Abstract
Nilotinib is a second-generation tyrosine kinase inhibitor that has been approved for the first-line treatment of chronic-phase chronic myeloid leukemia, based on the results of a prospective randomized study of nilotinib versus imatinib (ENESTnd). Apart from this registration study, very few data are currently available on first-line nilotinib treatment. We report here the long-term, 6-year results of the first investigator-sponsored, GIMEMA multicenter phase 2, single-arm trial with nilotinib 400 mg twice daily as first-line treatment in 73 patients with chronic-phase chronic myeloid leukemia. Six-year overall survival and progression-free survival rates were 96%, with one death after progression to blast phase. At 6 years, 75% of the patients were still on nilotinib. The cumulative incidence of major molecular response was 98%; only one patient had a confirmed loss of major molecular response. The cumulative incidence of deep molecular response (MR 4.0) was 76%. Deep molecular response was stable (≥2 years) in 34% of these patients. Cardiovascular adverse events, mainly due to arterial thrombosis, occurred in 11/73 patients (15%), after 24 to 76 months of therapy. They were more frequent in elderly patients, and in those with baseline cardiovascular risk factors. None was fatal, although there was a relevant morbidity. This is the study with the longest follow-up of a high dose of nilotinib (400 mg twice daily): it highlights the high efficacy and the cardiovascular toxicity of the drug (CTG.NCT.00481052).
Introduction
Nilotinib is a derivative of imatinib with greater potency and specificity against the tyrosine kinase activity of BCR-ABL1, the leukemogenic protein of chronic myeloid leukemia (CML).1 A randomized, phase 3 study (ENESTnd) showed that in newly diagnosed patients with chronic phase (CP) CML, nilotinib, both at 300 mg and 400 mg twice daily, induced more and deeper molecular responses than imatinib 400 mg once daily.2 On the basis of this study, nilotinib was approved for the first-line treatment of CML at the dose of 300 mg twice daily. The latest update of ENESTnd, with up to 5 years of follow-up, confirmed the high rates of deep molecular response, showed a slightly better progression-free survival, and no difference in overall survival, as compared to imatinib.3,4 Cardiovascular toxicity was not pointed out initially,2 but was reported in subsequent analyses.3–6 So far, only two small, single-arm studies of front-line nilotinib (400 mg twice daily) have been published by independent investigators.7,8 These studies, with a short follow-up (median <18 months), showed that nilotinib induced high response rates and that the responses were achieved quickly. Later on, some cardiovascular adverse events, mainly atherosclerotic, and particularly peripheral arterial occlusive disease,9–14 were reported in patients treated with nilotinib, as second- or third-line therapy, raising concern on the long-term safety of the drug. Currently the GIMEMA study has the longest followup with nilotinib, but only early data have been published so far, in 2009.7 Here we report the efficacy and safety results of this study, with a minimum follow-up of 6 years.
Methods
Seventy-three adult (≥18 years old) patients with newly diagnosed, Philadelphia chromosome-positive, BCR-ABL1-positive CP-CML were enrolled between June 2007 and February 2008 at 18 GIMEMA Centers in Italy, in a single-arm study of first-line nilotinib (CTG NCT 00481052), given at an initial dose of 400 mg twice daily. Guidelines for dose adaptation and for treatment discontinuation have been described in detail elsewhere.7 The study protocol was approved by the ethics committees of all participating centers and was conducted in accordance with the Declaration of Helsinki. All patients gave written informed consent. The main exclusion criteria were a Performance Status ≥2, uncontrolled serious medical conditions, and prior treatment with imatinib. Molecular response (MR) was evaluated on peripheral blood every 3 months until a major MR (MMR; MR 3.0) was achieved and confirmed, then at least every 6 months. MMR was assessed and expressed according to the International Scale, as a BCR-ABL1 transcript level ≤0.1%, corresponding to a 3-log decrease from the International Scale standard, in samples with more than 10,000 ABL1 copies. MR 4.0 was defined as a BCR-ABL1 transcript level ≤0.01%, corresponding to a 4-log reduction, in samples with more than 10,000 ABL1 copies.15 Stable MR 4.0 was defined by at least five evaluations with MR 4.0, with no value >0.01%, during the preceding 2 years of treatment. Molecular tests were performed in one center (Bologna, Italy) until other GIMEMA centers were standardized for the International Scale. Mutational screening of the BCR-ABL1 kinase domain was done, using conventional Sanger sequencing as previously reported,16 only in the case of resistance or progression. The baseline relative risk was calculated using Sokal, EURO, and EUTOS.17–19 Blast phase was defined according to European LeukemiaNet recommendations.20 Atherosclerotic adverse events were defined as peripheral arterial occlusive disease, acute coronary syndrome (acute myocardial infarction, instable angina), chronic ischemic heart disease (stable angina), and ischemic stroke. Cardiovascular risk factors [hypertension, diabetes, hypercholesterolemia, prior cardiovascular disease, obesity (body mass index ≥30)] were identified at baseline, prior to nilotinib treatment, on the basis of the medical history, and no specific suggestions for their management were provided in the protocol. Since this study was designed before the publication of data evidencing the cardiovascular toxicity of nilotinib, evaluation of serum lipids was not planned in the protocol (thus potentially leading to an underestimation of hypercholesterolemia), and no information on tobacco abuse was collected. Descriptive statistics are provided. The cumulative probabilities of events and survival were estimated using the Kaplan-Meier method.
Results
Patients
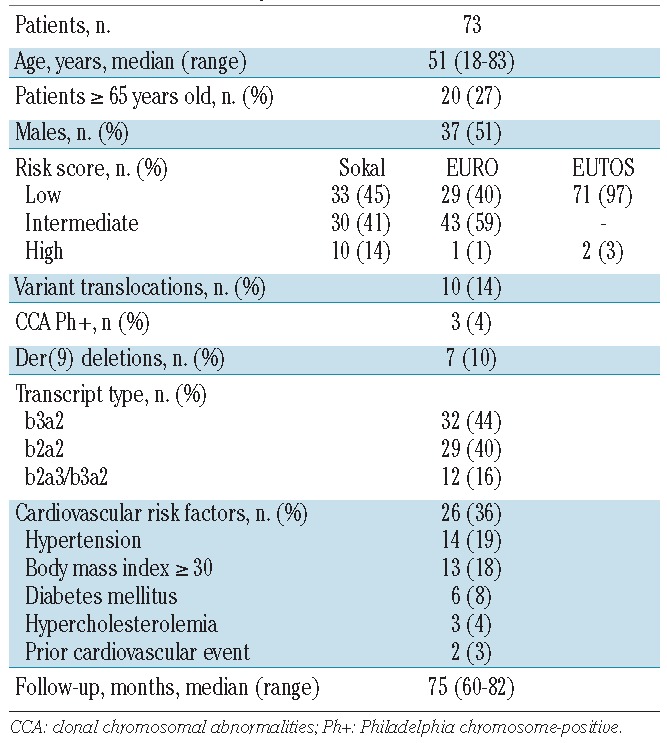
The baseline characteristics of the patients are shown in Table 1. Few patients were at high risk: Sokal 14%, EURO 1%, and EUTOS 3%. The median age was 51 years, with 27% of the patients aged 65 years or more. Thirty-six per cent of patients had one or more cardiovascular risk factors: hypertension 19%, obesity 18%, diabetes mellitus 8%, hypercholesterolemia 4%, and prior cardiovascular disease 3%.
Table 1.
Characteristics of patients.
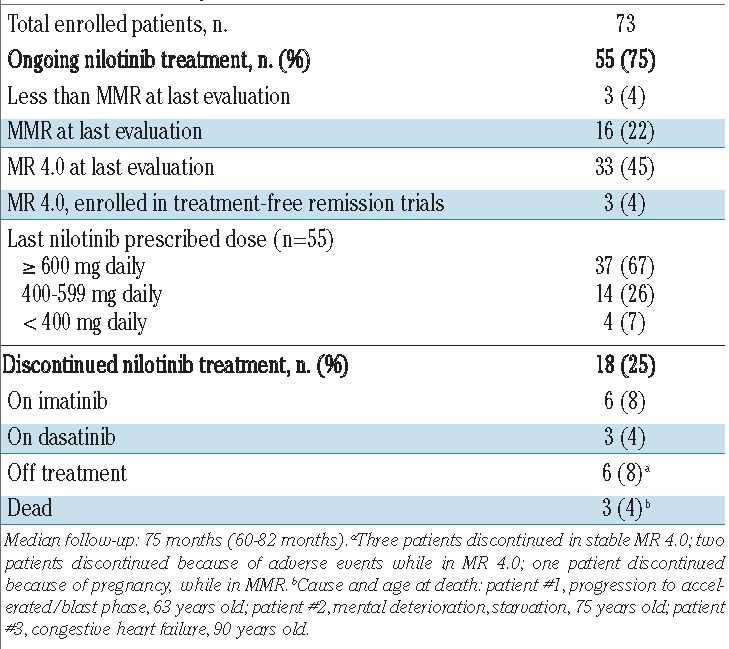
Outcome and patients’ disposition
No patient was lost to follow-up, which ranged between 60 and 82 months (median 75 months). The 6-year overall survival rate was 96% (Figure 1). One patient progressed to blast phase on therapy, at 6 months, and died of leukemia. Another two patients died in remission, while on nilotinib: one with dementia (75 years old), and one of congestive heart failure (90 years old). Apart from these three patients, 13 patients discontinued nilotinib: they are now on imatinib (6 patients) or dasatinib (3 patients) or in a trial of treatment-free remission (4 patients). Adverse events were the main reason for nilotinib discontinuation (11 patients, 15%): cardiovascular adverse events occurred in eight patients (peripheral arterial occlusive disease in 4, carotid stenosis in 2, angina pectoris in 1, and atrial fibrillation in 1), and recurrent grade 3 or 4 lipase elevation in three patients. At last contact, 55 patients (75%) were still on nilotinib, of whom 67% at doses of 300 mg twice daily, 26% at 400 mg once daily, and 7% at 300 mg once daily (Table 2).
Figure 1.
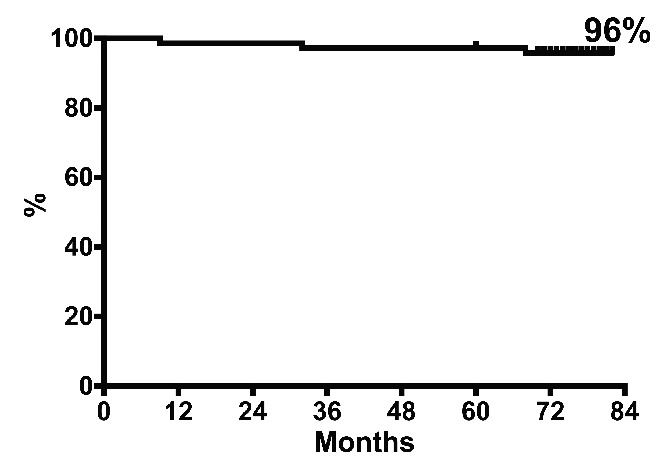
Overall survival. With a median follow-up of 75 months, 3/73 patients died, one of leukemia (after progression to blast phase) and two of other causes, in MMR. The 6-year overall survival rate was 96%.
Table 2.
Patients’ disposition.
Molecular response
Molecular response is shown in Figure 2. The cumulative 6-year probability of achieving a MMR was 98% (70/73 patients). The MMR was stable in almost all patients: occasional fluctuations above 0.1% were observed, but only one patient had a confirmed loss of MMR. Three patients never achieved MMR: one of them, Sokal high risk, progressed to lymphoid, T315I-mutated, blast phase after 6 months of treatment. The other two patients are still on nilotinib, with BCR-ABL1 levels fluctuating between 0.1 and 1%. The cumulative probability of achieving MR 4.0 was 76%. Of the 55 patients who were still on nilotinib at last contact, 18 (33%) were in stable MR 4.0, while in 24 (44%) the BCR-ABL1 transcript levels fluctuated between 0.1 and <0.01%.
Figure 2.
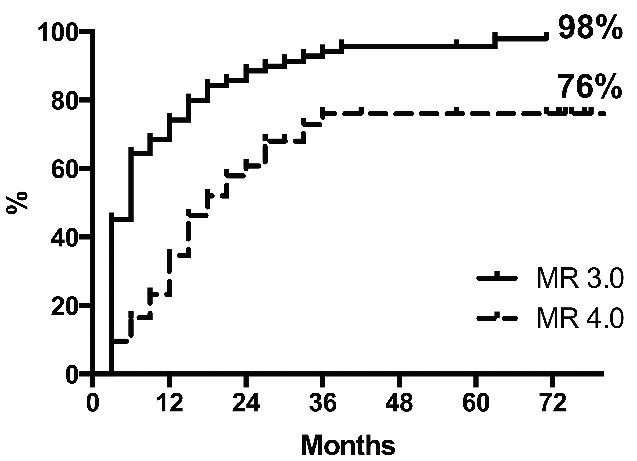
Cumulative incidences of MR 3.0 and MR 4.0. The median times to MR 3.0 and MR 4.0 were 6 and 18 months, respectively.
Atherosclerotic adverse events
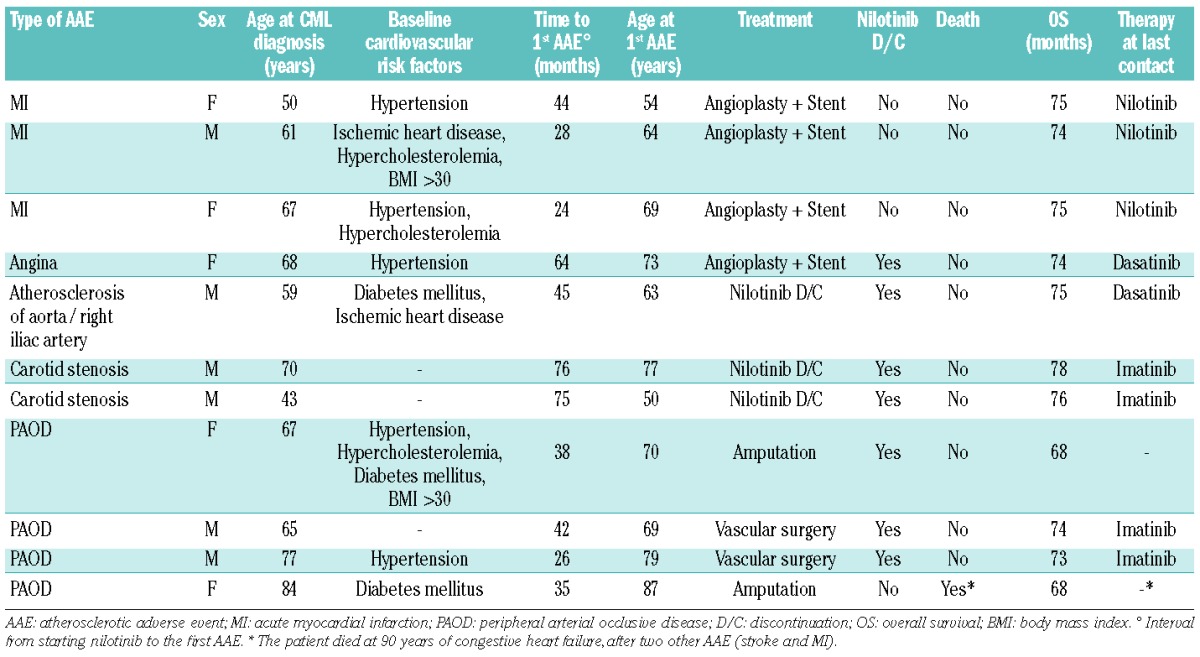
Thirteen atherosclerotic adverse events were recorded in 11/73 (15%) patients. The most common atherosclerotic adverse events were myocardial infarction (n=4) and peripheral arterial occlusive disease (n=4); details of the atherosclerotic adverse events are reported in Tables 3 and 4. The median time of developing these adverse events from starting nilotinib was 42 months (range, 24 – 76). The median age at first atherosclerotic adverse event was 69 years (range, 50 – 87 years). Seven patients permanently discontinued nilotinib after an atherosclerotic adverse event. Four patients, three with myocardial infarction and one with peripheral arterial occlusive disease, continued nilotinib at a reduced dose (200 – 400 mg once daily), together with anticoagulant or anti-platelet therapy: the cardiopathy was well controlled in the three patients with myocardial infarction, while two more vascular events (myocardial infarction and stroke) occurred in the patient with peripheral arterial occlusive disease. Atherosclerotic adverse events occurred more frequently in elderly patients [7/20 (35%) in patients ≥65 years old, versus 4/53 (8%) in patients <65 years old, P=0.003], as well as in patients with baseline cardiovascular risk factors [8/26 (31%) versus 3/55 (6%), P=0.002].
Table 3.
Atherosclerotic adverse events.
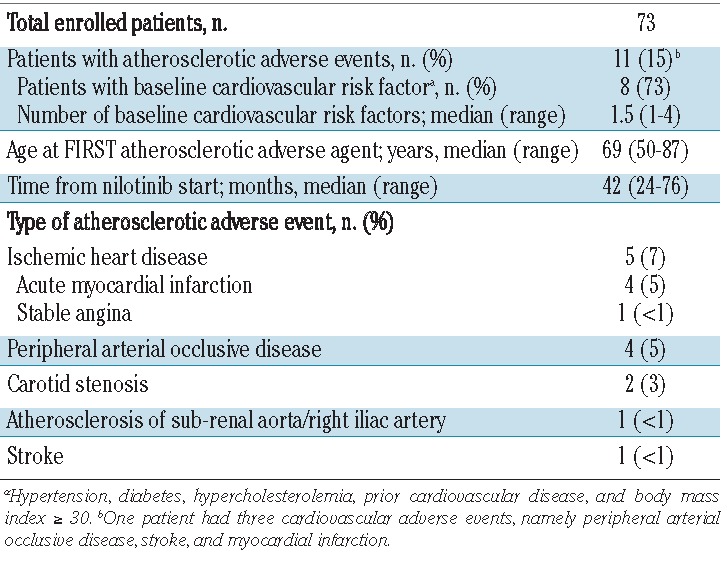
Table 4.
Details of atherosclerotic adverse events.
Discussion
This final report of a prospective, multicenter, investigator-sponsored, single arm study with a minimum follow-up of 6 years provides independent data on the rate, the depth, and the stability of molecular responses, on safety (particularly, the long-term cardiovascular safety), and on the long-term outcome of front-line treatment with nilotinib in newly diagnosed CP-CML.
All patients but one responded, and all patients but three became optimal responders according to European LeukemiaNet 2013 recommendations.20 Thirty-six of 73 (49%) patients were in MR 4.0 at last contact. Of all 73 patients, 18 (24%) were in stable MR 4.0 and were, therefore, eligible for a trial of treatment discontinuation. Six patients are currently enrolled in such a trial. One patient progressed to blast phase on therapy, with a T315I mutation. The particularly low rate of progression to blast phase observed in this study might have been partially determined by the low proportion of high Sokal risk patients (14%). Indeed, the progression-free survival rate (96% at 6 years) is similar to that observed in some trials with frontline imatinib, in the subset of not high-risk patients,21 or in those with a low proportion of high-risk patients.22
Two patients, 75 and 90 years old, died of other causes, while having a MMR. The 6-year overall survival rate was 96%. Due to some degree of selection of patients, as occurs in many clinical trials, the median age at CML diagnosis in this study was lower than that reported in most epidemiological registries, and, possibly, contributed to the high overall survival observed.
Overall, 75% of the patients were still on nilotinib at 6 years. Fifteen per cent (11/73) of patients experienced clinically relevant atherosclerotic adverse events. None died, but there was a relevant morbidity: two patients underwent amputation of a lower limb and four had angioplasty for coronary artery disease.
When comparing the efficacy and safety data of this study with corresponding data from the ENESTnd trial, it must be considered that there were two cohorts in the latter (one treated with 300 mg twice daily and one with 400 mg twice daily), the number of patients was higher (282 and 281, respectively), the proportion of Sokal high-risk patients was higher (28%), the age was lower (median, 46–47 years), and the follow-up was slightly shorter (median 5 years).22–6 Taking into account these differences, the proportions of patients still on nilotinib at 5 years were 60% (in patients treated with 300 mg twice daily) and 62% (in those treated with 400 mg twice daily) in ENESTnd versus 75% in this study. The cumulative probability of achieving MR 4.0 was 66% (at 300 mg twice daily) and 63% (at 400 mg twice daily) in ENESTnd versus 76% in this trial. The rate of progression to blast phase was 3.5% (at 300 mg twice daily) and 2.1% (at 400 mg twice daily) in ENESTnd versus 1.3% in this study. The rate of deaths from any cause was 6.4% (at 300 mg twice daily) and 3.5% (at 400 mg twice daily) in ENESTnd versus 4.1% in this trial. The rate of cardiovascular adverse events was 8% (at 300 mg twice daily) and 13% (at 400 mg twice daily) in ENESTnd versus 15% in this study. Overall, the results of this smaller clinical trial are well in line with the results of the ENESTnd study for efficacy. For safety, the initial nilotinib dose (400 mg twice daily) and the more advanced age of the patients likely account for a higher rate of cardiovascular adverse events. It should not be overlooked that the frequency of cardiovascular adverse events reported in the ENESTnd and in this trial may be lower than those reported in other studies, in which the analyses were retrospective and many patients had received prior treatment with other tyrosine kinase inhibitors.9–14 However, the rate of cardiovascular adverse events with the initial dose of 400 mg twice daily remains significant, and supports the use of the lower dose of 300 mg twice daily, as is currently registered for the front-line treatment of CP-CML.
In conclusion, this independent study of nilotinib in the first-line treatment of newly diagnosed CP-CML highlights the rapid and high anti-leukemic efficacy of this tyrosine kinase inhibitor and warns that in patients with cardiovascular risk factors, and particularly in elderly patients, the treatment can favor the development of clinically relevant, potentially disabling and life-threatening, cardiovascular complications, especially atherosclerotic complications. The balance between efficacy and safety will depend on the characteristics of the patient and on the goal of therapy, whether it is limited to survival or it is addressed, more ambitiously, to the achievement of a treatment-free remission.
Footnotes
Funding
This study was supported by GIMEMA Onlus, BolognAIL, and European LeukemiaNet (LSHC-CT-2004-503216). The authors thank Miriam Fogli for her precious assistance in the data management.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Weisberg E, Manley PW, Breitenstein W, et al. Characterization of AMN107, a selective inhibitor of native and mutant Bcr-Abl. Cancer Cell. 2005;7(2):129–141. [DOI] [PubMed] [Google Scholar]
- 2.Saglio G, Kim DW, Issaragrisil S, et al. Nilotinib versus imatinib for newly diagnosed chronic myeloid leukemia. N Engl J Med. 2010;362(24):2251–2259. [DOI] [PubMed] [Google Scholar]
- 3.Hughes T, le Coutre P, Jootar S, et al. ENESTnd 5-year follow-up: continued benefit of frontline nilotinib (NIL) compared with imatinib (IM) in patients (pts) with chronic myeloid leukemia in chronic phase (CML-CP). Haematologica. 2014;99(s1)(236–237. [Google Scholar]
- 4.Larson R, Kim D, Issaragrisil S, et al. Efficacy and safety of nilotinib (NIL) vs imatinib (IM) in patients (pts) with newly diagnosed chronic myeloid leukemia in chronic phase (CML-CP): long-term follow-up of ENESTnd. Blood. 2014;ASH Meeting, Abstract 4541. [Google Scholar]
- 5.Kantarjian HM, Hochhaus A, Saglio G, et al. Nilotinib versus imatinib for the treatment of patients with newly diagnosed chronic phase, Philadelphia chromosome-positive, chronic myeloid leukaemia: 24-month minimum follow-up of the phase 3 randomised ENESTnd trial. Lancet Oncol. 2011;12(9): 841–851. [DOI] [PubMed] [Google Scholar]
- 6.Larson RA, Hochhaus A, Hughes TP, et al. Nilotinib vs imatinib in patients with newly diagnosed Philadelphia chromosome-positive chronic myeloid leukemia in chronic phase: ENESTnd 3-year follow-up. Leukemia. 2012;26(10):2197–2203. [DOI] [PubMed] [Google Scholar]
- 7.Rosti G, Palandri F, Castagnetti F, et al. Nilotinib for the frontline treatment of Ph(+) chronic myeloid leukemia. Blood. 2009;114(24):4933–4938. [DOI] [PubMed] [Google Scholar]
- 8.Cortes JE, Jones D, O’Brien S, et al. Nilotinib as front-line treatment for patients with chronic myeloid leukemia in early chronic phase. J Clin Oncol. 2010;28(3):392–397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Aichberger KJ, Herndlhofer S, Schernthaner GH, et al. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in CML. Am J Hematol. 2011;86(7):533–539. [DOI] [PubMed] [Google Scholar]
- 10.Le Coutre P, Rea D, Abruzzese E, et al. Severe peripheral arterial disease during nilotinib therapy. J Natl Cancer Inst. 2011;103(17):1347–1348. [DOI] [PubMed] [Google Scholar]
- 11.Levato L, Cantaffa R, Kropp MG, Magro D, Piro E, Molica S. Progressive peripheral arterial occlusive disease and other vascular events during nilotinib therapy in chronic myeloid leukemia: a single institution study. Eur J Haematol. 2013;90(6):531–532. [DOI] [PubMed] [Google Scholar]
- 12.Giles FJ, Mauro MJ, Hong F, et al. Rates of peripheral arterial occlusive disease in patients with chronic myeloid leukemia in the chronic phase treated with imatinib, nilotinib, or non-tyrosine kinase therapy: a retrospective cohort analysis. Leukemia. 2013;27(6):1310–1315. [DOI] [PubMed] [Google Scholar]
- 13.Kim TD, Rea D, Schwarz M, et al. Peripheral artery occlusive disease in chronic phase chronic myeloid leukemia patients treated with nilotinib or imatinib. Leukemia. 2013;27(6):1316–1321. [DOI] [PubMed] [Google Scholar]
- 14.Valent P, Hadzijusufovic E, Schernthaner GH, Wolf D, Rea D, le Coutre P. Vascular safety issues in CML patients treated with BCR/ABL1 kinase inhibitors. Blood. 2015;125(6):901–906. [DOI] [PubMed] [Google Scholar]
- 15.Cross NC, White HE, Muller MC, Saglio G, Hochhaus A. Standardized definitions of molecular response in chronic myeloid leukemia. Leukemia. 2012;26(10):2172–2175. [DOI] [PubMed] [Google Scholar]
- 16.Soverini S, Martinelli G, Amabile M, et al. Denaturing-HPLC-based assay for detection of ABL mutations in chronic myeloid leukemia patients resistant to imatinib. Clin Chem. 2004;50(7):1205–1213. [DOI] [PubMed] [Google Scholar]
- 17.Sokal JE, Cox EB, Baccarani M, et al. Prognostic discrimination in “good-risk” chronic granulocytic leukemia. Blood. 1984;63(4):789–799. [PubMed] [Google Scholar]
- 18.Hasford J, Pfirrmann M, Hehlmann R, et al. A new prognostic score for survival of patients with chronic myeloid leukemia treated with interferon alfa. Writing Committee for the Collaborative CML Prognostic Factors Project Group. J Natl Cancer Inst. 1998;90(11):850–858. [DOI] [PubMed] [Google Scholar]
- 19.Hasford J, Baccarani M, Hoffmann V, et al. Predicting complete cytogenetic response and subsequent progression-free survival in 2060 patients with CML on imatinib treatment: the EUTOS score. Blood. 2011;118(3):686–692. [DOI] [PubMed] [Google Scholar]
- 20.Baccarani M, Deininger MW, Rosti G, et al. European LeukemiaNet recommendations for the management of chronic myeloid leukemia: 2013. Blood. 2013;122(6):872–884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hochhaus A, O’Brien SG, Guilhot F, et al. Six-year follow-up of patients receiving imatinib for the first-line treatment of chronic myeloid leukemia. Leukemia. 2009;23(6):1054–1061. [DOI] [PubMed] [Google Scholar]
- 22.Cervantes F, Lopez-Garrido P, Montero MI, et al. Early intervention during imatinib therapy in patients with newly diagnosed chronic-phase chronic myeloid leukemia: a study of the Spanish PETHEMA group. Haematologica. 2010;95(8):1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]


