Abstract
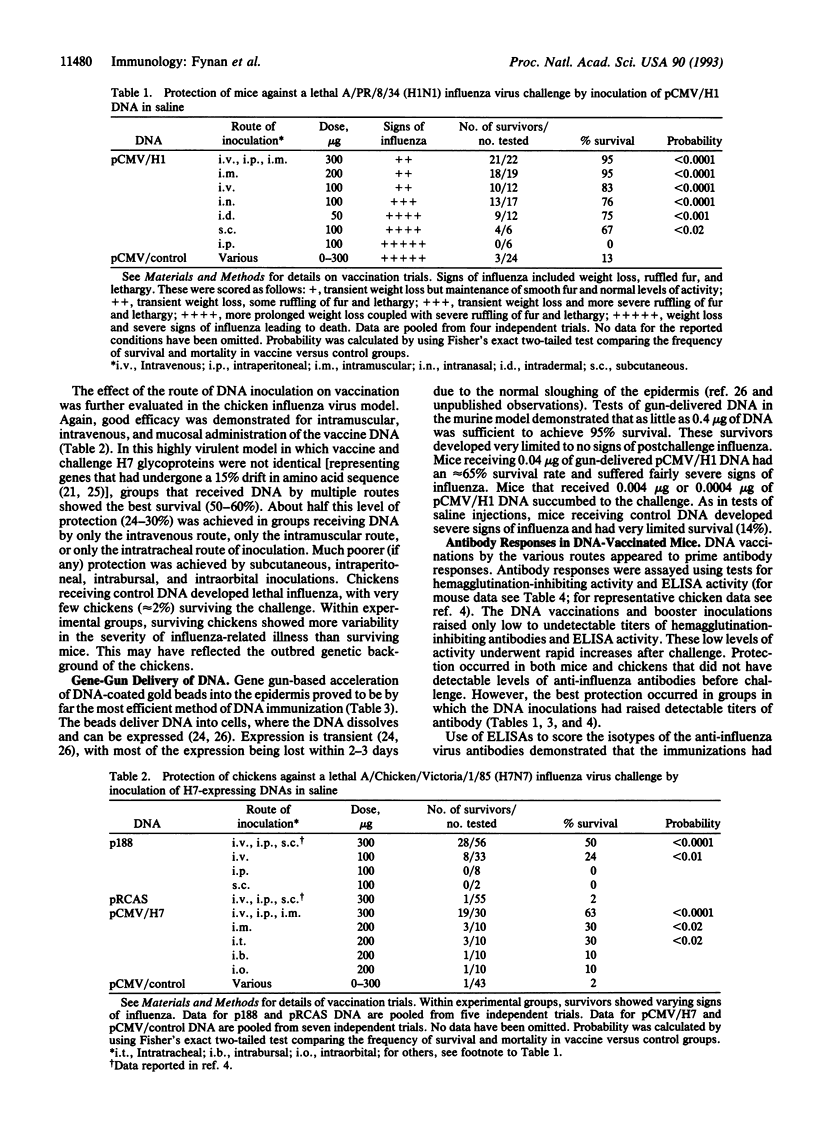
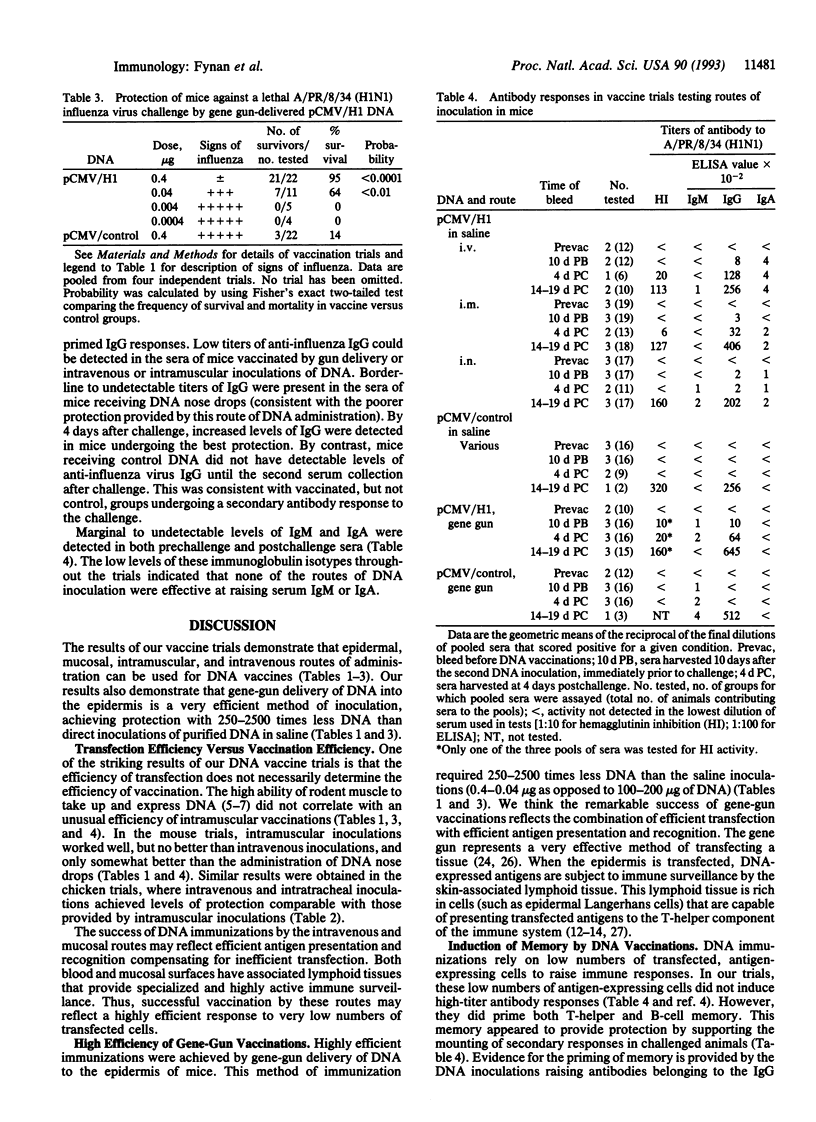
Plasmid DNAs expressing influenza virus hemagglutinin glycoproteins have been tested for their ability to raise protective immunity against lethal influenza challenges of the same subtype. In trials using two inoculations of from 50 to 300 micrograms of purified DNA in saline, 67-95% of test mice and 25-63% of test chickens have been protected against a lethal influenza challenge. Parenteral routes of inoculation that achieved good protection included intramuscular and intravenous injections. Successful mucosal routes of vaccination included DNA drops administered to the nares or trachea. By far the most efficient DNA immunizations were achieved by using a gene gun to deliver DNA-coated gold beads to the epidermis. In mice, 95% protection was achieved by two immunizations with beads loaded with as little as 0.4 micrograms of DNA. The breadth of routes supporting successful DNA immunizations, coupled with the very small amounts of DNA required for gene-gun immunizations, highlight the potential of this remarkably simple technique for the development of subunit vaccines.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Acsadi G., Jiao S. S., Jani A., Duke D., Williams P., Chong W., Wolff J. A. Direct gene transfer and expression into rat heart in vivo. New Biol. 1991 Jan;3(1):71–81. [PubMed] [Google Scholar]
- Bienenstock J., Johnston N., Perey D. Y. Bronchial lymphoid tissue. I. Morphologic characteristics. Lab Invest. 1973 Jun;28(6):686–692. [PubMed] [Google Scholar]
- Cox G. J., Zamb T. J., Babiuk L. A. Bovine herpesvirus 1: immune responses in mice and cattle injected with plasmid DNA. J Virol. 1993 Sep;67(9):5664–5667. doi: 10.1128/jvi.67.9.5664-5667.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cullen B. R. Trans-activation of human immunodeficiency virus occurs via a bimodal mechanism. Cell. 1986 Sep 26;46(7):973–982. doi: 10.1016/0092-8674(86)90696-3. [DOI] [PubMed] [Google Scholar]
- GOWANS J. L., KNIGHT E. J. THE ROUTE OF RE-CIRCULATION OF LYMPHOCYTES IN THE RAT. Proc R Soc Lond B Biol Sci. 1964 Jan 14;159:257–282. doi: 10.1098/rspb.1964.0001. [DOI] [PubMed] [Google Scholar]
- Guy-Grand D., Griscelli C., Vassalli P. The gut-associated lymphoid system: nature and properties of the large dividing cells. Eur J Immunol. 1974 Jun;4(6):435–443. doi: 10.1002/eji.1830040610. [DOI] [PubMed] [Google Scholar]
- Hughes S. H., Greenhouse J. J., Petropoulos C. J., Sutrave P. Adaptor plasmids simplify the insertion of foreign DNA into helper-independent retroviral vectors. J Virol. 1987 Oct;61(10):3004–3012. doi: 10.1128/jvi.61.10.3004-3012.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt L. A., Brown D. W., Robinson H. L., Naeve C. W., Webster R. G. Retrovirus-expressed hemagglutinin protects against lethal influenza virus infections. J Virol. 1988 Aug;62(8):3014–3019. doi: 10.1128/jvi.62.8.3014-3019.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israel M. A., Chan H. W., Hourihan S. L., Rowe W. P., Martin M. A. Biological activity of polyoma viral DNA in mice and hamsters. J Virol. 1979 Mar;29(3):990–996. doi: 10.1128/jvi.29.3.990-996.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Dertzbaugh M. T., Eldridge J. H., Hirasawa M., Kiyono H. The mucosal immune system: from fundamental concepts to vaccine development. Vaccine. 1992;10(2):75–88. doi: 10.1016/0264-410x(92)90021-b. [DOI] [PubMed] [Google Scholar]
- Naeve C. W., Webster R. G. Sequence of the hemagglutinin gene from influenza virus A/Seal/Mass/1/80. Virology. 1983 Sep;129(2):298–308. doi: 10.1016/0042-6822(83)90169-1. [DOI] [PubMed] [Google Scholar]
- Novak M., Moldoveanu Z., Schafer D. P., Mestecky J., Compans R. W. Murine model for evaluation of protective immunity to influenza virus. Vaccine. 1993;11(1):55–60. doi: 10.1016/0264-410x(93)90339-y. [DOI] [PubMed] [Google Scholar]
- Robinson H. L., Hunt L. A., Webster R. G. Protection against a lethal influenza virus challenge by immunization with a haemagglutinin-expressing plasmid DNA. Vaccine. 1993;11(9):957–960. doi: 10.1016/0264-410x(93)90385-b. [DOI] [PubMed] [Google Scholar]
- Steinman R. M. The dendritic cell system and its role in immunogenicity. Annu Rev Immunol. 1991;9:271–296. doi: 10.1146/annurev.iy.09.040191.001415. [DOI] [PubMed] [Google Scholar]
- Streilein J. W. Skin-associated lymphoid tissues (SALT): origins and functions. J Invest Dermatol. 1983 Jun;80 (Suppl):12s–16s. doi: 10.1111/1523-1747.ep12536743. [DOI] [PubMed] [Google Scholar]
- Tang D. C., DeVit M., Johnston S. A. Genetic immunization is a simple method for eliciting an immune response. Nature. 1992 Mar 12;356(6365):152–154. doi: 10.1038/356152a0. [DOI] [PubMed] [Google Scholar]
- Ulmer J. B., Donnelly J. J., Parker S. E., Rhodes G. H., Felgner P. L., Dwarki V. J., Gromkowski S. H., Deck R. R., DeWitt C. M., Friedman A. Heterologous protection against influenza by injection of DNA encoding a viral protein. Science. 1993 Mar 19;259(5102):1745–1749. doi: 10.1126/science.8456302. [DOI] [PubMed] [Google Scholar]
- Wang B., Ugen K. E., Srikantan V., Agadjanyan M. G., Dang K., Refaeli Y., Sato A. I., Boyer J., Williams W. V., Weiner D. B. Gene inoculation generates immune responses against human immunodeficiency virus type 1. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4156–4160. doi: 10.1073/pnas.90.9.4156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster R. G., Rott R. Influenza virus A pathogenicity: the pivotal role of hemagglutinin. Cell. 1987 Aug 28;50(5):665–666. doi: 10.1016/0092-8674(87)90321-7. [DOI] [PubMed] [Google Scholar]
- Wiley D. C., Skehel J. J. The structure and function of the hemagglutinin membrane glycoprotein of influenza virus. Annu Rev Biochem. 1987;56:365–394. doi: 10.1146/annurev.bi.56.070187.002053. [DOI] [PubMed] [Google Scholar]
- Williams R. S., Johnston S. A., Riedy M., DeVit M. J., McElligott S. G., Sanford J. C. Introduction of foreign genes into tissues of living mice by DNA-coated microprojectiles. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2726–2730. doi: 10.1073/pnas.88.7.2726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winter G., Fields S., Brownlee G. G. Nucleotide sequence of the haemagglutinin gene of a human influenza virus H1 subtype. Nature. 1981 Jul 2;292(5818):72–75. doi: 10.1038/292072a0. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Malone R. W., Williams P., Chong W., Acsadi G., Jani A., Felgner P. L. Direct gene transfer into mouse muscle in vivo. Science. 1990 Mar 23;247(4949 Pt 1):1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- Wolff J. A., Williams P., Acsadi G., Jiao S., Jani A., Chong W. Conditions affecting direct gene transfer into rodent muscle in vivo. Biotechniques. 1991 Oct;11(4):474–485. [PubMed] [Google Scholar]
- Yang N. S., Burkholder J., Roberts B., Martinell B., McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci U S A. 1990 Dec;87(24):9568–9572. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]




