Abstract
Serial studies have demonstrated that induction therapy with FLAM [flavopiridol (alvocidib) 50 mg/m2 days 1–3, cytarabine 667 mg/m2/day continuous infusion days 6–8, and mitoxantrone (FLAM) 40 mg/m2 day 9] yields complete remission rates of nearly 70% in newly diagnosed poor-risk acute myeloid leukemia. Between May 2011–July 2013, 165 newly diagnosed acute myeloid leukemia patients (age 18–70 years) with intermediate/adverse-risk cytogenetics were randomized 2:1 to receive FLAM or 7+3 (cytarabine 100 mg/m2/day continuous infusion days 1–7 and daunorubicin 90 mg/m2 days 1–3), across 10 institutions. Some patients on 7+3 with residual leukemia on day 14 received 5+2 (cytarabine 100 mg/m2/day continuous infusion days 1–5 and daunorubicin 45 mg/m2 days 1–2), whereas patients on FLAM were not re-treated based on day 14 bone marrow findings. The primary objective was to compare complete remission rates between one cycle of FLAM and one cycle of 7+3. Secondary end points included safety, overall survival and event-free survival. FLAM led to higher complete remission rates than 7+3 alone (70% vs. 46%; P=0.003) without an increase in toxicity, and this improvement persisted after 7+3+/−5+2 (70% vs. 57%; P=0.08). There were no significant differences in overall survival and event-free survival in both arms but post-induction strategies were not standardized. These results substantiate the efficacy of FLAM induction in newly diagnosed AML. A phase III study is currently in development. This study is registered with clinicaltrials.gov identifier: 01349972.
Introduction
Adults with acute myeloid leukemia (AML) have a poor prognosis with conventional chemotherapy agents. There are approximately 18,000 new cases of AML with close to 11,000 deaths yearly in the United States.1 Unfortunately, treatment of AML has changed little over the last four decades. “7+3” [7 days of continuous infusion (CI) cytarabine and 3 days of anthracycline] remains the standard induction chemotherapy regimen for newly diagnosed non-acute promyelocytic leukemia (APL) AML patients who are fit for intensive therapy.2 Despite many attempts to improve the 7+3 regimen with the addition and/or substitution of mechanistically diverse agents, no regimen has proven to be consistently superior to 7+3.3
Timed sequential therapy (TST) has been shown to improve outcomes in some newly diagnosed adults and children with AML.4–8 TST relies on the opportune timing of cell-cycle specific cytotoxic agents in order to exert maximal effect on leukemic cell death. In vitro studies of flavopiridol, a pan cyclin-dependent, multi-serine-threonine kinase inhibitor, followed by cytarabine in a TST manner demonstrated increased cytotoxicity compared to either agent alone.9 FLAM (flavopiridol followed by cytarabine and mitoxantrone) was evaluated in 138 newly diagnosed poor-risk AML patients in serial phase II trials, with overall complete remission (CR) rates of 67%–80% and reproducibly low rates of morbidity and mortality.10–12 These data suggest that FLAM might improve outcomes relative to 7+3 induction therapy in newly diagnosed AML patients. Therefore, we sought to compare FLAM to 7+3 in a multicenter randomized phase II trial in newly diagnosed adult AML patients with intermediate- and adverse-risk cytogenetics.
Methods
Patient eligibility
Between May 2011 and July 2013, newly diagnosed AML patients aged 18–70 years with pathological confirmation of bone marrow (BM) blasts 20% or more were enrolled in a multi-institutional study. Eligibility criteria were similar to those from previous studies.10–12 FISH for core-binding factor (CBF) AML (t(8;21); inv(16); (t(16;16)) was performed at each institution prior to enrollment, and patients were excluded if CBF positive. The study was conducted in accordance with the Declaration of Helsinki after approval by the ethics committee of each participating center.
Treatment
Patients were randomized by centralized computer-generated allocation procedure (REDCap13) 2:1 to receive FLAM (arm A): flavopiridol 50 mg/m2 IV days 1–3, cytarabine 2 gm/m2 CI IV days 6–8 (667 mg/m2/day), and mitoxantrone 40 mg/m2 IV day 9 or 7+3 (arm B): cytarabine 100 mg/m2/day CI IV days 1–7, and daunorubicin 90 mg/m2 IV days 1–3 (idarubicin 12 mg/m2 IV days 1–3 was substituted as needed for lack of daunorubicin availability). Patients were stratified according to the following risk factors: 1) age 50 years or over; 2) secondary AML (defined as treatment-related AML or AML from antecedent hematologic disorder) and/or known adverse cytogenetics;14 and 3) hyperleukocytosis [white blood cell (WBC) count >50×109/L].
All patients received a BM biopsy on day 14 unless medically contraindicated. Residual leukemia on day 14 was defined as BM blasts 5% or more morphologically with overall cellularity 10% or more. Arm B patients were eligible to receive an additional cycle of induction therapy, 5+2 (cytarabine 100 mg/m2/day CI IV days 1–5, daunorubicin 45 mg/m2 IV days 1–2) in the setting of residual leukemia on day 14. Post-induction treatment was performed according to physician preference.
Response and toxicity
Bone marrow (BM) aspirates and biopsies were performed before treatment, on day 14 of treatment, and at hematologic recovery or when leukemia regrowth was suspected. Response criteria were defined according to standard definitions.14 Adverse events were graded by NCI Common Terminology Criteria for Adverse Events (CTCAE) v. 4.0.
Statistical analysis
The study was designed to compare CR rates between FLAM and one cycle of 7+3, using a Bayesian approach for interim monitoring for futility. The primary analysis would conclude a significant benefit for FLAM if the one-sided P value from a Fisher’s exact test less than 0.10. A sample size of 165 patients, randomized 2:1 to FLAM or 7+3, respectively, yielded 85% power to detect an increase in the probability of CR from 55% with 7+315–17 to 75% with FLAM. In addition to the planned primary end point analysis, CR rates between FLAM and 7+3+/−5+2 were analyzed by Fisher’s exact test with a one-sided P value analogous to the primary end point analysis.
Secondary end points included toxicity comparisons, overall survival (OS), and event-free survival (EFS). OS was defined from date of randomization to death or last known follow up. EFS was defined as date of randomization to the first occurrence of persistent AML after one cycle of induction, relapse or death. Patients were censored for EFS if they had received non-protocol therapy or an allogeneic stem cell transplant (SCT). All significance tests for secondary end points were two-sided with P<0.05 considered significant. To explore heterogeneity of treatment effects between various subgroups, logistic regression models with terms for treatment, the patient subgroup, and their interaction were fit. P values were derived from likelihood ratio tests and are considered exploratory. OS, EFS, and time to hematologic recovery probabilities were calculated using the Kaplan-Meier method and tested for differences between treatment arms with the log rank test.
Further details of Methods are provided in the Online Supplementary Appendix.
Results
Patients’ characteristics
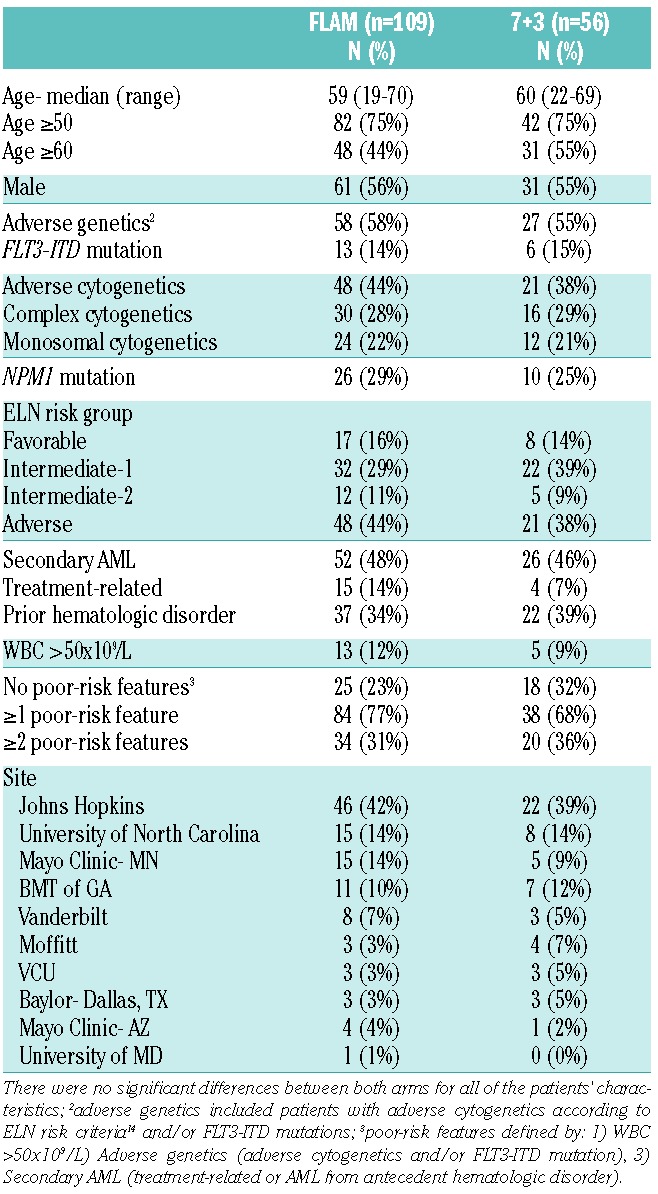
One hundred and sixty-five patients (FLAM: n=109, 7+3: n=56) from 10 institutions were randomized, treated, and included in the analysis, as shown in Figure 1. Clinical demographics and disease biological features of all patients are presented in Table 1. Adverse-risk according to ELN criteria14 was seen in 44% and 38% of patients on FLAM and 7+3, respectively. In addition, 47% of patients (FLAM: 48%, 7+3: 46%) had secondary AML. The majority of patients (FLAM: 77% vs. 7+3: 68%) had one or more poor-risk features, defined as: 1) WBC >50×109/L; 2) adverse genetics (ie. adverse cytogenetics and/or FLT3-ITD mutation); and/or 3) secondary AML.
Figure 1.
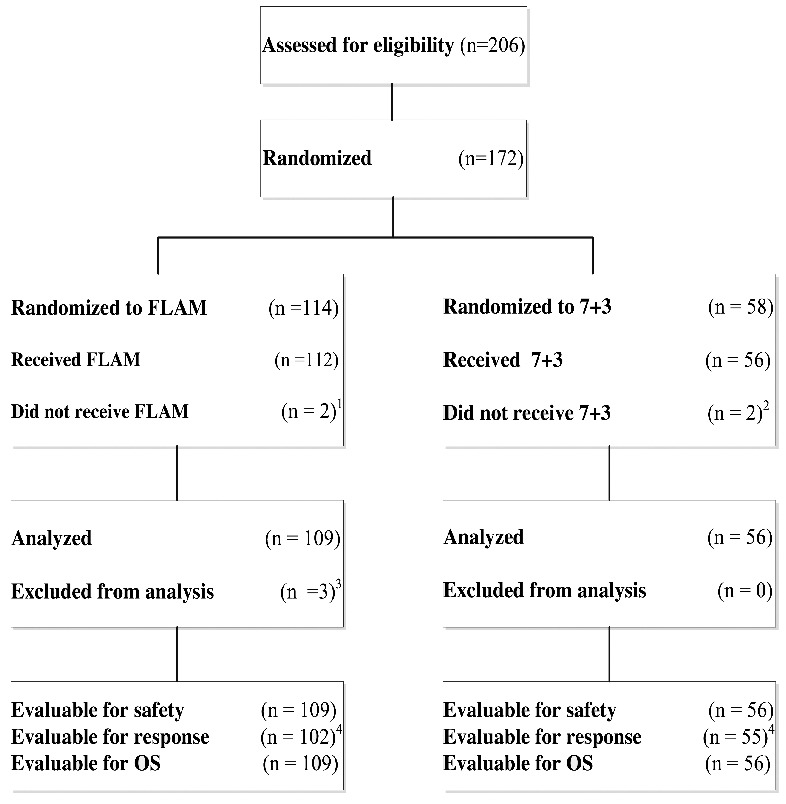
Consort Diagram. 172 patients were randomized between FLAM (n = 114) and 7+3 (n = 58), 109 patients on FLAM and 56 patients on 7+3 were analyzed for response and overall survival (OS).1 2 patients did not receive FLAM after randomization: progressive deterioration of performance status (n=1), exceeded maximum prior anthracycline dose (n=1)2 2 patients did not receive 7+3 after randomization: withdrawal of consent (n=1), death before intervention (n=1).3 3 patients were excluded from the analysis after initiating treatment with FLAM due to ineligibility: T-cell acute lymphoblastic leukemia (n=1), Favorable-risk cytogenetics: t(8;21) (n=1), Prior treatment for AML (n=1).4 7 patients treated with FLAM and one patient treated with 7+3 were non-evaluable (NE) for response due to early death.
Table 1.
Patients’ characteristics.
Toxicity
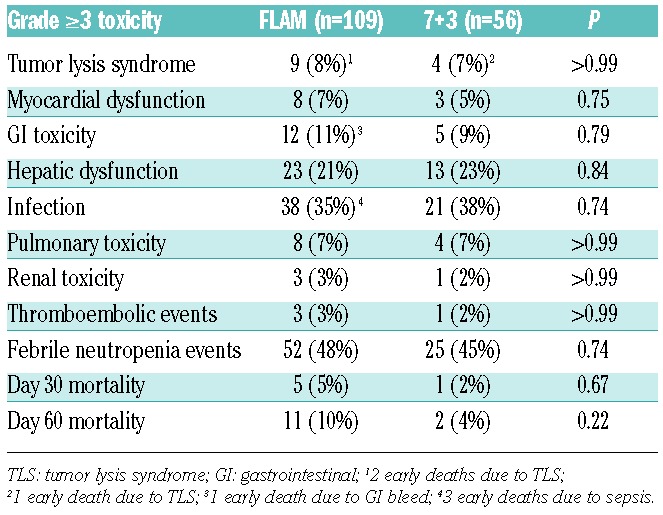
Grade 3 or more toxicities were similar in both arms (Table 2). Although the rates of tumor lysis syndrome (TLS) were similar between FLAM and 7+3 (FLAM: 8% vs. 7+3: 7%), there were 2 early deaths on the FLAM arm due to TLS (compared with 1 early death on 7+3), and 3 grade 4 TLS toxicities on FLAM: acute kidney injury requiring dialysis (n=2), and cytokine release syndrome (n=1). While there was no significant difference in treatment-related mortality between both arms (day 60 mortality: FLAM: 10%, 95%CI: 5%–17% vs. 7+3: 4%, 95%CI: 0–12%; P=0.22), the majority (8 of 11) of early deaths on FLAM were in patients aged 60 years or over. Causes of early mortality (day 60 mortality) on FLAM included refractory leukemia (n=4), infection/sepsis (n=3), TLS (n=2), multi-organ failure (n=1), and gastrointestinal (GI) bleed (n=1). Causes of early mortality on 7+3 included refractory leukemia (n=1) and TLS (n=1).
Table 2.
Toxicity comparisons.
Time to full hematologic recovery (ANC >1×109/L and platelet count >100×109/L) for all patients who achieved CR was similar for FLAM (37 days, 95%CI: 34–40 days) and 7+3 (34 days, 95%CI: 32–37 days) (P=0.30). Patients who received 5+2 (n=13) had a median time from start of initial therapy to full hematologic recovery of 49 days.
Clinical outcomes
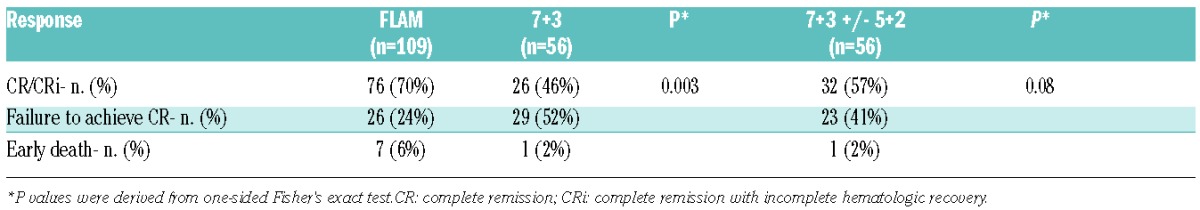
FLAM led to a 70% CR rate (71 CR + 5 CRi; 95%CI: 60%–78%), while 7+3 led to a 46% CR rate (25 CR + 1 CRi; 95%CI: 33%–60%); odds ratio = 2.64, 95%CI: 1.29–5.45, one-sided P=0.003 (Table 3). The treatment effect was of similar magnitude and significance after controlling for the randomization stratification factors (odds ratio =2.94, 95%CI: 1.44–5.99, one-sided P=0.001). Thirteen of 56 (23%) patients on the 7+3 arm received 5+2 for evidence of residual leukemia on day 14 (median % cellularity: 10%, range: 5%–50%; median % blasts: 33%, range: 12%–90%). Six of 13 (46%) patients achieved CR [including one complete remission with incomplete hematologic recovery (CRi)] after receiving 5+2. A comparison of CR rates between FLAM and 7+3+/−5+2 was 70% (95%CI: 60%–78%) versus 57% (95%CI: 43%–70%), respectively (one-sided P=0.08).
Table 3.
Response comparisons.
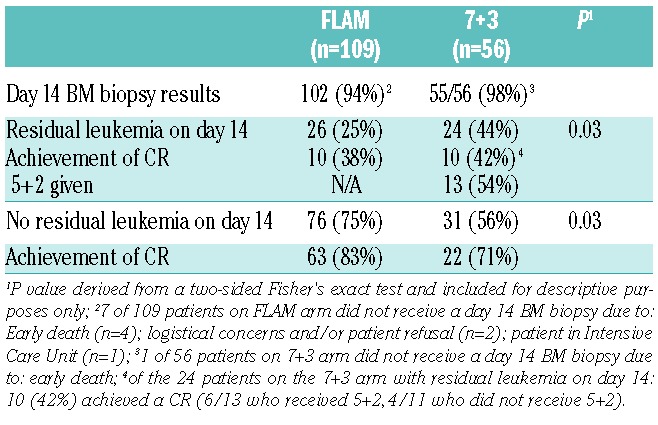
A day 14 BM biopsy was performed on 102 of 109 FLAM patients and 55 of 56 7+3 patients (Table 4). Residual leukemia on day 14 was significantly less with FLAM compared with 7+3 (25%, 95%CI: 17–35% vs. 44%, 95%CI: 30%–58%, respectively; P=0.03). Patients in CR received diverse post-induction treatment strategies based on physician/institution preference. Of the 76 FLAM patients who achieved CR, 35 (46%) underwent FLAM consolidation, 21 (28%) high-dose cytarabine (HiDAC) consolidation, 13 (17%) early allogeneic SCT without consolidation, 5 (7%) had poor performance status preventing further therapy, one had early relapse, and one underwent clofarabine treatment for persistent cytogenetic abnormalities. Of the 32 CR patients on 7+3 (with or without 5+2), 26 (81%) underwent HiDAC consolidation, 3 (9%) early allogeneic SCT without consolidation, 2 (6%) had poor performance status preventing further therapy, and one patient refused further therapy. A total of 82 (50%) patients underwent allogeneic SCT (FLAM: 55 of 109=51%; 7+3: 27 of 56=48%), and one patient underwent an autologous SCT in CR on the 7+3 arm. Of the 83 patients who underwent a SCT (autologous + allogeneic), myeloablative conditioning was performed on 44% and 64% of FLAM and 7+3 patients, respectively, based on institution preference. Donors on the FLAM arm included matched related (n=17), matched unrelated (n=19), haploidentical (n=16), and cord blood (n=3) donors. Donors on the 7+3 arm included matched related (n=7), matched unrelated (n=14), haploidentical (n=5), and cord blood (n=1) donors. Relapses were similar in patients who underwent SCT in CR1 (FLAM: 32% vs. 7+3: 38%).
Table 4.
Day 14 bone marrow biopsy comparisons.
Subset analyses
Although not powered to demonstrate significance, FLAM increased CR rates across different risk groups, as seen in exploratory ad hoc analyses (Figure 2). When testing for heterogeneity of treatment effect, patients under 50 years of age derived greater benefit from FLAM versus one cycle of 7+3 (CR rates: FLAM: 96% vs. 7+3: 57%; odds ratio = 19.5, 95%CI: 2.03–187.0) compared to those aged 50 years or over (FLAM: 61% vs. 7+3: 43%; odds ratio = 2.08, 95%CI: 0.98–4.43; P for interaction = 0.04). This difference also persisted for FLAM versus 7+3+/−5+2 (P for interaction = 0.007). Those with no poor-risk features also saw greater benefit from FLAM (CR rates: FLAM: 100% vs. 7+3: 72%; odds ratio = 20.8, 95%CI: 1.07–405.0) when compared with one or more poor-risk features (FLAM: 61% vs. 7+3: 34%; odds ratio = 2.9, 95%CI: 1.32–6.0; P for interaction = 0.04). Notably, patients with secondary AML (CR rates: FLAM: 60% vs. 7+3: 35%) derived similar benefit from FLAM as de novo AML (FLAM: 79% vs. 7+3: 57%).
Figure 2.
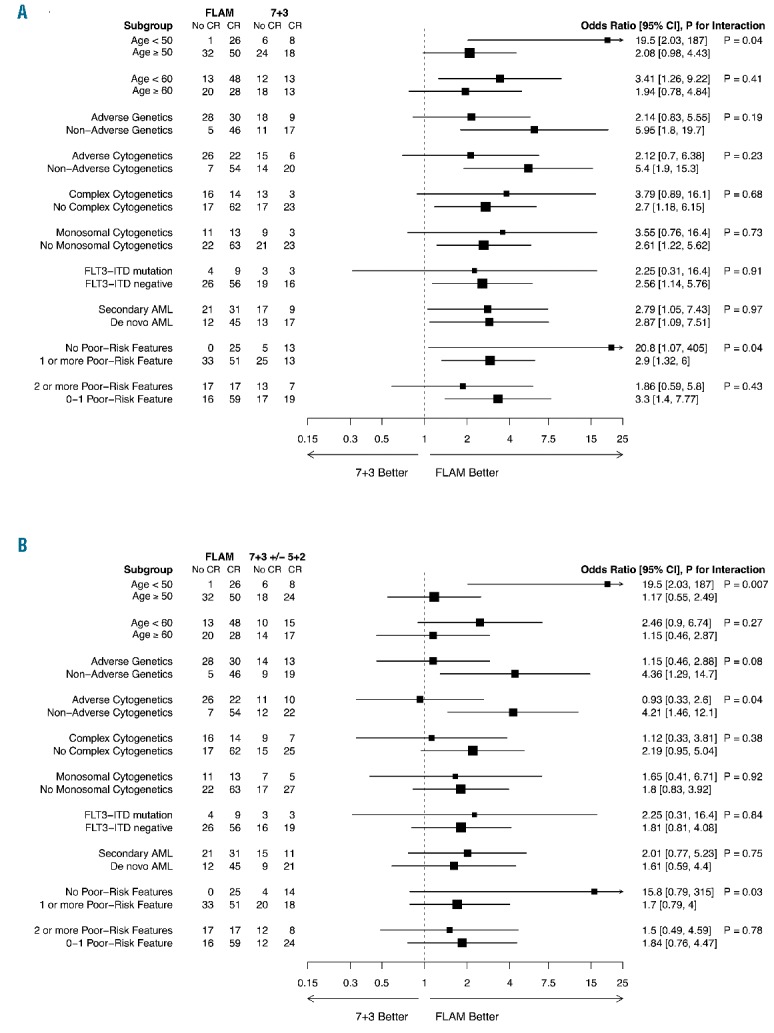
Subset analyses. Forest plot of odds ratios for complete remission in patient subgroups, plotted on a log scale. P values were derived from likelihood ratio tests of logistic regression models for interaction between treatment: (A) FLAM vs. 7+3 and (A) FLAM vs. 7+3+/−5+2, and patient subgroup on complete remission. To provide information about patients in the ‘no poor-risk features’ subgroup, 0.5 was added to all cell counts in this analysis for calculating odds ratios and 95% confidence intervals.
Overall survival and event-free survival outcomes
Median follow up was 553 days (range: 1–1217 days). To date, relapses were similar for FLAM and 7+3 (43% vs. 50%, respectively). There was no significant OS difference between FLAM and 7+3: median OS = 17.5 months, 95%CI: 12.7–25.4 months, on FLAM versus 22.2 months, 95%CI: 16.2–40.0 months, on 7+3 (P=0.39) (Figure 3A). Two-year OS on FLAM was 50% versus 59% on 7+3. Although not significantly different, there was an apparent clinical improvement with FLAM with a median EFS = 9.7 months (95%CI: 5.0–11.7 months) on FLAM versus 3.4 months (95%CI: 1.3–13.3 months) on 7+3 (P=0.15) (Figure 3B).
Figure 3.
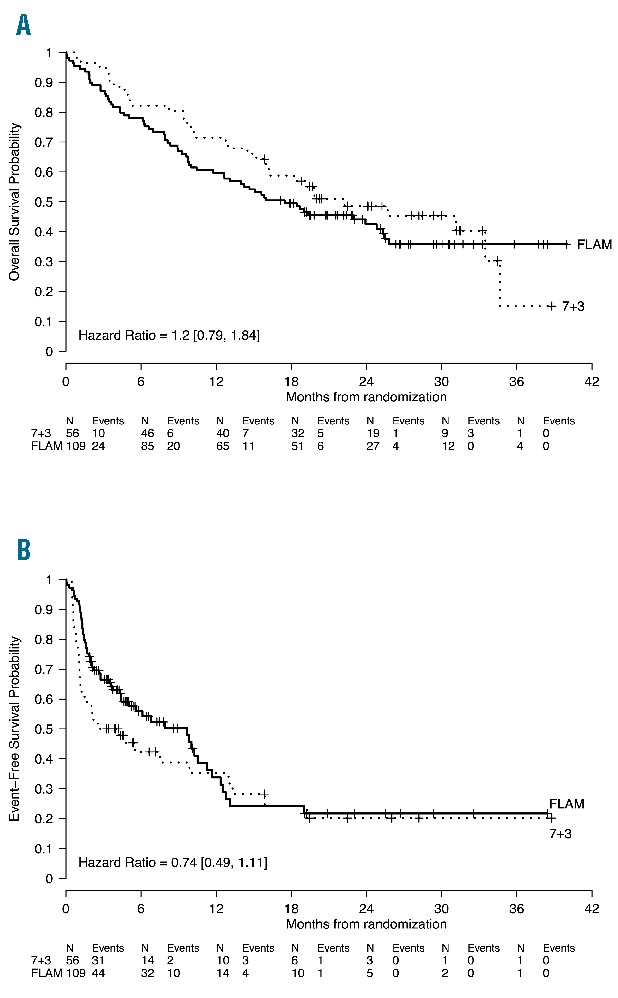
Kaplan-Meier estimates of overall survival (OS) and event-free survival (EFS). (A). OS for the entire cohort and treatment arm measured by Kaplan-Meier methodology. OS is defined as the date of randomization to date of last follow up or death. (B). EFS for the entire cohort and treatment arm measured by Kaplan-Meier methodology. EFS is defined as time from randomization to persistent AML after one cycle of induction therapy, relapse, death, or date of last follow up (censored).
Overall, 65 (60%) patients died on the FLAM arm compared with 32 (57%) deaths on the 7+3 arm. Causes of death on the FLAM arm included refractory leukemia (n=43), sepsis/infection (n=8), allogeneic SCT complications (n=6), hemorrhage/coagulopathy (n=3), TLS (n=2), stroke (n=1), multi-organ failure (n=1), and unknown causes (n=1), whereas causes of death on 7+3 included refractory leukemia (n=26), allogeneic SCT complications (n=4), TLS (n=1), and sepsis/infection (n=1). In addition, 13 (17%) patients on FLAM died while in CR compared with 3 (10%) on 7+3. Causes of death in CR on FLAM included allogeneic SCT complications (n=6), infections (n=3; including one patient who died of E. coli sepsis during HiDAC consolidation), progressive bone marrow failure (n=1), presumed central nervous system (CNS) leukemia (n=1), intracerebral hemorrhage (n=1), and unknown causes (n=1). Causes of death in CR on 7+3 included allogeneic SCT complications (n=2), and sepsis/infection (n=1).
Discussion
The main study objective of this phase II trial was to assess the relative activity of FLAM compared with 7+3 in order to determine whether there was justification for further development of FLAM based on CR rate. The study findings support the hypothesis that FLAM induction leads to superior CR rates compared with 7+3 therapy (70% vs. 46%; P=0.003) in patients with intermediate and adverse-risk cytogenetics. The cycle 1 CR rate was chosen as a more specific indicator of relative activity between the 2 regimens because of variations in the approach following one cycle of induction therapy in practice. For instance, many leukemia clinicians utilize a re-induction strategy (ie. 5+2) in some patients with persistent disease on day 14 after 7+3 induction. In contrast, FLAM is not intensified on the basis of early BM biopsy findings. We considered it to be important to compare the FLAM regimen to an appropriate contemporary standard control regimen of 7+3; thus, we allowed a re-induction strategy (ie. 5+2) in those patients with residual leukemia on day 14, as recommended by the National Comprehensive Cancer Network Guidelines in AML.18 There are a number of confounders when determining whether to give a second cycle of induction, such as patient age, performance status, toxicities, and day 14 BM findings.19 Therefore, a second cycle of induction was recommended, but not mandated, in those patients with residual leukemia on day 14. The comparison of FLAM to 7+3+/−5+2 (CR rates: 70% vs. 57%; P=0.08) also substantiates the improved efficacy with FLAM and further supports the development of this regimen.
The CR rates seen on this study were lower than other recently reported phase III studies in AML due to differences in the patient populations studied.16,20,21 Patients with favorable-risk cytogenetic features (ie. CBF AML) were excluded on this study. In addition, newly diagnosed elderly AML patients were included on this study up to 70 years of age, whereas other trials only included patients up to 60 years of age.16,20,21 Forty-seven percent of patients on this study had secondary AML, a subgroup with poor outcomes.22 A larger proportion of patients had adverse-risk cytogenetics (41% total) when compared with other contemporary phase III studies in AML.16,20,21 The relatively low CR rates seen on the 7+3 arm of this study were consistent with other recent reports in similar non-favorable risk patient populations.23,24
Importantly, there was no difference in overall toxicity between FLAM and 7+3. TLS was a major concern given the high rates of TLS with flavopiridol in chronic lymphocytic leukemia25–27 and consistent 7%–10% incidence of grade 3 or more TLS in our prior phase II studies.10–12 Three patients treated with FLAM on this study had grade 4 TLS, and 2 patients died due to complications of TLS. Patients with WBC more than 20×109/L, monocytic phenotypes (ie. M4/M5, or arising from pre-existing MPN), and baseline renal dysfunction appear to predispose to grade 4 or more TLS with FLAM. Though all patients received allopurinol and sevelamer prophylaxis, it may be necessary to use rasburicase and other supportive care measures to blunt cytokine release syndrome (eg. steroids) in order to decrease the severity of TLS in future trials with FLAM.
In addition, older age (>60 years) appears to predispose to FLAM toxicity. Although overall treatment-related mortality rates were similar between FLAM and 7+3, 8 of 11 early deaths (<60 days) on FLAM occurred in patients aged 60 years or over. On subset analyses, younger patients had higher CR rates with FLAM compared with 7+3, and this difference remained statistically significant after 7+3+/−5+2.
A major question in the management of AML patients is whether or not to give more chemotherapy for a day 14 BM biopsy revealing residual leukemia. There is, unfortunately, a lack of a consistent approach in managing these patients. Moreover, some patients with residual leukemia on a day 14 BM biopsy after 7+3 induction will ultimately achieve CR and may not need additional induction therapy.28–30 There is considerable investigator bias regarding whether or not to give a second cycle of induction therapy for patients with residual leukemia on day 14.19 In our study, 24 patients on the 7+3 arm had residual leukemia on day 14, but only 13 (54%) received 5+2, and 6 of 13 (46%) achieved CR (compared with 4 of 11 without 5+2). The proportion of patients achieving a CR with a second cycle of induction was similar to other reports.16,30 Although Rowe et al.31 reported that adults who achieve a CR after one or two courses of induction therapy have similar overall outcomes, this analysis was retrospective, and the patients included on this analysis received an identical course of induction therapy (ie. 7+3), as opposed to 5+2 for day 14 residual leukemia. In contrast, the GOELAMS study group32 performed a multicenter prospective study in which a second induction course was given to patients with 5% or more blasts on a day 15 BM biopsy, and showed that those with residual leukemia on day 15 had significantly worse outcomes, even after a second induction course. In the present study, patients on the FLAM arm did not receive more intensive therapy in the presence of residual leukemia on day 14, and a proportion of those (38%) still achieved CR.
Secondary AML represents a subgroup of patients with an extremely poor outcome who may benefit from FLAM induction. Secondary AML can be confirmed based on history alone in most cases. While 60% of patients with secondary AML in this current study achieved CR, we have now treated 158 newly diagnosed secondary AML patients with FLAM across all 4 phase II studies with an overall CR rate of 66% (105 of 158).10–12 The similar effect with FLAM in secondary versus de novo AML highlights FLAM activity in secondary AML, despite the overall decreased response rates and poor outcomes seen in this subpopulation. Moreover, although small numbers, all 6 patients with secondary AML and aged under 50 years treated with FLAM achieved a CR on this study (compared to 1 of 3 patients with secondary AML and under 50 years of age achieving a CR on 7+3). Thus, these data substantiate the benefit of FLAM in secondary AML, particularly in younger patients, and compare favorably to the promising findings seen with CPX-351.23
Despite the significant improvement in response rates seen with FLAM, our study did not show a difference in OS. These findings must be interpreted cautiously as the study was not powered to detect an OS difference. Other recently performed randomized studies in AML have also reported improved CR rates without OS advantage.33 Moreover, lack of an OS advantage from a randomized phase II study aimed primarily to assess activity does not diminish the pertinence of these findings. It also reinforces the importance of a larger randomized phase III study specifically powered to detect an OS advantage, as evidenced by the finding that the OS of the 56 patients on the 7+3 arm of this poor-risk patient population was significantly better than the results of recently reported phase III studies.16,20,21,34 An additional limitation of the OS/EFS analyses on this study was the lack of standardized post-induction treatment strategies in both arms. FLAM was initially developed as two cycles (induction and consolidation therapy) in patients who achieve CR but post-induction treatment for both arms in this study was not defined. For example, only 46% of CR patients on FLAM received FLAM consolidation, while 28% received HiDAC, and 17% received early allogeneic SCT without consolidation therapy. In addition, post-remission transplantation conditioning therapies and donor selections were also variable. This lack of a consistent treatment approach for patients who achieve CR is a major challenge in drug development in AML. It will be essential for these post-induction treatment strategies to be standardized for phase III studies in order to truly verify whether FLAM induction can improve overall outcomes compared with 7+3. Given the inherent variability and unclear utility of a second cycle of induction with 7+3, a phase III study comparing FLAM to one cycle of 7+3 (without early re-induction therapy) would eliminate any potential biases of re-treatment.
In conclusion, FLAM represents a promising induction regimen for AML patients with intermediate- and adverse-risk cytogenetic features. Our findings reveal that FLAM induction leads to superior CR rates when compared with 7+3. On subset analyses, younger patients (<50 years), and those with no poor-risk features, appeared to benefit more from FLAM than their 7+3 counterparts. Moreover, FLAM demonstrated promising activity in secondary AML. While the data clearly demonstrate that FLAM induction leads to superior CR rates compared with 7+3 alone, rigorous clinical trial designs are required to determine OS differences, particularly among select subgroups such as secondary AML.
Acknowledgments
The authors would like to thank the research support staff from each institution, the nurses and patient care teams who managed the study patients expertly, and all of the patients and their families who trusted their care to us. Without these partnerships, this trial could not have been done and our goal of improving the treatments for patients with AML would not be possible.
Footnotes
The online version of this article has a Supplementary Appendix.
Funding
This study was supported in part by NCI Cooperative Agreement U01 CA70095 and NCI Cancer Center Support Grant 2P30 CA06973-46.
JFZ received a 2013 Conquer Cancer Foundation Young Investigator Award, in memory of Dr. John R, Durant, and is a 2014–2017 Leukemia and Lymphoma Society Special Fellow in Clinical Research.
Authorship and Disclosures
Information on authorship, contributions, and financial & other disclosures was provided by the authors and is available with the online version of this article at www.haematologica.org.
References
- 1.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64(1): 9–29. [DOI] [PubMed] [Google Scholar]
- 2.Rai KR, Holland JF, Glidewell OJ, et al. Treatment of acute myelocytic leukemia: a study by cancer and leukemia group B. Blood. 1981;58(6):1203–1212. [PubMed] [Google Scholar]
- 3.Tefferi A, Letendre L. Going beyond 7 + 3 regimens in the treatment of adult acute myeloid leukemia. J Clin Oncol. 2012; 30(20):2425–2428. [DOI] [PubMed] [Google Scholar]
- 4.Burke PJ, Karp JE, Braine HG, Vaughan WP. Timed sequential therapy of human leukemia based upon the response of leukemic cells to humoral growth factors. Cancer Res. 1977;37(7 Pt 1):2138–2146. [PubMed] [Google Scholar]
- 5.Geller RB, Burke PJ, Karp JE, et al. A two-step timed sequential treatment for acute myelocytic leukemia. Blood. 1989; 74(5): 1499–1506. [PubMed] [Google Scholar]
- 6.Rytting M, Ravandi F, Estey E, et al. Intensively timed combination chemotherapy for the induction of adult patients with acute myeloid leukemia: long-term followup of a phase 2 study. Cancer. 2010; 116(22):5272–5278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Braess J, Spiekermann K, Staib P, et al. Dose-dense induction with sequential high-dose cytarabine and mitoxantone (SHAM) and pegfilgrastim results in a high efficacy and a short duration of critical neutropenia in de novo acute myeloid leukemia: a pilot study of the AMLCG. Blood. 2009;113(17):3903–3910. [DOI] [PubMed] [Google Scholar]
- 8.Woods WG, Kobrinsky N, Buckley JD, et al. Timed-sequential induction therapy improves postremission outcome in acute myeloid leukemia: a report from the Children’s Cancer Group. Blood. 1996; 87(12):4979–4989. [PubMed] [Google Scholar]
- 9.Karp JE, Ross DD, Yang W, et al. Timed sequential therapy of acute leukemia with flavopiridol: in vitro model for a phase I clinical trial. Clin Cancer Res. 2003; 9(1):307–15. [PubMed] [Google Scholar]
- 10.Karp JE, Smith BD, Levis MJ, et al. Sequential flavopiridol, cytosine arabinoside, and mitoxantrone: a phase II trial in adults with poor-risk acute myelogenous leukemia. Clin Cancer Res. 2007;13(15 Pt 1):4467–4473. [DOI] [PubMed] [Google Scholar]
- 11.Karp JE, Blackford A, Smith BD, et al. Clinical activity of sequential flavopiridol, cytosine arabinoside, and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Leuk Res. 2010;34(7):877–882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karp JE, Garrett-Mayer E, Estey EH, et al. Randomized phase II study of two schedules of flavopiridol given as timed sequen tial therapy with cytosine arabinoside and mitoxantrone for adults with newly diagnosed, poor-risk acute myelogenous leukemia. Haematologica. 2012; 97(11):1736–1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap): a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009;42(2):377–381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dohner H, Estey EH, Amadori S, et al. Diagnosis and management of acute myeloid leukemia in adults: recommendations from an international expert panel, on behalf of the European LeukemiaNet. Blood. 2010;115(3):453–474. [DOI] [PubMed] [Google Scholar]
- 15.Appelbaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–3485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fernandez HF, Sun Z, Yao X, et al. Anthracycline dose intensification in acute myeloid leukemia. N Engl J Med. 2009; 361(13):1249–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461). Blood. 2002;100(13):4325–4336. [DOI] [PubMed] [Google Scholar]
- 18.O’Donnell MR, Abboud CN, Altman J, et al. Acute myeloid leukemia. J Natl Compr Canc Netw. 2012;10(8):984–1021. [DOI] [PubMed] [Google Scholar]
- 19.Ueda M, Xie H, Sandhu RK, Walter RB, Pagel JM, Estey EH. Factors associated with early re-induction chemotherapy for adults with acute myeloid leukemia. Leuk Lymphoma. 2015;56(3):782–784. [DOI] [PubMed] [Google Scholar]
- 20.Mrozek K, Marcucci G, Nicolet D, et al. Prognostic significance of the European LeukemiaNet standardized system for reporting cytogenetic and molecular alter ations in adults with acute myeloid leukemia. J Clin Oncol. 2012;30(36):4515–4523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Holowiecki J, Grosicki S, Giebel S, et al. Cladribine, but not fludarabine, added to daunorubicin and cytarabine during induction prolongs survival of patients with acute myeloid leukemia: a multicenter, randomized phase III study. J Clin Oncol. 2012;30(20):2441–2448. [DOI] [PubMed] [Google Scholar]
- 22.Schoch C, Kern W, Schnittger S, Hiddemann W, Haferlach T. Karyotype is an independent prognostic parameter in therapy-related acute myeloid leukemia (t-AML): an analysis of 93 patients with t-AML in comparison to 1091 patients with de novo AML. Leukemia. 2004;18(1):20–125. [DOI] [PubMed] [Google Scholar]
- 23.Lancet JE, Cortes JE, Hogge DE, et al. Phase 2 trial of CPX-351, a fixed 5:1 molar ratio of cytarabine/daunorubicin, vs cytarabine/daunorubicin in older adults with untreated AML. Blood. 2014; 123(21):3239–3246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Stone RM, Mazzola E, Neuberg D, et al. Phase III Open-Label Randomized Study of Cytarabine in Combination With Amonafide L-Malate or Daunorubicin As Induction Therapy for Patients With Secondary Acute Myeloid Leukemia. J Clin Oncol. 2015;33(11):1252–1257. [DOI] [PubMed] [Google Scholar]
- 25.Ji J, Mould DR, Blum KA, et al. A pharma-cokinetic/pharmacodynamic model of tumor lysis syndrome in chronic lymphocytic leukemia patients treated with flavopiridol. Clin Cancer Res. 2013; 19(5):1269–1280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blum KA, Ruppert AS, Woyach JA, et al. Risk factors for tumor lysis syndrome in patients with chronic lymphocytic leukemia treated with the cyclin-dependent kinase inhibitor, flavopiridol. Leukemia. 2011;25(9):1444–1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Byrd JC, Lin TS, Dalton JT, et al. Flavopiridol administered using a pharmacologically derived schedule is associated with marked clinical efficacy in refractory, genetically high-risk chronic lymphocytic leukemia. Blood. 2007;109(2):399–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Morris TA, DeCastro CM, Diehl LF, et al. Re-induction therapy decisions based on day 14 bone marrow biopsy in acute myeloid leukemia. Leuk Res. 2013; 37(1):28–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hussein K, Jahagirdar B, Gupta P, Burns L, Larsen K, Weisdorf D. Day 14 bone marrow biopsy in predicting complete remission and survival in acute myeloid leukemia. Am J Hematol. 2008;83(6):446–450. [DOI] [PubMed] [Google Scholar]
- 30.Yezefski T, Xie H, Walter R, et al. Value of routine ‘day 14’ marrow exam in newly diagnosed AML. Leukemia. 2015;29(1):247–249. [DOI] [PubMed] [Google Scholar]
- 31.Rowe JM, Kim HT, Cassileth PA, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116(21):5012–5021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bertoli S, Bories P, Bene MC, et al. Prognostic impact of day 15 blast clearance in risk-adapted remission induction chemotherapy for younger patients with acute myeloid leukemia: long-term results of the multicenter prospective LAM-2001 trial by the GOELAMS study group. Haematologica. 2014;99(1):46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Burnett AK, Russell NH, Hills RK, et al. Optimization of chemotherapy for younger patients with acute myeloid leukemia: results of the medical research council AML15 trial. J Clin Oncol. 2013; 31(27):3360–3368. [DOI] [PubMed] [Google Scholar]
- 34.Lowenberg B, Ossenkoppele GJ, van Putten W, et al. High-dose daunorubicin in older patients with acute myeloid leukemia. N Engl J Med. 2009;361(13):1235–1248. [DOI] [PubMed] [Google Scholar]


