Abstract
Presented is the first case of acute immune reconstitution inflammatory syndrome (IRIS)-associated cryptococcal meningoencephalitis in a patient on natalizumab for multiple sclerosis. The patient developed acute cerebral edema after initiation of amphotericin B. We propose several mechanisms that explain the acuity of IRIS in this specific patient population and suggest possible therapies.
Keywords: Cryptococcus, fungal, IRIS, meningitis, natalizumab
Cryptococcus neoformans is an encapsulated yeast ubiquitous in nature that typically causes disease in patients with severe immunosuppressive conditions such as human immunodeficiency virus (HIV)/acquired immune deficiency syndrome (AIDS) or organ transplantation. Meningoencephalitis is the most common form of disseminated cryptococcosis and may cause severe inflammation in the central nervous system (CNS). Immune reconstitution inflammatory syndrome (IRIS) is a well described complication of effective treatment for CNS cryptococcosis, leading to an increased risk for morbidity and mortality secondary to increased intracranial pressure (ICP) [1, 2]. Corticosteroids are often used as an adjunct to antifungal therapy for treatment of IRIS [1].
Natalizumab is a monoclonal antibody against the cell adhesion molecule α4-integrin and prevents transmigration of leukocytes across the blood brain barrier, reducing the CNS leukocyte count and inflammation caused by autoimmune diseases, and it has US Food and Drug Administration approval to treat relapsing remitting multiple sclerosis (RRMS). Natalizumab has been associated with progressive multifocal leukoencephalopathy and gastrointestinal cryptosporidiosis [3].
CASE REPORT
A 46-year-old man with RRMS who had been receiving natalizumab for 2 years presented to the hospital with nausea, vomiting, headache, and poor appetite, which developed 2 months before this presentation. He was given a diagnosis of a viral illness by his physician. He did not improve, and over the next several weeks he developed progressive loss of vision in the right eye, horizontal diplopia, and tinnitus. He presented to an outside hospital the week before admission where magnetic resonance imaging (MRI) demonstrated bilateral thalamic lesions. He was treated with intravenous Solu-Medrol for presumed multiple sclerosis (MS) exacerbation. He was given a prednisone taper on discharge; however, he did not improve and was admitted to our institution. At that time, he reported no respiratory symptoms, and he had no other significant past medical, surgical, or family history. His last dose of natalizumab was 2 months before this admission.
At presentation he was alert, oriented, and afebrile with normal vital signs. His physical and neurologic exam was notable for decreased vision in the right eye, 6th nerve palsy on the right, and nuchal rigidity.
An MRI was performed and demonstrated abnormal T2/FLAIR deep gray matter hyperintense lesions most pronounced in the basal ganglia bilaterally. Lumbar puncture (LP) was performed and showed opening pressure of 32 cm water, cerebrospinal fluid (CSF) glucose 50 mg/dL, protein 27 mg/dL, and white blood cell count (WBC) 2041/cmm (88% lymphocytes, 12% macrophages). Cerebrospinal fluid cryptococcal antigen was positive at a titer of 1:5120; CSF culture was positive for C neoformans. Human immunodeficiency virus serum assay and JC virus CSF reverse transcription-polymerase chain reaction were negative.
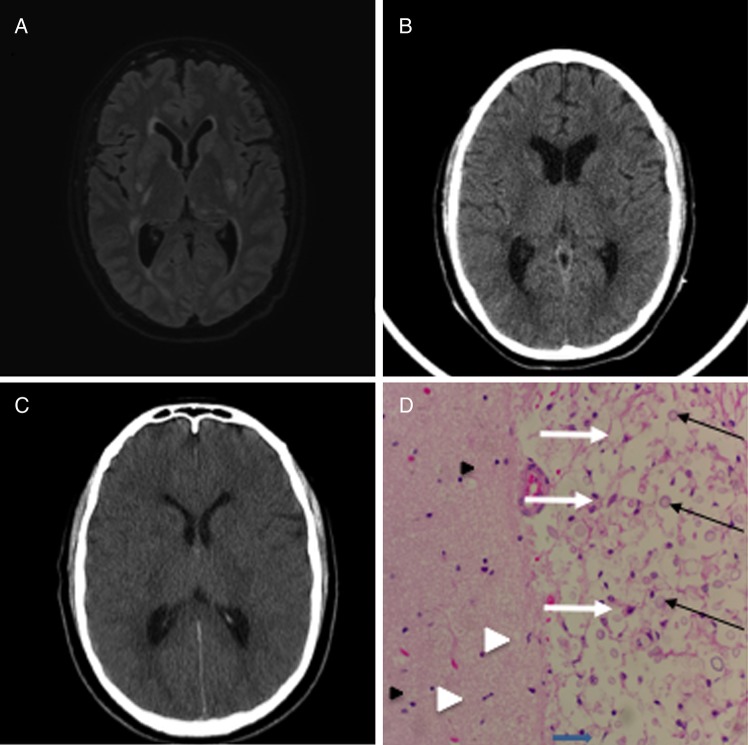
Figure 1.
(A) Axial noncontract 3D FLAIR-SENSE magnetic resonance image, hospital day 1, showing areas of hyperintensity in the basal ganglia bilaterally representing ischemic infarcts due to cryptococcal vasculopathy. (B) Axial noncontrast computed tomography (CT) head, hospital day 9, after the initial seizure-like event. (C) Axial noncontrast CT head, hospital day 10, after the acute decline in neurologic status showing the interval development of diffuse cerebral edema. (D) Representative hematoxylin and eosin stain of cerebral white matter from the frontal lobe showing Cryptococcus (black arrows) with classic “soap bubble” appearance and surrounding tissue destruction. Microglia (white arrowhead) and reactive astrocytes (white arrows) are also present. The left side shows intact neuropil (pink, strands in the background) as well as more microglia and scattered oligodendrocytes (black arrowhead).
Amphotericin B lipid complex and flucytosine were initiated on the day of admission, and systemic corticosteroids were tapered. Repeat LP performed on hospital day 3 demonstrated an opening pressure of 14 cm water, glucose of 23 mg/dL, protein of 41 mg/dL, WBC of 436/cmm (68% lymphocytes, 6% macrophages). He had persistent headaches but otherwise was stable until hospital day 5 when he became febrile and developed seizure-like activity. A repeat computed tomography (CT) scan of the head was unchanged. On hospital day 6, he developed more seizure-like activity together with rapid decline in mental status. He was transferred to the intensive care unit where he was intubated for airway protection and had a Glasgow Coma Scale of 3. Chest radiograph demonstrated diffuse right lower lobe infiltrates consistent with aspiration pneumonia, and his left lung was clear. He became hypertensive (blood pressure 200/120) and became progressively unresponsive. A repeat CT head scan at that point showed severe diffuse brain swelling. The patient did not recover brainstem reflexes and was declared brain dead on hospital day 7.
An autopsy was performed, which demonstrated bilateral fungal pneumonia. Gross examination of the brain revealed mucinous vacuolations within the basal ganglia. Microscopic examination of the brain revealed edema, reactive astrocytosis, and numerous budding yeasts in the leptomeninges and perivascular spaces of the cerebral cortex, hippocampus, basal ganglia, brainstem, cerebellum, pituitary gland, and spinal cord.
DISCUSSION
This is the first reported case of IRIS-associated with natalizumab and cryptococcal meningitis and the second case of cryptococcal meningitis associated with natalizumab [4]. Cryptococcal-associated IRIS is classically seen among HIV/AIDS patients after the initiation of antiretroviral therapy [2], but it is also occasionally seen in immunocompetent patients with cryptococcal meningitis [5]. Patients taking natalizumab are at high risk of IRIS upon discontinuation of the medicine independent of any opportunistic infections [6]. Treatment for IRIS after cryptococcal meningitis is not well defined, but induction therapy with glucocorticosteroids with a prolonged taper is commonly practiced [1].
Cryptococcus has unique affinity for the CNS and gains access by manipulating the host response at the cellular and molecular level via multiple independent mechanisms. Transcytosis seems to be the predominant process leading to CNS penetration, as demonstrated by murine studies using Cryptococcus-like polystyrene microspheres [7]. Trojan horse entry, which involves passive transfer of Cryptococcus into the CNS within host macrophages, has also been demonstrated. This method of CNS entry is supported by studies demonstrating that mice given Cryptococcus-infected macrophages elicited higher rates of cryptococcal meningitis than those given injections of free-living organisms [8]. More importantly, virulence factors also likely play an important role in allowing cryptococcal penetration with studies suggesting a role for cryptococcal urease, plasmin, and inositol [9, 12].
Natalizumab is indicated for the treatment of relapsing/remitting MS. Although the mechanisms responsible for its efficacy have yet to be completely elucidated, natalizumab causes a profound decrease in CNS CD4/CD8 cells to levels comparable to patients with advanced HIV/AIDS [9]. In addition, it may decrease the amount of antigen-presenting cells in the CSF, causing the immunosuppressive effect to persist months after discontinuation of therapy [10]. Such profound localized CNS immunosuppression has been demonstrated to increase the risk for CNS infections typically associated with HIV/AIDS, such as progressive multifocal leukoencephalopathy [3]. More importantly, natalizumab-mediated immunosuppression is unique in that it is compartmentalized to the CNS only, with patients exhibiting normal peripheral CD4 T-cell populations [9]. In stark contrast, patients with HIV/AIDS exhibit profound loss of both peripheral and CNS CD4 populations.
This patient developed cryptococcal meningitis likely as a result of immunomodulation with natalizumab and corticosteroids for MS. The worsening headache, vision loss, and MRI with bilateral thalamic lesions represented the initial presentation of cryptococcal disease because MS lesions are typically found in juxtacortical, periventricular, infratentorial, or spinal cord. He was given 5 days of high-dose glucocorticosteroids for presumed MS flair, and this was continued until his final admission, where they were tapered once the diagnosis of cryptococcosis was established. Although corticosteroid use for primary IRIS prophylaxis is not recommended [1], in retrospect, this might have prevented an IRIS phenomenon, such that dose reduction of corticosteroids in combination with the initiation of antifungal therapy resulted in signs of increased ICP, with development of cerebral edema and rapid progression to death.
Autopsy findings demonstrated substantial evidence of high fungal burden in both the meninges and lungs, which doubtlessly contributed to his ultimate demise. However, based on the rapidity of clinical deterioration and progression to brain death, we postulate that this patient developed a natalizumab-mediated IRIS-like phenomenon. This has not been described in patients receiving natalizumab. We know that natalizumab prevents CNS migration of peripheral T cells through the blood-brain barrier (BBB), and we hypothesize that rapid lysis of cryptococcal organisms in the CNS with subsequent presentation of those antigens to the CNS endothelial and antigen-presenting cells overwhelmed the inhibition of α4-integrin by natalizumab, allowing migration of peripheral CD4 T cells across the BBB. Consequently, because patients on natalizumab have been shown to exhibit normal peripheral CD4 T-cell populations, we believe that the patient experienced massive migration of activated T cells across the BBB, which led to significant inflammation and cerebral edema. In addition, the median time to development of IRIS in patients with HIV/AIDS is 4 weeks [11] and corresponds with the reconstitution of peripheral CD4 populations. In this patient, an IRIS-like clinical deterioration occurred in days, rather than weeks, which may be explained by the presence of normal peripheral CD4 T-cell populations at the time of induction antifungal therapy and was supported by the autopsy findings of severe cerebral edema.
CONCLUSIONS
To date, there is little clinical experience to guide treatment of cryptococcal IRIS in patients on natalizumab, and this case underscores the importance of understanding the risk of IRIS in these patients. We believe that these patients represent a unique population in that the CNS-limited immunosuppression places them at increased risk for IRIS after CNS opportunistic infections, and we recommend that clinicians maintain a low threshold for initiating high-dose glucocorticosteroids for those patients who experience clinical deterioration consistent with cerebral edema and IRIS.
Acknowledgments
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1.Perfect JR, Dismukes WE, Dromer F et al. Clinical practice guidelines for the management of cryptococcal disease: 2010 update by the Infectious Diseases Society Of America. Clin Infect Dis 2010; 50:291–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shelburne SA, Visnegarwala F, Darcourt J, Graviss EA et al. Incidence and risk factors for immune reconstitution inflammatory syndrome during highly active antiretroviral therapy. AIDS 2005; 19:399–406. [DOI] [PubMed] [Google Scholar]
- 3.Pucci E, Giuliani G, Solari A et al. Natalizumab for relapsing remitting multiple sclerosis. Cochrane Database Syst Rev 2011:CD007621. [DOI] [PubMed] [Google Scholar]
- 4.Valenzuela RM, Pula JH, Garwacki D et al. Cryptococcal meningitis in a multiple sclerosis patient taking natalizumab. J Neurol Sci 2014; 340:109–11. [DOI] [PubMed] [Google Scholar]
- 5.Somerville LK, et al. Successful treatment of Cryptococcus neoformans immune reconstitution inflammatory syndrome in an immunocompetent host using thalidomide. Med Mycol Case Rep 2015; 7:12–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Miravalle A, Jensen R, Kinkel RP. Immune reconstitution inflammatory syndrome in patients with multiple sclerosis following cessation of natalizumab therapy. Arch Neurol 2011; 68:186–91. [DOI] [PubMed] [Google Scholar]
- 7.Shi M, Li SS, Zheng C et al. Real-time imaging of trapping and urease-dependent transmigration of Cryptococcus neoformans in mouse brain. J Clin Invest 2010; 120:1683–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Charlier C, Nielsen K, Daou S et al. Evidence of a role for monocytes in dissemination and brain invasion by Cryptococcus neoformans. Infect Immun 2009; 77:120–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liu TB, Perlin DS, Xue C. Molecular mechanisms of cryptococcal meningitis. Virulence 2012; 3:173–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuve O, Marra CM, Bar-Or A et al. Altered CD4+/CD8+ T-cell ratios in cerebrospinal fluid of natalizumab-treated patients with multiple sclerosis. Arch Neurol 2006; 63:1383–7. [DOI] [PubMed] [Google Scholar]
- 11.Stuve O. The effects of natalizumab on the innate and adaptive immune system in the central nervous system. J Neurol Sci 2008; 274:39–41. [DOI] [PubMed] [Google Scholar]
- 12.Ratnam I, Chiu C, Kandala NB, Easterbrook PJ. Incidence and risk factors for immune reconstitution inflammatory syndrome in an ethnically diverse HIV type 1-infected cohort. Clin Infect Dis 2006; 42:418–27. [DOI] [PubMed] [Google Scholar]