Abstract
Myocardial fibrosis is a significant global health problem associated with nearly all forms of heart disease. Cardiac fibroblasts comprise an essential cell type in the heart that is responsible for the homeostasis of the extracellular matrix; however upon injury, these cells transform to a myofibroblast phenotype and contribute to cardiac fibrosis. This remodeling involves pathological changes that include chamber dilation, cardiomyocyte hypertrophy and apoptosis, and ultimately leads to the progression to heart failure. Despite the critical importance of fibrosis in cardiovascular disease, our limited understanding of this cell population impedes the development of potential therapies that effectively target this cell type and its pathological contribution to disease progression. This review summarizes current knowledge regarding the origins and roles of fibroblasts, mediators and signaling pathways known to influence fibroblast function after myocardial injury, as well as novel therapeutic strategies under investigation to attenuate cardiac fibrosis.
Keywords: Cardiac fibroblasts, cardiac fibrosis, heart failure, therapeutics
Introduction
Heart failure, the clinical manifestation of numerous forms of cardiovascular disease, is a devastating disorder characterized by interstitial fibrosis, chamber remodeling and reduced ventricular compliance. Heart disease remains the predominant cause of mortality in the United States, accounting for nearly 800,000 deaths per year.1 Furthermore, it presents a considerable economic burden, with estimated direct and indirect costs in 2011 of about $320 billion and predictions suggesting that costs will rise to about $918 billion by 2030.1 Despite substantial improvements in therapeutic strategies, cardiovascular disease remains the leading cause of death worldwide indicating an urgent need for innovative treatment strategies.
Nearly all etiologies of heart disease involve pathological myocardial remodeling characterized by excessive deposition of extracellular matrix (ECM) proteins by cardiac fibroblasts (CFs), which reduces tissue compliance and accelerates the progression to heart failure. The CF is an essential cell type, predominantly of embryonic epicardial and endothelial origins,2–4 that resides within the myocardial interstitium, epicardial and perivascular regions.5, 6 Physiologically, CFs are responsible for homeostasis of the ECM, which provides a structural scaffold for cardiomyocytes (CMs), distributes mechanical forces through the cardiac tissue, and mediates electrical conduction.6–8 Previously, identification of these cells was largely based on phenotypic observations; morphologically, fibroblasts are flat, spindle shaped cells with multiple processes when propagated on tissue culture plastic. The cardiac cellular milieu can vary greatly depending on the species being examined and between healthy and injured myocardium.9 Although prior studies suggested that CFs accounted for the majority of cells within the adult rodent and human myocardium,7, 9, 10 recent reports with more accurate delineation of the CF population have proposed that CFs may comprise less than 20% of the total cell population in the adult murine heart, substantially less than previously suggested.2, 11 While it is now appreciated that a majority of resident fibroblasts originate from the embryonic epicardium,12, 13 the contribution of various resident and infiltrating cells to the population of activated cardiac myofibroblasts (MFs) remains under active investigation, including further refinement of specific molecular markers for CFs.
Unlike other organs, the heart has very limited regenerative capacity following injury, and instead, repair processes involve the removal of necrotic CMs followed by fibrotic scar tissue replacement that acts to preserve myocardial structural and functional integrity. To perform these functions, CFs within the connective tissue convert to their activated form, often known as MFs, which secrete elevated levels of ECM proteins to promote a profibrotic environment. Cardiac fibrosis provokes pathological changes that culminate in chamber dilatation, CM hypertrophy and apoptosis, and ultimately lead to the development of congestive heart failure.14–16 While the sources of these activated fibroblasts remain under intense investigation and debate, the refinement of molecular markers and the development of new techniques for lineage tracing are helping to enhance our understanding of their origins.2, 4 Despite the pathophysiological importance of fibrosis in cardiovascular disease, the CF remains relatively mischaracterized and poorly understood; furthermore, there currently exist limited clinical interventions that effectively target this cell type and its pathological contributions to disease progression. While recent progress has improved our understanding of the CF, the absence of a universal marker or method for lineage mapping, combined with the heterogeneous nature of this cell population, have provided a formidable challenge in the interpretation of results.17 This review summarizes current knowledge regarding the origin and role of the CF in development and disease, and presents recent advances toward novel therapeutic interventions targeting this critical cell population.
Role of CFs in Injury
The CF is now recognized for its fundamental contributions to the heart’s response to various forms of injury (Figure 1). In general, the critical phases of this response consist of inflammation, proliferation of non-myocytes and scar maturation, with the CF intimately involved in all of these processes. Following an acute myocardial injury, the expression of various pro-inflammatory cytokines and pro-fibrotic factors is upregulated in CFs, leading to increased proliferation of these cells and ultimately, the transition to the MF phenotype.18 During this maturation phase, MFs begin to secrete elevated levels of collagens and other ECM proteins. The purpose of this adaptive fibrosis is to maintain the structural integrity and pressure generating capacity of the heart, as a loss of integrity in the mechanical strength of the ventricle may lead to myocardial dysfunction or rupture. In the advanced phases of fibrotic scar formation, the tensile strength of collagen increases within the site of injury.19 In this setting, a subset of activated MFs acquire new phenotypic characteristics, including the expression of the contractile protein α smooth muscle actin (αSMA), and contribute to pathologic cardiac remodeling.20, 21 While initially adaptive, these processes eventually lead to the development of adverse changes in ventricular structure and compliance, and a concurrent progression into overt heart failure.
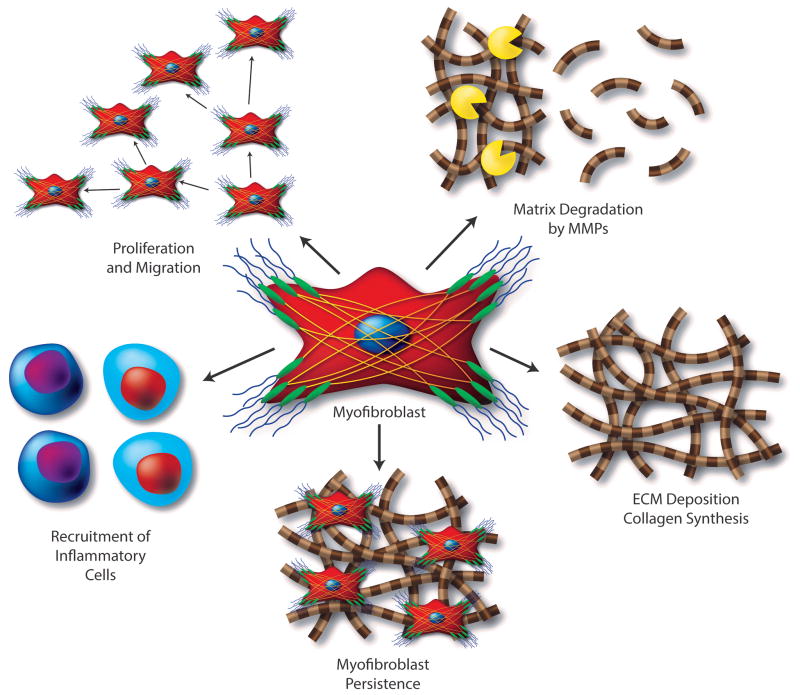
Figure 1. Characteristics and Functions of Activated Cardiac MFs.
Cardiac fibroblasts respond to pathologic stress and environmental stimuli by transforming into MFs that (1) express elevated levels of various pro-inflammatory and pro-fibrotic factors that directly contribute to inflammatory cell infiltration and fibroblast proliferation, (2) secrete high levels of matrix metalloproteinases and other ECM degrading enzymes that facilitate fibroblast migration and (3) contribute to the deposition of collagen and other ECM proteins leading to scar formation. While this adaptive fibrotic scar tissue maintains the structural integrity and pressure generating capacity of the heart, MF persistence eventually leads to the development of adverse changes in ventricular structure and compliance, leading to the progression to heart failure.
Pathologic cardiac remodeling is characterized by fibroblast accumulation and excessive deposition of ECM proteins, which leads to distorted organ architecture and has significant consequences on cardiac function.22 Fibrogenesis contributes to impaired cardiac function as fibrotic ECM increases ventricular stiffness and can lead to contractile dysfunction.23, 24 In addition, excess ECM and fibroblasts impair mechano-electric coupling of CMs, thus reducing cardiac contraction and increasing the risk of arrhythmogenesis and mortality.25, 26 This pathologic process can also include hypertrophy and dysfunction of CMs via paracrine mechanisms, which further contribute to impaired cardiac function.27 Furthermore, inflammation and fibrosis within perivascular regions may decrease tissue availability to oxygen and nutrients and increase the pathological remodeling response.28
Following the initial wound healing process in many tissues, a majority of collagen-secreting fibroblasts undergo apoptosis and leave a mature scar composed of cross-linked collagen and other matrix components.29 However, when this process persists in the heart, MFs within the cardiac scar tissue continue to release maladaptive proinflammatory and prohypertrophic signals, characterized by CM hypertrophy and necrosis followed by replacement fibrosis.8, 30–32 Importantly, following an acute injury such as myocardial infarction (MI), activated fibroblasts not only increase the synthesis of ECM proteins at the site of injury, but also within the healthy tissue remote from the immediate infarct,15, 33 commonly referred to as reactive fibrosis.5, 34 This potentiates the pathophysiologic response after acute myocardial injury by reducing chamber compliance and increasing stiffness of the ventricles.35 A portion of MFs remain embedded in mature cardiac scars long after the initial myocardial injury, likely due to a resistance to apoptosis, which perpetuates these pathologic processes.34
Cardiac fibrosis can be categorized into two forms, namely reactive interstitial fibrosis or replacement fibrosis, each which can be recapitulated by several models of heart failure.36, 37 Transverse aortic constriction (TAC) is an animal model of left ventricular pressure overload (similar to aortic stenosis) characterized initially by reactive interstitial fibrosis, an adaptive response to preserve cardiac structure and function, and followed by replacement fibrosis in areas of CM necrosis.38 In animal models of acute ischemic injury, such as MI or ischemia/reperfusion injuries, the initial injury is characterized by an immediate, robust inflammatory response and extensive CM death; replacement fibrosis restores this region devoid of viable CMs to prevent cardiac rupture.39 Cardiac fibrosis also occurs in the context of right ventricular (RV) volume overload that can result from congenital malformations, such as repaired tetralogy of Fallot or pulmonary hypertension. In a mouse model of RV volume overload, fibrosis is evident first in the subendocardial RV, but relatively little is known of the cellular origins or molecular mechanisms of fibrosis in this context.40 These models represent critical yet differing recapitulations of human disease, and as such, show distinct functions of the fibroblast response to injury.39
Cardiac Fibroblast Markers and Limitations
One major area of study is an attempt to quantify relative contributions of the various cardiac cell lineages to the CF population following injury. Such studies rely upon molecular biomarkers, but experimental interpretation is difficult as this cell population can include fibroblasts, endothelial cells, pericytes and immune cells. Further complicating this effort, while lineage-specific markers have long been established for several cell types within this group, robust markers of both quiescent CFs and activated cardiac MFs remain elusive. With numerous molecular markers now being recognized for their ability to identify particular populations of CFs, the controversy surrounding this cell population remains unresolved. In the heart, as with other organs, there is increasing interest in the investigation of novel CF and MF markers, and transcriptomics is proving an effective method for the uncovering of new markers.41 Aside from the immediate benefits for cell identification, biomarkers in use for other organs, for example the kidney, can be used as tools to diagnose the progression of disease and also as novel therapeutic targets. Here we present several commonly used molecular markers to identify quiescent and activated CFs, concerns regarding their use, and how the refinement of these markers may potentially provide for the identification of a purer fibroblast population (Table 1).
Table 1. Fibroblast Markers and Mouse Models for Gene Targeting.
This table lists a selection of molecular markers that have been utilized for the identification of quiescent cardiac fibroblasts and activated myofibroblasts; these markers have been translated into numerous mouse lines for lineage tracing and gene targeting studies. Limited expression in all cardiac fibroblasts or positive expression in alternate cardiac cells types are among the problems associated with the use of these markers. Multiple mouse lines exist for many of the markers listed with varying levels of specificity, and complications associated with some lines include Cre expression in the absence of induction, germ line expression, and low recombination efficiencies.
| Marker / Mouse Line | Function | Expression in CFs | Expression in Other Cell Types Found in the Heart | References |
|---|---|---|---|---|
| α-Smooth Muscle Actin | Contractile intermediate filament-associated protein | Expression limited to subset of activated CFs in fibrotic regions | Epicardium, smooth muscle cells, pericytes and cardiac muscle cells | 4, 8, 46, 68 |
| Collagen 1a1 | Extracellular matrix protein | Majority of CFs | Epicardium, adventitia of large vessels and valve interstitial cells | 4, 47, 65–67 |
| Discoidin Domain Receptor 2 | Tyrosine kinase receptor for several ECM proteins | Limited expression in resident CFs | Limited expression in epicardium | 9, 42 |
| Fibroblast-Specific Protein 1 | Calcium-binding protein Intermediate filament-associated protein |
Activated fibroblasts in ischemic and non-ischemic heart failure models (typically perivascular) | Endothelial, smooth muscle and immune cells | 4, 43–46 |
| Periostin | Matricellular protein secreted by activated CFs | CFs in development Re-expressed in activated CFs following injury |
Epicardium, vascular smooth muscle cells and valve interstitial cells | 17, 44, 69–77, 255 |
| Platelet-Derived Growth Factor Receptor-α | Mitogenic tyrosine kinase receptor | CFs during development and following injury | Epicardium | 47, 57, 61, 63 |
| The Transcription Factor 21 | Regulates mesenchymal cell transitions | Resident CFs in development and following myocardial injury | Epicardium | 47, 55, 56, 237 |
| Thymus Cell Antigen 1 | Membrane glycoprotein for cell adhesion | Limited expression in quiescent resident CFs | Endothelial cells, pericytes and immune cells | 11, 47–50 |
| Vimentin | Intermediate filament protein | CFs in healthy and injured heart | Endothelium | 2, 51–53 |
Among the first markers used to identify CFs was discoidin domain receptor 2 (DDR2), which acts as a receptor for several ECM proteins.42 While expression is limited in other cell types, including endothelial cells, smooth muscle cells and myocytes, it does not appear that all CFs are positive for DDR2.9 Fibroblast-specific protein 1 (FSP1, S100A4), one of the initial markers identified, was believed to be a reliable fibroblast indicator.43 FSP1 was commonly used to designate quiescent and activated CFs in myocardial injury, including pressure overload and infarction-induced fibrosis models. However, recent in vivo work utilizing FSP1-GFP and FSP1 immunostaining has demonstrated that it is not fibroblast-specific, based on positive expression in several cell types, including a subset of immune and endothelial cells.4, 44–46 Another marker used to identify CFs is Thymus cell antigen 1 (Thy1, CD90), a membrane glycoprotein expressed on the surface of CFs.47 While this receptor does appear to be expressed on all CFs, other cell types are known to express Thy1, including immune cells, lymphatic endothelial cells and pericytes.48–50 Vimentin is an intermediate filament protein that has been used extensively both in vitro and in vivo for the identification of fibroblasts, as it is expressed in the majority of fibroblasts both in the healthy and injured myocardium.2 While CFs are positive for vimentin, concerns with its expression in other cell types, including the endothelium, restrict its efficiency and specificity.51–53 In general, the major complications associated with many of the markers currently in use involve either expression in other cell types or limited expression in all forms of CFs.5, 54
The transcription factor TCF21 has been used successfully to trace the development of resident CFs from their epicardial precursors.47 In addition to its extensive expression during development, TCF21 is also broadly expressed in CFs both within interstitial and perivascular fibrotic regions in response to heart failure models of both pressure overload and ischemic etiologies.55 Importantly, TCF21 is not expressed in infiltrating immune cells identified by positive CD45 expression.55 Additionally, the TCF21 transcription factor appears to be required for CF cell fate determination in development.47 The inducible TCF21mERCremER transgenic mouse line represents one of the more powerful inducible lines for the study of CFs in development and disease.56 In development, TCF21 is essential for the formation of CFs in utero,47 and the TCF21 signal remains active in adult mice, which enables this line to provide significant insight into the contribution of resident fibroblasts to the development of fibrosis in the injured heart. Platelet derived growth factor receptor alpha (PDGFRα) has emerged as a reasonable marker of CFs both during development and in healthy and injured adult tissues.47, 57 It is also expressed in multiple other organs including lung fibroblast lineages,58 oligodendrocyte progenitors,59 and bladder interstitial cells.60 In the heart, PDGFRα is robustly expressed in fibroblasts, with relatively limited expression in other cell types, including smooth muscle cells61 and possible expression in cardiac progenitor cells;62 it has been utilized for the development of an inducible Cre system, the PDGFRα-GFPCreERT2 knock-in mouse line.63 This will likely prove a useful tool in characterizing CFs and their role in fibrosis. PDGFRβ has also been used as a marker of fibroblasts. However, expression of PDGFRβ has been observed in numerous other cell types including smooth muscle cells, pericytes, neurons, kidney mesangium, myoblasts and muscle lineages, thus restricting its specificity and efficiency.64
As CFs are likely the principal cells responsible for the secretion of ECM proteins, a new line of thinking involves the identification of CFs by the expression of markers of ECM production. As the predominant protein of the myocardial ECM, Collagen Iα promoter sequences linked to Green Fluorescent Protein (GFP) has been used to create a reporter mouse for identification of CFs.4, 47, 65 An inducible form of the Collagen1α2 Cre driver has also been developed, allowing for both genetic lineage tracing of Collagen1α2 Cre positive cells and conditional gene deletion for functional studies.66 However, the expression of collagens is increasingly recognized as not being restricted solely to the fibroblast lineage. Positive Collagen 1a1-GFP expression has been reported within cells of both the epicardium and adventitia of large vessels,4, 67 suggesting caution in the interpretation of fibroblast-centric conclusions from studies utilizing these lines.
In addition to the molecular markers discussed above, several are expressed following the transition to the activated MF. Identification of MFs in many tissues has almost universally relied on the expression of the contractile protein αSMA.8, 46, 68 However, recent work using a pressure-overload model of heart disease showed that this marker is restricted to a subset of activated fibroblasts and is not expressed in all fibroblasts associated with fibrosis.4, 46 Periostin (Postn) is an ECM protein expressed in development, which is also robustly upregulated by activated CFs following injury.44, 69–72 A Postn-Cre transgenic mouse line has been developed that possesses an EGFP/Cre fusion expression vector under the control of the endogenous periostin promoter that is expressed in resident CFs and epicardial cells in the developing heart,17, 73–75 making it well suited to developmental investigations.76 As Postn-Cre is robustly expressed within activated CFs/MFs and myocardial infarct sites following injury,75–77 it presents one of the more promising tools for lineage tracing and genetically manipulating CFs and MFs.75 The development of an inducible knock-in system in Postn expressing cells will likely be an enormously effective tool for the study of activated fibroblasts in disease. Of note, interstitial cells of the valves may also express some of the ECM proteins that mark fibroblasts, such as Col1α and periostin.78 Overall, advancements in the identification and reliability of CF molecular biomarkers remain essential areas of research, and will eventually allow for a greater understanding of this critical cell type.
Sources of Cardiac Fibroblasts in Disease
The source(s) of activated CFs that accumulate in response to various pathological insults remains somewhat unclear. In other organs, including the lung, kidney and liver in particular, the cellular and molecular mechanisms of fibrosis, and the origins and characteristics of MFs have also been extensively studied, yet some ambiguity remains.79–83 Lineage tracing has recently been utilized in cardiac fibrosis studies and numerous precursors to the fibroblast population in the injured heart have been proposed, including resident fibroblasts, cells of vascular origin, hematopoietic cells and pericytes (Figure 2).
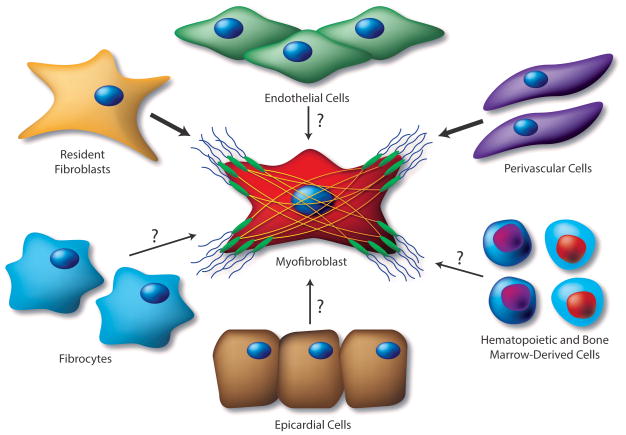
Figure 2. Proposed Sources of Cardiac MFs.
The source(s) of activated CFs that accumulate in response to various pathological insults remains under active investigation. Mounting evidence suggests that activated MFs in the fibrotic heart derive from the proliferation and activation of resident fibroblasts, as these cells are remarkably sensitive to pathologic insult and represent a feasible source of matrix-producing cells during cardiac fibrosis. Numerous additional precursors to the fibroblast population in the injured heart have been proposed; these include endothelial and epicardial cells, through EMT and EndMT, respectively, hematopoietic bone marrow-derived cells, perivascular cells and fibrocytes. While supporting evidence exists for cellular sources of activated fibroblasts denoted by question marks, controversy remains regarding the functional contribution of these cell types to the cardiac MF population. Biomarkers with greater specificity will be required to fully characterize the pathophysiologic relevance of these cell types in the cardiac fibrotic response, which will enable potential targeting for anti-fibrotic therapies.
Resident Fibroblasts
It is traditionally recognized that activated fibroblasts in the fibrotic heart derive from the proliferation and activation of resident fibroblasts, as these cells are remarkably sensitive to circulating pathologic stimuli.2, 4, 84, 85 This theory coincides with numerous studies in the kidney, which propose that following renal injury a majority of MFs originate from nephrogenic progenitors that give rise to resident fibroblasts as well as pericytes, and also discount the contribution of other cell types.79 Similarly, it is hypothesized that hepatic fibrosis is based on the activation of hepatic stellate cells and their transformation into MFs that express a broad spectrum of matrix components.80 The majority of resident CFs appear to arise from the embryonic epicardium, and recent lineage tracing studies suggest that these resident epicardial-derived CFs account for the majority of fibroblasts responsible for fibrotic response.2, 4 Studies have also revealed that cells of the endocardium, a specialized cardiac endothelial lining, undergo endothelial-mesenchymal transition (EndMT) during development to form the cardiac valves86 and resident fibroblasts of the interventricular septum.4 Proliferation of these endocardial-derived CFs has also been shown to contribute to the fibrotic response following pressure-overload.2 These studies also discount the contribution of cells other than resident fibroblasts to the development of fibrosis in a pressure overload model of heart failure, as well as in a model of parabiosis.2, 4 While these data contradict some prior studies, they indicate that MF accumulation following injury predominantly results from the proliferation of resident fibroblasts. In addition, resident fibroblasts have been suggested as a feasible source of matrix-producing cells during fibrosis, which could identify them as an important potential target for anti-fibrotic therapies. Several studies have evaluated the contribution of alternative sources to the activated fibroblast population in pathologic cardiac remodeling as individually outlined below.
Vascular Endothelium
Endothelial cells of the coronary vasculature have been proposed as contributors to the MF population following injury through EndMT. Lineage tracing studies utilizing the constitutive endothelial/hematopoietic restricted Tie1Cre mouse line have suggested that FSP1 positive cells contribute substantially to the fibroblast population in a pressure overload model of heart failure.46 These endothelial cells are believed to acquire a fibroblast-like phenotype and respond to pro-fibrotic stimuli in a manner similar to resident CFs.87, 88 This phenotypic conversion involves the migration of these cells into the interstitium, where they exhibit typical MF markers and are believed to contribute to the fibrotic response.46 However, recent studies have demonstrated that, in addition to fibroblasts, FSP1 also marks a substantial number of immune cells.4, 43–45 Furthermore, Tie1Cre is not specific to the endothelium, with fibroblasts being labeled in addition to immune cells and other cell types.89 In an attempt to resolve this controversy, several studies have since been performed using refined fibroblast markers, including tamoxifen-inducible Cre recombinase under the control of the vascular endothelial cadherin (VECad/Cdh5) promoter (VECad-Cre-ERT2), in concert with collagen-1α and PDGFRα as fibroblast markers.4, 90 This was complemented by a study using the Tie2Cre mouse line to label and identify cells with endothelial origins.2, 91 These studies concluded that, at least in the pressure overload model of heart failure, the endothelium does not significantly contribute to the MF population.
Epicardium
Resident fibroblasts of the cardiac interstitium are now generally recognized to derive predominantly from cells of the embryonic epicardium.92 During development, these cells undergo epithelial-mesenchymal transition (EMT) under the influence of several growth factors;12, 13, 93, 94 subsequently, a portion of these mesenchymal cells invade the myocardium to become the resident CFs.12, 13 Signals regulating the transition of epicardial cells to fibroblasts are tightly regulated and factors known to influence this transition can include fibroblast growth factors (FGFs) and members of the transforming growth factor β (TGF-β) superfamily.95 Once these cells have undergone EMT, platelet derived growth factor (PDGF) and TGF-β are thought to promote their transition into the CF phenotype.57, 93 As seen during development, a subset of epicardial cells can undergo EMT to generate CFs following an acute cardiac injury.96, 97 In pulmonary fibrosis, cells of the lung epithelium, particularly type II pneumocytes, play a key role in the initiation and propagation of fibrotic signaling in response to either endogenous or exogenous stress, leading to the activation of resident macrophages and fibroblasts.98 In addition, several studies have suggested that a heterogeneous pool of MFs may originate from EMT of cholangiocytes in hepatic fibrosis, however new work has demonstrated that EMT may not play a significant role.99, 100 Similarly, recent studies have indicated that cells of epicardial origin may contribute to MF accumulation following myocardial infarction to aid in the fibrotic response;96, 97 however, EMT does not appear to be functionally significant in the pressure-overload model of heart failure.4 These cells may present an interesting source of activated CFs, but more work is needed to characterize the particular functional contribution(s) of this cell type in vivo.
Perivascular Cells
Pericytes, which lie in the perivascular space of cardiac vessels, can differentiate into collagen-producing cells in models of dermal scarring,101 and may contribute to the fibroblast population following cardiac injury. This cell type has been defined in other tissues, including the retina and kidneys, where they have demonstrated phenotypical and functional overlap with fibroblasts,102, 103 however they are more thoroughly characterized in the central nervous system.104 Pericytes surround the neurovasculature and interact with other cell types including astrocytes and endothelial cells. Here they maintain the blood-brain barrier and regulate angiogenesis, immune responses and scar formation, which are affected by factors such as PDGF and TGF-β.104 The integrity of retinal microvessels is particularly dependent on pericytes, as loss of retinal pericytes contributes to diabetic retinopathy through the formation of microaneurysms.104 Recent work has revealed the presence of Gli1+ mesenchymal stem cell (MSC) – like cells within cardiac perivascular regions. Lineage tracing studies demonstrated that the proliferation of these resident cells following kidney, lung, liver or cardiac injury generates MFs that contribute to organ fibrosis. Furthermore, ablation of these cells ameliorates cardiac fibrosis and preserves overall cardiac function in a pressure overload model of heart failure, and attenuates renal fibrosis following unilateral ureteral obstruction injury.105 While these Gli1+ cells may not represent a distinct mesenchymal cell population, as they are also positive for a number of other markers including PDGFRα, these studies do indicate that perivascular cells appear to be an important source of cardiac MFs and may represent a potential therapeutic target for ameliorating cardiac and general organ fibrosis.105
Hematopoietic Bone-Marrow Derived Progenitor Cells
Bone marrow-derived progenitor cells are considered a potential source of fibroblasts in the fibrotic heart. This assertion is based on studies of GFP-labeled bone marrow transplants, which identified GFP-expressing cells within fibrotic regions following both pressure overload and ischemic myocardial injuries.46, 106, 107 It has been suggested that bone marrow-derived cells may account for up to 60% of all fibroblasts within the site of cardiac injury. However this cell population is significantly reduced after the initial reparatory process and therefore is unlikely to contribute to a persistent fibrotic response.107 CD45-positive monocytes have been identified as a potential source of fibroblasts, as they appear to co-express MF markers108 and inhibition of monocyte recruitment diminished the CF population and myocardial remodeling following MI.109
Several studies have proposed that infiltrating cells are instead likely to consist of a specific phenotype of inflammatory cells. Both a lineage tracing study using the hematopoietic specific Vav-Cre line and a study performing genetically labeled bone marrow transplants incorporating more specific markers of fibroblasts have refuted this idea, demonstrating that the contribution of circulating hematopoietic cells to the CF population is minimal.2, 4 Future studies will determine whether these data from a pressure-overload heart failure model will extend to models of ischemic heart failure etiology.
Fibrocytes
The contribution of cells of hematopoietic origin to the fibroblast population is also under intense investigation following the identification of fibrocytes in the circulation.110 In fact, subsequent studies have reported the contribution of these fibrocytes to the CF population, as well as to the development of cardiac fibrosis in several injury models.46, 107, 108, 111, 112 Their pathophysiologic relevance to cardiac fibrosis has been suggested in studies linking the inhibition of fibrocyte recruitment to reduced fibrosis and remodeling.108 Fibrocytes represent a unique fibroblast progenitor population that co-express fibroblast markers, such as procollagen I and vimentin, along with typical hematopoietic markers.113 These circulating fibrocytes appear to originate from hematopoietic stem cells in the bone marrow,113 however their pathophysiologic relevance in cardiac disease and fibrosis has yet to be fully characterized.
Alternate Growth Substrates
Mechanical stretching of the CF, which potentially occurs secondary to cardiac dilatation or changes in the hemodynamic burden of the heart, may induce expression of pro-fibrotic cytokines and expression of ECM proteins and receptors on CFs.114 Mechanical stress could play a contributory or preeminent role for the spontaneous activation observed when CFs are cultured on the stiff surface of standard tissue culture plastic. The elasticity (Young’s Modulus) of most cell culture plastic is >1 GPa, and for glass, this measure exceeds 70 GPa. In contrast, the Young’s Modulus of the normal heart is estimated to be less than 10 kPa. Thus, the natural growth environment for CFs is many orders of magnitude more elastic than standard cell culture conditions. Attempts to circumvent the issue of spontaneous activation in standard cell culture conditions have focused predominantly on the modulation of growth substrate tensile modulus. Polyethylene glycol-based hydrogels with a physiologically relevant elasticity (~7kPa) have shown promise in preserving the quiescent fibroblast phenotype of valvular interstitial cells when compared to standard tissue culture polystyrene. Cells grown on this substrate exhibited reduced levels of typical MF markers, including α-SMA and connective tissue growth factor (CTGF).115, 116 The PI3K/AKT pathway is upregulated when these cells are mechanically activated by stiff substrates, and activation of this pathway was significantly attenuated in cells grown on the more compliant substrate.116 Using tissue culture substrates with a physiologically relevant tensile modulus will aid in the characterization of fibroblast biology in vitro.
Therapeutic Targets in Cardiac Fibrosis
Numerous signaling pathways have been implicated in the early activation of CFs as well as in the pathologic remodeling that persists long after the initial injury. Modulation of these signals is of intense scientific interest as they represent potentially novel therapeutic targets or strategies. Here we explore mediators and signaling pathways known to influence fibroblast function after myocardial injury as well as developing therapeutic strategies to combat pathogenic cardiac fibrosis (Table 2; Figure 3).
Table 2.
Therapeutic Targets and Approaches in Cardiac Fibrosis
| Target | Approach | References |
|---|---|---|
| Transforming Growth Factor-β | TGF-β Receptor 1 (ALK5) Inhibition TGF-β Neutralizing Antibodies TGF-β-Activated Kinase (TAK) 1 Inhibition p38 Inhibition |
126–129, 135, 138–140 |
| Angiotensin System | Angiotensin II Receptor Blockers (ARBs) Angiotensin Converting Enzyme (ACE) Inhibition |
152–161 |
| Endothelin System | Endothelin Receptor Antagonists | 136, 173–178 |
| RhoA-SRF-MRTF Pathway | Serum Response Factor Inhibition RhoA Inhibition |
135, 256 |
| Transient Receptor Potential Channels | TRPC6 Inhibition Calcineurin Inhibition |
135, 188 |
| Connective Tissue Growth Factor | CTGF Neutralizing Antibodies | 195 |
| Platelet Derived Growth Factor | PDGF Receptor Antagonists PDGF Receptor Neutralizing Antibodies |
201, 202 |
| Integrins | αVβ1 Integrin Inhibition (Lung and Liver) | 207 |
| Inflammatory Signaling | Interferon-γ Inhibition | 215, 216 |
| Cardiomyocyte Regeneration | Induced Pluripotent Stem Cell Transplantation Fibroblast Reprogramming |
234, 236, 237, 239 |
| Electrical Communication | Cx43 – Expressing Cardiomyocyte Engraftment | 254 |
| Adrenergic System | Gβγ-GRK2 Inhibition (Paroxetine, βARKct and Gallein) RhoA Inhibition β-arrestin Inhibition |
224–233 |
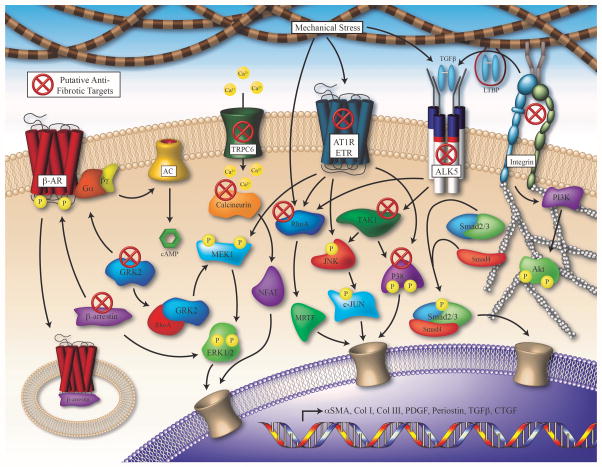
Figure 3. Selection of Signaling Pathways Regulating MF Activation and Potential Therapeutic Targets for Cardiac Fibrosis.
Numerous signaling pathways have been implicated in the activation of cardiac fibroblasts and the induction of pathological remodeling; targeting these signals is of intense scientific interest toward development of novel therapeutic strategies. Cardiac injury promotes the activation of receptors such as the β-AR, ALK5, AT1R, ETR, TRPC6 and Integrins, which induces pathologic signaling through numerous mediators, leading to the transcription of factors that regulate MF activation and fibrotic remodeling. Proposed antifibrotic therapeutic targets are marked with a red X. (β-AR: β-adrenergic receptor, AC: adenylate cyclase, TRPC6: transient receptor potential channel C6, AT1R: type 1 angiotensin II receptor, ETR: endothelin receptor: TGF-β: transforming growth factor β, LTBP: latent TGF-β binding protein, ALK5: activin receptor-like kinase 5 / TGF-β receptor, GRK2: g protein-coupled receptor kinase 2, ERK1/2: extracellular regulated kinase 1/2, MEK1: mitogen activated protein kinase kinase 1, NFAT: nuclear factor of activated T-cells, MRTF: myocardin-related transcription factor, TAK1: TGF-β – activated kinase 1, JNK: c-JUN N-terminal kinase, PI3K: phosphoinositide 3-kinase).
Transforming Growth Factor β
The TGF-β family of growth factors is perhaps the most extensively studied mediator of fibroblast activation, of which TGF-β1 is likely to play the greatest role in pathological fibrosis.117 TGF-β is known to play a major role in regulating fibrotic processes in many organs, and in fact, serum levels are utilized as diagnostic tools and also correlate with the severity of chronic liver and kidney diseases.81 TGF-β1 is initially secreted in a complex with latent TGF-β binding proteins that restrict its activity; this complex is proteolytically cleaved and can be activated in an integrin-mediated process.118, 119 The Type I TGF-β receptor, which is also known as activin receptor-like kinase (ALK) 5, represents the subtype thought to be predominantly responsible for the fibrotic activities of TGF-β1. The canonical pathway of TGF-β1 signaling involves the phosphorylation of Smad2/3, which subsequently bind Smad4 and translocate to the nucleus. The complex then acts as a transcription factor, inducing the activation of numerous pro-fibrotic genes.120, 121 The ability of TGF-β to induce the production of ECM proteins in vitro is thought to depend upon Smad3,120–122 and furthermore, fibroblasts isolated from Smad3-deficient mice appear resistant to TGF-β1-induced expression of ECM proteins.123, 124 Perhaps equally detrimental to overall cardiac function as the initial infarct, the canonical TGF-β pathway is also activated in the infarct border and remote zones where it mediates the formation of pathological reactive fibrosis.120–122 Finally, the persistence of activated fibroblasts is believed to be the result of perpetual TGF-β signaling, which may prevent CF apoptosis following the initial injury through Smad-mediated pathways.125
Inhibitors of the TGF-β receptor ALK5 are currently under investigation as potential antifibrotic mediators. ALK5 inhibitors can decrease TGF-β activity, rescuing cardiac dysfunction and ameliorating the remodeling that occurs post-MI.126 These inhibitors can also attenuate the development of fibrosis and expression of collagen following pressure overload by transverse aortic constriction, and reduce the expression of collagen in response to TGF-β stimulation in vitro.127 Several groups are also evaluating TGF-β neutralizing antibodies in experimental myocardial fibrosis models. While fibroblast activation and collagen transcript levels were reduced, there were no substantial improvements in overall cardiac function,128 and there is evidence that this strategy may actually increase mortality.129 While disparate, these results may have been the result of the disruption of the initial reparatory response leading to a reduction in cardiac function and increased mortality. Similar contradictory results have been observed in studies investigating TGF-β inhibition in animal models of chronic kidney disease (CKD). While there is a significant body of evidence in preclinical trials showing antifibrotic effects of TGF-β inhibition,130 the clinical translation of these studies has been limited. Furthermore, recent data show that TGF-β plays a role in renal autophagy as a cytoprotective mechanism,131 which suggests that therapies inhibiting TGF-β1 activity should be approached with caution. While inhibition of the canonical TGF-β signaling pathway remains appealing, this approach may require further investigation and refinement before it will have significant clinical impact.
In addition to the Smad-mediated pathways, TGF-β can also induce non-canonical signaling that involves several mitogen-activated protein kinases (MAPKs), including c-Jun N-terminal kinase (JNK) and p38.132–134 This pathway involves the activation of TGF-β – activated kinase (TAK) 1, which is believed to contribute to pathological cardiac remodeling, since cardiac overexpression of constitutively active TAK1 does induce cardiac hypertrophy and heart failure.132–134 While the MF-activating properties of TGF-β have previously been attributed to the canonical signaling pathway, growing evidence suggests the non-canonical pathway may actually be the dominant driving force.135, 136 Thus, attempts to prevent this non-canonical signaling may prove efficacious for the treatment of fibrosis and heart failure. The TGF-β non-canonical signaling pathway is believed to propagate primarily through the Type II TGF-β receptor (TGFβRII), given that CM-specific deletion of the TGFβRII has resulted in reduced fibrosis and remodeling in the TAC model of heart failure.137 As the main effector of the TGF-β non-canonical pathway, TAK1 also represents a viable therapeutic target, as inhibition of TAK1 reduced TGF-β – induced fibroblast ECM protein production.138 Further downstream, inhibition of p38 is being actively investigated for its antifibrotic potential. Inhibitors of p38 signaling can attenuate TGF-β-induced MF activation and reduce the expression of collagen, fibronectin and αSMA in mouse embryonic fibroblasts (MEFs); moreover, overexpression of p38 can induce the MF transition.135 In vivo, inhibition of p38 reduces fibrosis and the expression of αSMA following MI.139, 140 Several clinical trials evaluating the efficacy of p38 inhibition have begun in patients following acute MI. In the SOLSTICE Phase II trial, the p38 inhibitor losmapimod was well tolerated in non-ST segment elevation MI (NSTEMI) patients with no major liver toxicities observed. Although infarct size was non-significantly reduced, patients receiving losmapimod experienced fewer cardiac events.141 These findings provide strong evidence for the therapeutic potential of targeting the TGF-β non-canonical signaling pathway, specifically through modulation of TAK1 and p38, in the treatment of cardiac fibrosis.
Renin Angiotensin System
The renin-angiotensin-aldosterone system (RAAS), of which Angiotensin II (AngII) appears to be the predominant effector, promotes many physiological and pathological functions, including the development of cardiac fibrosis.142, 143 The AngII type 1 receptor (AT1R) mediates many of the effects of AngII in fibroblasts, including cell proliferation, migration and the induction of ECM protein synthesis.144, 145 Cardiac AngII levels are quickly elevated following injury, and stimulation of CFs by AngII induces proliferation and the expression of collagen.146, 147 Renal RAAS activity, which is reflected by urinary angiotensinogen levels, directly correlates with the extent of renal fibrosis and deterioration of renal function in patients with CKD.148 AngII is also intimately involved with the inflammatory response, as it is expressed and activated by macrophages and MFs.149 It is believed that AngII is also involved with TGF-β signaling, both in CMs and CFs. Specifically, AngII activation of ATR1 induces the expression TGF-β1, and it is thought that TGF-β is required for AngII to induce both cardiac hypertrophy and fibrosis.150, 151 Furthermore, AngII-induced expression of collagen in CFs requires TGF-β/Smad and MAPK signaling.146, 149
Hemodynamic burden, chronic fibrotic remodeling and tissue stiffening following a myocardial injury can stimulate the release of endogenous AngII from CM stores, however it is now recognized that this mechanical stress can contribute directly to the activation of the AT1R.152 While HEK293 and COS7 cells do not natively respond to mechanical stretch, expression of the AT1R confers this ability, as seen by an increase in the activation of ERK, suggesting that the AT1R is itself a mechanical sensor. Mechanical stretching of CMs isolated from ATG null mice, which do not express endogenous AngII, also resulted in ERK activation. Additionally, cardiac hypertrophy in ATG−/− mice was induced by pressure overload, indicating that mechanical stress can induce hypertrophy even in the absence of AngII.152 Of note, these mechanical sensing abilities appear to be specific to the AT1R, as other GPCRs do not respond to mechanical stretch.152 A similar phenomenon occurs in CFs, as these cells are highly susceptible to variations in their mechanical microenvironment.21 This stretch-induced activation of the AT1R can be inhibited by the AngII receptor blocker candesartan,152 revealing a potential antihypertrophic and antifibrotic role for this class of drugs. As candesartan will reduce blood pressure and thus cardiac load, the mechanism by which it attenuates fibrosis remains unclear and may not necessarily be due to direct inhibition of pathologically activated cardiac fibroblasts.
While not approved for the treatment of cardiac fibrosis, inhibitors of angiotensin signaling, including angiotensin converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs), have already demonstrated significant efficacy in the treatment of heart failure, frequently reducing cardiac fibrosis both in humans and animal models.153–155 One such angiotensin receptor inhibitor, losartan, is believed to reduce cardiac fibrosis through the inhibition of EndMT, as was demonstrated in mitral valve endothelial cells, by acting to block the AngII-elicited, TGF-β – induced phosphorylation of ERK1/2.156 Losartan reduced collagen I synthesis and fibroblast activation in response to stimulation by AngII.157 Stimulation of the AT1R promotes a pro-inflammatory environment, and losartan can decrease the expression of inflammatory markers, including TNF-α, IL-1β and IL-6.158–160 Clinically, losartan is thought to attenuate the progression of myocardial hypertrophy and fibrosis in patients with hypertrophic cardiomyopathy.161 These data present a compelling argument that AngII is a potent fibrotic mediator, and that the protective effects of AngII antagonists may partly be due to their ability to reduce cardiac fibrosis. Further studies will be needed to mechanistically establish the effects of AngII inhibition specifically in CFs, which may lead to refinement of ACE/AngII/receptor inhibitors and allow for more direct targeting of the pathway’s pathologic activation of this cell population.
Endothelin
The endothelin family of peptides is typically recognized for its vasoconstrictive properties, and is now beginning to be appreciated for its potential role in promoting tissue fibrosis. While originally believed to be secreted predominantly by endothelial cells, Endothelin-1 (ET-1) is now understood as a significant pro-fibrotic peptide released by both inflammatory cells and fibroblasts in the lung.162 The endothelin system also plays a major role in kidney disease, as ET-1 production is stimulated in disease downstream to both the sympathetic nervous system and RAAS.163 There exist two known receptors for ET-1 in the heart, the Endothelin-A (ETA) and Endothelin-B (ETB) receptors, which have been shown to play differing and sometimes opposing roles. These receptors are primarily expressed by endothelial cells, however new data define expression on multiple cell types including CMs and CFs, along with some immune cells, such as macrophages.164, 165 Importantly, ET-1 activation of the ETA receptor is known to increase collagen production in isolated human CFs.166 Furthermore, MFs isolated from scar tissue following experimental MI possess elevated levels of ET-1, suggesting an important function for endothelin within these cells.167 While known to have fibroblast-activating properties of its own, ET-1 also acts as a downstream mediator of both AngII and TGFβ to promote MF persistence in pulmonary fibrosis.136, 162, 168 ET-1 signaling is thought to interact with AngII as well. For example, the development of cardiac fibrosis and hypertrophy in response to AngII stimulation is impaired in mice in which ET-1 is ablated in endothelial cells. It is believed these factors are mechanistically linked through the activation of ERK1/2 and the myocardin-related transcription factor (MRTF) A.169–172
Endothelin antagonists are currently approved for the treatment of pulmonary hypertension, and many believe they will be additionally efficacious in the treatment of pathologic fibrosis in the heart.136 Endothelin receptor blockade has been incorporated extensively both experimentally and in the clinic for its salutary role in the treatment of hypertension. Bosentan, a nonselective endothelin receptor antagonist, while used clinically for the treatment of pulmonary hypertension, can improve cardiac function and reduce infarct size following myocardial ischemia/reperfusion injury in rats.173 Preliminary trials in humans had demonstrated beneficial hemodynamic and cardiac effects in patients with end stage heart failure.174 However subsequent clinical trials evaluating ET receptor antagonists on coronary artery disease and heart failure have not met primary endpoints, perhaps due to inefficient selection of potential responders prior to the initiation of major clinical trials.175–178 While the reasons for these discrepancies remain unclear, manipulation of ET-1 signaling appears promising. Next generation ETR subtype-specific antagonists, such as ambrisentan and darustentan that are proving to be highly beneficial in the treatment of pulmonary hypertension, may prove more efficacious and help define receptor specificity that may be required for salutary effects. Further studies will be necessary to determine if ET-1 and its receptors will become clinically viable antifibrotic therapeutic targets.
RhoA-MRTF-SRF Signaling Pathway
Serum response factor (SRF), a member of the MADS box-containing family of transcription factors, is critically involved in the regulation of MF activation, given that numerous SRF binding sites are located within the promoter regions of genes responsible for fibroblast activation.179, 180 Experiments in bleomycin-injured lung tissue have demonstrated upregulation of SRF gene expression in isolated MFs,181 and overexpression of SRF can induce the transformation of both lung and cardiac fibroblasts.135, 181 SRF interacts with cofactors such as members of the MRTF family of transcription factors, which translocate to the nucleus and bind SRF to regulate its transcriptional efficacy.182 In CFs, MRTF-A overexpression alone can induce the MF transition and the expression of αSMA; conversely, MRTF-A ablation in vivo reduces the fibrotic response following MI.182 SRF activation occurs downstream of stimulation by TGF-β, AngII and ET-1, and knockdown of SRF by siRNA can inhibit MF activation in response to these stimuli.135 The Rho family of GTPases is believed to mediate the activation of the SRF-MRTF signaling pathway; specifically, RhoA signaling is activated downstream of the TGF-β receptor, several G-protein coupled receptors (GPCR) and mechanical tension.183–185 Thus, TGF-β stimulation in fibroblasts can simultaneously activate Rho-MRTF-SRF signaling along with its canonical and non-canonical signaling pathways. Perhaps the inhibition of RhoA, using compounds such as fasudil, presents a potential antifibrotic strategy that complements those described above.
Transient Receptor Potential Channels
Receptor-activated Ca2+ entry is induced following the activation of G protein-coupled receptors and receptor tyrosine kinases, including the AT1R or TGF-β receptor, leading to the activation of calcineurin and nuclear translocation of the nuclear factor of activated T-cells (NFAT) family of transcription factors.186 The transient receptor potential (TRP) proteins are the channels responsible for this receptor-mediated Ca2+ entry; the canonical TRP (TRPC) channels specifically couple receptor-phospholipase C (PLC) signaling pathways to Ca2+ entry,187 and are upregulated in mice subjected to myocardial pressure overload.188 CM-specific overexpression of TRPC6 resulted in cardiomyopathy and promoted NFAT-dependent promoter activity. Furthermore, siRNA knockdown of TRPC6 reduced hypertrophic signaling in response to ET-1, suggesting a role of TRPC6 in the GPCR-dependent activation of the calcineurin-NFAT pathway.188
Recently, the TRPC6 channel was identified in an unbiased genome-wide screen as an important regulator of MF activation.135 In primary fibroblasts, TRPC6 overexpression induced the expression of MF marker genes, and genetic ablation conferred resistance to TGF-β- and AngII-dependent MF transformation.135 Furthermore, overexpression of a constitutively active calcineurin in CFs induced the MF transition, while inhibition of calcineurin blocked TRPC6-mediated transformation, indicating a functionally-relevant interaction of these proteins in CFs.135 These findings have revealed TRPC6-mediated Ca2+ entry and activation of the calcineurin/NFAT signaling pathway as novel regulatory mediators in the activation of fibroblasts, and suggest that their inhibition may represent an important antifibrotic therapeutic strategy.
Connective Tissue Growth Factor
CTGF belongs to the CCN family (the acronym comes from the first three members of the family that were found: CYR61 (cysteine-rich angiogenic inducer 61 or CCN1), CTGF (connective tissue growth factor or CCN2), and NOV (nephroblastoma overexpressed or CCN3) of matricellular proteins, which are dynamically expressed non-structural proteins of the ECM.189 This family of proteins is believed to play a significant regulatory role in the ECM to modulate cell surface receptors and their responses to cytokines, growth factors and proteins of the ECM.189 CTGF is a well-characterized mediator of TGF-β activity during the fibrotic response, and its expression is induced in fibroblasts stimulated by TGF-β.190, 191 Furthermore, CTGF is strongly upregulated both in human heart failure and animal models associated with myocardial fibrosis.190, 192 Interestingly, induction of CTGF following injury has been observed prior to the upregulation of TGF-β or deposition of ECM proteins, suggesting an important role in these processes.193 CTGF also appears to play a significant role directly in isolated CFs, as evidenced by a reduction of various chemokines, matrix metalloproteinases, ECM proteins and cell adhesion proteins in CFs in which CTGF is ablated using siRNA.194 While studies targeting CTGF in animal models of heart failure are relatively limited, preliminary studies in a pressure overload model have suggested efficacy of CTGF neutralizing antibodies in reducing cardiac remodeling and dysfunction, concomitant with attenuated collagen deposition.195 These data indicate that the pursuit of therapies targeting CTGF may be of clinical interest for the treatment of cardiac fibrosis.
These data are in stark contrast to a recent study investigating the mechanism of CTGF and definitively characterizing its role in cardiac injury. Several transgenic mouse lines were developed that included heart-specific CTGF knockouts and overexpressors in combination with constitutively active TGF-β and fibroblast-specific CTGF knockout mice. Surprisingly, neither gain nor loss of CTGF affected overall cardiac function or the fibrotic response in multiple models of cardiac injury. However, modulation of CTGF did appear to slightly impact the response to TGF-β in a pressure overload model, suggesting a potential interaction between these factors.196 As described above, CTGF is believed to play an important functional role with TGF-β to regulate the fibrotic response, but recent data suggest that CTGF is of limited importance as a TGF-β effector and thus may no longer represent an important therapeutic target for the treatment of cardiac fibrosis and heart failure.
Platelet Derived Growth Factor
The PDGF family of growth factors is a group of proteins originally recognized for their ability to regulate cell proliferation in smooth muscle cells. Expression of the PDGF family is significantly increased in endothelial cells, macrophages and MFs following MI.197 Enhanced expression of both the ligands and receptors occur concomitantly with inflammatory and fibrogenic responses following MI, identifying a potential role in the regulation of cardiac repair.197 Stimulation by PDGF is known to enhance CF proliferation, and also enhances TGF-β expression in vitro.198, 199 Similarly, cardiac-specific overexpression of PDGF significantly elevates TGF-β1 transcription and promotes the development of cardiac fibrosis.200
The disruption of PDGF signaling is currently under active investigation for the treatment of myocardial fibrosis. The tyrosine kinase inhibitor Imatinib, typically recognized for its anti-tumorigenic properties, can also suppress PDGF signaling by acting as an antagonist against the PDGF receptor. In a MI model of heart failure, Imatinib reduced the expression of fibrogenic mediators, including TGF-β and collagen I, significantly reduced fibrotic scar formation and mildly rescued cardiac dysfunction.201 A neutralizing PDGF receptor antibody has also been shown to attenuate atrial fibrosis in a pressure overload model of heart failure.202 Collectively, these data implicate PDGF in the development of cardiac fibrosis, likely through the induction of TGF-β, and additionally present PDGF inhibition as a potential approach for antifibrotic therapy.
Integrins
Integrins are transmembrane proteins comprised of α and β subunits that mediate interactions between the extracellular environment and the actin cytoskeleton. CFs express a wide variety of integrin subtypes that mediate multiple cellular functions including proliferation, migration, adhesion, differentiation and apoptosis.203–205 Furthermore, it has been reported in cultured MFs that integrins are required for the activation of latent TGF-β.119 The integrin-mediated transformation of MFs is believed to occur through activation of MAPK signaling cascades, including ERK1/2 and p38.206 This regulation of TGF-β activation may be one potential mechanism causing the persistence of MFs within fibrotic scars, and may offer a target for ameliorating adverse myocardial remodeling following injury.118, 125 Strategies aimed at blocking the function of integrins have shown preliminary success in limiting maladaptive remodeling. For example, small molecule targeting of the αVβ1 integrin, which is highly expressed in activated fibroblasts, attenuated pulmonary and liver fibrosis through a mechanism involving the inhibition of TGF-β activation.207 It is possible that therapeutic approaches targeting various integrin subtypes could be therapeutically useful for the treatment of numerous diseases characterized by excessive tissue fibrosis.
Inflammation/Interferon Receptors
Inflammation is an essential mediator of the reparative response to an acute cardiac insult. Numerous inflammatory cells, including neutrophils and macrophages, infiltrate the site of injury where various pro-inflammatory cytokines are released, such as TNFα, Interleukin-1β (IL-1β) and IL-6, and play an important role in the initial induction of resident fibroblast proliferation and MF activation.208 In addition to their direct release of ECM components, activated CFs also express numerous cytokines and growth factors that affect wound healing via autocrine and paracrine mechanisms.209 Cytokine expression by CFs is markedly upregulated following acute myocardial injury, including the expression of the pro-inflammatory cytokines IL-1β and IL-6. IL-1β promotes fibroblast migration through increasing the expression of proteins involved in ECM turnover, including matrix metalloproteinases,6, 210 and IL-6 is known to increase fibroblast proliferation and myocardial fibrosis.211
Interferons (IFNs) are a family of cytokines that cause a myriad of biological responses and, in addition to immune cells, they can be secreted by other cell types such as CFs.212, 213 However, the role of IFNs in fibrosis, specifically IFN-γ, remains somewhat controversial.214 In IFN-γ knockout mice challenged with AngII, there was a reduction in the MF marker α-SMA.215 Similarly, mice null for the IFN-γ receptor (IFNGR) exhibit a decrease in cardiac hypertrophy and fibrosis, as well as a reduction in the infiltration of macrophages and T cells.216 Since extensive data have demonstrated a critical role of inflammation in the fibrotic response, inhibiting these inflammatory mediators may prove efficacious in the treatment of cardiac fibrosis.
GPCR/Adrenergic Signaling
The adrenergic system plays a fundamental role in the physiologic regulation of the myocardium, but chronic overstimulation can induce both cardiac hypertrophy and fibrosis.217 While several subtypes of the β-adrenergic receptor (β-AR) are expressed in the heart, the β2-AR appears to be the form that is predominantly expressed by CFs.218 Chronic stimulation of this receptor can induce cell proliferation, collagen secretion, migration and transformation to the MF phenotype.218
It is well established that acute stimulation of the β2-AR increases the levels of cAMP, which can modulate proliferation219 as well as inhibit the synthesis and secretion of various forms of collagen.220, 221 In addition, elevated cAMP levels can inhibit the transformation of CFs to MFs induced by stimulation with TGF-β.221, 222 Recent studies indicated that failing human CFs isolated from patients with heart failure had higher baseline collagen synthesis that was not inhibited by β-agonist stimulation. Furthermore, β-AR signaling was markedly uncoupled and associated with increased expression and activity of G protein-coupled receptor kinase 2 (GRK2).223 Similarly, knockdown or inhibition of GRK2 restored β-agonist-stimulated inhibition of collagen synthesis and decreased collagen synthesis in response to TGF-β stimulation, indicating a significant role for GRK2 in the regulation of collagen synthesis and maladaptive ventricular remodeling.223 GRK2 is also recognized for its role as a RhoA-activated scaffold protein for the ERK MAPK cascade, which may have implications in the development of hypertension,224 and also suggests potential interactions with profibrotic mediators, including TGF-β, AngII and ET-1. GRK2 is typically recognized for its role in the downregulation of β-ARs, predominantly through the recruitment of β-arrestin, which is also significantly upregulated in adult human CFs isolated from failing left ventricles.225 Enhanced β-arrestin signaling in CFs appears to be deleterious in that it promotes a pro-fibrotic phenotype via the uncoupling of β-ARs and potentiates ERK and Smad signaling downstream of TGF-β.225 Targeting GRK2, RhoA and β-arrestin may represent plausible therapeutic strategies for the prevention of myocardial fibrosis.
Several approaches for specifically targeting GRK2 have been developed, all of which have shown some promise in reducing the fibrotic response concomitant with protection against overall cardiac dysfunction. The selective serotonin reuptake inhibitor paroxetine was recently identified as an inhibitor of GRK2.226 Paroxetine is known to reverse left ventricular dysfunction following MI, and reduce both immediate infarct and remote region fibrosis, albeit at a dose substantially in excess of that used in humans.227 These data suggest that targeting GRK2 signaling may be a potent anti-fibrotic approach. The idea of inhibiting the interaction between GRK2 and G-protein βγ subunits has been investigated in several models of heart failure, using either small molecules or a truncated form of GRK2 known as βARKct. The βARKct peptide has shown promise in preventing myocardial dysfunction in both small and large animal models of heart failure.228, 229 The small molecule Gβγ-GRK2 inhibitor gallein has demonstrated an uncanny effect to improve cardiac function and reduce the fibrotic response in several murine models of heart failure.230–233 Of further interest, the introduction of gallein attenuated pathologic renal abnormalities, including renal dysfunction, tissue damage and fibrosis, that occur secondary to myocardial pressure overload injury (in press). While the mechanism of GRK2 and Gβγ-GRK2 inhibition within CFs has yet to be fully delineated, GRK2 represents an exceptionally important target for therapeutic interventions directed against myocardial fibrosis.
Fibroblasts for CM Regeneration
With the advent of stem cell-based therapeutics for the treatment of cardiovascular disease, there is now a growing interest in the development of reprogramming techniques to induce the transformation of fibroblasts into functional CMs. While still in its infancy, this technique relies on the creation of induced pluripotent stem (iPS) cells, which can be differentiated in vitro to potentially generate patient-specific CMs for subsequent introduction into the failing or infarcted heart.234 Newer methods are beginning to allow for the direct reprogramming of fibroblasts into CMs in vivo through the administration of a specific combination of transcription factors.234–237 Unfortunately, with these current approaches, the number of fibroblasts that successfully transition into healthy cardiac muscle cells in vivo still remains quite small, and some reports suggest this may not be a viable approach.238 Additionally, there is arrhythmogenic potential resulting from newly generated CMs that do not correctly integrate with the surrounding cardiac muscle.239 Perhaps with the development of more efficient delivery systems and identification of novel factors,240 this technology may eventually allow for manipulation of chronic scar tissue as well as the development of new CMs and potentially attenuate or even reverse post infarct remodeling.239
Electrical Communication
A new area of study that has previously been thought to be of little physiological relevance is the electrical communication between CFs and CMs. The relatively large membrane resistance of interstitial CFs renders these cells suitable for the conduction of electrical signals. Seminal ex vivo studies have demonstrated a robust physical communication with adjoining CMs, although the physiological relevance of such interactions in vivo is still under investigation.241, 242 It is now understood that CFs are capable of electrically coupling with CMs which influences their electrical properties,243 and this likely occurs through the action of connexin 43 (Cx43), the subtype predominantly expressed by CMs.244–246 The belief is this will allow CFs to form bridges linking regions of myocytes to aid in the synchronization of myocyte contractions.247–251
The development of fibrosis is known to disrupt the normal arrangement of myocytes and fibroblasts, and as such, has serious implications in the development of ventricular arrhythmias in patients following an acute myocardial injury. Modulation of the interactions between CFs and CMs may prove to be an interesting therapeutic approach.243, 248, 252, 253 Of note, the risk of post-infarction ventricular tachycardia is significantly reduced following the engraftment of embryonic CMs engineered to express Cx43, likely due to the improved electrical coupling between the surrounding myocardium and the infarct region.254 This therapeutic avenue will need to be approached cautiously though, as there are conflicting data regarding the effect of CF density on the impact of CF-CM coupling.32 While the targeting of CF-CM electrical coupling holds potential for the effective treatment of cardiac arrhythmogenesis, the variable responses in different clinical settings and intricacies of this signaling will require full examination before these types of interventions can become clinically viable.
Conclusions
It is abundantly clear that a deeper understanding of CFs will be critical to achieving exciting advancements in the treatment of cardiac fibrosis and disease. A limited knowledge of the role of this essential and dynamic cell type continues to hinder progress in the design and application of meaningful new therapies. The development of more specific in vivo approaches along with the identification of novel biomarkers will allow for greater investigation of this important cell population. Furthermore, unraveling the intricate mechanisms underlying this fibrotic signaling is of utmost importance for the development of effective therapies for the treatment of cardiac fibrosis. Therapeutic interventions targeting CFs and the pathologic fibrosis they promote will inevitably lead to significant advancements in the treatment of heart failure.
Acknowledgments
We wish to acknowledge Jeffrey Molkentin for his astute suggestions during the development of this review.
Sources of Funding
This work was supported by National Institutes of Health grants R01 HL091475, R01 HL129772, R01 HL132551, R01 GM097347, U54 HL119810 (BCB), and P01 HL069779 (BCB, JR and KEY), an American Heart Association Postdoctoral Fellowship (FAK), and a Predoctoral Fellowship from the Pharmaceutical Research and Manufacturers of America Foundation (JGT).
Abbreviations
- ALK
Activin receptor-like kinase
- CF
Cardiac fibroblast
- CKD
Chronic kidney disease
- CM
Cardiomyocyte
- CTGF/CCN2
Connective tissue growth factor
- CYR61/CCN1
Cysteine-rich angiogenic inducer 61
- DDR2
Discoidin domain receptor 2
- EMT
Epithelial-mesenchymal transition
- EndMT
Endothelial-mesenchymal transition
- ET-1
Endothelin-1
- ETA/ETB
Endothelin receptor A/B
- FSP1
Fibroblast specific factor 1
- IL-1β
Interleukin-1β
- IFN
Interferon
- iPS
Induced pluripotent stem cell
- JNK
c-Jun N-terminal kinase
- MF
Myofibroblast
- MRTF
Myocardin-related transcription factor
- MSC
Mesenchymal stem cell
- NFAT
Nuclear factor of activated T-cells
- NOV/CCN3
Nephroblastoma overexpressed
- NSTEMI
Non-ST segment elevation MI
- PLC
Phospholipase C
- Postn
Periostin
- αSMA
α-smooth muscle actin
- SRF
Serum response factor
- TAC
Transverse aortic constriction
- TAK
TGF-β – activated kinase
- Thy1/CD90
Thymus cell antigen 1
- TRPC
Canonical transient receptor potential channel
- VECad/Cdh5
Vascular endothelial cadherin
Footnotes
Disclosures
None.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu SM, Mackey RH, Matchar DB, McGuire DK, Mohler ER, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB, Comm AHAS, Subcomm SS. Heart disease and stroke statistics-2015 update a report from the american heart association. Circulation. 2015;131:E29–E322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Ali SR, Ranjbarvaziri S, Talkhabi M, Zhao P, Subat A, Hojjat A, Kamran P, Muller AMS, Volz KS, Tang ZY, Red-Horse K, Ardehali R. Developmental heterogeneity of cardiac fibroblasts does not predict pathological proliferation and activation. Circ Res. 2014;115:625–U681. doi: 10.1161/CIRCRESAHA.115.303794. [DOI] [PubMed] [Google Scholar]
- 3.Mikawa T, Gourdie RG. Pericardial mesoderm generates a population of coronary smooth muscle cells migrating into the heart along with ingrowth of the epicardial organ. Dev Biol. 1996;174:221–232. doi: 10.1006/dbio.1996.0068. [DOI] [PubMed] [Google Scholar]
- 4.Moore-Morris T, Guimaraes-Camboa N, Banerjee I, Zambon AC, Kisseleva T, Velayoudon A, Stallcup WB, Gu YS, Dalton ND, Cedenilla M, Gomez-Amaro R, Zhou B, Brenner DA, Peterson KL, Chen J, Evans SM. Resident fibroblast lineages mediate pressure overload-induced cardiac fibrosis. J Clin Invest. 2014;124:2921–2934. doi: 10.1172/JCI74783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krenning G, Zeisberg EM, Kalluri R. The origin of fibroblasts and mechanism of cardiac fibrosis. Journal of cellular physiology. 2010;225:631–637. doi: 10.1002/jcp.22322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Souders CA, Bowers SLK, Baudino TA. Cardiac fibroblast the renaissance cell. Circ Res. 2009;105:1164–1176. doi: 10.1161/CIRCRESAHA.109.209809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Camelliti P, Borg TK, Kohl P. Structural and functional characterisation of cardiac fibroblasts. Cardiovasc Res. 2005;65:40–51. doi: 10.1016/j.cardiores.2004.08.020. [DOI] [PubMed] [Google Scholar]
- 8.Porter KE, Turner NA. Cardiac fibroblasts: At the heart of myocardial remodeling. Pharmacol Therapeut. 2009;123:255–278. doi: 10.1016/j.pharmthera.2009.05.002. [DOI] [PubMed] [Google Scholar]
- 9.Banerjee I, Fuseler JW, Price RL, Borg TK, Baudino TA. Determination of cell types and numbers during cardiac development in the neonatal and adult rat and mouse. Am J Physiol-Heart C. 2007;293:H1883–H1891. doi: 10.1152/ajpheart.00514.2007. [DOI] [PubMed] [Google Scholar]
- 10.Zak R. Development and proliferative capacity of cardiac-muscle cells. Circ Res. 1974;35:17–26. [PubMed] [Google Scholar]
- 11.Pinto AR, Ilinykh A, Ivey MJ, Kuwabara JT, D’Antoni M, Debuque RJ, Chandran A, Wang L, Arora K, Rosenthal N, Tallquist MD. Revisiting cardiac cellular composition. Circ Res. 2015 doi: 10.1161/CIRCRESAHA.115.307778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gittenberger-de Groot AC, Peeters MPFMV, Mentink MMT, Gourdie RG, Poelmann RE. Epicardium-derived cells contribute a novel population to the myocardial wall and the atrioventricular cushions. Circ Res. 1998;82:1043–1052. doi: 10.1161/01.res.82.10.1043. [DOI] [PubMed] [Google Scholar]
- 13.Munoz-Chapuli R, Perez-Pomares JA, Macias D, Garcia-Garrido L, Carmona R, Gonzalez-Iriarte M. The epicardium as a source of mesenchyme for the developing heart. Marc Malpig Symp Ser. 2001;7:187–196. [PubMed] [Google Scholar]
- 14.Baudino TA, Carver W, Giles W, Borg TK. Cardiac fibroblasts: Friend or foe? Am J Physiol-Heart C. 2006;291:H1015–H1026. doi: 10.1152/ajpheart.00023.2006. [DOI] [PubMed] [Google Scholar]
- 15.Cleutjens JPM, Verluyten MJA, Smits JFM, Daemen MJAP. Collagen remodeling after myocardial-infarction in the rat-heart. Am J Pathol. 1995;147:325–338. [PMC free article] [PubMed] [Google Scholar]
- 16.Cohn JN, Ferrari R, Sharpe N, Remodeling IFC. Cardiac remodeling-concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J Am Coll Cardiol. 2000;35:569–582. doi: 10.1016/s0735-1097(99)00630-0. [DOI] [PubMed] [Google Scholar]
- 17.Lajiness JD, Conway SJ. Origin, development, and differentiation of cardiac fibroblasts. J Mol Cell Cardiol. 2014;70:2–8. doi: 10.1016/j.yjmcc.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kawaguchi M, Takahashi M, Hata T, Kashima Y, Usui F, Morimoto H, Izawa A, Takahashi Y, Masumoto J, Koyama J, Hongo M, Noda T, Nakayama J, Sagara J, Taniguchi S, Ikeda U. Inflammasome activation of cardiac fibroblasts is essential for myocardial ischemia/reperfusion injury. Circulation. 2011;123:594. doi: 10.1161/CIRCULATIONAHA.110.982777. [DOI] [PubMed] [Google Scholar]
- 19.van den Borne SWM, Diez J, Blankesteijn WM, Verjans J, Hofstra L, Narula J. Myocardial remodeling after infarction: The role of myofibroblasts. Nat Rev Cardiol. 2010;7:30–37. doi: 10.1038/nrcardio.2009.199. [DOI] [PubMed] [Google Scholar]
- 20.Calderone A, Bel-Hadj S, Drapeau J, El-Helou V, Gosselin H, Clement R, Villeneuve L. Scar myofibroblasts of the infarcted rat heart express natriuretic peptides. Journal of cellular physiology. 2006;207:165–173. doi: 10.1002/jcp.20548. [DOI] [PubMed] [Google Scholar]
- 21.Tomasek JJ, Gabbiani G, Hinz B, Chaponnier C, Brown RA. Myofibroblasts and mechano-regulation of connective tissue remodelling. Nature reviews. Molecular cell biology. 2002;3:349–363. doi: 10.1038/nrm809. [DOI] [PubMed] [Google Scholar]
- 22.Weber KT. Fibrosis and hypertensive heart disease. Curr Opin Cardiol. 2000;15:264–272. doi: 10.1097/00001573-200007000-00010. [DOI] [PubMed] [Google Scholar]
- 23.Chaturvedi RR, Herron T, Simmons R, Shore D, Kumar P, Sethia B, Chua F, Vassiliadis E, Kentish JC. Passive stiffness of myocardium from congenital heart disease and implications for diastole. Circulation. 2010;121:979–988. doi: 10.1161/CIRCULATIONAHA.109.850677. [DOI] [PubMed] [Google Scholar]
- 24.Espira L, Czubryt MP. Emerging concepts in cardiac matrix biology. Can J Physiol Pharmacol. 2009;87:996–1008. doi: 10.1139/Y09-105. [DOI] [PubMed] [Google Scholar]
- 25.de Bakker JM, van Capelle FJ, Janse MJ, Tasseron S, Vermeulen JT, de Jonge N, Lahpor JR. Fractionated electrograms in dilated cardiomyopathy: Origin and relation to abnormal conduction. J Am Coll Cardiol. 1996;27:1071–1078. doi: 10.1016/0735-1097(95)00612-5. [DOI] [PubMed] [Google Scholar]
- 26.Spach MS, Boineau JP. Microfibrosis produces electrical load variations due to loss of side-to-side cell connections: A major mechanism of structural heart disease arrhythmias. Pacing Clin Electrophysiol. 1997;20:397–413. doi: 10.1111/j.1540-8159.1997.tb06199.x. [DOI] [PubMed] [Google Scholar]
- 27.Gray MO, Long CS, Kalinyak JE, Li HT, Karliner JS. Angiotensin ii stimulates cardiac myocyte hypertrophy via paracrine release of tgf-beta 1 and endothelin-1 from fibroblasts. Cardiovasc Res. 1998;40:352–363. doi: 10.1016/s0008-6363(98)00121-7. [DOI] [PubMed] [Google Scholar]
- 28.Kai H, Mori T, Tokuda K, Takayama N, Tahara N, Takemiya K, Kudo H, Sugi Y, Fukui D, Yasukawa H, Kuwahara F, Imaizumi T. Pressure overload-induced transient oxidative stress mediates perivascular inflammation and cardiac fibrosis through angiotensin ii. Hypertens Res. 2006;29:711–718. doi: 10.1291/hypres.29.711. [DOI] [PubMed] [Google Scholar]
- 29.Gurtner GC, Werner S, Barrandon Y, Longaker MT. Wound repair and regeneration. Nature. 2008;453:314–321. doi: 10.1038/nature07039. [DOI] [PubMed] [Google Scholar]
- 30.Takeda N, Manabe I. Cellular interplay between cardiomyocytes and nonmyocytes in cardiac remodeling. Int J Inflam. 2011;2011:535241. doi: 10.4061/2011/535241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Turner NA. Effects of interleukin-1 on cardiac fibroblast function: Relevance to post-myocardial infarction remodelling. Vasc Pharmacol. 2014;60:1–7. doi: 10.1016/j.vph.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 32.Vasquez C, Benamer N, Morley GE. The cardiac fibroblast: Functional and electrophysiological considerations in healthy and diseased hearts. J Cardiovasc Pharm. 2011;57:380–388. doi: 10.1097/FJC.0b013e31820cda19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Y, Weber KT. Infarct scar: A dynamic tissue. Cardiovasc Res. 2000;46:250–256. doi: 10.1016/s0008-6363(00)00032-8. [DOI] [PubMed] [Google Scholar]
- 34.Turner NA, Porter KE. Function and fate of myofibroblasts after myocardial infarction. Fibrogenesis & tissue repair. 2013;6:5. doi: 10.1186/1755-1536-6-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jugdutt BI. Ventricular remodeling after infarction and the extracellular collagen matrix: When is enough enough? Circulation. 2003;108:1395–1403. doi: 10.1161/01.CIR.0000085658.98621.49. [DOI] [PubMed] [Google Scholar]
- 36.Anderson KR, Sutton MG, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease. J Pathol. 1979;128:79–85. doi: 10.1002/path.1711280205. [DOI] [PubMed] [Google Scholar]
- 37.Weber KT. Cardiac interstitium in health and disease: The fibrillar collagen network. J Am Coll Cardiol. 1989;13:1637–1652. doi: 10.1016/0735-1097(89)90360-4. [DOI] [PubMed] [Google Scholar]
- 38.Isoyama S, Nitta-Komatsubara Y. Acute and chronic adaptation to hemodynamic overload and ischemia in the aged heart. Heart Fail Rev. 2002;7:63–69. doi: 10.1023/a:1013701923065. [DOI] [PubMed] [Google Scholar]
- 39.Hasenfuss G. Animal models of human cardiovascular disease, heart failure and hypertrophy. Cardiovasc Res. 1998;39:60–76. doi: 10.1016/s0008-6363(98)00110-2. [DOI] [PubMed] [Google Scholar]
- 40.Reddy S, Zhao M, Hu DQ, Fajardo G, Katznelson E, Punn R, Spin JM, Chan FP, Bernstein D. Physiologic and molecular characterization of a murine model of right ventricular volume overload. American journal of physiology. Heart and circulatory physiology. 2013;304:H1314–1327. doi: 10.1152/ajpheart.00776.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Furtado MB, Costa MW, Pranoto EA, Salimova E, Pinto AR, Lam NT, Park A, Snider P, Chandran A, Harvey RP, Boyd R, Conway SJ, Pearson J, Kaye DM, Rosenthal NA. Cardiogenic genes expressed in cardiac fibroblasts contribute to heart development and repair. Circ Res. 2014;114:1422–1434. doi: 10.1161/CIRCRESAHA.114.302530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Morales MO, Price RL, Goldsmith EC. Expression of discoidin domain receptor 2 (ddr2) in the developing heart. Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2005;11:260–267. doi: 10.1017/S1431927605050518. [DOI] [PubMed] [Google Scholar]
- 43.Strutz F, Okada H, Lo CW, Danoff T, Carone RL, Tomaszewski JE, Neilson EG. Identification and characterization of a fibroblast marker: Fsp1. The Journal of cell biology. 1995;130:393–405. doi: 10.1083/jcb.130.2.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kong P, Christia P, Saxena A, Su Y, Frangogiannis NG. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am J Physiol-Heart C. 2013;305:H1363–H1372. doi: 10.1152/ajpheart.00395.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Osterreicher CH, Penz-Osterreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, Sasik R, Hardiman G, Karin M, Brenner DA. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. P Natl Acad Sci USA. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zeisberg EM, Tarnavski O, Zeisberg M, Dorfman AL, McMullen JR, Gustafsson E, Chandraker A, Yuan XL, Pu WT, Roberts AB, Neilson EG, Sayegh MH, Izumo S, Kalluri R. Endothelial-to-mesenchymal transition contributes to cardiac fibrosis. Nat Med. 2007;13:952–961. doi: 10.1038/nm1613. [DOI] [PubMed] [Google Scholar]
- 47.Acharya A, Baek ST, Huang G, Eskiocak B, Goetsch S, Sung CY, Banfi S, Sauer MF, Olsen GS, Duffield JS, Olson EN, Tallquist MD. The bhlh transcription factor tcf21 is required for lineage-specific emt of cardiac fibroblast progenitors. Development. 2012;139:2139–2149. doi: 10.1242/dev.079970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crisan M, Yap S, Casteilla L, Chen CW, Corselli M, Park TS, Andriolo G, Sun B, Zheng B, Zhang L, Norotte C, Teng PN, Traas J, Schugar R, Deasy BM, Badylak S, Buhring HJ, Giacobino JP, Lazzari L, Huard J, Peault B. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell stem cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 49.Jurisic G, Iolyeva M, Proulx ST, Halin C, Detmar M. Thymus cell antigen 1 (thy1, cd90) is expressed by lymphatic vessels and mediates cell adhesion to lymphatic endothelium. Experimental cell research. 2010;316:2982–2992. doi: 10.1016/j.yexcr.2010.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Raff MC. Surface antigenic markers for distinguishing t and b lymphocytes in mice. Transplantation reviews. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- 51.Franke WW, Schmid E, Osborn M, Weber K. Intermediate-sized filaments of human endothelial cells. The Journal of cell biology. 1979;81:570–580. doi: 10.1083/jcb.81.3.570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lane EB, Hogan BL, Kurkinen M, Garrels JI. Co-expression of vimentin and cytokeratins in parietal endoderm cells of early mouse embryo. Nature. 1983;303:701–704. doi: 10.1038/303701a0. [DOI] [PubMed] [Google Scholar]
- 53.Mork C, van Deurs B, Petersen OW. Regulation of vimentin expression in cultured human mammary epithelial cells. Differentiation. 1990;43:146–156. doi: 10.1111/j.1432-0436.1990.tb00441.x. [DOI] [PubMed] [Google Scholar]
- 54.Zeisberg EM, Kalluri R. Origins of cardiac fibroblasts. Circ Res. 2010;107:1304–1312. doi: 10.1161/CIRCRESAHA.110.231910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Braitsch CM, Kanisicak O, van Berlo JH, Molkentin JD, Yutzey KE. Differential expression of embryonic epicardial progenitor markers and localization of cardiac fibrosis in adult ischemic injury and hypertensive heart disease. J Mol Cell Cardiol. 2013;65:108–119. doi: 10.1016/j.yjmcc.2013.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Acharya A, Baek ST, Banfi S, Eskiocak B, Tallquist MD. Efficient inducible cre-mediated recombination in tcf21 cell lineages in the heart and kidney. Genesis. 2011;49:870–877. doi: 10.1002/dvg.20750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Smith CL, Baek ST, Sung CY, Tallquist MD. Epicardial-derived cell epithelial-to-mesenchymal transition and fate specification require pdgf receptor signaling. Circ Res. 2011;108:E15–U28. doi: 10.1161/CIRCRESAHA.110.235531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ntokou A, Klein F, Dontireddy D, Becker S, Bellusci S, Richardson WD, Szibor M, Braun T, Morty RE, Seeger W, Voswinckel R, Ahlbrecht K. Characterization of the platelet-derived growth factor receptor-alpha-positive cell lineage during murine late lung development. Am J Physiol Lung Cell Mol Physiol. 2015;309:L942–958. doi: 10.1152/ajplung.00272.2014. [DOI] [PubMed] [Google Scholar]
- 59.Mitra SS, Feroze AH, Gholamin S, Richard C, Esparza R, Zhang M, Azad TD, Alrfaei B, Kahn SA, Hutter G, Guzman R, Creasey GH, Plant GW, Weissman IL, Edwards MS, Cheshier S. Neural placode tissue derived from myelomeningocele repair serves as a viable source of oligodendrocyte progenitor cells. Neurosurgery. 2015;77:794–802. doi: 10.1227/NEU.0000000000000918. discussion 802. [DOI] [PubMed] [Google Scholar]
- 60.Koh BH, Roy R, Hollywood MA, Thornbury KD, McHale NG, Sergeant GP, Hatton WJ, Ward SM, Sanders KM, Koh SD. Platelet-derived growth factor receptor-alpha cells in mouse urinary bladder: A new class of interstitial cells. J Cell Mol Med. 2012;16:691–700. doi: 10.1111/j.1582-4934.2011.01506.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chong JJH, Reinecke H, Iwata M, Torok-Storb B, Stempien-Otero A, Murry CE. Progenitor cells identified by pdgfr-alpha expression in the developing and diseased human heart. Stem Cells Dev. 2013;22:1932–1943. doi: 10.1089/scd.2012.0542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Noseda M, Harada M, McSweeney S, Leja T, Belian E, Stuckey DJ, Abreu Paiva MS, Habib J, Macaulay I, de Smith AJ, al-Beidh F, Sampson R, Lumbers RT, Rao P, Harding SE, Blakemore AI, Jacobsen SE, Barahona M, Schneider MD. Pdgfralpha demarcates the cardiogenic clonogenic sca1+ stem/progenitor cell in adult murine myocardium. Nat Commun. 2015;6:6930. doi: 10.1038/ncomms7930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Miwa H, Era T. Generation and characterization of pdgfralpha-gfpcreert2 knock-in mouse line. Genesis. 2015;53:329–336. doi: 10.1002/dvg.22853. [DOI] [PubMed] [Google Scholar]
- 64.Cuttler AS, LeClair RJ, Stohn JP, Wang QZ, Sorenson CM, Liaw L, Lindner V. Characterization of pdgfrb-cre transgenic mice reveals reduction of rosa26 reporter activity in remodeling arteries. Genesis. 2011;49:673–680. doi: 10.1002/dvg.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yata Y, Scanga A, Gillan A, Yang L, Reif S, Breindl M, Brenner DA, Rippe RA. Dnase i-hypersensitive sites enhance alpha 1(i) collagen gene expression in hepatic stellate cells. Hepatology. 2003;37:267–276. doi: 10.1053/jhep.2003.50067. [DOI] [PubMed] [Google Scholar]
- 66.Zheng B, Zhang Z, Black CM, de Crombrugghe B, Denton CP. Ligand-dependent genetic recombination in fibroblasts : A potentially powerful technique for investigating gene function in fibrosis. Am J Pathol. 2002;160:1609–1617. doi: 10.1016/S0002-9440(10)61108-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bochmann L, Sarathchandra P, Mori F, Lara-Pezzi E, Lazzaro D, Rosenthal N. Revealing new mouse epicardial cell markers through transcriptomics. Plos One. 2010;5:e11429. doi: 10.1371/journal.pone.0011429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Skalli O, Ropraz P, Trzeciak A, Benzonana G, Gillessen D, Gabbiani G. A monoclonal-antibody against alpha-smooth muscle actin - a new probe for smooth-muscle differentiation. Journal of Cell Biology. 1986;103:2787–2796. doi: 10.1083/jcb.103.6.2787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Katsuragi N, Morishita R, Nakamura N, Ochiai T, Taniyama Y, Hasegawa Y, Kawashima K, Kaneda Y, Ogihara T, Sugimura K. Periostin as a novel factor responsible for ventricular dilation. Circulation. 2004;110:1806–1813. doi: 10.1161/01.CIR.0000142607.33398.54. [DOI] [PubMed] [Google Scholar]
- 70.Kudo A. Periostin in fibrillogenesis for tissue regeneration: Periostin actions inside and outside the cell. Cell Mol Life Sci. 2011;68:3201–3207. doi: 10.1007/s00018-011-0784-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Stanton LW, Garrard LJ, Damm D, Garrick BL, Lam A, Kapoun AM, Zheng Q, Protter AA, Schreiner GF, White RT. Altered patterns of gene expression in response to myocardial infarction. Circ Res. 2000;86:939–945. doi: 10.1161/01.res.86.9.939. [DOI] [PubMed] [Google Scholar]
- 72.Visconti RP, Markwald RR. Recruitment of new cells into the postnatal heart - potential modification of phenotype by periostin. Ann Ny Acad Sci. 2006;1080:19–33. doi: 10.1196/annals.1380.003. [DOI] [PubMed] [Google Scholar]
- 73.Kruzynska-Frejtag A, Machnicki M, Rogers R, Markwald RR, Conway SJ. Periostin (an osteoblast-specific factor) is expressed within the embryonic mouse heart during valve formation. Mech Develop. 2001;103:183–188. doi: 10.1016/s0925-4773(01)00356-2. [DOI] [PubMed] [Google Scholar]
- 74.Lindsley A, Snider P, Zhou H, Rogers R, Wang J, Olaopa M, Kruzynska-Frejtag A, Koushik SV, Lilly B, Burch JBE, Firulli AB, Conway SJ. Identification and characterization of a novel schwann and outflow tract endocardial cushion lineage-restricted periostin enhancer. Dev Biol. 2007;307:340–355. doi: 10.1016/j.ydbio.2007.04.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Takeda N, Manabe I, Uchino Y, Eguchi K, Matsumoto S, Nishimura S, Shindo T, Sano M, Otsu K, Snider P, Conway SJ, Nagai R. Cardiac fibroblasts are essential for the adaptive response of the murine heart to pressure overload. J Clin Invest. 2010;120:254–265. doi: 10.1172/JCI40295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Snider P, Standley KN, Wang J, Azhar M, Doetschman T, Conway SJ. Origin of cardiac fibroblasts and the role of periostin. Circ Res. 2009;105:934–947. doi: 10.1161/CIRCRESAHA.109.201400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Conway SJ, Molkentin JD. Periostin as a heterofunctional regulator of cardiac development and disease. Curr Genomics. 2008;9:548–555. doi: 10.2174/138920208786847917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chakraborty S, Wirrig EE, Hinton RB, Merrill WH, Spicer DB, Yutzey KE. Twist1 promotes heart valve cell proliferation and extracellular matrix gene expression during development in vivo and is expressed in human diseased aortic valves. Dev Biol. 2010;347:167–179. doi: 10.1016/j.ydbio.2010.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Duffield JS. Cellular and molecular mechanisms in kidney fibrosis. J Clin Invest. 2014;124:2299–2306. doi: 10.1172/JCI72267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gressner AM, Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and tgf-beta as major players and therapeutic targets. J Cell Mol Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Gressner OA, Gao C. Monitoring fibrogenic progression in the liver. Clin Chim Acta. 2014;433:111–122. doi: 10.1016/j.cca.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 82.Hardie WD, Glasser SW, Hagood JS. Emerging concepts in the pathogenesis of lung fibrosis. Am J Pathol. 2009;175:3–16. doi: 10.2353/ajpath.2009.081170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kage H, Borok Z. Emt and interstitial lung disease: A mysterious relationship. Curr Opin Pulm Med. 2012;18:517–523. doi: 10.1097/MCP.0b013e3283566721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fredj S, Bescond J, Louault C, Potreau D. Interactions between cardiac cells enhance cardiomyocyte hypertrophy and increase fibroblast proliferation. Journal of cellular physiology. 2005;202:891–899. doi: 10.1002/jcp.20197. [DOI] [PubMed] [Google Scholar]
- 85.Teekakirikul P, Eminaga S, Toka O, Alcalai R, Wang LB, Wakimoto H, Nayor M, Konno T, Gorham JM, Wolf CM, Kim JB, Schmitt JP, Molkentin JD, Norris RA, Tager AM, Hoffman SR, Markwald RR, Seidman CE, Seidman JG. Cardiac fibrosis in mice with hypertrophic cardiomyopathy is mediated by non-myocyte proliferation and requires tgf-beta. J Clin Invest. 2010;120:3520–3529. doi: 10.1172/JCI42028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lincoln J, Alfieri CM, Yutzey KE. Development of heart valve leaflets and supporting apparatus in chicken and mouse embryos. Dev Dyn. 2004;230:239–250. doi: 10.1002/dvdy.20051. [DOI] [PubMed] [Google Scholar]
- 87.Krenning G, Moonen JR, van Luyn MJ, Harmsen MC. Vascular smooth muscle cells for use in vascular tissue engineering obtained by endothelial-to-mesenchymal transdifferentiation (enmt) on collagen matrices. Biomaterials. 2008;29:3703–3711. doi: 10.1016/j.biomaterials.2008.05.034. [DOI] [PubMed] [Google Scholar]
- 88.Moonen JR, Krenning G, Brinker MG, Koerts JA, van Luyn MJ, Harmsen MC. Endothelial progenitor cells give rise to pro-angiogenic smooth muscle-like progeny. Cardiovasc Res. 2010;86:506–515. doi: 10.1093/cvr/cvq012. [DOI] [PubMed] [Google Scholar]
- 89.Gustafsson E, Brakebusch C, Hietanen K, Fassler R. Tie-1-directed expression of cre recombinase in endothelial cells of embryoid bodies and transgenic mice. J Cell Sci. 2001;114:671–676. doi: 10.1242/jcs.114.4.671. [DOI] [PubMed] [Google Scholar]
- 90.Monvoisin A, Alva JA, Hofmann JJ, Zovein AC, Lane TF, Iruela-Arispe ML. Ve-cadherin-creer(t2) transgenic mouse: A model for inducible recombination in the endothelium. Dev Dynam. 2006;235:3413–3422. doi: 10.1002/dvdy.20982. [DOI] [PubMed] [Google Scholar]
- 91.Kisanuki YY, Hammer RE, Miyazaki J, Williams SC, Richardson JA, Yanagisawa M. Tie2-cre transgenic mice: A new model for endothelial cell-lineage analysis in vivo. Dev Biol. 2001;230:230–242. doi: 10.1006/dbio.2000.0106. [DOI] [PubMed] [Google Scholar]
- 92.Norris RA, Borg TK, Butcher JT, Baudino TA, Banerjee I, Markwald RR. Neonatal and adult cardiovascular pathophysiological remodeling and repair: Developmental role of periostin. Ann N Y Acad Sci. 2008;1123:30–40. doi: 10.1196/annals.1420.005. [DOI] [PubMed] [Google Scholar]
- 93.Olivey HE, Mundell NA, Austin AF, Barnett JV. Transforming growth factor-beta stimulates epithelial-mesenchymal transformation in the proepicardium. Dev Dyn. 2006;235:50–59. doi: 10.1002/dvdy.20593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Zhou B, von Gise A, Ma Q, Hu YW, Pu WT. Genetic fate mapping demonstrates contribution of epicardium-derived cells to the annulus fibrosis of the mammalian heart. Dev Biol. 2010;338:251–261. doi: 10.1016/j.ydbio.2009.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Morabito CJ, Dettman RW, Kattan J, Collier JM, Bristow J. Positive and negative regulation of epicardial-mesenchymal transformation during avian heart development. Dev Biol. 2001;234:204–215. doi: 10.1006/dbio.2001.0254. [DOI] [PubMed] [Google Scholar]
- 96.Duan JZ, Gherghe C, Liu DX, Hamlett E, Srikantha L, Rodgers L, Regan JN, Rojas M, Willis M, Leask A, Majesky M, Deb A. Wnt1/beta catenin injury response activates the epicardium and cardiac fibroblasts to promote cardiac repair. Embo J. 2012;31:429–442. doi: 10.1038/emboj.2011.418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Russell JL, Goetsch SC, Gaiano NR, Hill JA, Olson EN, Schneider JW. A dynamic notch injury response activates epicardium and contributes to fibrosis repair. Circ Res. 2011;108:51–U102. doi: 10.1161/CIRCRESAHA.110.233262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Young LR, Gulleman PM, Bridges JP, Weaver TE, Deutsch GH, Blackwell TS, McCormack FX. The alveolar epithelium determines susceptibility to lung fibrosis in hermansky-pudlak syndrome. Am J Respir Crit Care Med. 2012;186:1014–1024. doi: 10.1164/rccm.201207-1206OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ikegami T, Zhang Y, Matsuzaki Y. Liver fibrosis: Possible involvement of emt. Cells Tissues Organs. 2007;185:213–221. doi: 10.1159/000101322. [DOI] [PubMed] [Google Scholar]
- 100.Scholten D, Osterreicher CH, Scholten A, Iwaisako K, Gu G, Brenner DA, Kisseleva T. Genetic labeling does not detect epithelial-to-mesenchymal transition of cholangiocytes in liver fibrosis in mice. Gastroenterology. 2010;139:987–998. doi: 10.1053/j.gastro.2010.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Sundberg C, Ivarsson M, Gerdin B, Rubin K. Pericytes as collagen-producing cells in excessive dermal scarring. Lab Invest. 1996;74:452–466. [PubMed] [Google Scholar]
- 102.Covas DT, Panepucci RA, Fontes AM, Silva WA, Jr, Orellana MD, Freitas MC, Neder L, Santos AR, Peres LC, Jamur MC, Zago MA. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with cd146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 103.Humphreys BD, Lin SL, Kobayashi A, Hudson TE, Nowlin BT, Bonventre JV, Valerius MT, McMahon AP, Duffield JS. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Dalkara T, Alarcon-Martinez L. Cerebral microvascular pericytes and neurogliovascular signaling in health and disease. Brain Res. 2015;1623:3–17. doi: 10.1016/j.brainres.2015.03.047. [DOI] [PubMed] [Google Scholar]
- 105.Kramann R, Schneider RK, DiRocco DP, Machado F, Fleig S, Bondzie PA, Henderson JM, Ebert BL, Humphreys BD. Perivascular gli1+ progenitors are key contributors to injury-induced organ fibrosis. Cell stem cell. 2015;16:51–66. doi: 10.1016/j.stem.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kania G, Blyszczuk P, Stein S, Valaperti A, Germano D, Dirnhofer S, Hunziker L, Matter CM, Eriksson U. Heart-infiltrating prominin-1+/cd133+ progenitor cells represent the cellular source of transforming growth factor beta-mediated cardiac fibrosis in experimental autoimmune myocarditis. Circ Res. 2009;105:462–470. doi: 10.1161/CIRCRESAHA.109.196287. [DOI] [PubMed] [Google Scholar]
- 107.Van Amerongen MJ, Bou-Gharios G, Popa ER, Van Ark J, Petersen AH, Van Dam GM, Van Luyn MJA, Harmsen MC. Bone marrow-derived myofibroblasts contribute functionally to scar formation after myocardial infarction. J Pathol. 2008;214:377–386. doi: 10.1002/path.2281. [DOI] [PubMed] [Google Scholar]
- 108.Haudek SB, Xia Y, Huebener P, Lee JM, Carlson S, Crawford JR, Pilling D, Gomer RH, Trial J, Frangogiannis NG, Entman ML. Bone marrow-derived fibroblast precursors mediate ischemic cardiomyopathy in mice. P Natl Acad Sci USA. 2006;103:18284–18289. doi: 10.1073/pnas.0608799103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.van Amerongen MJ, Harmsen MC, van Rooijen N, Petersen AH, van Luyn MJ. Macrophage depletion impairs wound healing and increases left ventricular remodeling after myocardial injury in mice. Am J Pathol. 2007;170:818–829. doi: 10.2353/ajpath.2007.060547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Bucala R, Spiegel LA, Chesney J, Hogan M, Cerami A. Circulating fibrocytes define a new leukocyte subpopulation that mediates tissue-repair. Mol Med. 1994;1:71–81. [PMC free article] [PubMed] [Google Scholar]
- 111.Mollmann H, Nef HM, Kostin S, von Kalle C, Pilz I, Weber M, Schaper J, Hamm CW, Elsasser A. Bone marrow-derived cells contribute to infarct remodelling. Cardiovasc Res. 2006;71:661–671. doi: 10.1016/j.cardiores.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 112.Xu J, Lin SC, Chen JY, Miao YX, Taffet GE, Entman ML, Wang YL. Ccr2 mediates the uptake of bone marrow-derived fibroblast precursors in angiotensin ii-induced cardiac fibrosis. Am J Physiol-Heart C. 2011;301:H538–H547. doi: 10.1152/ajpheart.01114.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Abe R, Donnelly SC, Peng T, Bucala R, Metz CN. Peripheral blood fibrocytes: Differentiation pathway and migration to wound sites. J Immunol. 2001;166:7556–7562. doi: 10.4049/jimmunol.166.12.7556. [DOI] [PubMed] [Google Scholar]
- 114.van Wamel AJ, Ruwhof C, van der Valk-Kokshoom LE, Schrier PI, van der Laarse A. The role of angiotensin ii, endothelin-1 and transforming growth factor-beta as autocrine/paracrine mediators of stretch-induced cardiomyocyte hypertrophy. Molecular and cellular biochemistry. 2001;218:113–124. doi: 10.1023/a:1007279700705. [DOI] [PubMed] [Google Scholar]
- 115.Wang H, Haeger SM, Kloxin AM, Leinwand LA, Anseth KS. Redirecting valvular myofibroblasts into dormant fibroblasts through light-mediated reduction in substrate modulus. Plos One. 2012;7:e39969. doi: 10.1371/journal.pone.0039969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang H, Tibbitt MW, Langer SJ, Leinwand LA, Anseth KS. Hydrogels preserve native phenotypes of valvular fibroblasts through an elasticity-regulated pi3k/akt pathway. Proc Natl Acad Sci U S A. 2013;110:19336–19341. doi: 10.1073/pnas.1306369110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Leask A, Abraham DJ. Tgf-beta signaling and the fibrotic response. Faseb J. 2004;18:816–827. doi: 10.1096/fj.03-1273rev. [DOI] [PubMed] [Google Scholar]
- 118.Sarrazy V, Koehler A, Chow ML, Zimina E, Li CX, Kato H, Caldarone CA, Hinz B. Integrins alpha v beta 5 and alpha v beta 3 promote latent tgf-beta 1 activation by human cardiac fibroblast contraction. Cardiovasc Res. 2014;102:407–417. doi: 10.1093/cvr/cvu053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Wipff PJ, Rifkin DB, Meister JJ, Hinz B. Myofibroblast contraction activates latent tgf-beta 1 from the extracellular matrix. Journal of Cell Biology. 2007;179:1311–1323. doi: 10.1083/jcb.200704042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Bujak M, Frangogiannis NG. The role of tgf-beta signaling in myocardial infarction and cardiac remodeling. Cardiovasc Res. 2007;74:184–195. doi: 10.1016/j.cardiores.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Bujak M, Ren G, Kweon HJ, Dobaczewski M, Reddy A, Taffet G, Wang XF, Frangogiannis NG. Essential role of smad3 in infarct healing and in the pathogenesis of cardiac remodeling. Circulation. 2007;116:2127–2138. doi: 10.1161/CIRCULATIONAHA.107.704197. [DOI] [PubMed] [Google Scholar]
- 122.Dobaczewski M, Bujak M, Li N, Gonzalez-Quesada C, Mendoza LH, Wang XF, Frangogiannis NG. Smad3 signaling critically regulates fibroblast phenotype and function in healing myocardial infarction. Circ Res. 2010;107:418–U176. doi: 10.1161/CIRCRESAHA.109.216101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Verrecchia F, Chu ML, Mauviel A. Identification of novel tgf-beta/smad gene targets in dermal fibroblasts using a combined cdna microarray/promoter transactivation approach. J Biol Chem. 2001;276:17058–17062. doi: 10.1074/jbc.M100754200. [DOI] [PubMed] [Google Scholar]
- 124.Yang YC, Piek E, Zavadil J, Liang D, Xie DL, Heyer J, Pavlidis P, Kucherlapati R, Roberts AB, Bottinger EP. Hierarchical model of gene regulation by transforming growth factor beta. P Natl Acad Sci USA. 2003;100:10269–10274. doi: 10.1073/pnas.1834070100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Vivar R, Humeres C, Ayala P, Olmedo I, Catalan M, Garcia L, Lavandero S, Diaz-Araya G. Tgf-beta 1 prevents simulated ischemia/reperfusion-induced cardiac fibroblast apoptosis by activation of both canonical and non-canonical signaling pathways. Bba-Mol Basis Dis. 2013;1832:754–762. doi: 10.1016/j.bbadis.2013.02.004. [DOI] [PubMed] [Google Scholar]
- 126.Tan SM, Zhang Y, Connelly KA, Gilbert RE, Kelly DJ. Targeted inhibition of activin receptor-like kinase 5 signaling attenuates cardiac dysfunction following myocardial infarction. Am J Physiol-Heart C. 2010;298:H1415–H1425. doi: 10.1152/ajpheart.01048.2009. [DOI] [PubMed] [Google Scholar]
- 127.Engebretsen KVT, Skardal K, Bjornstad S, Marstein HS, Skrbic B, Sjaastad I, Christensen G, Bjornstad JL, Tonnessen T. Attenuated development of cardiac fibrosis in left ventricular pressure overload by sm16, an orally active inhibitor of alk5. J Mol Cell Cardiol. 2014;76:148–157. doi: 10.1016/j.yjmcc.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 128.Kuwahara F, Kai H, Tokuda K, Kai M, Takeshita A, Egashira K, Imaizumi T. Transforming growth factor-beta function blocking prevents myocardial fibrosis and diastolic dysfunction in pressure-overloaded rats. Circulation. 2002;106:130–135. doi: 10.1161/01.cir.0000020689.12472.e0. [DOI] [PubMed] [Google Scholar]
- 129.Frantz S, Hu K, Adamek A, Wolf J, Sallam A, Maier SKG, Lonning S, Ling H, Ertl G, Bauersachs J. Transforming growth factor beta inhibition increases mortality and left ventricular dilatation after myocardial infarction. Basic Res Cardiol. 2008;103:485–492. doi: 10.1007/s00395-008-0739-7. [DOI] [PubMed] [Google Scholar]
- 130.Sato M, Muragaki Y, Saika S, Roberts AB, Ooshima A. Targeted disruption of tgf-beta1/smad3 signaling protects against renal tubulointerstitial fibrosis induced by unilateral ureteral obstruction. J Clin Invest. 2003;112:1486–1494. doi: 10.1172/JCI19270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ding Y, Kim SL, Lee SY, Koo JK, Wang Z, Choi ME. Autophagy regulates tgf-beta expression and suppresses kidney fibrosis induced by unilateral ureteral obstruction. J Am Soc Nephrol. 2014;25:2835–2846. doi: 10.1681/ASN.2013101068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Liao P, Georgakopoulos D, Kovacs A, Zheng MZ, Lerner D, Pu HY, Saffitz J, Chien K, Xiao RP, Kass DA, Wang YB. The in vivo role of p38 map kinases in cardiac remodeling and restrictive cardiomyopathy. P Natl Acad Sci USA. 2001;98:12283–12288. doi: 10.1073/pnas.211086598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Yan W, Wang PH, Zhao CX, Tang JR, Xiao X, Wang DW. Decorin gene delivery inhibits cardiac fibrosis in spontaneously hypertensive rats by modulation of transforming growth factor-beta/smad and p38 mitogen-activated protein kinase signaling pathways. Hum Gene Ther. 2009;20:1190–1200. doi: 10.1089/hum.2008.204. [DOI] [PubMed] [Google Scholar]
- 134.Zhang D, Gaussin V, Taffet GE, Belaguli NS, Yamada M, Schwartz RJ, Michael LH, Overbeek PA, Schneider MD. Tak1 is activated in the myocardium after pressure overload and is sufficient to provoke heart failure in transgenic mice. Nat Med. 2000;6:556–563. doi: 10.1038/75037. [DOI] [PubMed] [Google Scholar]
- 135.Davis J, Burr AR, Davis GF, Birnbaumer L, Molkentin JD. A trpc6-dependent pathway for myofibroblast transdifferentiation and wound healing in vivo. Dev Cell. 2012;23:705–715. doi: 10.1016/j.devcel.2012.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Leask A. Potential therapeutic targets for cardiac fibrosis tgf beta, angiotensin, endothelin, ccn2, and pdgf, partners in fibroblast activation. Circ Res. 2010;106:1675–1680. doi: 10.1161/CIRCRESAHA.110.217737. [DOI] [PubMed] [Google Scholar]
- 137.Koitabashi N, Danner T, Zaiman AL, Pinto YM, Rowell J, Mankowski J, Zhang D, Nakamura T, Takimoto E, Kass DA. Pivotal role of cardiomyocyte tgf-beta signaling in the murine pathological response to sustained pressure overload. J Clin Invest. 2011;121:2301–2312. doi: 10.1172/JCI44824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Ono K, Ohtomo T, Ninomiya-Tsuji J, Tsuchiya M. A dominant negative tak1 inhibits cellular fibrotic responses induced by tgf-beta. Biochem Bioph Res Co. 2003;307:332–337. doi: 10.1016/s0006-291x(03)01207-5. [DOI] [PubMed] [Google Scholar]
- 139.Kompa AR, See F, Lewis DA, Adrahtas A, Cantwell DM, Wang BH, Krum H. Long-term but not short-term p38 mitogen-activated protein kinase inhibition improves cardiac function and reduces cardiac remodeling post-myocardial infarction. J Pharmacol Exp Ther. 2008;325:741–750. doi: 10.1124/jpet.107.133546. [DOI] [PubMed] [Google Scholar]
- 140.See F, Thomas W, Way K, Tzanidis A, Kompa A, Lewis D, Itescu S, Krum H. P38 mitogen-activated protein kinase inhibition improves cardiac function and attenuates left ventricular remodeling following myocardial infarction in the rat. J Am Coll Cardiol. 2004;44:1679–1689. doi: 10.1016/j.jacc.2004.07.038. [DOI] [PubMed] [Google Scholar]
- 141.Newby LK, Marber MS, Melloni C, Sarov-Blat L, Aberle LH, Aylward PE, Cai G, de Winter RJ, Hamm CW, Heitner JF, Kim R, Lerman A, Patel MR, Tanguay JF, Lepore JJ, Al-Khalidi HR, Sprecher DL, Granger CB, Investigators S. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-st-segment elevation myocardial infarction: A randomised phase 2 trial. Lancet. 2014;384:1187–1195. doi: 10.1016/S0140-6736(14)60417-7. [DOI] [PubMed] [Google Scholar]
- 142.Schnee JM, Hsueh WA. Angiotensin ii, adhesion, and cardiac fibrosis. Cardiovasc Res. 2000;46:264–268. doi: 10.1016/s0008-6363(00)00044-4. [DOI] [PubMed] [Google Scholar]
- 143.Zhang RX, Zhang YY, Huang XR, Wu Y, Chung ACK, Wu EX, Szalai AJ, Wong BCY, Lau CP, Lan HY. C-reactive protein promotes cardiac fibrosis and inflammation in angiotensin ii-induced hypertensive cardiac disease. Hypertension. 2010;55:953–U265. doi: 10.1161/HYPERTENSIONAHA.109.140608. [DOI] [PubMed] [Google Scholar]
- 144.Chen XQ, Liu X, Wang QX, Zhang MJ, Guo M, Liu F, Jiang WF, Zhou L. Pioglitazone inhibits angiotensin ii-induced atrial fibroblasts proliferation via nf-kappa b/tgf-beta 1/trif/traf6 pathway. Experimental cell research. 2015;330:43–55. doi: 10.1016/j.yexcr.2014.08.021. [DOI] [PubMed] [Google Scholar]
- 145.Villarreal FJ, Kim NN, Ungab GD, Printz MP, Dillmann WH. Identification of functional angiotensin-ii receptors on rat cardiac fibroblasts. Circulation. 1993;88:2849–2861. doi: 10.1161/01.cir.88.6.2849. [DOI] [PubMed] [Google Scholar]
- 146.Gao XR, He XY, Luo B, Peng LY, Lin J, Zuo ZY. Angiotensin ii increases collagen i expression via transforming growth factor-beta1 and extracellular signal-regulated kinase in cardiac fibroblasts. Eur J Pharmacol. 2009;606:115–120. doi: 10.1016/j.ejphar.2008.12.049. [DOI] [PubMed] [Google Scholar]
- 147.Schorb W, Booz GW, Dostal DE, Conrad KM, Chang KC, Baker KM. Angiotensin ii is mitogenic in neonatal rat cardiac fibroblasts. Circ Res. 1993;72:1245–1254. doi: 10.1161/01.res.72.6.1245. [DOI] [PubMed] [Google Scholar]
- 148.Yamamoto T, Nakagawa T, Suzuki H, Ohashi N, Fukasawa H, Fujigaki Y, Kato A, Nakamura Y, Suzuki F, Hishida A. Urinary angiotensinogen as a marker of intrarenal angiotensin ii activity associated with deterioration of renal function in patients with chronic kidney disease. J Am Soc Nephrol. 2007;18:1558–1565. doi: 10.1681/ASN.2006060554. [DOI] [PubMed] [Google Scholar]
- 149.Ma FF, Li YL, Jia LX, Han YL, Cheng JZ, Li HH, Qi YF, Du J. Macrophage-stimulated cardiac fibroblast production of il-6 is essential for tgf beta/smad activation and cardiac fibrosis induced by angiotensin ii. Plos One. 2012;7 doi: 10.1371/journal.pone.0035144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Campbell SE, Katwa LC. Angiotensin ii stimulated expression of transforming growth factor-beta(1) in cardiac fibroblasts and myofibroblasts. J Mol Cell Cardiol. 1997;29:1947–1958. doi: 10.1006/jmcc.1997.0435. [DOI] [PubMed] [Google Scholar]
- 151.Schultz JEJ, Witt SA, Glascock BJ, Nieman ML, Reiser PJ, Nix SL, Kimball TR, Doetschman T. Tgf-beta 1 mediates the hypertrophic cardiomyocyte growth induced by angiotensin ii. J Clin Invest. 2002;109:787–796. doi: 10.1172/JCI14190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zou Y, Akazawa H, Qin Y, Sano M, Takano H, Minamino T, Makita N, Iwanaga K, Zhu W, Kudoh S, Toko H, Tamura K, Kihara M, Nagai T, Fukamizu A, Umemura S, Iiri T, Fujita T, Komuro I. Mechanical stress activates angiotensin ii type 1 receptor without the involvement of angiotensin ii. Nat Cell Biol. 2004;6:499–506. doi: 10.1038/ncb1137. [DOI] [PubMed] [Google Scholar]
- 153.Diez J, Querejeta R, Lopez B, Gonzalez A, Larman M, Ubago JLM. Losartan-dependent regression of myocardial fibrosis is associated with reduction of left ventricular chamber stiffness in hypertensive patients. Circulation. 2002;105:2512–2517. doi: 10.1161/01.cir.0000017264.66561.3d. [DOI] [PubMed] [Google Scholar]
- 154.Pfeffer JM, Fischer TA, Pfeffer MA. Angiotensin-converting enzyme-inhibition and ventricular and remodeling after myocardial-infarction. Annu Rev Physiol. 1995;57:805–826. doi: 10.1146/annurev.ph.57.030195.004105. [DOI] [PubMed] [Google Scholar]
- 155.Yang FY, Chung ACK, Huang XR, Lan HY. Angiotensin ii induces connective tissue growth factor and collagen i expression via transforming growth factor-beta-dependent and -independent smad pathways the role of smad3. Hypertension. 2009;54:877–884. doi: 10.1161/HYPERTENSIONAHA.109.136531. [DOI] [PubMed] [Google Scholar]
- 156.Oliveira-Junior SA, Martinez PF, Guizoni DM, Campos DH, Fernandes T, Oliveira EM, Okoshi MP, Okoshi K, Padovani CR, Cicogna AC. At1 receptor blockade attenuates insulin resistance and myocardial remodeling in rats with diet-induced obesity. Plos One. 2014;9:e86447. doi: 10.1371/journal.pone.0086447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Galie PA, Russell MW, Westfall MV, Stegemann JP. Interstitial fluid flow and cyclic strain differentially regulate cardiac fibroblast activation via at1r and tgf-beta 1. Experimental cell research. 2012;318:75–84. doi: 10.1016/j.yexcr.2011.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Miguel-Carrasco JL, Zambrano S, Blanca AJ, Mate A, Vazquez CM. Captopril reduces cardiac inflammatory markers in spontaneously hypertensive rats by inactivation of nf-kb. J Inflamm-Lond. 2010;7 doi: 10.1186/1476-9255-7-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Duerrschmid C, Trial J, Wang YL, Entman ML, Haudek SB. Tumor necrosis factor a mechanistic link between angiotensin-ii-induced cardiac inflammation and fibrosis. Circ-Heart Fail. 2015;8:352. doi: 10.1161/CIRCHEARTFAILURE.114.001893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Sato H, Watanabe A, Tanaka T, Koitabashi N, Arai M, Kurabayashi M, Yokoyama T. Regulation of the human tumor necrosis factor-alpha promoter by angiotensin ii and lipopolysaccharide in cardiac fibroblasts: Different cis-acting promoter sequences and transcriptional factors. J Mol Cell Cardiol. 2003;35:1197–1205. doi: 10.1016/s0022-2828(03)00210-4. [DOI] [PubMed] [Google Scholar]
- 161.Shimada YJ, Passeri JJ, Baggish AL, O’Callaghan C, Lowry PA, Yannekis G, Abbara S, Ghoshhajra BB, Rothman RD, Ho CY, Januzzi JL, Seidman CE, Fifer MA. Effects of losartan on left ventricular hypertrophy and fibrosis in patients with nonobstructive hypertrophic cardiomyopathy. JACC Heart Fail. 2013;1:480–487. doi: 10.1016/j.jchf.2013.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Shi-Wen X, Rodriguez-Pascual F, Lamas S, Holmes A, Howat S, Pearson JD, Dashwood MR, du Bois RM, Denton CP, Black CM, Abraham DJ, Leask A. Constitutive alk5-independent c-jun n-terminal kinase activation contributes to endothelin-1 overexpression in pulmonary fibrosis: Evidence of an autocrine endothelin loop operating through the endothelin a and b receptors. Molecular and cellular biology. 2006;26:5518–5527. doi: 10.1128/MCB.00625-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Speed JS, Pollock DM. Endothelin, kidney disease, and hypertension. Hypertension. 2013;61:1142–1145. doi: 10.1161/HYPERTENSIONAHA.113.00595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Chen S, Evans T, Mukherjee K, Karmazyn M, Chakrabarti S. Diabetes-induced myocardial structural changes: Role of endothelin-1 and its receptors. J Mol Cell Cardiol. 2000;32:1621–1629. doi: 10.1006/jmcc.2000.1197. [DOI] [PubMed] [Google Scholar]
- 165.Dashwood MR, Abraham D. Endothelin: From bench to bedside and back. Pharmacol Res. 2011;63:445–447. doi: 10.1016/j.phrs.2011.04.005. [DOI] [PubMed] [Google Scholar]
- 166.Hafizi S, Wharton J, Chester AH, Yacoub MH. Profibrotic effects of endothelin-1 via the eta receptor in cultured human cardiac fibroblasts. Cell Physiol Biochem. 2004;14:285–292. doi: 10.1159/000080338. [DOI] [PubMed] [Google Scholar]
- 167.Katwa LC. Cardiac myofibroblasts isolated from the site of myocardial infarction express endothelin de novo. American journal of physiology. Heart and circulatory physiology. 2003;285:H1132–1139. doi: 10.1152/ajpheart.01141.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Xu SW, Kennedy L, Renzoni EA, Bou-Gharios G, du Bois RM, Black CM, Denton CP, Abraham DJ, Leask A. Endothelin is a downstream mediator of profibrotic responses to transforming growth factor beta in human lung fibroblasts. Arthritis Rheum-Us. 2007;56:4189–4194. doi: 10.1002/art.23134. [DOI] [PubMed] [Google Scholar]
- 169.Adiarto S, Heiden S, Vignon-Zellweger N, Nakayama K, Yagi K, Yanagisawa M, Emoto N. Et-1 from endothelial cells is required for complete angiotensin ii-induced cardiac fibrosis and hypertrophy. Life Sci. 2012;91:651–657. doi: 10.1016/j.lfs.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 170.Cheng TH, Cheng PY, Shih NL, Chen IB, Wang DL, Chen JJ. Involvement of reactive oxygen species in angiotensin ii-induced endothelin-1 gene expression in rat cardiac fibroblasts. J Am Coll Cardiol. 2003;42:1845–1854. doi: 10.1016/j.jacc.2003.06.010. [DOI] [PubMed] [Google Scholar]
- 171.Liou JY, Hong HJ, Sung LC, Chao HH, Chen PY, Cheng TH, Chan P, Liu JC. Nicorandil inhibits angiotensin-ii-induced proliferation of cultured rat cardiac fibroblasts. Pharmacology. 2011;87:144–151. doi: 10.1159/000323555. [DOI] [PubMed] [Google Scholar]
- 172.Weng X, Yu L, Liang P, Chen D, Cheng X, Yang Y, Li L, Zhang T, Zhou B, Wu X, Xu H, Fang M, Gao Y, Chen Q, Xu Y. Endothelial mrtf-a mediates angiotensin ii induced cardiac hypertrophy. J Mol Cell Cardiol. 2015;80:23–33. doi: 10.1016/j.yjmcc.2014.11.009. [DOI] [PubMed] [Google Scholar]
- 173.Singh AD, Amit S, Kumar OS, Rajan M, Mukesh N. Cardioprotective effects of bosentan, a mixed endothelin type a and b receptor antagonist, during myocardial ischaemia and reperfusion in rats. Basic Clin Pharmacol Toxicol. 2006;98:604–610. doi: 10.1111/j.1742-7843.2006.pto_405.x. [DOI] [PubMed] [Google Scholar]
- 174.Sutsch G, Kiowski W, Yan XW, Hunziker P, Christen S, Strobel W, Kim JH, Rickenbacher P, Bertel O. Short-term oral endothelin-receptor antagonist therapy in conventionally treated patients with symptomatic severe chronic heart failure. Circulation. 1998;98:2262–2268. doi: 10.1161/01.cir.98.21.2262. [DOI] [PubMed] [Google Scholar]
- 175.Rodriguez-Pascual F, Busnadiego O, Gonzalez-Santamaria J. The profibrotic role of endothelin-1: Is the door still open for the treatment of fibrotic diseases? Life Sci. 2014;118:156–164. doi: 10.1016/j.lfs.2013.12.024. [DOI] [PubMed] [Google Scholar]
- 176.Kohan DE, Cleland JG, Rubin LJ, Theodorescu D, Barton M. Clinical trials with endothelin receptor antagonists: What went wrong and where can we improve? Life Sci. 2012;91:528–539. doi: 10.1016/j.lfs.2012.07.034. [DOI] [PubMed] [Google Scholar]
- 177.Anand I, McMurray J, Cohn JN, Konstam MA, Notter T, Quitzau K, Ruschitzka F, Luscher TF investigators E. Long-term effects of darusentan on left-ventricular remodelling and clinical outcomes in the endothelina receptor antagonist trial in heart failure (earth): Randomised, double-blind, placebo-controlled trial. Lancet. 2004;364:347–354. doi: 10.1016/S0140-6736(04)16723-8. [DOI] [PubMed] [Google Scholar]
- 178.Prasad SK, Dargie HJ, Smith GC, Barlow MM, Grothues F, Groenning BA, Cleland JG, Pennell DJ. Comparison of the dual receptor endothelin antagonist enrasentan with enalapril in asymptomatic left ventricular systolic dysfunction: A cardiovascular magnetic resonance study. Heart. 2006;92:798–803. doi: 10.1136/hrt.2004.049734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Miano JM. Serum response factor: Toggling between disparate programs of gene expression. J Mol Cell Cardiol. 2003;35:577–593. doi: 10.1016/s0022-2828(03)00110-x. [DOI] [PubMed] [Google Scholar]
- 180.Zhang SX, Garcia-Gras E, Wycuff DR, Marriot SJ, Kadeer N, Yu W, Olson EN, Garry DJ, Parmacek MS, Schwartz RJ. Identification of direct serum-response factor gene targets during me2so-induced p19 cardiac cell differentiation. J Biol Chem. 2005;280:19115–19126. doi: 10.1074/jbc.M413793200. [DOI] [PubMed] [Google Scholar]
- 181.Yang Y, Zhe X, Phan SH, Ullenbruch M, Schuger L. Involvement of serum response factor isoforms in myofibroblast differentiation during bleomycin-induced lung injury. Am J Respir Cell Mol Biol. 2003;29:583–590. doi: 10.1165/rcmb.2002-0315OC. [DOI] [PubMed] [Google Scholar]
- 182.Small EM, Thatcher JE, Sutherland LB, Kinoshita H, Gerard RD, Richardson JA, Dimaio JM, Sadek H, Kuwahara K, Olson EN. Myocardin-related transcription factor-a controls myofibroblast activation and fibrosis in response to myocardial infarction. Circ Res. 2010;107:294–304. doi: 10.1161/CIRCRESAHA.110.223172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Lutz S, Shankaranarayanan A, Coco C, Ridilla M, Nance MR, Vettel C, Baltus D, Evelyn CR, Neubig RR, Wieland T, Tesmer JJ. Structure of galphaq-p63rhogef-rhoa complex reveals a pathway for the activation of rhoa by gpcrs. Science. 2007;318:1923–1927. doi: 10.1126/science.1147554. [DOI] [PubMed] [Google Scholar]
- 184.Numaguchi K, Eguchi S, Yamakawa T, Motley ED, Inagami T. Mechanotransduction of rat aortic vascular smooth muscle cells requires rhoa and intact actin filaments. Circ Res. 1999;85:5–11. doi: 10.1161/01.res.85.1.5. [DOI] [PubMed] [Google Scholar]
- 185.Vardouli L, Vasilaki E, Papadimitriou E, Kardassis D, Stournaras C. A novel mechanism of tgfbeta-induced actin reorganization mediated by smad proteins and rho gtpases. FEBS J. 2008;275:4074–4087. doi: 10.1111/j.1742-4658.2008.06549.x. [DOI] [PubMed] [Google Scholar]
- 186.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, Grant SR, Olson EN. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Hofmann T, Schaefer M, Schultz G, Gudermann T. Transient receptor potential channels as molecular substrates of receptor-mediated cation entry. J Mol Med (Berl) 2000;78:14–25. doi: 10.1007/s001099900070. [DOI] [PubMed] [Google Scholar]
- 188.Kuwahara K, Wang Y, McAnally J, Richardson JA, Bassel-Duby R, Hill JA, Olson EN. Trpc6 fulfills a calcineurin signaling circuit during pathologic cardiac remodeling. J Clin Invest. 2006;116:3114–3126. doi: 10.1172/JCI27702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Leask A, Abraham DJ. All in the ccn family: Essential matricellular signaling modulators emerge from the bunker. J Cell Sci. 2006;119:4803–4810. doi: 10.1242/jcs.03270. [DOI] [PubMed] [Google Scholar]
- 190.Chuva de Sousa Lopes SM, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S, ten Dijke P, Lyons KM, Goldschmeding R, Doevendans P, Mummery CL. Connective tissue growth factor expression and smad signaling during mouse heart development and myocardial infarction. Dev Dyn. 2004;231:542–550. doi: 10.1002/dvdy.20162. [DOI] [PubMed] [Google Scholar]
- 191.Mori T, Kawara S, Shinozaki M, Hayashi N, Kakinuma T, Igarashi A, Takigawa M, Nakanishi T, Takehara K. Role and interaction of connective tissue growth factor with transforming growth factor-beta in persistent fibrosis: A mouse fibrosis model. Journal of cellular physiology. 1999;181:153–159. doi: 10.1002/(SICI)1097-4652(199910)181:1<153::AID-JCP16>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 192.Koshman YE, Patel N, Chu MS, Iyengar R, Kim T, Ersahin C, Lewis W, Heroux A, Samarel AM. Regulation of connective tissue growth factor gene expression and fibrosis in human heart failure. J Card Fail. 2013;19:283–294. doi: 10.1016/j.cardfail.2013.01.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Rosin NL, Falkenham A, Sopel MJ, Lee TD, Legare JF. Regulation and role of connective tissue growth factor in angii-induced myocardial fibrosis. Am J Pathol. 2013;182:714–726. doi: 10.1016/j.ajpath.2012.11.014. [DOI] [PubMed] [Google Scholar]
- 194.Tank J, Lindner D, Wang X, Stroux A, Gilke L, Gast M, Zietsch C, Skurk C, Scheibenbogen C, Klingel K, Lassner D, Kuhl U, Schultheiss HP, Westermann D, Poller W. Single-target rna interference for the blockade of multiple interacting proinflammatory and profibrotic pathways in cardiac fibroblasts. J Mol Cell Cardiol. 2014;66:141–156. doi: 10.1016/j.yjmcc.2013.11.004. [DOI] [PubMed] [Google Scholar]
- 195.Szabo Z, Magga J, Alakoski T, Ulvila J, Piuhola J, Vainio L, Kivirikko KI, Vuolteenaho O, Ruskoaho H, Lipson KE, Signore P, Kerkela R. Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure. Hypertension. 2014;63:1235–1240. doi: 10.1161/HYPERTENSIONAHA.114.03279. [DOI] [PubMed] [Google Scholar]
- 196.Accornero F, van Berlo JH, Correll RN, Elrod JW, Sargent MA, York A, Rabinowitz JE, Leask A, Molkentin JD. Genetic analysis of connective tissue growth factor as an effector of transforming growth factor beta signaling and cardiac remodeling. Molecular and cellular biology. 2015;35:2154–2164. doi: 10.1128/MCB.00199-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Zhao W, Zhao T, Huang V, Chen Y, Ahokas RA, Sun Y. Platelet-derived growth factor involvement in myocardial remodeling following infarction. J Mol Cell Cardiol. 2011;51:830–838. doi: 10.1016/j.yjmcc.2011.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Ponten A, Folestad EB, Pietras K, Eriksson U. Platelet-derived growth factor d induces cardiac fibrosis and proliferation of vascular smooth muscle cells in heart-specific transgenic mice. Circ Res. 2005;97:1036–1045. doi: 10.1161/01.RES.0000190590.31545.d4. [DOI] [PubMed] [Google Scholar]
- 199.Zhao T, Zhao W, Chen Y, Li VS, Meng W, Sun Y. Platelet-derived growth factor-d promotes fibrogenesis of cardiac fibroblasts. American journal of physiology. Heart and circulatory physiology. 2013;304:H1719–1726. doi: 10.1152/ajpheart.00130.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Tuuminen R, Nykanen AI, Krebs R, Soronen J, Pajusola K, Keranen MA, Koskinen PK, Alitalo K, Lemstrom KB. Pdgf-a, -c, and -d but not pdgf-b increase tgf-beta1 and chronic rejection in rat cardiac allografts. Arterioscler Thromb Vasc Biol. 2009;29:691–698. doi: 10.1161/ATVBAHA.108.178558. [DOI] [PubMed] [Google Scholar]
- 201.Liu C, Zhao W, Meng W, Zhao T, Chen Y, Ahokas RA, Liu H, Sun Y. Platelet-derived growth factor blockade on cardiac remodeling following infarction. Molecular and cellular biochemistry. 2014;397:295–304. doi: 10.1007/s11010-014-2197-x. [DOI] [PubMed] [Google Scholar]
- 202.Liao CH, Akazawa H, Tamagawa M, Ito K, Yasuda N, Kudo Y, Yamamoto R, Ozasa Y, Fujimoto M, Wang P, Nakauchi H, Nakaya H, Komuro I. Cardiac mast cells cause atrial fibrillation through pdgf-a-mediated fibrosis in pressure-overloaded mouse hearts. J Clin Invest. 2010;120:242–253. doi: 10.1172/JCI39942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Hudon-David F, Bouzeghrane F, Couture P, Thibault G. Thy-1 expression by cardiac fibroblasts: Lack of association with myofibroblast contractile markers. J Mol Cell Cardiol. 2007;42:991–1000. doi: 10.1016/j.yjmcc.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 204.Ross RS, Borg TK. Integrins and the myocardium. Circ Res. 2001;88:1112–1119. doi: 10.1161/hh1101.091862. [DOI] [PubMed] [Google Scholar]
- 205.Talior-Volodarsky I, Connelly KA, Arora PD, Gullberg D, McCulloch CA. Alpha11 integrin stimulates myofibroblast differentiation in diabetic cardiomyopathy. Cardiovasc Res. 2012;96:265–275. doi: 10.1093/cvr/cvs259. [DOI] [PubMed] [Google Scholar]
- 206.Pechkovsky DV, Scaffidi AK, Hackett TL, Ballard J, Shaheen F, Thompson PJ, Thannickal VJ, Knight DA. Transforming growth factor beta1 induces alphavbeta3 integrin expression in human lung fibroblasts via a beta3 integrin-, c-src-, and p38 mapk-dependent pathway. J Biol Chem. 2008;283:12898–12908. doi: 10.1074/jbc.M708226200. [DOI] [PubMed] [Google Scholar]
- 207.Reed NI, Jo H, Chen C, Tsujino K, Arnold TD, DeGrado WF, Sheppard D. The alphavbeta1 integrin plays a critical in vivo role in tissue fibrosis. Sci Transl Med. 2015;7:288ra279. doi: 10.1126/scitranslmed.aaa5094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Christia P, Bujak M, Gonzalez-Quesada C, Chen W, Dobaczewski M, Reddy A, Frangogiannis NG. Systematic characterization of myocardial inflammation, repair, and remodeling in a mouse model of reperfused myocardial infarction. J Histochem Cytochem. 2013;61:555–570. doi: 10.1369/0022155413493912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Zhao L, Eghbali-Webb M. Release of pro- and anti-angiogenic factors by human cardiac fibroblasts: Effects on DNA synthesis and protection under hypoxia in human endothelial cells. Biochimica et biophysica acta. 2001;1538:273–282. doi: 10.1016/s0167-4889(01)00078-7. [DOI] [PubMed] [Google Scholar]
- 210.Mitchell MD, Laird RE, Brown RD, Long CS. Il-1beta stimulates rat cardiac fibroblast migration via map kinase pathways. American journal of physiology. Heart and circulatory physiology. 2007;292:H1139–1147. doi: 10.1152/ajpheart.00881.2005. [DOI] [PubMed] [Google Scholar]
- 211.Banerjee I, Fuseler JW, Intwala AR, Baudino TA. Il-6 loss causes ventricular dysfunction, fibrosis, reduced capillary density, and dramatically alters the cell populations of the developing and adult heart. American journal of physiology. Heart and circulatory physiology. 2009;296:H1694–1704. doi: 10.1152/ajpheart.00908.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Ivashkiv LB, Donlin LT. Regulation of type i interferon responses. Nat Rev Immunol. 2014;14:36–49. doi: 10.1038/nri3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Noppert SJ, Fitzgerald KA, Hertzog PJ. The role of type i interferons in tlr responses. Immunol Cell Biol. 2007;85:446–457. doi: 10.1038/sj.icb.7100099. [DOI] [PubMed] [Google Scholar]
- 214.Levick S, Goldspink P. Could interferon-gamma be a therapeutic target for treating heart failure? Heart Fail Rev. 2014;19:227–236. doi: 10.1007/s10741-013-9393-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Han YL, Li YL, Jia LX, Cheng JZ, Qi YF, Zhang HJ, Du J. Reciprocal interaction between macrophages and t cells stimulates ifn-gamma and mcp-1 production in ang ii-induced cardiac inflammation and fibrosis. Plos One. 2012;7 doi: 10.1371/journal.pone.0035506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Marko L, Kvakan H, Park JK, Qadri F, Spallek B, Binger KJ, Bowman EP, Kleinewietfeld M, Fokuhl V, Dechend R, Muller DN. Interferon-gamma signaling inhibition ameliorates angiotensin ii-induced cardiac damage. Hypertension. 2012;60:1430–U1154. doi: 10.1161/HYPERTENSIONAHA.112.199265. [DOI] [PubMed] [Google Scholar]
- 217.Sun Y, Weber KT. Animal models of cardiac fibrosis. Methods Mol Med. 2005;117:273–290. doi: 10.1385/1-59259-940-0:273. [DOI] [PubMed] [Google Scholar]
- 218.Turner NA, Porter KE, Smith WHT, White HL, Ball SG, Balmforth AJ. Chronic beta(2)-adrenergic receptor stimulation increases proliferation of human cardiac fibroblasts via an autocrine mechanism. Cardiovasc Res. 2003;57:784–792. doi: 10.1016/s0008-6363(02)00729-0. [DOI] [PubMed] [Google Scholar]
- 219.Meszaros JG, Gonzalez AM, Endo-Mochizuki Y, Villegas S, Villarreal F, Brunton LL. Identification of g protein-coupled signaling pathways in cardiac fibroblasts: Cross talk between g(q) and g(s) Am J Physiol-Cell Ph. 2000;278:C154–C162. doi: 10.1152/ajpcell.2000.278.1.C154. [DOI] [PubMed] [Google Scholar]
- 220.Liu XQ, Insel PA, Ostrom RS. Decreased sensitivity of fibroblasts cultured from human fibrotic lung to camp-mediated inhibition of cell proliferation and collagen svnthesis. Faseb J. 2004;18:A312–A313. [Google Scholar]
- 221.Swaney JS, Roth DM, Olson ER, Naugle JE, Meszaros JG, Insel PA. Inhibition of cardiac myofibroblast formation and collagen synthesis by activation and overexpression of adenylyl cyclase. P Natl Acad Sci USA. 2005;102:437–442. doi: 10.1073/pnas.0408704102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Liu XQ, Sun SQ, Hassid A, Ostrom RS. Camp inhibits transforming growth factor-beta-stimulated collagen synthesis via inhibition of extracellular signal-regulated kinase 1/2 and smad signaling in cardiac fibroblasts. Mol Pharmacol. 2006;70:1992–2003. doi: 10.1124/mol.106.028951. [DOI] [PubMed] [Google Scholar]
- 223.D’Souza KM, Malhotra R, Philip JL, Staron ML, Theccanat T, Jeevanandam V, Akhter SA. G protein-coupled receptor kinase-2 is a novel regulator of collagen synthesis in adult human cardiac fibroblasts. J Biol Chem. 2011;286:15507–15516. doi: 10.1074/jbc.M111.218263. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 224.Robinson JD, Pitcher JA. G protein-coupled receptor kinase 2 (grk2) is a rho-activated scaffold protein for the erk map kinase cascade. Cell Signal. 2013;25:2831–2839. doi: 10.1016/j.cellsig.2013.08.031. [DOI] [PubMed] [Google Scholar]
- 225.Li J, Philip JL, Xu X, Theccanat T, Abdur Razzaque M, Akhter SA. Beta-arrestins regulate human cardiac fibroblast transformation and collagen synthesis in adverse ventricular remodeling. J Mol Cell Cardiol. 2014;76:73–83. doi: 10.1016/j.yjmcc.2014.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Thal DM, Homan KT, Chen J, Wu EK, Hinkle PM, Huang ZM, Chuprun JK, Song J, Gao E, Cheung JY, Sklar LA, Koch WJ, Tesmer JJ. Paroxetine is a direct inhibitor of g protein-coupled receptor kinase 2 and increases myocardial contractility. ACS Chem Biol. 2012;7:1830–1839. doi: 10.1021/cb3003013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Schumacher SM, Gao E, Zhu W, Chen X, Chuprun JK, Feldman AM, JJGT, Koch WJ. Paroxetine-mediated grk2 inhibition reverses cardiac dysfunction and remodeling after myocardial infarction. Sci Transl Med. 2015;7:277ra231. doi: 10.1126/scitranslmed.aaa0154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Raake PW, Schlegel P, Ksienzyk J, Reinkober J, Barthelmes J, Schinkel S, Pleger S, Mier W, Haberkorn U, Koch WJ, Katus HA, Most P, Muller OJ. Aav6.Betaarkct cardiac gene therapy ameliorates cardiac function and normalizes the catecholaminergic axis in a clinically relevant large animal heart failure model. Eur Heart J. 2013;34:1437–1447. doi: 10.1093/eurheartj/ehr447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Rengo G, Lymperopoulos A, Zincarelli C, Donniacuo M, Soltys S, Rabinowitz JE, Koch WJ. Myocardial adeno-associated virus serotype 6-betaarkct gene therapy improves cardiac function and normalizes the neurohormonal axis in chronic heart failure. Circulation. 2009;119:89–98. doi: 10.1161/CIRCULATIONAHA.108.803999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Belmonte SL, Blaxall BC. G protein coupled receptor kinases as therapeutic targets in cardiovascular disease. Circ Res. 2011;109:309–319. doi: 10.1161/CIRCRESAHA.110.231233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Casey LM, Pistner AR, Belmonte SL, Migdalovich D, Stolpnik O, Nwakanma FE, Vorobiof G, Dunaevsky O, Matavel A, Lopes CM, Smrcka AV, Blaxall BC. Small molecule disruption of g beta gamma signaling inhibits the progression of heart failure. Circ Res. 2010;107:532–539. doi: 10.1161/CIRCRESAHA.110.217075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kamal FA, Mickelsen DM, Wegman KM, Travers JG, Moalem J, Hammes SR, Smrcka AV, Blaxall BC. Simultaneous adrenal and cardiac g-protein-coupled receptor-gbetagamma inhibition halts heart failure progression. J Am Coll Cardiol. 2014;63:2549–2557. doi: 10.1016/j.jacc.2014.02.587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Kamal FA, Smrcka AV, Blaxall BC. Taking the heart failure battle inside the cell: Small molecule targeting of gbetagamma subunits. J Mol Cell Cardiol. 2011;51:462–467. doi: 10.1016/j.yjmcc.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Ieda M, Fu JD, Delgado-Olguin P, Vedantham V, Hayashi Y, Bruneau BG, Srivastava D. Direct reprogramming of fibroblasts into functional cardiomyocytes by defined factors. Cell. 2010;142:375–386. doi: 10.1016/j.cell.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Ieda M, Tsuchihashi T, Ivey KN, Ross RS, Hong TT, Shaw RM, Srivastava D. Cardiac fibroblasts regulate myocardial proliferation through beta1 integrin signaling. Dev Cell. 2009;16:233–244. doi: 10.1016/j.devcel.2008.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Qian L, Huang Y, Spencer CI, Foley A, Vedantham V, Liu L, Conway SJ, Fu JD, Srivastava D. In vivo reprogramming of murine cardiac fibroblasts into induced cardiomyocytes. Nature. 2012;485:593. doi: 10.1038/nature11044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Song K, Nam YJ, Luo X, Qi X, Tan W, Huang GN, Acharya A, Smith CL, Tallquist MD, Neilson EG, Hill JA, Bassel-Duby R, Olson EN. Heart repair by reprogramming non-myocytes with cardiac transcription factors. Nature. 2012;485:599–604. doi: 10.1038/nature11139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, Gregoire S, Engels MC, Rajarajan K, Karra R, Abel ED, Wu JC, Milan D, Wu SM. Inefficient reprogramming of fibroblasts into cardiomyocytes using gata4, mef2c, and tbx5. Circ Res. 2012;111:50–55. doi: 10.1161/CIRCRESAHA.112.270264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Deb A, Ubil E. Cardiac fibroblast in development and wound healing. J Mol Cell Cardiol. 2014;70:47–55. doi: 10.1016/j.yjmcc.2014.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Zhao Y, Londono P, Cao Y, Sharpe EJ, Proenza C, O’Rourke R, Jones KL, Jeong MY, Walker LA, Buttrick PM, McKinsey TA, Song K. High-efficiency reprogramming of fibroblasts into cardiomyocytes requires suppression of pro-fibrotic signalling. Nat Commun. 2015;6:8243. doi: 10.1038/ncomms9243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Angst BD, Khan LU, Severs NJ, Whitely K, Rothery S, Thompson RP, Magee AI, Gourdie RG. Dissociated spatial patterning of gap junctions and cell adhesion junctions during postnatal differentiation of ventricular myocardium. Circ Res. 1997;80:88–94. doi: 10.1161/01.res.80.1.88. [DOI] [PubMed] [Google Scholar]
- 242.Baudino TA, McFadden A, Fix C, Hastings J, Price R, Borg TK. Cell patterning: Interaction of cardiac myocytes and fibroblasts in three-dimensional culture. Microscopy and microanalysis : the official journal of Microscopy Society of America, Microbeam Analysis Society, Microscopical Society of Canada. 2008;14:117–125. doi: 10.1017/S1431927608080021. [DOI] [PubMed] [Google Scholar]
- 243.Yue LX, Xie J, Nattel S. Molecular determinants of cardiac fibroblast electrical function and therapeutic implications for atrial fibrillation. Cardiovasc Res. 2011;89:744–753. doi: 10.1093/cvr/cvq329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kanno S, Saffitz JE. The role of myocardial gap junctions in electrical conduction and arrhythmogenesis. Cardiovasc Pathol. 2001;10:169–177. doi: 10.1016/s1054-8807(01)00078-3. [DOI] [PubMed] [Google Scholar]
- 245.Kizana E, Ginn SL, Smyth CM, Boyd A, Thomas SP, Allen DG, Ross DL, Alexander IE. Fibroblasts modulate cardiomyocyte excitability: Implications for cardiac gene therapy. Gene Ther. 2006;13:1611–1615. doi: 10.1038/sj.gt.3302813. [DOI] [PubMed] [Google Scholar]
- 246.Zhang Y, Kanter E, Laing J, Aprhys C, Johns D, Kardami E, Yamada K. Connexin43 expression levels influence intercellular coupling and cell proliferation of native murine cardiac fibroblasts. Cell Commun Adhes. 2008;15:289–303. doi: 10.1080/15419060802198736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Chilton L, Giles WR, Smith GL. Evidence of intercellular coupling between co-cultured adult rabbit ventricular myocytes and myofibroblasts. J Physiol. 2007;583:225–236. doi: 10.1113/jphysiol.2007.135038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Gaudesius G, Miragoli M, Thomas SP, Rohr S. Coupling of cardiac electrical activity over extended distances by fibroblasts of cardiac origin. Circ Res. 2003;93:421–428. doi: 10.1161/01.RES.0000089258.40661.0C. [DOI] [PubMed] [Google Scholar]
- 249.Kohl P. Heterogeneous cell coupling in the heart: An electrophysiological role for fibroblasts. Circ Res. 2003;93:381–383. doi: 10.1161/01.RES.0000091364.90121.0C. [DOI] [PubMed] [Google Scholar]
- 250.Miragoli M, Gaudesius G, Rohr S. Electrotonic modulation of cardiac impulse conduction by myofibroblasts. Circ Res. 2006;98:801–810. doi: 10.1161/01.RES.0000214537.44195.a3. [DOI] [PubMed] [Google Scholar]
- 251.Rohr S. Role of gap junctions in the propagation of the cardiac action potential. Cardiovasc Res. 2004;62:309–322. doi: 10.1016/j.cardiores.2003.11.035. [DOI] [PubMed] [Google Scholar]
- 252.Sachse FB, Moreno AP, Abildskov JA. Electrophysiological modeling of fibroblasts and their interaction with myocytes. Ann Biomed Eng. 2008;36:41–56. doi: 10.1007/s10439-007-9405-8. [DOI] [PubMed] [Google Scholar]
- 253.Zhang Y, Kanter EM, Yamada KA. Remodeling of cardiac fibroblasts following myocardial infarction results in increased gap junction intercellular communication. Cardiovasc Pathol. 2010;19:e233–240. doi: 10.1016/j.carpath.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Roell W, Lewalter T, Sasse P, Tallini YN, Choi BR, Breitbach M, Doran R, Becher UM, Hwang SM, Bostani T, von Maltzahn J, Hofmann A, Reining S, Eiberger B, Gabris B, Pfeifer A, Welz A, Willecke K, Salama G, Schrickel JW, Kotlikoff MI, Fleischmann BK. Engraftment of connexin 43-expressing cells prevents post-infarct arrhythmia. Nature. 2007;450:819–U813. doi: 10.1038/nature06321. [DOI] [PubMed] [Google Scholar]
- 255.Litvin J, Chen X, Keleman S, Zhu S, Autieri M. Expression and function of periostin-like factor in vascular smooth muscle cells. Am J Physiol Cell Physiol. 2007;292:C1672–1680. doi: 10.1152/ajpcell.00153.2006. [DOI] [PubMed] [Google Scholar]
- 256.Mack CP. Signaling mechanisms that regulate smooth muscle cell differentiation. Arterioscler Thromb Vasc Biol. 2011;31:1495–1505. doi: 10.1161/ATVBAHA.110.221135. [DOI] [PMC free article] [PubMed] [Google Scholar]