Abstract
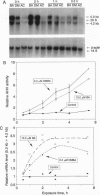
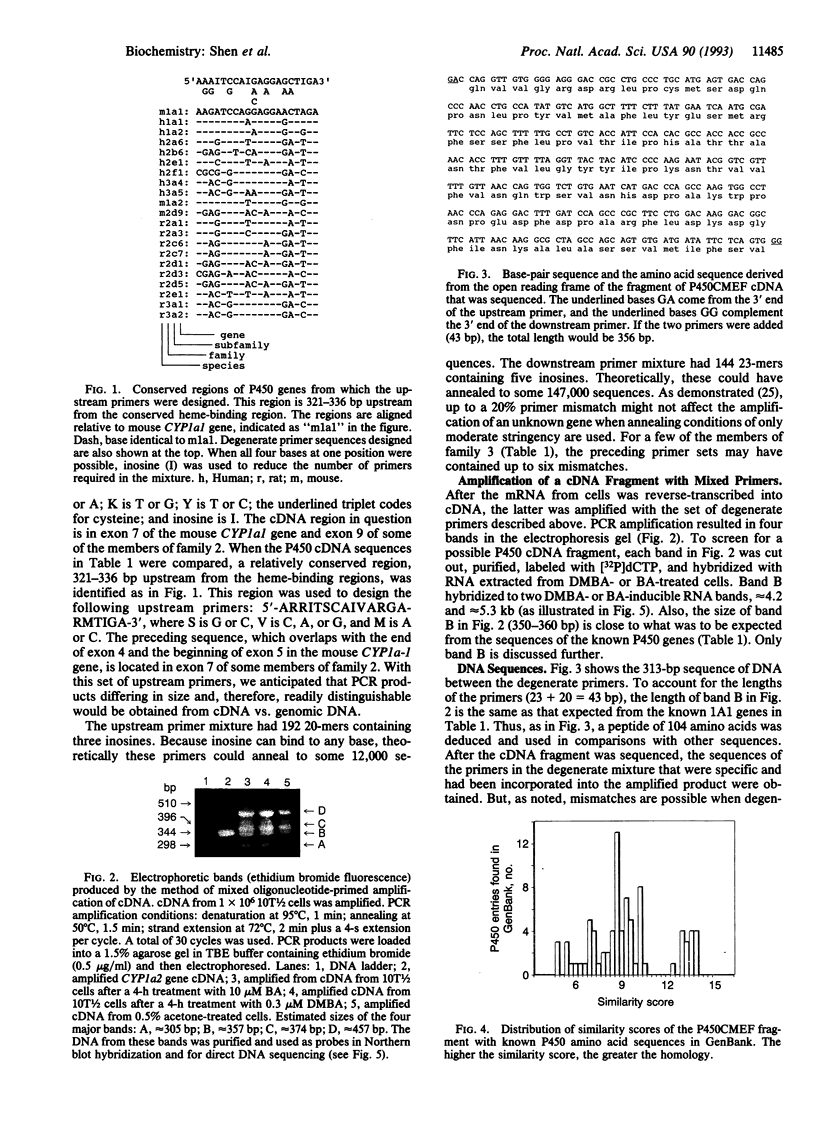
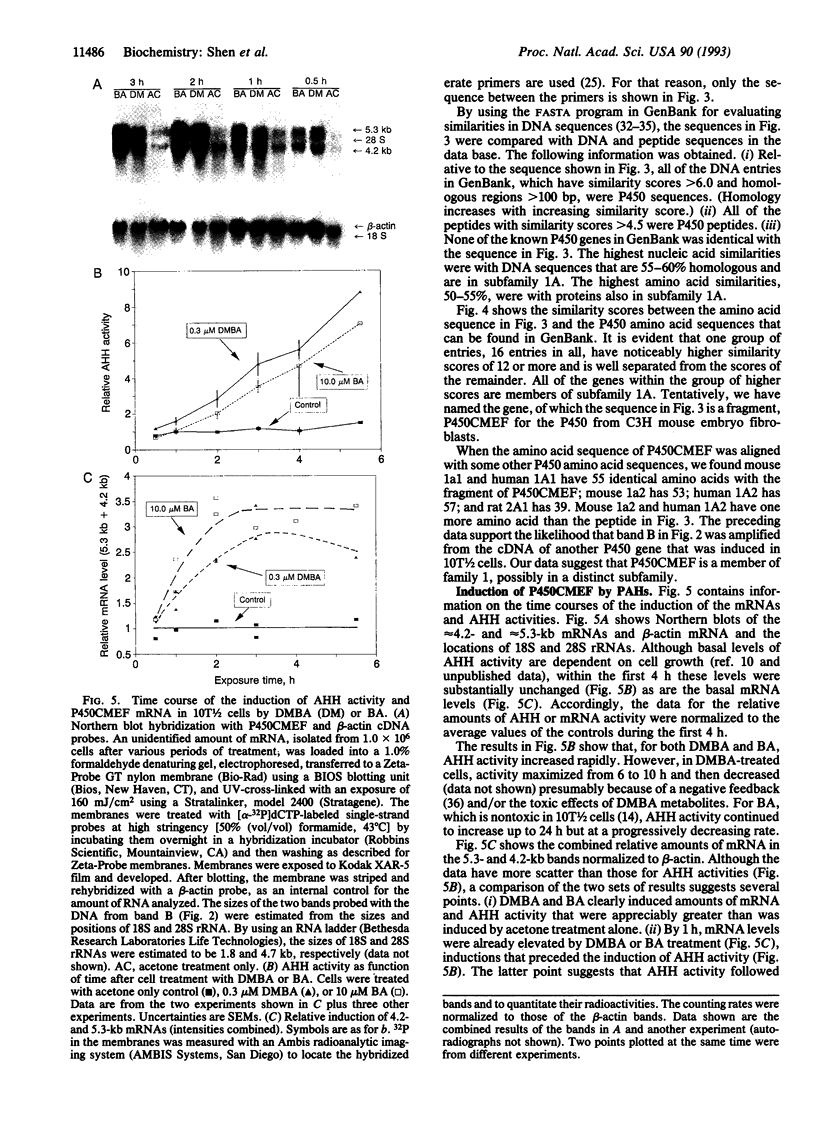
A cytochrome P450-like gene, tentatively named P450CMEF, was amplified by a mixed oligonucleotide-primed amplification of cDNA from C3H mouse embryo fibroblast cells, designated 10T1/2, that had been treated with 7,12-dimethylbenz[a]anthracene (DMBA) or benz[a]anthracene (BA). A set of inosine-containing degenerate primers that were targeted to two conserved regions of known cytochrome P450 cDNAs were used. One primer was coded for the well-described and conserved heme-binding region of P450 enzymes, and the second was designed based upon other considerations of homology among P450 molecules. One of the four PCR-amplified cDNA products hybridized to two major RNA bands, 4.2 and 5.3 kb, that were induced by DMBA or BA. The amino acid sequence of the fragment deduced from the base-sequence data indicate that the amplified cDNA has a 50-55% identity with the cytochrome P450 subfamily 1A. The induction of P450CMEF mRNA preceded the induction of aryl hydrocarbon hydroxylase activity after DMBA or BA treatment, suggesting that the product of P450CMEF is involved in the metabolism of these polycyclic aromatic hydrocarbons in 10T1/2 cells. From the partial sequence of the cDNA identified by this procedure, we propose that P450CMEF is a member of the P450 superfamily, possibly in a subfamily of family 1, that is induced in 10T1/2 cells by DMBA and BA. This method should be useful in identifying additional P450 genes and genes in other gene families.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altschul S. F., Erickson B. W. A nonlinear measure of subalignment similarity and its significance levels. Bull Math Biol. 1986;48(5-6):617–632. doi: 10.1007/BF02462327. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Christou M., Stewart P., Pottenger L. H., Fahl W. E., Jefcoate C. R. Differences in the modulation of P450IA1 and epoxide hydratase expression by benz[a]anthracene and 2,3,7,8-tetrachlorodibenzo-p-dioxin in mouse embryo versus mouse hepatoma-derived cell lines. Carcinogenesis. 1990 Oct;11(10):1691–1698. doi: 10.1093/carcin/11.10.1691. [DOI] [PubMed] [Google Scholar]
- Erlich H. A., Gelfand D., Sninsky J. J. Recent advances in the polymerase chain reaction. Science. 1991 Jun 21;252(5013):1643–1651. doi: 10.1126/science.2047872. [DOI] [PubMed] [Google Scholar]
- Gehly E. B., Fahl W. E., Jefcoate C. R., Heidelberger C. The metabolism of benzo(alpha)pyrene by cytochrome P-450 in transformable and nontransformable C3H mouse fibroblasts. J Biol Chem. 1979 Jun 25;254(12):5041–5048. [PubMed] [Google Scholar]
- Gonzalez F. J., Crespi C. L., Gelboin H. V. DNA-expressed human cytochrome P450s: a new age of molecular toxicology and human risk assessment. Mutat Res. 1991 Mar;247(1):113–127. doi: 10.1016/0027-5107(91)90039-q. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Kimura S., Song B. J., Pastewka J., Gelboin H. V., Hardwick J. P. Sequence of two related P-450 mRNAs transcriptionally increased during rat development. An R.dre.1 sequence occupies the complete 3' untranslated region of a liver mRNA. J Biol Chem. 1986 Aug 15;261(23):10667–10672. [PubMed] [Google Scholar]
- Gonzalez F. J., Mackenzie P. I., Kimura S., Nebert D. W. Isolation and characterization of full-length mouse cDNA and genomic clones of 3-methylcholanthrene-inducible cytochrome P1-450 and P3-450. Gene. 1984 Sep;29(3):281–292. doi: 10.1016/0378-1119(84)90057-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez F. J., Nebert D. W., Hardwick J. P., Kasper C. B. Complete cDNA and protein sequence of a pregnenolone 16 alpha-carbonitrile-induced cytochrome P-450. A representative of a new gene family. J Biol Chem. 1985 Jun 25;260(12):7435–7441. [PubMed] [Google Scholar]
- Gonzalez F. J. The molecular biology of cytochrome P450s. Pharmacol Rev. 1988 Dec;40(4):243–288. [PubMed] [Google Scholar]
- Ho D., Gill K., Fahl W. E. Benz[a]anthracene and 3-methylcholanthrene induction of cytochrome P-450 in C3H/10T1/2 mouse fibroblasts. Modulating role of cytotoxic 3-methylcholanthrene metabolites. Mol Pharmacol. 1983 Jan;23(1):198–205. [PubMed] [Google Scholar]
- Kawajiri K., Gotoh O., Sogawa K., Tagashira Y., Muramatsu M., Fujii-Kuriyama Y. Coding nucleotide sequence of 3-methylcholanthrene-inducible cytochrome P-450d cDNA from rat liver. Proc Natl Acad Sci U S A. 1984 Mar;81(6):1649–1653. doi: 10.1073/pnas.81.6.1649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoth K., Roberds S., Poteet C., Tamkun M. Highly degenerate, inosine-containing primers specifically amplify rare cDNA using the polymerase chain reaction. Nucleic Acids Res. 1988 Nov 25;16(22):10932–10932. doi: 10.1093/nar/16.22.10932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence C. B., Goldman D. A. Definition and identification of homology domains. Comput Appl Biosci. 1988 Mar;4(1):25–33. doi: 10.1093/bioinformatics/4.1.25. [DOI] [PubMed] [Google Scholar]
- Lawrence C. B., Goldman D. A., Hood R. T. Optimized homology searches of the gene and protein sequence data banks. Bull Math Biol. 1986;48(5-6):569–583. doi: 10.1007/BF02462324. [DOI] [PubMed] [Google Scholar]
- Miura T., Patierno S. R., Sakuramoto T., Landolph J. R. Morphological and neoplastic transformation of C3H/10T1/2 Cl 8 mouse embryo cells by insoluble carcinogenic nickel compounds. Environ Mol Mutagen. 1989;14(2):65–78. doi: 10.1002/em.2850140202. [DOI] [PubMed] [Google Scholar]
- Nagata K., Matsunaga T., Gillette J., Gelboin H. V., Gonzalez F. J. Rat testosterone 7 alpha-hydroxylase. Isolation, sequence, and expression of cDNA and its developmental regulation and induction by 3-methylcholanthrene. J Biol Chem. 1987 Feb 25;262(6):2787–2793. [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Jones J. E. Regulation of the mammalian cytochrome P1-450 (CYP1A1) gene. Int J Biochem. 1989;21(3):243–252. doi: 10.1016/0020-711x(89)90182-1. [DOI] [PubMed] [Google Scholar]
- Nebert D. W., Nelson D. R., Coon M. J., Estabrook R. W., Feyereisen R., Fujii-Kuriyama Y., Gonzalez F. J., Guengerich F. P., Gunsalus I. C., Johnson E. F. The P450 superfamily: update on new sequences, gene mapping, and recommended nomenclature. DNA Cell Biol. 1991 Jan-Feb;10(1):1–14. doi: 10.1089/dna.1991.10.1. [DOI] [PubMed] [Google Scholar]
- Nelson D. R., Kamataki T., Waxman D. J., Guengerich F. P., Estabrook R. W., Feyereisen R., Gonzalez F. J., Coon M. J., Gunsalus I. C., Gotoh O. The P450 superfamily: update on new sequences, gene mapping, accession numbers, early trivial names of enzymes, and nomenclature. DNA Cell Biol. 1993 Jan-Feb;12(1):1–51. doi: 10.1089/dna.1993.12.1. [DOI] [PubMed] [Google Scholar]
- Nesnow S., Leavitt S., Garland H., Vaughan T. O., Hyatt B., Montgomery L., Cudak C. Identification of cocarcinogens and their potential mechanisms of action using C3H10T 1/2 CL8 mouse embryo fibroblasts. Cancer Res. 1981 Aug;41(8):3071–3076. [PubMed] [Google Scholar]
- Nhamburo P. T., Kimura S., McBride O. W., Kozak C. A., Gelboin H. V., Gonzalez F. J. The human CYP2F gene subfamily: identification of a cDNA encoding a new cytochrome P450, cDNA-directed expression, and chromosome mapping. Biochemistry. 1990 Jun 12;29(23):5491–5499. doi: 10.1021/bi00475a012. [DOI] [PubMed] [Google Scholar]
- Okey A. B., Mason M. E., Gehly E. B., Heidelberger C., Muncan J., Dufresne M. J. Defective binding of 3-methylcholanthrene to the Ah receptor within C3H/1OT1/2 clone 8 mouse fibroblasts in culture. Eur J Biochem. 1983 May 2;132(2):219–227. doi: 10.1111/j.1432-1033.1983.tb07351.x. [DOI] [PubMed] [Google Scholar]
- Otto S., Bhattacharyya K. K., Jefcoate C. R. Polycyclic aromatic hydrocarbon metabolism in rat adrenal, ovary, and testis microsomes is catalyzed by the same novel cytochrome P450 (P450RAP). Endocrinology. 1992 Dec;131(6):3067–3076. doi: 10.1210/endo.131.6.1332854. [DOI] [PubMed] [Google Scholar]
- Otto S., Marcus C., Pidgeon C., Jefcoate C. A novel adrenocorticotropin-inducible cytochrome P450 from rat adrenal microsomes catalyzes polycyclic aromatic hydrocarbon metabolism. Endocrinology. 1991 Aug;129(2):970–982. doi: 10.1210/endo-129-2-970. [DOI] [PubMed] [Google Scholar]
- Patierno S. R., Banh D., Landolph J. R. Transformation of C3H/10T1/2 mouse embryo cells to focus formation and anchorage independence by insoluble lead chromate but not soluble calcium chromate: relationship to mutagenesis and internalization of lead chromate particles. Cancer Res. 1988 Sep 15;48(18):5280–5288. [PubMed] [Google Scholar]
- Pearson W. R., Lipman D. J. Improved tools for biological sequence comparison. Proc Natl Acad Sci U S A. 1988 Apr;85(8):2444–2448. doi: 10.1073/pnas.85.8.2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pottenger L. H., Christou M., Jefcoate C. R. Purification and immunological characterization of a novel cytochrome P450 from C3H/10T1/2 cells. Arch Biochem Biophys. 1991 May 1;286(2):488–497. doi: 10.1016/0003-9861(91)90070-y. [DOI] [PubMed] [Google Scholar]
- Pottenger L. H., Jefcoate C. R. Characterization of a novel cytochrome P450 from the transformable cell line, C3H/10T1/2. Carcinogenesis. 1990 Feb;11(2):321–327. doi: 10.1093/carcin/11.2.321. [DOI] [PubMed] [Google Scholar]
- Reznikoff C. A., Bertram J. S., Brankow D. W., Heidelberger C. Quantitative and qualitative studies of chemical transformation of cloned C3H mouse embryo cells sensitive to postconfluence inhibition of cell division. Cancer Res. 1973 Dec;33(12):3239–3249. [PubMed] [Google Scholar]
- Shen Z., Liu J., Wells R. L., Elkind M. M. Cycle sequencing using degenerate primers containing inosines. Biotechniques. 1993 Jul;15(1):82-4, 88-9. [PubMed] [Google Scholar]
- Wells R. L., Chen C. C., Elkind M. M. Abrogation of killing and neoplastic transformation of C3H10T1/2 cells due to 7,12-dimethylbenz[a]anthracene. Carcinogenesis. 1992 Apr;13(4):643–649. doi: 10.1093/carcin/13.4.643. [DOI] [PubMed] [Google Scholar]
- Wells R. L., Sneider T. W., Elkind M. M. Aryl hydrocarbon hydroxylase activity assayed in whole cell lysates using synchronous fluorescence spectroscopy. Anal Biochem. 1991 Nov 1;198(2):355–362. doi: 10.1016/0003-2697(91)90439-z. [DOI] [PubMed] [Google Scholar]