Abstract
In recent years, cancer immunotherapy made significant advances due to a better understanding of the principles underlying tumor biology and immunology. In this context, CD73 is a key molecule, since via degradation of adenosine monophosphate into adenosine, endorses the generation of an immunosuppressed and pro-angiogenic niche within the tumor microenvironment that promotes the onset and progression of cancer. Targeting CD73 results in favorable antitumor effects in pre-clinical models and combined treatments of CD73 blockade with other immune-modulating agents (i.e. anti-CTLA-4 mAb or anti-PD1 mAb) is particularly attractive. Although there is still a long way to go, anti-CD73 therapy, through the development of CD73 monoclonal antibodies, can potentially constitute a new biologic therapy for cancer patients. In this review, we discuss the link between CD73 and the onset, development and spread of tumors, highlighting the potential value of this molecule as a target and as a novel biomarker in the context of personalized cancer therapy
Keywords: CD73, adenosine, cancer, tumor microenvironment, immunotherapy
Neoplastic microenvironment: a leading role for adenosine
The neoplastic microenvironment is a dynamic niche consisting of cancer cells, immune cells, fibroblasts, myofibroblasts, cytokines, and blood vessels, as well as extracellular matrix[1]. The environment surrounding the tumor mass is void of oxygen, low in glucose and other nutrients, and it is usually characterized by low pH values[2]. Cancer cells reprogram their metabolic machinery to adapt to survive in a harsh environment and, in some cases, subvert these adverse conditions to their own benefit[1, 3]. Among these metabolic alterations, emerging evidence shows a key role for altered purine metabolism, particularly, for an increased expression of ecto-5'-nucleotidase, otherwise known as CD73[4, 5]. This catabolic enzyme (Box 1 summarizes the molecular structure and function of CD73), dephosphorylates extracellular adenosine monophosphate (AMP) (Figure 1) and plays a pivotal role in generating an ‘immunosuppressive and pro-angiogenic adenosine halo’ that contributes to cancer progression [4–6]. Extracellular adenosine, via interaction with specific G-protein-coupled receptors, shapes the activity of a variety of cell types in the neoplastic milieu, causing dysregulation of immune cell infiltrates and a pro-tumorigenic abnormal vascularization, which promotes the onset and progression of tumors, metastatic spread, and determines a poor disease outcome [5].
Box 1. Molecular biology and regulation of CD73.
Human CD73 is encoded by the NT5E gene, located on the long (q) arm of chromosome 6 between positions 14 and 21, from base pair (bp) 86159301 to bp 86205508[109]. There are two known transcript variants that encode two different isoforms. Variant 1 represents the longer transcript and encodes the longer isoform 1, with 9 exons, a transcript length of 3548 bp and translation length of 574 residues. Variant 2 lacks an alternate in-frame exon compared to variant 1, consists of 8 exons and has a transcript length of 3384 bp and translation length of 524 residues. NT5E is a classic hypoxia inducible factor (HIF) target gene, whose expression and function is stimulated by hypoxic conditions[110–113]. The expression and function of CD73 is up-regulated by several inflammatory mediators besides hypoxia, such as transforming growth factor (TGF)-β, interferons (IFNs), tumor necrosis factor (TNF), interleukin (IL)-1β and prostaglandin E2[5, 114]. In addition, signaling through the intracellular Wnt and cAMP pathways, as well as exposure of cells to metabolites such as polyunsaturated fatty acids can also regulate CD73 expression, thus highlighting that the expression of this enzyme can be modulated by different factors through a number of mechanisms[9]. CD73 is expressed to variable extents in different tissues, and is specially abundant in the colon, kidney, brain, liver, heart, lung, spleen, lymph nodes and the bone marrow[5]. In the vasculature, CD73 is predominantly associated with the endothelium of vessels such as the aorta, carotid and coronary artery, as well as on afferent lymphatic endothelium, where it participates in the regulation of leukocyte trafficking[91]. In the immune system, CD73 is found on the surface of macrophages, lymphocytes, regulatory T cells (Treg) and dendritic cells[5, 36].
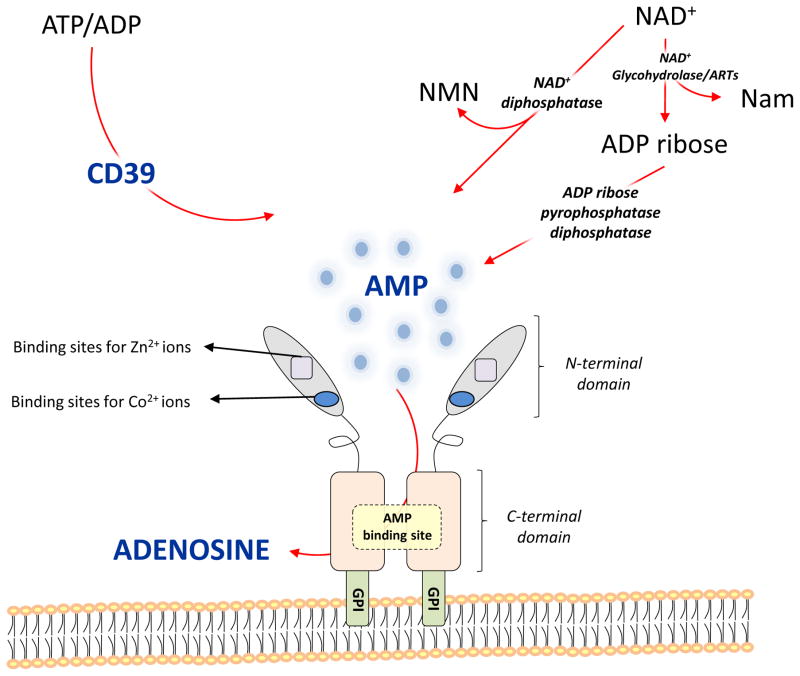
Structurally, CD73 consists of two identical 70-kD subunits tethered by non-covalent bonds and anchored to the plasma membrane via a C-terminal serine residue, which is linked to glycosylphosphatidyl inositol (GPI) (Figure 1). In the mature form, a 26-amino acid signal peptide in the N-terminal domain coordinates the binding of two catalytic divalent metal ions (Zn2+ and Co2+), whereas the C-terminal domain provides the binding pocket for AMP [115]. A portion of approximatively 25 hydrophobic aminoacid residues at the C-terminus is replaced by a GPI anchor, which mediates binding of the mature protein to the cell membrane via the C-terminal Ser-523 residue [115]. This Ser-523 residue is linked to a complex oligoglycan and a sphingolipidinositol group [115]. No protein segments are embedded within the cell membrane[115]. The active site is located at the interface of the two 70-kD subunits. Substrate-bound and substrate-free structures of CD73 indicate that a large domain motion is required for catalysis [115]. A truncated soluble form of CD73, that retains ecto-5′-nucleotidase activity, has also been described [5, 116]. This soluble form can be shed from the membrane by hydrolysis of the GPI anchor by phosphatidylinositol-specific phospholipase or proteolytic cleavage and can circulate freely in the bloodstream and other biological fluids [5, 117]. It has been suggested that soluble nucleotidases could represent an important auxiliary effector system for local inactivation of acutely elevated nucleotides, especially at sites of injury and inflammation [117].
Functionally, CD73 is involved in two important extracellular metabolic pathways: AMP and nicotinamide adenine dinucleotide (NAD+) metabolism. In the first pathway, extracellularly released ATP and ADP are degraded to AMP by CD39, and then CD73 mediates hydrolysis of AMP to adenosine and inorganic phosphate [6, 118]. Once released, extracellular adenosine binds specific G-protein-coupled cell surface adenosine receptors named A1, A2A, A2B and A3. The other pathway involves NAD+, an essential co-enzyme that can be released into the extracellular milieu[119] and then degraded by different enzymes, such as NAD+-glycohydrolases, ADP-ribosyltransferases and pyrophosphatases [120, 121]. Pyrophosphatases cleave NAD+ to yield AMP and β–nicotinamide mononucleotide, thereby providing the substrate for CD73[121] (Figure 1). It is important to note that in addition to its well-understood enzymatic function, CD73 also acts as adhesion molecule, participating in the migration of normal and neoplastic cells[122].
Figure 1.
Structure and role of CD73 in the extracellular metabolic pathways.
CD73 is characterized by an N-terminal domain containing binding sites for zinc and cobalt ions and a C-terminal domain containing the AMP binding site. The C-terminal domain is linked to the plasma membrane through a GPI anchor. These two domains are connected by a short α helix. Once released in the extracellular milieu, ATP and ADP are converted into AMP via ecto enzyme CD39 and further into adenosine through CD73. Extracellular NAD+ is converted into ADPribose and nicotinamide (Nam) by NAD+-glycohydrolases and ADP ribosyl-transferases (ARTs). ADP ribose can be further split to form AMP by ADP-ribose pyrophosphatase, subsequently converted to adenosine by CD73. In addition, NAD+ can also be degradated by NAD+ diphosphatase to produce nicotinamide mononucleotide (NMN) and AMP.
Over the past few years, studies on the modulation of CD73 expression and activity in various preclinical models of neoplastic disorders have highlighted the value of this enzyme as a potential therapeutic target for cancer treatment [7–12]. Engineered mice lacking CD73 display resistance to the onset of neoplasia and metastasis[13–15], while targeting CD73 with small molecule inhibitors or monoclonal antibodies shows antitumor effects[16–23]. In addition, pharmacological blockade of CD73 synergizes with other currently available antineoplastic agents, such as anthracycline[24], anti-cytotoxic T-lymphocyte antigen (CTLA)-4 mAb[25] and anti-programmed cell death protein (PD)-1 mAb[25].
This Review discusses the role of CD73 in tumorigenesis and its potential as a molecular target and biomarker in cancer immunotherapy.
Regulation of cancer immunity by CD73
Recent work suggests that tumor escape from immune surveillance is supported by the cellular and molecular characteristics of the local microenvironment and can occur by two major broad mechanisms [26]. In one, tumors display a T cell–inflamed phenotype consisting of infiltrating T cells, a broad chemokine profile and a type I interferon signature indicative of innate immune activation [26]. These tumors appear to resist against immune attack through the dominant inhibitory effects of immune system [26]. In particular, despite the influx of CD8+ effector T cells into the tumor microenvironment, these cells become functionally inhibited by the effects of programmed death-ligand-1(PD-L1), indoleamine 2,3-dioxygenase (IDO), regulatory T cells (Tregs) and anergy [26]. In the second, the tumors are characterized by poor expression of chemokines and lack the T cell–inflamed phenotype. It is speculated that these tumors appear to resist immune attack through immune system exclusion or ignorance via a denser stroma and alternative myeloid or macrophage populations [26].
Increasing line of evidence shows that CD73 participates in the process of tumor immunoescape by inhibiting the activation, clonal expansion and homing of tumor-specific T cells, and thus impairing tumor cell killing by cytolytic effector T lymphocytes, dictating a substantial component of the suppressive capabilities of Treg and Th17 cells, and enhancing the conversion of anti-tumor type 1 macrophages into pro-tumor type 2 macrophages [27–29].
Role of CD73 in T cell-infiltrated tumors
Patients with tumors characterized by high infiltration of cytotoxic T lymphocytes generally display enhanced survival rates [30]. Nevertheless, despite the presence of cytotoxic T cells into the tumor microenvironment, the cancer puts into play a series of immunosuppressive pathways (i.e. Tregs, a broad range of chemokines, and innate immune cells, such as dendritic cells (DCs) and macrophages) that provide a shield against anti-tumor immune response [26].
Tregs, which are highly enriched in the tumor microenvironment, can prevent an efficient effector T cell response against cancer cells, thus paving the way towards uncontrolled growth of the tumor [31]. The depletion of Tregs has been associated with increased infiltration of cytotoxic T cells [30]. An increasing body of evidence highlights a pivotal role of CD73 in the regulation of Treg activity [8, 15, 32–34]. Murine and human Treg cells express CD39, responsible for hydrolysis of ATP into AMP[35]. The subsequent hydrolysis of AMP into adenosine is mediated by CD73, which in mice is also expressed on the surface of Treg cells[35]. By contrast, in human Tregs, there are high levels of CD73 intracellularly, suggesting that these cells have the potential to up-regulate surface ectonucleotidase activity under appropriate circumstances[36].
CD73, facilitating the pericellular generation of adenosine, is responsible for a substantial component of the immunosuppressive and anti-inflammatory functions of Treg cells[5]. The immunosuppressive action of Treg-derived adenosine can be ascribed to the activation of A2A receptors expressed on T effector cells. In addition, adenosine triggers a self-reinforcing autocrine loop of Treg function, as the stimulation of A2A receptors on Tregs elicits cell expansion and increases their immunoregulatory activity[27]. In parallel, A2A receptor activation on effector cells inhibits T-cell–mediated cytotoxicity and causes a reduction of cytokine production and T-cell proliferation[27, 37]. CD73-derived adenosine, produced by Tregs, inhibits NF-κB activation in effector T cells through A2A receptors[38], thereby reducing the release of a broad range of pro-inflammatory cytokines and chemokines [39]. It has been observed that Treg-depleted mice, reconstituted with purified CD4+ CD25+ Tregs isolated from wild-type mice, but not from CD73-deficient mice, develop colon cancer, thus indicating a critical role for this enzyme in paving the way to the tumor immune escape [15].
The activation of mammalian target of rapamycin (mTOR), the catalytic subunit of complexes-1 and 2 (mTORC1,2), is a critical negative regulator of Treg de novo differentiation and population expansion [40]. Upon uptake into the cell via nucleoside transporters, adenosine inhibits the mTOR in Tregs by activating the AMP kinase (AMPK), thus promoting cell differentiation and expansion [36]. In particular, AMPK activation inhibits the mTORC1 both directly and indirectly via the tuberous sclerosis complexes 1 and 2 (TSC1, 2) and the Rheb protein. In addition, extracellular adenosine can also generate increased levels of AMP within the cell via activation of A2A and A2B receptors and also contribute to mTOR inhibition [36].
The classical T cell anergy, a condition of hyporesponsiveness during which T cells fail to respond to their cognate antigen, leads to a T cell–intrinsic dysfunction that contributes to cancer immune escape[26]. CD73 is increased in anergic T cells and contributes to the hyporesponsivity of these cells following in vitro stimulation[41].
CD73 expressed on stromal cells or tumor cells contributes substantially to tumor-induced immune suppression. Adenosine generated by CD73 expressed on tumor cells decreases the function of antitumor T-cell and promotes T-cell apoptosis, thereby contributing to tumor immune evasion [23, 24, 34] [42]. For example, the antigen-specific T cell responses, including both activation and effector function, are suppressed in the local tumor microenvironment of CD73-expressing peritoneal tumors [42]. The effector function of these cells is restored by knockdown of tumor CD73[42]. In hematopoietic and non-hematopoietic stromal cells, CD73 fosters distinct adenosine-dependent effects to regulate antitumor T cell responses. CD73 expression on non-hematopoietic cells, such as endothelial cells, is important in controlling T cell homing to the tumor mass via adenosine production, while adenosine generation by CD73 on leukocytes restricts both T cell proliferation and effector function[34]. In addition, CD73 can promote tumor growth through a non-enzymatic mechanism, by functioning as an adhesion molecule for immune cells[43, 44]. Indeed, CD73 Abs interfere with the adhesion of lymphocytes to cultured endothelial cells [43]. In particular, the engagement of lymphocyte CD73, but not the endothelial CD73, represents a critical step in enhancing lymphocyte binding to endothelial cells, an event that is mediated by the integrin lymphocyte function-associated antigen 1 (LFA-1)[44]. The engagement of CD73 does not induce a high-affinity state for LFA-1 receptors but increases the avidity of LFA-1 by calpain-dependent cluster formation, a critical adhesion-enhancing event[44].
While DCs are important for inducing and maintaining antitumor immunity, they often become inefficient within the cancer environment. For example, they can become compromised in their ability to present antigens to T cells due to incomplete maturation [45]. DCs can also be polarized into immunosuppressive/tolerogenic regulatory cells and this limits their stimulation of effector T cells and supports tumor growth and progression[45]. Among several factors that account for an abnormal function of DCs in cancer [45], adenosine is a primary candidate, as deletion of A2A and A2B receptors stimulates dendritic cells function and activates anti-tumor immunity [46, 47]. Thus, CD73 may also contribute to cancer progression by adenosine-mediated suppression of dendritic cells.
Macrophages are essential innate effector cells activated by a wide spectrum of host- or tumor-derived stimuli and polarized towards functionally different phenotypes[48]. They fall into two distinct subsets: i) classically activated macrophages (M1), expressing a series of pro-inflammatory cytokines, chemokines and effector molecules (i.e IL-12, IL-23, TNF, iNOS and MHCI/II)[48]; and ii) alternatively activated macrophages (M2), expressing a wide array of anti-inflammatory molecules, such as IL-10, TGF-β and arginase1[48]. In most cancers, infiltrating macrophages are polarized towards the M2 phenotype and provide an immunosuppressive microenvironment that promotes tumor growth[48]. CD73 is critically involved in the fine-tuning of macrophage differentiation and activity by regulation of adenosine concentration in the extracellular space[5]. Pro-inflammatory M1 macrophages display decreased CD73 expression and activity, which associates with reduced adenosine generation[49]. By contrast, M2 macrophages show increased expression and activity of CD73 and increased conversion of AMP into adenosine[49]. Since adenosine is a strong stimulator of M2 macrophage polarization[50–56], it is possible that CD73 pro-tumorigenic effects are also mediated by an enhanced alternative macrophage activation.
The ultimate goal in cancer immunotherapy is to drive tumour-specific T cells to tumors where they can kill malignant cells[30]. Ipilumimab, a novel checkpoint inhibitor antibody (CTLA-4 blocker) amplifies T cell infiltration into neoplastic tissue and thereby increases patience survival[30]. PD-1 receptor is another immune checkpoint molecule that promotes the apoptosis of antigen specific T-cells in lymph nodes and simultaneously decreases apoptosis of Tregs [57, 58]. Allard et al.[25] evaluated whether targeted blockade of CD73 can enhance the antitumor activity of anti-CTLA-4 and anti-PD-1 antibodies against transplanted and chemically induced mouse tumors. CD73 blockade enhances the activity of both drugs, suggesting that anti-CD73 antibodies potentiate the therapeutic activity of immune checkpoint inhibitors.
Role of CD73 in non-T cell-infiltrated tumors
Non-T cell-infiltrated tumors avoid immune attacks by scarce chemokine expression, lack of T-cell infiltration and a minimal presence of defined immune inhibitory pathways [26]. They have a denser stroma and an increased myeloid-derived suppressor cell (MDSC) and alternative macrophage populations[26]. In these tumors, the vasculature is non permissive to the entry of T cells and the dense stroma also prevents normal T cell trafficking and/or function [26].
MDSCs comprise a heterogeneous population of immature myeloid cells, including myeloid progenitors and precursors of macrophages, granulocytes, and dendritic cells [59] [60]. Once activated, MDSCs produce arginase 1, inducible nitric oxide synthase, IDO, NADPH oxidase and a plethora of immunosuppressive cytokines [59]. Studies showed an inverse correlation between MDSCs and T cell frequency in the peripheral blood of tumor bearing mice [61]. High levels of CD73 are expressed in granulocytic MDSCs and the ability of these cells to suppress T cell proliferation is facilitated by the presence of AMP (CD73 substrate), which is quickly converted into adenosine [62]. The activation of A2B receptors on myeloid precursors prompts the expansion of granulocytic MDSCs, thus creating a positive feedback mechanism that further facilitates the generation of adenosine and enhances immunosuppression[62]. TGF-β promotes the maturation of myeloid-derived suppressor cells into terminally differentiated myeloid mononuclear cells (TDMMC), and induces high levels of CD39/CD73 expression and increased adenosine-generating capacity[60]. The disruption of TGF-β signaling in myeloid cells decreases the accumulation of TDMMCs expressing CD39 and CD73, and is followed by increased infiltration of T lymphocytes, reduced density of blood vessels, and decreased cancer growth[60].
The cancer stromal compartment includes cancer-associated fibroblasts (CAFs), epithelial cells and mesenchymal stem cells (MSCs) as well as variable amounts of extracellular matrix (ECM). These stroma components promote tumor progression, angiogenesis, inflammation and metastasis via the release of growth factors and chemokines [63, 64] and impair host immune responses [26]. Non-T-cell infiltrated tumors are characterized by dense peritumoral ECM fibers surrounding tumor islets, which together with a lack of adhesion molecules and abnormal vessel structure, play an important role in limiting T cell access to cancer cells[65].
CAFs secrete different paracrine and autocrine factors involved in tumorigenesis[66]. Bone marrow mesenchymal stem cells (MSCs) can be recruited into tumours, where they proliferate and acquire a CAF-like phenotype[66]. CAFs and MSCs share immunophenotypic similarities, including the presence of cell surface factors, such as human leukocyte antigen-DR sub-region (HLA-DR), CD29, CD44, CD73, CD90, CD106 and CD117[66]. However, CAFs preferentially secrete vascular endothelial growth factor (VEGF), TGF-β1, interleukins (IL-4, IL-10) and TNF [66]. Huang et al. reported that CD117+/CD73+ fibroblast-like stromal cells are associated with worst clinicopathological features and poor survival in ovarian carcinomas [67]. CD73 expression in CAFs positively correlates with tumor progression and worse prognosis in breast cancer [68], rectal adenocarcinoma [69, 70] and high-grade serous ovarian cancer [71].
MSCs migrate and engraft into tumor sites where they affect cancer growth, invasion and metastasis [72]. Tumour MSC density was found to correlate directly with tumor volume and inversely with the density of tumor-infiltrating leukocytes[73]. MSCs contribute to blood- and lymph- neo-angiogenesis and myofibroblast generation, and display a range of immunomodulatory effects on both the innate and adaptive arms of the immune system[74]. Tumour-MSCs exert a suppressive effect on stimulated T lymphocytes, which is primarily mediated by indoleamine 2,3 dioxygenase activity [73], and express CD73 on their surface[73, 75, 76], which contributes to immunosuppression via adenosine production[77]. Although CD73 is a major cell surface marker for MSCs, it is surprising how little is known about the role of this molecule in the regulation of these cells [78].
Non-T-cell infiltrated tumors, which cannot support trafficking of activated T cells into the tumor microenvironment, are unlikely to respond clinically to current immunotherapies. For these reasons, therapeutic interventions for this subset of tumors will need to focus on strategies aimed at increasing cytotoxic T cell infiltration and activation. As CD73 appears to be important for preventing T cell infiltration into tumors, CD73 blockade may favor the priming of the immune system and a recruitment of T cells through a reduction of intratumoral adenosine levels.
CD73 in neoplastic cells
An increasing body of evidence supports a pivotal role of the adenosine pathway in the regulation of cancer growth and dissemination by interfering with cell proliferation, apoptosis and metastasis[4]. CD73, which is over-expressed in several cancer types (see Table I) [79], appears to critically modulate the magnitude and duration of intratumoral purinergic signaling [80–84]. The mechanisms underlying CD73 overexpression in tumor cells were recently summarized by Allard et al. [85] (see Box 1).
Table I.
CD73 in solid and blood neoplasias.
| Cancer types | CD73 Expression and Activity | Refs |
|---|---|---|
| Glioma | Increase of CD73 mRNA and activity in glioma cell lines | [83, 142] |
| Head Neck | Increase of CD73 expression and activity in Treg cells | [143] |
| Melanoma | Elevated CD73 expression is associated with a highly invasive phenotype of melanoma cell lines | [144] |
| Thyroid | Increase of CD73 expression and activity on the apical cell membrane of papillary carcinoma | [145] |
| Breast | Increase of CD73 mRNA and protein level and increased enzyme activity in breast carcinoma cell lines and in tumor stroma Involvement of CD73 in tumor growth and the suitability of this enzyme as a marker of breast cancer progression CD73 expression correlates positively with epidermal growth factor receptor expression/function |
[68, 80, 98, 100, 146] |
| Pancreas | Increase of CD73 expression in the cytoplasm of pancreatic ductal adenocarcinomas | [147] |
| Colon | Increase of CD73 expression in tumor cells and stroma of colorectal cancer | [70, 148] |
| Bladder | Increase of CD73 mRNA and activity in human tumor bladder cell lines | [149] |
| Ovarian | Increase of CD73 expression in epithelial cells of ovarian cancer | [135] |
| Prostate | Increase of CD73 expression in lymph node metastasizing prostate cancer | [95] |
| Leukemia | Increase of CD73 expression in proliferating chronic lymphocytic leukemia cells and in perivascular areas | [84] |
In vitro and in vivo studies showed that CD73 stimulates the growth of cancer cells [13, 14, 22, 23, 81, 83]. CD73 was especially efficient in boosting the growth of colon adenocarcinoma, melanoma and lymphoma [15, 34].
CD73 in angiogenesis and lymphangiogenesis
A role for CD73 in supporting tumor angiogenesis was established both in vitro and in vivo [11, 18, 86, 87]. CD73 promotes the formation of new vessels in C57BL/6 mice bearing B16F10 melanoma cells and tumor-induced angiogenesis in Matrigel plugs [18]. The pharmacological inhibition of CD73 decreases the development of melanoma vascular beds by reducing the number or the maturation of developing new blood vessels[18]. CD73 can also promote microvascular endothelial cell migration and the formation of tube-like structures[86]. These effects can be related to both non-enzymatic and enzymatic activities of CD73 [86]. CD73 induces endothelial cell growth by up-regulating the pro-proliferative protein cyclin D1[86]. In addition, the CD73-derived adenosine promotes the production and release of VEGF during hypoxia through A2A receptors, thus supporting angiogenesis [86]. Allard et al.[11] recently confirmed that CD73 expressed on cancer cells promotes VEGF production via its enzymatic activity, whereas CD73 expression on endothelial cells prompts tube formation and endothelial cell migration independently of extracellular adenosine generation[11] .
The tumor microenvironment contains many factors that promote lymphangiogenesis[88]. Recent experimental and clinical studies indicate that lymphangiogenic factors influence cancer spreading [89]. The lymphatic endothelium, like the vascular endothelium, also expresses CD73. However, the function of this enzyme in the lymphatic context remains fairly unknown. Recently, Yegutkin et al.[90] shed new light on the role of CD73 in lymphatic vessels. The authors reported that lymphatic CD73 is not needed for VEGF/bFGF-induced lymphangiogenesis or for the control of lymphatic migration of immune cells [90], in contrast to what is observed in blood vessels[91]. Intralymphatic lymphocytes express higher levels of CD73 than blood-borne lymphocytes[90]. Based on current evidence, it appears that CD73 activity does not play the same functional role in terms of leukocyte trafficking, vascular permeability and angiogenesis in the lymphatic and vascular systems. New efforts to examine further these differential roles should be focused on discovering the relevant roles of CD73 in the regulation of lymphatic endothelium and lymphocytes.
CD73 in metastasis
Increasing evidence indicates that adenosine generated via CD73 activity is implicated in cancer cell spread (see Box 2)[15, 19, 92–99]. A correlation between CD73 expression and metastasis-related markers is reported [99]. Expression of CD73 in increased in lymph node metastasizing prostate tumors compared with non-metastasizing ones, thus suggesting a critical role for this enzyme in enhancing metastatic propensity [95].
Box 2. CD73 and exosomes.
The metastatic process responsible for the spread of cancer cells from the primary site and formation of new tumors in distant organs via blood and/or lymphatic vessels accounts for as much as 90% of cancer-associated mortality and represents the most scantly understood stage of cancer pathophysiology[123]. A large body of experimental research and clinical data corroborates the “seed and soil” hypothesis formulated in 1889 by Stephen Paget, who proposed the organ-preference patterns of tumor metastasis as the result of favorable interactions between metastatic tumor cells (the “seed”), and their organ microenvironment (the “soil”)[124]. Emerging evidence suggests that cancer-derived exosomes may contribute to the recruitment and reprogramming of tumor stromal cells to form a pro-tumorigenic soil [125]. Exosomes are small membrane vesicles of endocytic origin, ranging from 50 to 100 nm in size, which can be released into the extracellular environment and be up-taken by neighboring cells[125, 126].
Several cell types, including immune and mesenchymal cells, as well as neoplastic cells, can release exosomes. Cancer-derived exosomes were described as sources of tumor antigens that can be used to induce antitumor immune responses[127]. It was demonstrated that tumor-derived exosomes are endowed with immunosuppressive properties, facilitating tumor growth, metastasis, and development of drug resistance[127], and thus contributing to the generation of pre-metastatic niches that are suitable for supporting the survival and growth of disseminated tumors[4].
Recently, it was reported that exosomes released by cancer cells are endowed with CD39 and CD73, which confer them the ability to dephosphorylate exogenous ATP and AMP into adenosine[128]. This mechanism induces an increase in adenosine concentration within the neoplastic microenvironment and may contribute to exosome-mediated immune evasion in cancer[128]. Exosomes released by Tregs following TCR activation also express CD39 and CD73 and contribute to immune suppression [129]. Schuler et al.[35] observed that CD4+CD39+ Tregs co-incubated with CD4+CD73+ T cells, B cells or CD39+ CD73+ exosomes produced adenosine, suggesting that a contact with membrane-tethered CD73 is necessary for production of immunosuppressive adenosine[35]. By investigating the effects of the internalization of mesenchymal stroma/stem cell-derived exosomes into different tumor types, a rearrangement of tumor microenvironment has been observed, with the acquisition of new tumor cell properties, such as an increased expression of CD73 and MMP-2. Along with being incorporated into exosomes, both CD39 and CD73 can freely circulate in the bloodstream as soluble enzymes modulating the metabolism of intravascular nucleotides[130].
The blockade of CD73 prevents the adhesion of human melanoma cells[99] and human breast cancer cells [19, 23, 81, 98] to ECM and subsequent migration. By contrast, forced over-expression of CD73 leads to increased epidermal growth factor receptor (EGFR) and IL-8 expression, and confers increased invasiveness and adhesiveness to ECM, processes that were prevented by blockade of CD73 and restored by exogenous adenosine[100]. The contribution of CD73 to the metastatic process has been observed in CD73-knockout mice, which display resistance against the development of melanoma metastases, in comparison with wild-type counterparts[15]. The pro-metastatic action of CD73 is independent from its immunosuppressive effects and it is instead ascribed to CD73 expression on endothelial cells. Therefore, the authors hypothesized that CD73 has a role in supporting transendothelial migration of tumour cells[15]. In other studies, however, the pro-metastatic effect of CD73 has an immune component, and CD73-derived adenosine increases melanoma metastasis by inhibiting perforin-dependent NK cell cytotoxicity through A2A receptors [94].
Epithelial-mesenchymal transition (EMT) is the process by which epithelial cells are reprogrammed to lose their polarity and adhesion, acquiring migratory and invasive properties [101]. Recently, Xiong et al. [102] reported that CD73 contributes to the initiation of EMT by regulating key factors such as cadherin-1 and vimentin.
Stagg et al. [23] showed that treatment of mice with the anti-CD73 (antibody clone TY/23) curbed the development of spontaneous 4T1.2 lung metastases[23], confirming the role of CD73 in promoting metastasis. In another study, treatment with AD2, an anti-human CD73 antibody with a moderate effect on CD73 catabolic activity, counteracted the ability of circulating human cancer cells to establish secondary tumor foci by promoting clustering and internalization of CD73 and therefore limiting the ability of tumor cells to extravasate and colonize other tissues[19]. The authors excluded a catabolic effect of CD73, since in in vitro assays AD2 displayed a negligible effect on the enzyme catalytic activity. However, the authors' conclusion that AD2 inhibits metastasis by a mechanism independent of CD73 catalytic activity should be taken with caution.
Different authors proposed that some of the tumorigenic effects attributed to CD73 are not related with its enzymatic activity[19] [37] [103]. These conclusions mainly rest on studies with the most potent competitive CD73 inhibitor known to date, α,β-methylene-ADP (APCP), which is capable of inhibiting AMP hydrolysis in different cells and tissues at a low micromolar range [6, 104]. Given that many tumor cells express extremely high CD73, it cannot be excluded that the remaining enzymatic activity could be sufficient for efficient breakdown of exogenously applied or endogenously released AMP in competitive functional assays. Probably, the employment of novel, more potent and selective CD73 inhibitors will allow to discriminate between enzymatic and non-enzymatic functions of CD73 in tumors[104].
Concluding remarks
CD73 plays multiple roles during cancer initiation and progression. Targeting CD73 with the small molecule APCP, as well as with novel and more potent APCP derivatives[104] or with neutralizing monoclonal antibodies shows anti-tumor effects in various pre-clinical models and, therefore, has translational potential (see Table II). Small molecules such as APCP or novel derivatives have several advantages over monoclonal antibody approaches such as better oral bioavailability, a greater exposure within the tumor microenvironment due to increased facility to cross physiological barriers (i.e. the blood–brain barrier), or the availability of diverse formulations that alleviate pharmacokinetic and/or pharmacodynamic challenges. Other advantage of small-molecules relates to the access of patients to these drugs, since they cost less and are easy to administrate (pill) than antibodies (infusion or injection). On the other hand, the use of neutralizing anti-CD73 mAbs can be of particular interest, since antibodies have generally higher specificity and longer half-life when compared with small molecule inhibitors. In addition, mAbs can exert both direct on-target and indirect immune-dependent antitumor effects, such as antibody-dependent cell-mediated cytotoxicity[105] through engagement of FcγR on immune cells[10], or antibody-dependent cell-mediated phagocytosis[105]. Moreover, several of the pro-tumorigenic effects of CD73 cannot be ascribed to its catalytic activity, suggesting that the use of anti-CD73 mAbs that trigger effects beyond blocking AMP degradation may be more useful than molecules that only inhibit the catalytic domain of CD73.
Table II.
In vivo effects of small molecule inhibitors and antibodies against CD73 in cancer
| Pharmacological tool | Animals | Tumor | Pharmacological effects | Refs |
|---|---|---|---|---|
| APCP (CD73 inhibitor) | C57BL/6 mice | Murine breast cancer cells | Inhibition of tumor migration | [94] |
| APCP | C57BL/6j mice | Murine melanoma cells | Tumor regression, by promoting the release of Th1- and Th17-associated cytokines, as well as CD8+ T cell infiltration in the tumor microenvironment. | [21] |
| APCP | C57BL/6 mice | Murine melanoma cells | Inhibition of tumor growth | [13] |
| APCP and short-hairpin RNA against CD73 | C57BL/6 mice | Murine ovarian cancer cells | Increased survival of tumor-bearing mice | [42] |
| APCP and anti-CD73 mAb | C57BL/6 mice | Murine melanoma cells | Inhibition of lung metastases | [15] |
| APCP | C57BL/6 mice | Murine breast cancer cells | Reduction of microvessels density in tumor | [150] |
| APCP | Nude/Balb/C mice | Human breast adenocarcinoma cells | Inhibition of tumor growth | [80] |
| APCP and anti-CD73 mAb | C57BL/6 mice | Murine melanoma cells | Inhibition of tumor growth and increased efficacy of adoptive T cell therapy | [34] |
| CD73 small interfering RNA RNA | Nude/Balb/C mice | Human breast adenocarcinoma cells | Increased apoptosis and inhibition of tumor growth | [81] |
| Anti-CD73 mAb and short- hairpin RNA against CD73 | Balb/C mice | Murine breast cancer cells | Inhibition of tumor growth and reduction of spontaneous lung metastasis | [23] |
| Anti-CD73 mAb | Athymic nude mice | Human breast cancer cells | Inhibition of tumor growth | [151] |
| Anti-CD73 mAb | C57BL/6 mice | Murine prostate cancer cells | Inhibition of tumor growth and metastasis | [14] |
| Anti-CD73 mAb | C57BL/6 mice | MCA-induced fibrosarcoma | Inhibition of tumor growth | [14] |
Taken together, this body of evidence indicates that CD73 blockade is a promising therapeutic option for the treatment of various cancers. In particular, CD73 blockade can magnify the efficacy of novel anticancer therapies based on the manipulation of effector T-cell activity through the blockade of immune checkpoint receptors with antibodies, such as ipilimumab (anti-CTLA-4 mAb) and nivolumab (anti-PD1 mAb). Indeed, despite the fact that many cancer patients display long-lasting clinical responses to immune checkpoint inhibitors, in some cases, the clinical benefits from these therapies are still modest. This failure may be ascribed to non-overlapping immunosuppressive mechanisms put into place by tumor to evade immune surveillance. The accumulation of adenosine seems to be one of these mechanisms. For this reason, the combination of CD73 blockade with novel immune checkpoint inhibitors as well as with the conventional cancer treatments (radiotherapy, chemotherapy, anti-angiogenic agents, or targeted therapies) could increase the efficacy of therapy across a wider range of cancers. In addition, the relationship between CD73 overexpression and cancer subtype, prognosis, and patient response to chemotherapy supports the potential value of CD73 as a biomarker to aid in personalized cancer therapy (see Box 3). However, many questions remain open (see Outstanding Questions).
Box 3. CD73 as a cancer biomarker.
Growing clinical evidence indicates a correlation between CD73 expression and the prognosis of oncologic patients, pointing out the relevance of this enzyme as a useful predictive biomarker [9]. In particular, CD73 overexpression has been associated with a worse prognosis in patients affected by digestive neoplasia (gastric, colorectal and gallbladder cancer)[70, 102, 131, 132], breast cancer (triple negative, luminal and HER2+ cancer)[24], prostate cancer [95], melanoma [133], and chronic lymphoblastic leukemia[84]. However, in some circumstances CD73 expression did not have a prognostic value (i.e. acute lymphoblastic leukemia[134]), while in some cases an increased CD73 expression is associated with a favorable prognosis, such as in epithelial ovarian carcinoma[135] or breast cancer (stage I-III)[136].
Besides having value as a prognostic marker, increased CD73 expression is also associated with chemotherapy resistance [137]. Pre-clinical[20, 138-140] and clinical studies [24, 141] indicate that CD73 has an important role in modulating anti-cancer chemoresistance mechanisms. Recent studies reported the involvement of CD73 in vincristine resistant glioblastoma multiforme cells[20], where a tight correlation between CD73 and the multiple drug protein-1 (Mrp1) (a transporter involved in conferring multiple drug resistance to cancer cells) was observed. The inhibition of CD73 expression in glioblastoma multiforme cells reduced Mrp1 expression, indicating that increased expression of CD73 can mediate drug resistance via Mrp1 modulation[139]. In breast cancer, CD73 overexpression in tumor cells confers chemoresistance to doxorubicin via increased adenosine generation and activation of A2A receptors [24].
Outstanding questions.
Is the expression of CD73 associated with resistance against cancer chemotherapy?
Will anti-CD73 antibodies be useful as therapeutic tools to treat non-T-cell infiltrated tumors?
Anti-CD73 antibodies offer a great promise to counteract cancer onset and development in combination with other therapies (i.e. anti-PD-1mAbs), but what is the appropriate timing, dosing and administration sequence of these agents to be successful in combinatorial approaches?
Nevertheless, there are some caveats to keep in mind when considering treatments based on CD73-blockade. First, CD73 is involved in the regulation, of several body homeostatic processes, such as the control of epithelial barrier function [106] and the regulation of secretive/reabsorptive processes at intestinal level [106]. Although animal models lacking CD73 or treated with anti-CD73 drugs do not show significant adverse events, most likely by virtue of compensatory mechanisms, the potential for adverse events following anti-CD73 therapy must be carefully taken into consideration before translating this therapeutic strategy into clinical practice. Second, anti-CD73 therapy could induce a supraphysiological immune activation that may overcome the body mechanisms of tolerance, thus leading to events mimicking autoimmune disorders. In addition, the extreme variability of local tumor microenvironments could deeply affect the response to anti-CD73 therapy. Teng et al.[107] suggested a stratification of tumors amenable to immunotherapy based on the immunoediting capacity of the tumor microenvironment (i.e. presence of T-cell infiltration and expression of the immunosuppressive pathways PD-1 and PDL-1). Accordingly, therapy with anti-CD73 mAbs might lead to better responses in patients with infiltrated cancers, since the reduction of CD73-derived adenosine in cancer milieu may spur the activity of infiltrating immune cells the tumor. Doubt persists regarding non-infiltrated cancer subtypes that are scarcely permeated by immune cells and, thereby, respond poorly to immune checkpoint therapy. In these tumors, blockade of CD73 can be of interest if there is any involvement of this enzyme on the interplay of non-immune cancer components, such as cancer cells and stroma [108].
Based on encouraging preclinical data, the clinical translation of anti-CD73 therapy is expected in the near future. At present, there are increasing efforts going into the generation of fully human or humanized anti-CD73 antibodies. Current Phase 1 multicenter clinical studies are evaluating the use of anti CD73 MEDI9447 alone or in combination with anti-PD-L1 antibody in patients with advanced solid tumors (ClinicalTrials.gov Identifier: NCT02503774).
In conclusion, anti-CD73 antibodies represent valuable anti-cancer tools that can be wisely combined and/or integrated with conventional chemo-radiation regimens, molecular targeted therapies or with other novel immunostrategies (see Table III).
Table III.
Generations of cancer immunotherapies
| First generation of cancer immunotherapies | ||
| Interferon-α (IFN-α) | FDA approved for hair cell leukemia | [152] |
| Interleukin-2 (IL-2) | FDA approved for metastatic melanoma and renal cell carcinoma | [152] |
| Second generation of cancer immunotherapies | ||
| Vaccines | Dendritic cell-based vaccines | [153] |
| Viral vector vaccines | [154] | |
| mRNA-based vaccines | [155] | |
| Multipeptide-based vaccines | [156] | |
| Locally released virotherapy | [157] | |
| Adoptive T cell therapy | Tumor-infiltrating lymphocyte | [158] |
| Chimeric antigen receptors | [159] | |
| Chimeric antigen receptor-transduced T lymphocytes | [160] | |
| Immune checkpoint inhibitors | mAbs against cytotoxic T lymphocyte-associated antigen 4 (CTLA4) (ipilimumab, tremelimumab) mAbs against programmed cell death protein 1 (PD1) (nivolumab, pembrolizumab, pidilizumab) tremelimumab) mAbs against programmed cell death protein ligand 1 (PDL1) (atezolizumab, MEDI4736, MSB0010718C) mAbs against lymphocyte activation gene 3 protein (LAG 3) (BMS-986016) |
[161] |
| Co-stimulatory mAbs | Agonistic CD137-specific mAbs (Urelumab, PF-05082566) Agonistic OX40-specific mAbs (MEDI6469, MEDI6383, MEDI0562, MOXR0916) Agonistic CD40-specific mAbs (CP870,893) Agonistic GITR-specific mAbs (TRX518, MK-4166) |
[161] |
Trends Box.
The immune system plays a critical role in the recognition and suppression of neoplastic cells. First generation immunotherapies have been used for decades with moderate success against a few tumors. However, the majority of such immunotherapies displayed a lack of either substantial efficacy or specificity, resulting in adverse events and thus a limited clinical employment (see Table III).
Over the last years, a better understanding of the complex interactions between the immune system and tumors has allowed the identification of key molecules (i.e. CTLA-4, PD-1, PD-L1) governing such interactions. This information has revitalized the interest in cancer immunotherapeutics designed to overcome the mechanisms exploited by tumors to evade immune-mediated destruction (see Table III).
The complementary modes of action of novel immunotherapies and conventional chemotherapy or targeted therapy suggest the possibility for therapeutic synergy in combination treatments.
Although the inhibition of CTLA-4, PD-1 or PD-L1 is being used successfully in the clinical practice, additional checkpoint pathways are being actively investigated. Among these additional immune checkpoint pathways, the ecto-5’nucleotidase (CD73) has been found to play a critical role in driving cancer immune evasion, thus representing a promising target for developing novel anticancer immunotherapies.
Acknowledgments
This work was supported by Nexus award “Marcello Tonini” (L.A.), by IBD Research Foundation (mini grant 2012) (L.A.), and by National Institutes of Health Grant R01GM66189 (G.H.).
Footnotes
Competing interests
The authors declare no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Weber CE, Kuo PC. The tumor microenvironment. Surg Oncol. 2012;21:172–177. doi: 10.1016/j.suronc.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Cairns RA, et al. Regulation of cancer cell metabolism. Nat Rev Cancer. 2011;11:85–95. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Antonioli L, et al. Immunity, inflammation and cancer: a leading role for adenosine. Nat Rev Cancer. 2013;13:842–857. doi: 10.1038/nrc3613. [DOI] [PubMed] [Google Scholar]
- 5.Antonioli L, et al. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;19:355–367. doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yegutkin GG. Enzymes involved in metabolism of extracellular nucleotides and nucleosides: functional implications and measurement of activities. Crit Rev Biochem Mol Biol. 2014;49:473–497. doi: 10.3109/10409238.2014.953627. [DOI] [PubMed] [Google Scholar]
- 7.Zhang B. CD73: a novel target for cancer immunotherapy. Cancer Res. 2010;70:6407–6411. doi: 10.1158/0008-5472.CAN-10-1544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beavis PA, et al. CD73: a potent suppressor of antitumor immune responses. Trends Immunol. 2012;33:231–237. doi: 10.1016/j.it.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 9.Gao ZW, et al. The roles of CD73 in cancer. Biomed Res Int. 2014;2014:460654. doi: 10.1155/2014/460654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang B. Opportunities and challenges for anti-CD73 cancer therapy. Immunotherapy. 2012;4:861–865. doi: 10.2217/imt.12.83. [DOI] [PubMed] [Google Scholar]
- 11.Allard B, et al. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2014;134:1466–1473. doi: 10.1002/ijc.28456. [DOI] [PubMed] [Google Scholar]
- 12.Cushman S, et al. Gene Expression Markers of Efficacy and Resistance to Cetuximab Treatment in Metastatic Colorectal Cancer: Results from CALGB 80203 (Alliance) Clin Cancer Res. 2015;21:1078–1086. doi: 10.1158/1078-0432.CCR-14-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yegutkin GG, et al. Altered purinergic signaling in CD73-deficient mice inhibits tumor progression. Eur J Immunol. 2011;41:1231–1241. doi: 10.1002/eji.201041292. [DOI] [PubMed] [Google Scholar]
- 14.Stagg J, et al. CD73-deficient mice are resistant to carcinogenesis. Cancer Res. 2012;72:2190–2196. doi: 10.1158/0008-5472.CAN-12-0420. [DOI] [PubMed] [Google Scholar]
- 15.Stagg J, et al. CD73-deficient mice have increased antitumor immunity and are resistant to experimental metastasis. Cancer Res. 2011;71:2892–2900. doi: 10.1158/0008-5472.CAN-10-4246. [DOI] [PubMed] [Google Scholar]
- 16.Figueiro F, et al. A monastrol-derived compound, LaSOM 63, inhibits ecto-5'nucleotidase/CD73 activity and induces apoptotic cell death of glioma cell lines. Anticancer Res. 2014;34:1837–1842. [PubMed] [Google Scholar]
- 17.Hausler SF, et al. Anti-CD39 and anti-CD73 antibodies A1 and 7G2 improve targeted therapy in ovarian cancer by blocking adenosine-dependent immune evasion. Am J Transl Res. 2014;6:129–139. [PMC free article] [PubMed] [Google Scholar]
- 18.Koszalka P, et al. Inhibition of CD73 stimulates the migration and invasion of B16F10 melanoma cells in vitro, but results in impaired angiogenesis and reduced melanoma growth in vivo. Oncol Rep. 2014;31:819–827. doi: 10.3892/or.2013.2883. [DOI] [PubMed] [Google Scholar]
- 19.Terp MG, et al. Anti-human CD73 monoclonal antibody inhibits metastasis formation in human breast cancer by inducing clustering and internalization of CD73 expressed on the surface of cancer cells. J Immunol. 2013;191:4165–4173. doi: 10.4049/jimmunol.1301274. [DOI] [PubMed] [Google Scholar]
- 20.Quezada C, et al. 5'-ectonucleotidase mediates multiple-drug resistance in glioblastoma multiforme cells. J Cell Physiol. 2013;228:602–608. doi: 10.1002/jcp.24168. [DOI] [PubMed] [Google Scholar]
- 21.Forte G, et al. Inhibition of CD73 improves B cell-mediated anti-tumor immunity in a mouse model of melanoma. J Immunol. 2012;189:2226–2233. doi: 10.4049/jimmunol.1200744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhi X, et al. RNAi-mediated CD73 suppression induces apoptosis and cell-cycle arrest in human breast cancer cells. Cancer Sci. 2010;101:2561–2569. doi: 10.1111/j.1349-7006.2010.01733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stagg J, et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc Natl Acad Sci U S A. 2010;107:1547–1552. doi: 10.1073/pnas.0908801107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Loi S, et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc Natl Acad Sci U S A. 2013;110:11091–11096. doi: 10.1073/pnas.1222251110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Allard B, et al. Targeting CD73 enhances the antitumor activity of anti-PD-1 and anti-CTLA-4 mAbs. Clin Cancer Res. 2013;19:5626–5635. doi: 10.1158/1078-0432.CCR-13-0545. [DOI] [PubMed] [Google Scholar]
- 26.Gajewski TF, et al. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–1022. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deaglio S, et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J Exp Med. 2007;204:1257–1265. doi: 10.1084/jem.20062512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eichin D, et al. CD73 Activity is Dispensable for the Polarization of M2 Macrophages. PLoS One. 2015;10:e0134721. doi: 10.1371/journal.pone.0134721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chalmin F, et al. Stat3 and Gfi-1 transcription factors control Th17 cell immunosuppressive activity via the regulation of ectonucleotidase expression. Immunity. 2012;36:362–373. doi: 10.1016/j.immuni.2011.12.019. [DOI] [PubMed] [Google Scholar]
- 30.Oelkrug C, JM Enhancement of T cell recruitment and infiltration into tumours. Clin Exp Immunol. 2014;178:1–8. doi: 10.1111/cei.12382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crimeen-Irwin B, et al. Failure of immune homeostasis -- the consequences of under and over reactivity. Curr Drug Targets Immune Endocr Metabol Disord. 2005;5:413–422. doi: 10.2174/156800805774912980. [DOI] [PubMed] [Google Scholar]
- 32.Li MQ, et al. CD4+Foxp3+ regulatory T cell differentiation mediated by endometrial stromal cell-derived TECK promotes the growth and invasion of endometriotic lesions. Cell Death Dis. 2014;5:e1436. doi: 10.1038/cddis.2014.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Salmi M, Jalkanen S. Host CD73 impairs anti-tumor immunity. Oncoimmunology. 2012;1:247–248. doi: 10.4161/onci.1.2.18310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wang L, et al. CD73 has distinct roles in nonhematopoietic and hematopoietic cells to promote tumor growth in mice. J Clin Invest. 2011;121:2371–2382. doi: 10.1172/JCI45559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schuler PJ, et al. Human CD4+ CD39+ regulatory T cells produce adenosine upon co-expression of surface CD73 or contact with CD73+ exosomes or CD73+ cells. Clin Exp Immunol. 2014;177:531–543. doi: 10.1111/cei.12354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Regateiro FS, et al. CD73 and adenosine generation in the creation of regulatory microenvironments. Clin Exp Immunol. 2013;171:1–7. doi: 10.1111/j.1365-2249.2012.04623.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ohta A, et al. A2A adenosine receptor may allow expansion of T cells lacking effector functions in extracellular adenosine-rich microenvironments. J Immunol. 2009;183:5487–5493. doi: 10.4049/jimmunol.0901247. [DOI] [PubMed] [Google Scholar]
- 38.Romio M, et al. Extracellular purine metabolism and signaling of CD73-derived adenosine in murine Treg and Teff cells. Am J Physiol Cell Physiol. 2011;301:C530–539. doi: 10.1152/ajpcell.00385.2010. [DOI] [PubMed] [Google Scholar]
- 39.Zarek PE, et al. A2A receptor signaling promotes peripheral tolerance by inducing T-cell anergy and the generation of adaptive regulatory T cells. Blood. 2008;111:251–259. doi: 10.1182/blood-2007-03-081646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zeng H, et al. mTORC1 couples immune signals and metabolic programming to establish T(reg)-cell function. Nature. 2013;499:485–490. doi: 10.1038/nature12297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Martinez RJ, et al. Arthritogenic self-reactive CD4+ T cells acquire an FR4hiCD73hi anergic state in the presence of Foxp3+ regulatory T cells. J Immunol. 2012;188:170–181. doi: 10.4049/jimmunol.1101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin D, et al. CD73 on tumor cells impairs antitumor T-cell responses: a novel mechanism of tumor-induced immune suppression. Cancer Res. 2010;70:2245–2255. doi: 10.1158/0008-5472.CAN-09-3109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Airas L, et al. CD73 is involved in lymphocyte binding to the endothelium: characterization of lymphocyte-vascular adhesion protein 2 identifies it as CD73. J Exp Med. 1995;182:1603–1608. doi: 10.1084/jem.182.5.1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Airas L, et al. CD73 engagement promotes lymphocyte binding to endothelial cells via a lymphocyte function-associated antigen-1-dependent mechanism. J Immunol. 2000;165:5411–5417. doi: 10.4049/jimmunol.165.10.5411. [DOI] [PubMed] [Google Scholar]
- 45.Ma Y, et al. Dendritic cells in the cancer microenvironment. J Cancer. 2013;4:36–44. doi: 10.7150/jca.5046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cekic C, et al. Adenosine A2B receptor blockade slows growth of bladder and breast tumors. J Immunol. 2012;188:198–205. doi: 10.4049/jimmunol.1101845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cekic C, et al. Myeloid expression of adenosine A2A receptor suppresses T and NK cell responses in the solid tumor microenvironment. Cancer Res. 2014;74:7250–7259. doi: 10.1158/0008-5472.CAN-13-3583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hao NB, et al. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zanin RF, et al. Differential macrophage activation alters the expression profile of NTPDase and ecto-5'-nucleotidase. PLoS One. 2012;7:e31205. doi: 10.1371/journal.pone.0031205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Csoka B, et al. Adenosine promotes alternative macrophage activation via A2A and A2B receptors. FASEB J. 2012;26:376–386. doi: 10.1096/fj.11-190934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Koscso B, et al. Adenosine augments IL-10-induced STAT3 signaling in M2c macrophages. J Leukoc Biol. 2013;94:1309–1315. doi: 10.1189/jlb.0113043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Csoka B, et al. A2B adenosine receptors prevent insulin resistance by inhibiting adipose tissue inflammation via maintaining alternative macrophage activation. Diabetes. 2014;63:850–866. doi: 10.2337/db13-0573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ferrante CJ, et al. The adenosine-dependent angiogenic switch of macrophages to an M2-like phenotype is independent of interleukin-4 receptor alpha (IL-4Ralpha) signaling. Inflammation. 2013;36:921–931. doi: 10.1007/s10753-013-9621-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hasko G, et al. Adenosine receptor agonists differentially regulate IL-10, TNF-alpha, and nitric oxide production in RAW 264.7 macrophages and in endotoxemic mice. J Immunol. 1996;157:4634–4640. [PubMed] [Google Scholar]
- 55.Nemeth ZH, et al. Adenosine augments IL-10 production by macrophages through an A2B receptor-mediated posttranscriptional mechanism. J Immunol. 2005;175:8260–8270. doi: 10.4049/jimmunol.175.12.8260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hasko G, et al. Adenosine inhibits IL-12 and TNF-[alpha] production via adenosine A2a receptor-dependent and independent mechanisms. FASEB J. 2000;14:2065–2074. doi: 10.1096/fj.99-0508com. [DOI] [PubMed] [Google Scholar]
- 57.Stadler S, et al. New therapeutic options for advanced non-resectable malignant melanoma. Adv Med Sci. 2014;60:83–88. doi: 10.1016/j.advms.2014.12.002. [DOI] [PubMed] [Google Scholar]
- 58.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Ryzhov SV, et al. Role of TGF-beta signaling in generation of CD39+CD73+ myeloid cells in tumors. J Immunol. 2014;193:3155–3164. doi: 10.4049/jimmunol.1400578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Talmadge JE, Gabrilovich DI. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13:739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ryzhov S, et al. Adenosinergic regulation of the expansion and immunosuppressive activity of CD11b+Gr1+ cells. J Immunol. 2011;187:6120–6129. doi: 10.4049/jimmunol.1101225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Madar S, et al. 'Cancer associated fibroblasts'--more than meets the eye. Trends Mol Med. 2013;19:447–453. doi: 10.1016/j.molmed.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 64.Lu P, et al. The extracellular matrix: a dynamic niche in cancer progression. J Cell Biol. 2012;196:395–406. doi: 10.1083/jcb.201102147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salmon H, et al. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122:899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Paunescu V, et al. Tumour-associated fibroblasts and mesenchymal stem cells: more similarities than differences. J Cell Mol Med. 2011;15:635–646. doi: 10.1111/j.1582-4934.2010.01044.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Huang R, et al. CD117 expression in fibroblasts-like stromal cells indicates unfavorable clinical outcomes in ovarian carcinoma patients. PLoS One. 2014;9:e112209. doi: 10.1371/journal.pone.0112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kruger KH, et al. Expression of ecto-5'-nucleotidase (CD73) in normal mammary gland and in breast carcinoma. Br J Cancer. 1991;63:114–118. doi: 10.1038/bjc.1991.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhang B, et al. The expression and clinical significance of CD73 molecule in human rectal adenocarcinoma. Tumour Biol. 2015:365459–5466. doi: 10.1007/s13277-015-3212-x. [DOI] [PubMed] [Google Scholar]
- 70.Wu XR, et al. High expression of CD73 as a poor prognostic biomarker in human colorectal cancer. J Surg Oncol. 2012;106:130–137. doi: 10.1002/jso.23056. [DOI] [PubMed] [Google Scholar]
- 71.Turcotte M, et al. CD73 is associated with poor prognosis in high-grade serous ovarian cancer. Cancer Res. 2015;75:4494–503. doi: 10.1158/0008-5472.CAN-14-3569. [DOI] [PubMed] [Google Scholar]
- 72.Hong IS, et al. Mesenchymal stem cells and cancer: Friends or enemies? Mutat Res Fundam Mol Mech Mutagen. 2014;768:98–10. doi: 10.1016/j.mrfmmm.2014.01.006. [DOI] [PubMed] [Google Scholar]
- 73.Liotta F, et al. Mesenchymal stem cells are enriched in head neck squamous cell carcinoma, correlates with tumour size and inhibit T-cell proliferation. Br J Cancer. 2015;112:745–754. doi: 10.1038/bjc.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Amarnath S, et al. Bone Marrow Derived Mesenchymal Stromal Cells Harness Purinergenic Signaling to Tolerize Human Th1 Cells In Vivo. Stem Cells. 2014;33:1200–1212. doi: 10.1002/stem.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Carothers AM, et al. Mesenchymal stromal cell mutations and wound healing contribute to the etiology of desmoid tumors. Cancer Res. 2012;72:346–355. doi: 10.1158/0008-5472.CAN-11-2819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Tanabe S. Role of mesenchymal stem cells in cell life and their signaling. World J Stem Cells. 2014;6:24–32. doi: 10.4252/wjsc.v6.i1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Haddad R, Saldanha-Araujo F. Mechanisms of T-cell immunosuppression by mesenchymal stromal cells: what do we know so far? Biomed Res Int. 2014;2014:216806. doi: 10.1155/2014/216806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Scarfi S. Purinergic receptors and nucleotide processing ectoenzymes: Their roles in regulating mesenchymal stem cell functions. World J Stem Cells. 2014;6:153–162. doi: 10.4252/wjsc.v6.i2.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Antonioli L, et al. CD39 and CD73 in immunity and inflammation. Trends Mol Med. 2013;2013 doi: 10.1016/j.molmed.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhou X, et al. Effects of ecto-5'-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol Rep. 2007;17:1341–1346. [PubMed] [Google Scholar]
- 81.Zhi X, et al. RNA interference of ecto-5'-nucleotidase (CD73) inhibits human breast cancer cell growth and invasion. Clin Exp Metastasis. 2007;24:439–448. doi: 10.1007/s10585-007-9081-y. [DOI] [PubMed] [Google Scholar]
- 82.Braganhol E, et al. Ecto-5'-nucleotidase/CD73 inhibition by quercetin in the human U138MG glioma cell line. Biochim Biophys Acta. 2007;1770:1352–1359. doi: 10.1016/j.bbagen.2007.06.003. [DOI] [PubMed] [Google Scholar]
- 83.Bavaresco L, et al. The role of ecto-5'-nucleotidase/CD73 in glioma cell line proliferation. Mol Cell Biochem. 2008;319:61–68. doi: 10.1007/s11010-008-9877-3. [DOI] [PubMed] [Google Scholar]
- 84.Serra S, et al. CD73-generated extracellular adenosine in chronic lymphocytic leukemia creates local conditions counteracting drug-induced cell death. Blood. 2011;118:6141–6152. doi: 10.1182/blood-2011-08-374728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Allard B, et al. CD73-generated adenosine: orchestrating the tumor-stroma interplay to promote cancer growth. J Biomed Biotechnol. 2012;2012:485156. doi: 10.1155/2012/485156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang L, et al. Ecto-5'-nucleotidase (CD73) promotes tumor angiogenesis. Clin Exp Metastasis. 2013;30:671–680. doi: 10.1007/s10585-013-9571-z. [DOI] [PubMed] [Google Scholar]
- 87.Burghoff S, et al. Growth and metastasis of B16-F10 melanoma cells is not critically dependent on host CD73 expression in mice. BMC Cancer. 2014;14:898. doi: 10.1186/1471-2407-14-898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Eklund L, et al. Mouse models for studying angiogenesis and lymphangiogenesis in cancer. Mol Oncol. 2013;7:259–282. doi: 10.1016/j.molonc.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Stacker SA. The role of tumor lymphangiogenesis in metastatic spread. FASEB J. 2002;16:922–934. doi: 10.1096/fj.01-0945rev. [DOI] [PubMed] [Google Scholar]
- 90.Yegutkin GG, et al. Ecto-5'-nucleotidase/CD73 enhances endothelial barrier function and sprouting in blood but not lymphatic vasculature. Eur J Immunol. 2015;45:562–573. doi: 10.1002/eji.201444856. [DOI] [PubMed] [Google Scholar]
- 91.Algars A, et al. Different role of CD73 in leukocyte trafficking via blood and lymph vessels. Blood. 2011;117:4387–4393. doi: 10.1182/blood-2010-11-321646. [DOI] [PubMed] [Google Scholar]
- 92.Pancione M, et al. Immune escape mechanisms in colorectal cancer pathogenesis and liver metastasis. J Immunol Res. 2014;2014:686879. doi: 10.1155/2014/686879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Qin L, et al. Requirement of NK cells for selective A2A receptor blockade to suppress CD73+ tumor metastasis. Immunotherapy. 2014;6:19–21. doi: 10.2217/imt.13.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Beavis PA, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A. 2013;110:14711–14716. doi: 10.1073/pnas.1308209110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang Q, et al. Overexpression of CD73 in prostate cancer is associated with lymph node metastasis. Pathol Oncol Res. 2013;19:811–814. doi: 10.1007/s12253-013-9648-7. [DOI] [PubMed] [Google Scholar]
- 96.Cappellari AR, et al. Characterization of ectonucleotidases in human medulloblastoma cell lines: ecto-5'NT/CD73 in metastasis as potential prognostic factor. PLoS One. 2012;7:e47468. doi: 10.1371/journal.pone.0047468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zhang B. CD73 promotes tumor growth and metastasis. Oncoimmunology. 2012;1:67–70. doi: 10.4161/onci.1.1.18068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wang L, et al. Ecto-5'-nucleotidase promotes invasion, migration and adhesion of human breast cancer cells. J Cancer Res Clin Oncol. 2008;134:365–372. doi: 10.1007/s00432-007-0292-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Sadej R, et al. Ecto-5'-nucleotidase (eN, CD73) is coexpressed with metastasis promoting antigens in human melanoma cells. Nucleosides Nucleotides Nucleic Acids. 2006;25:1119–1123. doi: 10.1080/15257770600894188. [DOI] [PubMed] [Google Scholar]
- 100.Zhou P, et al. Overexpression of Ecto-5'-nucleotidase (CD73) promotes T-47D human breast cancer cells invasion and adhesion to extracellular matrix. Cancer Biol Ther. 2007;6:426–431. doi: 10.4161/cbt.6.3.3762. [DOI] [PubMed] [Google Scholar]
- 101.Goubran HA, et al. Regulation of tumor growth and metastasis: the role of tumor microenvironment. Cancer Growth Metastasis. 2014;7:9–18. doi: 10.4137/CGM.S11285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Xiong L, et al. NT5E and FcGBP as key regulators of TGF-1-induced epithelial-mesenchymal transition (EMT) are associated with tumor progression and survival of patients with gallbladder cancer. Cell Tissue Res. 2014;355:365–374. doi: 10.1007/s00441-013-1752-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Chen F, et al. New horizons in tumor microenvironment biology: challenges and opportunities. BMC Med. 2015;13:45. doi: 10.1186/s12916-015-0278-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bhattarai S, et al. alpha,beta-Methylene-ADP (AOPCP) Derivatives and Analogues: Development of Potent and Selective ecto-5'-Nucleotidase (CD73) Inhibitors. J Med Chem. 2015;2015 doi: 10.1021/acs.jmedchem.5b00802. [DOI] [PubMed] [Google Scholar]
- 105.Young A, et al. Targeting cancer-derived adenosine: new therapeutic approaches. Cancer Discov. 2014;4:879–888. doi: 10.1158/2159-8290.CD-14-0341. [DOI] [PubMed] [Google Scholar]
- 106.Colgan SP, et al. Physiological roles for ecto-5'-nucleotidase (CD73. ) Purinergic Signal. 2006;2:351–360. doi: 10.1007/s11302-005-5302-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Teng MW, et al. Classifying Cancers Based on T-cell Infiltration and PD-L1. Cancer Res. 2015;75:2139–2145. doi: 10.1158/0008-5472.CAN-15-0255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pietras K, Ostman A. Hallmarks of cancer: interactions with the tumor stroma. Exp Cell Res. 2010;316:1324–1331. doi: 10.1016/j.yexcr.2010.02.045. [DOI] [PubMed] [Google Scholar]
- 109.Zimmermann H. 5'-Nucleotidase: molecular structure and functional aspects. Biochem J. 1992;285:345–365. doi: 10.1042/bj2850345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Synnestvedt K, et al. Ecto-5'-nucleotidase (CD73) regulation by hypoxia-inducible factor-1 mediates permeability changes in intestinal epithelia. J Clin Invest. 2002;110:993–1002. doi: 10.1172/JCI15337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hart ML, et al. Hypoxia-inducible factor-1alpha-dependent protection from intestinal ischemia/reperfusion injury involves ecto-5'-nucleotidase (CD73) and the A2B adenosine receptor. J Immunol. 2011;186:4367–4374. doi: 10.4049/jimmunol.0903617. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 112.Robinson A, et al. Mucosal protection by hypoxia-inducible factor prolyl hydroxylase inhibition. Gastroenterology. 2008;134:145–155. doi: 10.1053/j.gastro.2007.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Eltzschig HK, et al. Targeting hypoxia signalling for the treatment of ischaemic and inflammatory diseases. Nat Rev Drug Discov. 2014;13:852–869. doi: 10.1038/nrd4422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kiss J, et al. IFN-beta protects from vascular leakage via up-regulation of CD73. Eur J Immunol. 2007;37:3334–3338. doi: 10.1002/eji.200737793. [DOI] [PubMed] [Google Scholar]
- 115.Strater N. Ecto-5'-nucleotidase: Structure function relationships. Purinergic Signal. 2006;2:343–350. doi: 10.1007/s11302-006-9000-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Heuts DP, et al. Crystal structure of a soluble form of human CD73 with ecto-5'-nucleotidase activity. Chembiochem. 2012;13:2384–2391. doi: 10.1002/cbic.201200426. [DOI] [PubMed] [Google Scholar]
- 117.Yegutkin GG. Nucleotide- and nucleoside-converting ectoenzymes: Important modulators of purinergic signalling cascade. Biochim Biophys Acta. 2008;1783:673–694. doi: 10.1016/j.bbamcr.2008.01.024. [DOI] [PubMed] [Google Scholar]
- 118.Eltzschig HK, et al. Purinergic signaling during inflammation. N Engl J Med. 2012;367:2322–2333. doi: 10.1056/NEJMra1205750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Vaisitti T, et al. NAD+-metabolizing ecto-enzymes shape tumor-host interactions: the chronic lymphocytic leukemia model. FEBS Lett. 2011;585:1514–1520. doi: 10.1016/j.febslet.2011.04.036. [DOI] [PubMed] [Google Scholar]
- 120.Haag F, et al. Extracellular NAD and ATP: Partners in immune cell modulation. Purinergic Signal. 2007;3:71–81. doi: 10.1007/s11302-006-9038-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Horenstein AL, et al. A CD38/CD203a/CD73 ectoenzymatic pathway independent of CD39 drives a novel adenosinergic loop in human T lymphocytes. Oncoimmunology. 2013:2e26246. doi: 10.4161/onci.26246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sadej R, Skladanowski AC. Dual, enzymatic and non-enzymatic, function of ecto-5'-nucleotidase (eN, CD73) in migration and invasion of A375 melanoma cells. Acta Biochim Pol. 2012;59:647–652. [PubMed] [Google Scholar]
- 123.Chaffer CL, Weinberg RA. A perspective on cancer cell metastasis. Science. 2011;331:1559–1564. doi: 10.1126/science.1203543. [DOI] [PubMed] [Google Scholar]
- 124.Langley RR, I, Fidler J. The seed and soil hypothesis revisited--the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128:2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kahlert C, Kalluri R. Exosomes in tumor microenvironment influence cancer progression and metastasis. J Mol Med (Berl ) 2013;91:431–437. doi: 10.1007/s00109-013-1020-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Braicu C, et al. Exosomes as divine messengers: are they the Hermes of modern molecular oncology? Cell Death Differ. 2015;22:34–45. doi: 10.1038/cdd.2014.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Yang C, Robbins PD. The roles of tumor-derived exosomes in cancer pathogenesis. Clin Dev Immunol. 2011;2011:842849. doi: 10.1155/2011/842849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Clayton A, et al. Cancer exosomes express CD39 and CD73, which suppress T cells through adenosine production. J Immunol. 2011;187:676–683. doi: 10.4049/jimmunol.1003884. [DOI] [PubMed] [Google Scholar]
- 129.Smyth LA, et al. CD73 expression on extracellular vesicles derived from CD4+ CD25+ Foxp3+ T cells contributes to their regulatory function. Eur J Immunol. 2013;43:2430–2440. doi: 10.1002/eji.201242909. [DOI] [PubMed] [Google Scholar]
- 130.Yegutkin GG, et al. Metabolism of circulating ADP in the bloodstream is mediated via integrated actions of soluble adenylate kinase-1 and NTPDase1/CD39 activities. FASEB J. 2012;26:3875–3883. doi: 10.1096/fj.12-205658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu N, et al. CD73 as a novel prognostic biomarker for human colorectal cancer. J Surg Oncol. 2012;106:918–919. doi: 10.1002/jso.23159. [DOI] [PubMed] [Google Scholar]
- 132.Lu XX, et al. Expression and clinical significance of CD73 and hypoxia-inducible factor-1alpha in gastric carcinoma. World J Gastroenterol. 2013;19:1912–1918. doi: 10.3748/wjg.v19.i12.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Wang H, et al. NT5E (CD73) is epigenetically regulated in malignant melanoma and associated with metastatic site specificity. Br J Cancer. 2012;106:1446–1452. doi: 10.1038/bjc.2012.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Wieten E, et al. CD73 (5'-nucleotidase) expression has no prognostic value in children with acute lymphoblastic leukemia. Leukemia. 2011;25:1374–1376. doi: 10.1038/leu.2011.174. [DOI] [PubMed] [Google Scholar]
- 135.Oh HK, et al. Overexpression of CD73 in epithelial ovarian carcinoma is associated with better prognosis, lower stage, better differentiation and lower regulatory T cell infiltration. J Gynecol Oncol. 2012:23274–281. doi: 10.3802/jgo.2012.23.4.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Supernat A, et al. CD73 expression as a potential marker of good prognosis in breast carcinoma. Appl Immunohistochem Mol Morphol. 2012;20:103–107. doi: 10.1097/pai.0b013e3182311d82. [DOI] [PubMed] [Google Scholar]
- 137.Holohan C, et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 138.Ujhazy P, et al. Evidence for the involvement of ecto-5'-nucleotidase (CD73) in drug resistance. Int J Cancer. 1996;68:493–500. doi: 10.1002/(SICI)1097-0215(19961115)68:4<493::AID-IJC15>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 139.Garrido W, et al. FK506 confers chemosensitivity to anticancer drugs in glioblastoma multiforme cells by decreasing the expression of the multiple resistance-associated protein-1. Biochem Biophys Res Commun. 2011;411:62–68. doi: 10.1016/j.bbrc.2011.06.087. [DOI] [PubMed] [Google Scholar]
- 140.Ujhazy P, et al. Ecto-5'-nucleotidase (CD73) in multidrug-resistant cell lines generated by doxorubicin. Int J Cancer. 1994;59:83–93. doi: 10.1002/ijc.2910590117. [DOI] [PubMed] [Google Scholar]
- 141.Cushman SM, et al. Gene Expression Markers of Efficacy and Resistance to Cetuximab Treatment in Metastatic Colorectal Cancer: Results from CALGB 80203 (Alliance) Clin Cancer Res. 2015;21:1078–1086. doi: 10.1158/1078-0432.CCR-14-2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Xu S, et al. Synergy between the ectoenzymes CD39 and CD73 contributes to adenosinergic immunosuppression in human malignant gliomas. Neuro Oncol. 2013;15:1160–1172. doi: 10.1093/neuonc/not067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Mandapathil M, et al. Increased ectonucleotidase expression and activity in regulatory T cells of patients with head and neck cancer. Clin Cancer Res. 2009;15:6348–6357. doi: 10.1158/1078-0432.CCR-09-1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Sadej R, et al. Expression of ecto-5'-nucleotidase (eN, CD73) in cell lines from various stages of human melanoma. Melanoma Res. 2006;16:213–222. doi: 10.1097/01.cmr.0000215030.69823.11. [DOI] [PubMed] [Google Scholar]
- 145.Kondo T, et al. Expression of CD73 and its ecto-5'-nucleotidase activity are elevated in papillary thyroid carcinomas. Histopathology. 2006;48:612–614. doi: 10.1111/j.1365-2559.2005.02277.x. [DOI] [PubMed] [Google Scholar]
- 146.Zhi X, et al. Potential prognostic biomarker CD73 regulates epidermal growth factor receptor expression in human breast cancer. IUBMB Life. 2012;64:911–920. doi: 10.1002/iub.1086. [DOI] [PubMed] [Google Scholar]
- 147.Haun RS, et al. Bioorthogonal Labeling Cell-Surface Proteins Expressed in Pancreatic Cancer Cells to Identify Potential Diagnostic/Therapeutic Biomarkers. Cancer Biol Ther. 2015;16:1557–1556. doi: 10.1080/15384047.2015.1071740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Zhang B, et al. The expression and clinical significance of CD73 molecule in human rectal adenocarcinoma. Tumour Biol. 2015;2015 doi: 10.1007/s13277-015-3212-x. [DOI] [PubMed] [Google Scholar]
- 149.Stella J, et al. Differential ectonucleotidase expression in human bladder cancer cell lines. Urol Oncol. 2010;28:260–267. doi: 10.1016/j.urolonc.2009.01.035. [DOI] [PubMed] [Google Scholar]
- 150.Allard B, et al. Anti-CD73 therapy impairs tumor angiogenesis. Int J Cancer. 2013;2013 doi: 10.1002/ijc.28456. [DOI] [PubMed] [Google Scholar]
- 151.Rust S, et al. Combining phenotypic and proteomic approaches to identify membrane targets in a 'triple negative' breast cancer cell type. Mol Cancer. 2013;12:11. doi: 10.1186/1476-4598-12-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Kirkwood JM, et al. Next generation of immunotherapy for melanoma. J Clin Oncol. 2008;26:3445–3455. doi: 10.1200/JCO.2007.14.6423. [DOI] [PubMed] [Google Scholar]
- 153.Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity. 2013;39:38–48. doi: 10.1016/j.immuni.2013.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Larocca C, Schlom J. Viral vector-based therapeutic cancer vaccines. Cancer J. 2011;17:359–371. doi: 10.1097/PPO.0b013e3182325e63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McNamara MA, et al. RNA-Based Vaccines in Cancer Immunotherapy. J Immunol Res. 2015;2015:794528. doi: 10.1155/2015/794528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Slingluff CL., Jr The present and future of peptide vaccines for cancer: single or multiple, long or short, alone or in combination? Cancer J. 2011;17:343–350. doi: 10.1097/PPO.0b013e318233e5b2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Bartlett DL, et al. Oncolytic viruses as therapeutic cancer vaccines. Mol Cancer. 2013;12:103. doi: 10.1186/1476-4598-12-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Wu R, et al. Adoptive T-cell therapy using autologous tumor-infiltrating lymphocytes for metastatic melanoma: current status and future outlook. Cancer J. 2012;18:160–175. doi: 10.1097/PPO.0b013e31824d4465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Stauss HJ, et al. Cancer gene therapy with T cell receptors and chimeric antigen receptors. Curr Opin Pharmacol. 2015;24:113–118. doi: 10.1016/j.coph.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 160.Dotti G, et al. Design and development of therapies using chimeric antigen receptor-expressing T cells. Immunol Rev. 2014;257:107–126. doi: 10.1111/imr.12131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Melero I, et al. Evolving synergistic combinations of targeted immunotherapies to combat cancer. Nat Rev Cancer. 2015;15:457–472. doi: 10.1038/nrc3973. [DOI] [PubMed] [Google Scholar]