Dietary iron absorption offsets non-specific iron losses and is crucial for systemic iron balance. Inadequate iron acquisition leads to iron deficiency, a condition associated with anemia, poor pregnancy outcome and impaired cognitive and motor development.1 Heme derived from hemoglobin (Hb) and myoglobin of meat products is an important nutritional iron source.2 It is more bioavailable compared to inorganic iron, although less abundant in mixed diets. Thus, two-thirds of body iron stores are estimated to originate from heme, which accounts for only one-third of total dietary iron content in Western populations.3
The mechanism for inorganic iron absorption has been clarified from mouse studies.4 Ingested Fe(III) is reduced to Fe(II) in the intestinal lumen and then internalized by enterocytes via the apical transporter DMT1. The basolateral transporter ferroportin mediates Fe(II) efflux to the bloodstream.
Heme is absorbed by enterocytes as an intact molecule via receptor-mediated endocytosis or direct transport.3,5 The underlying mechanisms are not completely understood. Early studies suggested that internalized heme is catabolized to liberate Fe(II), which follows the fate of dietary Fe(II), although direct transport of intact heme to the plasma has also been reported.6 Enterocytes express heme-catabolic enzymes (heme oxygenases), the heme-inducible HO-1 and its constitutive homolog HO-2,7 as well as the low-affinity heme transporter HCP1 and the heme exporter FLVCR1.5
We generated intestinal-specific Hmox1−/− mice (Hmox1Vil-Cre) to dissect the role of HO-1 in heme iron assimilation (Online Supplementary Appendix). Because high-iron diets promote iron overload in mice,8 we reasoned that a high-heme diet (HHD) might elicit similar effects, or at least increase body iron stores in Hmox1fl/fl controls. Conversely, Hmox1Vil-Cre mice should be either spared and accumulate excess heme in enterocytes, or export heme to the bloodstream. To address these hypotheses, Hmox1fl/fl and Hmox1Vil-Cre mice were fed for two weeks with an iron-deficient diet (IDD) or a HHD.
Surprisingly, the HHD did not cause any appreciable increases in serum or hepatic iron in both genotypes. Likewise, mRNA levels of the iron regulator hepcidin were largely unaffected (Online Supplementary Figure S1). The absence of iron overload in HHD-fed control animals was not associated with inflammation or alterations in duodenal epithelial architecture (Online Supplementary Figure S2), suggesting that hemin may be a poor dietary iron source for mice. Nevertheless, the HHD intake promoted a profound increase of intestinal HO-1 in Hmox1fl/fl mice, consistent with hemin-mediated induction (Figure 1A and F). Thus, intestinal enterocytes are capable of internalizing a fraction of dietary hemin. Importantly, intestinal HO-1 expression was undetectable in Hmox1Vil-Cre mice and did not respond to HHD intake, demonstrating efficient tissue-specific ablation of the Hmox1 gene. The HHD did not affect expression of housekeeping HO-2 (Figure 1B and F), which was equal between Hmox1fl/fl and Hmox1Vil-Cre mice.
Figure 1.
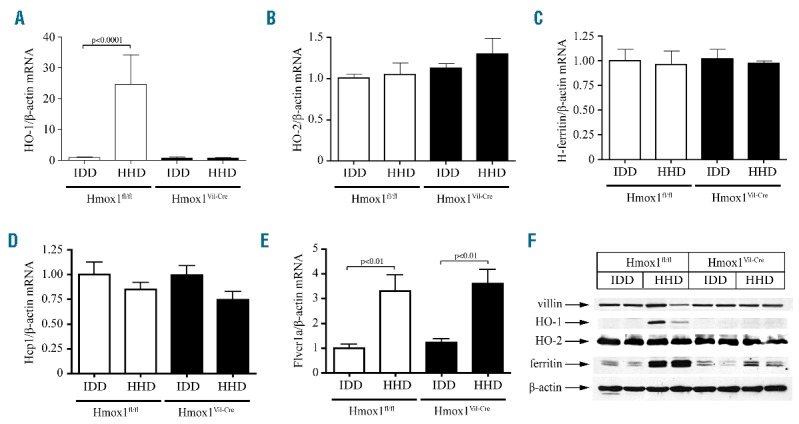
Effects of dietary heme excess in expression of intestinal heme and iron metabolism genes. 6-week old male Hmox1fl/fl and Hmox1Vil-Cre mice (n=5 for each genotype) were fed for two weeks with an IDD (containing only 2–6 ppm iron) or a HHD (IDD supplemented with 30 mmol/kg hemin, equivalent to 2% carbonyl iron in terms of iron quantity). At the end point the animals were sacrificed and duodenal RNA and protein extracts were prepared for qPCR and Western blot analysis, respectively. (A) The HHD promoted 23-fold induction of intestinal HO-1 mRNA in control Hmox1fl/fl but not in Hmox1Vil-Cre mice. (B–D) Intestinal HO-2 (B), H-ferritin (C) and Hcp1 (D) mRNA levels were not significantly affected by the diets and the genotypes. Constitutive expression of HO-2 mRNA was similar to that of HHD-induced HO-1 mRNA in Hmox1fl/fl mice. (E) The HHD stimulated intestinal Flvcr1a mRNA expression in both Hmox1fl/fl and Hmox1Vil-Cre mice. qPCR data in (A–E) are presented as the mean ± SEM. Statistical analysis was performed by one-way ANOVA. (F) The HHD induced intestinal HO-1 expression in control Hmox1fl/fl mice and intestinal ferritin expression in both Hmox1fl/fl and Hmox1Vil-Cre mice. Expression of HO-2, villin (intestinal marker) and β-actin (loading control) was not affected by the diets and the genotypes. Data from 2 representative mice are shown. Similar results were obtained with samples from the other mice used in the experiment.
Hcp1 mRNA was not significantly affected by the HHD in either genotype (Figure 1D). Flvcr1a mRNA did not differ substantially between Hmox1Vil-Cre and Hmox1fl/fl mice, but was similarly induced by HHD (Figure 1F), as reported in hemin-treated hepatocytes.9 This may indicate increased heme efflux independently of HO-1 expression. However, the lack of hemopexin or HO-1 mRNA induction in the liver and spleen (Figure 2A–C) suggests that heme export is negligible.
Figure 2.
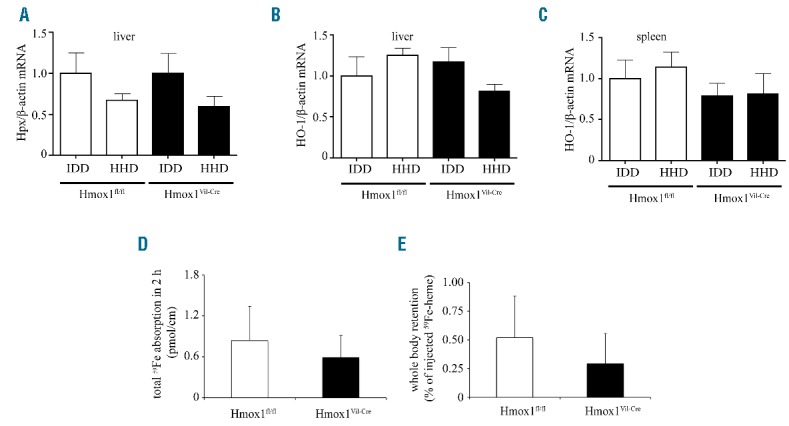
Effects of dietary heme excess in expression of hepatic and splenic heme metabolism genes, and analysis of intestinal heme transport. (A–C) Hepatic and splenic RNA was prepared from the mice described in Figure 1 and used for qPCR analysis. Dietary heme excess did not induce hemopexin (Hpx) mRNA in the liver (A), and HO-2 mRNA in the liver (B) or spleen (C). qPCR data are presented as the mean ± SEM. Statistical analysis was performed by one-way ANOVA. (D and E) Disruption of intestinal HO-1 did not affect absorption of 59Fe from 59Fe-Hb. A murine hemolysate containing 59Fe-Hb was administered to ligated intestinal loops from Hmox1fl/fl (n=5) and Hmox1Vil-Cre mice (n=6) for 2 h. (D) Total intestinal absorption of 59Fe. (E) Retention of 59Fe in the whole carcass (excluding the intestine). Data are presented as the mean ± SEM. Statistical analysis was performed by two-tailed Student’s t test.
Heme catabolism liberates Fe(II), which in turn post-transcriptionally up-regulates ferritin for iron storage.10 Intestinal ferritin (but not H-ferritin mRNA) was induced in HHD-fed Hmox1fl/fl mice (Figure 1C and F), presumably following enzymatic degradation of internalized hemin by HO-1. A similar response, although quantitatively less pronounced, was observed in HHD-fed Hmox1Vil-Cre mice. Considering that the HHD has virtually no inorganic iron, this indicates partial enzymatic degradation of internalized hemin by HO-2.
Conceivably, the failure of the HHD to elevate systemic iron levels is related to reduced bioavailability of chemically prepared hemin, which may polymerize and form aggregates.6 Therefore, we used a murine hemolysate containing 59Fe-Hb as physiological heme source. This was administered to ligated intestinal loops for 2 h; a prolonged incubation period to maximize signal-to-noise ratio. 59Fe absorption was slightly lower in Hmox1Vil-Cre mice but the difference was insignificant (Figure 2D). Due to limitations in the amount of 59Fe-Hb, we were not able to determine linear 59Fe absorption or calculate 59Fe lumen-to-body transfer rates. Assuming that plateau was not reached before 30 min, we estimate that iron absorption from 59Fe-Hb is at least 100-fold less efficient than from 59Fe-nitriloacetate.11 Consistently, there was very little whole body 59Fe retention (Figure 2E). These data reinforce the concept that mice cannot efficiently assimilate heme iron, independently of intestinal HO-1.
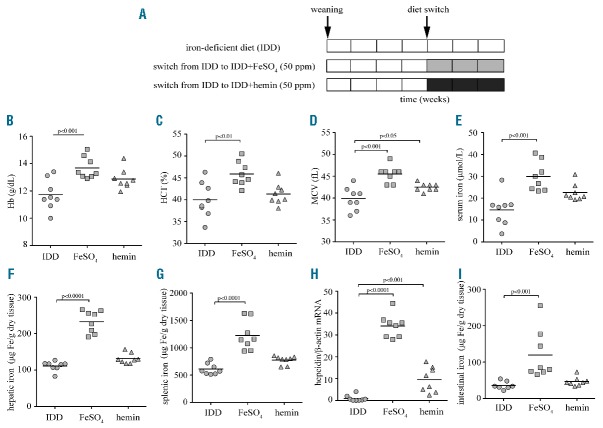
Since iron absorption is enhanced by erythropoietic drive,4 we examined heme bioavailability under anemia. Wild-type mice were subjected to a 4-week dietary iron restriction during their rapid growth period, immediately after weaning. Subsequently, they either remained on IDD, or switched to IDD supplemented with 50 ppm FeSO4 or hemin for three weeks (Figure 3A). The diets did not affect body weights (Online Supplementary Figure S3). At the end point, the IDD-fed mice exhibited reduced hematologic parameters and serum iron levels, consistent with iron deficiency anemia (Figure 3B–E). The switch to IDD+FeSO4 efficiently corrected anemia and increased serum iron. Even though the switch to IDD+hemin tended to slightly improve hematologic parameters and to increase serum iron, statistical significance was only reached for MCV values.
Figure 3.
Iron deficiency anemia is efficiently corrected by a diet supplemented with inorganic iron but not heme, due to low luminal heme transport in the gut. 3-week old female C57BL/6 mice (n=24) were fed with an IDD for four weeks. One group of the animals (n=8) remained on this diet for another three weeks; a second group (n=8) was switched to IDD supplemented with 50 ppm FeSO4 and a third group (n=8) was switched to IDD supplemented with 50 ppm hemin. At the end point, blood was prepared and used for hematologic and serum iron analysis, hepatic RNA was prepared for qPCR analysis, and tissues were used for assessment of total (non-heme and heme) iron content. (A) Schematic representation of experimental design. (B) Hb concentration. (C) Hematocrit (HCT). (D) Mean corpuscular volume (MCV). (E) Serum iron. (F) Hepatic iron. (G) Splenic iron. (H) Hepcidin mRNA was induced 34-fold by dietary FeSO4 and 9.5-fold by dietary hemin supplementation. (I) Intestinal iron levels were increased 3.3-fold following dietary FeSO4 supplementation and were not significantly affected by dietary hemin. All data are presented as the mean ± SEM. Statistical analysis was performed by one-way ANOVA.
Furthermore, FeSO4 intake significantly increased total hepatic and splenic iron, while the effects of hemin intake were marginal (Figure 3F,G). Nonetheless, both hemin and FeSO4 supplementation regimens promoted hepcidin mRNA induction, yet the hemin effect was weaker (Figure 3H). These findings further support the concept that heme is an inferior dietary iron source for mice, even under high iron demand. Notably, only FeSO4 but not hemin supplementation, significantly increased total intestinal iron content (Figure 3I). Thus, the limiting step for dietary heme iron acquisition is the transport of heme from the intestinal lumen across the apical membrane of brush border enterocytes.
We demonstrate that intestinal HO-1 is not essential for dietary heme iron assimilation in mice. Thus, Hmox1Vil-Cre mice do not exhibit any appreciable handicap in heme absorption relative to controls. The capacity of mice to acquire dietary heme iron is extremely low compared to inorganic iron sources. This holds true even under iron deficiency anemia, where iron absorption is stimulated to meet the high erythropoietic needs. The only limitation is in the apical transport of heme across the intestinal epithelium. This defect may be intrinsic to rodents, considering that also rats inefficiently absorb Hb iron.12 Our findings indicate that the mouse is not an appropriate model organism for intestinal heme transport studies. Given the established bioavailability of heme iron in humans, we speculate that carnivores and omnivores, including humans who became active predators in response to climate changes some 2 million years ago,13 have evolved more efficient mechanisms for heme absorption than mice, which feed mostly on grains, with some carrion if available.
We provide evidence that enzymatic heme degradation is obligatory for iron utilization in mice, and exclude significant export of intact heme. The potent induction of intestinal HO-1 and upregulation of ferritin in HHD-fed mice indicate a contribution of HO-1 in heme degradation. The persistence of ferritin upregulation, albeit relatively blunted, suggests partial compensation of HO-1 by HO-2.
The apparent functional redundancy of HO-1 and HO-2 in dietary heme processing is unexpected. Hmox1−/− and Hmox2−/− mice have distinct phenotypes, suggesting diverse physiological functions of HO-1 and HO-2. Hmox1−/− mice manifest severe pathological features including anemia, defective erythrophagocytosis, iron overload and inflammation, while Hmox2−/− mice exhibit sensitivity to hyperoxia.5 Importantly, HO-1 and HO-2 are expressed in distinct intracellular compartments. HO-1 is evenly distributed in rat enterocytes even following heme absorption, while HO-2 co-localizes extensively with endosomes.7 These data support the hypothesis that dietary heme is internalized via receptor-mediated endocytosis and undergoes degradation within endosomes via HO-2, without HO-1 involvement7
This model would provide an attractive mechanism for basal heme absorption. Nonetheless, the heme-mediated induction of intestinal HO-1 and subsequent upregulation of ferritin in control Hmox1fl/fl mice indicate a physiological function of both HOs in heme iron acquisition. We propose that HO-2 suffices to handle basal heme levels, while HO-1 is required for higher heme loads. Hence, HO-1 may be essential for efficient heme iron assimilation in carnivores/omnivores, even though it is dispensable in mice, which are poor heme absorbers. It is tempting to speculate that HO-2 may degrade basal heme internalized via receptor-mediated endocytosis; conversely, HO-1 may process excessive heme directly transferred via a transporter. While mice express Hcp15, it is likely that carnivores/omnivores express high-affinity heme transporter(s), which still have to be identified.
An important ramification of our findings is that the clinical use of HO-1-specific inhibitors would not affect basal HO-2-mediated heme iron absorption. This may prevent iron deficiency anemia, a side-effect of heme malabsorption in patients with Crigler-Najar type I syndrome subjected to prolonged therapy with tin-mesoporphyrin.14 Such non-selective HO substrate analogs have also received wider attention in the context of cancer therapy because HO-1 exerts anti-apoptotic and cytoprotective functions.15 However, these drugs diminish intestinal heme iron absorption and, in the long run, may promote iron deficiency anemia analogous to that observed in Crigler-Najar type I patients.
Acknowledgments
We are indebted to Dr. G. Kollias (BSRC Al. Fleming, Greece) for providing us the Hmox1fl/fl mice.
Footnotes
Funding: this work was supported by grants from the Canadian Institutes for Health Research (CIHR; MOP-86514 to KP and MOP-123246 to MMS) and the Natural Sciences and Engineering Research Council of Canada (NSERC; 298515-2011 to MMS). The intestinal absorption studies were co-funded by the Hildegard-Grunow Foundation of Munich, Germany. KP and MMS were recipients of Chercheur National and Chercheur Senior career awards, respectively, from the Fonds de la Recherche en Santé du Quebéc (FRSQ). KG was supported by doctoral awards from the J. Latsis and A. Onassis Public Benefit Foundations and from FRSQ.
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Zimmermann MB, Hurrell RF. Nutritional iron deficiency. Lancet. 2007;370(9586):511–520. [DOI] [PubMed] [Google Scholar]
- 2.Carpenter CE, Mahoney AW. Contributions of heme and nonheme iron to human nutrition. Crit Rev Food Sci Nutr. 1992;31(4):333–367. [DOI] [PubMed] [Google Scholar]
- 3.West AR, Oates PS. Mechanisms of heme iron absorption: current questions and controversies. World J Gastroenterol. 2008; 14(26):4101–4110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gulec S, Anderson GJ, Collins JF. Mechanistic and regulatory aspects of intestinal iron absorption. Am J Physiol Gastrointest Liver Physiol. 2014;307(4):G397–G409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Khan AA, Quigley JG. Control of intracellular heme levels: heme transporters and heme oxygenases. Biochim Biophys Acta. 2011; 1813(5):668–682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conrad ME, Weintraub LR, Sears DA, Crosby WH. Absorption of hemoglobin iron. Am J Physiol. 1966;211(5):1123–1130. [DOI] [PubMed] [Google Scholar]
- 7.West AR, Oates PS. Subcellular location of heme oxygenase 1 and 2 and divalent metal transporter 1 in relation to endocytotic markers during heme iron absorption. J Gastroenterol Hepatol. 2008; 23(1):150–158. [DOI] [PubMed] [Google Scholar]
- 8.Daba A, Gkouvatsos K, Sebastiani G, Pantopoulos K. Differences in activation of mouse hepcidin by dietary iron and parenterally administered iron dextran: compartmentalization is critical for iron sensing. J Mol Med (Berl). 2013;91:95–102. [DOI] [PubMed] [Google Scholar]
- 9.Vinchi F, Ingoglia G, Chiabrando D, et al. The Heme Exporter FLVCR1a Regulates Heme Synthesis and Degradation and Controls Activity of Cytochromes P450. Gastroenterology. 2014;146(5):1325–1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang J, Pantopoulos K. Regulation of cellular iron metabolism. Biochem J. 2011;434(3):365–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schümann K, Herbach N, Kerling C, et al. Iron absorption and distribution in TNFΔARE/+ mice, a model of chronic inflammation. J Trace Elem Med Biol. 2010;24(1):58–66. [DOI] [PubMed] [Google Scholar]
- 12.Cao C, Thomas CE, Insogna KL, O’Brien KO. Duodenal absorption and tissue utilization of dietary heme and nonheme iron differ in rats. J Nutr. 2014;144(11):1710–1717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cordain L, Watkins BA, Mann NJ. Fatty acid composition and energy density of foods available to African hominids. Evolutionary implications for human brain development. World Rev Nutr Diet. 2001; 90:144–161. [DOI] [PubMed] [Google Scholar]
- 14.Kappas A, Drummond GS, Galbraith RA. Prolonged clinical use of a heme oxygenase inhibitor: hematological evidence for an inducible but reversible iron-deficiency state. Pediatrics. 1993;91(3):537–539. [PubMed] [Google Scholar]
- 15.Was H, Dulak J, Jozkowicz A. Heme oxygenase-1 in tumor biology and therapy. Curr Drug Targets. 2010;11(12):1551–1570. [DOI] [PubMed] [Google Scholar]