Abstract
Heart failure affects approximately 5.7 million people in the United States alone. Angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, beta-blockers, and aldosterone antagonists have improved mortality in patients with heart failure and reduced ejection fraction, but mortality remains high. In July 2015, the FDA approved the first of a new class of drugs for the treatment of heart failure; valsartan/sacubitril (formerly known as LCZ696 and currently marketed by Novartis as Entresto) combines the angiotensin receptor blocker valsartan and the neprilysin inhibitor prodrug sacubitril in a 1:1 ratio in a sodium supramolecular complex. Sacubitril is converted by esterases to LBQ657, which inhibits neprilysin, the enzyme responsible for the degradation of the natriuretic peptides and many other vasoactive peptides. Thus, this combined angiotensin receptor antagonist and neprilysin inhibitor addresses two of the pathophysiologic mechanisms of heart failure - activation of the renin-angiotensin-aldosterone system and decreased sensitivity to natriuretic peptides. In the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial, valsartan/sacubitril significantly reduced mortality and hospitalization for heart failure, as well as blood pressure, compared to enalapril in patients with heart failure, reduced ejection fraction, and an elevated circulating level of brain natriuretic peptide or N-terminal pro-brain natriuretic peptide. Ongoing clinical trials are evaluating the role of valsartan/sacubitril in the treatment of heart failure with preserved ejection fraction and hypertension. We review here the mechanisms of action of valsartan/sacubitril, the pharmacologic properties of the drug, and its efficacy and safety in the treatment of heart failure and hypertension.
Keywords: natriuretic peptide, heart failure, drug, pharmacology
Heart failure affects approximately 5.7 million people in the United States, and the number is projected to grow to more than 8 million by the year 2030.1 Several compensatory mechanisms occur in patients with heart failure, including ventricular remodeling and neurohormonal activation. Over time, however, these compensatory mechanisms become maladaptive and lead to worsening heart failure. Angiotensin-converting enzyme (ACE) inhibitors, angiotensin AT1 receptor blockers (ARBs), beta-blockers, and aldosterone antagonists, designed to target these maladaptive compensatory changes, have all demonstrated a mortality benefit in patients with heart failure with reduced ejection fraction (HFrEF).2 Despite these many treatment options, mortality from heart failure remains high, and approximately 50% of individuals with heart failure die within five years of diagnosis.1 Recently, increasing the beneficial effects of natriuretic peptides in heart failure has gained considerable interest as a therapeutic approach for the treatment of heart failure. On July 7th 2015, the FDA approved valsartan/sacubitril, a novel complex of the ARB valsartan with an inhibitor of neprilysin (NEP, neutral endopeptidase-24.11), the enzyme responsible for the degradation of natriuretic peptides and many other vasoactive peptides (Figure 1). In this review, we will summarize the evidence for valsartan/sacubitril in the treatment of HFrEF and highlight the potential for its use in heart failure with preserved ejection fraction (HFpEF) and hypertension.
Figure 1.
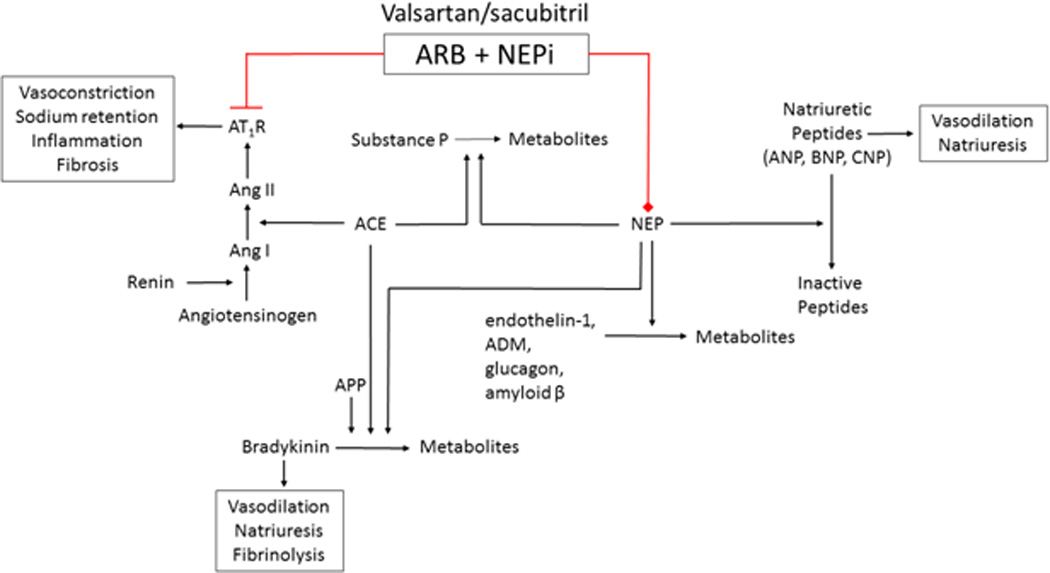
Effects of valsartan/sacubitril through inhibition of neprilysin (NEP) and blockade of the renin-angiotensin-aldosterone system. Red represents antagonism/inhibition. Ang I indicates angiotensin I; Ang II, angiotensin II; AT1R, angiotensin type 1 receptor; ARB, angiotensin receptor blocker; ACE, angiotensin-converting enzyme; APP, aminopeptidase P; ADM, adrenomedullin; NEPi, neprilysin inhibitor; ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; and CNP, C-type natriuretic peptide.
Mechanisms of Action of Valsartan/sacubitril
Valsartan/sacubitril is the first of a new class of drugs that has been referred to as angiotensin receptor-neprilysin inhibitors (ARNi).3 The class name is a misnomer, as ARBs are not enzyme inhibitors, and a more accurate name for the class would be ARANIs, angiotensin receptor antagonist/neprilysin inhibitors. The combined angiotensin receptor antagonist/neprilysin inhibitor was developed to address two pathophysiological mechanisms underlying heart failure – activation of the renin-angiotensin aldosterone system (RAAS) and decreased sensitivity to natriuretic peptides.
The mechanisms of action of ARBs such as valsartan have been studied extensively, are reviewed elsewhere, and will not be addressed in detail here.4 In brief, ARBs decrease vasoconstriction and aldosterone production, and increase natriuresis by blocking vascular and adrenal AT1 receptors. ARBs also decrease cardiac, vascular, and renal injury by decreasing AT1-, mineralocorticoid receptor (MR)-, or aldosterone- mediated hypertrophy, inflammation, and fibrosis. During angiotensin receptor blockade, angiotensin II (Ang II) concentrations are increased due to lack of feedback inhibition of renin production, and Ang II may exert favorable or unfavorable effects through non-AT1 receptors. With chronic administration of ARBs, as with chronic administration of ACE inhibitors, circulating aldosterone concentrations “escape” back to baseline levels, providing the rationale for the concurrent use of mineralocorticoid receptor antagonists.
Beneficial effects of NEP inhibition have been attributed to decreased degradation of the natriuretic peptides. Natriuretic peptides cause vasodilation by stimulating particulate guanylate cyclase to produce cGMP.5 In heart failure, administration of valsartan/sacubitril decreased N-terminal pro-brain natriuretic peptide (NT-proBNP), but increased brain natriuretic peptide (BNP), providing indirect evidence that BNP degradation was decreased.6 In addition, in a dose escalation study in healthy volunteers, there was a significant increase in cGMP at all doses of valsartan/sacubitril compared to baseline.3 Natriuretic peptides are cleared from circulation by binding to the natriuretic peptide receptor C (NPR-C) and degraded by neprilysin. Neprilysin has a higher affinity for ANP and CNP than for BNP7 (see Supplemental Table).
In the kidney, in addition to increasing renal blood flow and glomerular filtration, natriuretic peptides inhibit sodium reabsorption in the proximal and distal nephron. In addition, natriuretic peptides act to suppress the renin-angiotensin and sympathetic systems and decrease secretion of endothelin. The natriuretic peptides also exert anti-inflammatory, anti-fibrotic, and anti-hypertrophic effects in vitro in cardiomyocytes or cardiac fibroblasts.8–13 In cultured adipocytes, atrial natriuretic peptide (ANP) and BNP promote lipolysis and increase the synthesis and secretion of adiponectin.14, 15
As heart failure progresses, responsiveness to natriuretic peptides, in particular ANP and BNP, decreases.16, 17 Resistance to natriuretic peptides can result from down-regulation of natriuretic peptide receptors, increased clearance of BNP by NEP or the NPR-C receptor, or decreased downstream signaling.18 Expression of phosphodiesterase 5 (PDE5), which degrades cGMP, is also increased in experimental heart failure and PDE5 inhibition restores sensitivity to exogenous BNP.17 In addition, decreased processing of proBNP to active BNP 1–32 may contribute to diminished natriuresis and vasodilation in heart failure patients.19 Decreased degradation of natriuretic peptides with valsartan/sacubitril could overcome natriuretic resistance resulting from any one of these mechanisms.
Pharmacological strategies to enhance the actions of natriuretic peptides in humans have included exogenous administration of endogenous peptides or degradation-resistant peptides,20, 21 as well as the development of NEP inhibitors. Nesiritide or recombinant BNP was approved for the treatment of acutely decompensated heart failure in the United States in 2001. Concerns about hypotension, a lack of mortality benefit,22 its short half-life, and the requirement for intravenous administration have limited its use. NEP inhibitors thus offered an attractive alternative approach to increasing natriuretic peptides and, in humans, administration of a NEP inhibitor potentiates the natriuretic effect of ANP without altering vascular resistance.23
Early studies of neprilysin inhibition in the treatment of heart failure and hypertension provided disappointing results. For example, a dose-ranging study of ecadotril did not show benefit in heart failure, and there was a numeric increase in deaths in patients receiving the NEP inhibitor.24 Candoxatril, the first orally bioavailable neprilysin inhibitor, increased blood pressure as well as endothelin concentrations, in healthy volunteers.25 In addition to degrading vasodilator peptides, neprilysin catalyzes the conversion of Ang I to Ang (1–7) and degrades endothelin (Figure 1 and Supplemental Table).26, 27 The observation that candoxatril potentiates the pressor response to Ang II,28, 29 also suggested that increased Ang II offset increases in vasodilator peptides during NEP inhibition, and provided the impetus for combining drugs with activity against the RAAS with neprilysin inhibitors. An increased incidence of the side effect of angioedema during combined ACE and NEP inhibition (so called vasopeptidase inhibition),30 presumably due to decreased degradation bradykinin and substance P, led in turn to the development of valsartan/sacubitril.
In addition to cleaving the natriuretic peptides, neprilysin cleaves peptides such as bradykinin, substance P, vasoactive intestinal peptide (VIP), glucagon, neurotensin, adrenomedullin, and amyloid β peptide (Figure 1 and Supplemental Table). Bradykinin contributes to many beneficial effects of ACE inhibitors, including blood pressure reduction and endothelial fibrinolytic function, as well as to adverse events.31–33 The contribution of bradykinin to the effects of combined AT1 receptor blockade/NEP inhibition has not been studied. In studies in small resistance arteries from patients with coronary artery disease and preserved ejection fraction, the ACE inhibitor captopril, NEP inhibitor thiorphan, and the combined ACE/NEP inhibitor omapatrilat all significantly potentiated the vasodilator response to bradykinin.34 In the same model, none of the inhibitors potentiated the effects of C-type natriuretic peptide (CNP), substance P, or VIP. Omapatrilat potentiated the vasodilator response to adrenomedullin, whereas neither captopril nor thiorphan alone did.34 Adrenomedullin is a 52 amino acid peptide that was originally identified from a pheochromocytoma but is expressed by other tissues, particularly vascular endothelium, and present in the circulation. Infusion of adrenomedullin in the renal arteries of dogs causes increased renal blood flow, natriuresis, and diuresis.35 Neprilysin inhibition potentiates adrenomedullin induced natriuresis and diuresis in this model. In a heart failure model in sheep, neprilysin inhibition potentiated the vasodilator response to intravenous adrenomedullin while preserving natriuresis and diuresis.36
Pharmacokinetics of Valsartan/sacubitril
Valsartan/sacubitril is composed of the diprotic ARB valsartan and the neprilysin inhibitor prodrug sacubitril [chemical formula (2R,4S)-5-biphenyl-4-yl-4-(3-carboxy-propionylamino)-2-methyl-pentanoic acid ethyl ester] in a 1:1 ratio (Figure 2). It is synthesized by dissolution of the two drugs followed by the addition of aqueous sodium hydroxide solution to induce crystallization.37, 38 Crystal valsartan/sacubitril contains six sacubitril and six valsartan moieties in their anionic forms, 18 penta- and hexa- coordinated sodium cations, and 15 water molecules, and has been described as a sodium supramolecular complex.38 After ingestion, valsartan/sacubitril dissociates to valsartan and sacubitril, which is further converted by esterases to the active inhibitor of neprilysin LBQ657.
Figure 2.
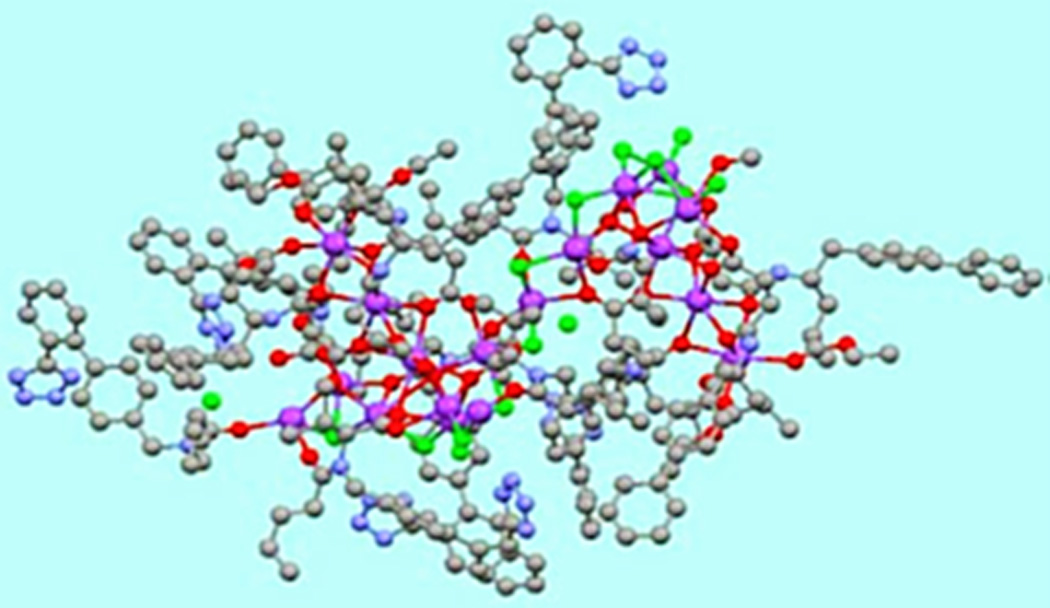
Structure of valsartan/sacubitril using a ball-and-stick model. Carbon atoms are represented in grey; sodium, purple; carboxylate and carbonyl oxygen, red; and water oxygen, green. Hydrogen atoms are not shown. Reproduced with permission from the publisher. Copyright © 2010, the American College of Clinical Pharmacology.
In multiple dosing studies in healthy volunteers, plasma concentrations of valsartan peak 1.6–4.9 hours, sacubitril 0.6–0.9 hours, and LBQ657 1.8 – 2.7 hours after dosing.3 Valsartan/sacubitril can be administered with or without food. Administration of valsartan as valsartan/sacubitril increases its bioavailability ~40% in humans. For example, the Cmax and area-under-the-concentration curve (AUC) of valsartan after a single dose of 400 mg of valsartan/sacubitril (194 mg of sacubitril and 206 mg of valsartan) is equivalent to that achieved after a dose of 320 mg of valsartan alone.3, 39 Similarly, the valsartan doses given in valsartan/sacubitril 100 mg (51 mg valsartan) and valsartan/sacubitril 200 mg (103 mg valsartan) are bioequivalent to 80 mg and 160 mg valsartan given alone.
After sacubitril is converted to LBQ657, it is not further metabolized; nor is valsartan. Following oral administration, 52% to 68% of sacubitril is excreted in the urine and 37–48% is excreted in the feces, in both cases primarily as LBQ657. Eighty-six percent of valsartan and its metabolites are excreted in feces. Valsartan, sacubitril, and LBQ657 have mean elimination half-lives of 9.9, 1.4, and 11.5 hours, respectively. The apparent volume of distribution of valsartan is 75 L, compared to 103 L for sacubitril. Approximately 94–97% of valsartan, sacubitril, and LBQ657 are bound to plasma proteins.
The pharmacokinetics of valsartan/sacubitril following a single dose of 400 mg are similar in males and females.40 The Cmax and AUC of valsartan in valsartan/sacubitril are increased 24% and 30%, respectively, in subjects 65 years of age or older compared to subjects 18–45 years old.40 The Cmax of LBQ657 is similar in the two age groups, whereas the AUC of LBQ657 is 42% greater in subjects 65 years of age or older compared to 18–45 year-old subjects. The Cmax and AUC of LBQ657 are increased in patients with renal insufficiency, and a lower starting dose of valsartan/sacubitril 50 mg (24/26 mg) is recommended in patients with severe renal impairment (estimated glomerular filtration rate less than 30 mL/min/1.73 m2).39 In moderate hepatic impairment (Child-Pugh Class B), the AUCs of valsartan and LBQ657 are increased and a low starting dose is advised.
Co-administration of valsartan/sacubitril with drugs that inhibit CYP450 does not significantly affect the pharmacokinetics of valsartan/sacubitril as neither of its components are metabolized by CYP450. In vitro studies reveal that sacubitril inhibits two members of the family of cellular uptake transporters called organic anion transporting polypeptides (OATP), namely OATP1B1 and OATP1B3.39 These two transporters are expressed on the basolateral membrane of hepatocytes and are involved in the hepatic clearance of many drugs including furosemide and statins.41–43 Valsartan/sacubitril appears to reduce the Cmaxof furosemide by approximately 50% and the AUC by 25%, whereas the Cmaxand AUC of atorvastatin are significantly increased.39 Valsartan/sacubitril also reduces the Cmax and AUC of hydrochlorothiazide and metformin. Given the widespread use of these drugs in patients with cardiovascular disease, further studies investigating drug-drug interactions with valsartan/sacubitril are warranted. Although OATP1 contributes to the clearance of valsartan,44 inhibition of OATP1 by sacubitril does not likely contribute to its increased bioavailability when administered as valsartan/sacubitril as concurrent administration of valsartan and sacubitril, not in valsartan/sacubitril, does not have the same effect.
Efficacy
Heart Failure with Reduced Ejection Fraction
The Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) trial was a prospective, multi-center trial in which 8442 patients with a reduced ejection fraction and New York Heart Association (NYHA) class II-IV were randomized to treatment groups of either valsartan/sacubitril 200 mg twice daily or enalapril 10 mg twice daily.45–47 Patients were required to have ejection fraction of 40% or less (later reduced to 35% or less) and to be taking a stable dose of a beta-blocker and an ACE inhibitor (6532 patients) or an ARB (1892 patients) for at least four weeks prior to screening. Patients were required to have a plasma BNP level of at least 150 pg/mL or an NT-proBNP concentration of at least 600 pg/mL. Patients who had been hospitalized previously for heart failure could have a BNP of at least 100 pg/mL or a NT-proBNP level of at least 400 pg/mL. The study included a single-blind run-in period in which each patient received enalapril for at least two weeks (median 15 days) and then valsartan/sacubitril for four to six weeks (median 29 days) to assess tolerability. The mean age of patients was 64 years old and 87% were male. The majority of patients were white (66%) or Asian (18%) and a small proportion were black (~5%). At randomization 82% of patients were taking a diuretic, 94% a beta-blocker, and 58% a mineralocorticoid receptor antagonist.
After a median follow up of 27 months, 21.8% of patients in the valsartan/sacubitril group experienced the primary composite outcome of either death from cardiovascular causes or hospitalization for heart failure, compared to 26.5% in the enalapril group, a relative risk reduction of 20% (p<0.001) (Figure 3).45 There was a significant interaction between NYHA class at randomization and the effect of treatment on this endpoint (p=0.03). Treatment with valsartan/sacubitril was associated with a 21% reduction in risk for heart failure admission (p<0.001) and an improved Kansas City Cardiomyopathy Questionnaire (KCCQ) symptom score compared to treatment with enalapril (p=0.001). All-cause mortality was significantly lower in the valsartan/sacubitril group (17% in the valsartan/sacubitril group versus 19.8% in the enalapril group, p=0.0009). There was no difference between the two treatment groups in the incidence of new onset atrial fibrillation or in decline in renal function.
Figure 3.
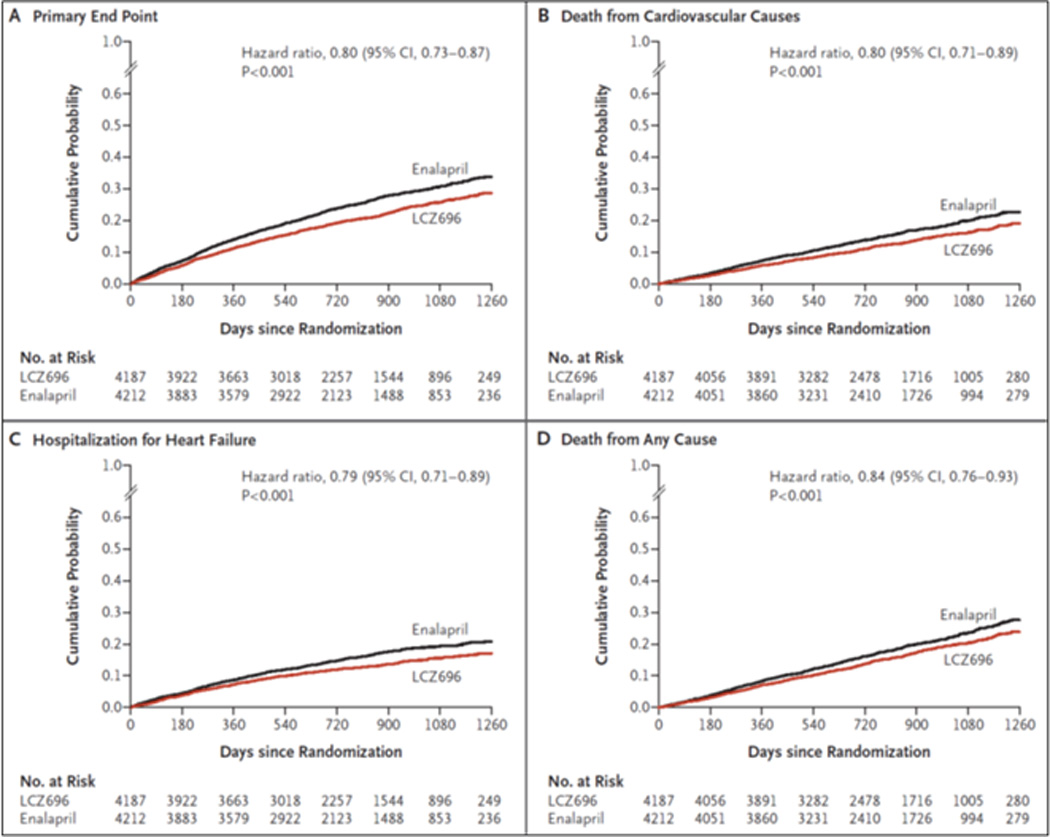
Kaplan-Meier curves from the PARADIGM-HF trial for the primary composite endpoint of cardiovascular death or first hospitalization for heart failure (A), death from cardiovascular causes (B), first hospitalization for heart failure (C), and death from any cause (D). LCZ696 is valsartan/sacubitril. Reproduced with permission from the publisher. Copyright © 2014, Massachusetts Medical Society. All rights reserved.
Mean systolic blood pressure was 3.2±0.4 mmHg lower in the valsartan/sacubitril group compared to the enalapril group (p<0.001), raising the possibility that the doses of valsartan/sacubitril and enalapril administered were not therapeutically equivalent. Inclusion of the between-group difference in blood pressure as a time-dependent covariate did not negate the benefit of valsartan/sacubitril, however. Of note, fewer than 1% of patients randomized had NYHA class IV heart failure, which limits inference of the mortality and morbidity benefit in this group. There was a higher rate of symptomatic hypotension in the valsartan/sacubitril group than in the enalapril group (14% versus 9.2%, p<0.001), whereas cough, elevated serum creatinine (2.5 mg/dL or greater), and hyperkalemia were significantly more frequent in the enalapril treatment group. Angioedema occurred in 19 patients in the valsartan/sacubitril group and 10 in the enalapril group during the double-blind treatment period.
Heart Failure with Preserved Ejection Fraction
Solomon et al. studied the effects of valsartan/sacubitril in patients with heart failure and preserved ejection fraction (HFpEF) in the Prospective comparison of ARni with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) trial. This trial randomized patients with NYHA class II-IV heart failure and an ejection fraction at least 45% and an NT-proBNP level greater than 400 pg/mL to receive either valsartan/sacubitril 200 mg bid or valsartan 160 mg bid. Randomization was preceded by a two-week, placebo run-in. The primary endpoint was change in NT-proBNP, which is not a substrate of neprilysin and therefore could serve as a biomarker of wall stress even in the presence of neprilysin inhibition. Increased NT-proBNP levels are associated with adverse outcomes in patients with chronic heart failure.48 Fifty-seven percent of randomized patients were women and the mean age was 71 years. Most patients were taking ACE inhibitors (54%) or ARBs (39%) prior to randomization.
NT-proBNP was significantly reduced after 12 weeks in the valsartan/sacubitril group compared to the valsartan group (ratio of change LCZ696/valsartan, 0.77 (95%CI 0.64–0.92), p=0.005).6 The difference in NT-proBNP between the two groups was not sustained at 36 weeks. Left atrial size at 36 weeks was significantly decreased in the valsartan/sacubitril group compared to the valsartan group (average left atrial volume decrease of 4.6 mL in the valsartan/sacubitril group versus an increase of 0.37 mL in the valsartan group, p=0.003). The decrease in left atrial size was postulated to reflect improved left ventricular filling pressures in patients receiving valsartan/sacubitril. It is important to note that blood pressure was reduced to a significantly greater degree in the valsartan/sacubitril group (−9.3±14/−4.9±10 mmHg versus −2.9±17/−2.1±11 mmHg in the valsartan group, p=0.001 for systolic and p=0.09 for diastolic pressure difference). The use of concomitant loop diuretics was increased in the valsartan group. There were no significant differences between treatment groups in other echocardiographic endpoints, in a clinical composite assessment, or in quality of life as measured by KCCQ. The incidence of adverse events was similar in the two groups. eGFR declined to a greater extent in the valsartan group, whereas urinary albumin/creatinine ratio increased to a greater extent in the valsartan/sacubitril group. There was one case of angioedema in the valsartan/sacubitril group and none in the valsartan group. Although this trial was not powered to evaluate for clinical outcomes, it set the stage for the Prospective comparison of ARni with Arb Global Outcomes in heart failure with preserved ejectioN fraction (PARAGON)-HF trial, a current study designed to evaluate the efficacy and safety of valsartan/sacubitril compared to valsartan in patients with HFpEF (NCT01920711, see Table 1 for a list of clinical trials involving valsartan/sacubitril).
Table 1.
Trials of combined angiotensin receptor antagonism/neprilysin inhibition with >100 patientsa.
| Trial Number | Title | Patient Population | Comparator | Results |
|---|---|---|---|---|
| NCT01035255 | Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure (PARADIGM-HF) | HFrEF (n=8442) | Enalapril | Published, Reference 45 |
| NCT00549770 | Efficacy and Safety of LCZ696A in Patients with Essential Hypertension | Essential Hypertension (n=1328) | Valsartan Sacubitril Placebo | Published, Reference 49 |
| NCT01193101 | Efficacy and Safety of LCZ696 Compared to Placebo in Patients with Essential Hypertension | Essential Hypertension (n=389) | Placebo | Published, Reference 50 |
| NCT00887588 | Prospective comparison of ARni with ARB on Management Of heart failUre with preserved ejectioN fracTion (PARAMOUNT) | HFpEF (n=301) | Valsartan | Published, Reference 6 |
| NCT01785472 | Efficacy and Safety of LCZ696 in Comparison to Olmesartan in Asian Patients with Essential Hypertension | Essential Hypertension (n=1438) | Olmesartan | Completed, no published results |
| NCT01599104 | Efficacy and Safety of LCZ696 in Comparison to Olmesartan in Japanese Patients with Essential Hypertension | Essential Hypertension (n=1161) | Olmesartan | Completed, no published results |
| NCT01281306 | An 8-week Study to Evaluate the Dose Response of AHU377 in Combination With Valsartan 320 mg in Patients With Mild-to-moderate Systolic Hypertension | Essential Hypertension (n=915) | Valsartan Sacubitril Placebo | Completed, no published results |
| NCT01615198 | Efficacy and Safety of LCZ696 in Comparison to Olmesartan in Elderly Patients with Essential Hypertension | Essential Hypertension (n=588) | Olmesartan | Completed, no published results |
| NCT01922089 | Safety and Tolerability of Initiating LCZ696 in Heart Failure Patients (TITRATION) | HFrEF (n=498) | NA | Completed, no published results |
| NCT01692301 | Study of the Safety and Efficacy of LCZ696 on Arterial Stiffness in Elderly Patients With Hypertension | Essential Hypertension (n=454) | Olmesartan | Completed, no published results |
| NCT01876368 | Efficacy and Safety of LCZ696 Compared to Olmesartan in Essential Hypertensive Patients Not Responsive to Olmesartan | Essential Hypertension (n=374) | Olmesartan | Completed, no published results |
| NCT01256411 | A Long-term (12 Months) Safety, Tolerability and Efficacy Study of LCZ696 in Patients With Essential Hypertension | Essential Hypertension (n=341) | NA | Completed, no published results |
| NCT01663233 | Efficacy and Safety of LCZ696 200 mg + Amlodipine 5 mg in Comparison With Amlodipine 5 mg in Hypertensive Patients Not Responding to Amlodipine | Essential Hypertension (n=266) | Amlodipine | Completed, no published results |
| NCT01920711 | Prospective comparison of ARni with Arb Global Outcomes in heart failure with preserved ejectioN fraction (PARAGON)-HF | HFpEF (n=4300)* | Valsartan | Currently Recruiting |
| NCT02226120 | Safety and Tolerability During Open-label Treatment With LCZ696 in Patients With CHF and Reduced Ejection Fraction | HFrEF (n=3714)* | NA | Currently Recruiting |
| NCT02468232 | Study of Efficacy and Safety of LCZ696 in Japanese Patients With Chronic Heart Failure and Reduced Ejection Fraction | HFrEF (n=220)* | Enalapril | Currently Recruiting |
| NCT01870739 | A Study to Evaluate the Effect of LCZ696 on Aortic Stiffness in Subjects With Hypertension | Essential Hypertension (n=140)* | Olmesartan | Currently Recruiting |
Trials registered with ClinicalTrials.gov.
HFrEF indicates heart failure with reduced ejection fraction; HFpEF, heart failure with preserved ejection fraction; ARNI, angiotensin receptor neprilysin inhibitor; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin receptor blocker; and NA, not applicable.
Estimated enrollment
Hypertension
Ruilope et al. examined the effect of eight-week treatment with valsartan/sacubitril in mild-to-moderate hypertension, defined by a mean sitting diastolic pressure of 90–109 mmHg after washout of antihypertensive medications or 95–109 mmHg in untreated patients. Eighty-seven percent of the patients studied were white and 57% were male. Patients underwent a two-week washout period, followed by two-week single-masked treatment with placebo, prior to randomization to one of eight treatment groups: placebo, sacubitril 200 mg, valsartan/sacubitril 100 mg, valsartan/sacubitril 200 mg, valsartan/sacubitril 400 mg, valsartan 80 mg, valsartan 160 mg, valsartan 320 mg.
The placebo-subtracted change in systolic and diastolic BP from baseline was similar following eight-week treatment of valsartan/sacubitril 100 mg and valsartan 80 mg.49 Valsartan/sacubitril 200 mg reduced SBP and DBP to a greater extent than the bioequivalent 160 mg valsartan (−11.00/−6.14 mmHg versus −5.69/−3.17 mmHg, p=0.0006 for SBP and p=0.0023 for DBP). Valsartan/sacubitril 400 mg reduced SBP and DBP significantly more than 320 mg valsartan (−12.50/−6.85 mmHg versus −6.44/−4.15 mmHg, p<0.0001 and p=0.0055 for SBP and DBP, respectively). A subset of patients also underwent ambulatory blood pressure monitoring. LCZ626 200 mg and 400 mg reduced ambulatory systolic blood pressure to a significantly greater extent than valsartan 160 mg and 320 mg, respectively. The 200 mg and 400 mg doses of valsartan/sacubitril reduced both placebo-subtracted sitting and ambulatory pulse pressure significantly more than valsartan 160 mg and 320 mg. In a trial of Asian patients with essential hypertension, Kairo et al. found similar reductions in SBP and DBP with valsartan/sacubitril 200 mg (−12.57/−7.29 mmHg, compared to placebo).50
Treatment with sacubitril 200 mg alone or the two higher doses of valsartan/sacubitril significantly increased ANP. Sacubitril and all doses of valsartan/sacubitril increased circulating concentrations of cGMP. Sacubitril alone did not affect plasma renin activity (PRA). Valsartan/sacubitril and bioequivalent doses of valsartan increased PRA similarly, moreover, suggesting that the effect of valsartan/sacubitril on PRA resulted solely from loss-of-feedback inhibition of renin production during angiotensin receptor blockade. Plasma aldosterone concentrations did not differ among active treatment groups or from placebo.
Safety and Adverse Effects
Treatment with the combined ACE and neprilysin inhibitor omapatrilat was associated with a three-fold increased risk of angioedema compared to ACE inhibitor alone.30 This has been attributed to concurrent inhibition of at least two pathways involved in the degradation of bradykinin and substance P, major effectors of angioedema. Bradykinin is inactivated primarily by ACE, but also by neprilysin and aminopeptidase P (APP), while substance P is inactivated by ACE, neprilysin and dipeptidyl peptidase IV. A polymorphism in the gene encoding for neprilysin has been also associated with ACE inhibitor-associated angioedema.51 Given that valsartan/sacubitril inhibits only neprilysin, the incidence of angioedema during valsartan/sacubitril may be expected to be lower than during vasopeptidase inhibition.
In the PARADIGM-HF trial, there were 19 confirmed cases of angioedema in patients treated with valsartan/sacubitril compared to 10 cases with enalapril (0.5% with valsartan/sacubitril versus 0.2% with enalapril, p=0.13). No patient in either group developed airway compromise or required endotracheal intubation. It should be noted, however, that 6532 of 8442 patients enrolled in the trial were previously treated with an ACE inhibitor and all patients were exposed to enalapril during the single-blind run-in period. The rate of angioedema during each run-in portion of the trial was 0.1%. Including a high proportion of patients who tolerated exposure to an ACE inhibitor or sacubitril, either prior to the study or during the run-in phase, may have introduced selection bias and led to an underrepresentation of angioedema in the trial, since more than 50% of cases of ACE inhibitor-associated angioedema occur within the first week of treatment initiation.52 In addition, the incidence of angioedema in the PARADIGM-HF trial may underestimate the incidence in the general population. Just 5% of patients in PARADIGM were African American, a group at increased risk of ACE-inhibitor and vasopeptidase-inhibitor associated angioedema.53, 54 Indeed, rates of angioedema were higher in African Americans treated with valsartan/sacubitril compared to non-African Americans.39 The use of valsartan/sacubitril in patients who were not previously taking ACE inhibitors may also lead to a higher rate of angioedema in clinical practice.
Rates of angioedema in patients taking valsartan/sacubitril may be increased by drug-drug interactions if patients inadvertently take an ACE inhibitor while taking valsartan/sacubitril. The current recommendation is to discontinue ACE inhibitor use at least 36 hours prior to starting valsartan/sacubitril; a longer washout period is desirable as ACE inhibitors have a prolonged duration of action.
Symptomatic hypotension was the most common adverse event reported with valsartan/sacubitril in the PARADIGM-HF and PARAMOUNT trials with a frequency of 18% and 19%, respectively. A similar rate of hypotension (19.5%) was found with omapatrilat in Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE), a trial designed to compare omapatrilat to enalapril in chronic heart failure.55 The higher rate of hypotension with valsartan/sacubitril compared to enalapril in the PARADIGM-HF trial did not lead to a significant difference in discontinuation of the drug (0.9% of valsartan/sacubitril patients discontinued the drug due to hypotension compared to 0.7% in the enalapril group, p=0.38). In a study assessing factors leading to the hypotensive effect of RAAS inhibition with captopril, Hodsman et al. found a greater blood pressure reduction in patients with renin-dependent states (i.e. low sodium intake and volume depletion).56 Therefore, it is recommended to correct volume and sodium depletion states before starting valsartan/sacubitril to minimize its hypotensive effects.
Like ACE inhibitors and ARBs, valsartan/sacubitril can cause hyperkalemia, cough, and worsening renal function. In the PARADIGM-HF trial, the frequency of hyperkalemia, defined as a serum potassium >5.5 mmol/L, was 16.1% in patients taking valsartan/sacubitril, which was not significantly different compared to enalapril. Concurrent use of potassium-sparing drugs increases the frequency of hyperkalemia. Valsartan/sacubitril had a significantly lower rate of cough than enalapril (11.3% with valsartan/sacubitril versus 14.3% with enalapril, p <0.001). Using data from the PARAMOUNT trial, Voors et al. found that patients treated with valsartan/sacubitril had a lower decline in eGFR compared to patients treated with enalapril at 36 weeks (−1.5 +/− 13.1 vs. −5.2 +/− 11.4 mL/min/1.73m2, p=0.008).57 There was a significant increase in the urine albumin to creatinine ratio (UACR) in the valsartan/sacubitril group from 2.4 at baseline to 2.9 mg/mmol at 36 weeks) while UACR in the enalapril group did not change significantly (2.1 at baseline to 2.0 mg/mmol at 36 weeks, p value between groups =0.016). Whether this reflects a marker disease progression or an effect of valsartan/sacubitril on vascular permeability remains unknown. It is not recommended to use an ACE inhibitor or ARB with valsartan/sacubitril due to the increased risk of adverse effects in trials of combined RAAS blockade. Valsartan/sacubitril does not affect the QT interval.
Drugs that inhibit the RAAS can cause harm to the fetus, and therefore valsartan/sacubitril should not be administered to pregnant women.
Neprilysin is involved in the clearance of amyloid-β (Aβ) from the brain and cerebrospinal fluid (CSF).39 Administration of valsartan/sacubitril 400 mg/d to healthy subjects for two weeks increased CSF Aβ1–38 compared to placebo, but CSF Aβ1–40 or CSF Aβ1–42 concentrations did not change. In young cynomolgus monkeys, two-week treatment with valsartan/sacubitril 50 mg/d increased CSF Aβ 1–40, 1–42, and 1–38 levels, but did not alter Aβ levels in the brain.39 In a 39-week toxicology study in cynomolgus monkeys, treatment with valsartan/sacubitril 300 mg/d did not result in amyloid-β accumulation in the brain.39
Cost
The wholesale price of valsartan/sacubitril is estimated to be $4500 per year, or $12.50 per day for twice daily dosing. By comparison, the cost of the enalapril dose used in the PARADIGM-HF trial is about $3.89 daily. Lisinopril is one of the least expensive ACE inhibitors on the market with a daily price of $1.56. Therefore, replacing a patient’s lisinopril with valsartan/sacubitril could potentially result in an 8-fold increase in drug costs. The Institute of Clinical and Economic Review (ICER) published a report evaluating the cost effectiveness of valsartan/sacubitril. Current estimates of cost savings due to a reduction of heart failure hospitalizations is $1,043 per patient after one year of treatment with valsartan/sacubitril, or approximately 25% of the annual drug cost.58 Assuming 390,000 patients receive valsartan/sacubitril in the first year, the estimated one-year budget impact is $1.4 billion.58
Conclusion
Valsartan/sacubitril, the first combined angiotensin receptor antagonist/neprilysin inhibitor, decreases mortality in patients with HFrEF. Ongoing clinical trials will determine the efficacy in other conditions, including hypertension and HFpEF. The coming years will bring additional information about this class of drugs. For example, is the beneficial effect of combined angiotensin receptor antagonism/neprilysin inhibition on mortality in HFrEF dependent on superior blood pressure and afterload reduction? Also, the long term renal effects of combined angiotensin receptor antagonism/neprilysin inhibition are yet to be determined. Is the clinical benefit of valsartan/sacubitril driven by decreased degradation of natriuretic peptides, or are there other mechanisms by which combined angiotensin receptor antagonism and neprilysin inhibition improve heart failure? As previously noted, neprilysin degrades a number of vasoactive peptides in addition to the natriuretic peptides, and the contribution of these peptides to the effects of valsartan/sacubitril remains to be studied. Related to this, what will be the incidence of angioedema among patients treated with valsartan/sacubitril in the real world setting? The answers to these questions are likely to be addressed in upcoming trials and will undoubtedly further our understanding of the pathophysiology of heart failure.
Supplementary Material
Acknowledgments
Funding Sources: This work was supported by National Institutes of Health grants T32-GM-108554 (Dr. Hubers) and R01-HL-125426 (Dr. Brown).
Disclosures: Dr. Brown served on an adjudication committee for angioedema for valsartan/sacubitril trials. In addition, Dr. Brown serves as a consultant to Novartis.
References
- 1.Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, Cushman M, de Ferranti S, Despres JP, Fullerton HJ, Howard VJ, Huffman MD, Judd SE, Kissela BM, Lackland DT, Lichtman JH, Lisabeth LD, Liu S, Mackey RH, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Muntner P, Mussolino ME, Nasir K, Neumar RW, Nichol G, Palaniappan L, Pandey DK, Reeves MJ, Rodriguez CJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Willey JZ, Woo D, Yeh RW, Turner MB. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation. 2015;131:e29–e322. doi: 10.1161/CIR.0000000000000152. [DOI] [PubMed] [Google Scholar]
- 2.Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;62:e147–e239. doi: 10.1016/j.jacc.2013.05.019. [DOI] [PubMed] [Google Scholar]
- 3.Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP. Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) J Clin Pharmacol. 2010;50:401–414. doi: 10.1177/0091270009343932. [DOI] [PubMed] [Google Scholar]
- 4.Burnier M. Angiotensin II type 1 receptor blockers. Circulation. 2001;103:904–912. doi: 10.1161/01.cir.103.6.904. [DOI] [PubMed] [Google Scholar]
- 5.Volpe M. Natriuretic peptides and cardio-renal disease. Int J Cardiol. 2014;176:630–639. doi: 10.1016/j.ijcard.2014.08.032. [DOI] [PubMed] [Google Scholar]
- 6.Solomon SD, Zile M, Pieske B, Voors A, Shah A, Kraigher-Krainer E, Shi V, Bransford T, Takeuchi M, Gong J, Lefkowitz M, Packer M, McMurray JJ. The angiotensin receptor neprilysin inhibitor LCZ696 in heart failure with preserved ejection fraction: a phase 2 double-blind randomised controlled trial. Lancet. 2012;380:1387–1395. doi: 10.1016/S0140-6736(12)61227-6. [DOI] [PubMed] [Google Scholar]
- 7.Kenny AJ, Bourne A, Ingram J. Hydrolysis of human and pig brain natriuretic peptides, urodilatin, C-type natriuretic peptide and some C-receptor ligands by endopeptidase-24.11. Biochem J. 1993;291(Pt 1):83–88. doi: 10.1042/bj2910083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hayashi D, Kudoh S, Shiojima I, Zou Y, Harada K, Shimoyama M, Imai Y, Monzen K, Yamazaki T, Yazaki Y, Nagai R, Komuro I. Atrial natriuretic peptide inhibits cardiomyocyte hypertrophy through mitogen-activated protein kinase phosphatase-1. Biochem Biophys Res Commun. 2004;322:310–319. doi: 10.1016/j.bbrc.2004.07.119. [DOI] [PubMed] [Google Scholar]
- 9.Fujita S, Shimojo N, Terasaki F, Otsuka K, Hosotani N, Kohda Y, Tanaka T, Nishioka T, Yoshida T, Hiroe M, Kitaura Y, Ishizaka N, Imanaka-Yoshida K. Atrial natriuretic peptide exerts protective action against angiotensin II-induced cardiac remodeling by attenuating inflammation via endothelin-1/endothelin receptor A cascade. Heart Vessels. 2013;28:646–657. doi: 10.1007/s00380-012-0311-0. [DOI] [PubMed] [Google Scholar]
- 10.Calderone A, Thaik CM, Takahashi N, Chang DL, Colucci WS. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 1998;101:812–818. doi: 10.1172/JCI119883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li P, Wang D, Lucas J, Oparil S, Xing D, Cao X, Novak L, Renfrow MB, Chen YF. Atrial natriuretic peptide inhibits transforming growth factor beta-induced Smad signaling and myofibroblast transformation in mouse cardiac fibroblasts. Circ Res. 2008;102:185–192. doi: 10.1161/CIRCRESAHA.107.157677. [DOI] [PubMed] [Google Scholar]
- 12.Kapoun AM, Liang F, O'Young G, Damm DL, Quon D, White RT, Munson K, Lam A, Schreiner GF, Protter AA. B-type natriuretic peptide exerts broad functional opposition to transforming growth factor-beta in primary human cardiac fibroblasts: fibrosis, myofibroblast conversion, proliferation, and inflammation. Circ Res. 2004;94:453–461. doi: 10.1161/01.RES.0000117070.86556.9F. [DOI] [PubMed] [Google Scholar]
- 13.Izumiya Y, Araki S, Usuku H, Rokutanda T, Hanatani S, Ogawa H. Chronic C-Type Natriuretic Peptide Infusion Attenuates Angiotensin II-Induced Myocardial Superoxide Production and Cardiac Remodeling. Int J Vasc Med. 2012;2012:246058. doi: 10.1155/2012/246058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moro C, Lafontan M. Natriuretic peptides and cGMP signaling control of energy homeostasis. Am J Physiol Heart Circ Physiol. 2013;304:H358–H368. doi: 10.1152/ajpheart.00704.2012. [DOI] [PubMed] [Google Scholar]
- 15.Sengenes C, Berlan M, De Glisezinski I, Lafontan M, Galitzky J. Natriuretic peptides: a new lipolytic pathway in human adipocytes. FASEB J. 2000;14:1345–1351. [PubMed] [Google Scholar]
- 16.Schmitt M, Gunaruwan P, Payne N, Taylor J, Lee L, Broadley AJ, Nightingale AK, Cockcroft JR, Struthers AD, Tyberg JV, Frenneaux MP. Effects of exogenous and endogenous natriuretic peptides on forearm vascular function in chronic heart failure. Arterioscler Thromb Vasc Biol. 2004;24:911–917. doi: 10.1161/01.ATV.zhq0504.7914. [DOI] [PubMed] [Google Scholar]
- 17.Forfia PR, Lee M, Tunin RS, Mahmud M, Champion HC, Kass DA. Acute phosphodiesterase 5 inhibition mimics hemodynamic effects of B-type natriuretic peptide and potentiates B-type natriuretic peptide effects in failing but not normal canine heart. J Am Coll Cardiol. 2007;49:1079–1088. doi: 10.1016/j.jacc.2006.08.066. [DOI] [PubMed] [Google Scholar]
- 18.Chen HH. Heart failure: a state of brain natriuretic peptide deficiency or resistance or both! J Am Coll Cardiol. 2007;49:1089–1091. doi: 10.1016/j.jacc.2006.12.013. [DOI] [PubMed] [Google Scholar]
- 19.Miller WL, Phelps MA, Wood CM, Schellenberger U, Van Le A, Perichon R, Jaffe AS. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ Heart Fail. 2011;4:355–360. doi: 10.1161/CIRCHEARTFAILURE.110.960260. [DOI] [PubMed] [Google Scholar]
- 20.Lee CY, Chen HH, Lisy O, Swan S, Cannon C, Lieu HD, Burnett JC., Jr Pharmacodynamics of a novel designer natriuretic peptide, CD-NP, in a first-in-human clinical trial in healthy subjects. J Clin Pharmacol. 2009;49:668–673. doi: 10.1177/0091270009336233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.McKie PM, Ichiki T, Burnett JC., Jr M-atrial natriuretic peptide: a novel antihypertensive protein therapy. Curr Hypertens Rep. 2012;14:62–69. doi: 10.1007/s11906-011-0244-5. [DOI] [PubMed] [Google Scholar]
- 22.O'Connor CM, Starling RC, Hernandez AF, Armstrong PW, Dickstein K, Hasselblad V, Heizer GM, Komajda M, Massie BM, McMurray JJ, Nieminen MS, Reist CJ, Rouleau JL, Swedberg K, Adams KF, Jr, Anker SD, Atar D, Battler A, Botero R, Bohidar NR, Butler J, Clausell N, Corbalan R, Costanzo MR, Dahlstrom U, Deckelbaum LI, Diaz R, Dunlap ME, Ezekowitz JA, Feldman D, Felker GM, Fonarow GC, Gennevois D, Gottlieb SS, Hill JA, Hollander JE, Howlett JG, Hudson MP, Kociol RD, Krum H, Laucevicius A, Levy WC, Mendez GF, Metra M, Mittal S, Oh BH, Pereira NL, Ponikowski P, Tang WH, Tanomsup S, Teerlink JR, Triposkiadis F, Troughton RW, Voors AA, Whellan DJ, Zannad F, Califf RM. Effect of nesiritide in patients with acute decompensated heart failure. N Engl J Med. 2011;365:32–43. doi: 10.1056/NEJMoa1100171. [DOI] [PubMed] [Google Scholar]
- 23.McDowell G, Nicholls DP. The endopeptidase inhibitor, candoxatril, and its therapeutic potential in the treatment of chronic cardiac failure in man. Expert Opin Investig Drugs. 1999;8:79–84. doi: 10.1517/13543784.8.1.79. [DOI] [PubMed] [Google Scholar]
- 24.Cleland JG, Swedberg K. Lack of efficacy of neutral endopeptidase inhibitor ecadotril in heart failure. The International Ecadotril Multi-centre Dose-ranging Study Investigators. Lancet. 1998;351:1657–1658. doi: 10.1016/s0140-6736(05)77712-6. [DOI] [PubMed] [Google Scholar]
- 25.Ando S, Rahman MA, Butler GC, Senn BL, Floras JS. Comparison of candoxatril and atrial natriuretic factor in healthy men. Effects on hemodynamics, sympathetic activity, heart rate variability, and endothelin. Hypertension. 1995;26:1160–1166. doi: 10.1161/01.hyp.26.6.1160. [DOI] [PubMed] [Google Scholar]
- 26.Ferro CJ, Spratt JC, Haynes WG, Webb DJ. Inhibition of neutral endopeptidase causes vasoconstriction of human resistance vessels in vivo. Circulation. 1998;97:2323–2330. doi: 10.1161/01.cir.97.23.2323. [DOI] [PubMed] [Google Scholar]
- 27.Stephenson SL, Kenny AJ. Metabolism of neuropeptides. Hydrolysis of the angiotensins, bradykinin, substance P and oxytocin by pig kidney microvillar membranes. Biochem J. 1987;241:237–247. doi: 10.1042/bj2410237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Richards AM, Wittert GA, Espiner EA, Yandle TG, Ikram H, Frampton C. Effect of inhibition of endopeptidase 24.11 on responses to angiotensin II in human volunteers. Circ Res. 1992;71:1501–1507. doi: 10.1161/01.res.71.6.1501. [DOI] [PubMed] [Google Scholar]
- 29.Richards AM, Wittert GA, Crozier IG, Espiner EA, Yandle TG, Ikram H, Frampton C. Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II. J Hypertens. 1993;11:407–416. doi: 10.1097/00004872-199304000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E. Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial. Am J Hypertens. 2004;17:103–111. doi: 10.1016/j.amjhyper.2003.09.014. [DOI] [PubMed] [Google Scholar]
- 31.Gainer JV, Morrow JD, Loveland A, King DJ, Brown NJ. Effect of bradykinin-receptor blockade on the response to angiotensin-converting-enzyme inhibitor in normotensive and hypertensive subjects. N Engl J Med. 1998;339:1285–1292. doi: 10.1056/NEJM199810293391804. [DOI] [PubMed] [Google Scholar]
- 32.Pretorius M, Rosenbaum D, Vaughan DE, Brown NJ. Angiotensin-converting enzyme inhibition increases human vascular tissue-type plasminogen activator release through endogenous bradykinin. Circulation. 2003;107:579–585. doi: 10.1161/01.cir.0000046268.59922.a4. [DOI] [PubMed] [Google Scholar]
- 33.Bas M, Hoffmann TK, Kojda G. Icatibant in ACE-inhibitor-induced angioedema. N Engl J Med. 2015;372:1867–1868. doi: 10.1056/NEJMc1503671. [DOI] [PubMed] [Google Scholar]
- 34.Dalzell JR, Seed A, Berry C, Whelan CJ, Petrie MC, Padmanabhan N, Clarke A, Biggerstaff F, Hillier C, McMurray JJ. Effects of neutral endopeptidase (neprilysin) inhibition on the response to other vasoactive peptides in small human resistance arteries: studies with thiorphan and omapatrilat. Cardiovasc Ther. 2014;32:13–18. doi: 10.1111/1755-5922.12053. [DOI] [PubMed] [Google Scholar]
- 35.Lisy O, Jougasaki M, Schirger JA, Chen HH, Barclay PT, Burnett JC., Jr Neutral endopeptidase inhibition potentiates the natriuretic actions of adrenomedullin. Am J Physiol. 1998;275:F410–F414. doi: 10.1152/ajprenal.1998.275.3.F410. [DOI] [PubMed] [Google Scholar]
- 36.Rademaker MT, Charles CJ, Cooper GJ, Coy DH, Espiner EA, Lewis LK, Nicholls MG, Richards AM. Combined endopeptidase inhibition and adrenomedullin in sheep with experimental heart failure. Hypertension. 2002;39:93–98. doi: 10.1161/hy0102.099197. [DOI] [PubMed] [Google Scholar]
- 37.Ksander GM, Ghai RD, deJesus R, Diefenbacher CG, Yuan A, Berry C, Sakane Y, Trapani A. Dicarboxylic acid dipeptide neutral endopeptidase inhibitors. J Med Chem. 1995;38:1689–1700. doi: 10.1021/jm00010a014. [DOI] [PubMed] [Google Scholar]
- 38.Feng L, Karpinski PH, Sutton P, Liu Y, Hook DF, Hu B, Blacklock TJ, Fanwick PE, Prashad M, Godtfredsen S, Ziltener C. LCZ696: a dual-acting sodium supramolecular complex. Tetrahedron Lett. 2012;53:275–276. [Google Scholar]
- 39.Entresto Prescribing Information. Drugs@FDA website. [Accessed August 21, 2015]; http://www.accessdata.fda.gov/drugsatfda_docs/label/2015/207620Orig1s000lbl.pdf. Updated July 7, 2015.
- 40.Gan L, Langenickel T, Petruck J, Kode K, Rajman I, Chandra P, Zhou W, Rebello S, Sunkara G. Effects of age and sex on the pharmacokinetics of LCZ696, an angiotensin receptor neprilysin inhibitor. J Clin Pharmacol. 2016;56:78–86. doi: 10.1002/jcph.571. Epub 2015 Aug 24. [DOI] [PubMed] [Google Scholar]
- 41.Abe T, Kakyo M, Tokui T, Nakagomi R, Nishio T, Nakai D, Nomura H, Unno M, Suzuki M, Naitoh T, Matsuno S, Yawo H. Identification of a novel gene family encoding human liver-specific organic anion transporter LST-1. J Biol Chem. 1999;274:17159–17163. doi: 10.1074/jbc.274.24.17159. [DOI] [PubMed] [Google Scholar]
- 42.Konig J, Cui Y, Nies AT, Keppler D. Localization and genomic organization of a new hepatocellular organic anion transporting polypeptide. J Biol Chem. 2000;275:23161–23168. doi: 10.1074/jbc.M001448200. [DOI] [PubMed] [Google Scholar]
- 43.Smith NF, Figg WD, Sparreboom A. Role of the liver-specific transporters OATP1B1 and OATP1B3 in governing drug elimination. Expert Opin Drug Metab Toxicol. 2005;1:429–445. doi: 10.1517/17425255.1.3.429. [DOI] [PubMed] [Google Scholar]
- 44.Maeda K, Ieiri I, Yasuda K, Fujino A, Fujiwara H, Otsubo K, Hirano M, Watanabe T, Kitamura Y, Kusuhara H, Sugiyama Y. Effects of organic anion transporting polypeptide 1B1 haplotype on pharmacokinetics of pravastatin, valsartan, and temocapril. Clin Pharmacol Ther. 2006;79:427–439. doi: 10.1016/j.clpt.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 45.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371:993–1004. doi: 10.1056/NEJMoa1409077. [DOI] [PubMed] [Google Scholar]
- 46.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau J, Shi VC, Solomon SD, Swedberg K, Zile MR. Dual angiotensin receptor and neprilysin inhibition as an alternative to angiotensin-converting enzyme inhibition in patients with chronic systolic heart failure: rationale for and design of the Prospective comparison of ARNI with ACEI to Determine Impact on Global Mortality and morbidity in Heart Failure trial (PARADIGM-HF) Eur J Heart Fail. 2013;15:1062–1073. doi: 10.1093/eurjhf/hft052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz M, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR. Baseline characteristics and treatment of patients in prospective comparison of ARNI with ACEI to determine impact on global mortality and morbidity in heart failure trial (PARADIGM-HF) Eur J Heart Fail. 2014;16:817–825. doi: 10.1002/ejhf.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Masson S, Latini R, Anand IS, Barlera S, Angelici L, Vago T, Tognoni G, Cohn JN. Prognostic value of changes in N-terminal pro-brain natriuretic peptide in Val-HeFT (Valsartan Heart Failure Trial) J Am Coll Cardiol. 2008;52:997–1003. doi: 10.1016/j.jacc.2008.04.069. [DOI] [PubMed] [Google Scholar]
- 49.Ruilope LM, Dukat A, Bohm M, Lacourciere Y, Gong J, Lefkowitz MP. Blood-pressure reduction with LCZ696, a novel dual-acting inhibitor of the angiotensin II receptor and neprilysin: a randomised, double-blind, placebo-controlled, active comparator study. Lancet. 2010;375:1255–1266. doi: 10.1016/S0140-6736(09)61966-8. [DOI] [PubMed] [Google Scholar]
- 50.Kario K, Sun N, Chiang FT, Supasyndh O, Baek SH, Inubushi-Molessa A, Zhang Y, Gotou H, Lefkowitz M, Zhang J. Efficacy and safety of LCZ696, a first-in-class angiotensin receptor neprilysin inhibitor, in Asian patients with hypertension: a randomized, double-blind, placebo-controlled study. Hypertension. 2014;63:698–705. doi: 10.1161/HYPERTENSIONAHA.113.02002. [DOI] [PubMed] [Google Scholar]
- 51.Pare G, Kubo M, Byrd JB, McCarty CA, Woodard-Grice A, Teo KK, Anand SS, Zuvich RL, Bradford Y, Ross S, Nakamura Y, Ritchie M, Brown NJ. Genetic variants associated with angiotensin-converting enzyme inhibitor-associated angioedema. Pharmacogenet Genomics. 2013;23:470–478. doi: 10.1097/FPC.0b013e328363c137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Sabroe RA, Black AK. Angiotensin-converting enzyme (ACE) inhibitors and angio-oedema. Br J Dermatol. 1997;136:153–158. [PubMed] [Google Scholar]
- 53.Brown NJ, Ray WA, Snowden M, Griffin MR. Black Americans have an increased rate of angiotensin converting enzyme inhibitor-associated angioedema. Clin Pharmacol Ther. 1996;60:8–13. doi: 10.1016/S0009-9236(96)90161-7. [DOI] [PubMed] [Google Scholar]
- 54.Kostis JB, Kim HJ, Rusnak J, Casale T, Kaplan A, Corren J, Levy E. Incidence and characteristics of angioedema associated with enalapril. Arch Intern Med. 2005;165:1637–1642. doi: 10.1001/archinte.165.14.1637. [DOI] [PubMed] [Google Scholar]
- 55.Packer M, Califf RM, Konstam MA, Krum H, McMurray JJ, Rouleau JL, Swedberg K. Comparison of omapatrilat and enalapril in patients with chronic heart failure: the Omapatrilat Versus Enalapril Randomized Trial of Utility in Reducing Events (OVERTURE) Circulation. 2002;106:920–926. doi: 10.1161/01.cir.0000029801.86489.50. [DOI] [PubMed] [Google Scholar]
- 56.Hodsman GP, Isles CG, Murray GD, Usherwood TP, Webb DJ, Robertson JI. Factors related to first dose hypotensive effect of captopril: prediction and treatment. Br Med J (Clin Res Ed) 1983;286:832–834. doi: 10.1136/bmj.286.6368.832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Voors AA, Gori M, Liu LC, Claggett B, Zile MR, Pieske B, McMurray JJ, Packer M, Shi V, Lefkowitz MP, Solomon SD. Renal effects of the angiotensin receptor neprilysin inhibitor LCZ696 in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2015;17:510–517. doi: 10.1002/ejhf.232. [DOI] [PubMed] [Google Scholar]
- 58.Ollendorf DA, Sandhu AT, Chapman R, Heidenreich PA, Russo E, Shore KK, Synnott P, Travers K, Weissberg J, Pearson SD. CardioMEMS™ HF System (St. Jude Medical) and Sacubitril/Valsartan (Entresto™, Novartis) for Management of Congestive Heart Failure: Effectiveness, Value, and Value-Based Price Benchmarks. California Technology Assessment Forum website. [Accessed November 14, 2015]; http://ctaf.org/sites/default/files/u148/CHF_Draft_Report_091115.pdf. Published September 11, 2015. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


