Case Presentation
A 75 year-old man with hypertension and a history of stable coronary artery disease (CAD) presents to your office 1 month prior to an elective total knee replacement. He states that he underwent percutaneous coronary intervention (PCI) with a drug eluting stent (DES) to his left anterior descending coronary artery 2 years ago. His medication regimen includes daily aspirin 81, clopidogrel 75mg, amlodipine 10mg, lisinopril 5mg, and atorvastatin 40mg. He does not smoke cigarettes. Recent laboratory data reveal normal renal function. He asks what he can do to reduce the cardiovascular risks of non-cardiac surgery.
The Clinical Problem
Perioperative cardiovascular complications are a source of morbidity and mortality for more than 200 million patients worldwide who undergo non-cardiac surgery each year. In large cohorts and randomized trials, perioperative myocardial infarction (MI) occurs in up to 6.2% of surgeries.1-4
Pathogenesis of Perioperative Cardiovascular Events
The pathogenesis of cardiovascular events in the postoperative period is complex (Figure 1). Induction of anesthesia, surgical trauma, bleeding, anemia, hypoxia, and post-operative pain lead to surges in catecholamines, cortisol production, and a hypercoaguable state. Inflammatory cytokines, including TNF-alpha, IL-1, IL-6, and CRP, rise in the post-operative period. Increased platelet activtation contributes to the thrombotic milieu.5 Tachycardia and elevations in blood pressure increase coronary artery sheer stress and can precipitate coronary plaque destabilization, plaque rupture, coronary thrombosis, and Type 1 MI. Post-operative myocardial necrosis and infarction may also be caused by imbalances in myocardial oxygen supply and demand from tachycardia, hypotension, hypoxia, or anemia in the setting of stable CAD (Type 2 MI). Microvascular coronary disease, endothelial dysfunction, and excess activation of inflammatory pathways may be contributing mechanisms but require further study.
Figure 1. Pathogenesis of perioperative cardiovascular events.
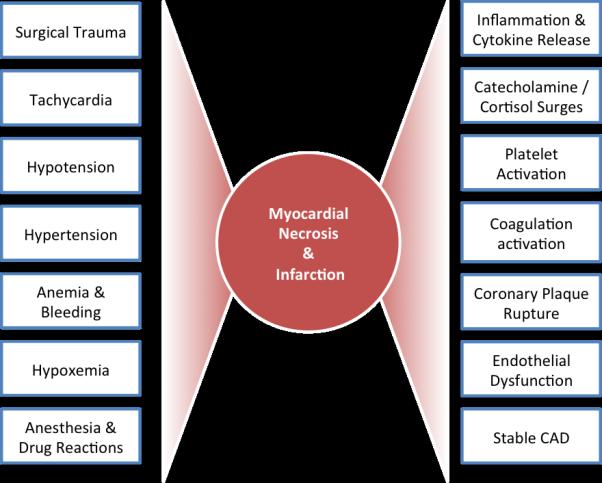
Multiple perioperative events and cardiovascular factors may contribute to the development of myocardial necrosis and infarction.
Methods of Risk Stratification
Systematic evaluation of perioperative cardiovascular risk is recommended prior to non-cardiac surgery. Risk prediction models provide quantitative estimates of risk (Table 1). Current AHA/ACC guidelines recommend pre-operative non-invasive risk stratification to evaluate for myocardial ischemia in patients with poor functional capacity and an elevated risk for non-cardiac surgery, since abnormal myocardial perfusion imaging and stress echocardiography are powerful predictors of post-operative cardiovascular events.6 Non-invasive anatomical testing with coronary computed tomographic angiography (CTA) prior to non-cardiac surgery is a promising approach that requires further study.
Table 1.
Comparison of Perioperative Risk Calculators
| Goldman Index of Cardiac Risk (1977) | Revised Cardiac Risk Index (1999) | NSQIP Perioperative MI and Cardiac Arrest (MICA) Risk Calculator (2011) | NSQIP Universal Surgical Risk Calculator (2013) | |
|---|---|---|---|---|
| Criteria | • Jugular venous distention or a third heart sound on auscultation • Recent MI within 6 months • ≥5 PVCs per minute, • Non-sinus cardiac rhythm or PACs on preoperative ECG • Age >70 • Aortic stenosis • Intra-peritoneal, intra-thoracic, or aortic surgery • Any emergency surgery |
• Cerebrovascular disease • Ischemic heart disease • History of congestive heart failure • Insulin therapy for diabetes • Serum creatinine ≥2.0 mg/dL • Planned high risk procedure (intra-peritoneal, intra-thoracic, or vascular surgery) |
• Age • ASA class • Creatinine • Preoperative Function • Procedure Type (Anorectal surgery, Aortic, Bariatric, Brain, Breast, Cardiac, ENT, Foregut/hepato-pancreatobiliary, Gallbladder/Appendix/Adrenal/Spleen, Intestinal, Neck, Obstetric/gynecologic, Orthopedic, Other abdomen, Peripheral vascular, Skin, Spine, Thoracic, Urology, Vein) |
• Age group, y • Sex • Functional status • Emergency case • ASA Class • Steroid use for chronic condition • Ascites within 30 d preoperatively • System sepsis within 48 h preoperatively • Ventilator dependent • Disseminated cancer • Diabetes • Hypertension requiring medication • Previous cardiac event • Congestive heart failure in 30 d preoperatively • Dyspnea • Current smoker within 1 y • History of COPD • Dialysis • Acute renal failure • BMI Class • CPT-specific linear risk |
| Outcome | Intraoperative/postoperative M, pulmonary edema, VT, cardiac death | MI, pulmonary edema, ventricular fibrillation, complete heart block, cardiac death | Intraoperative/postoperative MI or cardiac arrest within 30 days | Cardiac arrest, MI, all-cause mortality within 30 days |
| Derivation Set ROC | 0.61 | 0.76 | 0.88 | 0.90 (Cardiac arrest or MI) 0.94 (Mortality) |
| Validation Set ROC | 0.701 | 0.806 | 0.874 | Not reported |
Abbreviations: ASA: American Society of Anesthesiologists; BMI: body mass index; COPD: Chronic Obstructive Pulmonary Disease; CPT: Current Procedural Terminology; ECG: electrocardiogram; ENT: ear nose and throat; MI: myocardial infarction; ROC: Area under the receiver operating characteristic curve (C statistic); PAC: premature atrial contractions;
Perioperative Medical Therapy
Aspirin
Aspirin is a potent, irreversible inhibitor of COX-1 that blocks thromboxane A2 production, prevents platelet aggregation, and mitigates thrombotic risks at a cost of increased bleeding. Although aspirin has a clear role in the secondary prevention of vascular disease, uncertainty regarding the efficacy and safety of perioperative aspirin use prompted the second Perioperative Ischemic Evaluation (POISE-2) trial, which randomized 10,010 patients at risk for vascular complications to either perioperative aspirin or placebo prior to non-cardiac surgery.3 At 30 days, there was no difference in death or nonfatal MI (7.0% vs. 7.1%, p=0.92), but aspirin use was associated with excess of major bleeding (4.6% vs. 3.8%, p=0.04). No benefit of aspirin was observed in any pre-specified subgroup analyses, regardless of Revised Cardiac Risk Index (RCRI) score or aspirin use prior to randomization.3 However, fewer than a quarter of patients in POISE-2 had a history of CAD, only 4.7% had a history of PCI, and only 1.2% had DES, raising questions about whether patients had sufficient perioperative risk to demonstrate a benefit of perioperative aspirin. While POISE-2 trial results do not support routine perioperative aspirin initiation, there is insufficient evidence to conclude whether perioperative aspirin cessation is safe in patients with prior coronary stent implantation. Thus, individualized risks of thrombotic complications and perioperative bleeding must be considered when formulating a treatment strategy in certain high-risk groups.
Statins and Lipid-Lowering Therapy
Lipid lowering with statin therapy is a promising approach to reduce perioperative cardiovascular events. A retrospective, propensity-matched analysis of 204,885 patients undergoing non-cardiac surgery demonstrated that patients prescribed lipid-lowering agents in the first two days of hospitalization had a significantly reduced in-hospital mortality (adjusted OR 0.62, 95% CI, 0.58-0.67).7 A meta-analysis of randomized controlled trials (RCTs) and observational studies in vascular surgery, including the controversial DECREASE III study by Poldermanns et al. in which perioperative fluvastatin was associated with a 53% reduction in death or MI,8 reported perioperative statin use was associated with a significant reduction in the composite of MI, stroke, and death (OR, 0.45; 95% CI, 0.29-0.70).9 In contrast, a 2013 Cochrane review of RCTs of statins in unselected non-cardiac surgery that excluded studies by Poldermanns reported insufficient evidence to conclude that perioperative statins reduce adverse cardiovascular events.10 Based on available clinical trial data, clinically indicated statins should be continued in the perioperative period of non-cardiac surgery. Initiation of statin therapy prior to surgery should be considered for patients undergoing vascular surgery (Class IIa, LOE B), and may be considered for other patients with indications for lipid lowering therapy and elevated surgical risks (Class IIb, LOE C).6
Beta-Blockers
Beta-blockers prolong coronary diastolic filling time, decease myocardial wall stress, mitigate myocardial oxygen supply-demand mismatch, and can prevent MI. The Perioperative Ischemic Evaluation trial (POISE), the largest trial of perioperative beta blockade to date, randomized 8,351 patients to extended release metoprolol succinate 100mg or placebo within 2-4 hours prior to non-cardiac surgery, with continuation of therapy (with a metoprolol dose of 100-200mg/day or placebo) for 30 days.2 Although fewer patients in the group randomized to metoprolol had non-fatal MI, non-fatal cardiac arrest, or cardiovascular death (5.8% vs. 6.9%, p=0.039), beta-blocker therapy was associated with a higher incidence of all-cause mortality (3.1% vs. 2.3%, p=0.0317) and stroke (1.0% vs. 0.5%, p=0.0053), raising safety concerns.2 Only 43% of patients enrolled in POISE had a history of CAD, and fewer than 20% had ≥3 pre-operative RCRI risk factors. Indeed, in retrospective cohort studies of patients undergoing major non-cardiac surgery, perioperative beta-blocker therapy was associated with reduced in-hospital mortality among high-risk (RCRI ≥2), but not low-risk patients.11 In addition, all subjects in POISE began the study drug within 1 day of surgery, but studies suggest that longer durations of beta-blocker administration prior to surgery are associated with improved outcomes.12
In summary, the utility of perioperative beta-blocker therapy remains questionable. Patients prescribed outpatient beta-blockers should continue therapy in the perioperative period (Class I, LOE B) in the absence of bradycardia or hypotension.6 Patients with known ischemic heart disease at a high risk for perioperative MI, or those with ≥3 RCRI risk factors may warrant initiation of beta-blockers prior to surgery (Class IIb, LOE B). However, beta-blocker therapy should not be started on the day of surgery, a recommendation endorsed by current guidelines.6 The optimal timing of initiation and dose of perioperative beta-blockade is uncertain, and prospective trials are necessary. However, it is reasonable to begin therapy more than one week prior to surgery to determine safety and tolerability, and even longer pre-operative durations may be preferable.
Venous Thromboembolism Prophylaxis
Venous thromboembolism (VTE) is a significant adverse event following major orthopedic surgery. In addition to early mobilization and mechanical VTE prophylaxis in the post-operative period, a number of pharmacologic agents have been studied, including aspirin, warfarin, unfractionated heparin, low-molecular weight heparins (LMWH), fondaparinux, and new oral direct thrombin and factor Xa inhibitors, dabigatran, rivaroxaban, apixaban, and edoxaban. Guidelines from 2012 endorse the use of any of these agents for VTE prophylaxis after orthopedic surgery (Grade 1B), but recommend LMWH in preference to other alternatives (Grade 2B/C).13 In the Pulmonary Embolism Prevention (PEP) trial, which randomized 17,444 patients undergoing hip surgery to 35 days of aspirin or placebo, perioperative aspirin reduced the risk of post-operative VTE by 34% (17-47%, p=0.0003).14 Although aspirin was beneficial in comparison to placebo, there are few data comparing aspirin to anticoagulation. A meta-analysis (16 trials, n=38,747) comparing the new oral anticoagulants versus enoxaparin for VTE prophylaxis after total hip or knee replacement demonstrated a 35% significant reduction in symptomatic VTE with no significant difference in clinically relevant bleeding or net clinical benefit.15
Patients with Coronary Artery Disease
Coronary artery disease complicates the care of patients who require non-cardiac surgery. Routine perioperative coronary angiography and/or prophylactic revascularization is not recommended prior to non-cardiac surgery in contemporary guidelines, due to an absence of benefit.6 The Coronary Artery Revascularization Prophylaxis (CARP) trial compared coronary revascularization to a strategy of medical management in 510 patients with significant CAD who were scheduled to undergo non-urgent vascular surgery.16 In CARP, 59% of patients randomized to revascularization underwent PCI with early generations of bare metal stents (BMS), and 41% underwent coronary bypass surgery. The median delay to non-cardiac surgery was 36 days. There were no differences in 30-day (3.1% vs. 3.4%, p=0.87) and long-term (22% vs. 23%, p=0.92) mortality after 2.7 years of follow up. The CARP trial excluded patients with left main disease or severely reduced ejection fraction, and the effect of pre-operative revascularization prior to non-cardiac surgery in these populations is uncertain.
For patients with recent PCI, the timing of non-cardiac surgery also remains controversial. Surgery following placement of a coronary stent is associated with increased adverse cardiac events, attributed to the pro-thrombotic and inflammatory effects of surgery combined with premature cessation of antiplatelet therapy. Risks are highest when surgery is performed within 4-6 weeks of a coronary intervention, and recent guidelines recommend delaying elective surgery by ≥30 days after placement of a BMS or ≥1 year for DES (Class I, LOE B) based on early studies.17 A large retrospective cohort study of Veterans Affairs patients undergoing non-cardiac surgery within 2 years of a coronary stent demonstrated stable rates of cardiovascular events if surgery was performed at least 6 months after PCI.18 In this analysis, perioperative cardiovascular risk was similar across stent type (DES vs. BMS) but was higher in patients with PCI for recent MI versus those with revascularization for stable CAD.18 Based on these and other data,18, 19 elective noncardiac surgery may be considered ≥180 days after uncomplicated elective PCI with DES, if the risk of further surgical delay is greater than the risks of ischemic complications (Class IIb, LOE B).6 However, additional prospective studies are necessary to determine optimal antiplatelet management strategies for patients with recent DES who need elective non-cardiac surgery but cannot wait the recommended minimum 1-year delay.
Perioperative management of antiplatelet therapy after PCI is another common challenge. Clinical practice guidelines recommend continuation of aspirin monotherapy after PCI in the perioperative period, although this is largely based on expert opinion. In contrast, perioperative continuation of DAPT with aspirin and a P2Y12 inhibitor is associated with a substantial increase in moderate and severe bleeding.20 The timing of withdrawal of P2Y12 inhibitor therapy must be tailored to the pharmacokinetic and pharmacodynamic properties of each drug. Clopidogrel and ticagrelor should be discontinued ≥5 days prior to surgery, and prasugrel should be held for ≥7 days. Continuation of DAPT can be considered for patients with the highest risks of perioperative thrombotic events or those with minimal surgery-specific risks of bleeding. Clinical trials investigating the use of perioperative platelet activity testing and/or short-acting intravenous antiplatelet agents are needed in certain high-risk patients.
Novel Approaches to Risk Reduction
Efforts are currently underway to identify novel approaches to reduce perioperative cardiovascular events in high-risk patients and to manage patients who develop post-operative myocardial injury. Intensive medical management with high intensity statin therapy prior to non-cardiac surgery is a particularly promising strategy to reduce cardiovascular events. Ongoing trials will refine our understanding of perioperative cardiovascular events and will determine management of myocardial injury after non-cardiac surgery (Table 2).
Table 2.
Strategies to Prevent and/or Manage Perioperative Cardiovascular Events Under Investigation
| Table 2. Prevention and Treatment Strategies Under Investigation | |
|---|---|
|
Prevention of Perioperative Cardiovascular Events:
| |
| High Intensity Statin | Lowering the Risk of Operative Complications Using Atorvastatin Loading Dose (LOAD) (NCT01543555) |
| Ranolazine | Pathophysiology and Prevention of Perioperative Myocardial Injury (NCT01810796) |
| Ischemic Preconditioning | Preconditioning Shields Against Vascular Events in Surgery (SAVES) (NCT01691911) |
| Prevention of Myocardial Injury in Non-cardiac Surgery (PIXIE) (NCT02344797) | |
| Combination Therapy (ACEi, Beta-Blocker, Statin) | Optimization of Pre-surgical Testing With an Intensive Multifactorial Intervention to MinimiZe Cardiovascular Events in Orthopedic Surgery (NCT01837069) |
|
Post-operative Management of Perioperative Cardiovascular Events
| |
| Ticagrelor | Study of Ticagrelor Versus Aspirin Treatment in Patients With Myocardial Injury Post Major Non-cardiac Surgery (INTREPID) (NCT02291419) |
| Dabigatran | Management of Myocardial Injury After Noncardiac Surgery Trial (MANAGE) (NCT01661101) |
Abbreviations: ACEi: Angiotensin-converting-enzyme inhibitor
Case Resolution
Pre-operative cardiovascular risk stratification was performed. The patient had 1 RCRI risk factor, associated with a 0.9% risk of major perioperative cardiac complications; by the NSQIP calculator he had a 0.74% risk of MI or cardiac arrest and a 0.4% risk of death. In light of the low RCRI score, beta-blocker therapy was not initiated. Clopidogrel was discontinued 5 days prior to surgery, and aspirin and statin were continued in the perioperative period. The patient underwent an uncomplicated elective total knee replacement. On the first post-operative day, a one-month course of LMWH was initiated for VTE prophylaxis. The patient was discharged home with no post-operative cardiovascular complications.
Conclusions
Prevention of perioperative cardiovascular events is an important consideration for general practitioners, cardiologists, anesthesiologists, and surgeons alike, but substantial gaps in knowledge remain. Until additional data is available, mitigation and management of perioperative cardiovascular risk requires careful, individualized assessment of cardiovascular disease and the surgery-specific thrombotic and bleeding risks.
Acknowledgments
Funding Sources: Dr. Berger was partially funded by the National Heart and Lung Blood Institute of the NIH (HL114978), American Heart Association Clinical Research Program (13CRP14410042) and Doris Duke Charitable Foundation (2010055).
Footnotes
Disclosures: None.
References
- 1.Vascular Events In Noncardiac Surgery Patients Cohort Evaluation Study I. Devereaux PJ, Chan MT, Alonso-Coello P, Walsh M, Berwanger O, Villar JC, Wang CY, Garutti RI, Jacka MJ, Sigamani A, Srinathan S, Biccard BM, Chow CK, Abraham V, Tiboni M, Pettit S, Szczeklik W, Lurati Buse G, Botto F, Guyatt G, Heels-Ansdell D, Sessler DI, Thorlund K, Garg AX, Mrkobrada M, Thomas S, Rodseth RN, Pearse RM, Thabane L, McQueen MJ, VanHelder T, Bhandari M, Bosch J, Kurz A, Polanczyk C, Malaga G, Nagele P, Le Manach Y, Leuwer M, Yusuf S. Association between postoperative troponin levels and 30-day mortality among patients undergoing noncardiac surgery. JAMA. 2012;307:2295–2304. doi: 10.1001/jama.2012.5502. [DOI] [PubMed] [Google Scholar]
- 2.Group PS, Devereaux PJ, Yang H, Yusuf S, Guyatt G, Leslie K, Villar JC, Xavier D, Chrolavicius S, Greenspan L, Pogue J, Pais P, Liu L, Xu S, Malaga G, Avezum A, Chan M, Montori VM, Jacka M, Choi P. Effects of extended-release metoprolol succinate in patients undergoing non-cardiac surgery (poise trial): A randomised controlled trial. Lancet. 2008;371:1839–1847. doi: 10.1016/S0140-6736(08)60601-7. [DOI] [PubMed] [Google Scholar]
- 3.Devereaux PJ, Mrkobrada M, Sessler DI, Leslie K, Alonso-Coello P, Kurz A, Villar JC, Sigamani A, Biccard BM, Meyhoff CS, Parlow JL, Guyatt G, Robinson A, Garg AX, Rodseth RN, Botto F, Buse GL, Xavier D, Chan MT, Tiboni M, Cook D, Kumar PA, Forget P, Malaga G, Fleischmann E, Amir M, Eikelboom J, Mizera R, Torres D, Wang CY, Vanhelder T, Paniagua P, Berwanger O, Srinathan S, Graham M, Pasin L, Le Manach Y, Gao P, Pogue J, Whitlock R, Lamy A, Kearon C, Baigent C, Chow C, Pettit S, Chrolavicius S, Yusuf S, the P-I. Aspirin in patients undergoing noncardiac surgery. N Engl J Med. 2014;370:1494–503. doi: 10.1056/NEJMoa1401105. [DOI] [PubMed] [Google Scholar]
- 4.Oberweis BS, Smilowitz NR, Nukala S, Rosenberg A, Xu J, Stuchin S, Iorio R, Errico T, Radford MJ, Berger JS. Relation of perioperative elevation of troponin to long-term mortality after orthopedic surgery. Am J Cardiol. 2015;115:1643–8. doi: 10.1016/j.amjcard.2015.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneider GS, Rockman CB, Berger JS. Platelet activation increases in patients undergoing vascular surgery. Thromb Res. 2014;134:952–956. doi: 10.1016/j.thromres.2014.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fleisher LA, Fleischmann KE, Auerbach AD, Barnason SA, Beckman JA, Bozkurt B, Davila-Roman VG, Gerhard-Herman MD, Holly TA, Kane GC, Marine JE, Nelson MT, Spencer CC, Thompson A, Ting HH, Uretsky BF, Wijeysundera DN. 2014 acc/aha guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery: A report of the american college of cardiology/american heart association task force on practice guidelines. J Am Coll Cardiol. 2014;64:e77–e137. doi: 10.1016/j.jacc.2014.07.944. [DOI] [PubMed] [Google Scholar]
- 7.Lindenauer PK, Pekow P, Wang K, Gutierrez B, Benjamin EM. Lipid-lowering therapy and in-hospital mortality following major noncardiac surgery. JAMA. 2004;291:2092–2099. doi: 10.1001/jama.291.17.2092. [DOI] [PubMed] [Google Scholar]
- 8.Schouten O, Boersma E, Hoeks SE, Benner R, van Urk H, van Sambeek MR, Verhagen HJ, Khan NA, Dunkelgrun M, Bax JJ, Poldermans D. Dutch Echocardiographic Cardiac Risk Evaluation Applying Stress Echocardiography Study G. Fluvastatin and perioperative events in patients undergoing vascular surgery. N Engl J Med. 2009;361:980–989. doi: 10.1056/NEJMoa0808207. [DOI] [PubMed] [Google Scholar]
- 9.Antoniou GA, Hajibandeh S, Hajibandeh S, Vallabhaneni SR, Brennan JA, Torella F. Meta-analysis of the effects of statins on perioperative outcomes in vascular and endovascular surgery. J Vasc Surg. 2015;61:519–532. e511. doi: 10.1016/j.jvs.2014.10.021. [DOI] [PubMed] [Google Scholar]
- 10.Sanders RD, Nicholson A, Lewis SR, Smith AF, Alderson P. Perioperative statin therapy for improving outcomes during and after noncardiac vascular surgery. Cochrane Database Syst Rev. 2013;7:CD009971. doi: 10.1002/14651858.CD009971.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lindenauer PK, Pekow P, Wang K, Mamidi DK, Gutierrez B, Benjamin EM. Perioperative beta-blocker therapy and mortality after major noncardiac surgery. N Engl J Med. 2005;353:349–361. doi: 10.1056/NEJMoa041895. [DOI] [PubMed] [Google Scholar]
- 12.Flu WJ, van Kuijk JP, Chonchol M, Winkel TA, Verhagen HJ, Bax JJ, Poldermans D. Timing of pre-operative beta-blocker treatment in vascular surgery patients: Influence on post-operative outcome. J Am Coll Cardiol. 2010;56:1922–1929. doi: 10.1016/j.jacc.2010.05.056. [DOI] [PubMed] [Google Scholar]
- 13.Falck-Ytter Y, Francis CW, Johanson NA, Curley C, Dahl OE, Schulman S, Ortel TL, Pauker SG, Colwell CW, Jr., American College of Chest P Prevention of vte in orthopedic surgery patients: Antithrombotic therapy and prevention of thrombosis, 9th ed: American college of chest physicians evidence-based clinical practice guidelines. Chest. 2012;141:e278S–325S. doi: 10.1378/chest.11-2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Prevention of pulmonary embolism and deep vein thrombosis with low dose aspirin: Pulmonary embolism prevention (pep) trial. Lancet. 2000;355:1295–1302. [PubMed] [Google Scholar]
- 15.Gomez-Outes A, Terleira-Fernandez AI, Suarez-Gea ML, Vargas-Castrillon E. Dabigatran, rivaroxaban, or apixaban versus enoxaparin for thromboprophylaxis after total hip or knee replacement: Systematic review, meta-analysis, and indirect treatment comparisons. BMJ. 2012;344:e3675. doi: 10.1136/bmj.e3675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McFalls EO, Ward HB, Moritz TE, Goldman S, Krupski WC, Littooy F, Pierpont G, Santilli S, Rapp J, Hattler B, Shunk K, Jaenicke C, Thottapurathu L, Ellis N, Reda DJ, Henderson WG. Coronary-artery revascularization before elective major vascular surgery. N Engl J Med. 2004;351:2795–2804. doi: 10.1056/NEJMoa041905. [DOI] [PubMed] [Google Scholar]
- 17.Wilson SH, Fasseas P, Orford JL, Lennon RJ, Horlocker T, Charnoff NE, Melby S, Berger PB. Clinical outcome of patients undergoing non-cardiac surgery in the two months following coronary stenting. J Am Coll Cardiol. 2003;42:234–240. doi: 10.1016/s0735-1097(03)00622-3. [DOI] [PubMed] [Google Scholar]
- 18.Hawn MT, Graham LA, Richman JS, Itani KM, Henderson WG, Maddox TM. Risk of major adverse cardiac events following noncardiac surgery in patients with coronary stents. JAMA. 2013;310:1462–1472. doi: 10.1001/jama.2013.278787. [DOI] [PubMed] [Google Scholar]
- 19.Wijeysundera DN, Wijeysundera HC, Yun L, Wasowicz M, Beattie WS, Velianou JL, Ko DT. Risk of elective major noncardiac surgery after coronary stent insertion: A population-based study. Circulation. 2012;126:1355–1362. doi: 10.1161/CIRCULATIONAHA.112.102715. [DOI] [PubMed] [Google Scholar]
- 20.van Kuijk JP, Flu WJ, Schouten O, Hoeks SE, Schenkeveld L, de Jaegere PP, Bax JJ, van Domburg RT, Serruys PW, Poldermans D. Timing of noncardiac surgery after coronary artery stenting with bare metal or drug-eluting stents. Am J Cardiol. 2009;104:1229–1234. doi: 10.1016/j.amjcard.2009.06.038. [DOI] [PubMed] [Google Scholar]


