Abstract
Background. In this study, we analyzed the protective efficacy of a simian immunodeficiency virus (SIV) macaque 239 (SIVmac239) analogue of the clinically tested GOVX-B11 deoxyribonucleic acid (DNA)/modified vaccinia Ankara (MVA) human immunodeficiency virus vaccine.
Methods. The tested vaccine used a DNA immunogen mutated to mimic the human vaccine and a regimen with DNA deliveries at weeks 0 and 8 and MVA deliveries at weeks 16 and 32. Twelve weekly rectal challenges with 0.3 animal infectious doses of SIV sootey mangabey E660 (SIVsmE660) were administered starting at 6 months after the last immunization.
Results. Over the first 6 rectal exposures to SIVsmE660, <10-year-old tripartite motif-containing protein 5 (TRIM5)α-permissive rhesus macaques showed an 80% reduction in per-exposure risk of infection as opposed to a 46% reduction in animals over 10 years old; and, over the 12 challenges, they showed a 72% as opposed to a 10% reduction. Analyses of elicited immune responses suggested that higher antibody responses in the younger animals had played a role in protection.
Conclusions. The simian analogue of the GOVX-B11 HIV provided strong protection against repeated rectal challenges in young adult macaques.
Keywords: age-dependent protection, antibody, DNA/MVA vaccine, HIV vaccine, SIV
We have been advancing a prophylactic vaccine for clade B human immunodeficiency virus (HIV) termed GOVX-B11 that produces virus-like particles (VLPs) in the person being vaccinated. The vaccine uses recombinant deoxyribonucleic acid (DNA) to prime the immune response and recombinant modified vaccinia Ankara (MVA) to boost the response. Both components of the vaccine produce VLPs displaying trimeric membrane-bound envelope protein (Env). The DNA prime expresses the native glycoprotein (gp)160 Env [1], whereas the recombinant MVA boost expresses a 146-amino acid C-terminal truncated gp150 form of Env [2]. GOVX-B11 has had outstanding safety and reproducible immunogenicity in Phase 1 (HIV Vaccine Trials Network [HVTN] 065 n = 30) and 2a (HVTN 205 n = 150) clinical trials conducted by the HVTN [3, 4].
The current study was undertaken to provide additional preclinical efficacy data in support of the development of GOVX-B11. The primary goal of the study was to test the simian analogue of GOVX-B11 for protective potential in a rhesus macaque model using repeated rectal exposures to a heterologous virus. A secondary goal was to further investigate the ability of coexpressed granulocyte-macrophage colony-stimulating factor (GM-CSF) to enhance protection against repeated rectal challenges. In a prior study, coexpression of GM-CSF in the DNA prime of a vaccine expressing simian immunodeficiency virus (SIV) macaque 239 (SIVmac239) sequences had substantially enhanced protective efficacy against serial rectal challenges with SIVsmE660 [5]. The improved protection correlated with the avidity of the elicited antibody (Ab) for the Env of the challenge virus, which was enhanced in the GM-CSF-adjuvanted group [5].
In the current study, a larger number of rhesus macaques were used to obtain a more rigorous evaluation of the effects of coexpressed GM-CSF on elicited Ab and protection compared with the previous trial. In addition, the majority of animals expressed rhesus tripartite motif-containing protein 5α (TRIM5α) genotypes that are permissive for SIVsmE660 infection. This was important because rhesus TRIM5α has been shown to influence acquisition of SIVsmE660 infection [6–8]. Other conditions were also modified to make the DNA immunogen more like the DNA in GOVX-B11 and the regimen closer to the updated regimen being advanced with GOVX-B11. In particular, the DNA prime was modified by an inactivating point mutation in protease to enhance VLP production by preventing premature cleavage of overexpressed Gag [9]. The regimen was modified to favor avidity maturation of the Ab response by allowing 16 instead of 8 weeks between the 2 MVA boosts [10]. The regimen for the clinical advancement of GOVX-B11 is 2 DNA primes at 0 and 8 weeks followed by 3 MVA boosts at 16, 24, and 40 weeks. The trial also differed from prior trials by including 3- to 16-year-old male and female macaques as opposed to only 3- to 5-year-old males. Rhesus macaques reach puberty at 3 to 4 years of age with 1 year of rhesus life being approximately equivalent to 4 years of human life (http://genomics.senescence.info/species/entry.php?species=Macaca_mulatta). This means that trials that had been previously conducted in adolescents were now being conducted in young, middle-aged, and even elderly macaques.
The results of this study revealed the induction of higher avidity Abs than in the prior trial and similar avidity and protection in the GM-CSF-adjuvanted and nonadjuvanted groups. In TRIM5α-permissive animals <10 years old, an 80% reduction in per-exposure risk of infection occurred over the first 6 exposures, and a 72% reduction occurred over all 12 exposures. On the basis of these findings, the nonadjuvanted GOVX-B11 vaccine will undergo further development using a 16-week rest between the final 2 MVA boosts, and efficacy testing will limit participants to youths and young adults.
METHODS
Vaccines
The SIVmac239 DNA vaccines used for priming the immune response were modified from the Rama36 (non-GM-CSF coexpressing) and Rama42 (GM-CSF coexpressing vaccines) used in the prior study [5] by mutating the active site of protease from an aspartic acid to alanine (D26A) [9]. The resulting non-GM-CSF coexpressing DNA (Rama33) and GM-CSF coexpressing DNA (Rama47) expressed SIVmac239 Gag, PR, RT, Env, Tat, and Rev from a single ribonucleic acid (RNA) by subgenomic splicing and frame shifting. GM-CSF was expressed by the same mRNA as Env using the encephalomyocarditis virus internal ribosome entry site [5]. Levels of GM-CSF expression were measured on 48-hour supernatants of transiently transfected HEK293T cells using an enzyme-linked immunosorbent assay (ELISA) for human GM-CSF (Mabtech, Inc., Cincinnati, OH). The protease-inactivated Rama47 expressed slightly higher levels of GM-CSF than the protease-active Rama42 used in the previous study (617 ± 295 units compared with 407 ± 107 units). The same recombinant MVA as used before (SIVmac239-MVA formally designated DR1 or MVASIVgpe) expressed Gag, Pol, and Env but did not coexpress GM-CSF [11]. The DNA vaccines expressed the complete gp160 form of SIVmac239 Env, and the MVA vaccine encoded a gp150 form, which was truncated to remove 146 amino acids at the C-terminus of the transmembrane subunit to enhance expression and stabilize the insert [2].
Animals and Challenge Stock
Sixty Indian origin rhesus macaques (Macaca mulatta) weighing from 2.7 to 15.5 pounds were housed at Bioqual Inc. and cared for under guidelines established by the Animal Welfare Act and the National Institutes of Health Guide for the Care and Use of Laboratory Animals using protocols approved by the Institutional Animal Care and Use Committee. The challenge stock, SIVsmE660-ABL, was grown from the stock used in the prior trial (designated SIVsmE660-Hirsch 2000) [5] using peripheral blood mononuclear cells from the same pigtailed macaque used to produce the original challenge stock. All immunological tests and tests for infection were performed blinded. Sequence analysis and enumeration of transmitted-founder genomes was determined as previously described [12]
Antibody Responses
Simian immunodeficiency virus-specific Ab responses were assessed in serum by Env-specific ELISAs using commercially purchased SIVmac239 gp140 antigen (Immune Technology Corp, New York, NY) as previously described [13, 14]. The concentrations of immunoglobulin (Ig)G were estimated relative to a standard curve. Rectal secretions were collected using premoistened Weck-Cel Sponges as previously described [15]. For mucosal Abs, Env-specific IgG or IgA was measured and represented as nanograms of specific Ab per micrograms of total Ab [14]. TZM-bl luciferase-based neutralization assays were performed with tier 1 and tier 2 viruses as described previously [16]. Antibody-secreting cells were measured by enzyme-linked immunospot assays as previously described [17] using SIVmac239 gp140 as a coating antigen. Avidity of binding Ab was determined for SIVmac239 gp160 captured from VLP produced by transient transfection of HEK293T cells with Rama33 as described previously [14]. A reference standard of pooled sera was used in all assays. This standard had a mean avidity index of 52 and a standard deviation of 1.2. Antibody-dependent cellular phagocytosis (ADCP) assays were performed using THP-1 monocytic cells and SIVsmE660 gp140-coated fluorescent beads, and the phagocytic score was calculated as described previously [18]. Antibody-dependent cellular cytotoxicity (ADCC) assays were performed as described earlier [19] using SIVmac239-infected CEM.NKR-CCR5 CD4+ T cells as target cells.
T Cell Responses
Simian immunodeficiency virus-specific cellular immune responses were assessed by multiparameter intracellular cytokine staining (ICS) assays after stimulation with SIVmac239 peptides as previously described [20]. All values used in the analyses were background subtracted and 2 times higher than background levels.
Statistical Analysis
Kaplan-Meier curves and the log-rank Mantel Cox test were used to display and test for differences in infection curves. Per-exposure reductions in risk of infection were analyzed as described by Hudgens and Gilbert [21, 22]. The Wilcoxon Mann-Whitney U test was used to compare Ab and T-cell responses and viral RNA levels between groups. The Spearman rank correlation method was used for correlations. P values were not corrected for multiple comparisons, and a 2-sided P value of .05 was considered significant. Statistical analyses were performed using GraphPad Prism version 6.0 for Mac (GraphPad Software, San Diego, CA) and TIBCO Spotfire S 8.1 (TIBCO, Somerville, MA).
RESULTS
Study Design
Thirty male and 30 female animals were randomized by sex and weight into 2 vaccine and 1 control groups of 20 each. Rhesus in the vaccine groups were immunized intramuscularly in the quadriceps at 0 and 8 weeks with 3 mg of SIVmac239-DNA in phosphate-buffered saline (PBS) either coexpressing (Dg) or not coexpressing GM-CSF (D) followed by boosting with 1 × 108 plaque-forming units (pfu) of SIVmac239-MVA (M) in PBS at 16 and 32 weeks (DDMM or DgDgMM regimens) (Figure 1A). The control group received 1 × 108 pfu of parental MVA at 16 and 32 weeks. At 6 months after the final immunization, animals were entered into 12 weekly rectal exposures to 412 tissue culture infection dose (TCID50) of SIVsmE660-ABL. At each challenge, animals were tested for infection and considered infected when an animal scored for >1000 copies of viral RNA/mL plasma on 2 consecutive bleeds or >5000 copies of viral RNA/mL on a single bleed. Once an animal was infected, further challenges were stopped. Animals were typed for TRIM5α alleles [23, 24]. Animals that were TFP/TFP or TFP/CypA were considered TRIM5α restrictive, whereas animals that were TFP/Q, CypA/CypA, Q/CypA, or Q/Q were considered permissive.
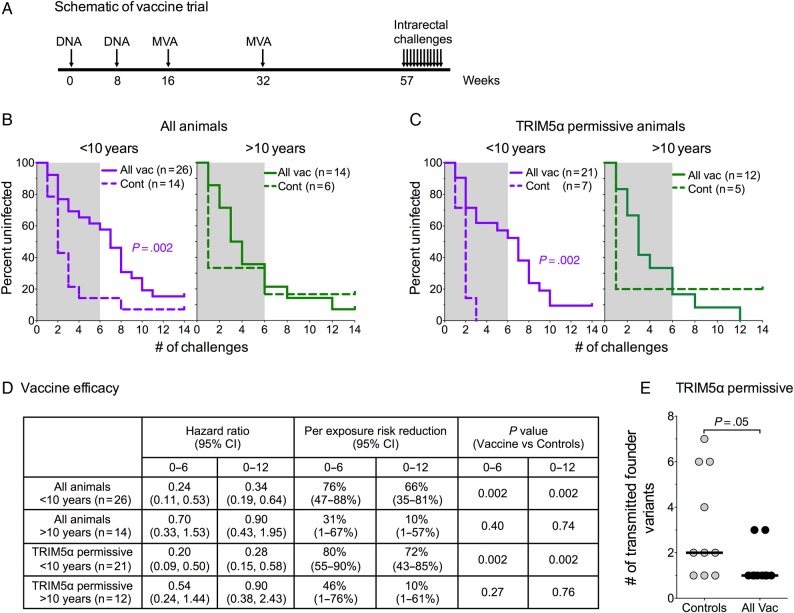
Figure 1.
Schematic of trial: Kaplan–Meier curves for vaccine-elicited prevention of infection and vaccine efficacy. (A) Schematic showing the vaccination and challenge schedule for the trial. (B) Kaplan-Meier curves for prevention of infection in all vaccinated and control animals separated by age. Granulocyte-macrophage colony-stimulating factor (GM-CSF) adjuvanted and nonadjuvanted vaccine groups were pooled for analysis. (C) Kaplan-Meier curves for prevention of infection in tripartite motif-containing protein 5 (TRIM5)α-permissive animals separated by age. (D) Vaccine efficacy. (E) Number of transmitted variants. For details, see Methods. Abbreviations: CI, confidence interval; Cont, controls; All Vac, combined groups; shaded area, first 6 challenges.
Strong Vaccine Mediated Protection in Younger but Not Older Tripartite Motif-Containing Protein 5α-Permissive Animals
Animals vaccinated with both the adjuvanted and nonadjuvanted vaccines were protected against serial rectal challenges with SIVsmE660 (Supplementary Figure 1). In contrast to our prior study, animals primed with the nonadjuvanted vaccine showed the same level of protection as those primed with the GM-CSF coexpressing vaccine (Supplementary Figure 1A). As expected, TRIM5α-restrictive animals showed better protection than TRIM5α-permissive animals (Supplementary Figure 1). Given that indistinguishable levels of protection had occurred in the adjuvanted and nonadjuvanted groups, the 2 groups were pooled for further evaluation.
Because age can significantly influence vaccine-induced immune responses [25–29], animals in the study were next analyzed for effects of age on protection (Figure 1). Two groups were established: the first more than 10 years old (n = 26) and the second <10 years old (n = 14).
The younger animals showed highly significant protection (P = .002), whereas the older animals did not (Figure 1B). In younger animals, per-challenge reductions in risk of infection were 76% over the first 6 challenges and 66% over all 12 challenges (Figure 1D). For younger TRIM5α-permissive animals, there was an 80% reduction in per-challenge risk of infection during the first 6 challenges and a 72% reduction in per-challenge risk over all 12 challenges (Figure 1C and 1D). In contrast, for TRIM5α-permissive animals more than 10 years old, the per-exposure risk reduction was 46% over the first 6 challenges and only 10% over all 12 challenges (Figure 1C and 1D). Analysis of 10 vaccinated and 10 unvaccinated TRIM5α-permissive animals for the number of transmitted-founder variants revealed a median of 1 (range, 1–3) in the vaccinated animals and a median of 2 (range, 1–7) in the unvaccinated animals (Figure 1E).
Immune Correlates for Protection
Elicited Ab responses for SIVmac239 gp140 had similar temporal magnitudes in the GM-CSF-adjuvanted and nonadjuvanted groups (Figure 2). Combining data for both vaccinated groups, the estimated median level of Env-specific IgG in sera at 2 weeks after the second and final MVA boost (week 34) was 241 µg/mL (Table 1). This level had contracted 6.5 fold to a median of 37 µg/mL at week 52, 5 weeks before the initiation of the serial rectal challenges (Table 1). Prechallenge levels of gp140-specific IgA in serum (0.6 µg/mL) were 60-times lower than prechallenge IgG. The specific activities of SIVmac239 gp140-specific rectal IgG were 11.2 ng/µg at peak response (Figure 2B). These specific activities had contracted by 7 fold to 1.6 ng/µg at week 52. The magnitudes of gp140-specific IgG- and IgA-secreting cells (Ab-secreting cells [ASCs]) were also similar in both groups at 5 days after the second MVA boost (Figure 2D) (Table 1). Specific activities for gp140-specific IgA in rectal secretions were measured, but are not presented because these values were at the background for detection.
Figure 2.
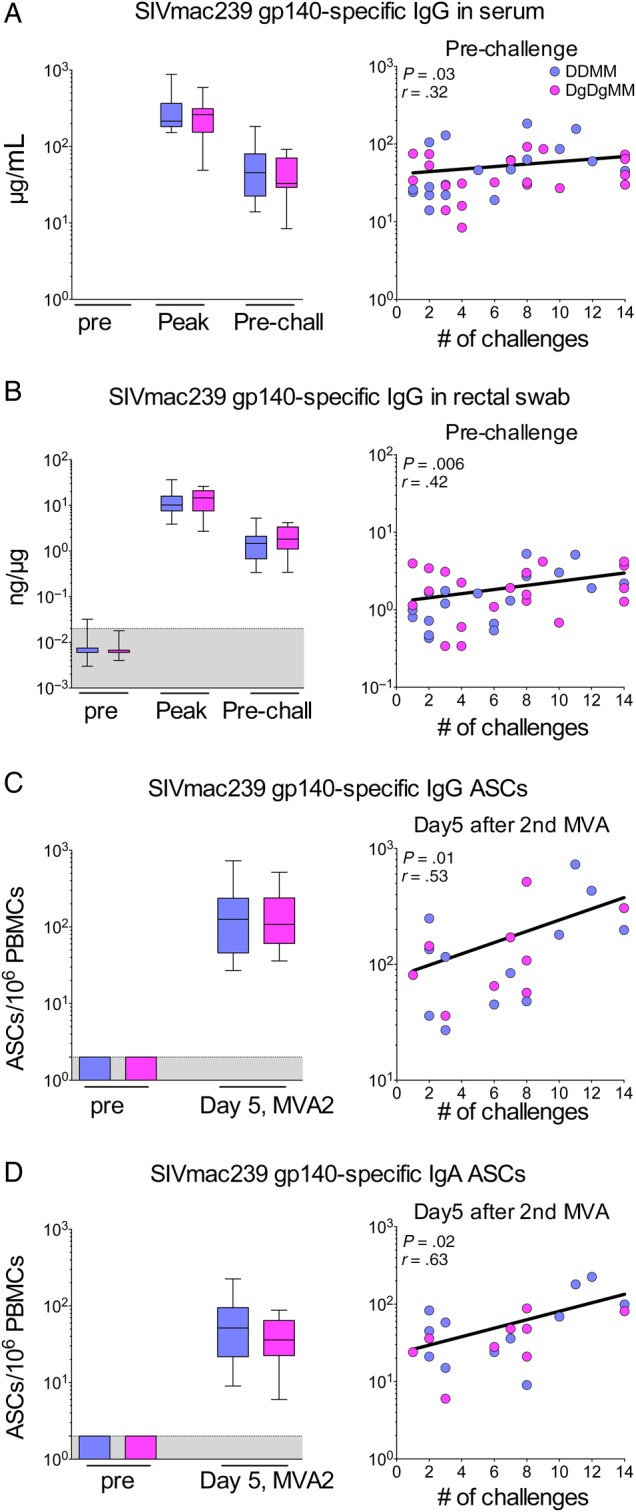
Simian immunodeficiency virus (SIV) macaque 239 (SIVmac239)-specific antibody (Ab) responses and their correlations with protection. (A) Levels of elicited glycoprotein (gp)140-specific immunoglobulin (Ig)G in serum. (B) Specific activity of elicited gp140-specific IgG in rectal secretions. (C) The gp140-specific IgG Ab-secreting cells (ASCs); (D) gp140-specific IgA ASCs. Antibody responses in serum and rectal secretions were measured at peak response (2 weeks after the final modified vaccinia Ankara [MVA] boost) and prechallenge (week 52). Antibody-secreting cells were measured at day 5 after the second (final) MVA boost. For each panel, the left figure shows the magnitudes of responses elicited by the GM-CSF coexpressing and non-coexpressing vaccines, and the right panel shows the correlation between the elicited Ab or ASC and the number of challenges to infection. Data are for all animals. For Ab to gp140 in serum, rectal secretions, and Ab-dependent cellular cytotoxicity, correlations are for prechallenge responses (week 52). For ASC, correlations are for 5 days after the second MVA boost. Abbreviations: DDMM, DNA at 0 and 2 months and MVA at 4 and 8 months; DgDgMM, same regimen for DNA coexpressing GMCSF. MVA2, second MVA inoculation; PBMC, peripheral blood mononuclear cells; peak, week 34; Pre, before first immunization; Pre-chall, week 52; shaded area, background.
Table 1.
Summary of Elicited Responses and Correlations With Number of Challenges to Infection
| Immunological Assay Performed | Peak |
Prechallenge |
||||
|---|---|---|---|---|---|---|
| Valuea | rb | P Valuec | Value | r | P Value | |
| SIVmac239 gp140-specific IgG in serum (μg/mL) | 241.5 (181, 327) | 0.32 | .04 | 37 (26, 37) | 0.32 | .03 |
| SIVmac239 gp140-specific IgG in rectal swabs (ng of specific IgG/μg IgG) | 11.2 (7.5, 18.23) | 0.17 | .30 | 1.6 (0.7, 2.9) | 0.42 | .006 |
| SIVmac239 gp140-specific IgG secreting ASCs (ASCs/10^6 PBMCs) | 116 (52.5, 223.5) | 0.53 | .01 | |||
| SIVmac239 gp140-specific IgA secreting ASCs (ASCs/106 PBMCs) | 45 (22.5, 82.0) | 0.63 | .02 | |||
| SIVmac251 gp140-specific IgA in serum (μg/mL) | 0.63 (0.38, 1.15) | 0.34 | .03 | |||
| SIVmac239 gp160-specific Avidity index | 49 (43.0, 53.7) | 0.01 | .9 | |||
| Neutralizing titers against challenge virus (SIVsmE660-ABL) | 3078 (1440, 4710) | 0.12 | .4 | 446 (168, 7.3) | 0.18 | .3 |
| ADCP score | 57 (51.7, 68.7) | 0.22 | .2 | |||
| ADCC activity (AUC values) | 2.06 (1.32, 2.57) | 0.24 | .13 | |||
| SIV-specific IFNγ+CD4+ T-cell response | 0.21 (0.11, 0.67) | 0.11 | .7 | 0.08 (0.05, 0.17) | 0.13 | .5 |
| SIV-specific IFNγ+CD8+ T-cell response | 0.35 (0.2, 1.2) | 0.01 | .9 | 0.04 (0.02, 0.15) | 0.05 | .8 |
Abbreviations: ADCC, antibody-dependent cellular cytotoxicity; ADCP, antibody-dependent cellular phagocytosis; ASCs, antibody-secreting cells; AUC, area under the curve; gp, glycoprotein; IFN, interferon; Ig, immunoglobulin; PBMCs, peripheral blood mononuclear cells; SIV, simian immunodeficiency virus; SIVmac239, SIV macaque 239.
a Median (25th, 75th percentiles).
b Spearman correlation coefficient.
c Two-tailed P value, unadjusted for Bonferroni correction.
Correlations conducted on the combined groups for elicited levels of binding Ab, or ASC, and the number of challenges to infection revealed modest but significant direct correlations between the levels of gp140-specific IgG and IgA in serum, gp140-specific IgG in rectal secretions and IgG and IgA ASCs, and the number of challenges to infection (Figure 2 and Table 1). The correlation coefficients were lowest for elicited IgG and IgA in prechallenge Sera (r = 0.32, P = .03 and r = 0.34, P = .03, respectively), slightly higher for gp140-specific IgG in rectal secretions prechallenge (r = 0.42, P = .006), and the highest for gp140-specific IgG and IgA ASCs at day 5 after the second MVA boost (r = 0.53, P = .01 and r = 0.63, P = .02, respectively).
Measurements of the avidity of the Ab response for the SIVmac239 gp160 revealed similar high avidities in the 2 groups (Figure 3A). The median avidity index for the pooled data was 49 with a range of 43 to 54. These values were higher than in the prior study in which the GM-CSF group had a median index of 39 with a range of 29 to 49 and the non-GM-CSF group had a median index of 29.5 with a range of 21 to 34 [5]. As in the prior study, the avidity for SIVmac239 gp160 did not correlate with protection.
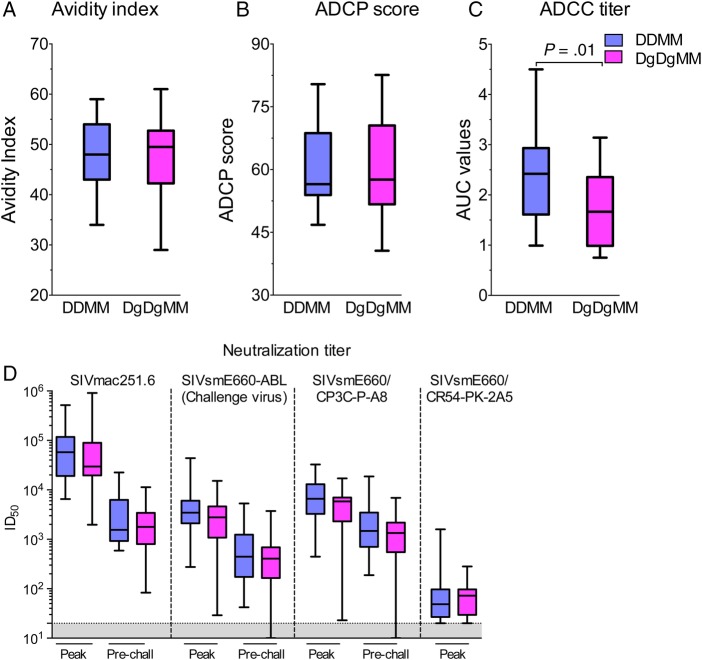
Figure 3.
Antibody (Ab) responses that did not show significant correlations with protection. (A) Avidity index measured against simian immunodeficiency virus (SIV) macaque 239 (SIVmac239) glycoprotein (gp)160. (B) Serum Ab-dependent cellular phagocytosis (ADCP) score measured against SIVsmE660gp140. (C) Serum Ab-dependent cellular cytotoxicity (ADCC) values measured at peak against SIVmac239 infected cells. (D) Serum neutralizations titers measured in TZM-bl cells against the indicated viruses at peak (2 weeks after the last modified vaccinia Ankara [MVA] boost) and prechallenge time points (52 weeks). Abbreviations: Cont, controls; DDMM, DNA at 0 and 8 weeks, MVA at 16 and 32 weeks; DgDgMM, same regimen for GM-CSF co-expressing DNA. ID50, inhibitory dose for 50% neutralization.
Measurements of functional activities for Ab included tests for neutralizing Ab, ADCP, and ADCC. With the exception of ADCC, which was lower in the GM-CSF-adjuvanted group, functional responses were similar in the plus and minus GM-CSF groups (Figure 3B–D). None of these activities, including neutralizing Ab to the challenge strain, showed a significant correlation with protection. Neutralizing Ab was measured using an easy to neutralize variant of SIVmac251 (SIVmac251.6), the challenge stock (SIVsmE660-ABL), and 2 variants of SIVsmE660, one of which is relatively easy to neutralize (SIVsmE660/CP3C-P-A8) and one of which is difficult to neutralize (SIVsmE660/CR54-PK-2A5) (Figure 3D). Median inhibitory doses (ID)50 for these different isolates ranged from a low of approximately 30 to a high of approximately 6 × 104.
Similar levels of SIV-specific cellular immune responses, assessed by ICS, were elicited in the adjuvanted and nonadjuvanted groups (Supplementary Figure 2) (Table 1). At 2 weeks after the last MVA dose, median levels of CD4+ T cells were 0.21% of total CD4+ T cells and median levels of CD8+ T cells were 0.35% of total CD8+ T cells. Prechallenge, these levels had contracted to 0.08% of total CD4+ T cells and 0.04% of total CD8+ T cells. The levels of elicited CD4+ and CD8+ T cells did not correlate with either the number of challenges to infection or the levels of postchallenge viremia.
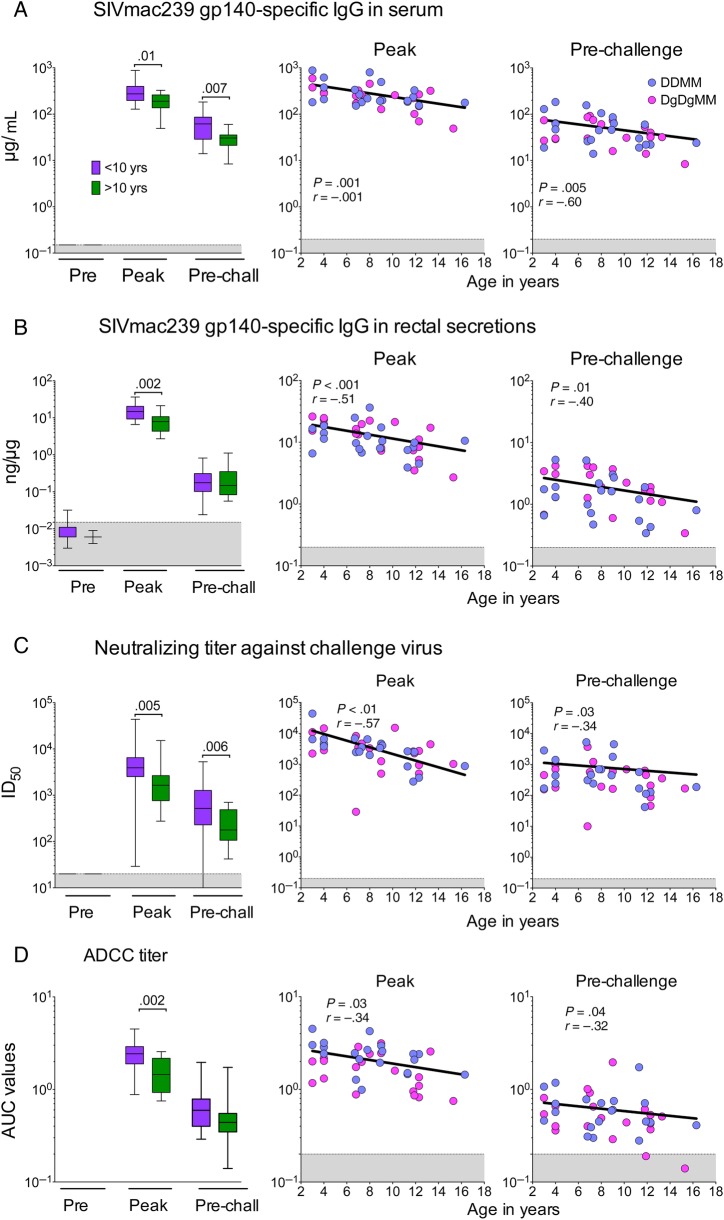
Diminished Vaccine-Elicited Humoral Immunity in Older Animals
We next investigated the immune mechanisms that contributed to the loss of vaccine protection in older animals. Because enhanced IgG responses had shown modest direct correlations with protection (Figure 2), we compared Ab responses in animals that were younger than or older than 10 years of age (Figure 4). These analyses, which included all animals, revealed significantly lower levels of elicited IgG in the older animals (Figure 4). The effect of age on Ab responses was evident in serum (Figure 4A) and rectal secretions (Figure 4B). In addition, older animals had significantly lower neutralizing Ab and ADCC responses (Figure 4C and D). In contrast, age did not significantly affect the levels of elicited T cells (Supplementary Figure 2).
Figure 4.
Effect of age on antibody (Ab) responses. Levels of elicited immunoglobulin (Ig)G are shown on the left and correlations with number of challenges to infection are on the right. (A) Levels of elicited glycoprotein (gp)140-specific IgG in serum. (B) Specific activity of elicited gp140-specific IgG in rectal secretions. (C) Magnitudes of neutralizing Ab against the challenge virus. (D) Antibody-dependent cellular cytotoxicity (ADCC) titer. Responses were measured at peak response (2 weeks after the final modified vaccinia Ankara boost) and prechallenge (week 52). For each panel, the left panels show the magnitudes of responses elicited for all animals in the 2 age groups, and the right panels show the correlation between the elicited Ab at peak and prechallenge timepoints and the number of challenges to infection. In the right panels, the color of points indicates whether data were from a GM-CSF-adjuvanted or nonadjuvanted animal. Abbreviations: AUC, area under the curve; ID50, inhibitory dose for 50% neutralization; Peak, week 34; Pre, preimmunization; pre-chall, week 52; shaded areas, background of detection; SIVmac239, simian immunodeficiency virus macaque 239.
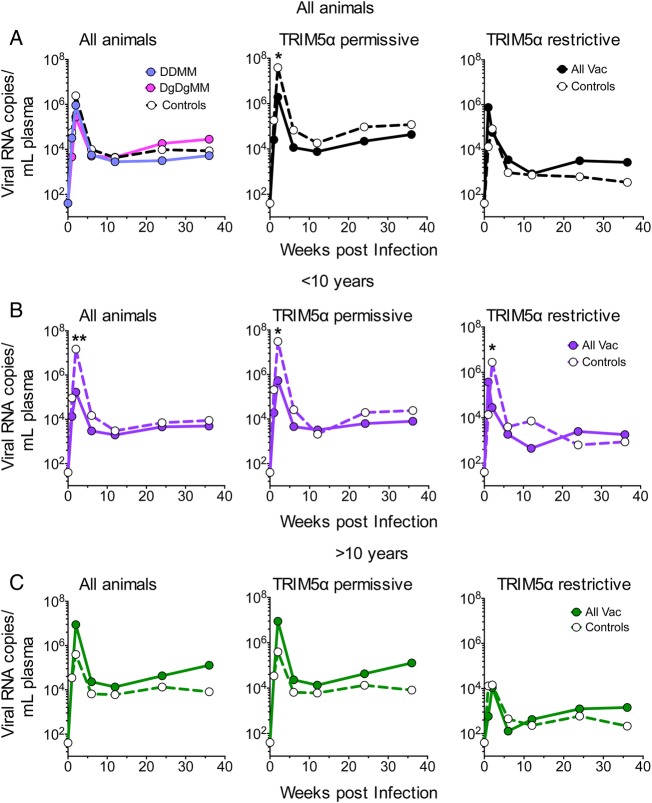
Finally, the effects of vaccination were analyzed for control of viremia during the first 6 months after infection (Figure 5). For these studies, infection was assumed to have taken place 1 week before the first detection of virus. One hundred-fold reductions in peak viremia occurred in the animals that were <10 years old (Figure 5B). These reductions occurred in the group representing all animals under 10 as well as in the TRIM5α-permissive group. No reduction in postchallenge viremia occurred in animals over 10 years of age (Figure 5C). The coexpressed GM-CSF did not appear to have an effect on postinfection control (Figure 5A, left panel). The TRIM5α-restrictive animals showed enhanced viral control compared with TRIM5α-permissive animals both in vaccinated and unvaccinated control groups. Analysis of male and female cohorts did not reveal substantial differences in elicited responses or protection (data not shown).
Figure 5.
Temporal postinfection viremia. (A) Temporal viremia for all animals based on vaccination in the presence or absence of coexpressed granulocyte-macrophage colony-stimulating factor in the deoxyribonucleic acid prime. (B) Temporal viremia for pooled vaccine groups that were <10 years old. (C) Temporal viremia for pooled vaccine groups that were more than 10 years old. In each panel, the left figure presents data for all animals, the middle figure presents data for tripartite motif-containing protein 5 (TRIM5)α-permissive animals, and the right figure presents data for TRIM5α-restrictive animals. Points with significant differences are indicted by asterisks: *P ≤ .05; **P ≤ .01. Abbreviations: All Vac, combined groups; DDMM, DNA at 0 and 8 weeks and MVA at 16 and 32 weeks; DgDgMM, the same regimen for the GM-CSF co-expressing DNA; RNA, ribonucleic acid.
DISCUSSION
In this trial, a SIVmac239 prototype of our GOVX-B11 HIV vaccine showed strong protection against a pathogenic heterologous SIVsmE660 mucosal challenge. More importantly, this protection was not dependent on the presence of restrictive TRIM5α alleles and did not require GM-CSF as an adjuvant. In addition, our trial showed a profound effect of age on the ability of a SIVmac239 vaccine to raise protective immunity for SIVsmE660. The reductions in per-exposure risk of infection were by far greater in animals <10 years old than in animals more than 10 years old. Over the first 6 rectal exposures, younger TRIM5α-permissive animals had 1.7 times higher reductions in per-challenge risk of infection than older animals (80% as opposed to 46%); and, over the 12 challenges administered in the trial, they had 7 times higher reductions in per-exposure risk of infection (72% as opposed to 10%). The challenge was a rigorous challenge as evidenced by the presence of a median of 2 transmitted variants in unvaccinated animals (range of 1 to 7) as opposed to 1 transmitted variant in vaccinated animals (range of 1 to 3). Most heterosexual transmissions have only 1 transmitted variant [30].
Elicited immune responses suggested that Ab, and not T cells, had played the key role in delaying infection. Levels of Env-specific IgG and IgA in serum, the specific activity of Env-specific IgG in rectal secretions, and IgG- and IgA-producing ASC showed modest correlations with the number of challenges to infection. Consistent with the poorer protection in older animals, each of these protection-associated Ab responses was significantly lower in older than younger animals.
Analyses for correlates of protection revealed weak correlations between binding Ab for Env in serum and rectal secretions and the number of challenges to infection. These correlations did not extend to functional assays for neutralizing and nonneutralizing activities. A failure of neutralizing Ab to protect is consistent with our prior studies in the SIV/macaque model [31]. However, others have found correlations between nonneutralizing Fc-mediated mechanisms of protection such as ADCP and ADCC and protection [32–39]. The fact that we did not observe these correlates may reflect the overall similar levels of responses in both vaccine groups, combined with the heterogeneity in age and sex having masked effects that have been observed for vaccines that raised a broader range of responses and were tested in more homogeneous groups [32–38].
The trial was undertaken to further test the hypothesis that avidity of elicited Ab for native Env was a correlate for protection and that avidity could be enhanced by coexpression of GM-CSF in the DNA prime. New features for this preclinical trial included mutating protease in the DNA prime so that it would be more efficient at producing VLPs [9] and changing the schedule to allow 16 instead of 8 weeks between the 2 MVA boosts to allow further avidity maturation of Ab [10]. Consistent with these changes favoring avidity maturation, the avidity of elicited Ab responses had a much higher median index than under prior conditions, and the coexpressed GM-CSF did not provide an immunological advantage for avidity maturation. We consider it unlikely for differences in the levels of GM-CSF expression between the 2 trials to have affected the outcome, because these were similar in both trials (see Methods). On the basis of these findings, the nonadjuvanted GOVX-B11 vaccine will undergo further development using the 16-week rest between the final 2 MVA boosts.
CONCLUSIONS
In this trial, age had a much more profound effect on protection and the elicitation of Ab responses than sex or the coexpression of GM-CSF in the DNA prime. Age has long been known to affect the efficiency of vaccination [25–29]. Thus, it is not surprising to find a strong effect of age in macaques on the efficacy of a simian prototype of an HIV vaccine. We have long recognized that Env is a poor immunogen [40]. This poor immunogenicity could magnify the effect of age on Ab responses. In contrast to most vaccines in which the elderly are primarily compromised; for HIV vaccines, both middle-aged (40–60 year olds) individuals and the elderly (>60 years old) may be compromised in their ability to generate protective Abs for Env. Of interest in this context, the 1 partially successful vaccine trial in humans was in a cohort of 18- to 30-year-old individuals [41]. Given the strong effect of age on protection, we plan to limit efficacy testing with GOVX-B11 to youths and young adults.
Supplementary Data
Supplementary material is available online at Open Forum Infectious Diseases online (http://OpenForumInfectiousDiseases.oxfordjournals.org/).
Acknowledgments
We thank Drs. Nancy Miller and Alan Schultz (Division of AIDS) for council on the design and execution of Simian Vaccine Evaluation Units study P165. We are indebted to Dr. Tianwei Yu for help with statistical analyses and to Robert L. Wilson for technical assistance in the measurement of mucosal antibodies. We also thank Dr. Hanne Andersen (Bioqual) for help in managing this trial. The CCR5+CD4+CEM.NKR T-cell line was obtained from the NIH AIDS Reagent Program and was contributed by Dr. Alexandra Trkola.
Financial support. This trial, P165, was funded by the Simian Vaccine Evaluation Unit of the Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID); the Primate Central Immunology Laboratory (Contract no. HHSN272201100016C; to D. C. M.); an Integrated Preclinical/Clinical AIDS Vaccine Development Grant (5U19AI074073; to H. L. R.); the Consortia for AIDS Vaccine Research in Nonhuman Primates Grant (U19AI096187; to R. R. A.); and funded in part with federal funds from the National Cancer Institute, National Institutes of Health (under Contract No. HHSN261200800001E) and the Division of Intramural Research, NIAID.
Potential conflicts of interest. H. L. R. and R. R. A. are inventors on the DNA/MVA technology that has been licensed to GeoVax Inc. by Emory University. H. L. R. is an employee of GeoVax and owns stock in GeoVax. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1.Smith JM, Amara RR, McClure HM et al. Multiprotein HIV-1 clade B DNA/MVA vaccine: construction, safety, andimmunogenicity in Macaques. AIDS Res Hum Retroviruses 2004; 20:654–65. [DOI] [PubMed] [Google Scholar]
- 2.Wyatt LS, Belyakov IM, Earl PL et al. Enhanced cell surface expression, immunogenicity and genetic stability resulting from a spontaneous truncation of HIV Env expressed by a recombinant MVA. Virology 2008; 372:260–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Goepfert PA, Elizaga ML, Sato A et al. Phase 1 safety and immunogenicity testing of DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2011; 203:610–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goepfert PA, Elizaga ML, Seaton K et al. Specificity and 6-month durability of immune responses induced by DNA and recombinant modified vaccinia Ankara vaccines expressing HIV-1 virus-like particles. J Infect Dis 2014; 210:99–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai L, Kwa S, Kozlowski PA et al. Prevention of infection by a granulocyte-macrophage colony-stimulating factor co-expressing DNA/modified vaccinia Ankara simian immunodeficiency virus vaccine. J Infect Dis 2011; 204:164–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yeh WW, Rao SS, Lim SY et al. The TRIM5 gene modulates penile mucosal acquisition of simian immunodeficiency virus in rhesus monkeys. J Virol 2011; 85:10389–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Letvin NL, Rao SS, Montefiori DC et al. Immune and genetic correlates of vaccine protection against mucosal infection by SIV in monkeys. Sci Transl Med 2011; 3:81ra36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds MR, Sacha JB, Weiler AM et al. The TRIM5{alpha} genotype of rhesus macaques affects acquisition of simian immunodeficiency virus SIVsmE660 infection after repeated limiting-dose intrarectal challenge. J Virol 2011; 85:9637–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Smith JM, Amara RR, Campbell D et al. DNA/MVA vaccine for HIV type 1: effects of codon-optimization and the expression of aggregates or virus-like particles on the immunogenicity of the DNA prime. AIDS Res Hum Retroviruses 2004; 20:1335–47. [DOI] [PubMed] [Google Scholar]
- 10.Ledgerwood JE, Wei CJ, Hu Z et al. DNA priming and influenza vaccine immunogenicity: two phase 1 open label randomised clinical trials. Lancet Infect Dis 2011; 11:916–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van Rompay KK, Greenier JL, Cole KS et al. Immunization of newborn rhesus macaques with simian immunodeficiency virus (SIV) vaccines prolongs survival after oral challenge with virulent SIVmac251. J Virol 2003; 77:179–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keele BF, Li H, Learn GH et al. Low-dose rectal inoculation of rhesus macaques by simian immunodeficiency virus smE660 or SIVmac251 recapitulates human mucosal infection by HIV-1. J Exp Med 2009; 206:1117–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwa S, Sadagopal S, Shen X et al. CD40L-adjuvanted DNA/modified vaccinia virus Ankara simian immunodeficiency virus (SIV) vaccine enhances protection against neutralization-resistant mucosal SIV infection. J Virol 2015; 89:4690–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lai L, Vodros D, Kozlowski PA et al. GM-CSF DNA: an adjuvant for higher avidity IgG, rectal IgA, and increased protection against the acute phase of a SHIV-89.6P challenge by a DNA/MVA immunodeficiency virus vaccine. Virology 2007; 369:153–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kozlowski PA, Lynch RM, Patterson RR et al. Modified wick method using Weck-Cel sponges for collection of human rectal secretions and analysis of mucosal HIV antibody. J Acquir Immune Defic Syndr 2000; 24:297–309. [DOI] [PubMed] [Google Scholar]
- 16.Montefiori DC. Evaluating neutralizing antibodies against HIV, SIV and SHIV in a luciferase reporter gene assay. In: Coligan JE, Kruisbeek AM, Margulies DH, Shevach EM, Strober W, Coico R, eds. Current Protocols in Immunology. New York: John Wiley and; Sons; Curr Protoc Immunol. 2005. Jan;Chapter 12:Unit 12.11. doi: 10.1002/0471142735.im1211s64. [DOI] [PubMed] [Google Scholar]
- 17.Silveira EL, Kasturi SP, Kovalenkov Y et al. Vaccine-induced plasmablast responses in rhesus macaques: phenotypic characterization and a source for generating antigen-specific monoclonal antibodies. J Immunol Methods 2015; 416:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ackerman ME, Moldt B, Wyatt RT et al. A robust, high-throughput assay to determine the phagocytic activity of clinical antibody samples. J Immunol Methods 2011; 366:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alpert MD, Heyer LN, Williams DE et al. A novel assay for antibody-dependent cell-mediated cytotoxicity against HIV-1- or SIV-infected cells reveals incomplete overlap with antibodies measured by neutralization and binding assays. J Virol 2012; 86:12039–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hulot SL, Cale EM, Korber BT, Letvin NL. Vaccine-induced CD8+ T lymphocytes of rhesus monkeys recognize variant forms of an HIV epitope but do not mediate optimal functional activity. J Immunol 2011; 186:5663–74. [DOI] [PubMed] [Google Scholar]
- 21.Hudgens MG, Gilbert PB, Mascola JR et al. Power to detect the effects of HIV vaccination in repeated low-dose challenge experiments. J Infect Dis 2009; 200:609–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hudgens MG, Gilbert PB. Assessing vaccine effects in repeated low-dose challenge experiments. Biometrics 2009; 65:1223–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kirmaier A, Wu F, Newman RM et al. TRIM5 suppresses cross-species transmission of a primate immunodeficiency virus and selects for emergence of resistant variants in the new species. PLoS Biol 2010; 8:e1000462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Reynolds MR, Weiler AM, Piaskowski SM et al. Macaques vaccinated with simian immunodeficiency virus SIVmac239Delta nef delay acquisition and control replication after repeated low-dose heterologous SIV challenge. J Virol 2011; 84:9190–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weinberger B, Herndler-Brandstetter D, Schwanninger A et al. Biology of immune responses to vaccines in elderly persons. Clin Infect Dis 2008; 46:1078–84. [DOI] [PubMed] [Google Scholar]
- 26.Fulton RB, Varga SM. Effects of aging on the adaptive immune response to respiratory virus infections. Aging health 2009; 5:775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dunn-Walters DK, Ademokun AA. B cell repertoire and ageing. Curr Opin Immunol 2010; 22:514–20. [DOI] [PubMed] [Google Scholar]
- 28.Frasca D, Landin AM, Riley RL, Blomberg BB. Mechanisms for decreased function of B cells in aged mice and humans. J Immunol 2008; 180:2741–6. [DOI] [PubMed] [Google Scholar]
- 29.Zheng B, Switzer K, Marinova E et al. Correction of age-associated deficiency in germinal center response by immunization with immune complexes. Clin Immunol 2007; 124:131–7. [DOI] [PubMed] [Google Scholar]
- 30.Keele BF, Giorgi EE, Salazar-Gonzalez JF et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc Natl Acad Sci USA 2008; 105:7552–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burton SL, Kilgore KM, Smith SA et al. Breakthrough ofsimian immunodeficiency virus strain smE660 challenge in SIV strain mac239-vaccinated rhesus macaques despite potent autologous neutralizing antibody responses. Proc Natl Acad Sci U S A 2015; 112:10780–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Florese RH, Demberg T, Xiao P et al. Contribution of nonneutralizing vaccine-elicited antibody activities to improved protective efficacy in rhesus macaques immunized with Tat/Env compared with multigenic vaccines. J Immunol 2009; 182:3718–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gomez-Roman VR, Patterson LJ, Venzon D et al. Vaccine-elicited antibodies mediate antibody-dependent cellular cytotoxicity correlated with significantly reduced acute viremia in rhesus macaques challenged with simian immunodeficiency virus SIV(mac251). J Immunol 2005; 174:2185–9. [DOI] [PubMed] [Google Scholar]
- 34.Hidajat R, Xiao P, Zhou Q et al. Correlation of vaccine-elicited systemic and mucosal nonneutralizing antibody activities with reduced acute viremia following intrarectal simian immunodeficiency virus SIVmac251 challenge of rhesus macaques. J Virol 2009; 83:791–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Xiao P, Zhao J, Patterson LJ et al. Multiple vaccine-elicited nonneutralizing antienvelope antibody activities contribute to protective efficacy by reducing both acute and chronic viremia following simian/human immunodeficiency virus SHIV89.6P challenge in rhesus macaques. J Virol 2010; 84:7161–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barouch DH, Liu J, Li H et al. Vaccine protection against acquisition of neutralization-resistant simian immunodeficiency virus challenges in rhesus monkeys. Nature 2012; 482:89–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pegu P, Vaccari M, Gordon S et al. Antibodies with high avidity to the gp120 envelope protein in protection from simian immunodeficiency virus SIV(mac251) acquisition in an immunization regimen that mimics the RV-144 Thai trial. J Virol 2013; 87:1708–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Barouch DH, Alter G, Broge T et al. HIV-1 vaccines. Protective efficacy of adenovirus/protein vaccines against SIV challenges in rhesus monkeys. Science 2015; 349:320–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Fouts TR, Bagley K, Prado IJ et al. Balance of cellular and humoral immunity determines the level of protection by HIV vaccines in rhesus macaque models of HIV infection. Proc Natl Acad Sci U S A 2015; 112: E992–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Richmond JF, Lu S, Santoro JC et al. Studies of the neutralizing activity and avidity of anti-human immunodeficiency virus type 1 Env antibody elicited by DNA priming and protein boosting. J Virol 1998; 72:9092–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med 2009; 361:2209–20. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.