Abstract
High expression of the chemokine receptor 4, CXCR4, associated with a negative prognosis in acute myeloid leukemia, is related to hypoxia. Because CXCR4 expression is under the post-transcriptional control of microRNA-146a in normal and leukemic monocytic cells, we first investigated the impact of hypoxia on microRNA-146a and CXCR4 expression during monocytopoiesis and in acute monocytic leukemia. We then analyzed the effects of hypoxia on drug sensitivity of CXCR4-expressing leukemic cells. We found that microRNA-146a is a target of hypoxia-inducible factor-1α or -2α in relation to the stage of monocytopoiesis and the level of hypoxia, and demonstrated the regulation of the microRNA-146a/CXCR4 pathway by hypoxia in monocytes derived from CD34+ cells. Thus, in myeloid leukemic cell lines, hypoxia-mediated control of the microRNA-146a/CXCR4 pathway depends only on the capacity of hypoxia-inducible factor-1α to up-regulate microRNA-146a, which in turn decreases CXCR4 expression. However, at variance with normal monocytic cells and leukemic cell lines, in acute monocytic leukemia overexpressing CXCR4, hypoxia up-modulates microRNA-146a but fails to down-modulate CXCR4 expression. We then investigated the effect of hypoxia on the response of leukemic cells to chemotherapy alone or in combination with stromal-derived factor-1α. We found that hypoxia increases stromal-derived factor-1α-induced survival of leukemic cells by decreasing their sensitivity to anti-leukemic drugs. Altogether, our results demonstrate that hypoxia-mediated regulation of microRNA-146a, which controls CXCR4 expression in monocytic cells, is lost in acute monocytic leukemia, thus contributing to maintaining CXCR4 overexpression and protecting the cells from anti-leukemic drugs in the hypoxic bone marrow microenvironment.
Introduction
The most primitive stem cells in the bone marrow are maintained in hypoxic niches that regulate the fate of these cells and hematopoietic progenitor cells in terms of quiescence, self-renewal and differentiation.1–6 Leukemic cells can infiltrate the niches leading to their enhanced self-renewal and proliferation, quiescence and resistance to chemotherapeutic agents.7,8 Hypoxia is, therefore, associated with poor prognosis in leukemia, especially in acute myeloid leukemia (AML),9–13 as also described in cancer and inflammatory diseases.8,14
Adaptation of leukemic cells to the bone marrow microenvironment is important in the clonal selection that leads to leukemia progression, drug resistance and relapse.7–11,14 To survive in hypoxia, cells activate adaptive responses, including gene regulation by hypoxia-inducible factors (HIF)-1alpha (HIF-1α) and -2alpha (HIF-2α).15,16 Hypoxia allows HIF to escape from normoxia-mediated degradation in the cytoplasm and to translocate into nuclei where they bind to hypoxia-response elements within the regulatory regions of target genes17–20 and microRNA.21,22
The use of HIF inhibitors represents a new strategy for the development of therapies targeting the hypoxic cancer microenvironment.23–27 Stabilization of HIF proteins by dimethyloxalyl glycine treatment, increases hematopoietic stem cell quiescence in vivo, improves blood recovery and enhances hematopoietic stem cell survival in the bone marrow of irradiated mice.23 Echinomycin, a DNA-intercalating agent that blocks HIF DNA-binding activity, selectively eradicates cancer stem cells in mouse models of lymphoma and human AML24 and selectively kills leukemia-initiating cells, inducing long-term remission in a murine model of relapsed AML.25
The chemokine receptor CXCR4 and its ligand stromal-derived factor-1α (SDF-1/CXCL12) are both major players in the cross-talk between leukemic cells and the bone marrow microenvironment.10–12 In patients with AML, hypoxia-induced CXCR4 expression is associated with poor prognosis, independently of the presence or not of the mutated FLT3 gene.12,28
CXCR4 is a target gene of microRNA-146a (miR-146a),29 among several other microRNA targeting this gene.30–32 miR-146a is a modulator of innate and adaptive immunity, is involved in tumor progression and in hematopoiesis, and is required for stem/progenitor cell differentiation into monocytes/macrophages.33–36 In malignant hematopoiesis, miR-146a is involved in the pathogenesis of myelodysplastic syndromes for its deletion on chromosome 537 and in the progression of chronic myeloid leukemia.38
We previously identified the molecular mechanism that regulates the level of CXCR4 protein expression by miR-146a targeting29 (miR-146a/CXCR4) during monocytopoiesis.39 We also reported that in acute monocytic leukemia (AML-M5), a high CXCR4 protein level is associated with low/absent miR-146a expression.39 We then showed that enforced miR-146a expression in leukemic cells decreases the level of CXCR4 protein and improves the sensitivity of these cells to drugs.39
CXCR4 is a target gene of HIF-1α and a post-transcriptional target of miR-146a,9,39 in monocytic cells, known to originate from a common myeloid precursor in the bone marrow giving rise to tissue macrophages and dendritic cells40 and subject to very different oxygen (O2) levels.41
In this study, we investigated the impact of chronic hypoxia on the miR-146a/CXCR4 regulatory axis during monocytopoiesis and in monocytic leukemic cells. The levels of hypoxia studied were mild (5% O2), such as that present in the sinusoidal cavity of bone marrow, and more severe (1% O2), such as that in hypoxic niches in bone marrow.
Altogether, our data demonstrated how the differential expression of HIF-1α and HIF-2α is mediated by O2 level (mild or severe) and down-regulates CXCR4 expression through a post-transcriptional mechanism mediated by up-regulation of miR-146a in normal monocytic cells and in monocytic leukemia cell lines, expressing a moderate level of CXCR4. However, this mechanism is dysregulated in primary AML-M5 blast cells that fail to decrease CXCR4 expression significantly in response to hypoxia. This dysregulation helps to explain why leukemic blasts express high CXCR4 levels, even in hypoxic conditions, and how they are protected from anti-leukemic drugs through CXCR4 activation mediated by SDF-1α secreted in the hypoxic bone marrow microenvironment.
Methods
Additional information is provided in the Online Supplement.
Cell cultures
After obtaining informed consent, human cord blood was taken from healthy donors and samples of blood were taken from patients with AML. This study was approved by the local ethical committees of the Italian National Institute of Health and the University of Tor Vergata.
Cord blood CD34+ hematopoietic progenitor cell purification, unilineage monocytic differentiation and morphological analyses were performed as previously described.39
Human primary AML-M5 blasts were maintained in culture in vitro in Iscove medium supplemented with 10% fetal calf serum, granulocyte-macrophage colony-stimulating factor (10 ng/mL), stem cell factor (50 ng/mL), and interleukin-3 (10 ng/mL) (PeproTech Inc. Rocky Hill, NJ, USA). RNA and protein samples were prepared as described elsewhere.39
Leukemic cell lines, U937 and HL-60, were grown in RPMI medium supplemented with 10% fetal calf serum (Gibco, Carlsbad, CA, USA).
Hypoxia
To provide a mild or more severe hypoxic environment, acute (1 to 6 h) or chronic (from 20 h to several days), cells were cultured and treated in sealed incubators calibrated for a constant hypoxic environment (mild hypoxia: 5% O2, 90% N2 and 5% CO2; severe hypoxia: 1% O2, 94% N2 and 5% CO2) at a temperature of 37°C. For physiological oxygenation or normoxia, cells were cultured in an incubator calibrated to 21% O2.
Gene silencing of hypoxia-inducible factors by small interfering RNA
U937 cells were transfected twice with 100 nM of short interfering ribonucleic acids (siRNA) of HIF-1α or HIF-2α (ON-TARGET plus SMART pool siR J004018-10 for HIF-1α and J004814-09 for HIF-2α, from Dharmacon, CO, USA), or non-targeting control siRNA (c-siRNA) (ON-TARGET plus Non-Targeting Pool D-001810-10-05, from Dharmacon) by using Lipofectamine 3000 as described elsewhere.29 Transfected U937(HIF-1α-siRNA), U937(HIF-2α-siRNA) and U937(c-siRNA) cells were cultured for 24 h under 1% O2 before analysis of HIFs-α, miR-146a and CXCR4 expression.
Dimethyloxalyl glycine and echinomycin treatment of cells
Dimethyloxalyl glycine (DMOG) treatment of U937 cells (25×104 cells/mL) was performed in normoxia, using 200 μM DMOG, (R&D Systems, Minneapolis, MN, USA) for 48 h, and findings compared to those of untreated U937 cells in hypoxia (48 h), used as a positive control of HIF-α activation.42,43
Echinomycin treatment of U937 cells (25×104 cells/mL) was performed under hypoxia (1% O2), with 0.1, 0.5, 1, 2, 5 or 10 nM echinomycin (Enzo Life Sciences, New York, USA) for 20 h, and results compared to those obtained in the normoxic state.
Results
Severe hypoxia delays monocytic proliferation and differentiation of hematopoietic progenitor cells, activates microRNA-146a and down-modulates CXCR4 protein expression
We investigated the effect of severe hypoxia (1% O2) on the proliferation and differentiation of monocytic cells derived from human CD34+ cells, as compared with the effect of mild (5% O2) hypoxia.
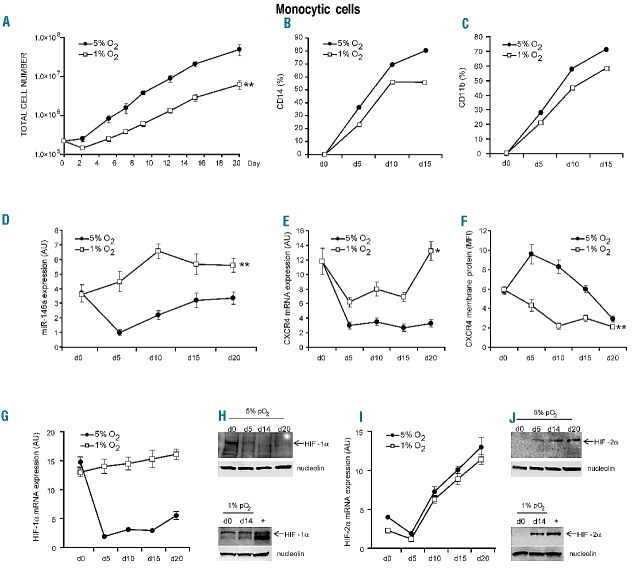
Severe hypoxia impaired cell growth (Figure 1A) and delayed monocytic differentiation/maturation, as shown by monocytic markers CD14 and CD11b, as compared to mild hypoxia (Figure 1B,C and Online Supplementary Figure S1A,B). Morphological analysis of monocytic cells showed less mature macrophagic elements in 1% O2 than in 5% O2 cultures, particularly on days 7 and 11 (Online Supplementary Figure S1C). Importantly, the percentage of apoptotic cells was similar on all days of culture for cells grown under mild and severe hypoxia (data not shown).
Figure 1.
Effect of mild or severe hypoxia on proliferation, differentiation, HIF-1α, HIF-2α, CXCR4 and miR-146a expression in hematopoietic progenitor cells induced to selective monocytic differentiation. (A–C) Severe hypoxia (1% O2) impairs cell proliferation (A) and delays monocytic differentiation of CD34+ hematopoietic progenitor cells (day 0), as shown by flow cytometry analysis of specific monocytic markers CD14 (B) and CD11b (C) expression, as compared to mild hypoxia (5% O2). (D, E) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) shows that 1% O2 up-regulates miR-146a (D) and CXCR4mRNA (E) expression in monocytic differentiating hematopoietic progenitor cells, as compared to 5% O2. (F) Flow cytometry analysis indicates that CXCR4 membrane protein levels are down-regulated by 1% O2, as compared to 5% O2 (G, I) qRT-PCR shows the differential expression of HIF-1α and HIF-2α mRNA during monocytic proliferation and differentiation of hematopoietic progenitor cells in hypoxia (mild or severe). (H, J) Western blot analysis of HIF-1α and HIF-2α nuclear protein expression during monocytic differentiation under 5% O2 and 1% O2 hypoxia. Nucleolin is shown as an internal control of nuclear proteins. (A, D–G, I) Mean ± SEM values from three independent experiments are shown. *P<0.05, **P<0.01. (B, C, H, J) One representative experiment of three is shown.
We analyzed the expression and regulation of miR-146a and CXCR4 in our monocytic cultures. Under mild hypoxia, miR-146a and CXCR4 mRNA expression levels decreased during the first days (d0–d5) of monocytopoiesis (Figure 1D–E), thus unblocking the translation of CXCR4 mRNA which resulted in an increase in CXCR4 protein levels (Figure 1F). Later, during monocytic maturation (d5–d20), the level of CXCR4 protein decreased (Figure 1F) while the level of CXCR4 mRNA remained constant (Figure 1E) and miR-146a increased (Figure 1D).
Severe hypoxia has a major effect on up-regulating miR-146a and CXCR4 mRNA expression levels, as compared to mild hypoxia (Figure 1D, E), indicating a hypoxia level-dependent transcriptional activation of these genes in monocytic differentiating hematopoietic progenitor cells. Thus, the decrease of CXCR4 protein levels observed under severe hypoxia (Figure 1F) is associated with a marked increase of miR-146a (Figure 1D) which blocks CXCR4 mRNA translation, according to the previously described mechanism of post-transcriptional control of CXCR4 expression by miR-146a targeting.39
Altogether, these data show that severe hypoxia impairs monocytic differentiation of hematopoietic progenitor cells and activates miR-146a/CXCR4 regulation to control CXCR4 level.
The differential expression of HIF-1α and HIF-2α depends on the level of hypoxia during monocytic proliferation and differentiation of hematopoietic progenitor cells
To investigate the molecular mechanisms involved in the hypoxia-mediated control of monocytopoiesis, we analyzed HIF expression in monocytic differentiating hematopoietic progenitor cells grown under conditions of mild and severe hypoxia.
Under conditions of mild hypoxia, HIF-1α expression at mRNA and nuclear protein levels is high in quiescent CD34+ cells (d0), then decreases during their monocytic differentiation (Figure 1G, H). In contrast, HIF-2α mRNA and HIF-2α nuclear protein expression, respectively low and undetectable in CD34+ cells (d0) and during the first days (d0–5) of monocytic culture, drastically increased during later stages of monocytic differentiation/maturation (Figure 1I,J).
Severe hypoxia activates HIF-1α mRNA expression and nuclear protein accumulation through monocytic differentiation (Figure 1G,H), whereas HIF-2α levels remain unchanged from those in a condition of mild hypoxia (Figure 1I,J).
Importantly, in normoxia CXCR4 and miR-146a expression levels were lower than in mild hypoxia and quite constant during the various steps of monocytic differentiation and HIF-1α and HIF-2α nuclear proteins were undetectable in monocytic cells in normoxia (data not shown), thus demonstrating that the accumulation of HIF nuclear proteins occurs only in hypoxic conditions.
In brief, we found that the differential expression of HIF-1α and HIF-2α observed throughout monocytopoiesis is dependent on the level of hypoxia, and severe hypoxia is required to activate HIF-1α in monocytic cells.
In monocytic differentiating hematopoietic progenitor cells, microRNA-146a is a new, shared target of HIF-1α and HIF-2α
We investigated whether miR-146a is a direct target of HIF-1α or HIF-2α in monocytic cells. We found a hypoxia-response element-related sequence 5′-GACTGGAGTGCAGTGGTGCAATCATAGC-3′ at position (−393; −422) in the promoter region of miR-146a29 (Prom-miR146a) (Figure 2A) which we identified as a new HIF-DNA binding site of both HIF-1α and HIF-2α, in vitro. Luciferase assays were performed using the reporter pGL3Basic-Prom146a vector previously described,29 in the presence or absence of HIF-1α or HIF-2α expression vectors (Figure 2B). Our data showed that both HIF-1α and HIF-2α expression caused a 40% activation of the Prom-miR146a, but not of the reporter pGL3Basic-Mut-Prom146a vector containing a mutant version of the hypoxia-response element in the Prom-miR146a (Mut-Prom146a) (Figure 2B). Overall, the newly identified hypoxia-response element in the promoter of miR-146a is able to bind in vitro HIF-1α and HIF-2α, both of which act as transcriptional activators of miR-146a.
Figure 2.
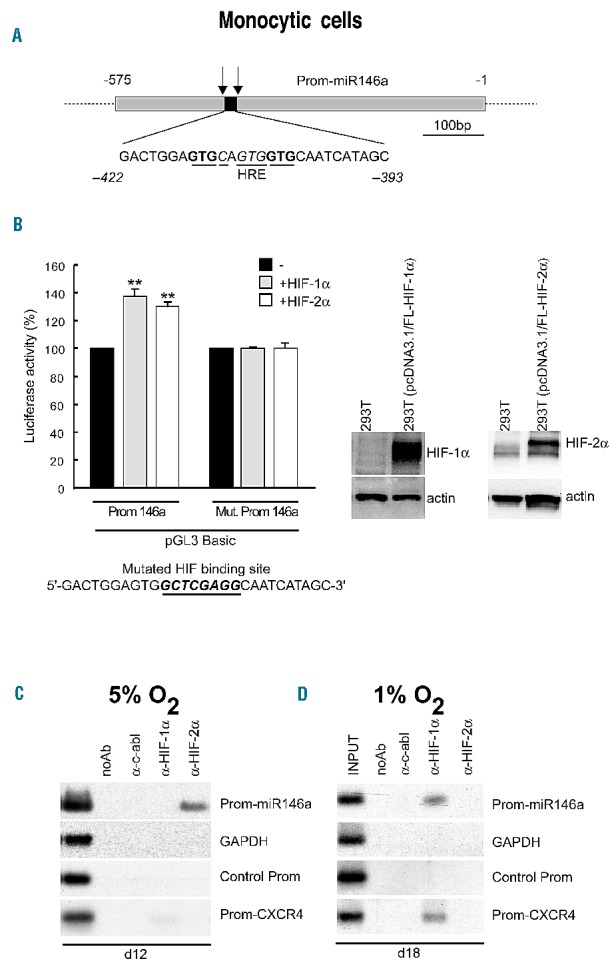
miR-146a is a target of HIF-2α, or HIF-1α, depending on hypoxic levels during monocytic differentiation of hematopoietic progenitor cells. (A) HIF-α putative binding site (HRE) identified on miR-146a Promoter (Prom-miR146a) cloned in a promoterless luciferase vector (pGL3Basic). The arrows indicate the chromatim immunoprecipitation (ChIP) primers used around HRE in Prom-miR146a. (B) Left panels: promoter activity assays performed in Phoenix cells transfected with the pGL3Basic-Prom146a vector alone (black bars), or in the presence of HIF-1α- (gray bars) or HIF-2α- (white bars) expression vectors, as compared to assays with the pGL3Basic-Mut. Prom146a, including the same region of Prom-miR146a but with a mutated HIF binding site (indicated below); Right panels: Western blot analysis of enforced expression of HIF-1α and HIF-2α protein in 293T (pcDNA3.1/HIFs-α) transfected cells, compared to untransfected 293T cells; actin is shown as an internal control of total protein extracts. Data are mean ± SEM values of three independent experiments. **P<0.01. (C) Under 5% O2, miR-146a is a target of HIF-2α, as shown by ChIP experiments performed using nuclear extracts from monocytic cells on day 12 of culture and analyzed by polymerase chain reaction (PCR) for Prom-miR146a and Prom-CXCR4 detection. (D) Under 1% O2, miR-146a is a target of HIF-1α, as shown by ChIP experiments performed on day 18 of culture in nuclear extracts from monocytic cells grown in 1% O2 and analyzed for Prom-miR146a and Prom-CXCR4 by PCR. (C, D) PCR of control for GAPDH used as internal control (GAPDH) and DNA sequence of Prom-146a without HRE (Control Prom) are shown. (B right panels, C, D) One representative experiment of three is shown.
We verified the recruitment of protein HIF-1α or HIF-2α to hypoxia-response element sites identified in CXCR4 and miR-146a promoters29,44 with chromatin immunoprecipitation experiments performed using nuclear extracts derived from monocytic cells grown under mild and severe hypoxia (Figure 2C,D).
We found that under mild hypoxia, in day 12 monocytic cells, Prom-miR-146a is a specific target of HIF-2α, but not of HIF-1α (Figure 2C: Prom-miR146a); in contrast, Prom-CXCR4 is a unique target of HIF-1α in the same monocytic cells, even if HIF-1α specific binding to Prom-CXCR4 is very weak (Figure 2C: Prom-CXCR4), as expected according to the low level of HIF-1α protein present in monocytic cells at this time.
Chromatin immunoprecipitation experiments indicated that, after 2 weeks of monocytic culture under severe hypoxia, miR-146a is a unique target of HIF-1α (Figure 2D: Prom-miR146a). In line with this finding, whereas HIF-1α and HIF-2α mRNA are both expressed in monocytic cells (d15–d20, Figure 1G, 1I), the level of HIF-1α nuclear protein is high compared to that of HIF-2α (Figure 1H). HIF-1α specific binding to Prom-CXCR4 was also detectable in day 18 monocytic cells (Figure 2D: Prom-CXCR4), in line with the CXCR4 mRNA level found in these cells (Figure 1E).
Our data show that chronic mild and severe hypoxia upregulate miR-146a and CXCR4mRNA expression by HIF-1α or HIF-2α differential targeting and HIF-1α targeting, respectively, during monocytic differentiation.
In monocytic leukemic cells, mild hypoxia is sufficient to activate HIF-1α-mediated control of microRNA-146a and CXCR4 mRNAα
To investigate the impact of hypoxia on miR-146a/CXCR4 regulation, we analyzed the effects of mild or severe hypoxia on U937 cells used as a model of monocytic leukemic cells.
Severe hypoxia, compared with mild hypoxia or normoxia, induced a decrease of U937 cell growth (Figure 3A, d0–3); apoptosis was observed only at late times in these culture (Figure 3B, d4), thus indicating the capacity of U937 cells to survive under severe hypoxia for a few days.
Figure 3.
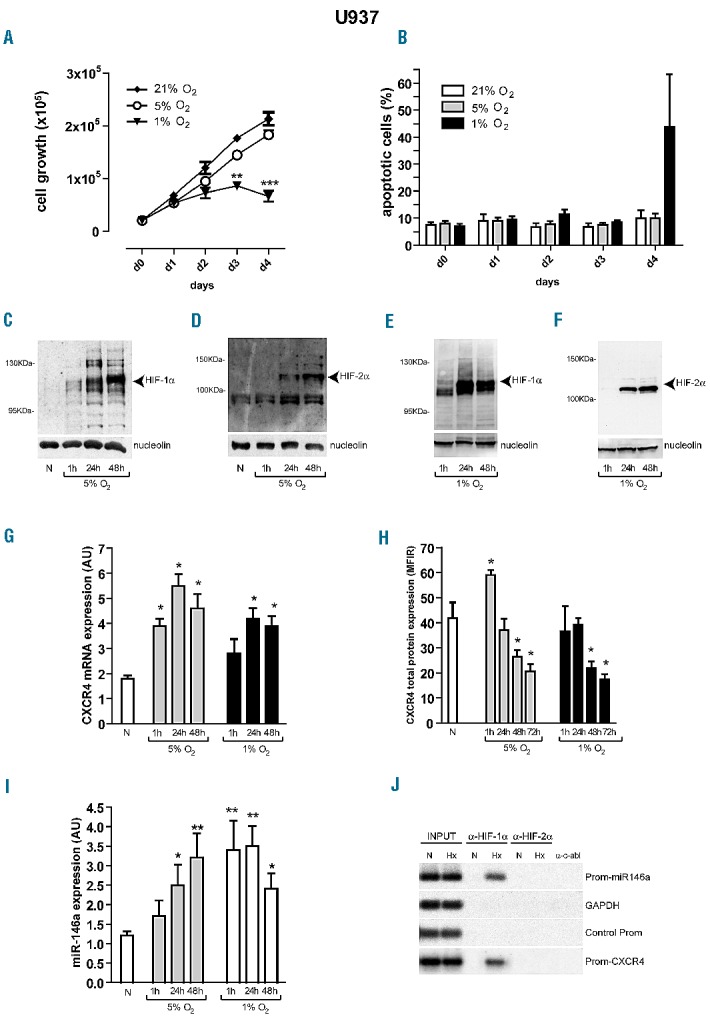
HIF-1α up-regulates CXCR4 mRNA and miR-146a expression that, in turn, decreases CXCR4 protein level in U937 cells. (A, B) Cell growth rate and apoptosis of U937 cells cultured under mild (5% O2) and severe (1% O2) hypoxia, as compared to normoxia (21% O2, N). (C–F) Western blot analysis shows that HIF-1α and HIF-2α nuclear proteins accumulate in U937 cells cultured under mild (C, D) or severe (E, F) hypoxia, as compared to normoxia (N). (C–F) Nucleolin expression is shown as an internal control of nuclear proteins; one representative experiment of three is shown. (G) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) shows that CXCR4 mRNA is up-regulated in U937 cells cultured from 1 h to 48 h, under either 5% O2 or 1% O2, as compared to normoxia (N). (H) Flow cytometry analysis shows that CXCR4 protein level, first up-regulated by acute (1 h) mild hypoxia (5% O2), is then significantly down-regulated in U937 cells cultured under chronic (from 48 h), mild or severe hypoxia, as compared to normoxia (N). (I) qRT-PCR indicates that miR-146a level, up-regulated in U937 cells cultured under chronic (24 h), mild hypoxia (5% O2), is up-regulated earlier (1 h) in U937 cells cultured under severe hypoxia (1%), as compared to normoxia (N). (G–I) Mean ± SEM values from three independent experiments are shown. *P<0.05, **P<0.01. (J) Chromatin immunoprecipitation experiments performed using nuclear extracts from U937 cells cultured under hypoxia (Hx), mild or severe, as compared to normoxia (N), identified the specific binding of HIF-1α, but not HIF-2α, to the hypoxia-responsive elements found in the promoter of miR-146a (Prom-146a) and of CXCR4mRNA (Prom-CXCR4) in leukemic cells.
We found that mild and severe hypoxia induced accumulation of both HIF-1α and HIF-2α nuclear proteins in leukemic cells (Figure 3C–F). It is important to note the biphasic modulation of CXCR4 by hypoxia in leukemic cells. After 1 h of mild or severe hypoxia, we observed an up-regulation of both CXCR4 mRNA and protein levels in U937 cells, related to a transcriptional control of CXCR4 (1 h, Figure 3G,H). However, after 24 h of hypoxia, we first observed the activation of miR-146a (24–48 h, Figure 3I) and then the down-modulation of CXCR4 protein level, indicating a post-transcriptional control of CXCR4 mRNA at these times (48–72 h, Figure 3H).
To verify whether HIF-1α or HIF-2α was recruited to the hypoxia-response elements in Prom-miR146a and Prom-CXCR4,44 we performed chromatin immunoprecipitation experiments using nuclear extracts from U937 cells cultured in conditions of mild and severe hypoxia, as compared to normoxia. The chromatin immunoprecipitation assays indicated that HIF-1α is the factor associated with both hypoxia-response elements, the one in Prom-miR146a and the other in Prom-CXCR4, in U937 cells in either mild (Figure 3J: Hx) or severe hypoxia (data not shown).
In order to demonstrate that hypoxia-induced changes in CXCR4 protein level are regulated by miR-146a, we silenced endogenous miR-146a expression, by transfecting antagomiR-146a or control antagomiR (C) oligonucleotides in hypoxic U937 cells, respectively U937(α-miR-146a) and U937(C) cells, as compared to normoxic cells (Online Supplementary Figure S2). We found that severe hypoxia similarly up-regulated HIF-1α nuclear protein expression in both U937(C) and U937(α-miR-146a) cells, as compared to normoxia (Online Supplementary Figure S2A), thus indicating that miR-146a silencing did not affect the capacity of hypoxia to induce HIF-1α stabilization. CXCR4 total protein level increased in U937(α-miR-146a) cells in both normoxic and hypoxic conditions, as compared to control U937(C) cells (Online Supplementary Figure S2B). It is important to note that CXCR4 levels observed in hypoxic U937(α-miR-146a) cells were comparable to those observed in normoxic U937(C) control cells (Online Supplementary Figure S2A). Importantly, miR-146a silencing reduced the inhibitory effect of hypoxia on CXCR4 expression (Online Supplementary Figure S2A).
Altogether, our data showed that in U937 cells cultured under conditions of mild or severe hypoxia, the transcriptional control of miR-146a and CXCR4 mRNA expression is regulated by HIF-1α.
DMOG stabilization of hypoxia-inducible factors and silencing of these factors demonstrate that HIF-1α activates microRNA-146a and CXCR4 mRNA in U937 cells
We used a prolyl hydroxylase inhibitor, DMOG,42,43 to pharmacologically stabilize HIF-α proteins in leukemic cells under normoxia and analyzed the effects on miR-146a and CXCR4 expression levels.
DMOG treatment of U937 cells in normoxia induced a decrease of cell growth (Figure 4A). HIF-1α nuclear protein accumulation in hypoxic and in DMOG-treated U937 cells was quite similar (Figure 4B). In normoxia, DMOG increased CXCR4 mRNA and miR-146a expression which, in turn, down-modulated CXCR4 protein level (Figure 4C–E), as found in hypoxia. DMOG treatment of leukemic cells in normoxia does, therefore, mimic the effects of hypoxia.
Figure 4.
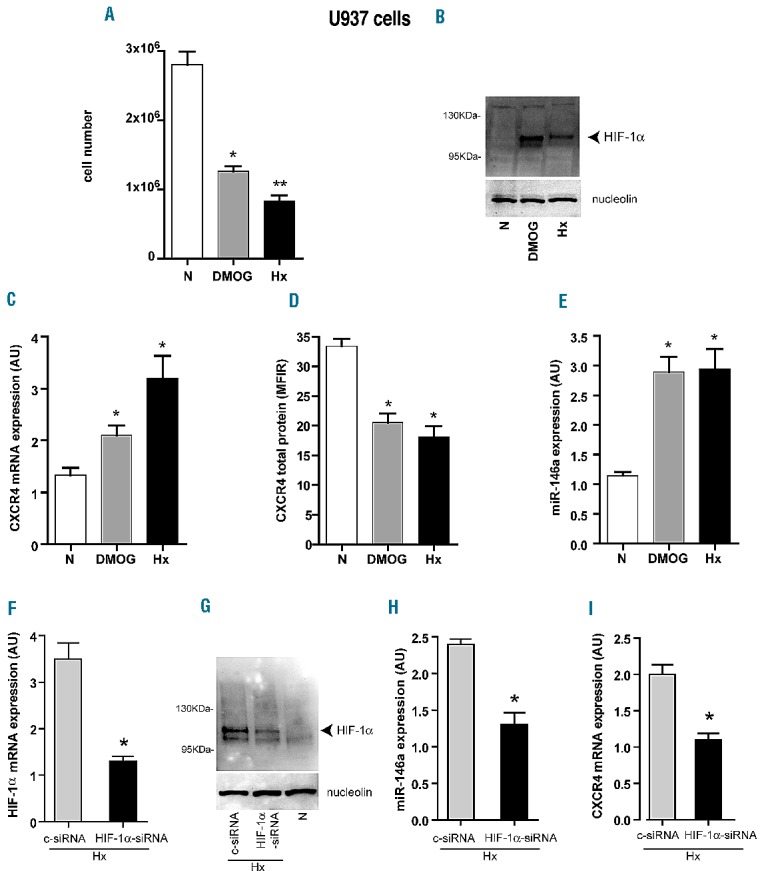
Effects of DMOG treatment of U937 cells in normoxia and HIF-1α-siRNA silencing in U937 cells in hypoxia, on miR-146a and CXCR4 mRNA expression. (A) Number of U937 cells treated by DMOG in normoxia (DMOG), as compared to untreated-U937 cells in normoxia (N) or in severe hypoxia (Hx). (B) Stabilization of HIF-1α protein by DMOG treatment of U937 cells in normoxia (DMOG) is shown by western blot analysis, as compared to untreated U937 cells cultured in normoxia (N) or in hypoxia (Hx). (C–E) Quantitative reverse transciptase polymerase chain reaction (qRT-PCR) analysis shows that DMOG treatment of U937 cells in normoxia increases both CXCR4mRNA (C) and miR-146a (E) expression level, as compared to untreated U937 cells in normoxia (N), as also found in U937 cells in hypoxia (Hx). (D) Flow cytometry analysis shows a decrease of CXCR4 protein level in U937 treated with DMOG (DMOG) as compared to normoxia (N), but as observed in hypoxic conditions (Hx). (F–G) qRT-PCR and western blot analysis show the decrease of HIF-1α mRNA (F) and protein (G) level in U937(HIF-α -siRNA) cells, as compared to U937(c-siRNA) cells in Hx. As a control, we show that HIF-1α protein is not detected in U937 cells in normoxia (N). Nucleolin is shown as an internal quantitative control of nuclear proteins. (H, I) qRT-PCR analysis indicates a significant decrease of both miR-146a (H) and CXCR4mRNA (I) in U937(HIF-1α-siRNA) cells, as compared to U937(c-siRNA) control cells, in the hypoxic condition. (A, C–F, H, I) Mean ± SEM values from three independent experiments are shown. *P<0.05, **P<0.01. (B, G) One representative experiment out of three is shown.
To confirm the specific binding of HIF-1α on its targets miR-146a and CXCR4 mRNA in U937 cells grown in hypoxia, we silenced HIFs-α expression in these cells. Specific silencing of HIF-1α, as shown by the decrease of HIF-1α mRNA and protein levels in HIF-1α-siRNA transfected U937cells (Figure 4F,G, HIF-1α-siRNA), reduced miR-146a and CXCR4mRNA expression (Figure 4H,I). Importantly, we did not detect any significant modulation of miR-146a and CXCR4 mRNA expression level by hypoxia in U937 cells transfected with HIF-2α-siRNA (data not shown).
Echinomycin inhibits HIF-1α-mediated activation of microRNA-146a and CXCR4 mRNA expression in monocytic leukemic cells
To inhibit the hypoxic-mediated activation of miR-146a and CXCR4mRNA expression in U937 cells, we used echinomycin to treat U937 cells grown under severe hypoxia, as compared to normoxia.
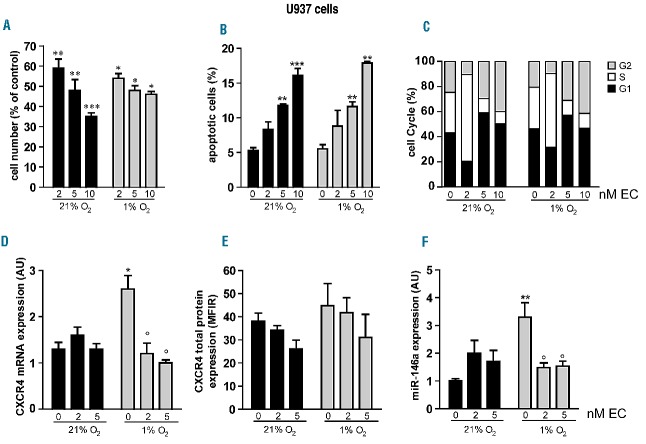
We showed that echinomycin treatment inhibited U937 cell growth rate in a significant way by using 2, 5 and 10 nM echinomycin for 20 h in both severe hypoxia and normoxia, and comparing the results with those of untreated cells (Figure 5A). For the other experiments we used 2 nM echinomycin since, at this concentration, we did not detect any significant apoptosis in either hypoxia or normoxia (Figure 5B). We also found an increased accumulation in S phase of U937 cells treated with 2 nM echinomycin, while cells treated with higher echinomycin concentrations (5–10 nM) displayed increased accumulation in G2 (Figure 5C).
Figure 5.
Echinomycin inhibits hypoxia-mediated up-regulation of CXCR4 mRNA and miR-146a in U937 cells grown for 20 h under severe hypoxia. (A–C) Dose response analysis of echinomycin treatment on leukemic cell growth (A), apoptosis (B) and cell cycle (C) of leukemic U937 cells cultured under severe hypoxia (1% O2), as compared to normoxia (21% O2). (D) Quantitative reverse transcriptase polymerase chain reaction (qRT-PCR) shows that in U937 cells, hypoxia-mediated activation of CXCR4mRNA (0 nM echinomycin) is inhibited by echinomycin treatment (1% O2, 2 and 5 nM). (E) Flow cytometry analysis shows that CXCR4 protein level is not significantly modulated by echinomycin treatment in U937 cells cultured in hypoxia, as compared to normoxia. (F) qRT-PCR shows that in U937 cells, hypoxia-mediated activation of miR-146a (0 nM echinomycin) is inhibited by echinomycin treatment (1% O2, 2 and 5 nM). (A, B, D–F) Mean ± SEM values from three independent experiments are shown. ***P<0.001; **P<0.01; * and ° P<0.05. (C): One representative experiment out of three is shown.
Altogether, we showed that echinomycin treatment (2 and 5 nM) of U937 cells grown under severe hypoxia inhibits CXCR4 mRNA and miR-146a up-regulation (Figure 5D–F), thus confirming the role of HIF-1α as a transcriptional activator of both targets, miR-146a and CXCR4 mRNA.
The control of CXCR4 expression mediated by the HIF-1α/microRNA-146a axis is deregulated in acute monocytic leukemia
We analyzed the impact of chronic, mild hypoxia on primary leukemic blast cells from patients with AML-M5. We previously reported that AML-M5 cells frequently express high levels of CXCR4 protein, associated with very low levels of miR-146a.39 We, therefore, cultured leukemic blast cells from eight cases of AML-M5 under mild hypoxia for 20 h or normoxia, and analyzed HIF-α nuclear proteins, miR-146a and CXCR4 expression in these cells.
As observed in U937 cells, we found that, compared to normoxia, mild hypoxia is sufficient to induce accumulation of both HIF-1α and HIF-2α nuclear proteins in leukemic blast cells (Figure 6A). Although the increased HIF-1α nuclear protein level was associated with an increased miR-146a level (Figure 6B), in AML-M5 cells we did not observe the significant decrease of CXCR4 protein level (Figure 6C) that we found in U937 cells in hypoxia.
Figure 6.
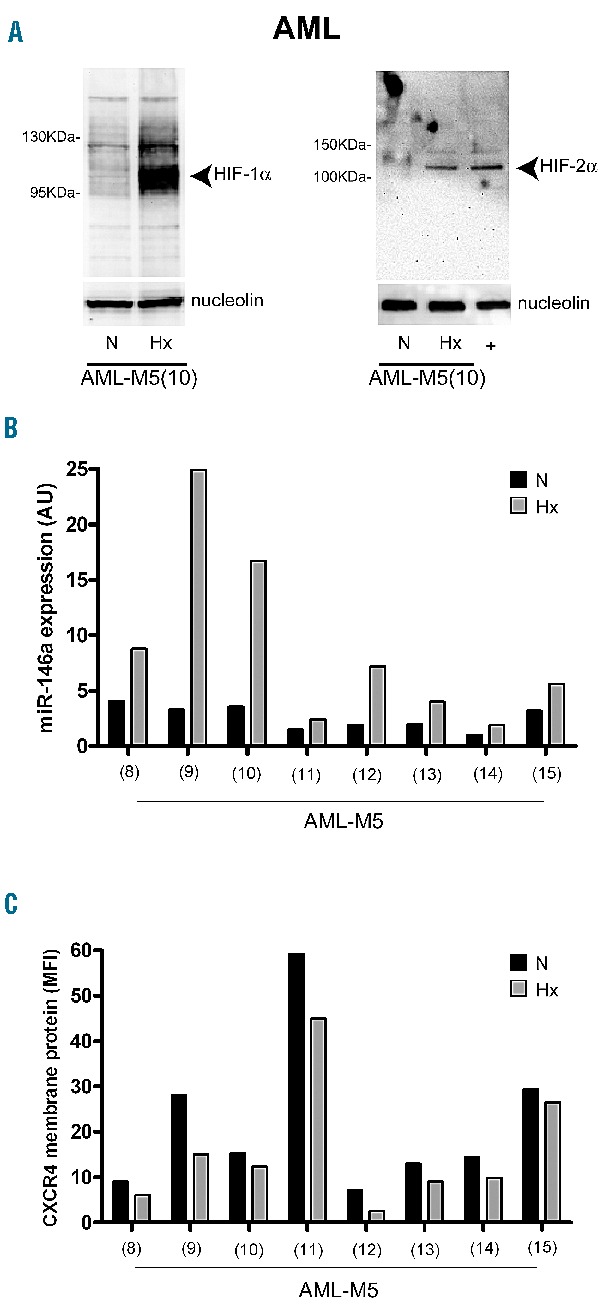
Mild hypoxia activates HIF-1α and HIF-2α, and up-regulates miR-146a expression without significant decrease of CXCR4 protein levels, in primary M5-AML. (A) Western blot analysis shows that HIF-1α and HIF-2α are activated in leukemic blast cells maintained 20 h under mild hypoxia (Hx), as compared to normoxia (N); Nucleolin is an internal control of nuclear proteins extracts. (B) Quantitative reverse transcriptase polymerase chain reaction shows that miR-146a is significantly up-regulated (mean ± SEM values are, in N: 2.6±0.4; in Hx: 8.9±2.8; and P=0.0424) in leukemic blast cells, obtained from eight patients with AML-M5, maintained in Hx, as compared to N. (C) Flow cytometry analysis of CXCR4 membrane protein level observed on leukemic blast cells in Hx (mean ± SEM values are, in N: 21.9±6.0; in Hx: 15.8±4.9; and P=0.4417), as compared to N. (A–C) One representative experiment out of three is shown.
These observations indicate that in AML-M5 the moderate miR-146a up-modulation elicited by hypoxia is not sufficient to induce a significant decrease of CXCR4 protein levels.
In order to understand whether CXCR4 can be down-modulated by higher levels of miR-146a expression in AML-M5 leukemic blasts, we transfected these cells with miR-146a mimics. A representative experiment reported in Online Supplementary Figure S3 showed that enforced miR-146a overexpression in AML-M5 cells induces moderate CXCR4 down-modulation.
Hypoxia increases the survival of SDF-1α-induced leukemic cells by decreasing the sensitivity of the leukemic cells to antileukemic drugs
To determine whether hypoxia and CXCR4 activation by SDF-1α protect leukemic cells from apoptosis induced by anti-leukemic drug treatment, we exposed U937 cells, HL-60(CXCR4) and K562(CXCR4) cells engineered to overexpress CXCR4, and AML-M5 cells to cytosine arabinoside (Ara-C, 1 μM), in combination or not with SDF-1α, and measured cell viability under normoxic and hypoxic conditions of culture (Figure 7 and Online Supplementary Figure S4). In K562(CXCR4) cells additional experiments were performed, incubating these cells with imatinib,38 an inhibitor of BCR-ABL, in combination or not with SDF-1α.
Figure 7.
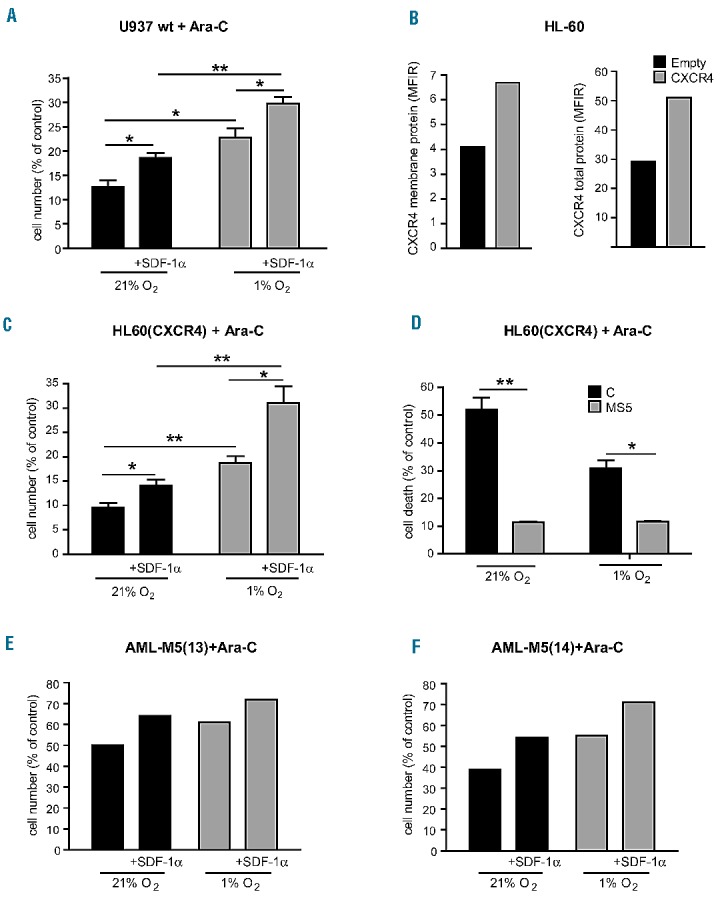
In combination with SDF-1α treatment, severe hypoxia improves leukemic cell survival of AML cell lines and AML-M5 cells treated with Ara-C. (A) SDF-1α significantly increases cell survival of Ara-C-treated U937 cells (+SDF-1α) compared to untreated cells, in normoxia and this effect is enhanced in hypoxia. (B) Flow cytometry analysis shows CXCR4 protein overexpression in HL-60(CXCR4) cells as compared to HL-60(Empty) control cells. (C) SDF-1α induces a significant increase in Ara-C-treated HL-60(CXCR4) cell survival (+SDF-1α) compared to untreated cells, in normoxia and this effect is enhanced in hypoxia. (D) Induction of apoptosis decreases in Ara-C-treated HL-60(CXCR4) cells grown on a layer of MS5 stromal cells (MS5), compared to standard culture (control, C). (E, F) In primary leukemic blast cells obtained from two patients with AML-M5, SDF-1α increases cell survival of Ara-C-treated AML-M5 (13) and (14) cells (+SDF-1α), compared to untreated cells, in both normoxia and hypoxia. (A, C, D) Mean ± SEM values from three independent experiments are shown. *P<0.05, **P<0.01. (B, E, F) One representative experiment is shown.
We found that hypoxia increases SDF-1α-induced leukemic cell survival of Ara-C-treated U937 cells (Figure 7A). In HL-60(CXCR4) cells (Figure 7B) we observed similar results (Figure 7C), as well as in K562(CXCR4) cells (Online Supplementary Figure S4). Indeed, we observed these effects again when we treated primary leukemic blast cells obtained from two cases of AML-M5 (13 and 14) incubated with Ara-C alone or in combination with SDF-1α in hypoxia as compared to normoxia. In both cases we observed that the survival of Ara-C-treated blast cells was increased when the cells were also treated with SDF-1α, and this effect increased under hypoxia, compared to normoxia (Figure 7E,F).
As CXCR4 is critically involved in the cross-talk between leukemic cells and bone marrow stromal cells, we also investigated response to chemotherapy of HL-60(CXCR4) cells grown on a MS5 stromal cell (MS5) layer, as compared to standard culture. Cells grown on a MS5 layer exhibited a markedly lower sensitivity to Ara-C under both normoxic and hypoxic conditions (Figure 7D).
Altogether, these findings show that severe hypoxia cooperates with CXCR4 activation by SDF-1α to induce leukemic cell survival by decreasing the sensitivity of CXCR4-expressing leukemic cells to chemotherapy-induced apoptosis.
Discussion
Like normal hematopoietic stem cells, leukemic cells proliferate in bone marrow where the O2 supply is limited. Leukemic cells survive by adapting their metabolism to hypoxic conditions.45
This adaptation to hypoxia is primarily mediated by two closely related hypoxia-inducible transcription factors, HIF-1α and HIF-2α, broadly expressed in many human cancers and frequently correlated with a poor prognosis. HIF-1α and HIF-2α exhibit shared or unique activities in processes that affect tumor growth and metastasis related to their targets genes, such as CXCR4 and its ligand SDF-1/CXCL12,9–11 but also microRNA.22
CXCR4 is involved in homing and mobilization of normal and leukemic cells and its high hypoxia-related expression is associated with a negative prognosis in AML.11,12 The impact of hypoxia on CXCR4 expression and function is currently under investigation in AML.
In this study, we first demonstrated that a variation of chronic hypoxia from 5% O2, seemingly representing a physiological mild hypoxic condition for differentiation, to 1% O2, a more severe hypoxia observed in hypoxic niches of the bone marrow, is a critical variable for HIF activation during monocytic differentiation/maturation.36
Under chronic, mild hypoxia, hematopoietic progenitor cells undergo a switch from the predominant HIF-1α expression and activation in undifferentiated CD34+ cells to the prevalent HIF-2α expression in differentiating monocytic precursors. This observation is in line with recent studies showing a key role of HIF-1α in maintaining the stemness of normal hematopoietic stem cells: in fact, in HIF-1α−/− mice hematopoietic stem cell numbers decrease during stress in association with a loss of quiescence.23,46 Hematopoietic stem cells in their niche use glycolysis to meet their energy demand, which depends on a Meis1-induced HIF-1α-signaling network.47
Associated with the differential HIF-1α and HIF-2α expression found in monocytic differentiating hematopoietic progenitor cells, we identified miR-146a as a new hypoxic-responsive microRNA. During monocytopoiesis under mild hypoxia, miR-146a is a direct target of HIF-2α in a new regulation loop which involves HIF-2α/miR-146a and miR-146a/CXCR4-specific interactions to control CXCR4 protein levels. Under severe hypoxia, HIF-1α is activated and miR-146a becomes its target.
These data shed light on how the preference between HIF-1α or HIF-2α binding to the promoter of miR-146a depends on the step of monocytic differentiation and on the level of hypoxia.
In leukemic monocytic U937 cells first, then in some primary AML-M5, we found that chronic, mild hypoxia up-regulates both HIF-1α and HIF-2α concomitantly with miR-146a up-modulation.
Interestingly in leukemic cell lines, severe hypoxia has the same effects as mild hypoxia and HIF-1α is the only hypoxia-associated factor that induces both miR-146a and CXCR4mRNA up-regulation, as demonstrated by DMOG stabilization of HIF and specific HIF-1α silencing.
In line with others studies,48 our results obtained in a small panel of primary AMLs-M5 indicate that both HIF-1α and HIF-2α are activated in leukemic blast cells. However, although hypoxia is able to increase miR-146a in some AML-M5, this is associated with only a slight decrease of CXCR4 protein levels; in fact, as we previously reported,39 most of these AML-M5 are characterized by low miR-146a and high CXCR4 protein levels, not modifiable by hypoxia. Thus, the loss (or reduction) of CXCR4 protein down-modulation by chronic hypoxia may represent one of the mechanisms contributing to explain the high constitutive CXCR4 expression in AML.
Altogether, our findings indicate that normal and monocytic leukemic cells are sensitive to hypoxia, even mild, by activating HIF and a set of hypoxia-related responses, including miR-146a up-modulation. The hypoxia-mediated control of miR-146a and CXCR4mRNA aims to decrease CXCR4 protein levels in normal monocytic cells. In primary monocytic AML blasts this mechanism is deregulated in that these cells up-regulate miR-146a in response to hypoxia, but fail to significantly down-regulate CXCR4: the dysregulation of this mechanism could explain the capacity of homing/mobilization of monocytic AML blasts and their capacity for migration/infiltration.
Hypoxia, together with CXCR4 activation by SDF-1α, significantly decreases the sensitivity of leukemic cells expressing CXCR4 to chemotherapy. According to these observations it is conceivable that the mild hypoxia present in the bone marrow microenvironment fails to induce a significant reduction of CXCR4 expression in leukemic cells and cooperates with SDF-1α present in this site to anchor leukemic cells in the bone marrow and to improve their survival and their resistance to cytotoxic drugs.
Furthermore, our data indicating that HIF-1α is a main player in the hypoxia-mediated control of miR-146a and CXCR4 support the findings of previous studies24,25 that used chemotherapy and HIF-inhibitors in combination to restore the drug sensitivity of leukemic cells in hypoxia, indicating a new therapeutic possibility to decrease leukemic cell resistance to cytotoxic drug treatment. Wang et al. showed that HIF-1α is constitutively expressed in enriched populations of leukemic stem cells purified from AML, and its targeting eliminates these cells.25 Interestingly, compared with normal hematopoietic stem cells,46 leukemic stem cells appear to be more dependent on the HIF-1α pathway.49 On the other hand, Zhang et al. showed that treatment of AML cells with CXCR4 inhibitors markedly reduced their leukemia-initiating capacity in xenotrans-plantation assays in NOD/SCID mice.50
In this context, it is important to consider the role of some microRNA, such as miR-146a, regulated by hypoxia and having a key role in the modulation of the expression of genes essential for hematopoietic and leukemic stem cell homing/migration, growth and survival such as CXCR4, to develop new therapeutic strategies that aim to manipulate these microRNA in leukemia.
Acknowledgments
The authors would like to thank A Boe for flow cytometer analysis and G. Loreto for graphics. This study was supported by Associazione Italiana per la Ricerca sul Cancro (AIRC 2009, IG# 9131) to CL and by an institutional grant from the Italian Ministry of Health to CL (RF-2010-2312222).
Footnotes
The online version of this article has a Supplementary Appendix.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci USA. 2007;104(13):5431–5436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eliasson P, Jönsson JI. The hematopoietic stem cell niche: low in oxygen but a nice place to be. J Cell Physiol. 2010;222(1):17–22. [DOI] [PubMed] [Google Scholar]
- 3.Simsek T, Kocabas F, Zheng J, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jing D, Wobus M, Poitz DM, Bornhäuser M, Ehninger G, Ordemann R. Oxygen tension plays a critical role in the hematopoietic microenvironment in vitro. Haematologica. 2012;97(3):331–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mimeault M, Batra SK. Hypoxia-inducing factors as master regulators of stemness properties and altered metabolism of cancer-and metastasis-initiating cells. J Cell Mol Med. 2013;17(1):30–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen Y, Jacamo R, Shi YX, et al. Human extramedullary bone marrow in mice: a novel in vivo model of genetically controlled hematopoietic microenvironment. Blood. 2012;119(21):4971–4980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bertout JA, Patel SA, Simon MC. The impact of O2 availability on human cancer. Nat Rev Cancer. 2008;8(12):967–975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mucaj V, Shay JE, Simon MC. Effects of hypoxia and HIFs on cancer metabolism. Int J Hematol. 2012;95(5):464–470. [DOI] [PubMed] [Google Scholar]
- 9.Schioppa T, Uranchimeg B, Saccani A, et al. Regulation of the chemokine receptor CXCR4 by hypoxia. J Exp Med. 2003;198(9):1391–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ratajczak MZ, Zuba-Surma E, Kucia M, Reca R, Wojakowski W, Ratajczak J. The pleiotropic effects of the SDF-1-CXCR4 axis in organogenesis, regeneration and tumorigenesis. Leukemia. 2006;20(11):1915–1924. [DOI] [PubMed] [Google Scholar]
- 11.Burger JA, Kipps TJ. CXCR4: a key receptor in the crosstalk between tumor cells and their microenvironment. Blood. 2006;107(5):1761–1767. [DOI] [PubMed] [Google Scholar]
- 12.Spoo AC, Lübbert M, Wierda WG, Burger JA. CXCR4 is a prognosis marker in acute myelogenous leukemia. Blood. 2007;109(2):786–791. [DOI] [PubMed] [Google Scholar]
- 13.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113(7):1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Semenza GL. Targeting HIF 1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. [DOI] [PubMed] [Google Scholar]
- 15.Majmundar AJ, Wong WJ, Simon MC. Hypoxia-inducible factors and the response to hypoxic stress. Mol Cell. 2010;40(2):294–309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wheaton WW, Chandel NS. Hypoxia. 2. Hypoxia regulates cellular metabolism. Am J Physiol Cell Physiol. 2011;300(3):C385–393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hu CJ, Wang LY, Chodosh LA, Keith B, Simon MC. Differential roles of hypoxia-inducible factor 1alpha (HIF-1alpha) and HIF-2alpha in hypoxic gene regulation. Mol Cell Biol. 2003;23(24):9361–9374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loboda A, Jozkowicz A, Dulak J. HIF-1 and HIF-2 transcription factors - similar but not identical. Mol Cells. 2010;29(5):435–442. [DOI] [PubMed] [Google Scholar]
- 19.Tsai YP, Wu KJ. Hypoxia-regulated target genes implicated in tumor metastasis. J Biomed Sci. 2012;19:102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Keith B, Johnson RS, Simon MC. HIF1 and HIF2: sibling rivalry in hypoxic tumour growth and progression. Nat Rev Cancer. 2012;12(1):9–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartel DP. MicroRNAs: genomics, biogenesis, mechanism, and function. Cell. 2004;116(2):281–297. [DOI] [PubMed] [Google Scholar]
- 22.Kulshreshtha R, Davuluri RV, Calin GA, Ivan M. A microRNA component of the hypoxic response. Cell Death Differ. 2008;15(4):667–671. [DOI] [PubMed] [Google Scholar]
- 23.Forristal CE, Winkler IG, Nowlan B, Barbier V, Walkinshaw G, Levesque JP. Pharmacologic stabilization of HIF-1 increases hematopoietic stem cell quiescence in vivo and accelerates blood recovery after severe irradiation. Blood. 2013;121(5): 759–769. [DOI] [PubMed] [Google Scholar]
- 24.Wang Y, Liu Y, Malek SN, Zheng P, Liu Y. Targeting HIF1 eliminates cancer stem cells in hematological malignancies. Cell Stem Cell. 2011;8(4):399–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang Y, Liu Y, Tang F, et al. Echinomycin protects mice against relapsed acute myeloid leukemia without adverse effect on hematopoietic stem cells. Blood. 2014;124(7):1127–1135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Onnis B, Rapisarda A, Melillo G. Development of HIF-1 inhibitors for cancer therapy. J Cell Mol Med. 2009;13(9A): 2780–2786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Semenza GL. Hypoxia-inducible factors: mediators of cancer progression and targets for cancer therapy. Trends Pharmacol Sci. 2012;33(4):207–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Peled A, Tavor S. Role of CXCR4 in the pathogenesis of acute myeloid leukemia. Theranostics. 2013;3(1):27–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Labbaye C, Spinello I, Quaranta MT, et al. A three-step pathway comprising PLZF/miR-146a/CXCR4 controls megakaryopoiesis. Nat Cell Biol. 2008;10(7):788–801. [DOI] [PubMed] [Google Scholar]
- 30.Tano N, Kim HW, Ashraf M. microRNA-150 regulates mobilization and migration of bone marrow-derived mononuclear cells by targeting Cxcr4. PLoS One. 2011;6(10): 23114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Liu Y, Zhou Y, Feng X, et al. MicroRNA-126 functions as a tumor suppressor in colorectal cancer cells by targeting CXCR4 via the AKT and ERK1/2 signaling pathways. Int J Oncol. 2014;44(1):203–210. [DOI] [PubMed] [Google Scholar]
- 32.Yu T, Liu K, Wu Y, et al. MicroRNA-9 inhibits the proliferation of oral squamous cell carcinoma cells by suppressing expression of CXCR4 via the Wnt/β-catenin signaling pathway. Oncogene. 2014;33(42):5017–5027. [DOI] [PubMed] [Google Scholar]
- 33.Rusca N, Monticelli S. MiR-146a in Immunity and disease. Mol Biol Int. 2011;2011:437301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Boldin MP, Taganov KD, Rao DS, et al. MiR-146a is a significant brake on autoimmunity, myeloproliferation, and cancer in mice. J Exp Med. 2011;208(6):1189–1201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Labbaye C, Testa U. The emerging role of MIR-146A in the control of hematopoiesis, immune function and cancer. J Hematol Oncol. 2012;5:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghan S, Riemke P, Schönheit J, et al. Macrophage development from HSCs requires PU.1-coordinated microRNA expression. Blood. 2011;118(8):2275–2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Starczynowski DT, Kuchenbauer F, Argiropoulos B, et al. Identification of miR-145 and miR-146a as mediators of the 5q-syndrome phenotype. Nat Med. 2010; 16(1):49–58. [DOI] [PubMed] [Google Scholar]
- 38.Ferreira AF, Moura LG, Tojal I, et al. ApoptomiRs expression modulated by BCR-ABL is linked to CML progression and imatinib resistance. Blood Cells Mol Dis. 2014;53(1–2):47–55. [DOI] [PubMed] [Google Scholar]
- 39.Spinello I, Quaranta MT, Riccioni R, et al. MicroRNA-146a and AMD3100, two ways to control CXCR4 expression in acute myeloid leukemias. Blood Cancer J. 2011; 1(6):e26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327(5966):656–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fangradt M, Hahne M, Gaber T, et al. Human monocytes and macrophages differ in their mechanisms of adaptation to hypoxia. Arthritis Res Ther. 2012;14(4): R181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pollard PJ, Loenarz C, Mole DR, et al. Regulation of Jumonji-domain-containing histone demethylases by hypoxia-inducible factor (HIF)-1. Biochem J. 2008;416(3):387–394. [DOI] [PubMed] [Google Scholar]
- 43.Ding H, Chen S, Song WQ, et al. Dimethyloxaloylglycine improves angiogenic activity of bone marrow stromal cells in the tissue-engineered bone. Int J Biol Sci. 2014;10(7):746–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Melchionna R, Romani M, Ambrosino V, et al. Role of HIF-1alpha in proton-mediated CXCR4 down-regulation in endothelial cells. Cardiovasc Res. 2010;86(2):293–301. [DOI] [PubMed] [Google Scholar]
- 45.Goto M, Miwa H, Suganuma K, et al. Adaptation of leukemia cells to hypoxic condition through switching the energy metabolism or avoiding the oxidative stress. BMC Cancer. 2014;14:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Takubo K, Goda N, Yamada W, et al. Regulation of HIF-1alpha level is essential for the hematopoietic stem cells. Cell Stem Cell. 2010;7(3):391–402. [DOI] [PubMed] [Google Scholar]
- 47.Kocabas F, Zheng J, Thet S, et al. Meis1 regulates the metabolic phenotype and oxidant defense of hematopoietic stem cells. Blood. 2012;120(25):4963–4972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hatfield KJ, Bedringsaas SL, Ryningen A, Gjertsen BT, Bruserud O. Hypoxia increases HIF-1 expression and constitutive cytokine release by primary human acute myeloid leukaemia cells. Eur Cytokine Netw. 2010;21(3):154–164. [DOI] [PubMed] [Google Scholar]
- 49.Zhang H, Li H, Xi HS, Li S. HIF-1 is required for survival maintenance of chronic myeloid leukemia stem cells. Blood. 2012;119(11):2595–2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Y, Patel S, Abdelouahab H, et al. CXCR4 inhibitors selectively eliminate CXCR4-expressing human acute myeloid leukemia cells in NOG mouse model. Cell Death Dis. 2012;3:e396. [DOI] [PMC free article] [PubMed] [Google Scholar]