Summary
Background
Treatment options for advanced, well-differentiated neuroendocrine tumours (NETs) remain scarce. Pazopanib is an orally bioavailable, small molecule, multitargeted kinase inhibitor that inhibits VEGF receptors 1, 2, and 3. We did a study of the efficacy of pazopanib with depot octreotide in patients with advanced NETs.
Methods
We did a parallel cohort study of patients with metastatic or locally advanced grade 1–2 carcinoid tumours or pancreatic NETs, by use of a single-group, two-stage design. Patients received pazopanib 800 mg orally once per day and octreotide at their preprotocol dosage. The primary endpoint was the proportion of patients achieving an objective response, as assessed by investigators, by intention-to-treat analysis. This study is registered with ClinicalTrials.gov, identifier NCT00454363, and was completed in March, 2014.
Findings
Between April 12, 2007, and July 2, 2009, we enrolled 52 patients, including 32 individuals with pancreatic NETs and 20 individuals with carcinoid tumours. Seven (21.9%, 95% CI 11.0–38.8) of 32 patients with pancreatic NETs achieved an objective response. We detected no responses in the first stage of the cohort with carcinoid tumours, and we terminated accrual at 20 patients. Toxic effects included one patient with grade 4 hypertriglyceridaemia and one with grade 4 thrombosis, with the most common grade three events being aminotransferase increases and neutropenia, each of which happened in 3 patients. In all 52 patients, the most frequently observed toxic effects were fatigue (39 [75%]), nausea (33 [63%]), diarrhoea (33 [63%]), and hypertension (28 [54%]).
Interpretation
Treatment with pazopanib is associated with tumour response for patients with pancreatic NETs, but not for carcinoid tumours; a randomised controlled phase 3 study to assess pazopanib in advanced pancreatic NETs is warranted.
Funding
US National Cancer Institute of the National Institutes of Health.
Introduction
Well-differentiated neuroendocrine tumours (NETs) include both pancreatic NETs (formerly known as islet cell carcinomas) and carcinoid tumours (NETs with any extrapancreatic primary site). Treatment options for both tumour subtypes remain scarce. Somatostatin analogues control symptoms of hormone hypersecretion, and evidence suggests that they also slow tumour growth.1,2 The mTOR inhibitor everolimus3 and the multitargeted kinase inhibitor sunitinib4 have shown activity in, and are approved for use for, patients with advanced pancreatic NETs. Lanreotide is the only drug currently approved for tumour control for patients with advanced carcinoid tumours.
Evidence suggests that VEGF pathway inhibitors might be promising treatments for NETs. VEGF receptor-2 is one of the main targets of sunitinib; treatment with sunitinib was reported to be associated with a significant improvement in progression-free survival compared with placebo for patients with advanced pancreatic NETs.4 Preliminary evidence of activity against NETs also exists for the tyrosine kinase inhibitor sorafenib,5–7 and bevacizumab,8–12 a monoclonal antibody that targets VEGF. Pazopanib, an oral multitargeted kinase inhibitor that targets VEGF receptors 1, 2, and 3, is approved for treatment of advanced renal cell carcinoma.13 Progression-free survival for patients with renal cell carcinoma treated with pazopanib is non-inferior compared with sunitinib, with a favourable toxic effect profile.14 Retrospective15 and prospective16 studies have suggested beneficial effects of pazopanib for patients with progressing advanced renal cell carcinoma, who were treated with VEGF inhibitors.
We tested whether pazopanib would have therapeutic activity in NETs. In view of evidence that carcinoid tumours and pancreatic NETs respond differently to systemic therapies,17 we tested this hypothesis in separate parallel cohorts for patients with pancreatic NETs and those with carcinoid tumours.
Methods
Study design and participants
We did a parallel cohort study of patients with metastatic or locally advanced grade 1–2 carcinoid tumours or pancreatic NETs. Patients were enrolled from the University of Texas MD Anderson Cancer Center and the Dana-Farber Cancer Institute, and were eligible if they were aged 21 years or older, with confirmed metastatic or unresectable grade 1–2 carcinoid tumours or pancreatic NETs, with or without previous treatment. Patients needed to have measurable disease as defined by Response Evaluation Criteria in Solid Tumors (RECIST) 1.0. We included patients who had received previous treatment, including cytotoxic chemotherapy (one regimen or fewer), surgery (if done ≥4 weeks before start of treatment), radiation (≥4 weeks), interferon therapy (≥4 weeks), and treatments that targeted pathways other than VEGF (>30 days). If patients had received octreotide, they needed to have received unchanged doses for at least 2 months before starting the treatment protocol, although octreotide treatment was not itself an inclusion criterion. To be eligible, patients needed Eastern Cooperative Oncology Group (ECOG) performance status of 1 or 0, with adequate organ function, as assessed by complete blood count, electrolytes, and liver function tests. Additionally, eligible patients needed to have resting systolic blood pressure 140 mm Hg or lower and diastolic blood pressure 90 mm Hg or lower, before they entered the study, with or without use of antihypertensive medications. We excluded patients if they were receiving chronic warfarin anticoagulation or drugs that interacted with the CYP450 system.
At the time of the study, estimated median overall survival of this population was 27 months for the cohort with pancreatic NETs and 46 months for the cohort with carcinoid tumours, based on a weighted average of the median survival durations of patients with NETs of the primary sites included in this study.18 The institutional review boards of the MD Anderson Cancer Center and Dana-Farber Cancer Institute approved the protocol. We did the study in accordance with the Declaration of Helsinki and Good Clinical Practice Guidelines. All patients provided written informed consent.
Procedures
After we received informed consent, patients were given pazopanib hydrochloride (GlaxoSmithKline, Collegeville, PA, USA) 800 mg orally once per day until disease progression or completion of 12 treatment cycles, of 28 days each. We discontinued pazopanib and took patients off protocol if they had grade 4 hypertension, symptomatic thrombosis, or haemorrhage, in accordance with the Common Terminology Criteria for Adverse Events (CTCAE) 4.0. For other grade 3 or 4 toxic effects, we stopped treatment until patients recovered to grade 1 or less, with dose reduction (by 400 mg if haematological toxicity, 200 mg if non-haematological toxicity) on resumption. Full details of dose reductions are included in the protocol section of the appendix. We removed patients from the study if toxic effects persisted after 21 days off treatment.
All patients who had previously received depot octreotide continued to receive it at their preprotocol dose without interruption, with the dose not exceeding 40 mg every 3 weeks. If any patients had been enrolled who had not previously received octreotide, they would have been given the standard dose of 30 mg intramuscularly every 28 days. We allowed depot octreotide dose reductions in 10 mg increments for CTCAE grade 2 or worse bradycardia and any other grade 3 or 4 toxic effects attributed to depot octreotide. We gave a maximum of 8 weeks for recovery, and patients continued to receive pazopanib during this period. If the patient did not recover, we discontinued depot octreotide but continued pazopanib.
Patients could remain in the study until disease progression or completion of 12 cycles, which could be extended at the discretion of the treating physician. Pretreatment and on-study assessments included history, physical examination, and laboratory tests, including complete blood count, electrolytes, and liver function tests. We measured tumours with CT scans or MRI at baseline and every three treatment cycles. We deemed patients to have progressive disease on study entry if baseline imaging showed RECIST-defined progression compared with a scan within the previous 6 months. We measured concentrations of chromogranin A, neuron-specific enolase, pancreatic polypeptide, gastrin, glucagon, vasoactive intestinal peptide, insulin, insulin-like growth factor I (IGF-1) at baseline and, if concentrations were increased, at each radiographic restaging, because these peptide hormones can serve as blood biomarkers of tumour volume when secreted by an individual patient's NET.
Outcomes
The primary endpoint of this study was objective response, defined as the proportion of patients in the cohort who had a 30% reduction in the sum of the longest diameters of up to five prospectively identified target lesions at any assessable timepoint. We assessed response with RECIST 1.019 and used CTCAE 4.0 to assess toxic effects.
The secondary endpoints were overall survival (defined as the time from study entry to death of the patient from any cause) and progression-free survival (defined as the time from study entry to tumour progression [a 20% increase in the sum of the longest diameters of the target lesions] or death from any cause). We assessed blood-based tumour marker levels as exploratory endpoints.
Statistical analysis
We stratified patients by primary site, with cohorts with pancreatic NETs and carcinoid tumours analysed separately for activity. We used a two-stage design, with the potential to stop enrolment into each cohort after 20 patients. For the cohort with pancreatic NETs, the null hypothesis was that 10% of patients would achieve an objective response, with an alternative hypothesis that 30% of patients would achieve an objective response. We continued accrual to 30 patients in total if more than three of the first 20 enrolled patients responded. We excluded the null hypothesis if more than four responses were detected in 30 patients. This design yields a type I error rate of 10% and 89% power to detect the difference between the null and alternative hypotheses. For the cohort with carcinoid tumours, the null hypothesis was that 5% of patients would achieve an objective response, with an alternative hypothesis that 20% of patients would achieve an objective response. We continued accrual to 30 patients in total if more than one of the first 20 patients responded; if we detected more than three responses, we would exclude the null hypothesis. This design yields a type I error rate of 5% and 86% power to detect the difference between the null and alternative hypotheses.
We measured best overall objective response, progression-free survival, and overall survival from the date of study entry to the date of last known contact. We calculated median duration of follow-up using the Kaplan-Meier method. We analysed the data jointly for all patients and as two separate cohorts (carcinoid tumours and pancreatic NETs). The intention-to-treat analysis was stratified by primary site of the tumour (ie, pancreatic NET vs carcinoid tumour), with all patients included in analysis. We did safety analyses on the whole population. For analysis of correlative laboratory studies, we tested baseline values of each biomarker association with RECIST-defined radiographic response using the Mann-Whitney U test. We tested reduction of chromogranin A by 30% or more at week 4 for association with objective radiographic response using the χ² test.
We did all statistical calculations with SPSS version 21.0. The study is registered with ClinicalTrials.gov, number NCT00454363.
Role of the funding source
The funder approved the study design, but had no role in the data collection, data analysis, or data interpretation. The funder had no role in the writing of the manuscript, but did review the manuscript before submission. All authors had access to the raw data. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
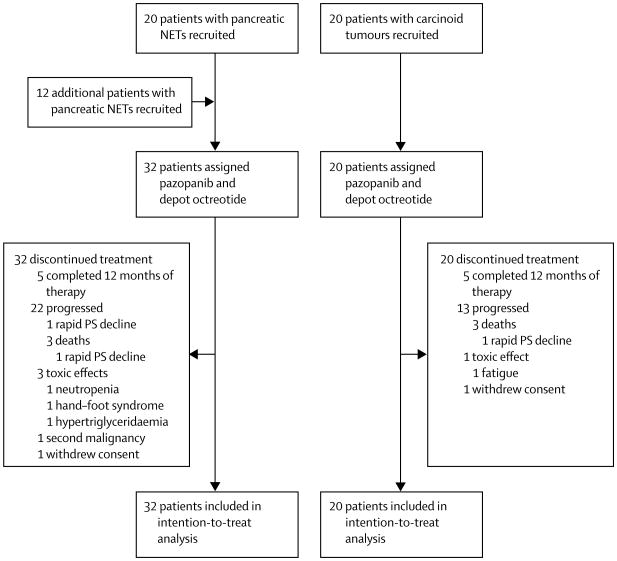
Between April 12, 2007, and July 2, 2009, we treated 52 patients on the protocol: 20 patients had carcinoid tumours and 32 patient had pancreatic NETs. The latest contact with any patient was June 2, 2014. One patient with a pancreatic NET and one patient with a carcinoid tumour withdrew consent before completing one cycle of protocol therapy, but we included them in the intention-to-treat analysis (figure 1). All patients received concurrent octreotide, and 21 (66%) of 32 patients with pancreatic NETs and 17 (85%) of 20 patients with carcinoid tumours had progressive disease at enrolment (table 1). Eight (25%) of 32 patients with pancreatic NETs and six (30%) of 20 patients with carcinoid tumours had received everolimus as part of therapy on other experimental protocols. 21 (66%) of 32 patients with pancreatic NETs and six (30%) of 20 patients with carcinoid tumours had received previous chemotherapy.
Figure 1. Trial profile.
PS=peformance status.
Table 1. Baseline characteristics of the intention-to-treat population.
| Patients with carcinoid tumours (n=20) | Patients with pancreatic NETs (n=32) | |
|---|---|---|
| Age (years) | 63 (60–69) | 54.5 (45–69) |
|
| ||
| Sex | ||
| Male | 12 (60%) | 22 (69%) |
| Female | 8 (40%) | 10 (31%) |
|
| ||
| Performance status | ||
| 0 | 9 (45%) | 16 (50%) |
| 1 | 11 (55%) | 16 (50%) |
|
| ||
| Ethnic origin | ||
| White | 18 (90%) | 26 (81%) |
| Black | 0 (0%) | 2 (6%) |
| Hispanic | 2 (10%) | 3 (9%) |
| Native American | 0 (0%) | 1 (3%) |
|
| ||
| Grade | ||
| 1 | 15 (75%) | 23 (72%) |
| 2 | 5 (25%) | 9 (28%) |
|
| ||
| Carcinoid tumour primary site | ||
| Small bowel | 11 (55%) | ‥ |
| Rectum | 1 (5%) | ‥ |
| Lung | 1 (5%) | ‥ |
| Appendix | 1 (5%) | ‥ |
| Stomach | 1 (5%) | ‥ |
| Caecum | 1 (5%) | ‥ |
| Kidney | 1 (5%) | ‥ |
| Unknown | 3 (15%) | ‥ |
|
| ||
| Baseline chromogranin A increased | ||
| Yes | 16 (80%) | 15 (47%) |
| No | 3 (15%) | 15 (47%) |
| Unknown | 1 (5%) | 2 (6%) |
|
| ||
| Time from diagnosis (months) | 44 (17–65) | 31 (14–70) |
|
| ||
| Extent of disease at enrolment | ||
| Unresectable | 0 (0%) | 4 (13%) |
| Metastatic | 20 (100%) | 28 (88%) |
|
| ||
| Progression at enrolment | ||
| Yes | 17 (85%) | 21 (66%) |
| No | 3 (15%) | 11 (34%) |
|
| ||
| Previous therapy | ||
| SSA | 20 (100%) | 32 (100%) |
| Chemotherapy | 5 (25%) | 21 (66%) |
| Everolimus | 6 (30%) | 8 (25%) |
| Liver-directed therapy | 6 (30%) | 3 (9%) |
Data are median (IQR) or n (%) unless otherwise stated. SSA=somatostatin analogue (octreotide).
The median number of treatment cycles was nine (IQR 3–12) for patients with carcinoid tumours and 11 (4–17.25) for patients with pancreatic NETs. The median time in the study was 9.7 months (IQR 2.9–12.2) for patients with carcinoid tumours and 10.8 months (3.9–16.8) for patients with pancreatic NETs. Median follow-up duration for all patients was 48.5 months (IQR 60.7–71.8); median follow-up was 71.8 months (IQR 49.5–79.4) for patients with carcinoid tumours and 64.3 months (60.7–68.1) for those with pancreatic NETs.
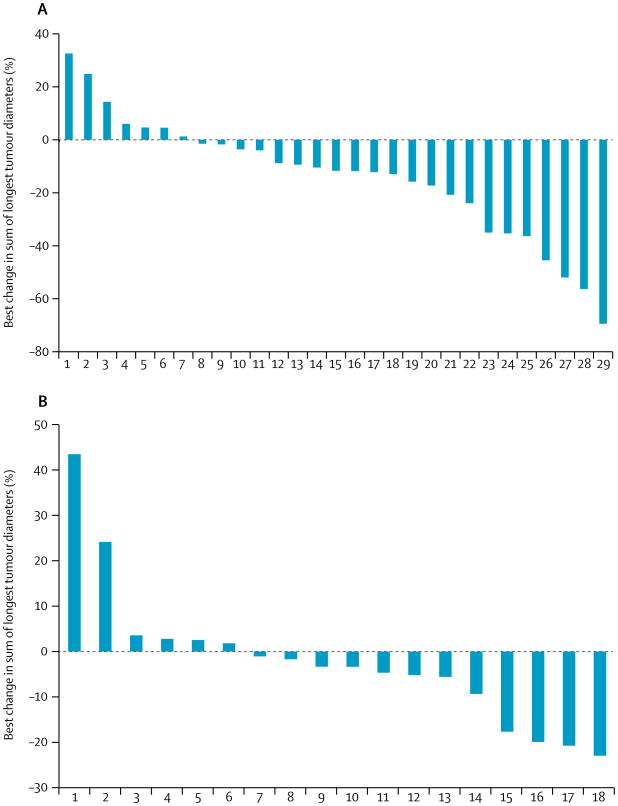
In the pancreatic NET cohort, we observed a response in five of the first 20 enrolled patients, and so enrolled 12 further patients into this cohort (32 patients accrued in total). In the cohort with carcinoid tumours, we saw no responses in the 20 patients in the first stage, so as per protocol we closed the cohort from further enrolment. In the intention-to-treat analysis of the pancreatic NET cohort seven (22%) of 32 patients achieved partial responses, giving an overall objective response of 21.9% (95% CI 11.0–38.8). In carcinoid tumours cohort, the overall objective response was 0% (95% CI 0.0–16.0). Because we understand patients who have progressive disease at study enrolment to present a more uniform and therapeutically relevant group20 than those who do not, we did a post-hoc analysis of this population. Notably, six (29%) of the 21 patients with pancreatic NETs who had progressive disease at study entry showed objective responses, yielding an overall objective response of 28.6% (95% CI 11.3–52.2) in this subgroup (table 2). Furthermore, responses were exclusively in the group of 26 patients who had liver metastases. 22 (76%) of 29 assessable patients with pancreatic NETs and 12 (67%) of 18 assessable patients with carcinoid tumours showed some degree of tumour shrinkage (figure 2). Seven (58%) of the 12 patients with carcinoid tumours who had some degree of tumour shrinkage had primary tumours of the small bowel.
Table 2. Baseline characteristics of the intention-to-treat population stratified by response to pazopanib.
| Patients with pancreatic NETs with response (n=7) | Patients with pancreatic NETs without response (n=25) | |
|---|---|---|
| Age (years) | 69 (65–76) | 53 (44–60) |
|
| ||
| Sex | ||
| Male | 3 (43%) | 19 (76%) |
| Female | 4 (57%) | 6 (24%) |
|
| ||
| Performance status | ||
| 0 | 2 (29%) | 14 (56%) |
| 1 | 5 (71%) | 11 (44%) |
|
| ||
| Ethnic origin | ||
| White | 7 (100%) | 19 (76%) |
| Black | 0 (0%) | 2 (8%) |
| Hispanic | 0 (0%) | 3 (12%) |
| Native American | 0 (0%) | 1 (4%) |
|
| ||
| Grade | ||
| 1 | 5 (71%) | 18 (72%) |
| 2 | 2 (29%) | 7 (28%) |
|
| ||
| Baseline chromogranin A increased | ||
| Yes | 6 (86%) | 9 (36%) |
| No | 1 (14%) | 14 (56%) |
| Unknown | 0 (0%) | 2 (8%) |
|
| ||
| Time from diagnosis (months) | 31 (18–56) | 30 (13–75) |
|
| ||
| Extent of disease at enrolment | ||
| Unresectable | 0 (0%) | 4 (16%) |
| Metastatic | 7 (100%) | 21 (84%) |
|
| ||
| Progression at enrolment | ||
| Yes | 6 (86%) | 15 (60%) |
| No | 1 (14%) | 10 (40%) |
|
| ||
| Previous therapy | ||
| SSA | 7 (100%) | 25 (100%) |
| Chemotherapy | 5 (71%) | 16 (64%) |
| Everolimus | 2 (29%) | 6 (24%) |
| Liver-directed therapy | 1 (14%) | 2 (8%) |
Data are median (IQR) or n (%) unless otherwise stated. SSA=somatostatin analogue (octreotide).
Figure 2. Best response for all patients with pancreatic neuroendocrine tumours (A) and carcinoid tumours (B).
Waterfall plots show data as percentage change in sum of longest tumour diameters.
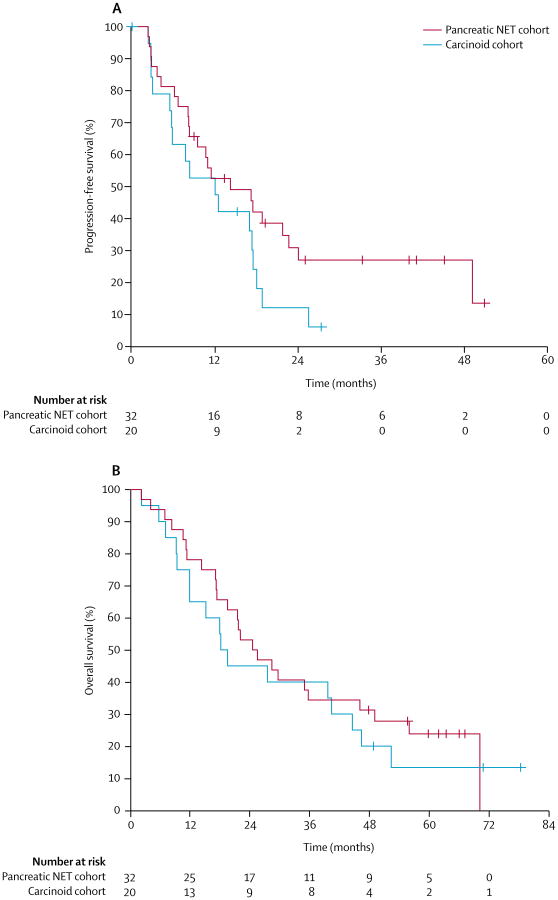
The median progression-free survival in the cohort with pancreatic NETs was 14.4 months (95% CI 5.9–22.9), and median overall survival in this cohort was 25 months (95% CI 15.5–34.4; figure 3). Median progression-free survival in the cohort with carcinoid tumours was 12.2 months (95% CI 5.3–19.0), and median overall survival was 18.5 months (95% CI 15.0–22.0; figure 3). When we did a post-hoc analysis on only patients with progressive disease at enrolment, patients with pancreatic NETs had a median progression-free survival of 14.4 months (95% CI 1.5–27.3) and a median overall survival of 22.1 months (95% CI 18.2–26.0). Patients with carcinoid tumours and progressive disease at enrolment had a median progression-free survival of 8.4 months (95% CI 0.0–16.9) and a median overall survival of 18.3 months (95% CI 9.8–26.8; appendix).
Figure 3. Progression-free survival (A) and overall survival (B) by cohort.
NET=neuroendocrine tumour.
Pazopanib was generally well tolerated. The only grade 4 adverse events were one asymptomatic thromboembolic event and an episode of hypertriglyceridaemia (table 3). The hyper triglyceridaemia resulted in the patient discontinuing protocol therapy, but the patient with thrombosis remained on protocol therapy. The most frequent grade 3 events were increases in aminotransferases and neutropenia, each of which was detected in three (6%) patients. In all 52 patients, the most frequently observed toxic effects were fatigue (39 [75%]), nausea (33 [63%]), diarrhoea (33 [63%]), and hypertension (28 [54%]; table 3). Four patients discontinued therapy because of toxic effects (one [2%] each of neutropenia, hand–foot syndrome, fatigue, and grade 4 hypertriglyceridaemia). 12 (23%) of 52 patients needed dose-reductions because of toxic effects, with seven reductions to 400 mg and five reductions to 600 mg. 18 (35%) patients had disease progression during the first 12 months on protocol, and three (6%) patients had symptomatic disease progression with reductions in performance status precluding their remaining on protocol. 25 (48%) patients completed at least 12 months of protocol therapy. Two patients withdrew consent for participation. No treatment-related deaths occurred.
Table 3. Adverse events.
| Grade 1–2 | Grade 3 | Grade 4 | |
|---|---|---|---|
| Haematological toxic effects | |||
|
| |||
| Anaemia | 12 (23%) | 1 (2%) | 0 |
| Neutrophil count decreased | 7 (13%) | 3 (6%) | 0 |
| Platelet count decreased | 11 (21%) | 0 | 0 |
| White blood cell decreased | 9 (17%) | 1 (2%) | 0 |
|
| |||
| Non-haematological toxic effects | |||
|
| |||
| Abdominal pain | 6 (12%) | 1 (2%) | 0 |
| Alanine aminotransferase increased | 14 (27%) | 3 (6%) | 0 |
| Alkaline phosphatase increased | 4 (8%) | 1 (2%) | 0 |
| Aspartate aminotransferase increased | 20 (38%) | 3 (6%) | 0 |
| Blood bilirubin increased | 11 (21%) | 0 | 0 |
| Confusion | 0 | 1 (2%) | 0 |
| Constipation | 8 (15%) | 0 | 0 |
| Dehydration | 0 | 1 (2%) | 0 |
| Diarrhoea | 30 (58%) | 3 (6%) | 0 |
| Fatigue | 35 (67%) | 4 (8%) | 0 |
| Headache | 9 (17%) | 0 | 0 |
| Hyperglycaemia | 6 (12%) | 0 | 0 |
| Hypertension | 22 (42%) | 6 (12%) | 0 |
| Hypertriglyceridaemia | 0 | 1 (2%) | 1 (2%) |
| Hypocalcaemia | 7 (13%) | 0 | 0 |
| Hypomagnesaemia | 9 (17%) | 0 | 0 |
| Hypophosphataemia | 5 (10%) | 0 | 0 |
| INR increased | 7 (13%) | 0 | 0 |
| Mucositis oral | 9 (17%) | 0 | 0 |
| Nausea | 32 (62%) | 1 (2%) | 0 |
| Hand–foot syndrome | 2 (4%) | 1 (2%) | 0 |
| Pancreatitis | 0 | 1 (2%) | 0 |
| Skin and subcutaneous tissue disorders (other) | 6 (12%) | 0 | 0 |
| Skin hypopigmentation | 7 (13%) | 0 | 0 |
| Thromboembolic event | 0 | 0 | 1 (2%) |
| Urinary tract infection | 0 | 1 (2%) | 0 |
| Vomiting | 11 (21%) | 0 | 0 |
Adverse events according to Common Terminology Criteria for Adverse Events 4.0, excluding grade 1–2 toxic effects that occurred in less than 10% of patients.
46 of 49 patients who could be assessed for RECIST had baseline measurements of chromogranin A and 45 (98%) of them had subsequent measurements. We had measure ments for 25 patients for baseline neuron-specific enolase, 25 patients for baseline vasoactive intestinal peptide, 30 patients for baseline glucagon, 25 patients for baseline pancreatic polypeptide, 11 patients for IGF-1, 18 patients for gastrin, and two patients for insulin. Baseline biomarkers were not clearly linked with likelihood of response. Because we identified no responses in the carcinoid cohort, no associations with likelihood of response could be made. In patients with pancreatic NETs, 15 (54%) of the 28 assessable patients had at least a 30% reduction in chromogranin A levels, as did seven (41%) of the 17 assessable patients with carcinoid tumours. In the cohort with pancreatic NETs, patients who had a 30% or more reduction in chromogranin A at week 4 were more likely to have an objective response to pazopanib than were those who did not (six [40%] of 15 patients with chromogranin A reduction vs one [8%] of 13 patients without chromogranin A reduction; p=0.047). Exploratory analysis of progression-free survival and overall survival did not reveal an association with early chromogranin A reduction (data not shown).
Discussion
Our data suggest that pazopanib is well tolerated in patients with advanced carcinoid tumours and pancreatic NETs. We saw clinical activity in patients with advanced pancreatic NETs.
In the development of pazopanib for renal cell carcinoma, results showed distinct adverse event profiles for pazopanib and sunitinib. Pazopanib had more frequent hair colour changes (168 [30%] of 554 patients), weight loss (84 [15%] of 554 patients), hypoglycaemia (83 [15%] of 548 patients), and hepatic enzyme abnormalities (at least 333 [61%] of 547 patients), but sunitinib had more frequent fatigue (344 [63%] of 548 patients), hand–foot syndrome (275 [50%] of 548 patients), and cytopenias (423 [78%] or more of 542 patients).14 Although only a small difference in quality-of-life metrics favoured pazopanib therapy,14 a crossover study showed that 70% of patients preferred to take pazopanib compared with sunitinib.21 In the phase 3 study of sunitinib for pancreatic NETs,4 adverse events were generally less common than in studies of sunitinib for renal cell carcinoma, possibly because of the foreshortened duration of drug exposure and data collection (median 4.6 months, range 0.4–17.5) caused by premature study termination. Although dosing schedules for pancreatic NETs and renal cell carcinoma differ, the results of a randomised study did not show any differences in toxic effects between the two schedules.22
Our results suggest that pazopanib has antitumour activity in pancreatic NETs. The objective responses and progression-free survival in our cohort with pancreatic NETs are similar to results seen in the phase 3 studies3,4 of other effective drugs for this disease. Because of overlapping confidence intervals, the median progression-free survival of 14.4 months (95% CI 5.9–22.9) that we saw for patients with pancreatic NETs in our study is not distinguishable from the 7.7 months (6.5–12.5) and 11.4 months (7.4–19.8) reported for sunitinib4,17 and the 9.7 months (8.3–13.3),23 11.0 months (8.4–13.9),3 and 16.7 months (11.1–not reached) for everolimus23 for similar patients enrolled at a similar time, although the CI surrounding the progression-free survival here is necessarily larger because of the small sample size of the study. As in the phase 3 trial of sunitinib,4 two-thirds of patients with pancreatic NETs in this study received previous chemotherapy. However, only 35% of patients in that study had received previous octreotide, which limits the comparison. In a phase 2 study23 of everolimus in patients with pancreatic NETs, more than 99% of whom had progressive disease at study entry, 9.6% (95% CI 4.9–16.5) of patients achieved an objective response, with a progression-free survival of 9.7 months (8.3–13.3) for patients receiving everolimus, and 4.4% (0.5–15.1) of patients receiving everolimus and octreotide achieved an objective response, with progression-free survival of 16.7 months (11.1–not reached). This result23 suggested that octreotide might also contribute to the prolonged progression-free survival seen in our study. However, in view of the fact that octreotide longacting repeatable (LAR) has consistently yielded an objective response less than 5%,24 the exclusion of the null hypothesis of an objective response of 10% in this study suggests that the response rate was not caused by the octreotide. Additionally, since six of the seven responses were in patients who had progressive disease while receiving octreotide, it seems unlikely that octreotide contributed significantly to the responses we saw.
Our findings differ in some respects from those reported for a separate phase 2 study25 of pazopanib mono therapy in 37 patients with NETs, 32 of whom had pancreatic primary sites. In that study,25 median progression-free survival was 9.1 months (95% CI 4.9–13.3) and objective responses were noted in 18.7% (8.0–35.2) of patients.25 However, unlike our study, 35% of patients had grade 3 tumours. Additionally, our study separately assessed pazopanib activity in pancreatic NETs and carcinoid tumours.
We detected no objective responses in the cohort with carcinoid tumours, which led to the early termination of the study in this group of patients. However, objective response might not be the optimum endpoint for NET trials. Proportions of patients achieving an overall response in randomised studies of sunitinib4 and everolimus3 for pancreatic NETs were less than 10%, despite significant differences in progression-free survival. Since the inception of this study, progression-free survival has therefore been accepted as a recommended endpoint for clinical trials of NETs, in which a delay in progression is expected in the absence of radiographically defined tumour response.20 In fact, the proportion of patients with tumour shrinkage and median progression-free survival in the subgroup of patients with carcinoid tumours in our study were encouraging. Although cross-trial comparisons should be drawn with caution, these results compare favourably with reported results for presumably inactive agents. For example, in a phase 2 study of the IGF-1 receptor antagonist ganitumab, tumour shrinkage was noted in only 37% (95% CI not reported) of patients and median progression-free survival was 10.5 months (95% CI 4.2–16.5).26 Median progression-free survival was even shorter, at 2.7 months (95% CI not reported, range 2–3) in patients with carcinoid tumours who received an IGF-1 receptor antagonist, MK 2206.27 Similarly, capecitabine monotherapy was associated with a median progression-free survival of 9.9 months (95% CI not reported, IQR 4.4–36.7), although progressive disease was not necessary for enrolment.28 We are therefore unwilling to exclude the possibility that pazopanib might have activity for advanced carcinoid tumours. Our data might also be limited by the fact that only 25 patients completed 12 months of therapy.
Lower proportions of patients achieving an overall response have been consistently reported for carcinoid tumours compared with pancreatic NETs. In a large phase 2 study17 of sunitinib, objective responses were noted in 16.8% (95% CI.8.6–27.9) of patients with pancreatic NETs, which led to a subsequent successful phase 3 study, while the objective responses were noted in 2.4% (0.1–12.9) of patients with carcinoid tumours. Similarly, although everolimus had a progression-free survival hazard ratio (HR) of 0.35 (0.27–0.45; p<0.001) relative to placebo in pancreatic NETs in the RADIANT-3 study,3 the benefit for patients with carcinoid tumours was less clear, with progression-free survival HR of 0.77 (0.59–1.00; p=0.026) relative to placebo in RADIANT-2, which was not significant.29 Genomic differences have also been reported: pancreatic NETs often have MEN1, ATRX, and DAXX mutations,30 whereas carcinoid tumours have only uncommon CDKN1 mutations.31 Why these differences should cause differential responsiveness to VEGF pathway inhibitors is unclear.
Also notable was the strong association between chromogranin A reduction and radiographic response to pazopanib, with patients who had a 30% decrease in chromogranin A by week 4 being more likely to have radiographic responses. This finding is consistent with previous data suggesting that chromogranin A is an early marker of response to everolimus.32 By use of compiled data from two studies22,33 of everolimus for pancreatic NETs, patients who had chromogranin A reductions while receiving everolimus had an increased likelihood of radiographic response and improved progression-free survival compared with those who had no reduction. Similarly, results from a study34 of fluorouracil, doxorubicin, and streptozocin as chemotherapy for pancreatic NETs suggested that decreases in chromogranin A of at least 30% from baseline within 4 months of starting therapy were associated with increased likelihood of radiographic response.
Our results suggest that pazopanib has antitumour activity in advanced pancreatic NETs. The effect of pazopanib on advanced carcinoid tumours cannot be fully assessed based on our results because progression-free survival is probably a better endpoint for this patient population than is radiographic response, which we did not appreciate when we designed the study. Further studies to assess the role of pazopanib in the management of both pancreatic and extrapancreatic NETs are warranted, such as the randomised phase 2 study of pazopanib versus placebo in patients with progressive NET (NCT01841736), and a planned randomised controlled phase 3 study of pazopanib versus best supportive care for patients with advanced pancreatic NETs who have had treatment failure with targeted agents.
Supplementary Material
Research in context.
Evidence before this study
We searched the PubMed database for the terms “neuroendocrine,” “carcinoid,” and “pazopanib,” between Jan 1, 2005, and Jan 1, 2015, with no language restrictions. We identified a single prospective study of pazopanib for patients with neuroendocrine tumours of mixed grade and primary site, which suggested the potential activity of this therapy. However, the study was limited by its inclusion of patients with tumours of all grades and primary sites in a single cohort.
Added value of this study
Our results suggest that pazopanib has potential therapeutic activity, specifically in well-differentiated pancreatic neuroendocrine tumours.
Implications of all the available evidence
Pazopanib might represent a new therapy for pancreatic neuroendocrine tumours, and a randomised phase 3 study of this therapy for these patients is a logical next step.
Acknowledgments
This study was fully funded by the United States National Cancer Institute of the National Institutes of Health (US NCI-CTEP N01CM-2011-00039).
Footnotes
Contributors: ATP, KRH, and JCY contributed to study design. ATP, DMH, DRF, PM, ER, CSN, JCY, and MHK contributed to the data collection and ATP, DMH, JAC, KRH, CSN, JCY, and MHK did the data analysis. ATP, DMH, JAC, DRF, KRH, PM, ER, CSN, JCY, and MHK wrote the manuscript, and all authors gave their final approval.
Declaration of interests: ATP, DMH, JAC, KRH, PM, ER, CSN, JCY, and MHK report grants from the National Cancer Institute, during the study. JAC reports grants from Novartis and Sanofi, consulting fees from Ipsen, and holds stock in Merck. CSN reports grants from GE Healthcare. MHK reports consulting fees from Ipsen and Novartis. DRF reports no competing interests.
See Online for appendix
Contributor Information
Alexandria T Phan, Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Daniel M Halperin, Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Jennifer A Chan, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
David R Fogelman, Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Prof Kenneth R Hess, Department of Biostatistics, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Paige Malinowski, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Eileen Regan, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
Prof Chaan S Ng, Department of Diagnostic Radiology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Prof James C Yao, Department of Gastrointestinal Medical Oncology, University of Texas MD Anderson Cancer Center, Houston, TX, USA.
Matthew H Kulke, Department of Medical Oncology, Dana-Farber Cancer Institute, Boston, MA, USA.
References
- 1.Caplin ME, Pavel M, Cwikla JB, et al. Lanreotide in metastatic enteropancreatic neuroendocrine tumors. N Engl J Med. 2014;371:224–33. doi: 10.1056/NEJMoa1316158. [DOI] [PubMed] [Google Scholar]
- 2.Rinke A, Müller HH, Schade-Brittinger C, et al. Placebo-controlled, double-blind, prospective, randomized study on the effect of octreotide LAR in the control of tumor growth in patients with metastatic neuroendocrine midgut tumors: a report from the PROMID Study Group. J Clin Oncol. 2009;27:4656–63. doi: 10.1200/JCO.2009.22.8510. [DOI] [PubMed] [Google Scholar]
- 3.Yao JC, Shah MH, Ito T, et al. Everolimus for advanced pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:514–23. doi: 10.1056/NEJMoa1009290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Raymond E, Dahan L, Raoul JL, et al. Sunitinib malate for the treatment of pancreatic neuroendocrine tumors. N Engl J Med. 2011;364:501–13. doi: 10.1056/NEJMoa1003825. [DOI] [PubMed] [Google Scholar]
- 5.Quintela-Fandino M, Krzyzanowska M, Duncan G, et al. In vivo RAF signal transduction as a potential biomarker for sorafenib Efficacy in patients with neuroendocrine tumours. Br J Cancer. 2013;108:1298–305. doi: 10.1038/bjc.2013.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chan JA, Mayer RJ, Jackson N, Malinowski P, Regan E, Kulke MH. Phase I study of sorafenib in combination with everolimus (RAD001) in patients with advanced neuroendocrine tumors. Cancer Chemother Pharmacol. 2013;71:1241–46. doi: 10.1007/s00280-013-2118-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Castellano D, Capdevila J, Sastre J, et al. Sorafenib and bevacizumab combination targeted therapy in advanced neuroendocrine tumour: a phase II study of Spanish Neuroendocrine Tumour Group (GETNE0801) Eur J Cancer. 2013;49:3780–87. doi: 10.1016/j.ejca.2013.06.042. [DOI] [PubMed] [Google Scholar]
- 8.Berruti A, Fazio N, Ferrero A, et al. Bevacizumab plus octreotide and metronomic capecitabine in patients with metastatic well-to-moderately differentiated neuroendocrine tumors: the xelbevoct study. BMC Cancer. 2014;14:184. doi: 10.1186/1471-2407-14-184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hadoux J, Ducreux M. Bevacizumab and subtype-adapted chemotherapy backbone in neuroendocrine tumors. J Clin Oncol. 2013;31:976–77. doi: 10.1200/JCO.2012.47.0351. [DOI] [PubMed] [Google Scholar]
- 10.Koumarianou A, Antoniou S, Kanakis G, et al. Combination treatment with metronomic temozolomide, bevacizumab and long-acting octreotide for malignant neuroendocrine tumours. Endocr Relat Cancer. 2012;19:L1–4. doi: 10.1530/ERC-11-0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chan JA, Stuart K, Earle CC, et al. Prospective study of bevacizumab plus temozolomide in patients with advanced neuroendocrine tumors. J Clin Oncol. 2012;30:2963–68. doi: 10.1200/JCO.2011.40.3147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kulke MH, Chan JA, Meyerhardt JA, et al. A prospective phase II study of 2-methoxyestradiol administered in combination with bevacizumab in patients with metastatic carcinoid tumors. Cancer Chemother Pharmacol. 2011;68:293–300. doi: 10.1007/s00280-010-1478-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28:1061–68. doi: 10.1200/JCO.2009.23.9764. [DOI] [PubMed] [Google Scholar]
- 14.Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369:722–31. doi: 10.1056/NEJMoa1303989. [DOI] [PubMed] [Google Scholar]
- 15.Rautiola J, Utriainen T, Peltola K, Joensuu H, Bono P. Pazopanib after sunitinib failure in patients with metastatic renal cell carcinoma. Acta Oncol. 2014;53:113–18. doi: 10.3109/0284186X.2013.794957. [DOI] [PubMed] [Google Scholar]
- 16.Hainsworth JD, Rubin MS, Arrowsmith ER, Khatcheressian J, Crane EJ, Franco LA. Pazopanib as second-line treatment after sunitinib or bevacizumab in patients with advanced renal cell carcinoma: a Sarah Cannon Oncology Research Consortium Phase II Trial. Clinical Genitourin Cancer. 2013;11:270–75. doi: 10.1016/j.clgc.2013.04.006. [DOI] [PubMed] [Google Scholar]
- 17.Kulke MH, Lenz HJ, Meropol NJ, et al. Activity of sunitinib in patients with advanced neuroendocrine tumors. J Clin Oncol. 2008;26:3403–10. doi: 10.1200/JCO.2007.15.9020. [DOI] [PubMed] [Google Scholar]
- 18.Yao JC, Hassan M, Phan A, et al. One hundred years after “carcinoid”: epidemiology of and prognostic factors for neuroendocrine tumors in 35,825 cases in the United States. J Clin Oncol. 2008;26:3063–72. doi: 10.1200/JCO.2007.15.4377. [DOI] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Kulke MH, Siu LL, Tepper JE, et al. Future directions in the treatment of neuroendocrine tumors: consensus report of the National Cancer Institute Neuroendocrine Tumor clinical trials planning meeting. J Clin Oncol. 2011;29:934–43. doi: 10.1200/JCO.2010.33.2056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Escudier B, Porta C, Bono P, et al. Randomized, controlled, double-blind, cross-over trial assessing treatment preference for pazopanib versus sunitinib in patients with metastatic renal cell carcinoma: PISCES Study. J Clin Oncol. 2014;32:1412–18. doi: 10.1200/JCO.2013.50.8267. [DOI] [PubMed] [Google Scholar]
- 22.Motzer RJ, Hutson TE, Olsen MR, et al. Randomized phase II trial of sunitinib on an intermittent versus continuous dosing schedule as first-line therapy for advanced renal cell carcinoma. J Clin Oncol. 2012;30:1371–77. doi: 10.1200/JCO.2011.36.4133. [DOI] [PubMed] [Google Scholar]
- 23.Yao JC, Lombard-Bohas C, Baudin E, et al. Daily oral everolimus activity in patients with metastatic pancreatic neuroendocrine tumors after failure of cytotoxic chemotherapy: a phase II trial. J Clin Oncol. 2010;28:69–76. doi: 10.1200/JCO.2009.24.2669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Schnirer II, Yao JC, Ajani JA. Carcinoid—-a comprehensive review. Acta Oncol. 2003;42:672–92. doi: 10.1080/02841860310010547. [DOI] [PubMed] [Google Scholar]
- 25.Ahn HK, Choi JY, Kim KM, et al. Phase II study of pazopanib monotherapy in metastatic gastroenteropancreatic neuroendocrine tumours. Br J Cancer. 2013;109:1414–19. doi: 10.1038/bjc.2013.470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Strosberg JR, Chan JA, Ryan DP, et al. A multi-institutional, phase II open-label study of ganitumab (AMG 479) in advanced carcinoid and pancreatic neuroendocrine tumors. Endocr Relat Cancer. 2013;20:383–90. doi: 10.1530/ERC-12-0390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reidy-Lagunes DL, Vakiani E, Segal MF, et al. A phase 2 study of the insulin-like growth factor-1 receptor inhibitor MK-0646 in patients with metastatic, well-differentiated neuroendocrine tumors. Cancer. 2012;118:4795–800. doi: 10.1002/cncr.27459. [DOI] [PubMed] [Google Scholar]
- 28.Medley L, Morel AN, Farrugia D, et al. Phase II study of single agent capecitabine in the treatment of metastatic non-pancreatic neuroendocrine tumours. Br J Cancer. 2011;104:1067–70. doi: 10.1038/bjc.2011.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pavel ME, Hainsworth JD, Baudin E, et al. Everolimus plus octreotide long-acting repeatable for the treatment of advanced neuroendocrine tumours associated with carcinoid syndrome (RADIANT-2): a randomised, placebo-controlled, phase 3 study. Lancet. 2011;378:2005–12. doi: 10.1016/S0140-6736(11)61742-X. [DOI] [PubMed] [Google Scholar]
- 30.Jiao Y, Shi C, Edil BH, et al. DAXX/ATRX, MEN1, and mTOR pathway genes are frequently altered in pancreatic neuroendocrine tumors. Science. 2011;331:1199–203. doi: 10.1126/science.1200609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Francis JM, Kiezun A, Ramos AH, et al. Somatic mutation of CDKN1B in small intestine neuroendocrine tumors. Nat Genet. 2013;45:1483–86. doi: 10.1038/ng.2821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yao JC, Pavel M, Phan AT, et al. Chromogranin A and neuron-specific enolase as prognostic markers in patients with advanced pNET treated with everolimus. J Clin Endocrinol Metab. 2011;96:3741–49. doi: 10.1210/jc.2011-0666. [DOI] [PubMed] [Google Scholar]
- 33.Yao JC, Phan AT, Chang DZ, et al. Efficacy of RAD001 (everolimus) and octreotide LAR in advanced low- to intermediate-grade neuroendocrine tumors: results of a phase II study. J Clin Oncol. 2008;26:4311–18. doi: 10.1200/JCO.2008.16.7858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kouvaraki MA, Ajani JA, Hoff P, et al. Fluorouracil, doxorubicin, and streptozocin in the treatment of patients with locally advanced and metastatic pancreatic endocrine carcinomas. J Clin Oncol. 2004;22:4762–71. doi: 10.1200/JCO.2004.04.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.