Abstract
Cardiovascular disease (CVD) and cancer are the two leading causes of death worldwide. Although commonly thought of as two separate disease entities, CVD and cancer possess various similarities and possible interactions, including a number of similar risk factors (e.g. obesity, diabetes), suggesting a shared biology for which there is emerging evidence. While chronic inflammation is an indispensible feature of the pathogenesis and progression of both CVD and cancer, additional mechanisms can be found at their intersection. Therapeutic advances, despite improving longevity, have increased the overlap between these diseases, but there are now millions of cancer survivors at risk of developing CVD. Cardiac risk factors have a major impact on subsequent treatment-related cardiotoxicity. In this review, we explore the risk factors common to both CVD and cancer, highlighting the major epidemiologic studies and potential biological mechanisms that account for them.
Keywords: cardio-oncology, cardiovascular disease risk factors
Introduction
Cardiovascular disease (CVD) and cancer are the largest contributors to the burden of chronic disease in the United States.1 With an estimated 15 and 14 million people with CVD (excluding hypertension) and a history of cancer, respectively, these numbers will undoubtedly rise as the population grows older and therapies enhance longevity.2,3 Emerging evidence suggests a relationship between CVD and cancer. A number of shared risk factors build the case for a shared biology. While inflammation appears to be a major unifying factor in the etiology and progression of these diseases, additional mechanisms have been described. Recent efforts in cardio-oncology have begun to revise the focus towards disease prevention and treatment, in terms of balancing the potential causal effects from one disease to the other.
Biological mechanisms common to CVD and cancer
Inflammation in CVD
Inflammation is a unifying theme amongst a variety of diseases, including both cardiovascular disease (CVD) and cancer.4,5 Common conditions such as obesity, hyperglycemia, hypertension, and hypertriglyceridemia induce inflammation6–9, and this may, in part, explain why CVD and cancer share several risk factors. Other sources of inflammation are widespread, including microbial and viral infections, allergen exposure, radiation, toxic chemicals, alcohol consumption, tobacco use, and other chronic and autoimmune diseases.10
Atherosclerosis was once viewed as a lipid storage disease, although it is now known that inflammation mediates all of its stages, from initiation to progression and, ultimately, thrombosis. Conditions such as hypertension, smoking, dyslipidemia and insulin resistance all appear to trigger atherosclerosis, in promoting the expression of adhesion molecules by endothelial cells, allowing leukocyte attachment to blood vessel walls that normally resist their attachment.5,11 Patients with elevated C-reactive protein (CRP), a biomarker of inflammation, have increased CVD events.12 Thus, immunotherapy for CVD reduction has become an area of intense interest. Statins, perhaps best-known for their cholesterol lowering effects, have been shown to also have anti-inflammatory benefits independent of cholesterol lowering (the use of CRP as a biomarker has validated this postulate).5 Additional clinical data with immunotherapy is anticipated, including the CANTOS study (ClinicalTrials.gov: CACZ885M2301), comparing Canakinumab, an inhibitor of interleukin-1β, a pro-inflammatory cytokine involved in atherosclerosis and the regulation of CRP, to placebo.
Inflammation in cancer
The role of inflammation in promoting carcinogenesis and tumor progression has been established. As early as the 19th century, Rudolf Virchow observed leukocytes within neoplastic tissue and speculated that cancer arises from inflammatory sites.13 In recent decades, extensive factual and circumstantial evidence has shown several cancer types to be induced by infection or chronic inflammatory disease (e.g. HPV and cervical cancer, H-pylori and stomach cancer, EBV and lymphoma).13 Inflammation within the tumor microenvironment has effects that promote malignant transformation of cells, carcinogenesis, and its progression.14 Furthermore, as tumors grow, their survival depends on the release of chemicals that signal immune cells to the tumor.
There may be an important role for the use of anti-inflammatory agents, such as statin therapy and non-steroidal anti-inflammatory drugs (NSAIDs), in the prevention and treatment of cancer, as discussed later.
Oxidative stress and reactive oxygen species
Accumulating evidence suggests that oxidative stress and its direct consequences, including lipid peroxidation, are involved in numerous pathological states including atherosclerosis, cancer, and inflammation.15 Biological systems are constantly exposed to oxidants, either from endogenous metabolic reactions or exogenous sources (e.g. toxins, smoking), and oxidative stress results from an imbalance of oxidant and antioxidant substances. Chronic inflammation, found in conditions common to both CVD and cancer (e.g. diabetes, hypertension, obesity), also induces oxidative stress.16
Other mechanistic hypotheses
Additional mechanisms may play a role in the shared biology between CVD and cancer. A number of hormones (e.g. leptin), cytokines, growth factors, and other metabolic reactions have been linked to both diseases, as discussed later.
Cardiovascular risk factors
Finding ways to predict and prevent CVD has become paramount to the field of cardiovascular medicine over previous decades. Risk assessment methods have become widely implemented. In clinical practice, risk models identify patients at risk for coronary heart disease (CHD) events, prompt clinician-patient discussions about lifestyle therapy, and justify the use of primary prevention medications. On a broader scale, the emphasis of CVD as an epidemic has increased public awareness and mobilized political interest in an effort to improve health.
The guidelines set forth by the American College of Cardiology and American Heart Association (AHA)17 utilize pooled cohort CVD risk equations to identify individuals at an increased absolute risk. An elevated 10-year risk of ≥ 7.5% in individuals aged 40–79 years requires blood cholesterol, weight and lifestyle management.
The AHA aims to reduce the incidence of cardiovascular-related deaths by 20% by the year 2020.18 The AHA endorses 7 metrics of ideal health that include a combination of health behaviors (not smoking, physical activity, low body mass index [BMI, defined as the weight in kilograms divided by the square of the height in meters], and healthy diet) and health factors (blood sugar, blood pressure, and total cholesterol). A strong inverse relationship exists between adherence to these health metrics and cardiovascular risk, as demonstrated by several studies including the National Health and Nutrition Examination Survey (NHANES),19 Cardiovascular Risk in Young Finns (YFS),20 and Atherosclerosis Risk in Communities (ARIC) studies.21
Cancer risk factors
Epidemiologic studies have identified many environmental and lifestyle risk factors for various types of cancer. In developed countries, lifestyle factors have been associated with many malignancies, including the four most common: lung, colorectal, breast, and prostate cancer. The World Health Organization (WHO) estimates that more than 30% of cancer deaths could be prevented by modifying or avoiding certain risk factors including tobacco use, obesity, unhealthy diets low in fruit and vegetable intake, inactivity, alcohol use, sexually transmitted HPV-infection, urban air pollution, and indoor smoke from solid fuels.22
Modifiable CVD risk factors and their cancer risk
Obesity
Obesity and CVD
Beyond Framingham data, which showed that obesity and CVD are related by the major risk factors (e.g. diabetes, smoking), this relationship is also mediated by several emerging risk factors found in obese persons, including atherogenic dyslipidemia, insulin resistance, a pro-inflammatory state, and a pro-thrombotic state.23 Obesity and CVD are also linked by the increased metabolic demands of cardiac output, compounded by decreased peripheral vascular resistance, and hypoventilation often due to apnea. To compensate for increased cardiac output in obesity, the stroke volume must increase, which leads to increased left ventricular (LV) filling pressure and volume overload. While early studies suggested that this state of volume overload led to eccentric hypertrophy, we now know that most obese subjects have some degree of concentric LV geometry, even in the absence of hypertension.24
Obesity and the risk of cancer
The body of epidemiological data suggests that up to 20% of malignancies could be related to weight, weight gain, and obesity.25 Based on numerous studies and meta-analyzed data (Figure 1),26,27 the American Institute for Cancer Research and World Cancer Research Fund (AICR/WCRF) believes there is convincing evidence linking obesity to esophageal adenocarcinoma, pancreatic, liver, colorectal, postmenopausal breast, endometrial, and kidney cancer. In addition, the AICR/WCRF reports probable evidence for other cancers. The risk of cancer with obesity appears to increase with increasing BMI, as demonstrated in a study of healthy never smokers (~1.46 million), where cancer risk was significantly increased by 12% with a BMI of 27.5–29.9 and up to 70% in those with a BMI of 40–49.9.37
Figure 1.
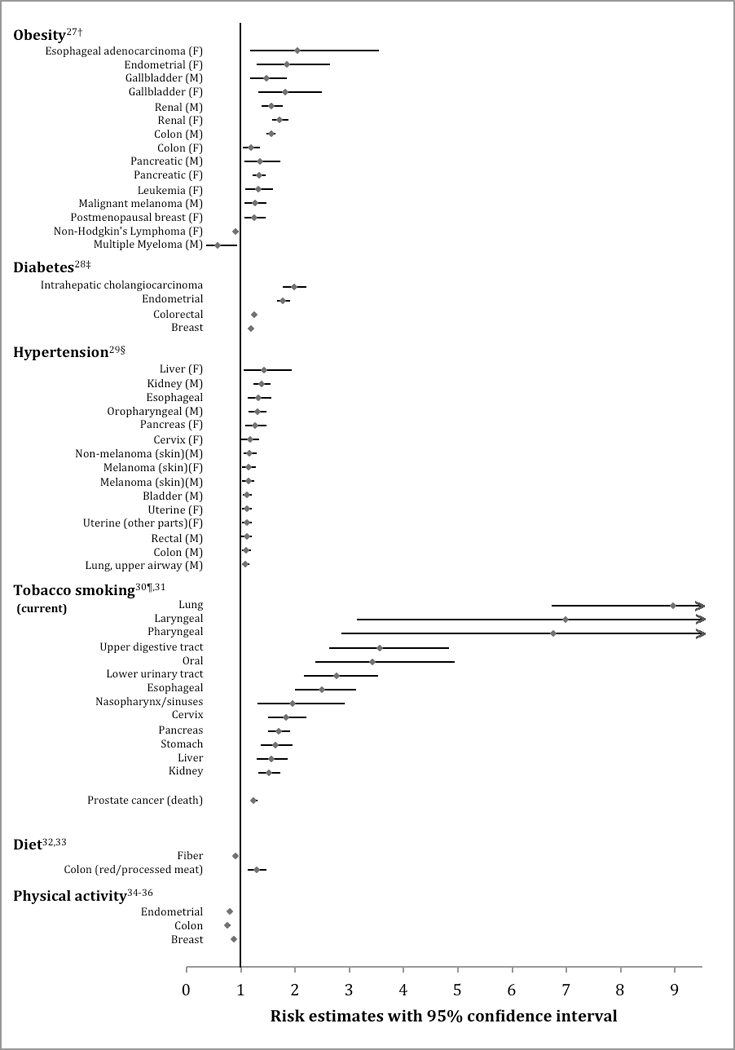
Modifiable cardiac risk factors with their estimated cancer risk. Figure limited to only the positive and/or negative associations using the most recently published meta-analysis or prospective cohort study investigating the associations between a cardiac risk factor and various cancer sites; hyperlipidemia was excluded from the figure due to inconclusive evidence that it is associated with cancer risk. All cancer estimates were reported as a relative risk except where noted below.
† Risk for BMI >30 kg/m2
‡ Note, there were several additional positive (bladder, esophageal, extrahepatic cholangiocarcinoma, gallbladder, gastric, hepatocellular carcinoma, kidney, leukemia, multiple myeloma, Non-Hodgkin’s lymphoma, ovarian cancer, pancreatic cancer) and negative (lung, prostate) associations by fixed effects models, but the authors did not deem these to be positive associations since their 95% prediction intervals included the null value.
§ Prospective observational cohort; Cox regression was used to calculate hazard ratios of cancer per 10 mmHg increments of midblood pressure.
¶ Only cancer sites with sufficient evidence of carcinogenicity related to tobacco exposure in humans were considered in the meta-analysis.
The carcinogenic effects of obesity on gender can differ, and this is most substantial for colon cancer. This gender difference is consistent throughout a number of studies, including the EPIC study, reporting a 55% increased risk of colorectal cancer in men compared to women.38 It has been hypothesized that estrogen, increased in obese women, inhibits inflammatory signaling and exerts an anti-tumor effect through selective activation of pro-apoptotic signaling through colonic estrogen receptors.39 This hypothesis was further supported by the Women’s Health Initiative in which women receiving post-menopausal estrogen replacement had a reduced risk of colorectal cancer.39
Obesity, cancer, and CVD: is there a shared biology?
Obesity, cancer, and CVD have a complex relationship mediated by several factors such as diet, body fat distribution, physical activity, hormones (sex hormones, insulin and insulin-like growth factor [IGF] signaling, and adipokines), chronic inflammatory burden, and oxidative stress. Pro-inflammatory cytokines and hormones, produced within adipose tissue, are elevated in the serum of obese people. Examples include IL-6, TNF-alpha, leptin, angiotensinogen, resistin (linking obesity to diabetes),40 and CRP, several of which have anti-apoptotic and pro-angiogenic properties that not only help sustain fat storage, but have tumorigenic effects at other sites. For example, leptin has been shown to be a critical regulator of hepatocellular carcinoma through its effects on telomerase reverse transciptase.41 Leptin also plays a pivotal role in obesity-related CVD, as demonstrated by numerous clinical and animal model investigations.42
One of the most abundant cytokines produced by adipose tissues is IL-6, which increases blood pressure and stimulates hepatic production of CRP, an inflammatory marker of CVD.12 Overexpression of IL-6 has been shown to inhibit cancer cell apoptosis, stimulate angiogenesis, and have a role in drug resistance, leading to tumor progression.43
Diabetes
Diabetes and CVD
Diabetes affects a number of different systems in the body, and its deleterious effects on the macrovasculature render it a coronary heart disease risk equivalent. The pathophysiology linking diabetes to atherosclerosis is multifaceted. Insulin resistance promotes dyslipidemia along with lipoprotein abnormalities through oxidative stress, glycosylation, and triglyceride enhancement. Endothelial dysfunction, an early marker for atherosclerosis, is stimulated by hyperglycemia-induced free radical damage within the vasculature.11 During hyperglycemia, IGF-1 stimulates the migration and proliferation of smooth muscle cells, a common mechanism of atherosclerosis, although additional mechanisms have also been described.44
Diabetes and the risk of cancer
Numerous studies link diabetes to cancer risk and its progression. In 2010, a consensus report by the American Diabetes Association concluded that there is convincing evidence associating diabetes with colorectal, breast, endometrial, liver, pancreatic and bladder cancers, and somewhat convincing evidence for other cancers such as kidney, leukemia, and esophageal.44
A recent systematic umbrella review of meta-analyses of observational studies (2015) assessing the association of type 2 diabetes with site-specific cancer incidences concluded that there is robust evidence for an association of type 2 diabetes with breast, intrahepatic cholangiocarcinoma, colorectal, and endometrial cancer whereas there is inconclusive or no evidence for an association with other cancer sites (Figure 1),28 although a number of stringent and perhaps debatable criteria contributed to this this largely negative conclusion.45
Diabetes, cancer, and CVD: is there a shared biology?
Diabetes influences CVD and the neoplastic process through several mechanisms including hyperinsulinemia, hyperglycemia, IGF, and inflammation. The insulin-cancer hypothesis postulates a central role for elevated levels of IGF, which promotes cell proliferation. Serum IGF levels are increased as chronic hyperinsulinemia leads to decreased levels of IGF-binding proteins.44 Tumor cells express both insulin receptors and IGF receptors. Meta-analyses have shown an increased risk of colorectal cancer, prostate cancer, and premenopausal breast cancer associated with high serum levels of IGF.46,47 Hyperinsulinemia also decreases hepatic synthesis of sex hormone binding globulins, increasing estrogen levels in men and women and testosterone levels in women. Elevated sex steroid levels are associated with an increased risk of post-menopausal breast and endometrial cancer, 48 although pleiotropic effects of estrogen on the cardiovascular system tend to be favorable.49
Finally, inflammation has been shown to promote insulin resistance and be involved in the pathogenesis of diabetes, further contributing to the complex network of interactions between inflammation, CVD, and cancer.50
Hypertension
Hypertension and CVD
Hypertension is a well-established CVD risk factor. Clinical and experimental studies demonstrate a causal relationship between hypertension and both vascular and structural cardiac remodeling. Hypertension induces oxidative stress on the arterial wall, thought to be the primary mechanism behind its atherogenic influence.51 Hypertensive heart disease occurs when the left ventricular wall thickens as a means of reducing wall stress due to elevated blood pressures.52 This can subsequently lead to both diastolic and systolic heart failure.
Hypertension and the risk of cancer
Observational studies assessing hypertension and cancer risk have shown mixed results. The largest study to date assessed over half a million people from the Metabolic Syndrome and Cancer Project over a median of 12 years and found that men had a total incident cancer HR (95% CI) of 1.07 (1.04–1.09) and for cancer deaths of 1.12 (1.08–1.15) per 10 mmHg blood pressure increment; women had an overall cancer mortality of 1.06 (1.02–1.11), but no association with incident cancer rates. Hypertension was associated with several specific cancer types (Figure 1).29
There is a particularly strong association between hypertension and renal cell carcinoma (RCC). Grossman and colleagues53 reported a pooled OR of 1.23 (95% CI 1.11–1.36) for all-cause cancer mortality and 1.75 (95% CI: 1.61–1.90) for RCC mortality associated with hypertension, after adjusting for age and tobacco use.
Hypertension, cancer, and CVD: is there a shared biology?
It is not known whether hypertension, per se, is a cancer-causing disease state, or if the association is through an alternative mechanism. A potential biological mechanism that relates hypertension and cancer may be through angiogenic factors. Elevated levels of plasma vascular endothelial growth factor (VEGF), a central hormone in the ability of tumors to induce new blood-vessel formation, is also evident in hypertensive subjects.54,55 Several studies suggest that angiogtensin II, a key hormone in vasoconstriction and hypertension, stimulates the production of VEGF.56 Thus, because hypertensive patients have higher levels of VEGF, it is plausible that this may potentiate the development or progression of cancer, accounting for the association. Also, hypertension affects arterial walls through oxidative stress, which is also associated with carcinogenesis, suggesting another possible shared biology between CVD and cancer.
Hyperlipidemia
Hyperlipidemia and CVD
Serum lipid levels have a well-known association with coronary artery disease.57 Atherogenesis begins when excess lipoproteins such as low-density lipoprotein (LDL) accumulate in the subendothelial space, are oxidatively modified, and are taken up selectively by macrophages and monocytes. LDL molecules are influenced by a number of factors, including the body’s metabolic profile and become more atherogenic in the setting of concomitant disease such as the metabolic syndrome.
Hyperlipidemia and the risk of cancer
Hyperlipidemia as a risk factor for cancer remains inconclusive based on heterogeneous data, though appears more convincing for breast cancer and less convincing for some other cancers.58 In contrast, several studies demonstrate an inverse association between LDL-cholesterol and cancer risk, although this may be due the malignancy itself (e.g. changes in cholesterol metabolism or absorption); also, tumor cells often express receptors that attract cholesterol metabolites necessary to support their growth, thus diminishing circulating levels in the plasma.59
Some animal models have found that a high cholesterol diet, in conjunction with other agents, increases colon adenomas.60 This may be related to hepatic bile acids, which are increased in the setting of chronic saturated fat intake, which have been shown to promote carcinogenesis.61
Hyperlipidemia, cancer, and CVD: is there a shared biology?
The cholesterol metabolite, 27-hydroxycholesterol (27HC), is similar in both structure and action to estradiol (an estrogen) and has been recently implicated in breast cancer.62 This forms the basis for an intriguing relationship between CVD and cancer through cholesterol and its metabolism. Furthermore, statin therapy (discussed later) was shown to attenuate the enhanced breast tumor growth associated with high fat diets in mice.14 Similar to estrogen, which has well-known pleiotropic effects on the cardiovascular system,49 emerging murine studies also demonstrate that 27HC has cardiovascular effects.
The intratumoral milieu has become an area of intense interest in many cancers. With regard to 27HC, the enzyme that produces this metabolite is abundant within tumor-associated macrophages,14 suggesting a probable role for inflammatory cells in the tumor process as well.
Tobacco
Tobacco and CVD
Cigarette smoking greatly impacts cardiovascular incidence and mortality, contributing to all stages of atherosclerosis via numerous mechanisms. Tobacco smoking affects the early stages of atherosclerosis by decreasing levels of nitric oxide, causing vasomotor dysfunction, and increasing oxidative stress, causing endothelial and structural changes. It plays a largely thrombotic role in acute coronary events.63
Tobacco and the risk of cancer
Tobacco usage, particularly smoking, is also a preventable and heavily weighted risk factor for multiple cancer types (Figure 1).30,31 The American Cancer Society estimates that smoking is responsible for 30% of all cancer-related deaths in the U.S.2 The main carcinogenic mechanism from smoking is repetitive injury to squamous cell epithelium, exceeding normal regenerative abilities, while a variety of other mechanism have also been described.
Tobacco, cancer, and CVD: is there a shared biology?
Tobacco smoking produces a number of irritants, carcinogens, pro-inflammatory stimuli, and oxidizing agents. These processes stimulate abnormal signaling pathways found within smoking-related cancer and CVD.64 Nicotine has also been implicated in the pathogenesis of both CVD and cancer. In vitro animal models have found that nicotine can inhibit apoptosis and enhance angiogenesis,63 which raises concern about the role of nicotine itself in both diseases.
Diet
Diet and CVD
Diet and nutrition are major determinants that influence CVD. Extensive investigations, in both animal and diverse human populations, have found strong and consistent associations between diet and CVD. This association is mediated by several intermediate CVD risk factors, such as body weight, blood pressure, and serum lipids.
Diet and the risk of cancer
The role of dietary composition in cancer risk ranges from known carcinogens in food sources (e.g. aflatoxins, nitrosamines) to dietary components impacting obesity, hypertension, hyperlipidemia, and chronic inflammatory patterns that mediate cancer risk. The AICR/WCRF reports several associations between diet and food, graded on a causal, probable, or moderate degree of evidence.65 They believe a causal relationship exists between the following foods and cancer types: red/processed meat with colorectal cancer;33 aflatoxins with liver cancer; and arsenic in drinking water and beta-carotenes with lung cancer risk. The AICR/WCRF reports a causal reduction in colorectal cancer risk with diets high in fiber.32 The AICR/WCRF reports a probable increased risk between the following: Cantonese-style salted fish with nasopharyngeal cancer; salty foods with stomach cancer; glycemic load with endometrial cancer; maté with esophageal cancer; and arsenic in drinking water and skin cancer. A probable decreased risk exists between the following: non-starchy vegetables with oropharygneal, laryngeal, esophageal and stomach cancer; garlic with colorectal cancer; fruits with oropharygneal, laryngeal, esophageal, lung and stomach cancer; and high calcium diets and colorectal cancer.65
Diet, cancer, and CVD: is there a shared biology?
Common biological pathways, or networks, between diet, CVD and cancer have been described. Genetic mutations in the folate metabolism pathway, in conjunction with inadequate folate intake, are associated with increased risk of both CVD and colorectal cancer.66 Aberrant methylation due to folate deficiency is hypothesized to contribute to atherosclerosis, as this may modulate the expression of a variety of genes involved in proliferation and migration capabilities within the smooth muscle of coronary vessels. In rapidly dividing tissues such as the epithelium of the gastrointestinal tract, inadequate folate causes inadequate thymidylate production, impairing DNA synthesis and producing genomic instability and subsequent carcinogenesis.
Conjugated linoleic acids (CLA), found primarily in foods derived from ruminant animals (e.g. beef and dairy products), have shown promising anti-atherosclerotic and anti-carcinogenic effects in experimental models;67 however, meat-dominated dietary patterns have been associated with several cancers, especially colorectal cancer.68 This cancer risk may be a result of chronic inflammation,69 increased dietary fat leading to carcinogenic bile acids, and several genotoxic substances (e.g. nitrosamines) that can act directly on DNA and cause point mutations, deletions, and insertions.
Consumption of polyphenols, found primarily in fruits, vegetables, and certain plants, has been associated with a reduction in both CVD and cancer, presumably due to their affect on several metabolic pathways, including MAP-kinases, P13-kinases, IGF-1, NF-kB and ROS.70
Neither vitamins nor antioxidants have been associated with a reduction in CVD or cancer in randomized controlled trials, despite positive effects within in vivo studies.70,71 Despite the positive cardiovascular effects of Omega-3 fatty acids, a major systemic review did not find a reduction in the risk of cancer.72
Alcohol
Alcohol and CVD
Moderate alcohol consumption has a well-established cardioprotective effect, although in the absence of any randomized controlled data.73 In patients with established cardiovascular disease, meta-analyzed data (8 observational studies) of 16,351 patients found moderate alcohol consumption to be associated with reduced CVD- and all-cause mortality.74 With healthy individuals free of CVD, there has been an extensively documented J-shaped dose-effect curve, with excessive alcohol use leading to increased cardiovascular events and all-cause mortality.20 Potential cardioprotective mechanisms include decreased inflammation, decreased platelet aggregation/function, reduced myocardial ischemia-reperfusion injury, effects on coagulation factors, endothelial events, elevated HDL-C levels, and effects on anti- and pro-apoptotic pathways.74,75 Higher alcohol levels are associated with increased mortality, CVD, elevated triglycerides, hypertension, atrial fibrillation, cardiomyopathy, and stroke.
Alcohol and the risk of cancer
There is believed to be a casual relationship, as a result of convincing evidence, between alcohol and oropharyngeal, laryngeal, esophageal, liver, colorectal, and pre- and postmenopausal breast cancers. There is a probable decreased risk of alcohol intake on kidney cancer.65,76 Compared to non-drinkers, regular consumption of about 50 g of alcohol per day confers a threefold risk of oral and pharyngeal cancers, a twofold risk of laryngeal and esophageal (squamous-cell) cancers, a 1.5-fold risk for female breast cancer, and a 1.4-fold risk for colon cancer. For regular consumption of 18 g of alcohol per day, the relative risk for breast cancer remains significant at 1.13. A meta-analysis77 also found that light drinking (up to 1 drink/day) was associated with the risk of oropharyngeal, esophageal squamous, and female breast cancer.
Mechanisms linking alcohol to cancer
Cancer risk with alcohol involves several biological mechanisms. These include polymorphisms in the genes related to ethanol metabolism, folate and methionine metabolism and DNA repair.77 Additional processes could involve the genotoxic effect of acetaldehyde (the primary metabolite of alcohol), increased estrogen levels, alcohol acting as a solvent for tobacco carcinogens, and the production of reactive oxygen species and nitrogen species.78
Physical activity
Physical activity and CVD
The cardiovascular benefits of physical activity are indisputable based on numerous scientific reports. Exercise favorably affects other established risk factors of CVD such as hypertension, obesity, diabetes, and hyperlipidemia. Additional effects include improvements in bone health, aerobic capacity, blood vessel capacitance and vascular wall function.79
Physical activity and the risk of cancer
There is accumulating epidemiological evidence on physical activity and reduced cancer risk. The AICR/WCRF reports that there is convincing evidence that physical activity reduces colorectal cancer risk, and there is probable evidence that it reduces postmenopausal breast and endometrial cancers.34,36,65 A recent meta-analysis80 that included 71 cohort studies found that individuals who participated in the most physical activity had an HR of 0.83 (95% CI 0.79 to 0.87) and 0.78 (95% CI 0.74 to 0.84) for cancer mortality in both the general population and among cancer survivors, respectively.
Physical activity, cancer, and CVD: is there a shared biology?
The biological mechanisms hypothesized to reduce cancer and CVD risk with increased exercise are multiple and tend to overlap with those discussed in previous sections (e.g. diet, obesity). Weight control appears to play a particularly important role. The reduction of adipose tissue through physical activity lowers the production of circulating sex and metabolic hormones, insulin, leptin, and inflammatory markers, several of which are potentially carcinogenic.81
Nonmodifiable CVD risk factors linked to cancer
Nonmodifiable risk factors, including age, sex, and race/ethnicity, are uncontrollable features that influence incidence rates of both cancer and CVD. While environment and cultural practices may impart disease patterns among members of specific ethnicities, genetic components of race pre-dispose individuals toward some of the aforementioned modifiable risk factors. Single nucleotide polymorphisms in linkage disequilibrium often demonstrate higher allele frequencies in certain populations that may be linked to these diseases.82 Disease screening and health care availability within socioeconomic groups affect the statistical diagnostics as well. Within the United States in 2009, cancer incidence rates were highest among blacks, largely driven by prostate and female breast cancers.83 Premature death from stroke and CVD was also higher among blacks.84
Regarding sex, there are obvious differences between male versus female organs and hormonal fluctuations that influence both CVD and cancer progression. While men are more likely to be diagnosed with CVD at an earlier age, they are also more frequently diagnosed with cancer. In the United States during 2010, the top three cancer types diagnosed across men of all racial/ethnic cohorts were prostate, lung, and colorectal cancers; whereas the most common cancer type for women of all racial/ethnic cohorts were breast cancer, lung, and colorectal cancer.83
Of all nonmodifiable risk factors, age is a steady independent variable with regard to CVD and cancer, yet the associations between age and disease onset can be highly influenced by lifestyle parameters, such as diet, physical activity, BMI, and smoking. Despite some cancer types that notoriously strike during childhood, increasing age ≥55 years is associated with 78% of the new cancer diagnoses in developed countries.44
Cancer risk related to the treatment of CVD and CVD risk factors
Anti-glycemic agents
Anti-glycemic agents have both positive and negative associations with cancer. Metformin has been thought to reduce the risk of cancer and has a plausible biological mechanism, although population studies have demonstrated mixed results.85,86 Metformin activates adenosine monophosphate-activated protein kinase (AMPK) in hepatocytes, a major cellular regulator of lipid and glucose metabolism, and AMPK is associated with several tumor suppressors. In contrast, a potential increased risk is associated with insulin analogues.87,88 The general patterns demonstrated across several studies support the hyperinsulinemia hypothesis, where therapies that increase circulating insulin levels increase cancer risk, and those that lower insulin resistance and circulating insulin levels reduce cancer risk.85
Antihypertensive agents
Several anti-hypertensive medication classes have been reported to increase cancer risk in various reports and retrospective analyses. Animal studies have described marginal increases in RCC, adenomas, and nephropathy with furosemide and hydrochlorothiazide.89–91 A meta-analysis by Grossman and colleagues showed an elevated RCC risk with diuretic use (OR 1.55; 95% CI 1.42–1.71), and both female gender and longer duration of treatment conferred a greater risk. Additional studies have adjusted for hypertension, still showing a weakened, albeit statistically significant, association between diuretics and RCC; more convincingly, diuretics have been shown to increase the risk of RCC in normotensive patients taking diuretics for alternative indications.92–94
Patients are often exposed to antihypertensive drugs for decades, much longer than the original clinical trials testing their safety. Thus, there is heightened interest in the chronic use of these medications and how they may influence cancer risk or cancer progression. For example, Ganz and colleagues95 investigated the association between breast cancer recurrence and the use of angiotensin-converting enzyme (ACE) inhibitors and/or β-blockers in the Life After Cancer Epidemiology (LACE) Study cohort. They found that ACE inhibitor exposure was associated with breast cancer recurrence (HR 1.56, 95% CI 1.02–2.39) while β-blockers exposure had a non-significantly lower HR of 0.86 (95% CI 0.57–1.32) for breast cancer recurrence. While potential mechanisms are only speculative, they bring greater attention to the potential role of these medications in breast cancer survivors.
Statin therapy
Statins, best known for reducing morbidity and mortality from CVD, have pleiotropic effects. Many in vitro experiments have demonstrated antitumor effects of statins against cancer stem cells and certain cell lines through suppression of cell proliferation and/or apoptosis.96 Statin inhibition of HMG-CoA reductase decreases levels of mevalonate and its downstream products, including not only cholesterol but additional factors that are critical for cancer growth and progression.96 Statins also have potent anti-inflammatory properties could also have protective effects against cancer.
Although an early cardiovascular clinical trial found a potential link between statins and cancer, a number of subsequent meta-analyses of statin trials showed no overall increased risk of cancer incidence.97 Meanwhile, emerging clinical studies looking specifically at the impact of statin exposure on cancer and tumor growth have found encouraging results. Strong evidence demonstrates improvement in disease-free survival in breast cancer survivors who were on statins at the time of diagnosis.14,98 Similarly, mounting evidence shows that prediagnostic statin use reduces the risk of lethal prostate cancer.99 A recent meta-analysis found statin use either before or after the diagnosis of cancer to be associated with improved cancer-specific and overall survival.100 The three largest cancer subgroups including colorectal, breast, and prostate, all showed a benefit from statin use. A number of ongoing trials are investigating the role of statins in breast and other cancer types. Findings from these studies may further elucidate the shared biology between CVD and cancer.
Aspirin and NSAIDs
There may be an important role for the use of anti-inflammatory agents in the prevention and treatment of cancer. A large prospective study showed NSAIDs to be associated with reduced risk of several alcohol-related, infection-related, obesity-related, and smoking-related cancers. 101 A meta-analysis looking at the association between aspirin and colorectal cancer found that aspirin reduced the risk of colorectal cancer by 24%.102 While aspirin’s benefits on the cardiovascular system has largely been attributed to its antiplatelet effects, the mechanism of aspirin’s antineoplastic effects is less clear, although emerging evidence suggests it may be related to both COX-dependent and COX-independent mechanisms.103
CVD risk in the treatment of cancer
Cancer treatment-related cardiotoxicity is a major cause of treatment-associated mortality and morbidity in cancer survivors. While this topic is outside the scope of the present discussion, it is an area of intense interest with emerging data.
CVD risk factors predict cardiotoxicity related to cancer therapy
Cardiac risk factors have a major influence on toxicity from cancer therapy. Age, prior cardiac dysfunction, coronary disease, hypertension, smoking, and obesity are well-described risk factors for anthracycline-related cardiotoxicity.104,105 Additionally, because chemotherapy doses are based on weight, overweight patients will also require higher cumulative doses that compound their overall complication risk. Baseline hypertension has predicted anti-VEGF therapy-induced blood pressure elevation.106 In patients treated with trastuzumab, hypertension had a 1.89-fold increased risk of a cardiac events, although not statistically significant.107
Drugs that target growth factor receptors, including those from the TGF-β family such as anti-EGFR, pose additional toxicity risk when compounded with cardiovascular risk factors, such as hypertension or diabetes. These drugs inhibit cardiomyocyte differentiation, function, and repair, exacerbating pre-existing issues or leading to cardiac or vascular remodeling.108
Ezaz and colleagues109 developed a risk-factor scoring tool for patients on trastuzumab to help identify those at highest risk of developing heart failure or cardiomyopathy (HF/CM). A 7-factor risk (age, adjuvant chemotherapy, CAD, atrial fibrillation or flutter, DM, HTN, and renal failure) score was derived and validated. Low (0 to 3 points), medium (4 to 5 points), and high (≥ 6 points) risk strata had 3-year rates of HF/CM of 16.2%, 26%, and 39.5%, respectively.
CVD risk factors affect outcomes in cancer survivors
Results from a Childhood Cancer Survivor Study (CCSS) analysis, which included patients treated with chemotherapy and/or radiation that survived beyond age 35, found a 5-fold increased risk of stroke or myocardial infarction compared to healthy sibling counterparts.110 This risk intensified in those with dyslipidemia, diabetes, and obesity. In multivariable models, hypertension alone significantly increased risk for all major cardiac events among survivors exposed to both chest-directed RT and anthracyclines.111
Controlling CVD risk factors can reduce the risk of cancer
The European Prospective Investigation Into Cancer and Nutrition-Potsdam (EPIC) study followed 23,153 participants aged 35 to 65 years. Healthy lifestyle factors were defined as never smoking, BMI <30, physical activity >3.5 hours weekly, and healthy diet. After a mean follow-up of 7.8 years, participants who adhered to all four healthy lifestyle factors compared to none had an adjusted HR (95% CI) of 0.22 (0.17–0.28) for chronic disease; 0.07 (0.05–0.12) for diabetes; 0.19 (0.07–0.53) for myocardial infarction; 0.50 (0.21–1.18) for stroke; and 0.64 (0.43–0.95) for cancer.112
A study by Rasmussen-Torvik and colleagues21 also examined whether better adherence to the 7 ideal cardiovascular health metrics defined by the AHA (described in section 3.1) was associated with incident cancer. This longitudinal analysis from 1987 to 2006 within the ARIC cohort (age 45–64 years at baseline) identified incident cancer (excluding non-melanoma skin cancer) in 2,880 of the 13,253 participants. Adherence to at least 6 of the 7 ideal health metrics (2.7% of the overall population) resulted in a 51% lower risk of incident cancer compared to subjects meeting 0 ideal health metrics (2.8% of the population), after adjusting for age, sex, race, and ARIC center. Incident cancer rates increased with each graded decrease in ideal health metrics.
Although the 7 ideal health metrics were chosen because of their strong associations with cardiovascular risk, a number of these metrics already have established associations with cancer, such as diet,65 physical activity,81 BMI,26 and smoking.113 Nonetheless, the results from the EPIC and Rasmussen-Torvik studies demonstrate that adherence to several of the health measures combined is associated with a reduced risk of incident cancer over time. In addition, such studies may help build the case for combined CVD and cancer prevention guidelines, which could have major public health implications.
Conclusion
The extensive overlap in risk factors and disease prevention for CVD and cancer suggests that these seemingly diverse diseases have some common basic molecular pathways or networks. Chronic inflammation may have a considerable role as it contributes to both diseases and occurs in conditions such as obesity, diabetes, hypertension, and dyslipidemia. Controlling CVD risk factors can help reduce the risk of cancer. There is an urgent need to improve the health status of the population to reduce the prevalence of disease. Further understanding of the delicate interaction between CVD and cancer may lead to better prevention, earlier detection and safer treatment strategies.
Acknowledgments
Funding Sources: Anna Prizment was supported by the National Center for Advancing Translational Sciences of the NIH Award Number UL1 TR000114.
Footnotes
Conflict of Interest Disclosures: None
References
- 1.Hoyert DL, Xu J. Deaths: preliminary data for 2011. Natl Vital Stat Reports From Centers Dis Control Prev Natl Cent Heal Stat Natl Vital Stat Syst. 2012;61:1–51. [PubMed] [Google Scholar]
- 2.American Cancer Society. Cancer Facts & Figures 2014. Atlanta: American Cancer Society; 2014. [Google Scholar]
- 3.Heidenreich PA, Trogdon JG, Khavjou OA, Butler J, Dracup K, Ezekowitz MD, Finkelstein EA, Hong Y, Johnston SC, Khera A, Lloyd-Jones DM, Nelson SA, Nichol G, Orenstein D, Wilson PWF, Woo YJ. Forecasting the Future of Cardiovascular Disease in the United States A Policy Statement From the American Heart Association. Circulation. 2011;123:933–944. doi: 10.1161/CIR.0b013e31820a55f5. [DOI] [PubMed] [Google Scholar]
- 4.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Libby P. Inflammation and cardiovascular disease mechanisms. Am J Clin Nutr. 2006;83:456S–460S. doi: 10.1093/ajcn/83.2.456S. [DOI] [PubMed] [Google Scholar]
- 6.Berg AH, Scherer PE. Adipose tissue, inflammation, and cardiovascular disease. Circ Res. 2005;96:939–949. doi: 10.1161/01.RES.0000163635.62927.34. [DOI] [PubMed] [Google Scholar]
- 7.Frayn K, Bernard S, Spalding K, Arner P. Adipocyte triglyceride turnover is independently associated with atherogenic dyslipidemia. J Am Heart Assoc. 2012;1:e003467. doi: 10.1161/JAHA.112.003467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rodriguez-Iturbe B, Pons H, Quiroz Y, Johnson RJ. The Immunological Basis of Hypertension. Am J Hypertens. 2014 Nov;27(11):1327–1337. doi: 10.1093/ajh/hpu142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Esposito K, Nappo F, Marfella R, Giugliano G, Giugliano F, Ciotola M, Quagliaro L, Ceriello A, Giugliano D. Inflammatory cytokine concentrations are acutely increased by hyperglycemia in humans: role of oxidative stress. Circulation. 2002;106:2067–2072. doi: 10.1161/01.cir.0000034509.14906.ae. [DOI] [PubMed] [Google Scholar]
- 10.Aggarwal BB, Vijayalekshmi RV, Sung B. Targeting inflammatory pathways for prevention and therapy of cancer: short-term friend, long-term foe. Clin Cancer Res. 2009;15:425–430. doi: 10.1158/1078-0432.CCR-08-0149. [DOI] [PubMed] [Google Scholar]
- 11.Tesfamariam B, Cohen RA. Free radicals mediate endothelial cell dysfunction caused by elevated glucose. Am J Physiol. 1992;263:H321–326. doi: 10.1152/ajpheart.1992.263.2.H321. [DOI] [PubMed] [Google Scholar]
- 12.Ridker PM. Novel risk factors and markers for coronary disease. Adv Intern Med. 2000;45:391–418. [PubMed] [Google Scholar]
- 13.Kamp DW, Shacter E, Weitzman SA. Chronic inflammation and cancer: the role of the mitochondria. Oncology (Williston Park) 2011;25:400–410. 413. [PubMed] [Google Scholar]
- 14.Nelson ER, Wardell SE, Jasper JS, Park S, Suchindran S, Howe MK, Carver NJ, Pillai RV, Sullivan PM, Sondhi V, Umetani M, Geradts J, McDonnell DP. 27-Hydroxycholesterol links hypercholesterolemia and breast cancer pathophysiology. Science. 2013;342:1094–1098. doi: 10.1126/science.1241908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Barrera G. Oxidative stress and lipid peroxidation products in cancer progression and therapy. ISRN Oncol. 2012;2012:137289. doi: 10.5402/2012/137289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Thanan R, Oikawa S, Hiraku Y, Ohnishi S, Ma N, Pinlaor S, Yongvanit P, Kawanishi S, Murata M. Oxidative stress and its significant roles in neurodegenerative diseases and cancer. Int J Mol Sci. 2015;16:193–217. doi: 10.3390/ijms16010193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stone NJ, Robinson J, Lichtenstein AH, Merz CNB, Blum CB, Eckel RH, Goldberg AC, Gordon D, Levy D, Lloyd-Jones DM, McBride P, Schwartz JS, Shero ST, Smith SC, Watson K, Wilson PWF. 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:S1–S45. doi: 10.1161/01.cir.0000437738.63853.7a. [DOI] [PubMed] [Google Scholar]
- 18.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger V, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD. Defining and Setting National Goals for Cardiovascular Health Promotion and Disease Reduction The American Heart Association’s Strategic Impact Goal Through 2020 and Beyond. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 19.Barreira TV, Harrington DM, Katzmarzyk PT. Cardiovascular health metrics and accelerometer-measured physical activity levels: National Health and Nutrition Examination Survey, 2003–2006. Mayo Clin Proc. 2014;89:81–86. doi: 10.1016/j.mayocp.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Laitinen TT, Pahkala K, Magnussen CG, Viikari JSA, Oikonen M, Taittonen L, Mikkila V, Jokinen E, Hutri-Kahonen N, Laitinen T, Kahonen M, Lehtimaki T, Raitakari OT, Juonala M. Ideal cardiovascular health in childhood and cardiometabolic outcomes in adulthood: the Cardiovascular Risk in Young Finns Study. Circulation. 2012;125:1971–1978. doi: 10.1161/CIRCULATIONAHA.111.073585. [DOI] [PubMed] [Google Scholar]
- 21.Rasmussen-Torvik LJ, Shay CM, Abramson JG, Friedrich CA, Nettleton JA, Prizment AE, Folsom AR. Ideal cardiovascular health is inversely associated with incident cancer: the Atherosclerosis Risk In Communities study. Circulation. 2013;127:1270–1275. doi: 10.1161/CIRCULATIONAHA.112.001183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer. World Health Organization; Retrieved from http://www.who.int/mediacentre/factsheets/fs297/en/ [Google Scholar]
- 23.Grundy SM. Obesity, metabolic syndrome, and coronary atherosclerosis. Circulation. 2002;105:2696–2698. doi: 10.1161/01.cir.0000020650.86137.84. [DOI] [PubMed] [Google Scholar]
- 24.Litwin SE. Cardiac remodeling in obesity: time for a new paradigm. JACC Cardiovasc Imaging. 2010;3:275–277. doi: 10.1016/j.jcmg.2009.12.004. [DOI] [PubMed] [Google Scholar]
- 25.Wolin KY, Carson K, Colditz GA. Obesity and cancer. Oncologist. 2010;15:556–565. doi: 10.1634/theoncologist.2009-0285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Renehan AG, Tyson M, Egger M, Heller RF, Zwahlen M. Body-mass index and incidence of cancer: a systematic review and meta-analysis of prospective observational studies. Lancet. 2008;371:569–578. doi: 10.1016/S0140-6736(08)60269-X. [DOI] [PubMed] [Google Scholar]
- 27.Dobbins M, Decorby K, Choi BCK. The Association between Obesity and Cancer Risk: A Meta-Analysis of Observational Studies from 1985 to 2011. ISRN Prev Med. 2013;2013:680536. doi: 10.5402/2013/680536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsilidis KK, Kasimis JC, Lopez DS, Ntzani EE, Ioannidis JPA. Type 2 diabetes and cancer: umbrella review of meta-analyses of observational studies. BMJ. 2015;350:g7607. doi: 10.1136/bmj.g7607. [DOI] [PubMed] [Google Scholar]
- 29.Stocks T, Van Hemelrijck M, Manjer J, Bjorge T, Ulmer H, Hallmans G, Lindkvist B, Selmer R, Nagel G, Tretli S, Concin H, Engeland A, Jonsson H, Stattin P. Blood pressure and risk of cancer incidence and mortality in the Metabolic Syndrome and Cancer Project. Hypertension. 2012;59:802–810. doi: 10.1161/HYPERTENSIONAHA.111.189258. [DOI] [PubMed] [Google Scholar]
- 30.Gandini S, Botteri E, Iodice S, Boniol M, Lowenfels AB, Maisonneuve P, Boyle P. Tobacco smoking and cancer: a meta-analysis. Int J Cancer. 2008;122:155–164. doi: 10.1002/ijc.23033. [DOI] [PubMed] [Google Scholar]
- 31.Islami F, Moreira DM, Boffetta P, Freedland SJ. A systematic review and meta-analysis of tobacco use and prostate cancer mortality and incidence in prospective cohort studies. Eur Urol. 2014;66:1054–1064. doi: 10.1016/j.eururo.2014.08.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aune D, Chan DSM, Lau R, Vieira R, Greenwood DC, Kampman E, Norat T. Dietary fibre, whole grains, and risk of colorectal cancer: systematic review and dose-response meta-analysis of prospective studies. BMJ. 2011;343:d6617. doi: 10.1136/bmj.d6617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Magalhães B, Peleteiro B, Lunet N. Dietary patterns and colorectal cancer: systematic review and meta-analysis. Eur J Cancer Prev. 2012;21:15–23. doi: 10.1097/CEJ.0b013e3283472241. [DOI] [PubMed] [Google Scholar]
- 34.Schmid D, Behrens G, Keimling M, Jochem C, Ricci C, Leitzmann M. A systematic review and meta-analysis of physical activity and endometrial cancer risk. Eur J Epidemiol. 2015;30:397–412. doi: 10.1007/s10654-015-0017-6. [DOI] [PubMed] [Google Scholar]
- 35.Robsahm TE, Aagnes B, Hjartåker A, Langseth H, Bray FI, Larsen IK. Body mass index, physical activity, and colorectal cancer by anatomical subsites: a systematic review and meta-analysis of cohort studies. Eur J Cancer Prev. 2013;22:492–505. doi: 10.1097/CEJ.0b013e328360f434. [DOI] [PubMed] [Google Scholar]
- 36.Wu Y, Zhang D, Kang S. Physical activity and risk of breast cancer: a meta-analysis of prospective studies. Breast Cancer Res Treat. 2013;137:869–882. doi: 10.1007/s10549-012-2396-7. [DOI] [PubMed] [Google Scholar]
- 37.Berrington de Gonzalez A, Hartge P, Cerhan JR, Flint AJ, Hannan L, MacInnis RJ, Moore SC, Tobias GS, Anton-Culver H, Freeman LB, Beeson WL, Clipp SL, English DR, Folsom AR, Freedman DM, Giles G, Hakansson N, Henderson KD, Hoffman-Bolton J, Hoppin JA, Koenig KL, Lee I-M, Linet MS, Park Y, Pocobelli G, Schatzkin A, Sesso HD, Weiderpass E, Willcox BJ, Wolk A, Zeleniuch-Jacquotte A, Willett WC, Thun MJ. Body-mass index and mortality among 1.46 million white adults. N Engl J Med. 2010;363:2211–2219. doi: 10.1056/NEJMoa1000367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pischon T, Lahmann PH, Boeing H, Friedenreich C, Norat T, Tjonneland A, Halkjaer J, Overvad K, Clavel-Chapelon F, Boutron-Ruault M-C, Guernec G, Bergmann MM, Linseisen J, Becker N, Trichopoulou A, Trichopoulos D, Sieri S, Palli D, Tumino R, Vineis P, Panico S, Peeters PHM, Bueno-de-Mesquita HB, Boshuizen HC, Van Guelpen B, Palmqvist R, Berglund G, Gonzalez CA, Dorronsoro M, Barricarte A, Navarro C, Martinez C, Quiros JR, Roddam A, Allen N, Bingham S, Khaw K-T, Ferrari P, Kaaks R, Slimani N, Riboli E. Body size and risk of colon and rectal cancer in the European Prospective Investigation Into Cancer and Nutrition (EPIC) J Natl Cancer Inst. 2006;98:920–931. doi: 10.1093/jnci/djj246. [DOI] [PubMed] [Google Scholar]
- 39.Caiazza F, Ryan EJ, Doherty G, Winter DC, Sheahan K. Estrogen Receptors and Their Implications in Colorectal Carcinogenesis. Front Oncol. 2015;5:19. doi: 10.3389/fonc.2015.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steppan CM, Bailey ST, Bhat S, Brown EJ, Banerjee RR, Wright CM, Patel HR, Ahima RS, Lazar MA. The hormone resistin links obesity to diabetes. Nature. 2001;409:307–312. doi: 10.1038/35053000. [DOI] [PubMed] [Google Scholar]
- 41.Stefanou N, Papanikolaou V, Furukawa Y, Nakamura Y, Tsezou A. Leptin as a critical regulator of hepatocellular carcinoma development through modulation of human telomerase reverse transcriptase. BMC Cancer. 2010;10:442. doi: 10.1186/1471-2407-10-442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hou N, Luo J-D. Leptin and cardiovascular diseases. Clin Exp Pharmacol Physiol. 2011;38:905–913. doi: 10.1111/j.1440-1681.2011.05619.x. [DOI] [PubMed] [Google Scholar]
- 43.Guo Y, Xu F, Lu T, Duan Z, Zhang Z. Interleukin-6 signaling pathway in targeted therapy for cancer. Cancer Treat Rev. 2012;38:904–910. doi: 10.1016/j.ctrv.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 44.Giovannucci E, Harlan DM, Archer MC, Bergenstal RM, Gapstur SM, Habel LA, Pollak M, Regensteiner JG, Yee D. Diabetes and Cancer: A consensus report. Diabetes Care. 2010;33:1674–1685. doi: 10.2337/dc10-0666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Satija A, Spiegelman D, Giovannucci E, Hu FB. Type 2 diabetes and risk of cancer. BMJ. 2015;350:g7707. doi: 10.1136/bmj.g7707. [DOI] [PubMed] [Google Scholar]
- 46.Renehan AG, Zwahlen M, Minder C, O’Dwyer ST, Shalet SM, Egger M. Insulin-like growth factor (IGF)-I, IGF binding protein-3, and cancer risk: systematic review and meta-regression analysis. Lancet. 2004;363:1346–1353. doi: 10.1016/S0140-6736(04)16044-3. [DOI] [PubMed] [Google Scholar]
- 47.Chen W, Wang S, Tian T, Bai J, Hu Z, Xu Y, Dong J, Chen F, Wang X, Shen H. Phenotypes and genotypes of insulin-like growth factor 1, IGF-binding protein-3 and cancer risk: evidence from 96 studies. Eur J Hum Genet EJHG. 2009;17:1668–1675. doi: 10.1038/ejhg.2009.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaaks R, Lukanova A, Kurzer MS. Obesity, endogenous hormones, and endometrial cancer risk: a synthetic review. Cancer Epidemiol Biomarkers Prev A Publ Am Assoc Cancer Res Cosponsored by Am Soc Prev Oncol. 2002;11:1531–1543. [PubMed] [Google Scholar]
- 49.Murphy E. Estrogen signaling and cardiovascular disease. Circ Res. 2011;109:687–696. doi: 10.1161/CIRCRESAHA.110.236687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shoelson SE, Lee J, Goldfine AB. Inflammation and insulin resistance. J Clin Invest. 2006;116:1793–1801. doi: 10.1172/JCI29069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Alexander RW. Theodore Cooper Memorial Lecture. Hypertension and the pathogenesis of atherosclerosis. Oxidative stress and the mediation of arterial inflammatory response: a new perspective. Hypertension. 1995;25:155–161. doi: 10.1161/01.hyp.25.2.155. [DOI] [PubMed] [Google Scholar]
- 52.Drazner MH. The progression of hypertensive heart disease. Circulation. 2011;123:327–334. doi: 10.1161/CIRCULATIONAHA.108.845792. [DOI] [PubMed] [Google Scholar]
- 53.Grossman E, Messerli FH, Boyko V, Goldbourt U. Is there an association between hypertension and cancer mortality? Am J Med. 2002;112:479–486. doi: 10.1016/s0002-9343(02)01049-5. [DOI] [PubMed] [Google Scholar]
- 54.Felmeden DC, Spencer CGC, Belgore FM, Blann AD, Beevers DG, Lip GYH. Endothelial damage and angiogenesis in hypertensive patients: relationship to cardiovascular risk factors and risk factor management. Am J Hypertens. 2003;16:11–20. doi: 10.1016/s0895-7061(02)03149-7. [DOI] [PubMed] [Google Scholar]
- 55.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 56.Kang YS, Park YG, Kim BK, Han SY, Jee YH, Han KH, Lee MH, Song HK, Cha DR, Kang SW, Han DS. Angiotensin II stimulates the synthesis of vascular endothelial growth factor through the p38 mitogen activated protein kinase pathway in cultured mouse podocytes. J Mol Endocrinol. 2006;36:377–388. doi: 10.1677/jme.1.02033. [DOI] [PubMed] [Google Scholar]
- 57.Stamler J, Wentworth D, Neaton JD. Is relationship between serum cholesterol and risk of premature death from coronary heart disease continuous and graded? Findings in 356,222 primary screenees of the Multiple Risk Factor Intervention Trial (MRFIT) JAMA. 1986;256:2823–2828. [PubMed] [Google Scholar]
- 58.Alexopoulos CG, Blatsios B, Avgerinos A. Serum lipids and lipoprotein disorders in cancer patients. Cancer. 1987;60:3065–3070. doi: 10.1002/1097-0142(19871215)60:12<3065::aid-cncr2820601234>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 59.Gabitova L, Gorin A, Astsaturov I. Molecular pathways: sterols and receptor signaling in cancer. Clin Cancer Res An Off J Am Assoc Cancer Res. 2014;20:28–34. doi: 10.1158/1078-0432.CCR-13-0122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tseng TH, Hsu JD, Chu CY, Wang CJ. Promotion of colon carcinogenesis through increasing lipid peroxidation induced in rats by a high cholesterol diet. Cancer Lett. 1996;100:81–87. doi: 10.1016/0304-3835(95)04073-0. [DOI] [PubMed] [Google Scholar]
- 61.Reddy BS. Diet and excretion of bile acids. Cancer Res. 1981;41:3766–3768. [PubMed] [Google Scholar]
- 62.Warner M, Gustafsson J-A. On estrogen, cholesterol metabolism, and breast cancer. N Engl J Med. 2014;370:572–573. doi: 10.1056/NEJMcibr1315176. [DOI] [PubMed] [Google Scholar]
- 63.Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, El-Chami MF, Bhakta S, Winchester DE, Al-Mallah MH, Sanchez Shields M, Deedwania P, Mehta LS, Phan BAP, Benowitz NL. Cardiovascular Effects of Exposure to Cigarette Smoke and Electronic Cigarettes. J Am Coll Cardiol. 2015;66:1378–1391. doi: 10.1016/j.jacc.2015.07.037. [DOI] [PubMed] [Google Scholar]
- 64.Leikauf GD, Borchers MT, Prows DR, Simpson LG. Mucin apoprotein expression in COPD. Chest. 2002;121:166S–182S. doi: 10.1378/chest.121.5_suppl.166s. [DOI] [PubMed] [Google Scholar]
- 65.Scientific Report of the 2015 Dietary Guidelines Advisory. ww.health.gov/dietaryguidelines/2015-scientific-report[Internet]. [cited 2015 Oct 10];Available from: http://health.gov/dietaryguidelines/2015-scientific-report/PDFs/07-Part-D-Chapter-2.pdf.
- 66.Stampfer M, Jahn JL. Partnerships for promoting prevention. Circulation. 2013;127:1267–1269. doi: 10.1161/CIRCULATIONAHA.113.001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wang T, Lee HG. Advances in research on cis-9, trans-11 conjugated linoleic acid: a major functional conjugated linoleic acid isomer. Crit Rev Food Sci Nutr. 2015;55:720–731. doi: 10.1080/10408398.2012.674071. [DOI] [PubMed] [Google Scholar]
- 68.English DR, MacInnis RJ, Hodge AM, Hopper JL, Haydon AM, Giles GG. Red meat, chicken, and fish consumption and risk of colorectal cancer. Cancer Epidemiol Biomarkers Prev. 2004;13:1509–1514. [PubMed] [Google Scholar]
- 69.Wood AD, Strachan AA, Thies F, Aucott LS, Reid DM, Hardcastle AC, Mavroeidi A, Simpson WG, Duthie GG, Macdonald HM. Patterns of dietary intake and serum carotenoid and tocopherol status are associated with biomarkers of chronic low-grade systemic inflammation and cardiovascular risk. Br J Nutr. 2014;112:1341–1352. doi: 10.1017/S0007114514001962. [DOI] [PubMed] [Google Scholar]
- 70.Baena Ruiz R, Salinas Hernández P. Diet and cancer: risk factors and epidemiological evidence. Maturitas. 2014;77:202–208. doi: 10.1016/j.maturitas.2013.11.010. [DOI] [PubMed] [Google Scholar]
- 71.Chang YJ, Myung S-K, Chung ST, Kim Y, Lee E-H, Jeon Y-J, Park C-H, Seo HG, Huh BY. Effects of vitamin treatment or supplements with purported antioxidant properties on skin cancer prevention: a meta-analysis of randomized controlled trials. Dermatology. 2011;223:36–44. doi: 10.1159/000329439. [DOI] [PubMed] [Google Scholar]
- 72.MacLean CH, Newberry SJ, Mojica WA, Khanna P, Issa AM, Suttorp MJ, Lim Y-W, Traina SB, Hilton L, Garland R, Morton SC. Effects of omega-3 fatty acids on cancer risk: a systematic review. JAMA. 2006;295:403–415. doi: 10.1001/jama.295.4.403. [DOI] [PubMed] [Google Scholar]
- 73.Ronksley PE, Brien SE, Turner BJ, Mukamal KJ, Ghali WA. Association of alcohol consumption with selected cardiovascular disease outcomes: a systematic review and meta-analysis. BMJ. 2011;342:d671. doi: 10.1136/bmj.d671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Costanzo S, Di Castelnuovo A, Donati MB, Iacoviello L, de Gaetano G. Cardiovascular and overall mortality risk in relation to alcohol consumption in patients with cardiovascular disease. Circulation. 2010;121:1951–1959. doi: 10.1161/CIRCULATIONAHA.109.865840. [DOI] [PubMed] [Google Scholar]
- 75.Albert MA, Glynn RJ, Ridker PM. Alcohol consumption and plasma concentration of C-reactive protein. Circulation. 2003;107:443–447. doi: 10.1161/01.cir.0000045669.16499.ec. [DOI] [PubMed] [Google Scholar]
- 76.Alcohol consumption and ethyl carbamate. IARC Monogr Eval Carcinog Risks Hum. 2010;96:3–1383. [PMC free article] [PubMed] [Google Scholar]
- 77.Bagnardi V, Rota M, Botteri E, Tramacere I, Islami F, Fedirko V, Scotti L, Jenab M, Turati F, Pasquali E, Pelucchi C, Bellocco R, Negri E, Corrao G, Rehm J, Boffetta P, La Vecchia C. Light alcohol drinking and cancer: a meta-analysis. Ann Oncol. 2013;24:301–308. doi: 10.1093/annonc/mds337. [DOI] [PubMed] [Google Scholar]
- 78.Boffetta P, Hashibe M. Alcohol and cancer. Lancet Oncol. 2006;7:149–156. doi: 10.1016/S1470-2045(06)70577-0. [DOI] [PubMed] [Google Scholar]
- 79.Myers J. Exercise and Cardiovascular Health. Circulation. 2003;107:2e–5. doi: 10.1161/01.cir.0000048890.59383.8d. [DOI] [PubMed] [Google Scholar]
- 80.Li T, Wei S, Shi Y, Pang S, Qin Q, Yin J, Deng Y, Chen Q, Wei S, Nie S, Liu L. The dose-response effect of physical activity on cancer mortality: findings from 71 prospective cohort studies. Br J Sports Med. 2015 doi: 10.1136/bjsports-2015-094927. [DOI] [PubMed] [Google Scholar]
- 81.Friedenreich CM, Orenstein MR. Physical activity and cancer prevention: etiologic evidence and biological mechanisms. J Nutr. 2002;132:3456S–3464S. doi: 10.1093/jn/132.11.3456S. [DOI] [PubMed] [Google Scholar]
- 82.Sur I, Tuupanen S, Whitington T, Aaltonen LA, Taipale J. Lessons from functional analysis of genome-wide association studies. Cancer Res. 2013;73:4180–4184. doi: 10.1158/0008-5472.CAN-13-0789. [DOI] [PubMed] [Google Scholar]
- 83.Henley SJ, Singh S, King J, Wilson R, Ryerson B. Invasive cancer incidence - United States, 2010. MMWR Morb Mortal Wkly Rep. 2014;63:253–259. [PMC free article] [PubMed] [Google Scholar]
- 84.Frieden TR. CDC Health Disparities and Inequalities Report - United States, 2013. Foreword. MMWR Surveill Summ. 2013;62(Suppl 3):1–2. [PubMed] [Google Scholar]
- 85.Johnson JA, Carstensen B, Witte D, Bowker SL, Lipscombe L, Renehan AG Diabetes Cancer Research C. Diabetes and cancer (1): evaluating the temporal relationship between type 2 diabetes and cancer incidence. Diabetologia. 2012;55:1607–1618. doi: 10.1007/s00125-012-2525-1. [DOI] [PubMed] [Google Scholar]
- 86.Pernicova I, Korbonits M. Metformin--mode of action and clinical implications for diabetes and cancer. Nat Rev Endocrinol. 2014;10:143–56. doi: 10.1038/nrendo.2013.256. [DOI] [PubMed] [Google Scholar]
- 87.Mannucci E, Monami M, Balzi D, Cresci B, Pala L, Melani C, Lamanna C, Bracali I, Bigiarini M, Barchielli A, Marchionni N, Rotella CM. Doses of insulin and its analogues and cancer occurrence in insulin-treated type 2 diabetic patients. Diabetes Care. 2010;33:1997–2003. doi: 10.2337/dc10-0476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hemkens LG, Grouven U, Bender R, Gunster C, Gutschmidt S, Selke GW, Sawicki PT. Risk of malignancies in patients with diabetes treated with human insulin or insulin analogues: a cohort study. Diabetologia. 2009;52:1732–1744. doi: 10.1007/s00125-009-1418-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Grossman E, Messerli FH, Goldbourt U. Does diuretic therapy increase the risk of renal cell carcinoma? Am J Cardiol. 1999;83:1090–1093. doi: 10.1016/s0002-9149(99)00021-1. [DOI] [PubMed] [Google Scholar]
- 90.Lijinsky W, Reuber MD. Pathologic effects of chronic administration of hydrochlorothiazide, with and without sodium nitrite, to F344 rats. Toxicol Ind Health. 1987;3:413–422. doi: 10.1177/074823378700300313. [DOI] [PubMed] [Google Scholar]
- 91.National Toxicology P. Toxicology and Carcinogenesis Studies of Furosemide (CAS No. 54-31-9) in F344/N Rats and B6C3F1 Mice (Feed Studies) Natl Toxicol Program Tech Rep Ser. 1989;356:1–190. [PubMed] [Google Scholar]
- 92.Chow WH, McLaughlin JK, Mandel JS, Wacholder S, Niwa S, Fraumeni JF. Risk of renal cell cancer in relation to diuretics, antihypertensive drugs, and hypertension. Cancer Epidemiol Biomarkers Prev A Publ Am Assoc Cancer Res Cosponsored by Am Soc Prev Oncol. 1995;4:327–331. [PubMed] [Google Scholar]
- 93.McLaughlin JK, Chow WH, Mandel JS, Mellemgaard A, McCredie M, Lindblad P, Schlehofer B, Pommer W, Niwa S, Adami H-O. International renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertension. Int J Cancer. 1995;63:216–221. doi: 10.1002/ijc.2910630212. [DOI] [PubMed] [Google Scholar]
- 94.Finkle WD, McLaughlin JK, Rasgon SA, Yeoh HH, Low JE. Increased risk of renal cell cancer among women using diuretics in the United States. Cancer causes Control CCC. 1993;4:555–558. doi: 10.1007/BF00052431. [DOI] [PubMed] [Google Scholar]
- 95.Ganz PA, Habel LA, Weltzien EK, Caan BJ, Cole SW. Examining the influence of beta blockers and ACE inhibitors on the risk for breast cancer recurrence: results from the LACE cohort. Breast Cancer Res Treat. 2011;129:549–556. doi: 10.1007/s10549-011-1505-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Warita K, Warita T, Beckwitt CH, Schurdak ME, Vazquez A, Wells A, Oltvai ZN. Statin-induced mevalonate pathway inhibition attenuates the growth of mesenchymal-like cancer cells that lack functional E-cadherin mediated cell cohesion. Sci Rep. 2014;4:7593. doi: 10.1038/srep07593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Dale KM, Coleman CI, Henyan NN, Kluger J, White CM. Statins and cancer risk: a meta-analysis. JAMA. 2006;295:74–80. doi: 10.1001/jama.295.1.74. [DOI] [PubMed] [Google Scholar]
- 98.Nielsen SF, Nordestgaard BG, Bojesen SE. Statin use and reduced cancer-related mortality. N Engl J Med. 2012;367:1792–1802. doi: 10.1056/NEJMoa1201735. [DOI] [PubMed] [Google Scholar]
- 99.Mucci LA, Stampfer MJ. Mounting evidence for prediagnostic use of statins in reducing risk of lethal prostate cancer. J Clin Oncol. 2014;32:1–2. doi: 10.1200/JCO.2013.53.2770. [DOI] [PubMed] [Google Scholar]
- 100.Zhong S, Zhang X, Chen L, Ma T, Tang J, Zhao J. Statin use and mortality in cancer patients: Systematic review and meta-analysis of observational studies. Cancer Treat Rev. 2015;41:554–567. doi: 10.1016/j.ctrv.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 101.Shebl FM, Hsing AW, Park Y, Hollenbeck AR, Chu LW, Meyer TE, Koshiol J. Non-steroidal anti-inflammatory drugs use is associated with reduced risk of inflammation-associated cancers: NIH-AARP study. PLoS One. 2014;9:e114633. doi: 10.1371/journal.pone.0114633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rothwell PM, Wilson M, Elwin C-E, Norrving B, Algra A, Warlow CP, Meade TW. Long-term effect of aspirin on colorectal cancer incidence and mortality: 20-year follow-up of five randomised trials. Lancet (London, England) 2010;376:1741–1750. doi: 10.1016/S0140-6736(10)61543-7. [DOI] [PubMed] [Google Scholar]
- 103.Garcia-Albeniz X, Chan AT. Aspirin for the prevention of colorectal cancer. Best Pract Res Clin Gastroenterol. 2011;25:461–472. doi: 10.1016/j.bpg.2011.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10:697–710. doi: 10.1038/nrclinonc.2013.195. [DOI] [PubMed] [Google Scholar]
- 105.Barrett-Lee PJ, Dixon JM, Farrell C, Jones A, Leonard R, Murray N, Palmieri C, Plummer CJ, Stanley A, Verrill MW. Expert opinion on the use of anthracyclines in patients with advanced breast cancer at cardiac risk. Ann Oncol Off J Eur Soc Med Oncol/ESMO. 2009;20:816–827. doi: 10.1093/annonc/mdn728. [DOI] [PubMed] [Google Scholar]
- 106.Hamnvik O-PR, Choueiri TK, Turchin A, McKay RR, Goyal L, Davis M, Kaymakcalan MD, Williams JS. Clinical risk factors for the development of hypertension in patients treated with inhibitors of the VEGF signaling pathway. Cancer. 2015;121:311–319. doi: 10.1002/cncr.28972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Morris PG, Hudis CA. Trastuzumab-related cardiotoxicity following anthracycline-based adjuvant chemotherapy: how worried should we be? J Clin Oncol. 2010;28:3407–3410. doi: 10.1200/JCO.2009.26.0125. [DOI] [PubMed] [Google Scholar]
- 108.Hayes DF, Picard MH. Heart of darkness: the downside of trastuzumab. J Clin Oncol. 2006;24:4056–4058. doi: 10.1200/JCO.2006.07.5143. [DOI] [PubMed] [Google Scholar]
- 109.Ezaz G, Long JB, Gross CP, Chen J. Risk prediction model for heart failure and cardiomyopathy after adjuvant trastuzumab therapy for breast cancer. J Am Heart Assoc. 2014;3:e000472. doi: 10.1161/JAHA.113.000472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Armstrong GT, Kawashima T, Leisenring W, Stratton K, Stovall M, Hudson MM, Sklar CA, Robison LL, Oeffinger KC. Aging and risk of severe, disabling, life-threatening, and fatal events in the childhood cancer survivor study. J Clin Oncol. 2014;32:1218–27. doi: 10.1200/JCO.2013.51.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Armstrong GT, Oeffinger KC, Chen Y, Kawashima T, Yasui Y, Leisenring W, Stovall M, Chow EJ, Sklar CA, Mulrooney DA, Mertens AC, Border W, Durand J-B, Robison LL, Meacham LR. Modifiable risk factors and major cardiac events among adult survivors of childhood cancer. J Clin Oncol. 2013;31:3673–3680. doi: 10.1200/JCO.2013.49.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Nothlings U, Ford ES, Kroger J, Boeing H. Lifestyle factors and mortality among adults with diabetes: findings from the European Prospective Investigation into Cancer and Nutrition-Potsdam study*. J Diabetes. 2010;2:112–117. doi: 10.1111/j.1753-0407.2010.00069.x. [DOI] [PubMed] [Google Scholar]
- 113.Sasco AJ, Secretan MB, Straif K. Tobacco smoking and cancer: a brief review of recent epidemiological evidence. Lung Cancer. 2004;45(Suppl 2):S3–9. doi: 10.1016/j.lungcan.2004.07.998. [DOI] [PubMed] [Google Scholar]


