Abstract
Ecdysteroids are important hormones that regulate moulting in arthropods. Three-host ixodid ticks normally moult to the next stage after finishing their blood meal, in the off-host environment. Presumably, three-host ticks that feed on the blood of insectivorous vertebrate hosts can be exposed to high levels of exogenous ecdysteroids causing them to initiate apolysis (the first step of moulting) on the vertebrate host. The aim of the present study was to investigate whether ticks undergo apolysis on insectivorous song birds, and if this phenomenon is associated with the seasonal variation in the availability of moths and with the presence of naturally acquired ecdysteroids in avian blood. During a triannual survey, 3330 hard tick larvae and nymphs were collected from 1164 insectivorous song birds of 46 species. A noteworthy proportion of ticks, 20.5%, showed apolysis. The occurrence of apolytic ticks on birds was correlated with the known seasonality of lepidopteran caterpillars. In addition, 18 blood samples of tick-infested birds were analysed with liquid chromatography – tandem mass spectrometry. Eight samples contained ecdysteroids or their derivatives, frequently in high concentrations, and the presence of these was associated with tick apolysis. In conclusion, naturally acquired ecdysteroids may reach high levels in the blood of insectivorous passerine birds, and will affect ticks (feeding on such blood) by shortening their parasitism.
Birds exhibit the most diverse range of ecological functions among vertebrates, because they participate in seed dispersal, pollination, pest control (by consuming insect parasites), carcass and waste disposal1. At the same time, birds are also known to play a significant role in the epidemiology of infectious diseases, e.g. by short- and long-distance dissemination of ixodid ticks and tick-borne pathogens2,3. The latter include several important zoonotic disease agents, such as Borrelia burgdorferi sensu lato that causes Lyme disease4, Anaplasma phagocytophilum responsible for granulocytic anaplasmosis5, and tick-borne encephalitis virus6. Although frequently studied separately, these two facets of avian life are necessarily interrelated, i.e. the niche birds occupy in an ecosystem will influence the risk they may pose as a source of ticks and tick-borne pathogens towards humans and their domestic animals.
In this host-parasite relationship, ticks may affect birds in several ways. For instance, infestation with certain tick species may cause disease in avian hosts7,8. Ticks may also inoculate tick-borne pathogens into birds, with or without pathological consequences4,5,9,10. On the other hand, the species-specific characteristics of birds will influence their tick infestation. The feeding level of birds (ground vs. arboreal) will have a significant impact on their tick burden, depending on the questing height different tick species occupy on the vegetation10,11. In addition, bird species may differ in how they interact with their ixodid ticks at the site of the tick bite. While ticks are known to inject salivary components to promote their prolonged blood feeding, the host’s immune system also mounts a local immune response against the tick12.
In the temperate climate zone, birds usually harbour larvae and nymphs of three-host ixodid ticks3. After blood feeding, these immature stages fall from their host, and moult to the next stage in the off-host environment. The moulting process is under control of moulting hormones, the so-called ecdysteroids13. However, on-host initiated moulting, i.e. apolysis (detachment of the previous cuticle) of three-host ticks has recently been reported in ticks feeding on goats and bats14,15. A plausible explanation for this phenomenon was that goats may ingest phytoecdysteroids with the plants in their forage14, whereas bats feed on insects that may contain ecdysteroids.
To evaluate this phenomenon further in the present study, ticks collected from insectivorous song birds (Aves: Passeriformes) during a tri-annual survey were examined for signs of apolysis, and blood samples of tick-infested birds were analysed for the presence of ecdysteroid moulting hormones. In this context, passerine birds appear to be particularly suitable subjects to study, because caterpillars constitute a significant portion of their diet (especially during the nesting period)16, and caterpillars are known for their high ecdysteroid concentrations17.
Results
During the three-year period, a total of 3330 ixodid ticks were collected from 1164 passerine birds (representatives of 46 mainly or partly insectivorous species). Larval and nymphal ticks of Ixodes spp. predominated, followed by Haemaphysalis concinna, accounting for 70.3% (2341 out of 3330, CI: 68.7–71.9%) and 29.7% (989 out of 3330, CI: 28.2–31.3%) of all collected ticks, respectively.
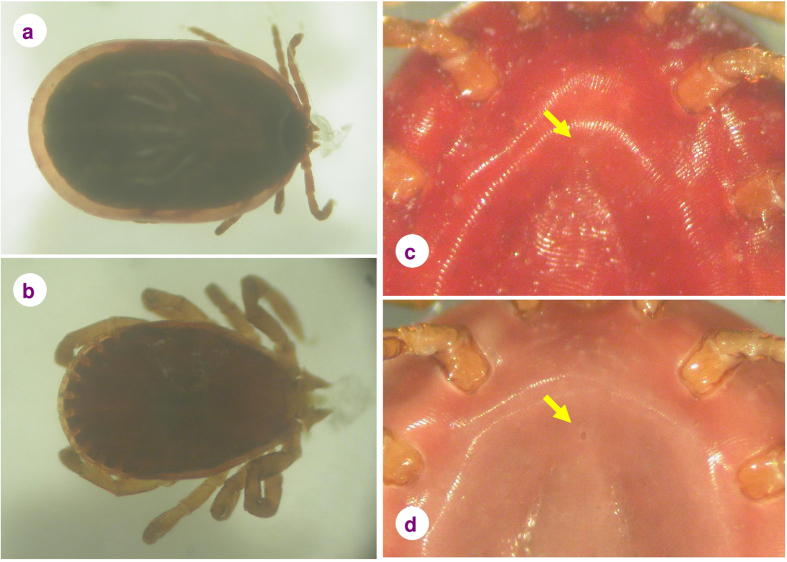
A noteworthy proportion, 20.5% (683 out of 3330, CI: 19.2–21.9%) of tick larvae and nymphs collected from birds showed apolysis, the initiative act of moulting (Fig. 1a). In the case of engorged apolytic nymphs, the place of the genital pore (which will open only in the adult stage) was frequently darker and more visible (Fig. 1c,d). The signs of apolysis were also observed in the case of unengorged ticks, i.e. at the beginning of their blood meal (Fig. 1b). The percentage of apolytic ticks on birds was the highest in July (35.5% = 250/705, CI: 31.9–39.1%). The monthly proportion of apolytic larvae and nymphs was highly associated with the reported regional monthly population density of lepidopterans (Spearman’s rank correlation: r = 0.93, P = 0.00001) (Fig. 2).
Figure 1.
Haemaphysalis concinna nymphs showing apolysis: (a) when close to full engorgement, (b) at the beginning of engorgement. Compared to nymphs that did not show the signs of apolysis (c), the place of the genital pore (arrow) was more apparent on apolytic nymphs (d).
Figure 2. The monthly regional population density of lepidopterans and the percentage of ticks showing apolysis.
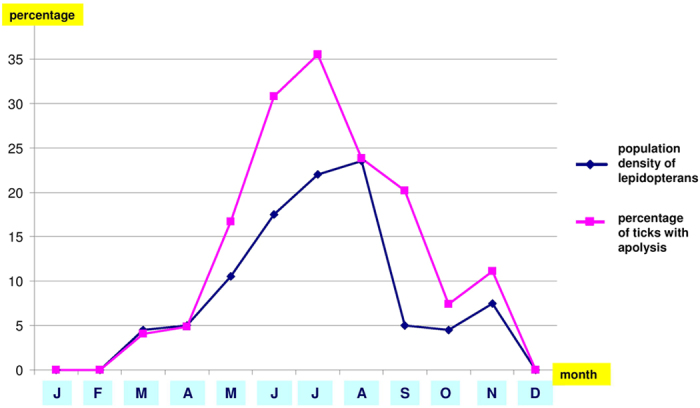
The latter indicates the number of apolytic ticks expressed as the percentage of all ticks removed from birds, calculated for each month.
Blood samples of 18 tick-infested birds were tested for the presence of ecdysteroids. In eight samples, up to seven ecdysteroids or their derivatives were present in detectable quantities (Fig. 3; Table 1). Structures of the ecdysteroids tested in the blood samples are summarized in Fig. 4. The proportion of ecdysteroid-positive samples was higher in the summer (61.5% = 8/13) than the spring (0.0% = 0/5) and this difference was statistically significant (χ2 = 5.539, df = 1, P = 0.019). The proportion of ecdysteroid-positive samples in birds with apolytic ticks (87.5% = 7/8) was almost nine times higher than in birds with no apolytic ticks (10.0% = 1/10) and this difference was statistically significant (χ2 = 10.811, df = 1, P = 0.001).
Figure 3. MRM chromatogram of sample No. 6.
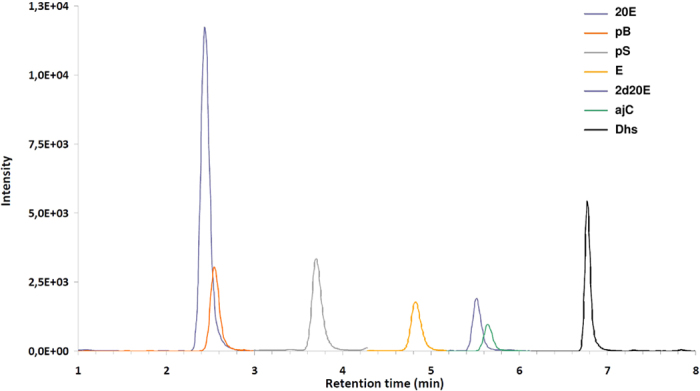
Abbreviations can be found in the legend of Table 2, sample data are shown in Table 1.
Table 1. Data of eighteen tick-infested birds: the presence/absence of ticks showing apolysis and ecdysteroid concentrations in corresponding blood samples.
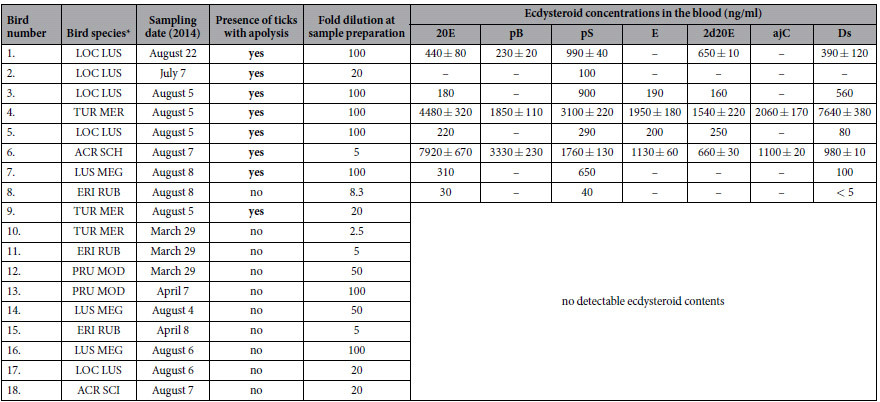
Abbreviations of compounds can be found in the legend of Table 2; “<” symbol denotes detectable ecdysteroid content below the limit of quantification. *Abbreviations: LOC LUS = Locustella luscinioides, TUR MER = Turdus merula, ACR SCH = Acrocephalus schoenobaenus, LUS MEG = Luscinia megarhynchos, ERI RUB = Erithacus rubecula, PRU MOD = Prunella modularis, ACR SCI = Acrocephalus scirpaceus.
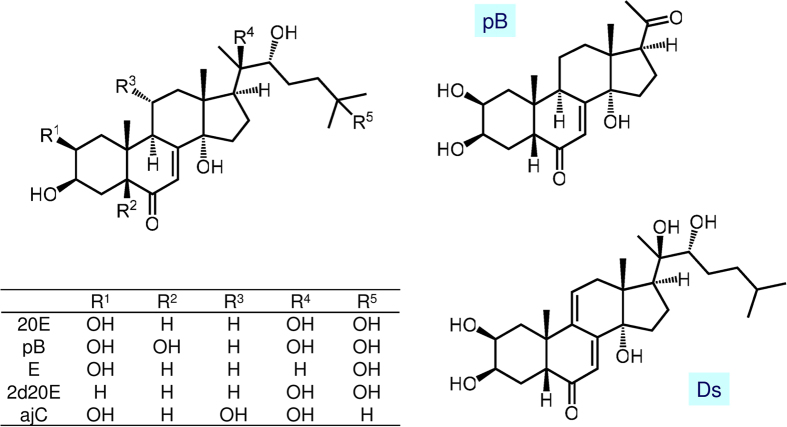
Figure 4. Structures of the ecdysteroids tested in the blood samples.
Discussion
Moulting hormones (ecdysteroids) have a similar role (i.e. triggering and controlling moulting) in ticks as in other arthropod groups13. It was observed that when ecdysteroids are produced in tick tissues, their levels rose slowly during the seven days of blood feeding and during the first ten days after detachment from the host13. During this period, ecdysteroids reached a concentration of only about 50 ng/ml haemolymph. However, this was followed by a sharp rise of ecdysteroid level, which peaked at 500 ng/ml at ~15 days after drop off from the vertebrate host13. As the first step of moulting, apolysis of ticks is induced by (and occurs concomitantly with) such highly elevated ecdysteroid titers13. Thus in nature, moulting of three-host ticks occurs exclusively in the off-host environment. On the other hand, when ticks are provided with ecdysteroid-containing blood (i.e. an exogenous source of moulting hormones), it will accelerate their moulting and will induce apolysis in a dose-dependent way18. Investigation of the natural occurrence of the latter phenomenon, to the best of our knowledge, has never been reported.
In the present survey, the percentage of ticks showing apolysis on birds was the highest in July, following the regional peak activity of caterpillars (May-June)16. Caterpillars predominate in the food of forest-dwelling passerine birds19, and their proportion can exceed 90% in the diet of nestlings16. Caterpillars are also known to contain high titres of ecdysteroids, up to 780 ng/g17. Throughout the study, a highly significant seasonal correlation was demonstrated between the percentage of apolytic ticks on birds, and the monthly population density of lepidopterans. This association can be interpreted in light of one common “preceding factor”, i.e. the presence of ecdysteroids in both the caterpillars and the blood of the birds. In other words, both the emergence of adult lepidopterans and the apolysis of ticks feeding on insectivorous birds can be regarded as a consequence of moulting hormones in caterpillars. The level of ecdysteroids in caterpillars effecting metamorphosis is likely to have similar physiological effects on immature ticks, based on the universal signal (analogous functions) they represent among arthropods (in general) and in ticks20. However, we recognize that the results from the present study are all correlative in nature and further experimental work is therefore required to establish a causal relationship between insect-derived ecdysteroids in the avian diet and on-host apolysis of hard ticks.
A second, lower peak of bird tick apolysis was observed in the month of November following its steady decline from the summer peak. One possible explanation for the November peak is that several song bird species switch from fully insectivorous to partly frugivorous and/or granivorous diet during the autumn, when insects are less available21. Relevant fruits (e.g. various berries) typically ripen from August in Central-Eastern Europe, and some (e.g. Malus spp.) were reported as ecdysteroid-positive by means of radioimmunoassay22.
Prior to apparent apolysis, untimely ingestion of large amounts of exogenous ecdysteroids may have detrimental consequences for ticks, because these hormones are known to have an antifeedant effect and to induce (earlier) salivary gland degeneration18,23, thus shortening the duration of blood feeding. These effects are particularly relevant in the case of ticks exposed to high levels of ecdysteroids at the beginning of their blood meal, as suggested by unengorged ticks showing apolysis in the present study. As the duration of blood feeding after tick attachment increases the risk of transmission of tick-borne pathogens24,25, the present findings may have epidemiological implications and are relevant for designing future strategies to control tick infestations and the risk of tick-borne diseases.
A large-scale feeding assay on newly-hatched Japanese quails found that foods containing high levels of ecdysteroids, such as Leuzea seeds, exerted anabolic activity and had beneficial effects on the birds26. A subsequent study with pure 20-hydroxyecdysone (20E) could also connect this dose-dependent anabolic activity in the birds to the ecdysteroid content of the diet27. In the group of birds with ad libitum access to the seeds during the 50-day experiment, the 20E levels reached a concentration of 80 ng/ml in the blood serum (as measured by a radioimmunoassay). In addition, the levels of 20E in the blood serum of the birds were proportional to the amount of seeds consumed26. This result indicates that the metabolism and/or elimination of ecdysteroids cannot be as rapid in birds as previously described for rodents (8.15 min half-life of 20E in mice)28, and that the accumulation of these compounds in birds is in fact possible. Our results on insectivorous wild passerine birds showed a much greater accumulation of 20E as well as of ecdysone in several individuals, reaching very high levels of up to ca. 8 μg/ml and 2 μg/ml of these two compounds, respectively.
In addition to this result, the presence of five other ecdysteroids as well as their unexpectedly high levels within several blood samples raise a number of questions concerning their origin and the mechanism of accumulation. These compounds occur in plant species belonging to different families including the Asteraceae (e.g. Serratula, Leuzea), Lamiaceae (e.g. Ajuga), and Caryophyllaceae (e.g. Silene)29, but the concentration of these compounds is typically an order of magnitude lower than that of 20E. Among them, the presence of poststerone and 2-deoxy-20-hydroxyecdysone might have a straightforward possible explanation. Poststerone has previously been identified as a major in vivo metabolite of 20E in mice30. Moreover, the 20,22 side-chain cleavage of cholesterol, initiated by the build-up of the corresponding vicinal diol, is the first step in the biosynthesis of the mammalian steroid hormone, which is catalysed by the enzyme cytochrome P450 11A1 (CYP11A1)31. Considering that the machinery for steroidogenesis appears to be highly conserved throughout the entire animal kingdom32, such a transformation of the 20,22-diol containing steroid 20E is also likely to occur in birds. However, as 2-deoxy-20-hydroxyecdysone was identified as a metabolite in human urine after consuming 20E33, it cannot be excluded that this compound was also present in the blood of the birds as a metabolite of dietary 20E.
On the other hand, the significant amounts of polypodine B, ajugasterone C, and particularly dacryhainansterone in the blood samples is very surprising: their structure makes it unlikely that they are the metabolic products of 20E in the diet of the birds. We therefore assume that these phytoecdysteroids made their way from plant sources through caterpillars to the birds, and, eventually, into the ticks. Amazingly, these compounds were detected in amounts comparable to or even higher than that of 20E, which strongly suggests that their metabolism and/or elimination is much slower. Based on the few available studies on the metabolism of ecdysone in mice34,35 and of 20E in rodents30,36 and in humans33,37, reduction at the B-ring is among the major metabolic routes of ecdysteroids. Moieties like a 5α-OH forming intramolecular H-bond with the 6-oxo group (polypodine B) or a conjugated 7(9,11)-dien-6-one (dacryhainansterone) might interfere with this process, and the lack of OH-25 (ajugasterone C and dacryhainansterone) can possibly decrease phase II metabolism i.e. sulphate or glucuronide conjugation. It should also be noted, that all these compounds, and mainly dacryhainansterone, are more lipophilic than 20E, based on which other pharmacokinetic properties (absorption, plasma protein binding etc.) can also significantly contribute to a relatively higher accumulation rate. To the best of our knowledge, no related studies are available with ecdysteroids other than 20E and ecdysone. Nevertheless, it is clear that the biological importance of the minor phytoecdysteroids is much greater than previously thought.
In summary, this is the first report on the presence of naturally acquired arthropod moulting hormones (ecdysteroids) in the blood of insectivorous passerine birds. Based on these results, exogenous ecdysteroids affected bird ticks by inducing on-host apolysis, which does not normally take place in the case of three-host ticks. On-host apolysis would shorten the average duration of the tick blood meal suggesting that an insectivorous diet protects birds from the full negative cost of tick feeding.
Methods
Sample collection
During a three year period (from January, 2012 until December, 2014), ixodid ticks were collected from passerine birds at three ringing stations in Hungary (Ócsa: 47° 17′ 54.3″ N, 19° 13′ 52.1″ E; Fenékpuszta: 46° 42′ 31.7″ N, 17° 14′ 33.8″ E; Bódva-völgy: 48° 17′ 36.3″ N, 20° 44′ 18.8″ E). Birds were mist-netted using standard Ecotone mist-nets (Gdynia, Poland), 12 m in length, 2.5 m in height and with a mesh diameter of 16 mm. The whole body of each captured bird was scrutinized for the presence of ticks. All ticks were removed with fine forceps, and put into 70% ethanol in separate vials according to their hosts. Tick species were determined according to standard keys38, and were consequently stored at room temperature.
In 2014, blood samples were taken from the brachial vein of some of the tick-infested birds using a fine (28G) needle and a 0.5 ml syringe (Kendall Monoject: Tyco Healthcare Group Lp., Mansfield, MA, USA). Blood samples were collected into EDTA-containing microtubes and stored frozen at −20 °C. Eighteen blood samples (from eight birds with ticks showing apolysis, and from ten birds with ticks not showing apolysis) were randomly selected for the analysis of ecdysteroids with liquid chromatography-coupled tandem mass spectrometry (LC-MS/MS) as described below.
Sample preparation
A volume of 100 μl or 250 μl of physiological saline solution was added to the frozen blood samples. After careful homogenization, each sample was transferred to Eppendorf tubes with a Hamilton syringe. The difference between the total volume, read from the syringe, and that of the volume added was considered as the original volume of blood. Following this step, the same volume of methanol was added, the solution was homogenized by shaking and left at room temperature for at least half an hour. The precipitate was subsequently centrifuged at 10,000 rpm for 10 min at 8 °C, and the clear supernatant was utilized for LC-MS/MS studies.
Calibration
Standard ecdysteroids 20-hydroxyecdysone (20E), poststerone (pS), ecdysone (E), 2-deoxy-20-hydroxyecdysone (2d20E), ajugasterone C (ajC) and dacryhainansterone (Ds) were obtained from previous phytochemical studies39,40, and possessed a purity of >95%. Standard stock solutions of each ecdysteroid were prepared in methanol at 1.0 mg/ml and stored at 4 °C before use. Equal volumes of the stock solutions were mixed and the obtained mixture (142.8 μg/ml for each analyte) was diluted first 100-fold and then 4-fold in serial with methanol to obtain 8 concentration levels for calibration (1428.60; 357.14; 89.29; 22.32; 5.58; 1.40; 0.35 and 0.09 ng/ml, respectively). Each calibration curve was constructed from at least six appropriate concentrations in triplicate. The limit of detection (LOD) and the limit of quantification (LOQ) were determined at the signal-to-noise ratio of about 3 and 10, respectively (Table 2).
Table 2. Calibration data for each standard ecdysteroid.
| Compound | Regression equation | R2 | Linear range (ng/mL) | LODa (ng/mL) | LOQb (ng/mL) |
|---|---|---|---|---|---|
| 20E | y = 59.27x + 44.0 | 0.9988 | 1.40–1428.6 | 0.24 | 0.79 |
| pB | y = 36.61x + 19.1 | 0.9998 | 1.40–1428.6 | 0.20 | 0.66 |
| pS | y = 78.44x + 28.3 | 0.9998 | 1.40–1428.6 | 0.34 | 1.12 |
| E | y = 67.86x + 22.0 | 0.9995 | 5.58–1428.6 | 0.77 | 2.56 |
| 2d20E | y = 98.14x + 66.2 | 0.9996 | 1.40–1428.6 | 0.27 | 0.89 |
| ajC | y = 32.42x–47.7 | 0.9999 | 1.40–1428.6 | 0.38 | 1.27 |
| Ds | y = 134.57x + 26.8 | 0.9995 | 0.35–1428.6 | 0.14 | 0.47 |
Abbreviations: 20-hydroxyecdysone (20E), polypodine B (pB), poststerone (pS), ecdysone (E), 2-deoxy-20-hydroxyecdysone (2d20E), ajugasterone C (ajC), dacryhainansterone (Ds). aLOD: limit of detection.
bLOQ: limit of quantification.
LC-MS/MS analysis
Experiments were carried out on an Agilent 1200 liquid chromatography system equipped with a vacuum degasser, a binary pump, an autosampler, a column temperature controller and a diode array detector. Chromatographic analysis was performed at 40 °C on a Kinetex XB-C18 column (100 × 2.1 mm, 2.6 μm) (Phenomenex, Torrance, CA, USA), with a mobile phase flow rate of 0.5 ml/min. The optimum separation was obtained under gradient elution with two isocratic time segments using 0.1% (v/v) formic acid in water as solvent A and 0.1% (v/v) formic acid in pure acetonitrile as solvent B. The linear gradient profile was: 0–0.5 min, 12% B; 0.5–2.0 min, 12–20% B; 2–3 min, 20% B; 3–9 min, 20–90% B. Post time was 6.0 min. The injection volume was set to 25 μL and the needle was rinsed and washed 3 times with methanol between injections in order to minimize carryover.
Mass spectrometry detection was performed using a 6410A triple quadrupole MS (Agilent Technologies, Palo Alto, CA, USA) equipped with an electrospray ionization (ESI) source used in positive ionization mode. The source settings were as follows: drying gas temperature, 350 °C; gas flow rate, 11 L/min; nebulizer, 40 psig; capillary voltage, 4000 V. Analyte detection was performed by multiple reaction monitoring (MRM) using an electron multiplier voltage (EMV) of 700 volts. Fragmentor voltage and collision energy (CE) were optimized individually for each target compound and are listed in Table 3. MassHunter B.04.01 was used for data acquisition and for qualitative analysis.
Table 3. Optimized LC-MS/MS conditions for each standard ecdysteroid.
| Compound | Retention time (min) | Quantitative MRM transition | CE (eV) | Qualitative MRM transition | CE (eV) | Fragmentor voltage (V) |
|---|---|---|---|---|---|---|
| 20E | 2.43 | 481 > 445 | 16 | 481 > 165 | 24 | 135 |
| pB | 2.53 | 497 > 443 | 20 | 497 > 369 | 24 | 135 |
| pS | 3.7 | 363 > 345 | 12 | 363 > 215 | 22 | 100 |
| E | 4.82 | 447 > 429 | 20 | 447 > 109 | 28 | 135 |
| 2d20E | 5.52 | 465 > 429 | 16 | 465 > 355 | 20 | 135 |
| ajC | 5.65 | 481 > 427 | 16 | 481 > 299 | 22 | 135 |
| Ds | 6.77 | 463 > 299 | 20 | 463 > 209 | 26 | 135 |
Abbreviations of compounds can be found in the legend of Table 2.
Ethical approval
The study was carried out according to the national animal welfare regulations of Hungary (28/1998). Bird ringing was approved by the National Inspectorate for Environment and Nature (under licence number 14/3858-9/2012).
Statistical analyses
Exact confidence intervals (CI) for the percentage abundances were calculated at the level of 95%. The monthly regional population density of lepidopterans was obtained from the mean monthly number of moths (Insecta: Lepidoptera), and was expressed as a percentage of the total yearly number. These data are based on the records of the Hungarian Plant Protection and Forestry Light Trap Network that were collected between 1974 and 2006, as reported41. Spearman rank correlation was used to test the association between the monthly proportion of apolytic ticks and the population density of lepidopterans. The association of blood ecdysteroids with season and tick apolysis (Table 1) was compared by using Chi-square test, and the differences were considered significant if P < 0.05.
Additional Information
How to cite this article: Hornok, S. et al. An unexpected advantage of insectivorism: insect moulting hormones ingested by song birds affect their ticks. Sci. Rep. 6, 23390; doi: 10.1038/srep23390 (2016).
Acknowledgments
Part of the study was funded by OTKA 115854. The authors would like to acknowledge networking contribution by the COST Action CM1407 ‘Challenging organic syntheses inspired by nature - from natural products chemistry to drug discovery’. Efforts of Dr. Zoltán Kele and Dr. Nikoletta Jedlinszki (University of Szeged, Szeged, Hungary) for testing samples on LC-MS/MS systems of relatively lower sensitivity, as well as technical assistance from Ibolya Hevérné Herke are highly appreciated. The authors thank Ármin Csipak and Bianka Jaksa for their indispensable participation in tick collection.
Footnotes
Author Contributions S.H. initiated the study, participated in tick identification, supervised parasitological work, wrote part of the manuscript. D.K. collected blood samples, participated in tick collection and provided ornithological information. B.F. participated in tick collection and identification. T.Cs. participated in tick collection, supervised ornithological work, and contributed with logistic support to the study. Á.K. performed quantitative LC-MS/MS analyses. Gy.T.B. provided analytical instrumentation and partly supervised the analytical work. A.Cs. developed LC-MS/MS method. A.H. designed and supervised the analytical studies, wrote part of the manuscript.
References
- Sekercioglu C. H. Increasing awareness of avian ecological function. Trends. Ecol. Evol. 21, 464–471 (2006). [DOI] [PubMed] [Google Scholar]
- Hornok S., Karcza Zs. & Csörgő T. Birds as disseminators of ixodid ticks and tick-borne pathogens: note on the relevance to migratory routes. Ornis Hung. 20, 86–89 (2012). [Google Scholar]
- Hasle G. Transport of ixodid ticks and tick-borne pathogens by migratory birds. Front. Cell. Infect. Microbiol. 3, 48 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gylfe A., Bergström S. & Lundström J. & Olsen, B. Reactivation of Borrelia infection in birds. Nature 403, 724–725 (2000). [DOI] [PubMed] [Google Scholar]
- Keesing F. et al. Reservoir competence of vertebrate hosts for Anaplasma phagocytophilum. Emerg. Infect. Dis. 18, 2013–2016 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldenström J. et al. Migrating birds and tickborne encephalitis virus. Emerg. Infect. Dis. 13, 1215–1218 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monks D., Fisher M. & Forbes N.A. Ixodes frontalis and avian tick-related syndrome in the United Kingdom. J. Small Anim. Pract. 47, 451–455 (2006). [DOI] [PubMed] [Google Scholar]
- Norte A. C. et al. Do ticks and Borrelia burgdorferi s.l. constitute a burden to birds ? Parasitol. Res. 112, 1903–1912. (2013) [DOI] [PubMed] [Google Scholar]
- Chambert T. et al. Exposure of black-legged kittiwakes to Lyme disease spirochetes: dynamics of the immune status of adult hosts and effects on their survival. J. Anim. Ecol. 81, 986–95 (2012). [DOI] [PubMed] [Google Scholar]
- Hornok S. et al. Birds as potential reservoirs of tick-borne pathogens: first evidence of bacteraemia with Rickettsia helvetica. Parasit. Vectors 7, 128 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Labruna M. B. et al. Ticks collected on birds in the State of São Paulo, Brazil. Exp. Appl. Acarol. 43, 147–160 (2007). [DOI] [PubMed] [Google Scholar]
- Singh S. K. & Girschick, H.J. Tick– host interactions and their immunological implications in tick-borne diseases. Current Science 85, 1284–1298 (2003). [Google Scholar]
- Diehl P. A. & Germond J. E. & Morici, M. Correlations between ecdysteroid titers and integument structure in nymphs of the tick, Amblyomma hebraeum Koch (Acarina: Ixodidae). Revue Suisse De Zoologie 89, 859–868 (1982). [Google Scholar]
- Hornok S., Horváth G. & Jongejan F. & Farkas, R. Ixodid ticks on ruminants, with on-host initiated moulting (apolysis) of Ixodes, Haemaphysalis and Dermacentor larvae. Vet. Parasitol. 187, 350–353 (2012). [DOI] [PubMed] [Google Scholar]
- Hornok S. et al. Bat ticks revisited: Ixodes ariadnae sp. nov. and allopatric genotypes of I. vespertilionis in caves of Hungary. Parasit. Vectors 7, 202 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bereczki K., Ódor P., Csóka Gy., Mag Zs. & Báldi A. Effects of forest heterogeneity on the efficiency of caterpillar control service provided by birds in temperate oak forests. Forest Ecol. Management 327, 96–105 (2014). [Google Scholar]
- Sehnal F., Maroy P. & Mala J. Regulation and significance of ecdysteroid titre fluctuations in lepidopterous larvae and pupae. J. Insect. Physiol. 27, 535–544 (1981). [Google Scholar]
- Rees H. H. Hormonal control of tick development and reproduction. Parasitology 129 (Suppl.), S127–S143 (2004). [DOI] [PubMed] [Google Scholar]
- Cholewa M. & Wesołowski T. Nestling food of European hole-nesting passerines: do we know enough to test the adaptive hypotheses on breeding seasons? Acta Ornithol. 46, 105–116 (2011). [Google Scholar]
- Palmer M. J., Harmon M. A. & Laudet V. Characterization of EcR and RXR homologues in the ixodid tick, Amblyomma americanum (L.). Am. Zool. 39, 747–757 (1999). [Google Scholar]
- Jordano P. Diet variation among wintering frugivorous Robins, Erithacus rubecula: effects on body condition. Ardeola 36, 161–183 (1989). [Google Scholar]
- Dinan L., Savchenko T. & Whiting P. On the distribution of phytoecdysteroids in plants. Cell. Mol. Life Sci. 58, 1121–1132 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman W. R. Further investigations on the action of ecdysteroids on the salivary glands of the female tick Amblyomma americanum. Exp. Appl. Acarol. 10, 259–265 (1991). [DOI] [PubMed] [Google Scholar]
- Wilhelmsson P. et al. Prevalence, diversity, and load of Borrelia species in ticks that have fed on humans in regions of Sweden and Åland Islands, Finland with different Lyme borreliosis incidences. PLoS One 8, e81433 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook M. J. Lyme borreliosis: a review of data on transmission time after tick attachment. Int. J. Gen. Med. 8, 1–8 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koudela K., Tenora J., Bajer J., Mathova A. & Slama K. Stimulation of Stimulation of growth and development in Japanese quails after oral administration of ecdysteroid-containing diet. Eur. J. Enthomol. 92, 349–354 (1995). [Google Scholar]
- Slama K., Koudela K., Tenora J. & Mathova A. Insect hormones in vertebrates: Anabolic effects of 20-hydroxyecdysone in Japanese quail. Experientia 52, 702–706 (1996). [DOI] [PubMed] [Google Scholar]
- Dzukharova M. K. et al. Pharmacokinetic experiments with ecdysterone. Khimiko-Farmatsevticheskii Zhurnal. 21, 1163–1167 (1987). [Google Scholar]
- Lafont R., Harmatha J., Marion-Poll F., Dinan L. & Wilson I. D. Ecdybase−The Ecdysone Handbook, available on-line at http://ecdybase.org.
- Kumpun S. et al. The metabolism of 20-hydroxyecdysone in mice: relevance to pharmacological effects and gene switch applications of ecdysteroids. J. Steroid Biochem. Mol. Biol. 126, 1–9 (2011). [DOI] [PubMed] [Google Scholar]
- Mast N. et al. Structural basis for three-step sequential catalysis by the cholesterol side chain cleavage enzyme CYP11A1. J. Biol. Chem. 286, 5607–5613 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan J. & Papadopoulos V. Evolutionary origin of the mitochondrial cholesterol transport machinery reveals a universal mechanism of steroid hormone biosynthesis in animals. PLoS One 8, e76701 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsitsimpikou C., Tsami G. D., Siskos P. A. & Spyridaki M. H. & Georgakopoulos, C.G. Study of excretion of ecdysterone in human urine. Rapid Commun. Mass Spectrom. 15, 1796–1801 (2001). [DOI] [PubMed] [Google Scholar]
- Girault J. P., Lafont R. & Kerb U. Ecdysone catabolism in the white mouse. Drug Metab. Dispos. 16, 716–720 (1988). [PubMed] [Google Scholar]
- Lafont R., Girault J. P. & Kerb U. Excretion and metabolism of injected ecdysone in the white mouse. Biochem. Pharmacol. 37, 1174–1177 (1988). [DOI] [PubMed] [Google Scholar]
- Ramazanov N., Saatov Z. & Syrov V. N. Study of ecdysterone metabolites isolated from rat urine. Khim. Prir. Soedin. 4, 558–564 (1996). [Google Scholar]
- Brandt F. Pharmakokinetik und Metabolismus des 20-Hydroxyecdysons im Menschen. PhD Thesis, University of Marburg (Germany) (2003). [Google Scholar]
- Babos S. Die Zeckenfauna Mitteleuropas. Akadémiai Kiadó, Budapest, pp. 410 (1964). [Google Scholar]
- Hunyadi A. et al. Preparative-scale chromatography of ecdysteroids of Serratula wolffii Andrae. J. Chromatogr Sci. 45, 76–86 (2007). [DOI] [PubMed] [Google Scholar]
- Tóth N. et al. 26-Hydroxylated Ecdysteroids from Silene viridiflora. J. Nat. Prod. 71, 1461–1463 (2008). [DOI] [PubMed] [Google Scholar]
- Gimesi L., Homoródi R., Hirka A., Szabóki C. & Hufnagel L. The effect of climatechange on the phenology of moth abundance and diversity. Appl. Ecol. Environ. Res. 10, 349–363 (2012). [Google Scholar]