Abstract
Although nanotherapeutics offer a targeted and potentially less toxic alternative to systemic chemotherapy in cancer treatment, nanotherapeutic transport is typically hindered by abnormal characteristics of tumor tissue. Once nanoparticles targeted to tumor cells arrive in the circulation of tumor vasculature, they must extravasate from irregular vessels and diffuse through the tissue to ideally reach all malignant cells in cytotoxic concentrations. The enhanced permeability and retention effect can be leveraged to promote extravasation of appropriately sized particles from tumor vasculature; however, therapeutic success remains elusive partly due to inadequate intra-tumoral transport promoting heterogeneous nanoparticle uptake and distribution. Irregular tumor vasculature not only hinders particle transport but also sustains hypoxic tissue kregions with quiescent cells, which may be unaffected by cycle-dependent chemotherapeutics released from nanoparticles and thus regrow tumor tissue following nanotherapy. Furthermore, a large proportion of systemically injected nanoparticles may become sequestered by the reticuloendothelial system, resulting in overall diminished efficacy. We review recent work evaluating the uptake and distribution of gold nanoparticles in pre-clinical tumor models, with the goal to help improve nanotherapy outcomes. We also examine the potential role of novel layered gold nanoparticles designed to address some of these critical issues, assessing their uptake and transport in cancerous tissue.
Introduction
Nanotechnology has garnered tremendous research attention in the past two decades. In particular, gold nanoparticles (NPs) have been extensively studied for targeted cancer diagnosis, imaging, photohermal therapy, and drug delivery. Gold NP shape configurations have been various, including nanospheres, nanorods, nanocages, and nanoshells. Issues such as tumor targeting efficacy, pharmacokinetics and pharmacodynamics, toxicity, and biocompatibility have unfortunately hindered clinical application. Even if these issues were resolved, however, the abnormal structure of cancerous tissue presents serious challenges to effective nanotherapy. We review recent studies specifically focusing on overcoming tumor tissue transport barriers, first summarizing the main biological and physiological barriers to NP transport, then giving a brief overview of gold nanospheres and nanoshells and their construction, followed by a description of in vitro and in vivo experimental techniques to evaluate NP uptake and distribution in cancerous tissue. We then review gold NP uptake and distribution in 3D cell culture and in vivo animal studies, as well as recent computational modeling designed to complement the experimental analysis of NP transport in cancerous tissue.
Biological and physiological barriers to nanoparticle transport
Upon systemic injection, NPs first have to navigate the body’s circulation while avoiding sequestration by the reticuloendothelial system (RES), which as part of the immune system is designed to remove pathogenic entities from the body. The RES, first reported by [1], comprises a network of cells and tissues throughout the body, including in the blood, lymph nodes, liver, spleen, lungs, bone marrow, and connective tissue. Surface modifications are typically applied to NPs to minimize RES sequestering while pursuing passive and active targeting to tumor cells. In particular, coating with the hydrophilic polymer poly(ethylene)-glycol (PEG) has been shown to prolong circulation and avoid RES uptake [2,3], while promoting extravasation from tumor capillaries and diffusion in tumor tissue [4, 5]. PEG is currently the most widely employed FDA-approved polymeric platform for treating various cancers and related diseases, including Oncaspar for acute lymphoblastic leukemia, Genexol-RM for metastatic breast cancer, and Neulasta for chemotherapy-associated neutropenia [6]. While PEGylation enhances drug delivery, adverse effects have included potential immunogenicity, which is still controversial [7]. Coating of NPs with ligands specific for receptors over-expressed in cancer cells (such as the high-density lipoprotein receptor, HDLR) has also been shown to enhance NP uptake and accumulation in tumor tissue [3].
Cancer cells typically exhibit unscheduled proliferation resulting in tissue growth with inadequate vascular supply. The resulting hypoxia triggers angiogenesis [8–10], which is malfunctional due to uncoordinated stimulation from tumor cells [11,12], leading to irregular and leaky vessels [13], with pore sizes larger than normal (typically ~ 400 nm [14,15]). Consequently, appropriately sized NPs administered systemically can preferentially leak out of tumor vessels through the enhanced permeability and retention (EPR) effect [16–19]. NP transport and accumulation can be further affected by NP surface charge, morphology, and size [20,21].
The irregular tumor vasculature hinders therapeutic efficacy even if NP systemic travel and targeted accumulation were successful [22]. Tumor cells distant from vasculature are exposed to lower levels of diffusible substances (such as glucose, oxygen, NPs, and drug molecules) [23–27]. Hypovascularization promotes cells that invoke survival mechanisms to overcome low nutrient and oxygen conditions, including cell cycle arrest (quiescence) leading to cell cycle-dependent chemotherapeutic resistance [23,28–30]. Transport may be further hindered by an acidic microenvironment, increased interstitial fluid pressure (IFP), and a dense and disorganized extra-cellular matrix (ECM) [4,22,31–33]. As a result, cancer tissue may have almost twice the volume of interstitial space compared to normal tissue [23,26]. These biological and physiological barriers, coupled with intrinsic cellular resistance mechanisms, are a major reason underlying NP inefficacy to treat tumor tissue.
Gold nanoparticles as therapeutic agents
Physics- and engineering-based approaches have been applied to help address NP transport barriers. NPs can be constructed of inorganic or organic materials, or a combination of both. In particular, metallic NPs have been shown useful in various applications including in vivo imaging, photothermal therapy, and drug delivery. NPs have been synthesized from metals such as gold [34–39], silver [40–43], copper [44–46], palladium [47], ruthenium [48], or nickel [49–51]. Gold NPs have undergone extensive research, and they have been shaped in various ways and functionalized with moities for targeting cancer cell-specific receptors [52–54]. Previous studies have shown cellular uptake of gold NPs to be proportional to NP size [55–57]. Accordingly, NP size and surface modifications have been modulated in the quest to overcome transport barriers and deliver NPs more homogeneously into tumor tissue. Gold is an inert material, with NPs larger than 5 nm exhibiting little cytotoxicity [58,59] depending on the surface charge. Systemic toxicity can be further alleviated by coating with PEG, which can help hide the NPs from sequestering by the RES [20,59].
The clinical use of gold NPs has been hampered by poor biocompatibility and uncertain fate in vivo [60]. In lung cancer, for example, it was shown that gold NPs coated with 1-stearoyl-2-oleoyl-sn-glycero-3-phospho-(1′-rac-glycerol) were readily uptaken by lung adenocarcinoma cells, but they also seemed to induce lamellar bodies which could export the NPs out of the cell, thus generating acquired cell resistance [61]. Gold NPs have been effective in diagnosing lung cancer in exhaled breath by detecting elevated concentrations of volatile organic compounds [62] and through histological classification by binding to biomarkers of various lung cancer types [63].
Nanoshells are a special case of gold NP [64], composed of an outer metallic shell coating an inner core of a material such as silica, polystyrene, poly (lactic-co-glycolic) acid, or Fe3O4. In particular, silica-gold nanoshells have an outer gold shell with a dielectric silica core, and have been extensively studied for cancer applications [65,66]. Varying the thickness of the metallic shell enables optical tuning of nanoshells through their extinction spectra and hydrodynamic size. They can be designed to exhibit maximum absorbance in the near infrared (NIR)-spectral range, thus providing potential use for both photothermal therapy and multi-modality imaging. NIR-absorbing gold nanoshells have proven effective at reducing cell viability in solid tumors via hyperthermia when exposed to laser radiation [67,68].
Construction of gold NPs
To illustrate the physical process of gold NP construction, we describe as an example the methods employed in our previous work [69,70] to synthesize nanospheres and nanoshells.
Synthesis of gold nanospheres
Citrate-stabilized gold NPs are typically synthesized using a method in which chloroauric acid is reduced by trisodium citrate [71]. For example, 2.2–2.4 mL 1% wt/v citrate and 200 mL 0.01% wt/v HAuCl4 are mixed and boiled for 10 min. The solution is then concentrated using a rotovapor to ~ 20 mL. Although citrate-stabilized gold NPs may be easier for designing a system for transport modeling studies, they cannot be used for thermal ablation since the wavelength of light (~ 540 nm) required for activation will cause injury to live tissue. Their application is thus limited compared to silica-core gold nanoshells, which exhibit tunable optical properties and provide energy absorption in the NIR range required for photothermal ablation.
Synthesis of silica gold nanoshells
The synthesis of nanoshells consists of four steps: production of a colloid of small gold particles (2–4 nm) through reduction and aging of gold colloid following the recipe of Vogel et al. [72], fabrication of monodispersed silica cores via the Stöber method [73,74], attachment of the seeds to the silica surface, and gold shell growth via reduction of additional gold. The gold colloid solution is created using the THPC (Tetrakis(hydroxymethyl)phosphonium chloride) method [68]. Growth of the silica cores can be achieved via the combination of 7.5 mL tetraethyl-orthosilicate (TEOS), 225 mL absolute ethanol, and 12.5–13.5 mL ammonia. The ammonia is adjusted to achieve the desired silica core sizes. After removal of the paraffin cover and evaporation of the ammonia, the cores are coated with 3%–4% aminopropyltriethoxysilane (APTES). This allows for slightly positive cores for deposition of small colloidal gold particles, thus forming a seed particle. The seeds are washed and a 10% gold solution is added to complete the shell. After reaction time, the seeds are washed and re-dispersed in distilled water. The seeds are diluted to 0.3–0.5 optical density (OD) at 530 nm. A sweep is performed to optimize the chemical ratio between the seeds, K2CO3-HAuCl4, and formaldehyde. Limiting the concentration of gold in the final reduction determines the thickness of the shell.
Functionalization of gold nanoparticles
In [69], gold nanospheres (45–60 nm) and silica gold nanoshells (160–175 nm) were functionalized with phosphatidylcholine (PC) and alkanethiol (two-layer), or high-density lipoprotein (HDL) and phosphatidylcholine/alkanethiol (three-layer), in the formation of layered NPs. The uptake and distribution of the layered NPs was evaluated in 3D cell cultures of various cancer cell lines, and their performance was compared to PEGylated NPs. PC is the most abundant phospholipid in the body and is a component of cell membranes, thus presenting less immunogenicity and offering an alternative to PEG. For example, PC has been used as a passivating agent for gold nanorods, significantly reducing their cytotoxicity [75]. Previous work has shown that thiol groups can help stabilize gold NPs due to the strong binding affinity between thiol and gold in comparison to the electrostatic binding with citrate [76–78]; a comprehensive review regarding the covalent interaction between gold and sulfur can be found in [78]. This functionalization creates a hydrophobic particle, with the thiol hydrocarbon chains pointing away from the core. Layering with the PC then yields a properly oriented layer surrounding the core as the hydrocarbon chains interact with the fatty acids of the PC. Such a two-layer approach may yield better biocompatibility as well as improved penetration into under-vascularized tumor tissue when compared to PEGylated particles. Additionally, the hydrophobic region between the PC and the thiol could potentially hold hydrophobic drugs.
In [69], the first layer applied to the gold nanospheres and silica-gold nanoshells was 1-Hexadecanethiol dissolved in ethanol. While stirring, 20 mL pure ethanol was placed in a beaker followed by 60 µL 1-Hexadecanethiol. The NPs were added to the sample slowly over the next few minutes. The sample was sonicated for 2 hours, and then placed for 12 hours on an orbital rocker. The sample was spun down at 5000 × g for 15 min, and the pellet was washed twice with ethanol and sonicated before resuspension in 9 mL chloroform. The second functionalization was the addition of L-α-Phosphatidylcholine (PC). The stock solution was made by diluting the PC in chloroform (2mg/mL), and 100 µL (molar ratio 2000 PC: 1 nanoparticle) was added to the particles coated with thiol and allowed to set for 12 hours on an orbital rocker. The solutions were transferred to glass tubes and the chloroform evaporated at ambient temperature. After removal of chloroform, PC-coated gold nanospheres and silica-gold nanoshells were reconstituted in ddH2O to 2 OD. Three-layered particles were created by optimizing the ratio of HDL to NP optical density via an overnight reaction after 2 hours sonication. PEGylated NPs and nanoshells were created to compare their efficacy to the two- and three-layer NPs. To this end, 2000 MW PEG was added to gold nanospheres or nanoshells at a molar ratio of 2500:1. The solution was set on a rocker and allowed to react for 12 hours. Excess PEG was removed by centrifugation at 13000 × g for 20 min, followed by resuspension in phosphate buffered saline (PBS).
Characterization of gold NPs
Gold NP surface modifications are typically assessed via optical, charge, and extinction spectra measurements (see table 1 for an example dataset). In [79] gold nanospheres had a peak absorbance in the range 533–541 nm, consistent with previous studies [80]. Size measured via scanning electron microscopy (SEM) was ~ 55 nm, while hydrodynamic size measured via DLS was ~ 80 nm. It has previously been shown that NPs sized ~ 50–60 nm can optimally recruit sufficient cellular receptors to induce cell internalization [81]. Further, gold NPs of this size have shown high uptake in orthotopic in vivo tumors [82]. In [79] silica-gold nanoshells had a peak absorbance in the range 820–860 nm, which corresponds to nanoshells with 110 nm diameter silica cores and ~ 15 nm gold coating [83]. With this set of parameters, the nanoshells exhibited ideal optical properties for NIR application [68,84].
Table 1.
Layered Gold NP Characteristics (data from [69]). Two-layer includes coating with phosphatidylcholine (PC) and alkanethiol, while three-layer additionally includes high-density lipoprotein (HDL). Maximum wavelength was obtained using UV-Visible Spectroscopy, while size was evaluated via Scanning Electron Microscopy (SEM) and Dynamic Light Scattering (DLS, with hydrodynamic diameter representing z-average of the intensity distribution). Parentheses denote S.D. (n = 3).
| Nanoparticle type | Functionalization | Max. wavelength (nm) |
Diameter (nm) (via SEM) |
Diameter (nm) (via DLS) |
|---|---|---|---|---|
| Gold nanospheres | PEGylated | 533 | 52.6 (15.4) | 82.81 (13.4) |
| Two layer | 540 | 47.1 (12.6) | 74.91 (13.3) | |
| Three Layer | 541 | 62.8 (14.9) | 85.26 (18.7) | |
| Silica gold nanoshells | PEGylated | 820 | 146.2 (9.1) | 161.82 (12.2) |
| Two layer | 855 | 144.9 (6.5) | 155.11 (13.9) | |
| Three layer | 860 | 158.1 (4.6) | 169.41 (11.8) |
The zeta potential of the NPs is measured in order to evaluate surface modifications. In [79], PC and thiol additions decreased the charge compared to PEGylated particles. In contrast, HDL increased the zeta potential to yield slightly negatively charged particles. PEGylated nanospheres had a zeta potential of −9 mV, while the two-layer version had −20 mV and the three-layer had −2 mV. In general, the nanoshells had higher surface charge than the nanospheres, due to the larger size of the nanoshells. The PEGylated versions had a zeta potential of −18 mV, the two-layer version had −29 mV, and the three-layer had −6.8 mV. These measurements were in agreement with previous studies [85–87].
Fourier Transform Infrared (FTIR) spectroscopy can be performed to ensure the presence of NP surface modifications. In [79], PEGylated NPs showed a C-O-C stretch of the PEG ether at ~ 1050 cm−1 and vibration at ~ 1350 cm−1, with −CH2 and −CH3 bending vibrations at ~ 1450 cm−1. This stretch was smaller than previously observed [88], possibly due to the coating with PEG onto the silica-core nanoshells. The two-layer version with an outer layer of PC had PO43− group vibrations between ~ 820–1000 cm−1, C-O-C stretch ~ 1100 cm−1, with the largest band corresponding to the asymmetric and symmetric −CH2 (2880 cm−1) and −CH3 (2950 cm−1) stretch and vibration, respectively. The HDL-coated versions had asymmetric and symmetric −CH2 (2880 cm−1) and −CH3 (2950 cm−1) stretch and vibration, with C=O from the lipid ester ~ 1750 cm−1, amide bonds in the range 1600–1700 cm−1, and a phospholipid P=O2 stretch ~ 1250 cm−1. The two and three layers showed overlapping spectra possibly due to the layering process.
NP size and morphology can be determined via SEM (table 1). NP uptake into tissues has been shown to depend on particle shape [59]. In [79], nanoshells were observed to be spherical, as were most of the nanospheres, which is a desirable attribute to attain optimized optical properties for potential NIR therapy. PEGylated versions were mostly spherical measuring ~ 150–170 nm in diameter. Hydrodynamic size influences NP performance in vivo. Accordingly, dynamic light scattering (DLS) values are also usually measured. In [79], nanospheres were substantially smaller than nanoshells, measuring ~ 50 nm in diameter (table 1). Addition of hexadecanethiol and PC adds ~ 5–7 nm to the diameter due to the size of PC (~ 2.5–3 nm) and the –thiol (~ 2.5 nm) [89,90], while HDL adds ~ 10 nm [91].
Setup for experimental evaluation
The following provides examples of in vitro cell culture and in vivo animal experiments that have been typically used to assess gold NP uptake and distribution in tumor tissue. The detection of NPs using hyperspectral imaging is also described.
Cell culture and nanoparticle experiments in vitro
In [69], the gold NP performance was evaluated with three different types of cancer cell lines in tumor spheroid 3D cell culture: liver hepatocellular carcinoma (HEPG2), human lung adenocarcinoma (A-549), and pancreatic adenocarcinoma (S2VP10). The spheroids ranged between 0.5 and 2 mm in diameter, while the S2VP10 cells formed smaller, grape-like tumors. The spheroids were incubated with 40 µL NPs at 25 OD for 6 hours. Spheroids were allowed to set for 2 hours in cryomolds made of tissue freezing medium, and then sectioned in 5 µm slices for histological analysis.
Mouse model experiments in vivo
For the study in [70], severe combined immunodeficiency (SCID) female mice received an orthotopic pancreatic injection of S2VP10 metastatic pancreatic adenocarcinoma cells expressing luciferase, resulting in pancreatic tumors within 7 days. An intravenous (tail vein) injection of 200 µL 2 OD NP solution was given to each mouse 9 days after tumor cell implantation. As there were five mice in each cohort, the first cohort received an injection of PC-coated gold nanospheres; the second cohort received an injection of PC-coated silica-gold nanoshells, and the third cohort received no injection (negative control). NPs were allowed to circulate for 48 hours before mice were euthanized. The pancreatic tumor, liver, and spleen were removed from each mouse for histological processing, with the liver and spleen providing a measure of NP uptake by two major components of the RES.
NP detection using hyperspectral imaging
Silver enhancement staining can be used to detect gold NPs in tissue samples using brightfield microscopy, although the staining intensity also depends on particle size and shape in addition to particle number, thus providing a qualitative assessment. A more quantitative evaluation can be performed via scanning electron microscopy (SEM) or transmission electron microscopy (TEM); however, these techniques by themselves cannot conclusively detect NPs. Elemental analysis in combination with other techniques (such as energy dispersive X-ray (EDX) microanalysis) provides further confirmation of NP identity [92]. Other techniques for gold NP detection in tissue have included confocal microscopy [93], atomic force microscopy (AFM) [94], fluorescence microscopy [95], dark-field microscopy [96], reflectance imaging [93], photothermal interference contrast [97], and micro computed tomography (micro-CT) [98]. Recently, hyperspectral imaging in combination with dark-field microscopy was used in [69,70,99] to assess NP uptake and distribution within histology tissue sections. In this approach, spectral libraries of known NPs are created to enable their detection in tissue. The combination of hyperspectral imaging with dark-field microscopy thus enables automated NP detection. Previous work illustrates the use of this technology for NP detection in biological tissues. For example in lung cancer, the deposition patterns of cisplatin aerosol therapy in surgically resected stage II lymph nodes was analyzed using hyperspectral imaging [100]. Hyperspectral imaging was also used to analyze the morphology and particle distribution of liposomes encapsulating recombinant human epidermal growth factor [101]. This type of imaging has also been utilized in patients for rapid histological classification of lung cancer type [102].
The CytoViva Hyperspectral Imaging System uses a Dage camera with a microscope with dark-field capability. Hyperspectral profiles are acquired using a Pixelfly camera and visualized using ENVI 4.8 software. To confirm the identity of NPs, spectral libraries are created using z-spectral profiles and compared to the tissue samples. The spectral mapping is able to detect and confirm the NPs within tissue samples by using multiple tissue images (> 10) containing either NPs or no NPs. Data analysis can be performed with ImageJ. NPs are counted in various regions of tissue (e.g., tumor spheroids [69,70] or multilayered cell cultures [99]) to determine concentration. However, light scattering from different components in dense tissue samples may hinder detection. Thus, to computationally extract the NPs, spectral matching should be mapped for each image to provide a clear identification of pixels associated with NPs. NP spectral profiles may be altered by backscatter from other sources, agglomeration status, and other mechanisms. For the tissue obtained from in vivo experiments, regions of interest can be randomly determined and particles within these regions counted to determine concentration. In this case, a sufficient number of regions should be evaluated to ensure an acceptable level of accuracy (e.g., based on stereological analysis).
Evaluation of gold NP uptake in cell culture
Small gold NP uptake by cancer cells in monolayer, 3D cell culture, and tumors in vivo was assessed in [76]. Two NP sizes (50 and 100 nm) were coated with tiopronin, a thiol drug used to control the rate of cysteine precipitation and excretion in patients suffering from cystinuria [103]. Tiopronin can be used as a stabilizing agent for metal NPs because its thiol groups prevent NP agglomeration [104], while its carboxyl groups can be modified with peptides, molecules, or drugs. It was previously shown that tiopronin-coated NPs functionalized with targeted and therapeutic peptides evinced enhanced NP uptake and distribution in 3D cell cultures of MCF-7 human breast cancer cells and in mice in vivo [105].
Exposure to the tiopronin-coated NPs for 24 hours was not toxic to the MCF-7 cells, indicating good biocompatibility [76]. Human breast cancer MCF-7 cells were then treated with 50 and 100 nm NPs at a concentration of 1 nmol/L for 24 hours. Comparison of the number of NPs in each cell versus NP size indicated that cellular uptake occurred in a size-dependent manner. More than 5× uptake was observed for the 50 nm compared to the 100 nm NPs, in agreement with a previously reported maximum uptake of 50 nm citrate-stabilized gold NPs [106]. The intracellular distribution of NPs after treatment was assessed by Bio-TEM, showing that both NP sizes localized in the cellular cytoplasm in an aggregated state. The density of 50 nm NPs was significantly higher than that of 100 nm NPs, which was in accordance with results from inductively coupled plasma mass spectrometry (ICP-MS).
In the 3D cell culture spheroid model, positively charged NPs were taken up mostly by proliferating cells [76], while negatively charged NPs were able to deliver their payload deeper into tissue due to fast diffusion [107]. Arginineglycine-aspartic acid peptide-conjugated dendrimers were developed to enhance NP delivery into tissue. These dendrimers exhibited significantly enhanced penetration of dendrimer/siRNA NPs into U87 malignant glioma 3D cell cultures. The mechanism seems to be based on decreased integrin-binding affinity [108,109]. Accordingly, 50 and 100 nm tiopronin-coated gold NPs with the same surface properties were prepared, in order to assess the effect of size on tissue penetration. The 3D cell cultures were exposed to the NPs at a concentration of 1 nmol/L for 3 and 24 hours. Evaluation of HE staining with dark-field microscopy showed that at 3 hours the NPs were primarily located at the periphery of the spheroids [76]. The penetration of the 50 nm NPs increased with extended incubation time from 3 to 24 hours, the amount being about 4× higher than that of the 100 nm NPs. A corresponding size-dependent differential in NP concentration was also observed. These results are in agreement with previous work showing that NP penetration into spheroids is limited to NP sizes smaller than 100 nm [110]. Similarly, NPs sized ~ 20 nm showed better penetration into spheroids than larger NPs [110].
In another study [111], ultra-small gold (2 and 6 nm) NPs were shown to have superior uptake and penetration of MCF-7 breast cancer cells in monolayer, 3D cell culture, and tumors in vivo compared to larger (15 nm) NPs. The NP uptake in cells in monolayer and their penetration in tumor spheroids was inversely proportional to the NP size. Intriguingly, 2 and 6 nm NPs were found in both cell cytoplasm and nuclei in vitro and in vivo, while 15 nm NPs aggregated in the cytoplasm. The gold content increased significantly with incubation time from 3 to 24 hours for both 2 and 6 nm NPs, but not for the larger 15 nm NPs [111]. The 2 and 6 nm NPs were distributed throughout the spheroids, suggesting deep penetration by the smaller particles, while the 15 nm NPs were found mostly at the spheroid periphery. ICP-MS analysis demonstrated that the number of NPs detected in 3D cell culture was inversely proportional to their size. This observation is consistent with previous work showing that NPs smaller than 20 nm have superior tissue penetration compared to larger (40, 100, or 200 nm) NPs [110].
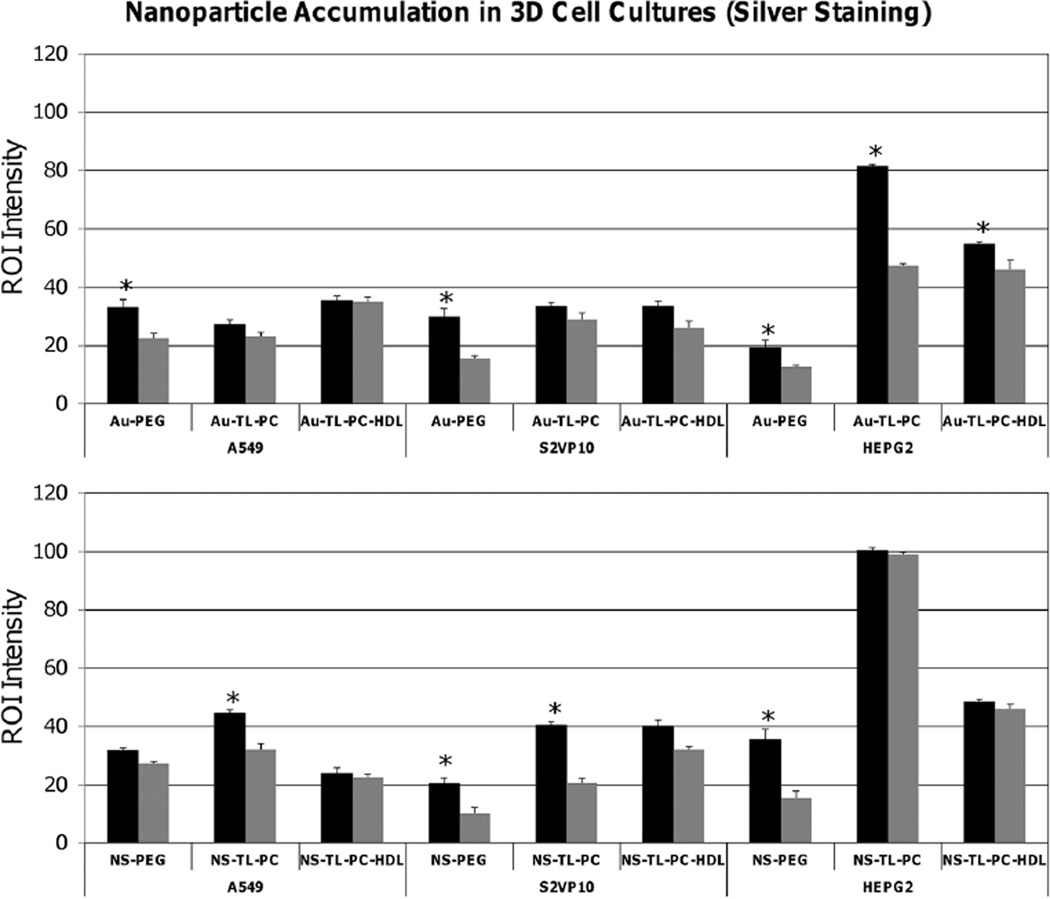
In [69], tumor spheroid sections representing hypo-vascularized tumor tissue from three different cell lines (A549 non-small cell lung cancer, S2VP10 pancreatic adenocarcinoma, and HEPG2 liver cancer) were treated with silver enhancement staining to assess NP uptake and distribution after exposure to layered nanospheres or silica gold nanoshells. Region of interest (ROI) measurements were taken at the periphery and center of the spheroid cultures. Samples exposed to two- and three-layer nanoshells had higher ROI intensity than PEGylated versions for all three cell lines. For the A549 cell line, the three-layer nanospheres had more uniform distribution compared to the PEGylated version (fig. 1, top left). In contrast, an increased accumulation of two-layer compared to PEGylated nanoshells was observed for the A549 cell line (fig. 1, bottom left). The two-layer nanoshells had more of a differential between periphery and tumor interior compared to the three-layer case (fig. 1, bottom left). For the S2VP10 cells, all of the two- and three-layer particle versions had significantly higher ROI in the interior than the PEGylated versions, suggesting increased penetration (fig. 1, middle). A more uniform particle distribution was suggested by the two- and three-layer nanospheres, which presented similar ROI intensity values between spheroid periphery and interior compared to the PEGylated versions (fig. 1, top middle). Two- and three-layer nanoshells had ~ 2× the ROI intensity values at the periphery for the S2VP10 cell line compared to the PEGylated version (fig. 1, bottom middle), with the three-layer showing ~ 50% higher values in the interior compared to the two-layer.
Fig. 1.
Uptake of gold NPs in 3D cell culture measured via silver enhancement (data from [69]). Top: gold nanospheres; bottom; silica gold nanoshells. ROI intensity measurements are shown for peripheral (black bars) and inner regions (grey bars) of A549 (non-small cell lung cancer), S2VP10 (pancreatic), and HEPG2 (liver) 3D cell cultures. Peripheral regions generally showed equal or higher number of NPs than the interior. Error bars represent standard deviation from n = 3 measurements; asterisk indicates statistically significant difference (p < 0.05) evaluated with Student’s t-test with α = 0.05.
For the HEPG2 cell culture, the two-layer nanospheres attained a peripheral ROI intensity almost 2× that of the interior, indicating a steep diffusion gradient into the 3D culture (fig. 1, top right). In comparison, the three-layer nanoshells showed similar intensities between periphery and interior, and at similar values than the nanospheres, indicating more uniform but poorer penetration (fig. 1 right). The two-layer nanoshells had ROI intensity values statistically the same between the periphery and interior of the HEPG2 cell culture, indicating uniform distribution into the tissue. The particles had an ROI intensity nearly 2× that of the three layer and 3× that of the PEGylated versions (fig. 1, bottom). The PEGylated version had higher ROI intensity at the periphery than the interior while having the lowest particle uptake (fig. 1, bottom). The ROI intensities recorded for the HEPG2 cell line were higher than both the S2VP10 and A549 cell lines, indicating a higher number of particles uptaken in the HEPG2 cell culture for all particle types.
Application of spectral libraries for the detection of gold NPs
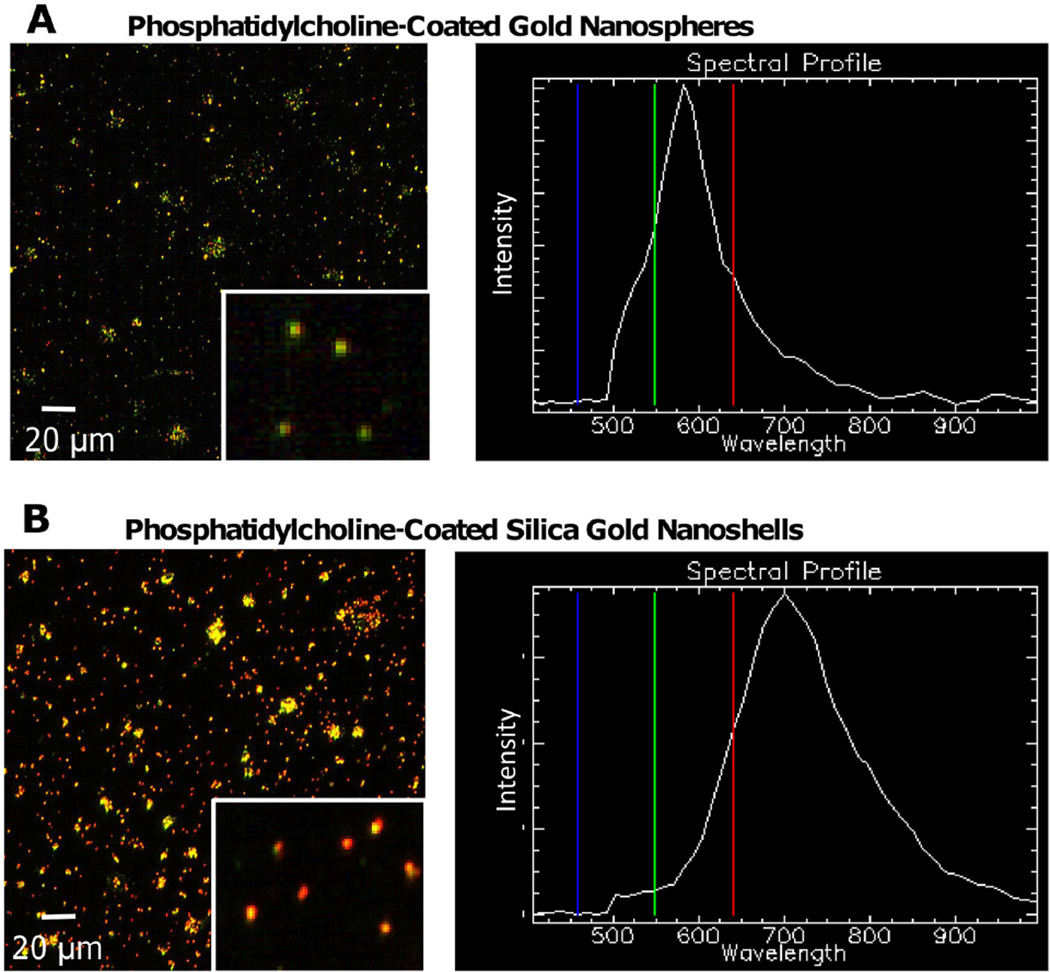
In [70], spectral libraries were created of PC-coated silica-gold nanoshells and nanospheres before detection in tissue samples. These libraries could later be matched to the tissue images for NP detection. In fig. 2, visible color changes (green to yellow to red) illustrate the shift of the maximum absorbance wavelength. The majority of nanospheres in solution exhibited green color, indicating maximum absorbance values within the lower range (500–600 nm) of the spectrum (fig. 2A). This was confirmed through the z-profile spectra mapping showing a maximum band at ~ 550–570 nm. The nanoshells contained a significant concentration of red NPs, indicative of absorbance at higher wavelengths (> 700 nm) (fig. 2B).
Fig. 2.
Hyperspectral imaging of PC-coated gold NPs [70]. (A) Gold nanospheres were primarily green, with a spectral profile indicating a maximum wavelength of 570 nm. (B) Silica-gold nanoshells displayed an abundance of red and yellow, with a spectral profile maximum of 700 nm. While the actual maximum wavelength of silica-gold nanoshells in solution is 820–860 nm, hyperspectral imaging is known to underestimate entities containing higher wavelength maxima. Bar, 20 µm. Reprinted from England et al., PLoS One 10, e0129172 (2015); used in accordance with Creative Commons Attribution (CC BY) license.
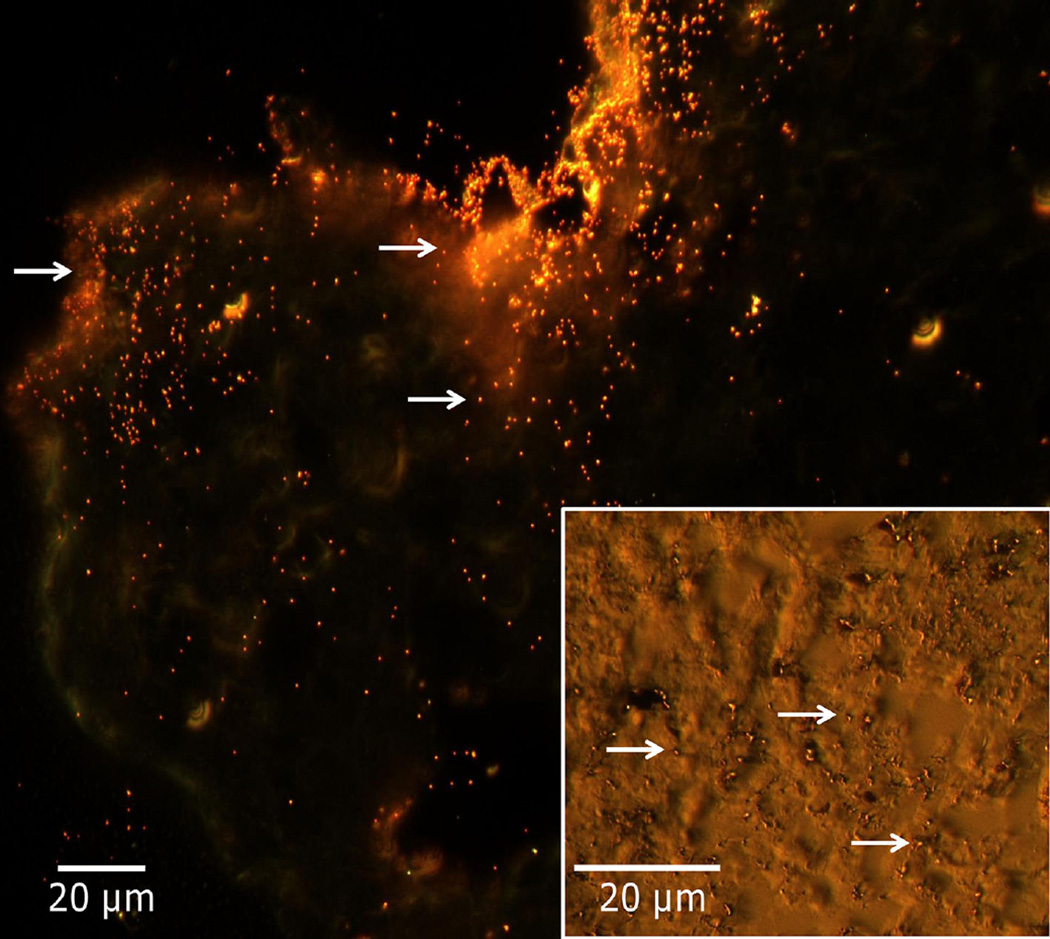
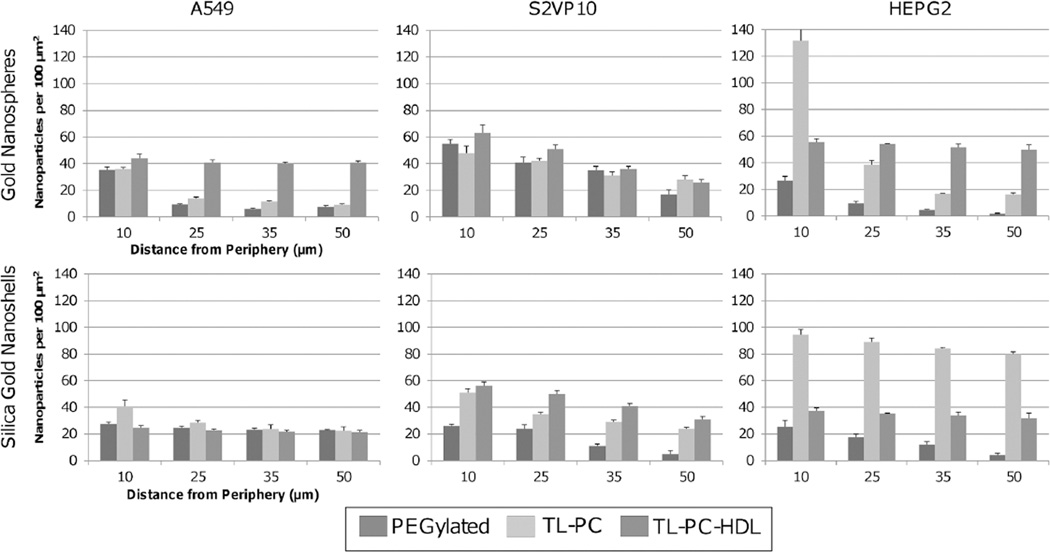
Dark-field microscopy enables observation of the NPs within tumor spheroid histology sections [70]. The light reflected by the particles provides sharp contrast with the surrounding tissue, enabling counting of the particles. Figure 3 shows a representative image, displaying small red-orange dots indicating particles within the tissue. Hyperspectral mapping was used to filter out the tissue to highlight the NPs [112]. NPs were thus identified, and their uptake and distribution assessed within the samples (fig. 4). The three-layer nanospheres in the A549 tumors and the two-layer silica gold nanoshells in the HEPG2 tumors showed the best performance, in agreement with silver staining measurements. Both particle types behaved similarly in the S2VP10 tissue, evincing steeper diffusion gradients and lower uptake.
Fig. 3.
Representative dark-field microscopy to assess gold NP uptake in 3D cell culture tissue histology, with hyperspectral imaging used to confirm particle identity (data from [69]). Image (60×) shows increased particle concentration around periphery of HEPG2 histology section (upper left), with a decreasing number towards the interior. Inset: Image of A549 histology section (100×) shows particles (arrows) distributed through the tissue.
Fig. 4.
Gold NP penetration and distribution in 3D cell cultures measured by dark-field microscopy (data from [69]). Particle uptake was measured as a function of distance from the tumor spheroid periphery for gold nanospheres (top) and silica gold nanoshells (bottom) for A549 (non-small cell lung cancer), S2VP10 (pancreatic), and HEPG2 (liver), 3D cell cultures. The three-layer gold nanospheres in the A549 tumors and the two-layer silica gold nanoshells in the HEPG2 tumors had the best performance, while both of these particle types behaved similarly in the S2VP10 culture, with steeper diffusion gradients and lower uptake. The PEGylated particles showed poorer penetration and lower uptake. All error bars represent standard deviation from at least n = 3 measurements.
The three-layer formulation showed slightly higher uptake in the S2VP10 tumors, possibly due to a looser morphology and to other pancreatic cell cancer-specific interactions with the HDL layer. Overall, the PEGylated particles had poorer penetration and lower uptake. Comparing the nanoshell performance across 3D cell cultures, the three-layer formulation exhibited an average 50% lower uptake compared to the two-layer [70]. The nanospheres had ~ 30% higher penetration at the spheroid periphery compared to the nanoshells, which may be mostly attributed to the larger size of the nanoshells (3× that of the nanospheres).
The penetration and uptake dynamics of gold NPs has very recently been studied using a Multilayered Cell Culture Model (MCL) [99], showing that NP transport and uptake are highly dependent on the tumor cell type. Gold NPs sized 20 nm were able to penetrate deeper into tissue composed of MDA-MB-231 cells than of tissue of MCF-7 cells, both human mammary carcinoma cell lines. The intracellular and extracellular spatial distribution of the NPs was mapped using hyperspectral imaging, illustrating the differential NP uptake between monolayer and tissue-like MCL models.
Evaluation of gold nanoparticle uptake in vivo
Accumulation of NPs in an organ is primarily a function of the associated tissue vasculature. Both the spleen and liver are highly vascularized tissue and thus offer a high number of extravasation sites, while tumors with impaired vascular development offer limited extravasation sites. The accumulation of 50 and 100 nm gold NPs was evaluated in mice in vivo in [76]. The pharmacokinetic behavior was first investigated after a single 5 mg NP/kg intravenous injection, with the results showing that the NPs were rapidly eliminated from circulation: the NP concentration decreased to 11.0 µg/mL at 10 min and 1.9 and 0.2 µg/mL at 8 and 24 hours for the 50 nm NPs, respectively [76]. The 100 nm NPs were cleared faster from circulation than the 50 nm NPs. The pharmacokinetic behavior is affected by many variables, including NP size and surface properties influencing the extent of opsonization and clearance. Since the NPs were coated with tiopronin, they were easily opsonized and cleared from circulation. A higher amount of gold was detected at 24 hr post injection for the 50 nm NPs (0.42 µg/g) and less for the 100 nm NPs (0.14 µg/g) [76]. The NPs accumulated predominantly in the spleen and the liver, uptaking more than 80% of the injected dose. The spatial distribution of the NPs was observed to be heterogeneous within the tumor tissue, with most of the NPs apparently remaining in the vicinity of the blood vessels after extravasation.
Gold NP pharmacokinetics was investigated after a single intravenous dose of 5 mg NP/kg in mice in vivo [111], finding that the NPs were rapidly eliminated from circulation. The concentration of gold in the blood for 2 nm NPs was 26.2 µg/mL at 10 min post injection, 7.57 µg/mL at 8 hr, and 0.94 µg/mL at 24 hours. For 6 nm NPs, the corresponding levels were 9.0 µg/mL, 2.8 µg/mL, and 0.51 µg/mL. The larger 15 nm NPs were cleared even faster, since the coating with tiopronin enhanced opsonization and clearance from circulation. These results indicate that smaller NPs extravasate from circulation and diffuse in tissue easier than the larger NPs. It has been shown that NPs with the capability to “shrink” from 100 nm to 10 nm exhibit deep tumor penetration and high accumulation in tumors in vivo [113]. In another study, the delivery of NPs with 12 nm diameter was enhanced by vascular normalization in mammary tumors, whereas larger 125 nm NPs were hindered [114]. The 2 and 6 nm NPs were widely distributed in different organs of the body, accumulating preferentially in the kidneys and with smaller amounts detected in the spleen and the liver. This is consistent with the distribution of 1.9 nm NPs [115,116]. In contrast, > 70% of the injected dose of 15 nm NPs was found in the spleen and the liver [111].
Systemic gold NP uptake in vivo based on NP size was evaluated in [117]. The NPs were traced after 1, 4 or 24 hours post intravenous (IV) (2 or 40 nm NPs) or intraperitoneal (IP) (with 40 nm NPs) injection. The NPs were primarily detected in macrophages only, and at moderate exposure mainly in the Kupffer cells in the liver. The IV injections yielded rapid NP uptake by the Kupffer cells, followed by slower uptake by macrophages in the spleen. In contrast, the IP injections yielded delayed uptake in the liver along with a moderate uptake in macrophages at the spleen, small intestine, and mesenteric lymph nodes. The NPs were not found in other organs, including lungs, brain, adrenal glands, kidneys and ovaries, and placenta and fetal liver for pregnant subjects. The results indicate that inert gold NPs penetrate cell membranes by endocytosis. Further, independent of size, the NPs were mainly uptaken by Kupffer cells in the liver and secondarily by macrophages in other organs. The blood-brain barrier apparently prevents the NPs from penetration into the central nervous system. The 2 nm NPs also seemed to be removed through urinary excretion via filtration in the renal glomeruli.
The uptake of a delivery system composed of 5 nm gold NPs loaded with cetuximab (C225) as a targeting agent, and gemcitabine as a chemotherapeutic drug was evaluated with an orthotopic pancreatic tumor growth in vivo using metastatic AsPC-1 cells [118]. Cetuximab is a monoclonal antibody that inhibits EGFR expressed on the cell surface of some cancers. The growth was monitored non-invasively via bioluminescence measurements, showing the advantage of a targeted system. Uptake of gold in various tissues was measured via inductively coupled plasma (ICP) analysis. The gold content in the tumor indicated that C225 was an efficient targeting agent, and showed that the loading with gemcitabine did not affect this targeting. Gold uptake by the liver and kidney was minimal, further highlighting the targeting efficacy. Treated tumors showed significant regression compared to untreated controls, with volumes reduced by ~ 75%. These results were confirmed in vitro with three pancreatic cancer cell lines (PANC-1, AsPC-1, and MIA Paca2) having variable epidermal growth factor receptor (EGFR) expression [118]. The testing showed that gold uptake correlated with EGFR expression, while evaluation of efficacy indicated significant inhibition of cell proliferation in vitro.
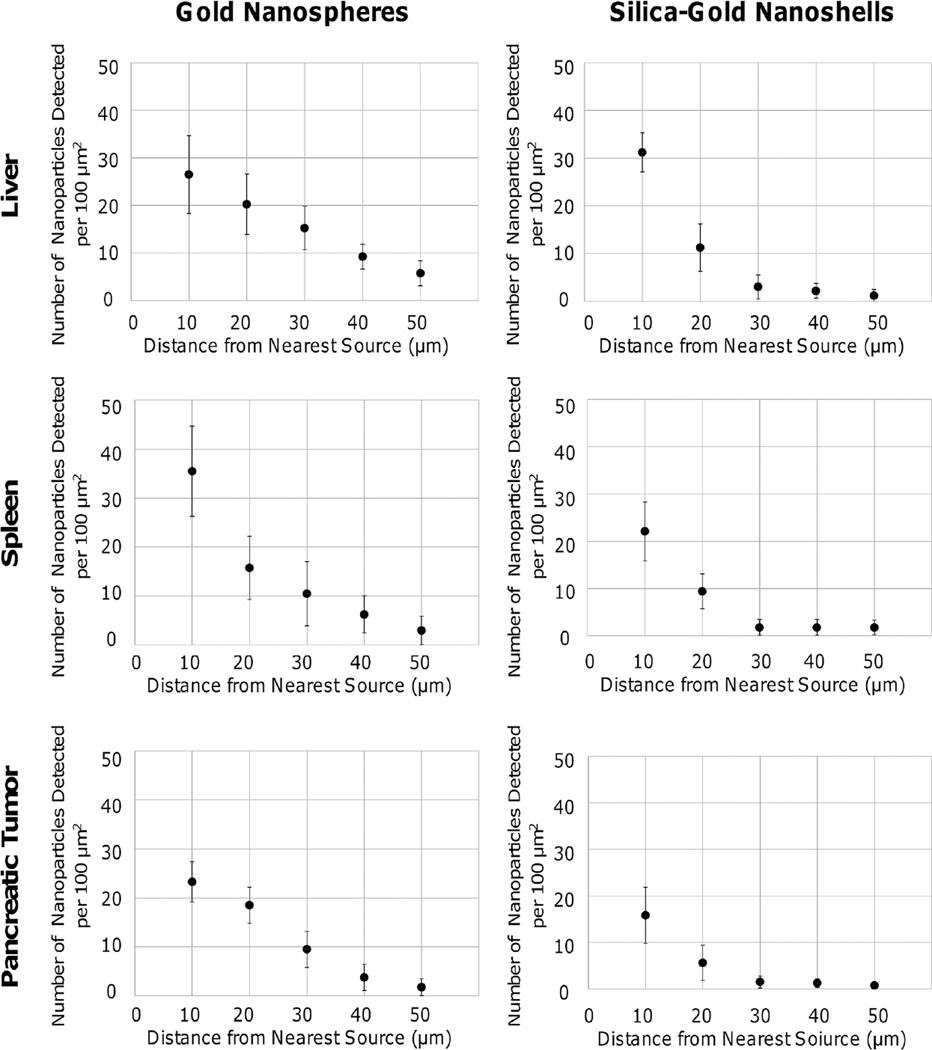
The biodistribution and uptake of PC-coated gold nanospheres and silica gold nanoshells in vivo was examined in [70]. The NPs were tail-vein injected into mice bearing orthotopic pancreatic tumors to enable systemic evaluation. Using dark-field microscopy in combination with hyperspectral imaging applied to histology samples, NP counts were obtained in the vicinity of extravasation sites to assess the distribution and uptake in various organs and tissues, including liver, spleen, and tumor. For nanospheres, the spleen exhibited a higher uptake of 35.5 ± 9.3 particles/100 µm2 within 10 µm of the source, followed by an exponentially decaying penetration pattern which was limited to ~ 50 µm (fig. 5). The liver and pancreatic tumor displayed a linear decline in concentration, with 26.5 ± 8.2 and 23.3 ± 4.1 particles/100 µm2, respectively, within 10 µm of the source and few NPs penetrating beyond 50 µm (fig. 5). NP concentration was highest in the liver (1.09 ± 0.14 NPs per µm2) in comparison to the spleen (0.74 ± 0.12 NPs per µm2) and pancreatic tumor (0.43 ± 0.07 NPs per µm2).
Fig. 5.
Penetration of PC-coated gold NPs in liver, spleen, and pancreatic tumor tissues [70]. Particles were visualized using hyperspectral imaging, and their distances measured from nearest source points. In the liver and pancreatic tumor, gold nanospheres had linear diffusion, while in the spleen it was an exponential decline. Silica gold nanoshells had an exponential decline for all tissue types, suggesting limited diffusivity. Their extravasation was highest in the liver, while for gold nanospheres it was highest in the spleen. All error bars denote standard deviation (n = 3). Reprinted from England et al., PLoS One 10, e0129172 (2015); used in accordance with Creative Commons Attribution (CC BY) license.
In this same study [70], silica-gold nanoshells exhibited limited penetration beyond 30 µm in the spleen and pancreatic tumor (fig. 5), with uptake of 22.1 ± 6.2 and 15.8 ± 6.1 particles/100 µm2, respectively, within 10 µm. The nanoshells were uptaken mostly within the liver and displayed on average 31.1 ± 4.1 particles/100 µm2 within 10 µm of the nearest source, with penetration hindered beyond 30 µm (fig. 5). The nanoshells exhibited a decaying exponential pattern in the liver, spleen, and pancreatic tumor, implying limited diffusion possibly due to size. NP concentration in the liver was 0.43 ± 0.07 NPs per µm2, and 0.30 ± 0.06 NPs per µm2 and 0.20 ± 0.04 NPs per µm2 in the spleen and the pancreatic tumor, respectively.
Evaluation of nanotherapy using computational modeling
In general, the large number of parameters available to tune NP performance coupled with the variability in tumor tissue makes it challenging to evaluate these complex interactions solely through experimental observation. Hence, mathematical modeling and computational simulation have been applied to complement the empirical effort in order to gain further insight into these phenomena. Nanotherapeutic response can be simulated by describing tumors as physical objects subject to physical laws, such as conservation of mass and momentum. Extensive modeling work studying tumor growth as well as angiogenesis has been published in recent years, as reviewed in [119–137] and associated references, with a subset of studies focusing on nanotherapy [24,27,138–148]. For example, the interaction between NP vascular extravasation, uptake, and distribution with heterogeneous tumor interstitial, vascular, and lymphatic conditions was studied in [140], finding that an elevated interstitial hydraulic conductivity leads to low NP accumulation regardless of NP capacity for vascular extravasation. The results further showed that an elevated vascular hydraulic conductivity modulates NP accumulation in a manner inversely proportional to the capacity for extravasation.
Some modeling studies have examined how tissue biological factors, including tumor size and vascularity, as well as NP physiochemical properties, such as size and surface modifications, affect NP performance [27,139,140,143,147,149]. The models may provide insight into NP uptake and distribution in vivo, which could help define NP and therapy parameters. In particular, a mathematical model was developed in [143] to predict NP diffusive transport and spatial distribution in 3D cell culture representing under-vascularized tissue, based on NP parameters measured from monolayer cell culture including diffusivity, cell surface binding capacity, and rate constants of association, dissociation, and cellular internalization. In [142], in vitro experiments were combined with mathematical modeling to study the synergistic effect of nanoparticle geometry (size, shape) and molecular targeting on specificity of delivery to tumor vasculature. An integrated approach was proposed in [144] for the rational design of NPs for intravascular delivery of imaging contrast agents and therapeutics. The approach combines in vitro experiments and in vivo intravital microscopy (IVM) with mathematical modeling to identify combinations of NP size, shape, and surface properties that maximize nanoparticle uptake within diseased vasculature.
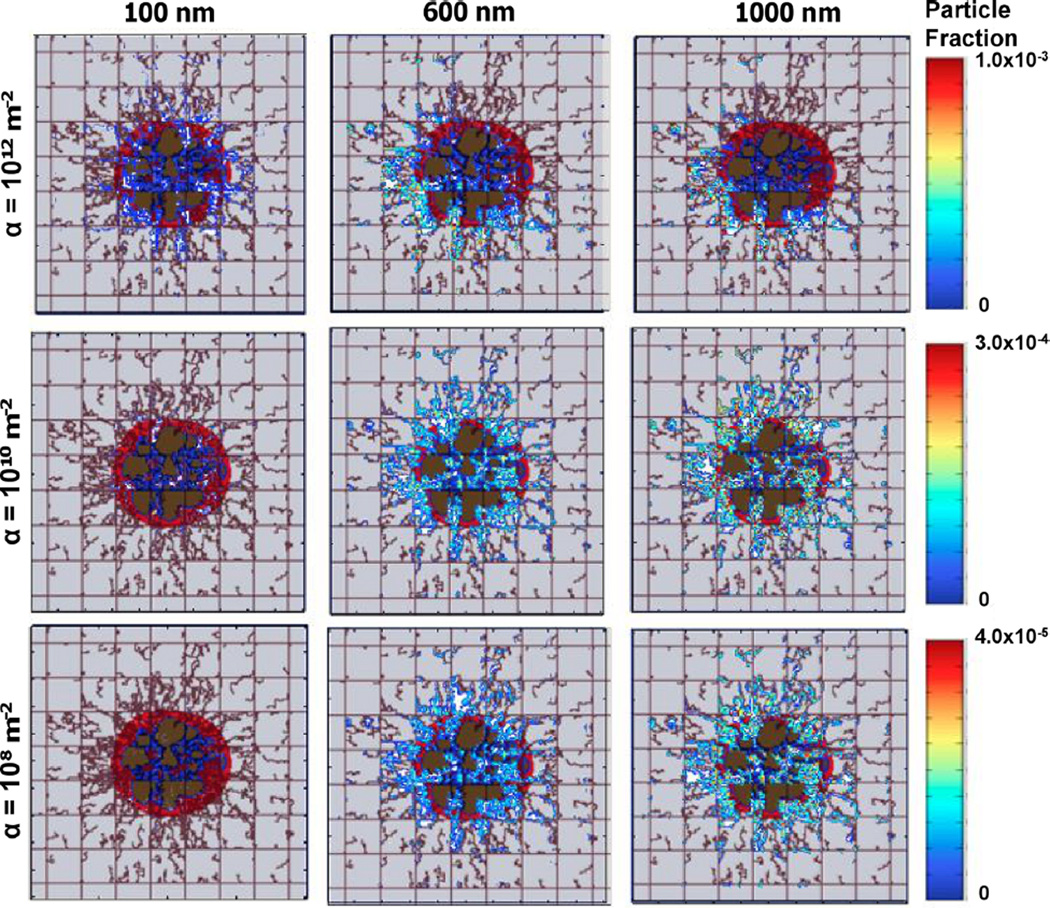
The transport and accumulation of NPs targeted to tumor vasculature has recently been modeled based on size, surface density and molecular affinity of surface ligands, and the expression of surface receptors on tumor endothelium [139]. The simulations showed that with higher vascular affinity, smaller NPs (100 nm) exhibited a more uniform distribution within tumor tissue, while larger NPs (600 and 100 nm) distributed inhomogeneously as their larger size with increased vascular affinity hindered their diffusivity (fig. 6). In contrast, smaller NPs with lower vascular affinity did not accumulate in the tumor. Low vascular affinity for larger NPs increased their accumulation and more homogeneous distribution. Optimal distribution and uptake were achieved for medium vascular affinity and intermediate-sized particles (600 nm), for which vascular and receptor affinity synergistically combined to yield higher accumulation and more homogeneous distribution [139].
Fig. 6.
Example of computational modeling to assess generic NP uptake in heterogeneously vascularized tumor tissue [139]. The columns denote three different NP sizes (100, 600, and 1000 nm), while the rows denote three different values for the model parameter α related to NP vascular affinity. The color scale provides a measure of fraction of injected NPs adhering at blood vessel walls at ~ 100 minutes after injection upstream of the tumor mass (bottom left corner in each panel). The tumor lesions (~ 750 µm diameter) have distinct regions denoting viable, hypoxic, and necrotic tissue, respectively represented by red, blue, and brown colors. Pre-existing capillary vessels, shown as straight lines, are laid out in a regular grid establishing normoxic conditions in the surrounding tissue. Angiogenesis-induced capillaries (irregular lines) sprout from the pre-existing vessels in response to pro-angiogenic factors produced by the hypoxic tissue. Field of view is 2 × 2 mm. Reprinted from Frieboes et al., PLoS One 8, e56876 (2013); used in accordance with Creative Commons Attribution (CC BY) license.
Conclusion
We have reviewed recent work studying the uptake and distribution of gold NPs in solid tumors, and examined in detail the performance of gold nanospheres and nanoshells in tissue in vitro and in vivo. The goal of these efforts is to evaluate how gold NP design in terms of size and surface modifications can help to overcome transport barriers in the tumor microenvironment in order to improve nanotherapy efficacy, and thus ultimately improve patient outcomes. Results in vivo illustrate the sequestering by the RES, with a high concentration of NPs uptaken in the spleen and the liver. Smaller gold NPs overall had enhanced tumor tissue accumulation compared to larger NPs. Functionalization of gold NPs has been shown to possibly improve targeting, transport, and uptake. As would be expected, low tissue vascularization hinders NP uptake regardless of size, e.g., as in pancreatic tumors [70].
3D cell culture offers the possibility to study diffusion into under-vascularized tumor tissue, as occurs locally in tumors. As reviewed here, the uptake and distribution of NPs within avascular/hypoxic regions of solid tumors has been studied using such in vitro models [69,70,99]. However, tissue within live subjects is more complex than that formed in 3D cell culture, typically having a fully developed ECM and multiple cell types (including immune system cells) in proximity of blood and lymph vessels, which may affect the deposition patterns and uptake of NPs beyond the mere effects of diffusion in 3D space. NP extravasation in tumor tissue in vivo is affected by interstitial fluid pressure, as evidenced in experimental and modeling studies [22,32,140]. The RES and other systemic factors in vivo may also significantly affect NP performance beyond what can be discerned from these experimental models.
Electron microscopy techniques (TEM, SEM, STEM) have traditionally been employed to detect NPs in tissue samples [150]. Other techniques including confocal microscopy have also been employed to assess NP translocation in co-culture cancer models [151]. Newer NP detection methodologies present opportunities for improved detection. In particular, hyperspectral imaging has shown the feasibility of determining particle size, wavelength differentiation, spatial location, and agglomeration status of NPs in solution [152]. In [70,99], dark-field microscopy in combination with hyperspectral imaging was used to evaluate NP uptake and distribution within tissue samples harvested from mice.
Further elucidation of gold NP uptake and distribution in tumor tissue could advance the potential for photothermal and targeted drug delivery therapies [153], as well as novel diagnostic imaging techniques such as multispectral optoacoustic tomography (MSOT) [154–157]. In combination with mathematical modeling, the experimental efforts could thus provide a suitable progression towards systematic development of more effective cancer nanotherapy.
Footnotes
Contribution to the Focus Point on “The Physics of Cancer” edited by M. Ben Amar.
References
- 1.Aschoff L, Kiyono K. Folia Haematol. 1913;15:383. [Google Scholar]
- 2.De Jong WH, Borm PJA. Int. J. Nanomed. 2008;3:133. [Google Scholar]
- 3.Zhang Z, Chen J, Ding L, et al. Small. 2010;6:430. doi: 10.1002/smll.200901515. [DOI] [PubMed] [Google Scholar]
- 4.Wang J, Lu Z, Gao Y, Wientjes MG, Au JLS. Nanomedicine. 2011;6:1605. doi: 10.2217/nnm.11.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li SD, Huang L. Biochim. Biophys. Acta-Biomembr. 2009;1788:2259. doi: 10.1016/j.bbamem.2009.06.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhang L, Gu FX, Chan JM, Wang AZ, Langer RS, Farokhzad OC. Clinical Pharmacol. Therapeutics. 2008;83:761. doi: 10.1038/sj.clpt.6100400. [DOI] [PubMed] [Google Scholar]
- 7.Schellekens H, Hennink WE, Brinks V. Pharm. Res. 2013;30:1729. doi: 10.1007/s11095-013-1067-7. [DOI] [PubMed] [Google Scholar]
- 8.Algire GH, Chalkley HW. J. Nat. Cancer Inst. 1945;6:73. [PubMed] [Google Scholar]
- 9.Goldman E. Lancet. 1907;2:1236. [Google Scholar]
- 10.Ide AG, Baker NH, Warren SL. Am. J. Radiol. 1939;42:891. [Google Scholar]
- 11.Greenblatt M, Shubik P. J. Natl. Cancer Inst. 1968;41:111. [PubMed] [Google Scholar]
- 12.Ehrmann RL, Knoth M. J. Natl. Cancer Inst. 1968;41:1329. [PubMed] [Google Scholar]
- 13.Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Science. 1983;219:983. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- 14.Yuan F, Dellian M, Fukumura D, et al. Cancer Res. 1995;55:3752. [PubMed] [Google Scholar]
- 15.Hashizume H, Baluk P, Morikawa S, et al. Am. J. Pathol. 2000;156:1363. doi: 10.1016/S0002-9440(10)65006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kong G, Braun RD, Dewhirst MW. Cancer Res. 2000;60:4440. [PubMed] [Google Scholar]
- 17.Libutti SK, Paciotti GF, Byrnes AA, et al. Clin. Cancer Res. 2010;16:6139. doi: 10.1158/1078-0432.CCR-10-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsumura Y, Maeda H. Cancer Res. 1986;46:6387. [PubMed] [Google Scholar]
- 19.Maeda H. Adv. Enzyme Regul. 2001;41:189. doi: 10.1016/s0065-2571(00)00013-3. [DOI] [PubMed] [Google Scholar]
- 20.Murphy CJ, Gole AM, Stone JW, et al. Acc. Chem. Res. 2008;41:1721. doi: 10.1021/ar800035u. [DOI] [PubMed] [Google Scholar]
- 21.Zamboni WC, Torchilin V, Patri AK, et al. Clin. Cancer Res. 2012;18:3229. doi: 10.1158/1078-0432.CCR-11-2938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jain RK, Stylianopoulos T. Nat. Rev. Clin. Oncol. 2010;7:653. doi: 10.1038/nrclinonc.2010.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chabner BA. Cancer Chemotherapy and Biotherapy: Principles and Practice. 5th. Philadelphia, PA: Lippincott Williams & Wilkins; 2011. [Google Scholar]
- 24.van de Ven AL, Wu M, Lowengrub J, et al. AIP Adv. 2012;2:11208. doi: 10.1063/1.3699060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brigger I, Dubernet C, Couvreur P. Adv. Drug Delivery Rev. 2002;54:631. doi: 10.1016/s0169-409x(02)00044-3. [DOI] [PubMed] [Google Scholar]
- 26.Jain RK. Cancer Res. 1987;47:3039. [PubMed] [Google Scholar]
- 27.van de Ven AL, Abdollahi B, Martinez CJ, et al. New J. Phys. 2013;15:55004. doi: 10.1088/1367-2630/15/5/055004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cairns RA, Harris IS, Mak TW. Nat. Rev. Cancer. 2011;11:85. doi: 10.1038/nrc2981. [DOI] [PubMed] [Google Scholar]
- 29.Vinci M, Gowan S, Boxall F, et al. Bmc Biol. 2012;10:29. doi: 10.1186/1741-7007-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krock BL, Skuli N, Simon MC. Genes Cancer. 2011;2:1117. doi: 10.1177/1947601911423654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Raghunand N, Gatenby RA, Gillies RJ. Br. J. Radiol. 2003;76:S11. doi: 10.1259/bjr/12913493. [DOI] [PubMed] [Google Scholar]
- 32.Baxter LT, Jain RK. Microvasc. Res. 1989;37:77. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- 33.Baxter LT, Jain RK. Microvasc. Res. 1990;40:246. doi: 10.1016/0026-2862(90)90023-k. [DOI] [PubMed] [Google Scholar]
- 34.Conde J, Tian FR, Hernandez Y, et al. Biomaterials. 2013;34:7744. doi: 10.1016/j.biomaterials.2013.06.041. [DOI] [PubMed] [Google Scholar]
- 35.Bhat R, Sharanabasava VG, Deshpande R, Shetti U, Sanjeev G, Venkataraman A. J. Photochem. Photobiol. B-Biology. 2013;125:63. doi: 10.1016/j.jphotobiol.2013.05.002. [DOI] [PubMed] [Google Scholar]
- 36.Liu M, Gu XH, Zhang K, et al. J. Nanoparticle Res. 2013;15:1745. [Google Scholar]
- 37.Choi YJ, Kim YJ, Lee JW, et al. J. Nanosci. Nanotechnol. 2013;13:4437. doi: 10.1166/jnn.2013.7176. [DOI] [PubMed] [Google Scholar]
- 38.Kao HW, Lin YY, Chen CC, et al. Bioorg. Med. Chem. Lett. 2013;23:3180. doi: 10.1016/j.bmcl.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 39.Ren F, Bhana S, Norman DD, et al. Bioconjug. Chem. 2013;24:376. doi: 10.1021/bc300442d. [DOI] [PubMed] [Google Scholar]
- 40.Fellahi O, Sarma RK, Das MR, et al. Nanotechnology. 2013;24:495101. doi: 10.1088/0957-4484/24/49/495101. [DOI] [PubMed] [Google Scholar]
- 41.Sankar R, Karthik A, Prabu A, Karthik S, Shivashangari KS, Ravikumar V. Colloids Surf. B-Biointerfaces. 2013;108:80. doi: 10.1016/j.colsurfb.2013.02.033. [DOI] [PubMed] [Google Scholar]
- 42.Gengan RM, Anand K, Phulukdaree A, Chuturgoon A. Colloids Surf. B-Biointerfaces. 2013;105:87. doi: 10.1016/j.colsurfb.2012.12.044. [DOI] [PubMed] [Google Scholar]
- 43.Govender R, Phulukdaree A, Gengan RM, Anand K, Chuturgoon AA. J. Nanobiotechnol. 2013;11:5. doi: 10.1186/1477-3155-11-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vyas KM, Jadeja RN, Patel D, Devkar RV, Gupta VK. Polyhedron. 2013;65:262. [Google Scholar]
- 45.Magaye R, Zhao JS, Bowman L, Ding M. Exp. Ther. Med. 2012;4:551. doi: 10.3892/etm.2012.656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Thounaojam MC, Jadeja RN, Valodkar M, Nagar PS, Devkar RV, Thakore S. Food Chemical Toxicol. 2011;49:2990. doi: 10.1016/j.fct.2011.07.055. [DOI] [PubMed] [Google Scholar]
- 47.Kankala S, Kankala RK, Balaboina R, Thirukovela NS, Vadde R, Vasam CS. Bioorg. Med. Chem. Lett. 2014;24:1180. doi: 10.1016/j.bmcl.2013.12.108. [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Fu Y, Mei Y, Jiang F, Lakowicz JR. Anal Chem. 2010;82:4464. doi: 10.1021/ac100241f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wan R, Mo Y, Chien S, et al. Nanotoxicology. 2011;5:568. doi: 10.3109/17435390.2010.537791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Glista-Baker EE, Taylor AJ, Sayers BC, Thompson EA, Bonner JC. Am. J. Respir. Cell Mol. Biol. 2012;47:552. doi: 10.1165/rcmb.2012-0023OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ahamed M, Akhtar MJ, Alhadlaq HA, et al. Chemosphere. 2015;135:278. doi: 10.1016/j.chemosphere.2015.03.079. [DOI] [PubMed] [Google Scholar]
- 52.Cai W, Gao T, Hong H, Sun J. Nanotechnol. Sci. Appl. 2008;2008:17. doi: 10.2147/NSA.S3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dykman L, Khlebtsov N. Chem. Soc. Rev. 2012;41:2256. doi: 10.1039/c1cs15166e. [DOI] [PubMed] [Google Scholar]
- 54.Huang X, El-Sayed MA. J. Adv. Res. 2010;1:13. [Google Scholar]
- 55.Garrett N, Whiteman M, Moger J. Opt. Express. 2011;19:17563. doi: 10.1364/OE.19.017563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Liang ZS, Liu Y, Li XY, et al. J. Biomed. Mater. Res. Part A. 2011;98A:479. doi: 10.1002/jbm.a.33068. [DOI] [PubMed] [Google Scholar]
- 57.Chithrani BD, Ghazani AA, Chan WCW. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 58.Alkilany AM, Murphy CJ. J. Nanoparticle Res. 2010;12:2313. doi: 10.1007/s11051-010-9911-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kennedy LC, Bickford LR, Lewinski NA, et al. Small. 2011;7:169. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 60.Tang M, Russell PJ, Khatri A. Discov. Med. 2007;7:68. [PubMed] [Google Scholar]
- 61.Wang M, Petersen NO. Biochim. Biophys. Acta. 2013;1831:1089. doi: 10.1016/j.bbalip.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 62.Peng G, Tisch U, Adams O, et al. Nat Nano. 2009;4:669. doi: 10.1038/nnano.2009.235. [DOI] [PubMed] [Google Scholar]
- 63.Barash O, Peled N, Tisch U, Bunn PA, Jr, Hirsch FR, Haick H. Nanomedicine. 2012;8:580. doi: 10.1016/j.nano.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Oldenburg SJ, Averitt RD, Westcott SL, Halas NJ. Chem. Phys. Lett. 1998;288:243. [Google Scholar]
- 65.Cheng FY, Chen CT, Yeh CS. Nanotechnology. 2009;20:425104. doi: 10.1088/0957-4484/20/42/425104. [DOI] [PubMed] [Google Scholar]
- 66.Liu HY, Chen D, Tang FQ, et al. Nanotechnology. 2008;19:455101. doi: 10.1088/0957-4484/19/45/455101. [DOI] [PubMed] [Google Scholar]
- 67.Lowery AR, Gobin AM, Day ES, Halas NJ, West JL. Int. J. Nanomed. 2006;1:149. doi: 10.2147/nano.2006.1.2.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gobin AM, Lee MH, Halas NJ, James WD, Drezek RA, West JL. Nano Lett. 2007;7:1929. doi: 10.1021/nl070610y. [DOI] [PubMed] [Google Scholar]
- 69.England CG, Priest T, Zhang G, et al. Int. J. Nanomed. 2013;8:3603. doi: 10.2147/IJN.S51668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.England CG, Kurtis J, Huang J, Zhang X, Gobin AM, Frieboes HB. PLoS One. 2015;10:e0129172. doi: 10.1371/journal.pone.0129172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frens G. Nat. Phys. Sci. 1973;241:20. [Google Scholar]
- 72.Vogel W, Duff DG, Baiker A. Langmuir. 1995;11:401. [Google Scholar]
- 73.Preston TC, Signorell R. ACS Nano. 2009;3:3696. doi: 10.1021/nn900883d. [DOI] [PubMed] [Google Scholar]
- 74.Stöber W, Fink A, Bohn E. J. Colloid Interface Sci. 1968;26:62. [Google Scholar]
- 75.Takahashi H, Niidome Y, Niidome T, Kaneko K, Kawasaki H, Yamada S. Langmuir. 2006;22:2. doi: 10.1021/la0520029. [DOI] [PubMed] [Google Scholar]
- 76.Huo S, Ma H, Huang K, et al. Cancer Res. 2013;73:319. doi: 10.1158/0008-5472.CAN-12-2071. [DOI] [PubMed] [Google Scholar]
- 77.Bain CD, Biebuych HA, Whitesides GM. Langmuir. 1989;5:723. [Google Scholar]
- 78.Häkkinen H. Nat. Chem. 2012;4:443. doi: 10.1038/nchem.1352. [DOI] [PubMed] [Google Scholar]
- 79.England CG, Priest T, Zhang G, et al. Int. J. Nanomed. 2013;8:3603. doi: 10.2147/IJN.S51668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kimling J, Maier M, Okenve B, Kotaidis V, Ballot H, Plech A. J. Phys. Chem. B. 2006;110:15700. doi: 10.1021/jp061667w. [DOI] [PubMed] [Google Scholar]
- 81.Shang L, Nienhaus K, Nienhaus GU. J. Nanobiotechnol. 2014;12:5. doi: 10.1186/1477-3155-12-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sykes EA, Chen J, Zheng G, Chan WC. ACS Nano. 2014;8:5696. doi: 10.1021/nn500299p. [DOI] [PubMed] [Google Scholar]
- 83.Leung K. Molecular Imaging and Contrast Agent Database (MICAD) Bethesda, MD: NCBI; 2004. Gold-polyethylene glycol nanoshells. [Google Scholar]
- 84.O’Neal DP, Hirsch LR, Halas NJ, Payne JD, West JL. Cancer Lett. 2004;209:171. doi: 10.1016/j.canlet.2004.02.004. [DOI] [PubMed] [Google Scholar]
- 85.Jokerst JV, Lobovkina T, Zare RN, Gambhir SS. Nanomedicine (London) 2011;6:715. doi: 10.2217/nnm.11.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Maldiney T, Richard C, Seguin J, Wattier N, Bessodes M, Scherman D. ACS Nano. 2011;5:854. doi: 10.1021/nn101937h. [DOI] [PubMed] [Google Scholar]
- 87.Gu X, Zhang W, Liu J, et al. AAPS Pharm. Sci. Tech. 2011;12:1200. doi: 10.1208/s12249-011-9668-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wen S, Zheng F, Shen M, Shi X. J. Appl. Polym. Sci. 2013;128:3807. [Google Scholar]
- 89.Lewis BA, Engelman DM. J. Mol. Biol. 1983;166:211. doi: 10.1016/s0022-2836(83)80007-2. [DOI] [PubMed] [Google Scholar]
- 90.Nagle JF, Tristram-Nagle S. Biochim. Biophys. Acta. 2000;1469:159. doi: 10.1016/s0304-4157(00)00016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.El Harchaoui K, Arsenault BJ, Franssen R, et al. Ann. Intern. Med. 2009;150:84. doi: 10.7326/0003-4819-150-2-200901200-00006. [DOI] [PubMed] [Google Scholar]
- 92.Zheng J, Nagashima K, Parmiter D, de la Cruz J, Patri AK. Methods Mol. Biol. 2011;697:93. doi: 10.1007/978-1-60327-198-1_9. [DOI] [PubMed] [Google Scholar]
- 93.Sokolov K, Follen M, Aaron J, et al. Cancer Res. 2003;63:1999. [PubMed] [Google Scholar]
- 94.Yang PH, Sun X, Chiu JF, Sun H, He QY. Bioconj. Chem. 2005;16:494. doi: 10.1021/bc049775d. [DOI] [PubMed] [Google Scholar]
- 95.de la Fuente JM, Berry CC, Riehle MO, Curtis ASG. Langmuir. 2006;22:3286. doi: 10.1021/la053029v. [DOI] [PubMed] [Google Scholar]
- 96.El-Sayed IH, Huang X, El-Sayed MA. Nano Lett. 2005;5:829. doi: 10.1021/nl050074e. [DOI] [PubMed] [Google Scholar]
- 97.Boyer D, Tamarat P, Maali A, Lounis B, Orrit M. Science. 2002;297:1160. doi: 10.1126/science.1073765. [DOI] [PubMed] [Google Scholar]
- 98.Hainfeld JF, O’Connor MJ, Dilmanian FA, Slatkin DN, Adams DJ, Smilowitz HM. Br. J. Radiol. 2011;84:526. doi: 10.1259/bjr/42612922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yohan D, Cruje C, Lu X, Chithrani D. Nano-Micro Lett. 2015;7:127. doi: 10.1007/s40820-014-0025-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zarogoulidis P, Darwiche K, Krauss L, et al. Future Oncol. 2013;9:1307. doi: 10.2217/fon.13.111. [DOI] [PubMed] [Google Scholar]
- 101.Jeon SO, Hwang HJ, Oh DH, et al. J. Microencapsulat. 2012;29:234. doi: 10.3109/02652048.2011.646327. [DOI] [PubMed] [Google Scholar]
- 102.Darwiche K, Zarogoulidis P, Krauss L, et al. Int. J. Nanomed. 2013;8:4533. doi: 10.2147/IJN.S54418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Goldsborough AS, Handley MD, Dulcey AE, Pluchino KM, Kannan P, Brimacombe KR. J. Med. Chem. 2011;54:4987. doi: 10.1021/jm2001663. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104.Dahl JA, Maddux BL, Hutchison JE. Chem. Rev. 2007;107:2228. doi: 10.1021/cr050943k. [DOI] [PubMed] [Google Scholar]
- 105.Kumar A, Ma H, Zhang X, et al. Biomaterials. 2012;33:1180. doi: 10.1016/j.biomaterials.2011.10.058. [DOI] [PubMed] [Google Scholar]
- 106.Chithrani BD, Ghazani AA, Chan WC. Nano Lett. 2006;6:662. doi: 10.1021/nl052396o. [DOI] [PubMed] [Google Scholar]
- 107.Kim JA, Aberg C, Salvati A, Dawson KA. Nat. Nanotechnol. 2012;7:62. doi: 10.1038/nnano.2011.191. [DOI] [PubMed] [Google Scholar]
- 108.Walte CL, Roth CM. Bioconj. Chem. 2009;20:1908. doi: 10.1021/bc900228m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Walte CL, Roth CM. Biotechnol. Bioeng. 2011;108:2999. doi: 10.1002/bit.23255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Goodman TT, Olive PL, Pun SH. Int. J. Nanomed. 2007;2:265. [PMC free article] [PubMed] [Google Scholar]
- 111.Huang K, Ma H, Liu J, et al. ACS Nano. 2012;6:4483. doi: 10.1021/nn301282m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Yen JC, Chang FJ, Chang S. IEEE Trans Image Process. 1995;4:370. doi: 10.1109/83.366472. [DOI] [PubMed] [Google Scholar]
- 113.Wong C, Stylianopoulos T, Cui JA, et al. Proc. Natl. Acad. Sci. U.S.A. 2011;108:2426. doi: 10.1073/pnas.1018382108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Chauhan VP, Stylianopoulos T, Martin JD, et al. Nat. Nanotechnol. 2012;7:383. doi: 10.1038/nnano.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Hirn S, Semmler-Behnke M, Schleh C, et al. Eur. J. Pharm. Biopharm. 2011;77:407. doi: 10.1016/j.ejpb.2010.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Hainfeld JF, Slatkin DN, Focella TM, Smilowitz HM. Br. J. Radiol. 2006;79:248. doi: 10.1259/bjr/13169882. [DOI] [PubMed] [Google Scholar]
- 117.Sadauskas E, Wallin H, Stoltenberg M, et al. Part. Fibre Toxicol. 2007;4:10. doi: 10.1186/1743-8977-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Patra CR, Bhattacharya R, Wang EF, et al. Cancer Res. 2008;68:1970. doi: 10.1158/0008-5472.CAN-07-6102. [DOI] [PubMed] [Google Scholar]
- 119.Wang Z, Butner JD, Cristini V, Deisboeck TS. J. Pharmacokinet. Pharmacodyn. 2015;42:179. doi: 10.1007/s10928-015-9403-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Deisboeck TS, Wang Z, Macklin P, Cristini V. Annu. Rev. Biomed. Eng. 2011;13:127. doi: 10.1146/annurev-bioeng-071910-124729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Wang Z, Deisboeck TS. Drug Discov. Today. 2014;19:145. doi: 10.1016/j.drudis.2013.06.015. [DOI] [PubMed] [Google Scholar]
- 122.Bachmann J, Raue A, Schilling M, Becker V, Timmer J, Klingmuller U. J. Int. Med. 2012;271:155. doi: 10.1111/j.1365-2796.2011.02492.x. [DOI] [PubMed] [Google Scholar]
- 123.Andasari V, Gerisch A, Lolas G, South AP, Chaplain MA. J. Math. Biol. 2011;63:141. doi: 10.1007/s00285-010-0369-1. [DOI] [PubMed] [Google Scholar]
- 124.Chaplain MAJ. Ima J. Appl. Math. 2011;76:371. [Google Scholar]
- 125.Michor F, Liphardt J, Ferrari M, Widom J. Nat. Rev. Cancer. 2011;11:657. doi: 10.1038/nrc3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Rejniak KA, Anderson ARA. WIREs Syst. Biol. Med. 2011;3:115. doi: 10.1002/wsbm.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Swanson KR, Rockne RC, Claridge J, Chaplain MA, Alvord EC, Anderson ARA. Cancer Res. 2011;71:7366. doi: 10.1158/0008-5472.CAN-11-1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Frieboes HB, Chaplain MA, Thompson AM, Bearer EL, Lowengrub JS, Cristini V. Cancer Res. 2011;71:298. doi: 10.1158/0008-5472.CAN-10-2676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Byrne HM. Nat. Rev. Cancer. 2010;10:221. doi: 10.1038/nrc2808. [DOI] [PubMed] [Google Scholar]
- 130.Kreeger PK, Lauffenburger DA. Carcinogenesis. 2010;31:2. doi: 10.1093/carcin/bgp261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Rejniak KA, McCawley LJ. Exp. Biol. Med. 2010;235:411. doi: 10.1258/ebm.2009.009230. [DOI] [PubMed] [Google Scholar]
- 132.Lowengrub JS, Frieboes HB, Jin F, et al. Nonlinearity. 2010;23:R1. doi: 10.1088/0951-7715/23/1/r01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Vineis P, Schatzkin A, Potter JD. Carcinogenesis. 2010;31:1703. doi: 10.1093/carcin/bgq087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Osborne JM, Walter A, Kershaw SK, et al. Philos. Trans. R. Soc. A-Math. Phys. Engin. Sci. 2010;368:5013. doi: 10.1098/rsta.2010.0173. [DOI] [PubMed] [Google Scholar]
- 135.Edelman LB, Eddy JA, Price ND. WIREs Syst. Biol. Med. 2010;2:438. doi: 10.1002/wsbm.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Palladini A, Nicoletti G, Pappalardo F, et al. Cancer Res. 2010;70:7755. doi: 10.1158/0008-5472.CAN-10-0701. [DOI] [PubMed] [Google Scholar]
- 137.Cristini V, Lowengrub J. Multiscale modeling of cancer: an integrated experimental and mathematical modeling approach. Cambridge University Press; 2010. [Google Scholar]
- 138.Kaddi CD, Phan JH, Wang MD. Nanomedicine (London) 2013;8:1323. doi: 10.2217/nnm.13.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Frieboes HB, Wu M, Lowengrub J, Decuzzi P, Cristini V. PloS One. 2013;8:e56876. doi: 10.1371/journal.pone.0056876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wu M, Frieboes HB, Chaplain MA, McDougall SR, Cristini V, Lowengrub J. J. Theor. Biol. 2014;355:194. doi: 10.1016/j.jtbi.2014.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Li M, Reineke J. Int. J. Nano Biomater. 2011;3:222. [Google Scholar]
- 142.Decuzzi P, Pasqualini R, Arap W, Ferrari M. Pharm. Res. 2009;26:235. doi: 10.1007/s11095-008-9697-x. [DOI] [PubMed] [Google Scholar]
- 143.Gao Y, Li M, Chen B, et al. AAPS J. 2013;15:816. doi: 10.1208/s12248-013-9478-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Godin B, Driessen WH, Proneth B, et al. Adv. Genetics. 2010;69:31. doi: 10.1016/S0065-2660(10)69009-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Frieboes HB, Sinek JP, Nalcioglu O, Fruehauf JP, Cristini V. Nanotechnology in Cancer Drug Therapy: A Biocomputational Approach. In: Ferrari M, Lee AP, Lee LJ, editors. BioMEMS and Biomedical Nanotechnology. US: Springer; 2006. p. 435. [Google Scholar]
- 146.Li M, Panagi Z, Avgoustakis K, Rineke J. Int. J. Nanomed. 2012;7:1345. doi: 10.2147/IJN.S23758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li M, Al-Jamal KT, Kostarelos K, Reineke J. ACS Nano. 2010;4:6303. doi: 10.1021/nn1018818. [DOI] [PubMed] [Google Scholar]
- 148.Li M, Czyszczon EA, Reineke JJ. Drug Deliv. Transl. Res. 2013;3:551. doi: 10.1007/s13346-013-0162-y. [DOI] [PubMed] [Google Scholar]
- 149.Li M, Reineke J. Methods Mol. Biol. 2012;926:369. doi: 10.1007/978-1-62703-002-1_24. [DOI] [PubMed] [Google Scholar]
- 150.Kempen PJ, Thakor AS, Zavaleta C, Gambhir SS, Sinclair R. Microsc. Microanal. 2013;19:1290. doi: 10.1017/S143192761300192X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Costa PM, Cardoso AL, Mendonca LS, et al. Mol. Ther. Nucleic Acids. 2013;2:e100. doi: 10.1038/mtna.2013.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weinkauf H, Brehm-Stecher BF. Biotechnol. J. 2009;4:871. doi: 10.1002/biot.200800358. [DOI] [PubMed] [Google Scholar]
- 153.Kennedy LC, Bickford LR, Lewinski NA, et al. Small. 2011;7:169. doi: 10.1002/smll.201000134. [DOI] [PubMed] [Google Scholar]
- 154.Guo CS, Yin S, Yu HJ, et al. Nanoscale. 2013;5:6469. doi: 10.1039/c3nr01025b. [DOI] [PubMed] [Google Scholar]
- 155.Li Y, Lu W, Huang Q, Huang M, Li C, Chen W. Nanomedicine (London) 2010;5:1161. doi: 10.2217/nnm.10.85. [DOI] [PubMed] [Google Scholar]
- 156.Taruttis A, Herzog E, Razansky D, Ntziachristos V. Opt. Express. 2010;18:19592. doi: 10.1364/OE.18.019592. [DOI] [PubMed] [Google Scholar]
- 157.Grosges T, Barchiesi D, Kessentini S, Grehan G, de la Chapelle ML. Biomed. Opt. Express. 2011;2:1584. doi: 10.1364/BOE.2.001584. [DOI] [PMC free article] [PubMed] [Google Scholar]