Abstract
Purpose
To describe the relationship between the waist-to-height ratio (WHtR) and cardiometabolic risk factors (CMRFs) and to evaluate the validity of WHtR in identifying adolescents with metabolic syndrome.
Materials and Methods
We analyzed data from a pooled population of 4068 adolescents aged 10–19 years from the Korean National Health and Nutrition Examination Surveys conducted between 1998 and 2008. Overweight individuals were defined by body mass index (BMI) ≥85th percentile. Those with at least 2 CMRFs among hypertension, hyperglycemia, hypertriglyceridemia, and decreased high-density lipoprotein cholesterol (HDL-C) were classified as having multiple CMRFs.
Results
WHtR was significantly related to systolic blood pressure, HDL-C, and triglycerides in both non-overweight and overweight adolescents (all p<0.01). Among overweight adolescents, the area under the curve (AUC) for WHtR in identifying multiple CMRFs was significantly greater than that for BMI (p=0.014). Metabolic syndrome was more common in overweight adolescents with a WHtR of ≥0.5 than in those with a WHtR of <0.5 (p<0.001). In non-overweight adolescents, the prevalences of multiple CMRFs (p=0.001) and metabolic syndrome (p<0.001) were higher in those with a WHtR of ≥0.5 than in those with a WHtR of <0.5. Among those without central obesity, the prevalence of multiple CMRFs was higher in those with a WHtR of ≥0.5 than in those with a WHtR of <0.5 (p=0.021).
Conclusion
WHtR is a simple and valid index for identifying adolescents with increased cardiometabolic risk and is related to CMRFs even in non-overweight adolescents. In adolescents already screened via BMI and waist circumference (WC), WHtR seems to be of additional help in discriminating those at higher cardiometabolic risk.
Keywords: Waist, height, body mass index, metabolic syndrome, obesity
INTRODUCTION
With the increase of obesity and sedentary lifestyles, the prevalences of diabetes and cardiovascular diseases are also rapidly increasing.1 It has been reported that atherosclerotic changes begin in childhood, and the presence of cardiometabolic risk factors (CMRFs) in adolescents has also been reported to be associated with premature cardiovascular disease and diabetes.2
Metabolic syndrome is defined by the presence of three or more CMRFs including central adiposity, hypertension, hyperglycemia, hypertriglyceridemia, and decreased high-density lipoprotein-cholesterol (HDL-C).3 It is rare in non-obese children; however, the prevalence increases rapidly in overweight or obese children and adolescents.1,4 Metabolic syndrome in children and adolescents can lead to increased morbidity and mortality later in life; thus, it is important to find those at higher risk for metabolic syndrome.2,5
Body mass index (BMI) and waist circumference (WC) are commonly used to define obesity in children and adolescents.6 However, BMI is not a measure of fat distribution, and different cutoffs are used according to age and sex.7,8 Although WC is a good proxy for central obesity, age-dependent WC cutoffs in this age group are also required.7
Waist-to-height ratio (WHtR) is an alternative tool for identifying people at higher metabolic risk. It has been reported that WHtR is superior to BMI in detecting cardiovascular risk factors in adults.9 Overweight children with a higher WHtR are more likely to have higher CMRFs.10,11 A single WHtR cutoff of 0.5 seems to be acceptable in children as well as in adults, suggesting that tables for age-dependent cutoffs are not required.12,13
This study was performed 1) to describe the relationship between WHtR and CMRFs in adolescents, 2) to evaluate the validity of WHtR in identifying metabolic syndrome14 when compared to BMI and WC, and 3) to determine if the additional use of WHtR is helpful in screening adolescents with higher cardiometabolic risk.
MATERIALS AND METHODS
Participants
This study was based on data from the Korea National Health and Nutrition Examination Survey (KNHANES), conducted by the Korean Ministry of Health and Welfare between 1998 and 2008. Of the 5733 participants aged 10 to 19, 4068 participants (2139 boys and 1929 girls) whose data for all components of metabolic syndrome were available and from whom samples were taken after fasting for more than 10 hours were enrolled.
Overweight individuals were defined by BMI ≥85th percentile for age and sex, and non-overweight individuals were defined by BMI <85th percentile. To determine whether the use of WHtR in addition to BMI or WC helps to discriminate those at higher cardiometabolic risk, participants were categorized according to BMI (overweight or non-overweight) and WC (with or without central obesity), and each of the BMI and WC groups were further stratified by WHtR (WHtR <0.5 vs. WHtR ≥0.5).
This study was approved by the Korean Ministry of Health and Welfare and by the Institutional Review Board of the National Health Insurance Service, Ilsan Hospital (NHIMC 2015-04-027).
Definition of cardiometabolic risk factors and metabolic syndrome
WC was measured at the narrowest point between the lower borders of the rib cage and the iliac crest at the end of normal expiration. WHtR was calculated by dividing WC (cm) by height (cm), and the WHtR cutoff of 0.5 was used, as it was previously suggested as a universal WHtR cutoff.12 Central obesity was defined by WC ≥90th percentile using Korean waist reference data for those of <16 years of age.15 For those ≥16 years of age, a WC of ≥90 cm in boys and of ≥85 cm in girls was used to define central obesity, according to the Korean-specific WC cutoff points.16
Hypertension was defined by systolic blood pressure (SBP) ≥130 mm Hg or diastolic BP (DBP) ≥85 mm Hg. Hyperglycemia was defined by fasting glucose ≥100 mg/dL, and hypertriglyceridemia was defined by fasting triglyceride level ≥150 mg/dL. Decreased HDL-C was defined as an HDL-C level of <40 mg/dL for all boys and girls of <16 years of age and of <50 mg/dL in girls of ≥16 years of age.14 The presence of two or more CMRFs among hypertension, hyperglycemia, hypertriglyceridemia, and decreased HDL-C was classified as multiple CMRFs.
Metabolic syndrome was defined by the International Diabetes Federation (IDF) criteria for children and adolescents.14 A WC of at least the 90th percentile using Korean waist reference data15 was a mandatory criterion for metabolic syndrome. For those of at least 16 years, a WC of ≥90 cm in boys or of ≥85 cm in girls was used to define central obesity.16 Two or more of the following components were also required: a fasting triglyceride level of 150 mg/dL, a fasting glucose concentration of 100 mg/dL or specific treatment, SBP of 130 mm Hg or DBP of 85 mm Hg, or an HDL-C level of <40 mg/dL for both boys and girls of <16 years or of <50 mg/dL in girls of ≥16 years.
Statistical analysis
Continuous data are expressed as mean±standard deviation and were compared using Wilcoxon's rank sum test. Categorical data are expressed as proportions (%) and were compared using chi-square and Fisher's exact tests. The relationship between WHtR and CMRFs was evaluated by performing a sex-and age-adjusted linear regression analysis. To validate the effectiveness of WHtR in identifying multiple CMRFs, the area under the curve (AUC) was calculated from the receiver operating characteristic (ROC) curve.17 Comparisons between subgroups were performed using Wilcoxon's rank sum test. All statistical analyses were performed using SAS 9.4 software (SAS Institute, Cary, NC, USA). p<0.05 was considered significant.
RESULTS
Clinical and cardiometabolic characteristics of participants are summarized in Table 1. On linear regression analysis adjusted for age and gender, WHtR was significantly associated with WC (p<0.001), SBP (p<0.001 in non-overweight group and p=0.001 in overweight group), triglycerides (p<0.001), and HDL-C (p<0.001) in both non-overweight and overweight adolescents (Table 2). In overweight adolescents, the AUC of WHtR in identifying multiple CMRFs was significantly higher than that of BMI (p=0.014) yet did not differ from that of WC (Table 3).
Table 1. Clinical and Cardiometabolic Characteristics of the Study Population.
| Boys | Girls | p value | |
|---|---|---|---|
| N | 2139 | 1929 | |
| Age (yrs) | 13.6±2.5 | 13.8±2.6 | 0.011 |
| Height (cm) | 161.8±13.1 | 156.1±8.4 | <0.001 |
| Height z score | 0.39±1.03 | 0.26±1.02 | <0.001 |
| Weight (kg) | 54.5±15.1 | 49.2±10.8 | <0.001 |
| Weight z score | 0.14±1.05 | 0.08±1.07 | 0.144 |
| BMI (kg/m2) | 20.5±3.7 | 20.0±3.3 | 0.004 |
| BMI z score | -0.06±1.08 | -0.02±1.08 | 0.201 |
| WC (cm) | 70.9±10.4 | 67.0±8.2 | <0.001 |
| WHtR | 0.44±0.06 | 0.43±0.05 | <0.001 |
| SBP (mm Hg) | 110.2±11.8 | 105.9±10.6 | <0.001 |
| DBP (mm Hg) | 66.2±10.7 | 65.0±9.5 | <0.001 |
| Fasting glucose (mg/dL) | 92.0±9.6 | 90.3±9.4 | <0.001 |
| Triglycerides (mg/dL) | 88.0±53.2 | 92.2±44.1 | <0.001 |
| HDL-C (mg/dL) | 48.6±11.4 | 50.5±11.0 | <0.001 |
| Central obesity (%) | 8.1 | 7.7 | 0.629 |
| Hypertension (%) | 8.0 | 2.9 | <0.001 |
| Hyperglycemia (%) | 18.3 | 15.2 | 0.008 |
| Hypertriglyceridemia (%) | 9.1 | 9.7 | 0.495 |
| Decreased HDL-C (%) | 21.6 | 26.0 | 0.001 |
| Multiple CMRFs (%) | 10.9 | 9.6 | 0.144 |
| Metabolic syndrome (%) | 2.4 | 1.7 | 0.084 |
WC, waist circumference; WHtR, waist to height ratio; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, highdensity lipoprotein-cholesterol; CMRF, cardiometabolic risk factor.
Table 2. Correlations between WHtR and Cardiometabolic Risk Factors.
| Non-overweight* | Overweight† | |||
|---|---|---|---|---|
| Adjusted R2‡ | p value | Adjusted R2‡ | p value | |
| WC | 0.7998 | <0.001 | 0.8207 | <0.001 |
| SBP | 0.1276 | <0.001 | 0.1269 | 0.001 |
| DBP | 0.1171 | 0.566 | 0.0613 | 0.028 |
| Glucose | 0.0222 | 0.132 | 0.0260 | 0.694 |
| Triglycerides | 0.0343 | <0.001 | 0.0527 | <0.001 |
| HDL-C | 0.0296 | <0.001 | 0.0447 | <0.001 |
WHtR, waist to height ratio; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein-cholesterol; BMI, body mass index.
*BMI <85th percentile, †BMI ≥85th percentile, ‡After adjustment for age and gender.
Table 3. Receiver Operating Characteristic Curve Analysis for Identifying Multiple Cardiometabolic Risk Factors.
| BMI | WC | WHtR | |
|---|---|---|---|
| AUC (95% CI) | AUC (95% CI) | AUC (95% CI) | |
| Non-overweight* | 0.835 (0.637, 1.000) | 0.995 (0.988, 1.000) | 0.998 (0.996, 1.000) |
| Overweight† | 0.742 (0.684, 0.800) | 0.820 (0.773, 0.867)‡ | 0.807 (0.763, 0.850)§ |
BMI, body mass index; WC, waist circumference; WHtR, waist-to-height ratio; AUC, area under the curve; CI, confidence interval.
*BMI <85th percentile, †BMI ≥85th percentile, ‡p<0.001 vs. BMI, §p=0.014 vs. BMI.
Table 4 summarizes the clinical characteristics of adolescents grouped by BMI and further classifies them according to WHtR. In non-overweight adolescents, the prevalence of multiple CMRFs was 7.9% in those with a WHtR of <0.5, whereas it was 17.8% in those with a WHtR of ≥0.5 (p=0.001) (Fig. 1A). The prevalence of metabolic syndrome was higher in those with a WHtR of ≥0.5 than in those with a WHtR of <0.5 in both non-overweight and overweight adolescents (both p<0.001) (Fig. 1B).
Table 4. Clinical Characteristics of Participants Grouped by BMI and Stratified According to WHtR.
| Non-overweight* | p value | Overweight† | p value | |||
|---|---|---|---|---|---|---|
| WHtR<0.5 | WHtR≥0.5 | WHtR<0.5 | WHtR≥0.5 | |||
| n (M:F) | 3260 (1706:1554) | 73 (43:30) | 293 (99:194) | 441 (290:151) | ||
| Age (yrs) | 13.7±2.5 | 12.8±2.5 | 0.002 | 13.9±2.6 | 13.5±2.7 | 0.019 |
| Height (cm) | 158.9±11.6 | 151.5±10.4 | <0.001 | 161.0±9.8 | 159.9±10.9 | 0.191 |
| Height z score | 0.29±1.01 | -0.19±1.02 | 0.001 | 0.62±1.15 | 0.52±1.03 | 0.352 |
| Weight (kg) | 48.7±11.0 | 49.6±9.1 | 0.521 | 64.3±10.7 | 68.9±15.0 | 0.001 |
| Weight z score | -0.22±0.85 | 0.34±0.66 | <0.001 | 1.41±0.52 | 1.68±0.62 | <0.001 |
| BMI (kg/m2) | 19.0±2.3 | 21.4±1.6 | <0.001 | 24.6±1.8 | 26.6±3.0 | <0.001 |
| BMI z-score | -0.42±0.82 | 0.57±0.48 | <0.001 | 1.36±0.28 | 1.71±0.45 | <0.001 |
| WC (cm) | 65.9±6.7 | 79.4±6.4 | <0.001 | 75.8±6.5 | 86.2±8.1 | <0.001 |
| SBP (mm Hg) | 107.1±11.2 | 109.8±9.8 | 0.018 | 111.0±11.3 | 113.3±12.1 | 0.008 |
| DBP (mm Hg) | 65.2±10.1 | 64.5±10.7 | 0.711 | 67.3±10.1 | 68.4±10.2 | 0.143 |
| Fasting glucose (mg/dL) | 91.0±9.5 | 92.5±13.2 | 0.599 | 92.0±9.5 | 91.7±8.9 | 0.834 |
| Triglycerides (mg/dL) | 83.7±41.1 | 113.6±56.0 | <0.001 | 100.7±59.3 | 125.4±72.5 | <0.001 |
| HDL-C (mg/dL) | 50.6±11.3 | 47.2±9.4 | 0.025 | 47.0±10.9 | 43.7±9.2 | <0.001 |
BMI, body mass index; WHtR, waist-to-height ratio; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density lipoprotein-cholesterol.
*BMI <85th percentile, †BMI ≥85th percentile.
Fig. 1. The prevalence of multiple CMRFs (A) and metabolic syndrome (B) in overweight (BMI ≥85 P) and non-overweight (BMI <85 P) adolescents further stratified by WHtR. *p<0.05 vs. BMI <85 P/WHtR <0.5 group, †p<0.05 vs. BMI ≥85 P/WHtR <0.5 group. WHtR, waist to height ratio; BMI, body mass index; CMRFs, cardiometabolic risk factors.
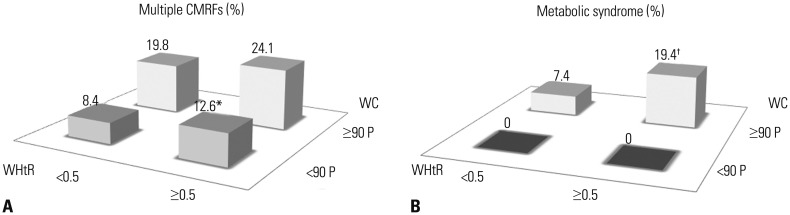
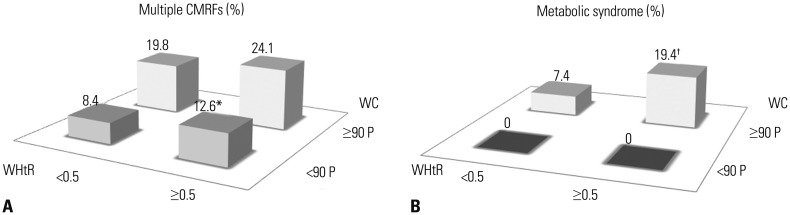
About 3% of adolescents without central obesity had a WHtR of ≥0.5. These adolescents were significantly shorter than their low-WHtR counterpart (Table 5). Among adolescents without central obesity, the prevalence of multiple CMRFs was significantly higher in the WHtR≥0.5 group compared to the WHtR <0.5 group (12.6% vs. 8.4%, p=0.021) (Fig. 2A).
Table 5. Clinical Characteristics of Participants Grouped by the Presence of Central Obesity and Further Stratified by WHtR.
| Non-overweight* | p value | Overweight† | p value | |||
|---|---|---|---|---|---|---|
| WHtR<0.5 | WHtR≥0.5 | WHtR<0.5 | WHtR≥0.5 | |||
| n (M:F) | 3473 (1778:1695) | 111 (90:21) | 81 (28:53) | 403 (243:160) | ||
| Age (yrs) | 13.7±2.5 | 12.8±2.6 | 0.001 | 13.7±2.3 | 13.5±2.6 | 0.392 |
| Height (cm) | 158.9±11.5 | 151.1±10.7 | <0.001 | 167.8±8.5 | 160.9±10.5 | <0.001 |
| Height z score | 0.29±1.01 | -0.45±1.04 | <0.001 | 1.68±0.86 | 0.66±0.93 | <0.001 |
| Weight (kg) | 49.5±11.5 | 55.0±12.4 | <0.001 | 69.1±9.6 | 69.2±15.3 | 0.592 |
| Weight z score | -0.13±0.9 | 0.73±0.62 | <0.001 | 1.76±0.49 | 1.70±0.68 | 0.313 |
| BMI (kg/m2) | 19.4±2.7 | 23.8±2.5 | <0.001 | 24.5±2.1 | 26.4±3.4 | <0.001 |
| BMI z-score | -0.31±0.91 | 1.13±0.48 | <0.001 | 1.31±0.48 | 1.66±0.59 | <0.001 |
| WC (cm) | 66.4±6.9 | 78.0±5.2 | <0.001 | 81.2±4.5 | 87.2±7.8 | <0.001 |
| SBP (mm Hg) | 107.4±11.3 | 109.2±10.3 | 0.060 | 110.3±11.2 | 113.8±12.1 | 0.017 |
| DBP (mm Hg) | 65.3±10.1 | 66.1±10.6 | 0.264 | 67.4±11.1 | 68.3±10.3 | 0.556 |
| Fasting glucose (mg/dL) | 91.1±9.5 | 93.1±10.7 | 0.034 | 92.7±10.1 | 91.4±9.2 | 0.325 |
| Triglycerides (mg/dL) | 84.6±42.5 | 110.2±58.6 | <0.001 | 106.7±60.6 | 127.5±73.0 | 0.006 |
| HDL-C (mg/dL) | 50.4±11.3 | 47.8±10.3 | 0.020 | 44.8±10.6 | 43.2±8.7 | 0.315 |
BMI, body mass index; WHtR, waist-to-height ratio; WC, waist circumference; SBP, systolic blood pressure; DBP, diastolic blood pressure; HDL-C, high-density li-poprotein-cholesterol.
*WC <90th percentile if age <16 years and WC <90 cm in boys and WC <85 cm in girls if age ≥16 years, †WC ≥90th percentile if age <16 years and if age ≥16 years, WC ≥90 cm in boys and WC ≥85 cm in girls.
Fig. 2. The prevalence of multiple CMRFs (A) and metabolic syndrome (B) in adolescents those with central obesity (WC ≥90 P) and without central obesity (WC <90 P) further stratified by WHtR. *p<0.05 vs. WC <90 P/WHtR <0.5 group, †p<0.05 vs. WC ≥90 P/WHtR <0.5 group. WHtR, waist to height ratio; WC, waist circumference; CMRFs, cardiometabolic risk factors.
Eighty-one (16.7%) adolescents with central obesity had a WHtR of <0.5. These adolescents were significantly taller than their high-WHtR counterpart (Table 5). Among those with central obesity, the prevalence of metabolic syndrome was 7.4% in adolescents with a WHtR of <0.5 and 19.4% in those with a WHtR of ≥0.5 (p=0.017) (Fig. 2B).
DISCUSSION
Our study demonstrates that WHtR is an easy-to-use and reliable index for identifying adolescents at higher metabolic risk. Additional use of WHtR can be helpful when screening obesity with BMI in adolescents, as WHtR represents central adiposity. Considering that WHtR is an index adjusted for height, it might be useful when used together with WC.
In a recent study in which BMI percentile categories were further stratified on the basis of WHtR, increasing WHtR was significantly associated with increased cardiometabolic risk in overweight and obese children.11 Furthermore, in a recent prospective study, those with a WHtR of >0.65 at ages 12 to 39 had a 139% greater risk of death before 55 years of age than those with a WHtR of <0.5.18 Taken together, increased WHtR might be related to increased prevalence of metabolic syndrome, which may in turn result in premature morbidity and mortality. Our data provide additional evidence that higher WHtR is closely related to increased cardiometabolic risk from adolescence.
BMI is commonly used for defining overweight and obese children and adolescents.6 However, BMI cannot distinguish individuals with excess body fat from those with high muscle mass, nor can it reflect the distribution of adipose tissue.7,8 In non-overweight adolescents, the WHtR ≥0.5 group showed higher prevalences of multiple CMRFs and metabolic syndrome than the WHtR <0.5 group in the present study. Among overweight adolescents, the prevalence of metabolic syndrome was only 2% in those with a WHtR of <0.5, and the prevalence increased to 17% in those with a WHtR of ≥0.5. These results suggest that central adiposity is closely associated with CMRFs in non-overweight as well as overweight adolescents and that an index for central adiposity, such as WHtR, should be used in addition to BMI when screening those with higher metabolic risk.7,11,13
Although the WC is a good proxy for central adiposity, tables for WC cutoffs according to age and sex are required in children and adolescents to define central obesity. In busy outpatient clinics or during mass screening, it is cumbersome to use tables for each patient. The advantage of WHtR is that a single cutoff can be used, which means that tables for age-dependent cutoffs are not required. In Korean children, the WHtR percentile curve almost plateaus at 10–19 years of age.19 A study on Hong Kong Chinese children also showed a similar WHtR percentile curve,20 suggesting that a single WHtR cutoff might be available for adolescents.
The limitation of WC is that it does not consider height. There is a concern that the cardiometabolic risk may differ between people who have the same WC yet differ in height. It was reported that shorter people have higher cardiometabolic risk than taller people with the same WC.21 WC is a height-dependent variable, and shorter children tend to have a lower WC than taller children.21 The tempo of growth and maturation vary from child to child, and tall children tend to fall into higher WC percentiles than their peers, which may overestimate central adiposity. In contrast, central obesity in children with constitutional delay of growth can be underestimated when it is defined by age-dependent WC cutoffs. Height should be taken into account when defining obesity in children and adolescents, and the WHtR is an alternative parameter that considers height. In adolescents with central obesity, the prevalence of metabolic syndrome was more than double in those with a WHtR of ≥0.5 compared to those with a WHtR of <0.5 in the present study. WHtR seems to be useful in discriminating those at higher metabolic risk, even when it is used in combination with WC, likely due to its consideration of height.
Consistent with previous reports,9,12,13,22 the validity of WHtR was comparable to that of WC, and the AUC for identifying multiple CMRFs was significantly higher than that of BMI. It is not surprising that WC shows the best result, as the IDF criteria for metabolic syndrome require a WC of ≥90 P as a mandatory criterion, although WHtR also seems to be an excellent parameter in screening adolescents at higher metabolic risk.
This study had several strengths and weaknesses. It was one of the largest studies in which all components of metabolic syndrome were directly measured in more than 4000 adolescents. Nevertheless, a limitation of our study was that the secular trend between 1998 and 2008 and the sampling weights were not considered.
In conclusion, WHtR is a simple and valid index for identifying adolescents with metabolic syndrome, and WHtR was related to CMRFs even in non-overweight adolescents. In adolescents screened via BMI and WC, WHtR seems to be of additional help in determining those at higher cardiometabolic risk.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Duncan GE, Li SM, Zhou XH. Prevalence and trends of a metabolic syndrome phenotype among us Adolescents, 1999-2000. Diabetes Care. 2004;27:2438–2443. doi: 10.2337/diacare.27.10.2438. [DOI] [PubMed] [Google Scholar]
- 2.Han JC, Lawlor DA, Kimm SY. Childhood obesity. Lancet. 2010;375:1737–1748. doi: 10.1016/S0140-6736(10)60171-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults. Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, And Treatment of High Blood Cholesterol In Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–2497. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 4.Park MJ, Boston BA, Oh M, Jee SH. Prevalence and trends of metabolic syndrome among Korean adolescents: from the Korean NHANES survey, 1998-2005. J Pediatr. 2009;155:529–534. doi: 10.1016/j.jpeds.2009.03.063. [DOI] [PubMed] [Google Scholar]
- 5.Morrison JA, Friedman LA, Wang P, Glueck CJ. Metabolic syndrome in childhood predicts adult metabolic syndrome and type 2 diabetes mellitus 25 to 30 years later. J Pediatr. 2008;152:201–206. doi: 10.1016/j.jpeds.2007.09.010. [DOI] [PubMed] [Google Scholar]
- 6.WHO Expert Consultation. Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 7.Zimmet P, Magliano D, Matsuzawa Y, Alberti G, Shaw J. The metabolic syndrome: a global public health problem and a new definition. J Atheroscler Thromb. 2005;12:295–300. doi: 10.5551/jat.12.295. [DOI] [PubMed] [Google Scholar]
- 8.Yajnik CS, Yudkin JS. The Y-Y paradox. Lancet. 2004;363:163. doi: 10.1016/S0140-6736(03)15269-5. [DOI] [PubMed] [Google Scholar]
- 9.Lee CM, Huxley RR, Wildman RP, Woodward M. Indices of abdominal obesity are better discriminators of cardiovascular risk factors than BMI: a meta-analysis. J Clin Epidemiol. 2008;61:646–653. doi: 10.1016/j.jclinepi.2007.08.012. [DOI] [PubMed] [Google Scholar]
- 10.Spolidoro JV, Pitrez Filho ML, Vargas LT, Santana JC, Pitrez E, Hauschild JA, et al. Waist circumference in children and adolescents correlate with metabolic syndrome and fat deposits in young adults. Clin Nutr. 2013;32:93–97. doi: 10.1016/j.clnu.2012.05.020. [DOI] [PubMed] [Google Scholar]
- 11.Khoury M, Manlhiot C, McCrindle BW. Role of the waist/height ratio in the cardiometabolic risk assessment of children classified by body mass index. J Am Coll Cardiol. 2013;62:742–751. doi: 10.1016/j.jacc.2013.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Ashwell M, Hsieh SD. Six reasons why the waist-to-height ratio is a rapid and effective global indicator for health risks of obesity and how its use could simplify the international public health message on obesity. Int J Food Sci Nutr. 2005;56:303–307. doi: 10.1080/09637480500195066. [DOI] [PubMed] [Google Scholar]
- 13.Maffeis C, Banzato C, Talamini G Obesity Study Group of the Italian Society of Pediatric Endocrinology and Diabetology. Waist-to-height ratio, a useful index to identify high metabolic risk in overweight children. J Pediatr. 2008;152:207–213. doi: 10.1016/j.jpeds.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 14.Zimmet P, Alberti KG, Kaufman F, Tajima N, Silink M, Arslanian S, et al. The metabolic syndrome in children and adolescents - an IDF consensus report. Pediatr Diabetes. 2007;8:299–306. doi: 10.1111/j.1399-5448.2007.00271.x. [DOI] [PubMed] [Google Scholar]
- 15.Moon JS, Lee SY, Nam CM, Choi JM, Choe BK, Seo JW, et al. 2007 Korean national growth charts: review of developmental process and an outlook. Korean J Pediatr. 2008;51:1–25. [Google Scholar]
- 16.Lee SY, Park HS, Kim DJ, Han JH, Kim SM, Cho GJ, et al. Appropriate waist circumference cutoff points for central obesity in Korean adults. Diabetes Res Clin Pract. 2007;75:72–80. doi: 10.1016/j.diabres.2006.04.013. [DOI] [PubMed] [Google Scholar]
- 17.Akobeng AK. Understanding diagnostic tests 3: receiver operating characteristic curves. Acta Paediatr. 2007;96:644–647. doi: 10.1111/j.1651-2227.2006.00178.x. [DOI] [PubMed] [Google Scholar]
- 18.Saydah S, Bullard KM, Imperatore G, Geiss L, Gregg EW. Cardiometabolic risk factors among US adolescents and young adults and risk of early mortality. Pediatrics. 2013;131:e679–e686. doi: 10.1542/peds.2012-2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gil JH, Lee MN, Lee HA, Park HS, Seo JW. Usefulness of the waist circumference-to-height ratio in screening for obesity in Korean children and adolescents. Korean J Pediatr Gastroenterol Nutr. 2010;13:180–192. [Google Scholar]
- 20.Sung RY, So HK, Choi KC, Nelson EA, Li AM, Yin JA, et al. Waist circumference and waist-to-height ratio of Hong Kong Chinese children. BMC Public Health. 2008;8:324. doi: 10.1186/1471-2458-8-324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hsieh SD, Yoshinaga H. Do people with similar waist circumference share similar health risks irrespective of height? Tohoku J Exp Med. 1999;188:55–60. doi: 10.1620/tjem.188.55. [DOI] [PubMed] [Google Scholar]
- 22.Kromeyer-Hauschild K, Neuhauser H, Schaffrath Rosario A, Schienkiewitz A. Abdominal obesity in German adolescents defined by waist-to-height ratio and its association to elevated blood pressure: the KiGGS study. Obes Facts. 2013;6:165–175. doi: 10.1159/000351066. [DOI] [PMC free article] [PubMed] [Google Scholar]