Abstract
The Ministry of Health (MOH) has developed the clinical practice guidelines on Prevention, Diagnosis and Management of Tuberculosis to provide doctors and patients in Singapore with evidence-based treatment for tuberculosis. This article reproduces the introduction and executive summary (with recommendations from the guidelines) from the MOH clinical practice guidelines on Prevention, Diagnosis and Management of Tuberculosis, for the information of SMJ readers. The chapters and page numbers mentioned in the reproduced extract refer to the full text of the guidelines, which are available from the Ministry of Health website: http://www.moh.gov.sg/content/moh_web/healthprofessionalsportal/doctors/guidelines/cpg_medical.html. The recommendations should be used with reference to the full text of the guidelines. Following this article are multiple choice questions based on the full text of the guidelines.
INTRODUCTION
Tuberculosis continues to be a disease of public health importance in Singapore and worldwide. According to the World Health Organisation’s Global Tuberculosis Report 2014, an estimated 9 million people developed tuberculosis and 1.5 million died from the disease. Issues like delayed detection and missed opportunities for treatment, and the emergence of drug-resistance are also of increasing concern.
1.1 Aim
The target audience is all healthcare practitioners in Singapore. These guidelines aim to:
Increase knowledge and awareness of tuberculosis so as to facilitate the early detection of active tuberculosis.
Serve as an evidence-based resource to provide guidance on the use of tuberculosis diagnostic tools and treatment regimens.
Inform regarding the public health measures necessary for the control of tuberculosis control in Singapore.
1.2 Scope
These guidelines will cover tuberculosis referral and diagnosis, treatment of active and latent tuberculosis, and public health actions required on the part of treating physicians. The standards of diagnosis and treatment, which are outlined in the International Standards for Tuberculosis Care, will also be referenced in the clinical practice guidelines (CPG).
1.3 Target group
The content of the guidelines will be useful for all healthcare professionals and public health service providers who encounter patients with tuberculosis. The CPG will be applicable to the diagnosis and management of both adult and paediatric patients. The doctor evaluating the patient is ultimately responsible for clinical decisions made after reviewing the individual patient’s history, clinical presentation and treatment options available.
1.4 Development of guidelines
These guidelines have been produced by a committee of respiratory physicians, infectious disease consultants, and representatives from polyclinics and the College of Family Physicians Singapore, as well as representatives from Ministry of Health (MOH) and Tuberculosis Control Unit appointed by the MOH. They were developed by the adaptation of existing guidelines, by the review of relevant literature and by expert clinical consensus with consideration of local practice.
The following principles underlie the development of these guidelines:
Treatment recommendations are supported by scientific evidence and expert clinical consensus.
Treatment should maximise therapeutic and public health benefits and minimise side effects.
1.5 Review of guidelines
Evidence-based CPGs are only as current as the evidence that supports them. Users must keep in mind that new evidence could supersede recommendations in these guidelines. The workgroup advises that these guidelines be scheduled for review five years after publication, or when new evidence appears that requires substantive changes to the recommendations.
EXECUTIVE SUMMARY OF RECOMMENDATIONS
Details of recommendations can be found on the indicated pages. Key recommendations are highlighted in grey.
Tuberculosis Transmission and Pathogenesis

Clinical Diagnosis of Tuberculosis
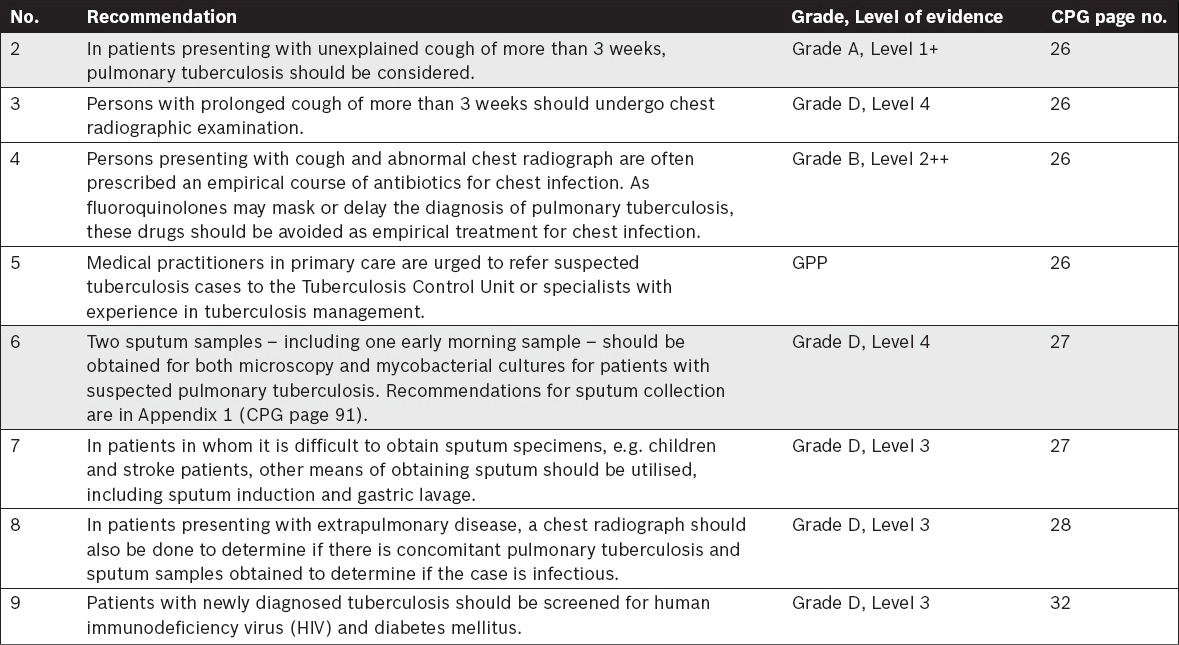
Imaging in Tuberculosis
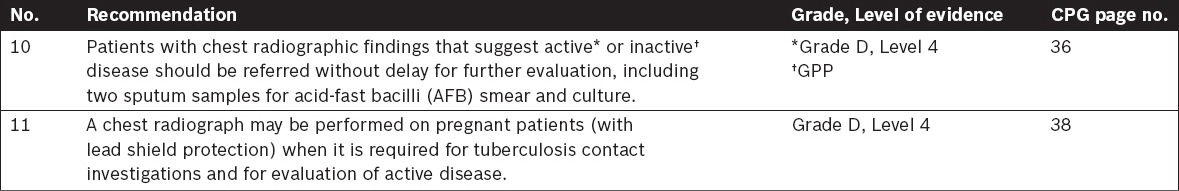
Tuberculosis Laboratory Diagnosis
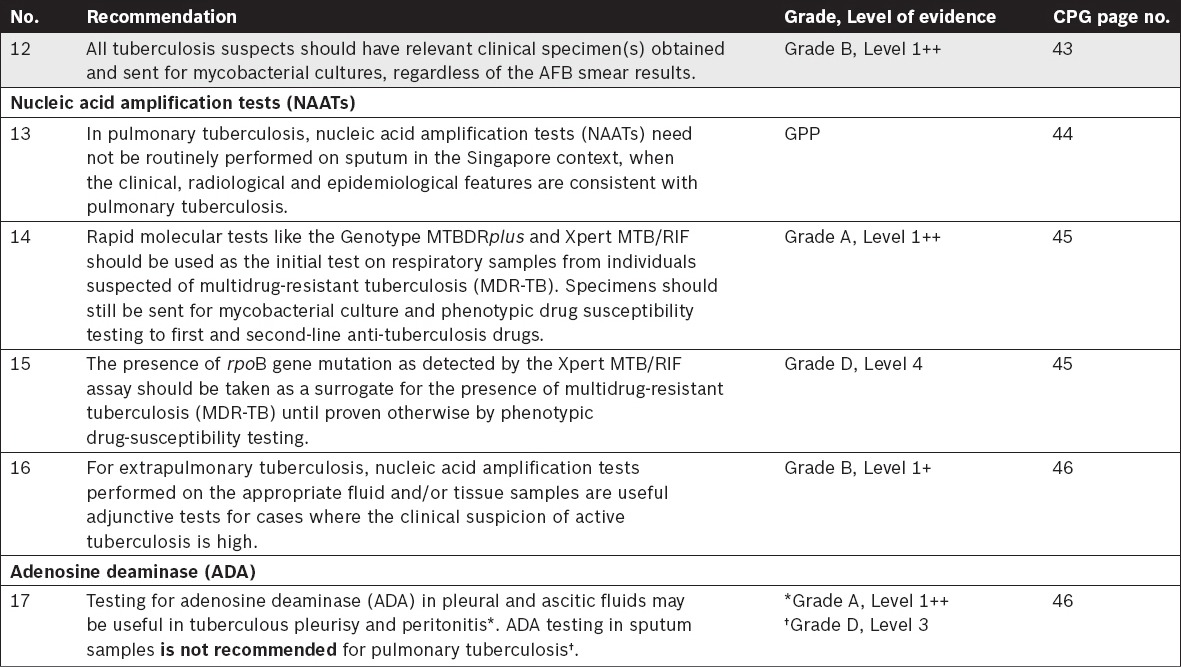
Treatment of Tuberculosis
Public Health Screening and Infection Control
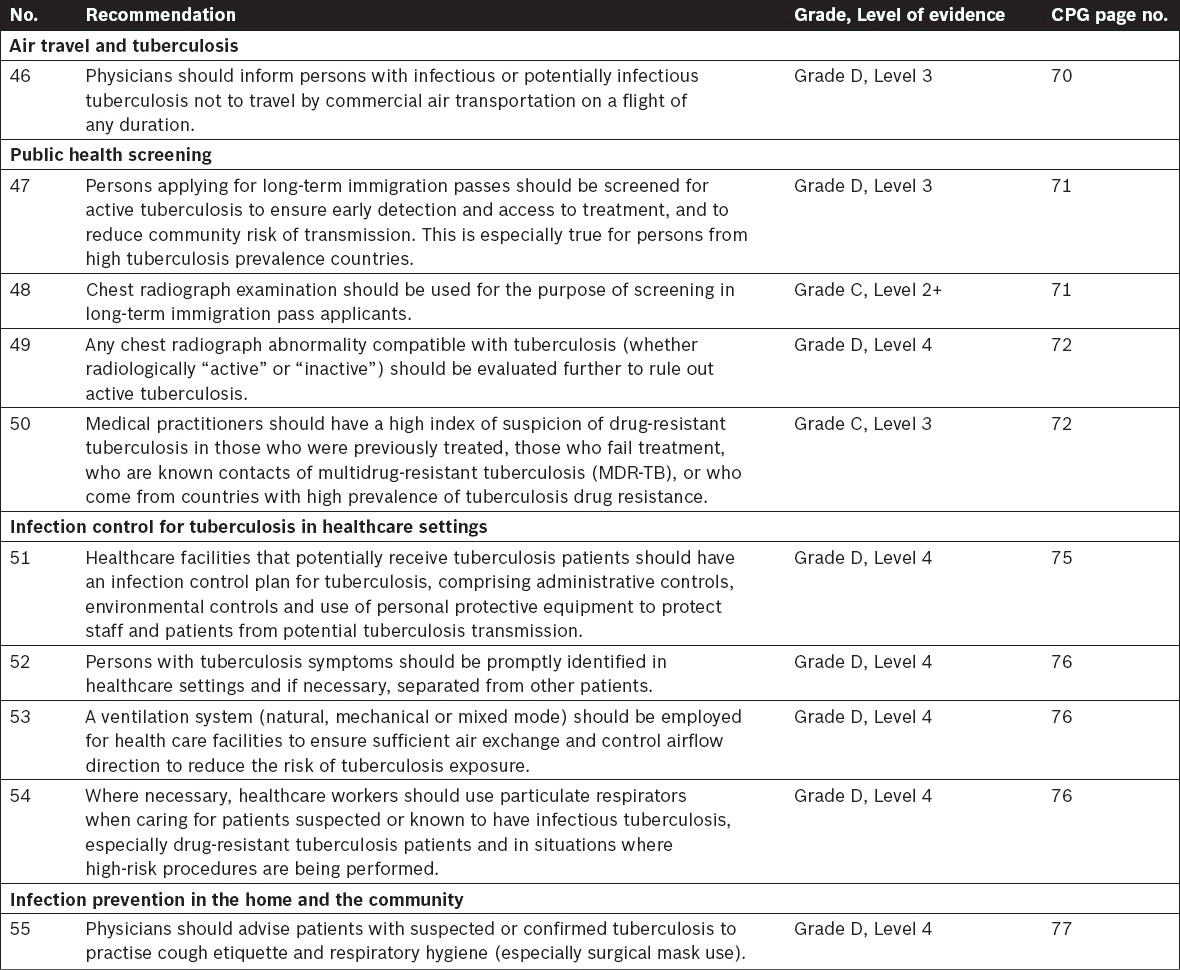
Tuberculosis Contact Investigations and Screening
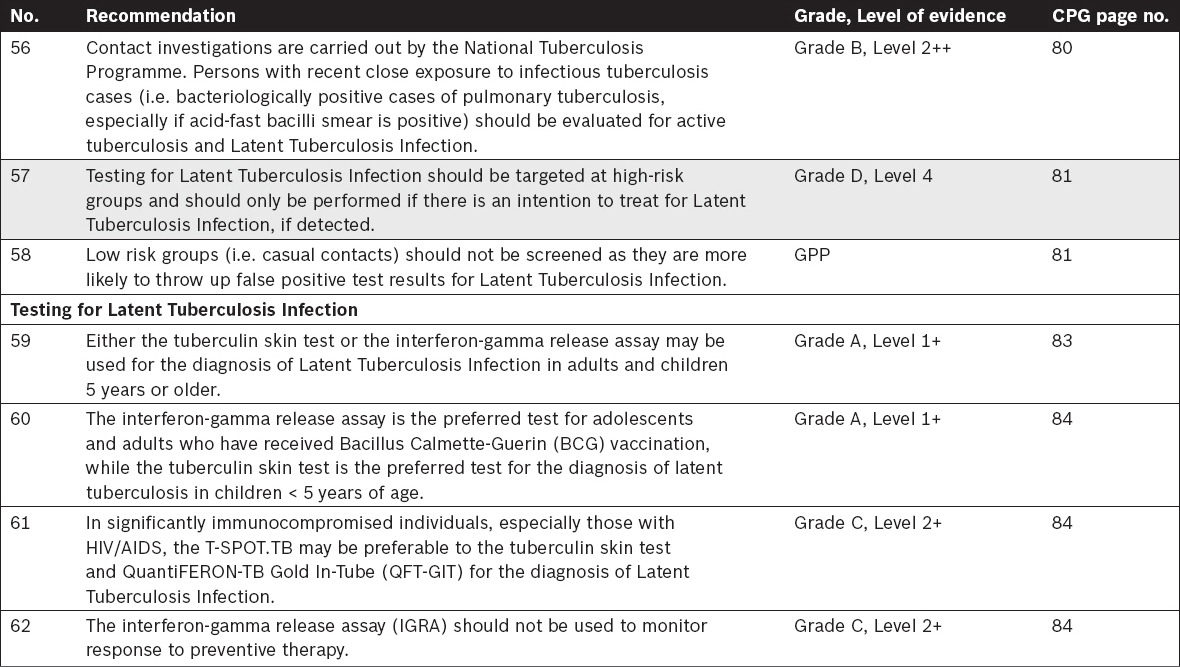
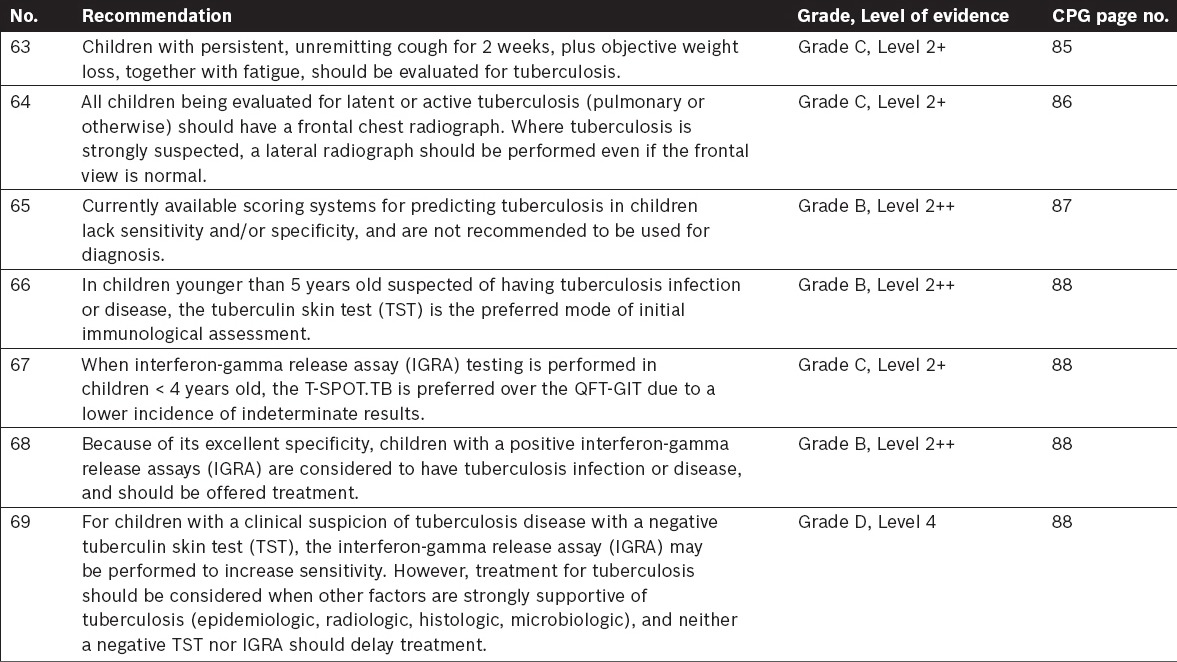
Tuberculosis in Children – Specific Considerations
APPENDIX 1
Recommendations for sputum collection
1. General
Specimens should be collected before starting patients on anti-tuberculosis drug therapy.
Sputum specimens should be collected in a well-ventilated area and precautions should be taken to ensure that healthcare workers and others are not exposed to infectious aerosols and materials. Contaminated materials should be disposed of in accordance with standard biosafety procedures.
Specimens should be obtained under the direct supervision of a healthcare worker.
2. Procedure for sputum collection
Sputum must be collected in sterile, screw-capped, leak-proof, disposable, plastic containers. Containers must be free from paraffin and other waxes or oils. The container should be clear so the specimen can be visualised without opening the container.
Sputum collection containers should be labelled with the patient’s name, NRIC number, nature of specimen, date and time of collection. The label should be on the side of the container instead of the lid.
-
Patients should be instructed to:
- Collect the specimen in the morning before any oral intake.
- Rinse his/her mouth with water before starting to collect the specimen to remove contamination such as food particles and bacteria. Patients with postnasal discharge should clear these passages before beginning sputum collection.
-
Cough from as deep inside the chest as possible, as it is important to collect sputum and not saliva.
- Instruct patient to take a deep breath, hold his/her breath for a few seconds, and then exhale slowly.
- Do this twice.
- The third time, inhale deeply, hold his/her breath, and then forcefully exhale through the mouth.
- The fourth time, inhale deeply and cough. Instruct patient to carefully direct the sputum into the container to minimise contamination of the outside of the container for safe handling.
- Patient is to repeat the process until at least 5 ml of specimen has been obtained.
The healthcare worker supervising the sputum collection may rap gently and firmly on the applicant’s back to help induce coughing and sputum production.
The supervising healthcare worker should inspect the specimen to ensure that it contains sputum and not saliva. Sputum is frequently thick and mucoid, but may consist of dull while or light green fluid with fine chunks of dead tissue that show up like solid flakes. Blood may or may not be present. In contrast, saliva appears thin and nearly clear; and should not be accepted.
The specimen container should be capped tightly to avoid leakage. Wipe off the outside of the container with a clean tissue before placing into a biohazard-labelled plastic specimen bag. Each specimen should be accompanied by a request with relevant patient and clinical data.
The healthcare worker and patient should practise hand hygiene after specimen collection to prevent transmission of microorganisms.
The specimen should be delivered to the laboratory as soon as possible after collection to minimise overgrowth of commensal bacteria or deterioration of the mycobacteria. Grade D, Level 4
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


