Abstract
Aims/hypothesis
Canagliflozin, a sodium glucose co-transporter 2 inhibitor, reduces HbA1c, body weight and systolic BP (SBP) in patients with type 2 diabetes. As weight loss is known to reduce both HbA1c and SBP, these analyses were performed to evaluate the contribution of weight loss resulting from treatment with canagliflozin to HbA1c and SBP reductions in patients with type 2 diabetes.
Methods
Pooled data from four placebo-controlled Phase 3 studies (N=2,250) in patients with type 2 diabetes were used in the analyses. In each study, patients were treated with placebo, canagliflozin 100 mg or canagliflozin 300 mg, once daily for 26 weeks. Changes from baseline in body weight, HbA1c and SBP were measured at week 26, and the contribution of weight loss to the lowering of HbA1c and SBP was obtained using ANCOVA.
Results
Canagliflozin 100 and 300 mg reduced mean body weight, HbA1c and SBP compared with placebo (p<0.001 for each), and more patients had body-weight reductions >0%, ≥5% and ≥10% with canagliflozin treatment than with placebo. Weight-loss-independent and weight-loss-associated mechanisms contributed to HbA1c and SBP lowering with canagliflozin: ~85% of HbA1c lowering and ~60% of SBP lowering was independent of weight loss.
Conclusions/interpretation
In patients with type 2 diabetes, canagliflozin provided clinically meaningful body-weight reductions, and the weight loss contributed to reductions in HbA1c and SBP.
Trial registration
ClinicalTrials.gov NCT01081834; NCT01106625; NCT01106677; and NCT01106690
Keywords: Body weight, Canagliflozin, Sodium glucose co-transporter 2 (SGLT2) inhibitor, Type 2 diabetes mellitus
Introduction
Overweight/obesity confer additional comorbidities to individuals with type 2 diabetes mellitus, and most patients with type 2 diabetes are overweight or obese [1]. Recently, the American Medical Association recognised obesity as a complex disease associated with comorbidities, including type 2 diabetes and cardiovascular disease (CVD) [2]. In this regard, achieving weight loss of 5–10% can improve glycaemic control and CVD risk factors, including BP, HDL-cholesterol and triacylglycerol in patients with type 2 diabetes [1, 3–5]. In addition, clinically meaningful improvements in glycaemic control can be seen even with weight loss of 2–5% [5]. Unfortunately, it is difficult to both achieve and maintain weight loss, particularly as some anti-hyperglycaemic agents ([AHAs] e.g. sulfonylureas, insulin and pioglitazone) are associated with weight gain [1]. Even when lifestyle modifications lead to initial weight loss, weight regain is common [6–8]. Therefore, clinical strategies that help achieve and maintain weight loss remain unmet needs in type 2 diabetes management.
Newer AHAs, such as sodium glucose co-transporter 2 (SGLT2) inhibitors, have been proposed to address unmet clinical needs (e.g. weight loss) beyond improving glycaemic control. Canagliflozin is an SGLT2 inhibitor recently approved for the treatment of patients with type 2 diabetes that lowers the renal threshold for glucose and increases urinary glucose excretion (UGE). These actions result in decreased plasma glucose in patients with hyperglycaemia and a mild osmotic diuresis [9–12]. Canagliflozin as monotherapy and in combination with other AHAs improves glycaemic control and reduces body weight and BP in patients with type 2 diabetes [9–12].
Given the observed weight loss with canagliflozin, an unanswered question relates to the weight-loss-associated mechanisms with this therapy. We used a pooled dataset of four placebo-controlled Phase 3 studies to characterise the changes in body weight, HbA1c and systolic BP (SBP) observed with canagliflozin treatment and estimated the relative contributions of weight-loss-associated mechanisms to the overall reductions in HbA1c and SBP in patients with type 2 diabetes.
Methods
Study design, patient population and treatments
Data were pooled from patients with type 2 diabetes enrolled in four randomised, double-blind, placebo-controlled Phase 3 studies with similar designs. Each study had a primary assessment at 26 weeks and evaluated canagliflozin 100 and 300 mg vs placebo as monotherapy, add-on to metformin, add-on to metformin plus sulfonylurea, or add-on to metformin plus pioglitazone [9–12]. Eligible patients were aged 18–80 years, and generally had HbA1c ≥7.0% (53 mmol/mol) and ≤10.5% (91 mmol/mol) and estimated glomerular filtration rate ≥55 ml min−1 1.73 m−2 at screening.
All studies were conducted in accordance with ethical principles that comply with the Declaration of Helsinki and are consistent with Good Clinical Practice and applicable regulatory requirements. Study protocols and amendments were approved by ethical committees or institutional review boards at participating institutions. All patients provided written informed consent prior to participation.
Study assessments
Change in body weight, HbA1c and SBP were assessed at several time points between baseline and week 26 in each study [9–12]. The proportion of patients achieving >0%, ≥5% and ≥10% weight loss and changes in body weight by quartiles of weight loss at week 26 were assessed. The last observation carried forward (LOCF) approach was used to impute any missing data at week 26.
Statistical analyses
Mean per cent change from baseline in body weight and mean changes from baseline in HbA1c and SBP at week 26, as well as corresponding cumulative distribution plots, are reported for each variable. For the analysis of weight-loss-independent and weight-loss-associated effects of canagliflozin on HbA1c and SBP, patients within each treatment group were divided into deciles based on weight change, and mean change in HbA1c and SBP were calculated within each decile. ANCOVA models were used with HbA1c or SBP change as the response variable and body-weight change as a covariable. The slope of the relationship between body-weight change and HbA1c or SBP change was determined and the differences in slopes between each treatment group were calculated. If the slopes were not statistically different (p<0.05) between treatment groups, a single slope was used for all groups. Weight-loss-independent components of HbA1c and SBP changes were calculated as placebo-subtracted least squares (LS) mean differences from ANCOVA models (i.e. between-group differences observed at the same weight loss). All analyses were performed in Matlab, v8.4 (MathWorks, Natick, MA, USA).
Results
Patients
Baseline demographic and disease characteristics are summarised in electronic supplementary material (ESM) Table 1. Patients had mild to moderate hyperglycaemia at baseline (mean HbA1c, 8.0% [64 mmol/mol] across groups). Of the patients included in these analyses, ≥30% of patients were overweight (BMI ≥25 and <30 kg/m2) and ≥50% of patients in each study were obese (BMI ≥30 kg/m2).
Changes in body weight, HbA1c and SBP
Dose-dependent reductions were seen in body weight, HbA1c and SBP with canagliflozin compared with placebo (Fig. 1a–c). Most patients treated with canagliflozin 100 and 300 mg had a reduction in HbA1c: 86% and 92%, respectively, compared with 55% of patients treated with placebo (Fig. 1d). Similarly, most canagliflozin-treated patients experienced weight loss with canagliflozin 100 and 300 mg: 82% and 85%, respectively, compared with 55% of placebo-treated patients (Fig. 1e and ESM Fig. 1). More patients treated with canagliflozin 100 and 300 mg vs placebo had ≥ 5% weight loss (25%, 33% and 6%, respectively) and ≥ 10% weight loss (3%, 3% and 1%, respectively; ESM Fig. 1). Body-weight reductions were seen in the highest three quartiles of weight loss with canagliflozin 100 and 300 mg compared with a small increase in the lowest quartile (ESM Fig. 2). Decreases in body weight vs placebo were seen with both canagliflozin doses across quartiles. The proportions of patients experiencing some reduction in SBP were also greater with canagliflozin 100 and 300 mg compared with placebo (64%, 67% and 50%, respectively; Fig. 1f).
Fig. 1.
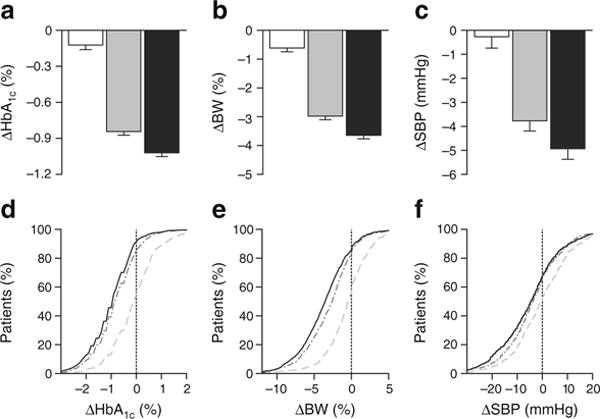
Mean (SE) reduction in (a) HbA1c, (b) body weight and (c) SBP. White bars, placebo; grey bars, canagliflozin 100 mg; black bars, canagliflozin 300 mg. (d–f) The cumulative distribution of change from baseline values for each measure. In each of these figures, the y coordinate represents the percentage of patients whose change from baseline value is lower than the change from baseline value represented by the x coordinate. Light grey dashed line, placebo; medium grey dashed–dotted line, canagliflozin 100 mg; solid black line, canagliflozin 300 mg. To convert values for change in HbA1c in % into mmol/mol, multiply by 10.929. BW, body weight
Fig. 2.
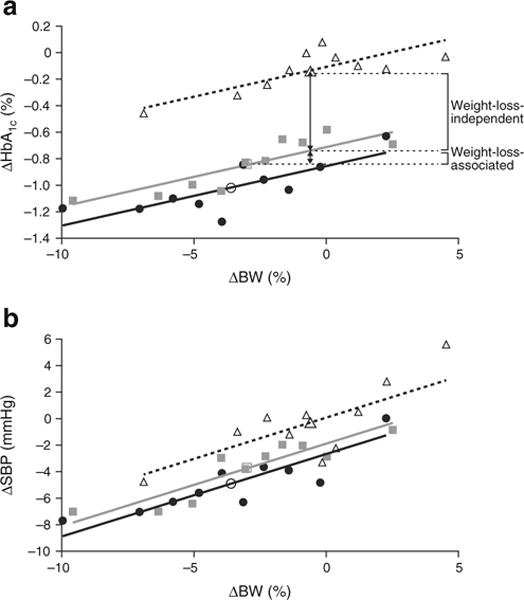
Relationship between changes in body weight and (a) HbA1c and (b) SBP at week 26. Larger open symbols represent the mean change for the entire treatment group, and smaller symbols represent mean changes within each decile of weight change; white triangles, placebo; grey squares, canagliflozin 100 mg; black circles, canagliflozin 300 mg. To convert values for change in HbA1c in % into mmol/mol, multiply by 10.929. BW, body weight
Effect of body-weight change on HbA1c and SBP
Patients with greater weight loss had greater reductions in HbA1c and SBP; slopes for the effect of weight loss on HbA1c and SBP were similar across groups (Fig. 2). Each 1% reduction in body weight was associated with a 0.045% (0.5 mmol/mol) reduction in HbA1c and a 0.62 mmHg reduction in SBP. The weight-loss-independent and weight-loss-associated effects of canagliflozin were evaluated using the ANCOVA models, as illustrated in Fig. 2. Approximately 85% of the placebo-subtracted reductions in HbA1c with canagliflozin were weight-loss-independent, and 15% were weight-loss-associated (Fig. 2). Approximately 58% of the placebo-subtracted reductions in SBP with canagliflozin were weight-loss-independent, with 42% being weight-loss-associated.
Discussion
Most AHA therapies are considered to be either weight-loss neutral (e.g. biguanides, dipeptidyl peptidase-4 inhibitors, α-glucosidase inhibitors, bile acid sequestrants, bromocriptine) or to potentially increase body weight (e.g. sulfonylureas, insulin, glinides, pioglitazone) [1]. Thus, prior to the introduction of SGLT2 inhibitors, glucagon-like peptide-1 receptor agonists were the only class consistently associated with body-weight reduction in patients with type 2 diabetes [1]; however, patient-to-patient variability in weight loss has been observed [1, 13]. In this pooled analysis, we demonstrate that canagliflozin treatment provided dose-dependent reductions in body weight in patients with type 2 diabetes. Of note, patients in the highest weight-loss quartile had weight loss of ~7.4–8.0%, demonstrating some variability in canagliflozin-associated weight loss. In addition, consistent HbA1c reductions were observed, whereas SBP changes were more variable.
HbA1c and SBP reductions have previously been associated with weight loss in patients with type 2 diabetes [14]. In this analysis, greater HbA1c and SBP reductions with canagliflozin were seen in patients with greater body-weight reductions over 26 weeks and occurred through both weight-loss-associated and weight-loss-independent mechanisms. Most of the HbA1c-lowering effect of canagliflozin (~85%) was independent of weight loss, and is likely attributable to increased UGE. Weight loss contributed ~40% to the overall reduction in SBP seen with canagliflozin. The estimated effects of weight loss on HbA1c and SBP in this analysis are slightly lower than corresponding estimates obtained following intensive diet/exercise modification for 1 year in the Look AHEAD study (estimated mean reductions of 0.074% [0.8 mmol/mol] in HbA1c and 0.79 mmHg in SBP per each 1% reduction in body weight [7]). Differences in estimated effects of weight loss on HbA1c and SBP may be attributable to different study durations; a comparable analysis was not performed for canagliflozin at 52 weeks because the placebo groups in most of the canagliflozin studies were switched to an active comparator for the 26 week extension. In addition, these differences may also be related to potential study effects to reduce HbA1c and SBP independent of weight loss in the Look AHEAD study.
The exact mechanism of weight-loss-independent BP reduction is not completely understood, but may partially be related to the mild osmotic diuresis or alterations in sodium reabsorption. If osmotic diuresis was considered as a major mechanism, it might be expected that the BP-lowering effect would be less in patients with renal impairment. Interestingly, we have observed that in patients with stage 3 chronic kidney disease, canagliflozin was associated with less weight loss and HbA1c lowering, but similar SBP lowering. However, the contribution of weight loss to HbA1c and SBP reductions was similar to the present analysis (unpublished data).
In summary, we have provided novel observations regarding the clinical effect of SGLT2 inhibition. As previously reported, canagliflozin treatment was associated with reductions in body weight, HbA1c and SBP. However, in this report, we provide data that show reductions in HbA1c and SBP with canagliflozin occurred by both weight-loss-independent and weight-loss-associated mechanisms, with a greater proportion of the reduction in HbA1c, relative to SBP, determined to be independent of weight loss.
Supplementary Material
Acknowledgments
The authors thank all investigators, study teams and patients for participating in these studies. The authors acknowledge C. Tong of Janssen Research & Development, LLC for her contribution to the data analyses. WTC is supported in part by 1 U54 GM104940 from the National Institute of General Medical Sciences of the National Institutes of Health. Editorial support was provided by K. McClendon of MedErgy, and was funded by Janssen Global Services, LLC. Canagliflozin has been developed by Janssen Research & Development, LLC, in collaboration with Mitsubishi Tanabe Pharma Corporation.
This study was previously presented, in part, in abstract form at the 49th Annual Meeting of the European Association for the Study of Diabetes, Barcelona, Spain, 23–27 September 2013; and the 73rd Scientific Sessions of the American Diabetes Association, Chicago, IL, USA, 21–25 June 2013.
Funding The studies described in this manuscript were sponsored by Janssen Research & Development, LLC.
Abbreviations
- AHA
Anti-hyperglycaemic agent
- CVD
Cardiovascular disease
- SBP
Systolic BP
- SGLT2
Sodium glucose co-transporter 2
- UGE
Urinary glucose excretion
Footnotes
Electronic supplementary material The online version of this article (doi:10.1007/s00125-015-3547-2) contains peer-reviewed but unedited supplementary material, which is available to authorised users.
Duality of interest WTC has served as principal investigator on clinical research grants received by his institutions from AstraZeneca, Janssen, MannKind Corporation, GlaxoSmithKline, Sanofi and Lexicon Pharmaceuticals; served as a consultant for Intarcia Therapeutics, Inc; and served as a consultant to Sanofi through an institutional agreement. KS has no proprietary interest in the tested product, does not have a significant equity interest in the sponsor of the covered study and has not received significant payments of other sorts from the sponsor. LAL has received research funding from, has provided continuing medical education on behalf of, or has served as a consultant to AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Lilly, GlaxoSmithKline, Janssen, Merck, Novo Nordisk, Sanofi, Servier and Takeda. JPHW has served as a consultant for Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Janssen and Novo Nordisk; served as a speaker for AstraZeneca, Bristol-Myers Squibb, Janssen, Lilly and Novo Nordisk; and received research support from AstraZeneca, Lilly, Novo Nordisk and Merck Sharp & Dohme. LB has served as an investigator for Lilly, Novo Nordisk and Sanofi; served as a speaker for Novo Nordisk, Sanofi, Merck, Janssen and AstraZeneca; and served as a consultant for Novo Nordisk, Sanofi, Merck, Janssen, Quest Diagnostics, AstraZeneca and GlaxoSmithKline. DP, JX, DS, KU, WC and GM are current or former full-time employees of Janssen Research & Development, LLC.
Contribution statement WTC, KS, LAL, JPHW and LB contributed to the conduct of the study and the acquisition, analysis and interpretation of data, and drafted, reviewed and approved the manuscript. DP, KU, WC and GM contributed to the design and conduct of the study and the acquisition, analysis and interpretation of data, and drafted, reviewed and approved the manuscript. JX and DS contributed to the analysis and interpretation of data, and drafted, reviewed and approved the manuscript. All authors approved the final version of the manuscript. WTC and GM are the guarantors of this work.
Contributor Information
William T. Cefalu, Email: william.cefalu@pbrc.edu, Pennington Biomedical Research Center, Louisiana State University, 6400 Perkins Road, Baton Rouge, LA 70808-4124, USA.
Kaj Stenlöf, Clinical Trial Center, Sahlgrenska University Hospital, Gothenburg, Sweden.
Lawrence A. Leiter, Li Ka Shing Knowledge Institute and Keenan Research Centre for Biomedical Science, St Michael’s Hospital, Division of Endocrinology and Metabolism, University of Toronto, Toronto, ON, Canada
John P. H. Wilding, Department of Obesity and Endocrinology, University of Liverpool, Liverpool, UK
Lawrence Blonde, Department of Endocrinology, Diabetes, and Metabolic Diseases, Ochsner Medical Center, New Orleans, LA, USA.
David Polidori, Janssen Research & Development, LLC, San Diego, CA, USA.
John Xie, Janssen Research & Development, LLC, Raritan, NJ, USA.
Daniel Sullivan, Janssen Research & Development, LLC, Raritan, NJ, USA.
Keith Usiskin, Janssen Research & Development, LLC, Raritan, NJ, USA.
William Canovatchel, Janssen Research & Development, LLC, Raritan, NJ, USA.
Gary Meininger, Janssen Research & Development, LLC, Raritan, NJ, USA.
References
- 1.Inzucchi SE, Bergenstal RM, Buse JB, et al. Management of hyperglycemia in type 2 diabetes: a patient-centered approach. Position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD) Diabetes Care. 2012;35:1364–1379. doi: 10.2337/dc12-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.American Medical Association House of Delegates. Resolution 420 (A-13) Recognition of obesity as a disease. 2013 www.ama-assn.org/assets/meeting/2013a/a13-addendum-refcomm-d.pdf. Accessed 22 July 2013.
- 3.Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129:S102–S138. doi: 10.1161/01.cir.0000437739.71477.ee. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Noria SF, Grantcharov T. Biological effects of bariatric surgery on obesity-related comorbidities. Can J Surg. 2013;56:47–57. doi: 10.1503/cjs.036111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.The Diabetes Prevention Program Research Group. Long-term safety, tolerability, and weight loss associated with metformin in the Diabetes Prevention Program Outcomes Study. Diabetes Care. 2012;35:731–737. doi: 10.2337/dc11-1299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pi-Sunyer X, Blackburn G, Brancati FL, et al. Reduction in weight and cardiovascular disease risk factors in individuals with type 2 diabetes: one-year results of the look AHEAD trial. Diabetes Care. 2007;30:1374–1383. doi: 10.2337/dc07-0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wing RR, Bolin P, Brancati FL, et al. Cardiovascular effects of intensive lifestyle intervention in type 2 diabetes. N Engl J Med. 2013;369:145–154. doi: 10.1056/NEJMoa1212914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stenlöf K, Cefalu WT, Kim K-A, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lavalle-González FJ, Januszewicz A, Davidson J, et al. Efficacy and safety of canagliflozin compared with placebo and sitagliptin in patients with type 2 diabetes on background metformin monotherapy: a randomised trial. Diabetologia. 2013;56:2582–2592. doi: 10.1007/s00125-013-3039-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wilding JP, Charpentier G, Hollander P, et al. Efficacy and safety of canagliflozin in patients with type 2 diabetes mellitus inadequately controlled with metformin and sulphonylurea: a randomised trial. Int J Clin Pract. 2013;67:1267–1282. doi: 10.1111/ijcp.12322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forst T, Guthrie R, Goldenberg R, et al. Efficacy and safety of canagliflozin over 52 weeks in patients with type 2 diabetes on background metformin and pioglitazone. Diabetes Obes Metab. 2014;16:467–477. doi: 10.1111/dom.12273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Monami M, Dicembrini I, Marchionni N, Rotella CM, Mannucci E. Effects of glucagon-like peptide-1 receptor agonists on body weight: a meta-analysis. Exp Diabetes Res. 2012;2012:672658. doi: 10.1155/2012/672658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.de las Cruces Souto-Gallardo M, Bacardi Gascon M, Jimenez Cruz A. Effect of weight loss on metabolic control in people with type 2 diabetes mellitus: systematic review. Nutr Hosp. 2011;26:1242–1249. doi: 10.1590/S0212-16112011000600008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


