins in men and women separately (Supplementary Table 1, only online). Positive expression of ApoA-1, ApoC-III, and ApoE in SCLC tissues was noted in 76.7%, 58.3%, and 76.7% of men and 72.4%, 58.6%, and 79.3% of women, respectively. No significant difference in apolipoprotein expression was observed between men and women; therefore, for all other analysis, we excluded gender as a parameter.
The percentage of SCLC tissues that showed positive expression of ApoA-1, ApoC-III, and ApoE was 73.0%, 58.4%, and 77.5%, respectively, compared to 57.9%, 68.4%, and 73.7% in NSCLC and 50%, 75%, and 75% in normal lung tissues. Among the NSCLC samples, 50%, 75%, and 75% of squamous cell carcinoma tissues showed positive expression of ApoA-1, ApoC-III, and ApoE, while 36.6%, 63.6%, and 72.6% of adenocarcinomas showed positive expression thereof (Table 2). Statistical analysis indicated that expression of ApoA-1 in SCLC was significantly higher than that in NSCLC or normal lung tissues, while the expression of ApoC-III was significantly lower in SCLC than in NSCLC or normal lung tissue (p<0.05).
Expression of ApoA-1 and ApoC-III is correlated with SCLC recurrence
One malignant feature of SCLC is a high recurrence rate. To test whether apolipoproteins are related with SCLC recurrence, we compared the expression of apolipoproteins in 13 cases of recurrence and 76 cases without recurrence (Table 3). The expression of ApoA-1 (61.5%) was significantly lower in the recurrence group than in the non-recurrence group (75%), while the expression of ApoC-III (69.2%) was significantly higher in the recurrence group than in the non-recurrence group (56.6%). The expression of ApoE was not affected by recurrence.
Table 3. Expression of ApoA-1 and ApoC-III Are Correlated with SCLC Recurrence.
| Group | ApoA-1 | ApoC-III | ApoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | |||||||
| Recurrence (13) | 38.5% (5/13) | 61.5% (8/13)* | 30.8% (4/13) | 69.2% (9/13)* | 23.1% (3/13) | 77.0% (10/13) | ||||||
| Control (76) | 25% (19/76) | 75% (57/76) | 43.4% (33/76) | 56.6% (43/76) | 22.4% (17/76) | 77.6% (59/76) | ||||||
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | |
| Recurrence (13) | 38.5% (5/13) |
23.3% (3/13) |
38.5% (5/13) |
23% (3/13) |
30.8% (4/13) |
38.5% (5/13) |
23.1% (3/13) |
7.7% (1/13) |
23.1% (3/13) |
15.4% (2/13) |
46.2% (6/13) |
15.4% (2/13) |
| Control (76) | 25% (19/76) |
19.7% (15/76) |
52.6% (40/76) |
23.4% (17/76) |
43.4% (33/76) |
35.5% (27/76) |
14.5% (11/76) |
6.6% (5/76) |
22.4% (17/76) |
27.6% (21/76) |
34.2% (26/76) |
15.8% (12/76) |
SCLC, small cell lung cancer.
*p<0.05.
Expression of ApoA-1 and ApoC-III altered in patients who underwent neoadjuvant chemotherapy before surgery
As a major supplementary treatment of SCLC, neoadjuvant chemotherapy before surgery has been reported to significantly increase the survival rate of SCLC patients.14 Since apolipoproteins are predictive in SCLC, we hypothesized that the expression of apolipoproteins may be used to monitor treatment efficacy. Among the 89 SCLC patients, twenty underwent neoadjuvant chemotherapy, and the percentages of positive expression of ApoA-1, ApoC-III, and ApoE therein were 60%, 80%, and 95%, respectively (Table 4). In contrast, the 69 patients who did not receive neoadjuvant chemotherapy showed significantly higher levels of ApoA-1 (76.8%), as well as lower levels of ApoC-III (52.2%), and ApoE (72.5%).
Table 4. The Effect of Neoadjuvant Chemotherapy on the Expression of Apolipoproteins.
| Group | ApoA-1 | ApoC-III | ApoE | |||
|---|---|---|---|---|---|---|
| Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | |
| Neoadjuvant chemotherapy (20) | 40% (8/20) | 60% (16/20)* | 20% (4/20) | 80% (16/20)* | 5% (1/20) | 95% (19/20)* |
| Control (69) | 23.2% (16/69) | 76.8% (53/69) | 47.8% (33/69) | 52.2% (36/69) | 27.5% (19/69) | 72.5% (50/69) |
*p<0.05.
ApoA-1, ApoC-III, and ApoE are not predictive of SCLC staging, metastasis, or tumor size
To test whether apolipoproteins can be used to monitor the progression of SCLC, we compared the expressions of ApoA-1, ApoC-III, and ApoE at different stages of disease (Supplementary Table 1, only online). No significant differences in ApoA-1, ApoC-III, and ApoE expression were observed between stages I, II, III, or IV. However, the levels of the three apolipoproteins increased between subgroups IA and IB, and IIA and IIB (Table 5). The positive expression of ApoA-1, ApoC-III, and ApoE was not significantly altered between stages N0, N1, N2, and N3 or between stages T1a, T1b, T2a, T2b, T3s, and T4 (Supplementary Table 1, only online).
Table 5. Expression of ApoA-1, ApoC-III, and ApoE Are Correlated with SCLC Sub-Stages.
| Stage | ApoA-1 | ApoC-III | ApoE | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | Negative (n/%) | Positive (n/%) | |||||||
| - | + | ++ | +++ | - | + | ++ | +++ | - | + | ++ | +++ | |
| I A (5) | 0% (0/5) | 40% (2/5)* | 60% (3/5) | 0% (0/5)* | 60% (3/5) | 20% (1/5)* | 20% (1/5)* | 0% (0/5)* | 20% (1/5) | 40% (2/5) | 40% (2/5)* | 0% (0/5)* |
| I B (7) | 14.3% (1/7) | 0% (0/7) | 71.4% (5/7) | 14.3% (1/7) | 42.9% (3/7) | 42.9% (3/7) | 0% (0/7) | 14.3% (1/7) | 28.6% (2/7) | 42.9% (3/7) | 14.3% (1/7) | 14.3% (1/7) |
| II A (14) | 14.3% (2/14) | 21.4% (3/14) | 42.9% (6/14) | 21.4% (3/14)* | 35.7% (5/14) | 35.7% (5/14) | 21.4% (3/14)* | 7.1% (1/14)* | 50% (7/14) | 21.4% (3/14)* | 14.3% (2/14)* | 14.3% (2/14)* |
| II B (5) | 0% (0/5) | 20% (1/5) | 40% (2/5) | 40% (2/5) | 20% (1/5) | 40% (2/5) | 40% (2/5) | 0% (0/5) | 0% (0/5) | 40% (2/5) | 40% (2/5) | 20% (1/5) |
| III A (29) | 10.3% (3/29) | 20.7% (6/29) | 51.7% (15/29) | 17.2% (5/29) | 48.3% (14/29) | 27.6% (8/29) | 20.7% (6/29) | 3.4% (1/29) | 17.2% (5/29) | 27.6% (8/29) | 41.4% (12/29) | 13.8% (4/29) |
| III B (23) | 0% (0/23) | 21.7% (5/23) | 47.8% (11/23) | 30.4% (7/23) | 47.8% (11/23) | 43.5% (10/23) | 4.3% (1/23) | 4.3% (1/23) | 17.4% (4/23) | 17.4% (4/23) | 43.5% (10/23) | 21.7% (5/23) |
| IV (4) | 0% (0/6) | 25% (1/4) | 75% (3/4) | 0% (0/4) | 0% (0/4) | 50% (2/4) | 50% (2/4) | 0% (0/4) | 25% (1/4) | 0% (0/4) | 75% (3/4) | 0% (0/4) |
SCLC, small cell lung cancer.
-, <25%; +, 25–50%; ++, 50–75%; +++, >75%.
*p<0.05 (comparison between I A and I B, II A and II B).
DISCUSSION
Altered expression of apolipoproteins in SCLC
Small cell lung cancers account for approximately 10–15% of all lung cancers.3 Despite a low prevalence, the disease is highly malignant. Current treatment for SCLC involves combined chemo radiation, which gradually loses effectiveness due to the increasing resistance of cancer cells to chemicals, resulting in almost invariable recrudescence and metastases.3 When diagnosed at a limited stage, 20–25% of the SCLC patients upon treatment survive longer than five years.15 Accordingly, the precise diagnose of SCLC, particularly at early stages, is crucial for the disease management. SCLC cells likely originate from bronchial epithelial cells or epithelial argyrophil cells, and are associated with signaling pathways, such as SOX2, FGFR1, RB, and Myc, which have been proposed as potential gene therapy targets.16,17 Other studies have focused on preventing blood vessel growth in SCLC tissue, although such applications are still in the theoretical and research phase.18 Therefore, additional diagnose and treatment options are required to improve the outcomes of SCLC patients.
Currently, SCLC diagnoses are based on pathological morphology and immunohistochemistry. Traditionally, standard tumor markers for SCLC include neuron specific enolase, CD56, chromogranin A, synaptophysin, and thyroid transcription factor-1. However, these tumor markers each carry their own limitations, and not all of them are suitable for early screening.4 Results from individual markers are highly susceptible to external conditions (e.g., hemolysis, smoking), types of samples tested (blood or plasma), and the protocols performed. Meanwhile, increasing lines of evidence suggest that apolipoproteins are associated with a wide variety of cancers, and may be potential targets for cancer treatment. Whether they are involved in SCLC development, however, remains unclear. In our study, we used immunohistochemistry to investigate the expression apolipoproteins in SCLC patients.
As a major structural component of high-density lipoproteins (HDL), ApoA-1 is widely associated with chylomicron (CM), HDL2, and HDL3, and is responsible for cholesterol excretion from tissues to the liver.19 Accumulating studies have shown that changes in ApoA-1 are associated with cancer progression and treatment outcomes, although controversial results have been frequently reported. For instance, down-regulation of serum ApoA-1 was reported in patients with pancreatic cancer, colorectal cancer, and ovarian cancer,20,21 while ApoA-1 expression was up-regulated in patients with lung cancer, bladder cancer, and recurrent head and neck squamous cell carcinoma.22,23,24 Recent molecular studies have demonstrated that ApoA-1 peptides may suppress the vascular endothelial growth factor 1 through the fibroblast growth factor signaling pathway, functioning as a tumor suppressor gene.25 Our present study showed that ApoA-1 is significantly overexpressed in SCLC tissue samples, compared to NSCLC and normal lung tissues, indicating the potential application of ApoA-1 in SCLC diagnosis.
Unlike ApoA-1, ApoC-III is a small soluble protein residing on the surface of apoA and apoB, and can be found in HDL, very-low-density lipoprotein (VLDL), and CM. ApoC-III suppresses lipoprotein lipase (LPL), and displaces LPL from triglyceride-rich lipoproteins.26 ApoC-III also affects liver production of lipoproteins, probably by inducing the expression of microsomal triglyceride transfer protein.27 Apart from the well-characterized function of ApoC-III in triglyceride metabolism and insulin resistance, emerging evidence suggests that ApoC-III is also linked to various cancers. Fan, et al.28 screened serum samples from patients with papillary thyroid carcinoma (PTC) and identified ApoC-III as a biomarker for PTC and an indicator for PTC staging. Honda, et al.10 showed that plasma ApoC-III is significantly decreased in patients with pancreatic cancer. Molecular studies also suggest that human ApoC-III promoter is synergistically activated by hepatocyte nuclear factor 4, Mdm2, and Smad proteins.29,30 Mdm2 antagonizes the indirect inhibition of p53 and SHP on ApoC-III, which is probably the underlying mechanism for the involvement of ApoC-III in tumorigenesis and cancer progression. In our study, ApoC-III expression was significantly reduced in SCLC tissues, compared to NSCLC and normal lung samples. The results indicated that ApoC-III could be used as a differentiating marker for SCLC.
ApoE is associated with CM, VLDL, and HDL, and is widely expressed in multiple tissues and cell types, such as liver, kidney, lung, and ovary cells. ApoE mediates the transportation of CM, VLDL, and their remnants to the liver, and defects in ApoE are closely related with hyperlipidemia and cardiovascular diseases.31 Moreover, accumulating evidence suggests that ApoE is involved in multiple signaling pathways that play a role in tumorigenesis. Martínez-Clemente, et al.32 showed that ApoE could inhibit TNF-alpha. ApoE was found to be overexpressed in malignant pleural effusions (MPE)-associated lung adenocarcinoma.33 ApoE knockdown in MPE-derived lung adenocarcinoma cell lines inhibited cancer cell growth, enhanced the sensitivity to cisplatin, and impaired cell migration.33 Trost, et al.34 showed decreased serum levels of ApoE and upregulated ApoE expression in tumor tissues in NSCLC patients, although they were not correlated with cancer stages or prognosis of NSCLC. In the present study, we observed equally high expressions of ApoE in normal lung and tumor samples from SCLC and NSCLC patients, indicating the lack of discriminating power of ApoE in SCLC diagnosis.
Apolipoprotein expression is not associated with SCLC progression
The first standard staging of SCLC, VALSG, was developed in the 1950s, and divides SCLC into limited stage and extensive stage disease, mainly based on the range of cancer spread.12,35 The IASLC/AJCC later proposed a new staging system for SCLC based on the TNM information.13 This updated TNM system is more applicable for patients undergoing surgical treatment, and provides additional prognostic information. The precise staging of SCLC is critical for the choice of subsequent treatment plans. Since apolipoproteins exhibit differential expression in SCLC tissue, compared to other cancer types and normal tissue, we anticipated that they may be of use in SCLC staging as well. Our study results, however, did not show that the expressions of ApoA-1, ApoC-III, and ApoE differ significantly between limited and ES disease or between different T/N stages according to the TNM system. We concluded that ApoA-1, ApoC-III, and ApoE are not suitable for differentiating or predicting SCLC staging. A recent study showed that serum levels of ApoA-1 were negatively correlated with cancer progression and positively correlated with survival.36 In our study, decreased expression of ApoA-1 was observed in tumor samples of SCLC patients with recurrence. These results implicate a complicated role for ApoA-1 in regulating cancer development. In another study, expression of Apo-CI in lung samples from patients with lung cancer, including NSCLC (n=23), SCLC (n=6), and undifferentiated cancer (n=1), was examined.37 The study showed that Apo-CI expression was upregulated in late stages of all lung cancer types. These results indicate that, despite the similar functions apolipoproteins have in lipid transport, they might play different roles in cancer development and progression.
Apolipoproteins are predictive of SCLC prognosis and can be used for monitoring the efficacy of chemotherapy
Currently the consensus treatment strategies for SCLC include combined radio chemotherapy for limited stage disease and combined chemotherapy for extensive disease.3 Patients at the limited stage have a 5-year survival rate of less than 15%.15 The median survival period for ES is 7–11 months, with a 2-year survival rate of less than 5%.6 As a systemic disease, surgery is not recommended.38 However, emerging studies have shown that surgery can significantly prolong the survival rates of patients with limited stage disease. Surgical treatment is recommended for stage I (T1–T2, N0) patients and stage II–IIIa patients who have undergone the first two-three cycles of chemotherapy and reached stage I.39,40 Recently Ploenes, et al.14 reported that neoadjuvant treatment following surgical treatment significantly improved the median overall survival (89.4 months compared to an average of 20.7 months of all surveyed patients) of patients with limited stage disease, which indicated that Karnofsky performance status (PS) and neoadjuvant chemotherapy have positive effect on the outcome of SCLC patients. In our study, patients were categorized into two groups, depending on whether they had undergone neoadjuvant chemotherapy before surgery. ApoA-1 and ApoC-III were found to be differentially expressed between the two groups, indicating that ApoA-1 and ApoC-III can be applied to monitor the efficacy of chemotherapy.
The 5-year survival rate of limited-stage SCLC patients after chemotherapy is approximately 19–23%.15,41 However, local recurrence as high as 30–70% has frequently been reported. Studies have shown that SCLC tissues often contain other types of cancer cells that are insensitive to chemotherapy. Typically, SCLCs contain abundant neuroendocrine granules and dopamine beta-hydroxylase, and generally are associated with better prognosis.42 The other SCLC is the small cell carcinoma with short doubling time, highly efficient cloning and insensitivity to radiation therapy, resulting in poor prognosis.3 Important risk factors for SCLC prognosis include staging, PS, lactate dehydrogenase, etc.43,44 In our study, we showed that ApoA-1 and ApoE expression differed between patients with and without recurrence, indicating that the two apolipoproteins are predictive for SCLC prognosis.
Footnotes
The authors have no financial conflicts of interest.
SUPPLEMENTARY MATERIAL
The Expression of Apolipoproteins in SCLC Tissues Under Different Conditions
References
- 1.Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–2917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 3.Jackman DM, Johnson BE. Small-cell lung cancer. Lancet. 2005;366:1385–1396. doi: 10.1016/S0140-6736(05)67569-1. [DOI] [PubMed] [Google Scholar]
- 4.Harmsma M, Schutte B, Ramaekers FC. Serum markers in small cell lung cancer: opportunities for improvement. Biochim Biophys Acta. 2013;1836:255–272. doi: 10.1016/j.bbcan.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 5.Pillai RN, Owonikoko TK. Small cell lung cancer: therapies and targets. Semin Oncol. 2014;41:133–142. doi: 10.1053/j.seminoncol.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Meerbeeck JP, Fennell DA, De Ruysscher DK. Small-cell lung cancer. Lancet. 2011;378:1741–1755. doi: 10.1016/S0140-6736(11)60165-7. [DOI] [PubMed] [Google Scholar]
- 7.Talmud PJ, Futema M, Humphries SE. The genetic architecture of the familial hyperlipidaemia syndromes: rare mutations and common variants in multiple genes. Curr Opin Lipidol. 2014;25:274–281. doi: 10.1097/MOL.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 8.Schilling S, DeStefano AL, Sachdev PS, Choi SH, Mather KA, De-Carli CD, et al. APOE genotype and MRI markers of cerebrovascular disease: systematic review and meta-analysis. Neurology. 2013;81:292–300. doi: 10.1212/WNL.0b013e31829bfda4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Podzielinski I, Saunders BA, Kimbler KD, Branscum AJ, Fung ET, DePriest PD, et al. Apolipoprotein concentrations are elevated in malignant ovarian cyst fluids suggesting that lipoprotein metabolism is dysregulated in epithelial ovarian cancer. Cancer Invest. 2013;31:258–272. doi: 10.3109/07357907.2013.789896. [DOI] [PubMed] [Google Scholar]
- 10.Honda K, Okusaka T, Felix K, Nakamori S, Sata N, Nagai H, et al. Altered plasma apolipoprotein modifications in patients with pancreatic cancer: protein characterization and multi-institutional validation. PLoS One. 2012;7:e46908. doi: 10.1371/journal.pone.0046908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shi J, Liu J, Liao L, Guo Y, Wang H, Hu W, et al. Identification of candidate serum biomarkers for small cell lung cancer by proteomics analysis. Minerva Med. 2014;105:137–147. [PubMed] [Google Scholar]
- 12.Bradley JD, Dehdashti F, Mintun MA, Govindan R, Trinkaus K, Siegel BA. Positron emission tomography in limited-stage small-cell lung cancer: a prospective study. J Clin Oncol. 2004;22:3248–3254. doi: 10.1200/JCO.2004.11.089. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd FA, Crowley J, Van Houtte P, Postmus PE, Carney D, Chansky K, et al. The International Association for the Study of Lung Cancer lung cancer staging project: proposals regarding the clinical staging of small cell lung cancer in the forthcoming (seventh) edition of the tumor, node, metastasis classification for lung cancer. J Thorac Oncol. 2007;2:1067–1077. doi: 10.1097/JTO.0b013e31815bdc0d. [DOI] [PubMed] [Google Scholar]
- 14.Ploenes T, Osei-Agyemang T, Krohn A, Kayser G, Burger M, Passlick B. Surgical treatment of early stage small cell lung cancer. Asian Cardiovasc Thorac Ann. 2012;20:694–698. doi: 10.1177/0218492312453348. [DOI] [PubMed] [Google Scholar]
- 15.Lee CB, Morris DE, Fried DB, Socinski MA. Current and evolving treatment options for limited stage small cell lung cancer. Curr Opin Oncol. 2006;18:162–172. doi: 10.1097/01.cco.0000208790.45312.25. [DOI] [PubMed] [Google Scholar]
- 16.Cooper WA, Lam DC, O'Toole SA, Minna JD. Molecular biology of lung cancer. J Thorac Dis. 2013;5(Suppl 5):S479–S490. doi: 10.3978/j.issn.2072-1439.2013.08.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhang Y, He J. The development of targeted therapy in small cell lung cancer. J Thorac Dis. 2013;5:538–548. doi: 10.3978/j.issn.2072-1439.2013.07.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lund EL, Thorsen C, Pedersen MW, Junker N, Kristjansen PE. Relationship between vessel density and expression of vascular endothelial growth factor and basic fibroblast growth factor in small cell lung cancer in vivo and in vitro. Clin Cancer Res. 2000;6:4287–4291. [PubMed] [Google Scholar]
- 19.Chetty PS, Mayne L, Lund-Katz S, Stranz D, Englander SW, Phillips MC. Helical structure and stability in human apolipoprotein A-I by hydrogen exchange and mass spectrometry. Proc Natl Acad Sci U S A. 2009;106:19005–19010. doi: 10.1073/pnas.0909708106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.TuftStavnes H, Nymoen DA, HetlandFalkenthal TE, Kærn J, Tropé CG, Davidson B. APOA1 mRNA expression in ovarian serous carcinoma effusions is a marker of longer survival. Am J Clin Pathol. 2014;142:51–57. doi: 10.1309/AJCPD8NBSHXRXQL7. [DOI] [PubMed] [Google Scholar]
- 21.Ehmann M, Felix K, Hartmann D, Schnölzer M, Nees M, Vorderwülbecke S, et al. Identification of potential markers for the detection of pancreatic cancer through comparative serum protein expression profiling. Pancreas. 2007;34:205–214. doi: 10.1097/01.mpa.0000250128.57026.b2. [DOI] [PubMed] [Google Scholar]
- 22.Yang HH, Chen XF, Hu W, Lv DQ, Ding WJ, Tang LJ, et al. Lipoprotein (a) level and its association with tumor stage in male patients with primary lung cancer. Clin Chem Lab Med. 2009;47:452–457. doi: 10.1515/CCLM.2009.094. [DOI] [PubMed] [Google Scholar]
- 23.Li C, Li H, Zhang T, Li J, Liu L, Chang J. Discovery of Apo-A1 as a potential bladder cancer biomarker by urine proteomics and analysis. Biochem Biophys Res Commun. 2014;446:1047–1052. doi: 10.1016/j.bbrc.2014.03.053. [DOI] [PubMed] [Google Scholar]
- 24.Gourin CG, Zhi W, Adam BL. Proteomic identification of serum biomarkers for head and neck cancer surveillance. Laryngoscope. 2009;119:1291–1302. doi: 10.1002/lary.20279. [DOI] [PubMed] [Google Scholar]
- 25.Gao F, Vasquez SX, Su F, Roberts S, Shah N, Grijalva V, et al. L-5F, an apolipoprotein A-I mimetic, inhibits tumor angiogenesis by suppressing VEGF/basic FGF signaling pathways. Integr Biol (Camb) 2011;3:479–489. doi: 10.1039/c0ib00147c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Larsson M, Vorrsjö E, Talmud P, Lookene A, Olivecrona G. Apolipoproteins C-I and C-III inhibit lipoprotein lipase activity by displacement of the enzyme from lipid droplets. J Biol Chem. 2013;288:33997–34008. doi: 10.1074/jbc.M113.495366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yao Z, Wang Y. Apolipoprotein C-III and hepatic triglyceride-rich lipoprotein production. Curr Opin Lipidol. 2012;23:206–212. doi: 10.1097/MOL.0b013e328352dc70. [DOI] [PubMed] [Google Scholar]
- 28.Fan Y, Shi L, Liu Q, Dong R, Zhang Q, Yang S, et al. Discovery and identification of potential biomarkers of papillary thyroid carcinoma. Mol Cancer. 2009;8:79. doi: 10.1186/1476-4598-8-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kardassis D, Pardali K, Zannis VI. SMAD proteins transactivate the human ApoCIII promoter by interacting physically and functionally with hepatocyte nuclear factor 4. J Biol Chem. 2000;275:41405–41414. doi: 10.1074/jbc.M007896200. [DOI] [PubMed] [Google Scholar]
- 30.Fraser JD, Martinez V, Straney R, Briggs MR. DNA binding and transcription activation specificity of hepatocyte nuclear factor 4. Nucleic Acids Res. 1998;26:2702–2707. doi: 10.1093/nar/26.11.2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Reilly M, Rader DJ. Apolipoprotein E and coronary disease: a puzzling paradox. PLoS Med. 2006;3:e258. doi: 10.1371/journal.pmed.0030258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martínez-Clemente M, Ferré N, González-Périz A, López-Parra M, Horrillo R, Titos E, et al. 5-lipoxygenase deficiency reduces hepatic inflammation and tumor necrosis factor alpha-induced hepatocyte damage in hyperlipidemia-prone ApoE-null mice. Hepatology. 2010;51:817–827. doi: 10.1002/hep.23463. [DOI] [PubMed] [Google Scholar]
- 33.Su WP, Chen YT, Lai WW, Lin CC, Yan JJ, Su WC. Apolipoprotein E expression promotes lung adenocarcinoma proliferation and migration and as a potential survival marker in lung cancer. Lung Cancer. 2011;71:28–33. doi: 10.1016/j.lungcan.2010.04.009. [DOI] [PubMed] [Google Scholar]
- 34.Trost Z, Marc J, Sok M, Cerne D. Increased apolipoprotein E gene expression and protein concentration in lung cancer tissue do not contribute to the clinical assessment of non-small cell lung cancer patients. Arch Med Res. 2008;39:663–667. doi: 10.1016/j.arcmed.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 35.Zelen M. Keynote address on biostatistics and data retrieval. Cancer Chemother Rep 3. 1973;4:31–42. [PubMed] [Google Scholar]
- 36.Cheng T, Dai X, Zhou DL, Lv Y, Miao LY. Correlation of apolipoprotein A-I kinetics with survival and response to first-line platinum-based chemotherapy in advanced non-small cell lung cancer. Med Oncol. 2015;32:407. doi: 10.1007/s12032-014-0407-8. [DOI] [PubMed] [Google Scholar]
- 37.Ko HL, Wang YS, Fong WL, Chi MS, Chi KH, Kao SJ. APOC1 as a novel diagnostic and prognostic biomarker for lung cancer: a marker phase i trial. Thoracic Cancer. 2014;5:500–508. doi: 10.1111/1759-7714.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fox W, Scadding JG. Medical Research Council comparative trial of surgery and radiotherapy for primary treatment of small-celled or oat-celled carcinoma of bronchus. Ten-year follow-up. Lancet. 1973;2:63–65. doi: 10.1016/s0140-6736(73)93260-1. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd FA, Ginsberg RJ, Evans WK, Feld R, Cooper JD, Ilves R, et al. Reduction in local recurrence and improved survival in surgically treated patients with small cell lung cancer. J Thorac Cardiovasc Surg. 1983;86:498–506. [PubMed] [Google Scholar]
- 40.Koletsis EN, Prokakis C, Karanikolas M, Apostolakis E, Dougenis D. Current role of surgery in small cell lung carcinoma. J Cardiothorac Surg. 2009;4:30. doi: 10.1186/1749-8090-4-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Turrisi AT, 3rd, Kim K, Blum R, Sause WT, Livingston RB, Komaki R, et al. Twice-daily compared with once-daily thoracic radiotherapy in limited small-cell lung cancer treated concurrently with cisplatin and etoposide. N Engl J Med. 1999;340:265–271. doi: 10.1056/NEJM199901283400403. [DOI] [PubMed] [Google Scholar]
- 42.Naranjo Gómez JM, Gómez Román JJ. Behaviour and survival of high-grade neuroendocrine carcinomas of the lung. Respir Med. 2010;104:1929–1936. doi: 10.1016/j.rmed.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 43.Yip D, Harper PG. Predictive and prognostic factors in small cell lung cancer: current status. Lung Cancer. 2000;28:173–185. doi: 10.1016/s0169-5002(00)00105-7. [DOI] [PubMed] [Google Scholar]
- 44.Foster NR, Mandrekar SJ, Schild SE, Nelson GD, Rowland KM, Jr, Deming RL, et al. Prognostic factors differ by tumor stage for small cell lung cancer: a pooled analysis of North Central Cancer Treatment Group trials. Cancer. 2009;115:2721–2731. doi: 10.1002/cncr.24314. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The Expression of Apolipoproteins in SCLC Tissues Under Different Conditions