Abstract
Introduction
Early recognition and treatment of trauma patients requiring massive transfusion (MT) has been shown to reduce mortality. While many risk factors predicting MT have been demonstrated, there is no universally accepted method or algorithm to identify these patients. We hypothesized that even among experienced trauma surgeons, the clinical gestalt of identifying patients who will require MT is unreliable.
Methods
Transfusion and mortality outcomes after trauma were observed at 10 U.S. Level-1 trauma centers in patients who survived ≥30 minutes after admission and received ≥1 unit of RBC within 6 hours of arrival. Subjects who received ≥ 10 units within 24 hours of admission were classified as MT patients. Trauma surgeons were asked the clinical gestalt question “Is the patient likely to be massively transfused?” ten minutes after the patients arrival. The performance of clinical gestalt to predict MT was assessed using chi-square tests and ROC analysis to compare gestalt to previously described scoring systems.
Results
Of the 1,245 patients enrolled, 966 met inclusion criteria and 221 (23%) patients received MT. 415 (43%) were predicted to have a MT and 551(57%) were predicted to not have MT. Patients predicted to have MT were younger, more often sustained penetrating trauma, had higher ISS scores, higher heart rates, and lower systolic blood pressures (all p < 0.05). Gestalt sensitivity was 65.6% and specificity was 63.8%. PPV and NPV were 34.9% and 86.2% respectively.
Conclusion
Data from this large multicenter trial demonstrates that predicting the need for MT continues to be a challenge. Because of the increased mortality associated with delayed therapy, a more reliable algorithm is needed to identify and treat these severely injured patients earlier.
Level of Evidence
II; Diagnostic study - Development of diagnostic criteria on basis of consecutive patients (with universally applied reference standard)
Keywords: trauma, gestalt, massive transfusion
INTRODUCTION
Predefined massive transfusion (MT) protocols initiate a sequence of events in trauma centers that facilitate rapid and on-going delivery of blood products to critically injured patients. Implementation and maturation of such protocols is associated with decreased time to plasma availability, reduction in overall delivery times, decreased provider variability, reduction in overall blood product usage and waste, and a reduction in mortality, irrespective of the ratios delivered [1–7]. Despite the increased adoption of MT protocols at many trauma centers, criteria for protocol activation remain ill defined and highly variable, and early identification of patients who will require MT remains a challenge.
The relative inability to reliably predict MT has resulted in the development of several algorithms to help identify MT patients, however most have originated from retrospective databases and often require limited or unavailable laboratory data, injury severity scores, or complicated calculated values. Others are not adopted because of the accuracy or user-dependence of some components (FAST). The limited accuracy, complexity, and feasibility issues of these models have resulted in no universally accepted predictive model currently in use. Given these limitations, many trauma surgeons rely on patient characteristics and clinical reasoning skills to formulate an overall clinical gestalt in order to quickly make treatment decisions and predict who will require a MT.
Gestalt theory was proposed as a concept in psychology that suggests that the nature of a unified whole is not understood by analyzing its parts [8]. Gestalt experiments have shown that the brain does not act like a sponge but rather it actively filters, structures, and matches incoming information against known patterns to make sense of it [9–11]. In healthcare decision-making, clinical gestalt is the heuristic approach to quickly forming a diagnosis and treatment plan, often within seconds of data collection, via pattern recognition and organization of clinical observations and our perception of those observations [12, 13]. Intuitively, experience positively influences pattern recognition skills and decision-making accuracy and this is supported in the literature [12, 14]. However there are several important pitfalls in gestalt-based decision-making, such as increased attention to obvious details and grouping of findings that are in close proximity or are similar to one another [15].
Many surgeons argue that defined scoring systems are unnecessary and that their own judgment or gestalt is quite reliable in identifying the extremes of overt hemorrhage (or lack thereof) with a relatively high degree of certainty. For remaining group of severely injured patients, in which the need for MT is less obvious early after arrival, the reliability of clinical gestalt is unknown and has not previously been evaluated. Furthermore, it is unclear how this skillset compares to existing scoring systems. We hypothesized that even among experienced trauma surgeons, the ability of clinical gestalt to predict patients who will require MT is unreliable.
METHODS
The PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) Study was conducted at ten level-1 trauma centers in the United States from July 2009 to October 2010 [16]. The primary objective of the PROMMTT study was to investigate in-hospital mortality to early transfusion of time-varying ratios of blood products. Patients were eligible if they required the highest level of trauma activation, were aged 16 years or older, and received a transfusion of at least one unit of RBCs in the first six hours after arrival. Because the premise of the study was built around observing outcomes in relation to transfusion practices, the inclusion criteria ensured that the patients selected for the study were very likely to have some degree of internal hemorrhage and/or hemodynamic instability suggesting hemorrhage. At each study site and the Data Coordinating Center, the local institutional review board approved the study. All participating centers had MT protocols in place [17].
Data was recorded in real-time via direct bedside observation by research assistants beginning at the time of trauma team activation and continuing until active resuscitation ended. As part of the study protocol, each trauma attending was asked to answer “yes” or “no” to the gestalt question “Is this patient likely to be massively transfused” ten minutes after the patient’s arrival. After direct observation ended, outcomes were recorded daily while the patient was in the intensive care unit and weekly thereafter during hospitalization. Individual site clinicians ascribed cause of in-hospital death and data collectors ascertained sites of bleeding.
Scoring Systems
Some severely injured patients did not undergo routine baseline assessments owing to the emergent nature of their injuries. Despite not having some of the critical elements required to calculate published MT scores in every patient, we evaluated three previously validated MT scoring systems. The TASH (Trauma Associated Severe Hemorrhage) score uses seven independent variables to identify patients who will require a MT. The variables are weighted and rely on laboratory values that may make the scoring system difficult to apply in a real-time fashion at some trauma centers. [18]. The McLaughlin score is a product of retrospective combat data that uses fewer variables and dichotomous outcomes for simplicity. However, like the TASH score, it requires lab values that may not be immediately available for calculating variables in any MT algorithm [19, 20]. The Assessment of Blood Consumption (ABC) score uses immediately available data and (no laboratory values or injury scores) but is limited in some centers by the variable use of and highly operator-dependent FAST examination [21, 22].
Statistical Analysis
Demographic evaluation and incidence of MT at each institution was calculated. Univariate comparison was performed for MT status, gestalt positive (patients predicted to receive an MT) versus gestalt negative (those not predicted to receive an MT), and those correctly and incorrectly classified within the gestalt negative group. MT was defined as receiving 10 units of RBCs within 24 hours of arrival. Alternatively, a separate analysis was conducted including those who died of hemorrhagic deaths within the first 24 hours of arrival to address concerns of potential survivor bias, as well as those receiving MT at 6 hours and those classified as having substantial bleeding (≥ 5 PRBCs) at 4 hours. The primary outcome of the study was the performance of trauma surgeon clinical gestalt to predict MT, as estimated by the AUROC curve and sensitivity, specificity, positive predictive value (PPV), and negative predictive value (NPV). Secondary outcomes included evaluating gestalt performance using alternative definitions of MT (MT or hemorrhagic death in 24 hours, MT at 6 hours, and substantial bleeding) as well as comparing gestalt performance of MT prediction to other previously described scoring systems.
Purposeful regression modeling was used to construct a multivariate logistic regression model to identify the risk factors for incorrectly classified gestalt negative patients (false negatives). In an effort to minimize the risk of falsely identifying significant results with multiple comparisons, all variables were pre-specified and judged a priori to be clinically sound. Variables with significance of <0.20 were used in the final model and included the following: injury mechanism, pelvic bleeding, limb bleeding, presence of isolated traumatic head injury, abbreviated injury scores (AIS) for the chest, abdomen, and extremities, base deficit, and blood pH. Calculation of the TASH, McLaughlin, and ABC scores was performed for patients who had the necessary variables required for all three scores (39% of PROMMTT Study patients and 50% who had gestalt question answered). AUROC curves were generated for each scoring system and compared to clinical gestalt using tests of equality for multiple ROC areas with adjustment using Sidak’s method.
Continuous data were presented as medians and interquartile ranges (IQR) and tested for significance using the Wilcoxon rank sum test (Mann-Whitney U test). Categorical data were reported as proportions and tested for significance using χ2 or Fisher exact tests. All statistical tests were two tailed with p < 0.05 set as significant. STATA version 12.1 (College Station, TX) statistical software was used for data management and analysis.
RESULTS
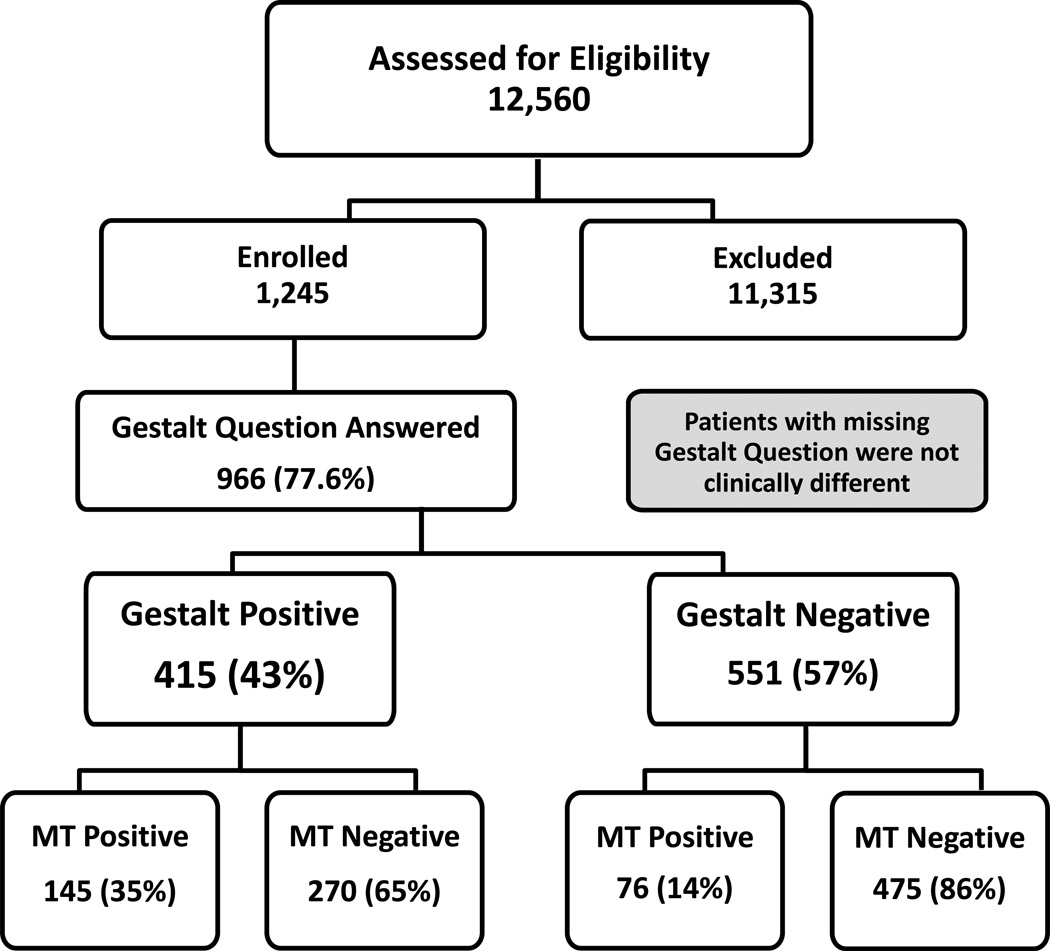
There were 34,362 trauma admissions at the 10 participating centers from July 2009 to October 2010. Data collection was initiated for 12,560 patients; of these, 11,315 became ineligible and were withdrawn from the study and 1,245 met all PROMMTT Study eligibility criteria. Of these, the clinical gestalt question was answered in 966 (78%) patients. No major clinical differences were detected between patient groups in which the gestalt question was answered versus not answered (FIGURE 1).
Figure 1.
CONSORT diagram for patient inclusion and exclusion in this study.
The study cohort was predominantly young to middle-aged (38 years; IQR 24–54) males (73.8%) sustaining blunt trauma (64.8%) with moderate to severe injury (ISS 25; IQR 14–34). The overall MT rate was 23 % (n = 221) and the overall in-hospital mortality rate was 21 % (n=203). Of those who died in the study, 54% died in the first 24 hours and 4% died between 30 minutes and 1 hour after arrival. Demographics and clinical characteristics by MT group are summarized in TABLE 1. Patients receiving MT were more critically injured and had a higher degree of physiologic derangement as indicated by lower arrival blood pressures, higher heart rates, lower GCS, and more extreme laboratory values. MT patients had more severe injuries to the chest and abdomen and more often had a positive FAST exam. The predicted rate of MT according to gestalt was 43% (n = 415). Similar to the comparison of patients by MT group, patients predicted to receive MT were also more critically injured and had a higher degree of physiologic derangements. TABLE 2 summarizes the clinical characteristics and outcomes of patients by clinical gestalt group.
Table 1.
Admission, transfusion, and mortality characteristics of patients by MT group
| MT negative (n=745) | MT positive (n=221) | ||||
|---|---|---|---|---|---|
| Nonmissing, No. |
No. (%) or Median (IQR) |
Nonmissing, No. |
No. (%) or Median (IQR) |
p-value | |
| Age, years | 744 | 38 (24–54) | 221 | 38 (24–52) | 0.508 |
| Male gender | 745 | 547 (73.4) | 221 | 166 (75.1) | 0.616 |
| Blunt Mechanism | 745 | 481 (64.6) | 221 | 145 (65.6) | 0.775 |
| White Race | 745 | 496 (66.6) | 221 | 153 (69.2) | 0.461 |
| Positive FAST | 482 | 127 (26.3) | 154 | 65 (42.2) | <0.001 |
| Systolic Blood Pressure | 736 | 108 (89–128) | 208 | 90 (73–120) | <0.001 |
| Heart Rate | 734 | 103 (85–122) | 212 | 114 (93–133) | <0.001 |
| GCS | 688 | 14 (3–15) | 195 | 8 (3–15) | <0.001 |
| Head AIS | 745 | 0 (0–3) | 221 | 0 (0–3) | 0.050 |
| Chest AIS | 745 | 3 (0–3) | 221 | 3 (0–4) | 0.001 |
| Abdomen AIS | 745 | 2 (0–3) | 221 | 3 (1–4) | <0.001 |
| Bleeding Sites | |||||
| Chest | 745 | 172 (23.1) | 221 | 66 (29.9) | 0.04 |
| Abdomen | 745 | 214 (28.7) | 221 | 93 (42.1) | <0.001 |
| Pelvis | 745 | 70 (9.4) | 221 | 50 (22.6) | <0.001 |
| Limb | 745 | 251 (33.7) | 221 | 99 (44.8) | 0.003 |
| Hemoglobin | 720 | 11.9 (10.6–13.6) | 208 | 10.8 (8.95–12.5) | <0.001 |
| INR | 641 | 1.2 (1.1–1.4) | 182 | 1.4 (1.2–1.7) | <0.001 |
| pH | 606 | 7.29 (7.21–7.35) | 185 | 7.19 (7.08–7.28) | <0.001 |
| Base deficit | 596 | −6 (–9 – −3) | 183 | −9 (−13 – −5) | <0.001 |
| 24-hr RBC, U | 745 | 4 (2–6) | 221 | 17 (12–27) | <0.001 |
| 24-hr plasma, U | 745 | 2 (0–5) | 221 | 14 (8–22) | <0.001 |
| 24-hr platelets, U | 745 | 0 (0–0) | 221 | 12 (6–18) | <0.001 |
| 24-hour mortality | 745 | 49 (6.6) | 221 | 60 (27.1) | <0.001 |
| 30-day mortality | 745 | 114 (15.3) | 221 | 89 (40.3) | <0.001 |
Abbreviations: IQR, interquartile range; FAST, focused assessment for the sonography of trauma; AIS, Abbreviated Injury Severity score; INR, International Normalized Ratio; RBC, red blood cells.
Table 2.
Admission, transfusion, and mortality characteristics of patients by Gestalt prediction group
| Gestalt negative (n=551) | Gestalt positive (n=415) | p-value | |||
|---|---|---|---|---|---|
| Nonmissing, No. |
No. (%) or Median (IQR) |
Nonmissing, No. |
No. (%) or Median (IQR) |
p-value | |
| Age, years | 551 | 40 (24–57) | 414 | 36 (23–51) | 0.004 |
| Male gender | 551 | 413 (75.0) | 415 | 300 (72.3) | 0.351 |
| Blunt Mechanism | 551 | 375 (68.1) | 415 | 251 (60.5) | 0.015 |
| White Race | 551 | 361 (65.5) | 415 | 288 (69.4) | 0.204 |
| Positive FAST | 358 | 85 (23.7) | 278 | 107 (38.5) | <0.001 |
| Systolic Blood Pressure | 548 | 110 (90–130) | 396 | 95 (80–119) | <0.001 |
| Heart Rate | 542 | 102 (85–120) | 404 | 111 (88–131) | 0.001 |
| GCS | 507 | 14 (3–15) | 376 | 10.5 (3–15) | 0.020 |
| Head AIS | 551 | 0 (0–3) | 415 | 0 (0–3) | 0.381 |
| Chest AIS | 551 | 3 (0–3) | 415 | 3 (0–4) | 0.013 |
| Abdomen AIS | 551 | 2 (0–3) | 415 | 2 (0–3) | 0.002 |
| Bleeding Sites | |||||
| Chest | 551 | 103 (18.7) | 415 | 135 (32.5) | <0.001 |
| Abdomen | 551 | 151 (27.4) | 415 | 156 (37.6) | 0.001 |
| Pelvis | 551 | 57 (10.3) | 415 | 63 (15.2) | 0.024 |
| Limb | 551 | 200 (36.3) | 415 | 150 (36.1) | 0.961 |
| Hemoglobin | 541 | 12.1 (10.7–13.6) | 387 | 11.2 (9.7–12.8) | <0.001 |
| INR | 485 | 1.2 (1.1–1.4) | 338 | 1.3 (1.2–1.5) | <0.001 |
| pH | 461 | 7.29 (7.21–7.35) | 330 | 7.24 (7.13–7.32) | <0.001 |
| Base deficit | 458 | –5 (−8–−2) | 321 | −9 (−12–−5) | <0.001 |
| 24-hr RBC, U | 551 | 4 (2–6) | 415 | 7 (4–13) | <0.001 |
| 24-hr plasma, U | 551 | 2 (0–6) | 415 | 5 (2–10) | <0.001 |
| 24-hr platelets, U | 551 | 0 (0–1) | 415 | 0 (0–6) | <0.001 |
| 24-hour mortality | 551 | 37 (6.7) | 415 | 72 (17.3) | <0.001 |
| 30-day mortality | 551 | 87 (15.8) | 415 | 116 (28.0) | <0.001 |
Abbreviations: IQR, interquartile range; FAST, focused assessment for the sonography of trauma; AIS, Abbreviated Injury Severity score; INR, International Normalized Ratio; RBC, red blood cells.
Of the 415 patients predicted to receive MT, only 145 (35%) actually received MTs, while of the 551 patients predicted not to receive MT, 475 (86%) were correctly classified (FIGURE 1). This indicates that gestalt more reliably predicts patients who will not require MT while over triaging those who might require a MT. Because exsanguinating patients who died in the first 24 hours might not have lived long enough to receive a full MT, a sensitivity analysis was conducted in which gestalt predicted either MT or hemorrhagic deaths within 24 hours. Even when accounting for this survivor bias, results were unchanged and gestalt performed nearly identical. TABLE 3 summarizes the sensitivity, specificity, positive and negative predictive values for clinical gestalt in predicting the need for MT at 24 hours, MT or hemorrhagic death.
Table 3.
Performance of Clinical Gestalt at Predicting MT and Significant Bleeding
| MT at 24 hrs* (n=966) |
MT or Hemorrhagic Death in 24 hrs* (n=966) |
|
|---|---|---|
| Prevalence, % | 23.0 (20.0–25.7) | 24.0 (22.0–27.3) |
| Sensitivity, % | 65.6 (58.9–71.9) | 66.5 (60.1–72.5) |
| Specificity, % | 63.8 (60.2–67.2) | 64.7 (61.1–68.1) |
| PPV, % | 34.9 (30.4–39.7) | 37.8 (33.1–42.7) |
| NPV, % | 86.2 (83.0–89.0) | 85.7 (82.5–88.5) |
| AUROC | 0.65 (0.61–0.68) | 0.66 (0.62–0.69) |
Abbreviations: PPV, positive predictive value; NPV, negative predictive value; AUROC, area under receiver operating curve
MT: denotes transfusion of ≥ 10 units red blood cells (RBC) in specified time period
Gestalt accurately predicted only two thirds of patients who would require MT and 76 (14%) patients who were predicted to not receive MT were incorrectly classified and received a MT. To better understand these false negatives or “missed” MT patients, comparison was made between false negatives and true negatives (TABLE 4). Among the gestalt negative group, false negatives more often had a positive FAST exam and were three times as likely to have bleeding in the pelvis than those who did not receive a MT. False negatives were also more critically injured with higher degrees of physiologic derangements and the mortality rate was twice as high for false negatives than true negatives (27.6 vs. 13.9%; p=0.002). Using multivariate logistic regression to adjust for potential confounders, bleeding in the pelvis (OR 2.04; 95%CI 1.07–3.90; p=0.03), increasing pH (OR 0.003; 95%CI 0.0001–0.0944–3.90; p=0.001), and more positive base values (OR 1.10; 95%CI 1.02–1.19; p=0.02) were found to be independently predictive of MT gestalt failure. This indicates that patients with less severe acidosis (each 0.1 increase in pH) and less severe base deficit (each 1 point positive increase) were more likely to be incorrectly predicted to not receive a MT.
Table 4.
Admission, Transfusion, and Mortality Characteristics of Gestalt Negative Patients by MT group
| True negative (n=475) | False negative (n=76) | ||||
|---|---|---|---|---|---|
| Nonmissing, No. |
No. (%) or Median (IQR) |
Nonmissing, No. |
No. (%) or Median (IQR) |
p-value | |
| Age, years | 475 | 40 (25–57) | 76 | 37.5 (24–55.5) | 0.450 |
| Male gender | 475 | 352 (74.1) | 76 | 61 (80.3) | 0.250 |
| Blunt Mechanism | 475 | 319 (67.2) | 76 | 56 (73.7) | 0.257 |
| White Race | 475 | 306 (64.4) | 76 | 55 (72.4) | 0.176 |
| Positive FAST | 303 | 66 (21.8) | 55 | 19 (34.5) | 0.041 |
| Systolic Blood Pressure | 472 | 110 (90–132) | 76 | 96 (81–123) | 0.003 |
| Heart Rate | 469 | 101 (84–120) | 73 | 113 (97–127) | 0.001 |
| GCS | 442 | 14 (3–15) | 65 | 12 (3–15) | 0.088 |
| Head AIS | 475 | 0 (0–3) | 76 | 0 (0–3) | 0.403 |
| Chest AIS | 475 | 3 (0–3) | 76 | 3 (0–4) | 0.023 |
| Abdomen AIS | 475 | 2 (0–3) | 76 | 3 (0–4) | <0.001 |
| Bleeding Sites | |||||
| Chest | 475 | 87 (18.3) | 76 | 16 (21.1) | 0.570 |
| Abdomen | 475 | 125 (26.3) | 76 | 26 (34.2) | 0.152 |
| Pelvis | 475 | 39 (8.2) | 76 | 18 (23.7) | <0.001 |
| Limb | 475 | 165 (34.7) | 76 | 35 (46.1) | 0.057 |
| Hemoglobin | 465 | 12.1 (10.8–13.7) | 76 | 11.9 (10.1–13.5) | 0.133 |
| INR | 416 | 1.2 (1.1–1.3) | 69 | 1.3 (1.2–1.6) | <0.001 |
| pH | 390 | 7.3 (7.23–7.35) | 71 | 7.22 (7.11–7.3) | <0.001 |
| Base deficit | 388 | −5 (−8 – −2) | 70 | −7 (−11 – −4) | <0.001 |
| 24-hr RBC, U | 475 | 3 (2–5) | 76 | 14 (11–21.5) | <0.001 |
| 24-hr plasma, U | 475 | 2 (0–4) | 76 | 12 (8.5–18) | <0.001 |
| 24-hr platelets, U | 475 | 0 (0–0) | 76 | 12 (6–18) | <0.001 |
| 24-hour mortality | 475 | 25 (5.3) | 76 | 12 (15.8) | 0.001 |
| 30-day mortality | 475 | 66 (13.9) | 76 | 21 (27.6) | 0.002 |
Abbreviations: IQR, interquartile range; FAST, focused assessment for the sonography of trauma; AIS, Abbreviated Injury Severity score; INR, International Normalized Ratio; RBC, red blood cells.
In an effort to put prediction of MT using clinical gestalt in perspective in this subset of trauma patients, the performance of gestalt was compared to three previously proposed scoring systems (FIGURE 2). Because of missing data in at least one of the several variables required to calculate all of the scores, this analysis was limited to only 486 patients that had complete data for all necessary variables. The associated variables and performance of gestalt and the ABC, TASH, and McLaughlin scores are listed in Table 5. Each of the scores performed significantly lower in the current study than in the original or validated datasets from which they originated. Among the 486 patients analyzed for comparison, the TASH score correctly classified more patients than gestalt (0.72 vs. 0.62; p=0.01), however no statistically significant difference in predictive ability was detected between clinical gestalt and the ABC or McLaughlin scores (TABLE 5).
Figure 2.
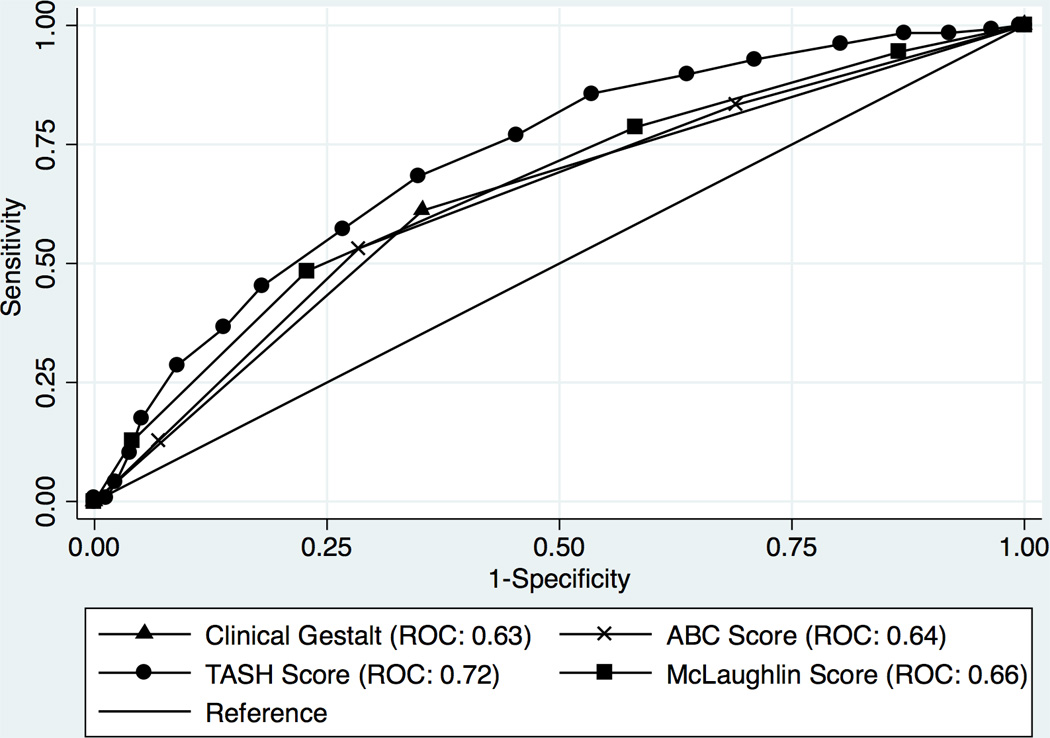
Table 5.
Comparison of gestalt predictive ability to scoring systems within PROMMTT and by published standards.
| Physiology | Laboratory | Anatomic | PROMMTT AUROC |
Original AUROC |
|
|---|---|---|---|---|---|
| Gestalt | -- | -- | -- | 0.62 (0.57–0.67) | -- |
| ABC Score | HR, SBP | -- | FAST, mechanism | 0.63 (0.58–0.69) | 0.86 (0.82–0.90) |
| TASH | HR, SBP | Base value, Hgb | Male, FAST or abdominal AIS, long bone or pelvic fracture | 0.72 (0.67–0.77) | 0.89 (0.88–0.91) |
| McLaughlin | HR, SBP | pH, Hct | -- | 0.66 (0.61–0.72) | 0.84 (0.79–0.89) |
Note: only 486 patients (50%) had complete data to run scoring systems
Abbreviations:; AUROC, area under receiver operating curve; FAST, focused assessment for the sonography of trauma; SBP, systolic blood pressure; HR, heart rate; GCS, Glascow Coma Score; INR, International Normalized Ratio AIS, Abbreviated Injury Severity score; Hgb, hemoglobin; Hct, hematocrit.
DISCUSSION
This study is the first to prospectively evaluate the ability of clinical gestalt in predicting the need for massive transfusion in bleeding trauma patients. After observing 966 trauma patients across ten U.S. trauma centers who received at least one unit of PRBC within the first six hours after injury, we found that clinical gestalt is an unreliable predictor of MT in trauma patients. With a sensitivity of only 66%, gestalt functioned poorly as a screening test for MT, missing over one third of patients who ultimately required MT. This is further underscored by the fact that the tendency is to significantly over-triage patients, as indicated by the fact that only 35% (PPV) of those predicted to receive MT actually received it. Ultimately this indicates that trauma surgeons’ threshold for MT activation is low yet one third of patients are still missed and potentially under resuscitated.
The performance of clinical gestalt and its potential pitfalls have perhaps best been described in determining the pretest probability of pulmonary embolism. Previous studies have shown gestalt to be a relatively poor diagnostic tool however its performance is comparable to existing algorithms [7, 23]. The same has been demonstrated in the trauma setting [22, 24]. When used to identify trauma patients at risk for thoracic injury, the sensitivity of gestalt was only 58.7% however this was still significantly higher than two other statistical models with which it was compared. Similar to our findings, the NPV was much higher at 94.4%, indicating gestalt was more accurate in ruling out the diagnosis [25, 26]. In a separate trauma study evaluating the ability to predict survival, gestalt performed poorly with sensitivities as low as 34.8% in nurses and 51.7% in attending trauma surgeons [27].
In the setting of a well-developed MT protocol and trauma system, over-triaging is an acceptable consequence, as it is much easier to return unused blood than manage the patients awaiting product delivery.[6] The patients of interest, then, are those who “fool us” and go undetected despite this tendency to over-triage. After adjusting for confounders, these false negatives or “missed” MT patients were more often bleeding in the pelvis and had less deviation in their blood pH and base deficits. This is similar to the original description of the ABC score’s false negative patients (pelvic fractures, severe chest trauma, multiple long bone fractures) [21]. While it is unclear how often these labs were available at the time of the gestalt decision, it is possible that the patients with less critical lab values appeared to be less “sick” compared to those who were predicted to need MT. This may suggest that our threshold should be lower for patients with significant pelvic injuries.
There are several important limitations of this study. The first and perhaps most important is that the PROMMTT inclusion criteria was designed to include in the study patients receiving at least some blood (one unit in first six hours) but not patients so sick they died within 30 minutes. This inherently selected out the two extremes of patients in which clinical gestalt likely performed best: those who succumbed early to massive exsanguination and those who were not bleeding at all. If clinical gestalt had been evaluated in these patients, it may have performed with much higher accuracy. Therefore it is important to interpret these data as an assessment of clinical gestalt in bleeding patients who survive to 30 minutes. This selection bias is also likely responsible for the poor performance of the other MT scoring systems calculated in this cohort. Finally, a more relevant and important question may be is “what really defines a massive transfusion?” Clinical gestalt and existing algorithms may perform poorly because we are asking the wrong question. Upon initial assessment of a critically injured patient arriving to the ED, it is likely that physicians are actually considering immediate transfusion needs rather than 10 units of RBCs over the next 24 hours. If the goal of MT protocols is to give blood early and give it fast, then the utility of clinical gestalt is most important in the first several hours, not in the first 24 hours. Future studies should asses these endpoints.
The results of this study also rest on the inherent assumption that the MTs provided to patients were appropriate and beneficial, however the indications and benefits cannot be ascertained from this dataset. In addition, the issue of missing data limited several of the analyses and was an additional source of bias, as missing data was likely not missing at random for some variables. The most obvious case was the fact that the gestalt question was not answered in 22% of PROMMTT patients, however no clinically important differences between these two patient groups were detected. Missing data also limited our ability to calculate and compare the three other MT scoring systems with gestalt in all 966 cohort patients. In addition to the aforementioned selection-bias, the much smaller sample size of patients used for this secondary analysis is likely another contributor to the low performance of MT scoring systems in our study. Despite this limitation and deviation from the potential higher performance of these scores in larger, unbiased datasets, the purpose of this analysis was primarily to aid in the interpretation of results by evaluating the performance of gestalt relative to other scoring systems in this particular subset of trauma patients. Scores were compared to each other in 486 patients with complete datasets, and this still represents the only comparison of clinical gestalt to other MT scoring systems using prospectively collected data. It is important to note that the presence of missing data also limits our ability to dismiss the performance of these scores as equally poor in their predictive ability. As illustrated in TABLE 5, these well-described and validated scoring systems perform much better when data necessary for their calculation is complete.
The gestalt question may have also influenced the attending surgeon’s thought process or decision to activate their institution’s MT protocol. When measuring provider compliance of individual MT protocol components, failure to activate MT protocols in the ED has been shown to be an independent predictor of early mortality and have the highest noncompliance rate [1]. The gestalt question may have actually reminded the physician to activate an MT protocol in situations where they had not initially thought to do so, possibly increasing the rate of protocol activation and contributing to over-triage.
Finally, the question of “why” trauma surgeons predicted MT was not addressed in this study and may provide an additional critical element to understanding MT prediction and care of the critically injured patient. However, if clinical gestalt is, in fact, more complicated than the sum of its parts, then the answer to this question would likely be multiple and highly variable, not only by surgeon, but also for each patient. The surgeon’s assessment of hemorrhage and need for MT is constantly changing as new information becomes available and the patients condition changes. Therefore, a single assessment of the need for MT is likely insufficient and the clinical gestalt question likely needs to be readdressed at several different points during the resuscitation process.
Despite these limitations, clinical gestalt is by no means irrelevant or unreliable to the extent it should not be used in the clinical setting. In the setting of mass casualty or disaster scenarios, where laboratory and imaging capabilities may be scarce, clinical gestalt is undoubtedly the primary, if not only, method of triaging patients and prioritizing utilization of resources. Furthermore, clinical gestalt may perform even better if used in conjunction with lab-driven algorithms or as an added component to existing scoring systems to further increase the early identification and treatment of severely injured patients in need of MT.
CONCLUSION
Many deaths after trauma occur early and result from exsanguination. Despite many advances in trauma resuscitation, early identification of patients who require a MT remains a challenge and current algorithms are limited. While there may be limitations to predicting MT after trauma using clinical gestalt, the marginal performance and limitations of existing algorithms continue to prevent widespread use or universal acceptance of any single, high-performing alternative. More work is needed to develop an accurate, reliable, and standardized way of identifying the need for MT in critically injured patients.
Acknowledgments
Funding/Support: This project was funded by the U.S. Army Medical Research and Materiel Command subcontract W81XWH-08-C-0712. Infrastructure for the Data Coordinating Center was supported by CTSA funds from NIH grant UL1 RR024148.
Role of the Sponsor: The sponsors did not have any role in the design and conduct of the study; collection, management, analysis and interpretation of the data; preparation, review or approval of the manuscript; or the decision to submit this manuscript for publication.
PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) Study Group
Data Coordinating Center: Mohammad H. Rahbar, PhD (principal investigator); John B. Holcomb, MD (co-investigator); Erin E. Fox, PhD (co-investigator and study coordinator); Deborah J. del Junco, PhD (co-investigator); Bryan A. Cotton, MD, MPH (co-investigator); Charles E. Wade, PhD (co-investigator); Jiajie Zhang, PhD (co-investigator); Nena Matijevic, PhD (co-investigator); Yu Bai, MD, PhD (co-investigator); Weiwei Wang, PhD (co-investigator); Jeanette Podbielski, RN (study coordinator); Sarah J. Duran, MSCIS (data manager); Ruby Benjamin-Garner, PhD (data manager); Robert J. Reynolds, MPH (data manager); Xuan Zhang, MS (data analyst); Aisha Dickerson, MSPH (graduate assistant); Elizabeth S. Camp, MSPH (data analyst).
PROMMTT Clinical Sites:
Brooke Army Medical Center: Christopher E. White, MD (principal investigator); Kimberly L. Franzen, MD (co-investigator); Elsa C. Coates, MS, RN (study coordinator).
Medical College of Wisconsin: Karen J. Brasel, MD, MPH (principal investigator); Pamela Walsh (study coordinator).
Oregon Health and Sciences University: Martin A. Schreiber, MD (principal investigator); Samantha J. Underwood, MS (study coordinator); Jodie Curren (study coordinator).
University of California, San Francisco: Mitchell J. Cohen, MD (principal investigator); M. Margaret Knudson, MD (co-investigator); Mary Nelson, RN, MPA (study coordinator); Mariah S. Call, BS (study coordinator).
University of Cincinnati: Peter Muskat, MD (principal investigator); Jay A. Johannigman, MD (co-investigator); Bryce RH Robinson, MD (co-investigator); Richard Branson (co-investigator); Dina Gomaa, BS, RRT (study coordinator); Cendi Dahl (study coordinator).
University of Pittsburgh Medical Center: Louis H. Alarcon, MD (principal investigator); Andrew B. Peitzman, MD (co-investigator); Stacy D. Stull, MS, CCRC (study coordinator); Mitch Kampmeyer MPAS, CCRC, PA-C, (study coordinator); Barbara J. Early, RN, BSN, CCRC (study coordinator); Helen L. Shnol, BS, CRC (study coordinator); Samuel J. Zolin, BS (research associate); Sarah B. Sears, BS (research associate).
University of Texas Health Science Center at Houston: John B. Holcomb, MD (co-principal investigator); Bryan A. Cotton, MD, MPH (co-principal investigator); Marily Elopre, RN (study coordinator); Quinton M. Hatch, MD (research associate); Michelle Scerbo (research associate); Zerremi Caga-Anan, MD (research associate).
University of Texas Health Science Center at San Antonio: John G. Myers, MD (co-principal investigator); Ronald M. Stewart, MD (co-principal investigator); Rick L. Sambucini, RN, BS (study coordinator); Marianne Gildea, RN, BSN, MS (study coordinator); Mark DeRosa CRT (study coordinator); Rachelle Jonas, RN, BSN (study coordinator); Janet McCarthy, RN (study coordinator).
University of Texas Southwestern Medical Center: Herbert A. Phelan, MD, MSCS (principal investigator); Joseph P. Minei, MD (co-investigator); Elizabeth Carroll, MD (study coordinator).
University of Washington: Eileen M. Bulger, MD (principal investigator); Patricia Klotz, RN (study coordinator); Keir J. Warner, BS (research coordinator).
Footnotes
Presented at the 72nd Annual Meeting of the American Association for the Surgery of Trauma, September 18–21, San Francisco, California
Disclaimer: The views and opinions expressed in this manuscript are those of the authors and do not reflect the official policy or position of the Army Medical Department, Department of the Army, the Department of Defense, or the United States Government.
Author contributions:
JBH, CEW, EEF, DJD, KJB, EMB, MJC, LHA, MAS, JGM, HAP, PM, MR and BAC designed the study; MJP, MDG and BAC conducted the literature search; JBH, CEW, EEF, DJD, KJB, EMB, MJC, LHA, MAS, JGM, HAP, PM, MR and BAC collected the data; MJP, EEF, DJD and BAC analyzed the data; MJP, EEF, DJD, CEW, JBH and BAC interpreted the data; All authors participated in the writing, revising and editing of the manuscript.
Contributor Information
Matthew J. Pommerening, Email: matthew.j.pommerening@uth.tmc.edu.
Michael D. Goodman, Email: md-goodman@hotmail.com.
John B. Holcomb, Email: john.holcomb@uth.tmc.edu.
Charles E. Wade, Email: charles.e.wade@uth.tmc.edu.
Erin E. Fox, Email: erin.e.fox@uth.tmc.edu.
Deborah J. del Junco, Email: Deborah.J.DelJunco@uth.tmc.edu.
Karen J. Brasel, Email: kbrasel@mcw.edu.
Eileen M. Bulger, Email: ebulger@u.washington.edu.
Mitch J. Cohen, Email: mcohen@sfghsurg.ucsf.edu.
Louis H. Alarcon, Email: alarconl@upmc.edu.
Martin A. Schreiber, Email: schreibm@ohsu.edu.
John G. Myers, Email: myersjg@uthscsa.edu.
Herb A. Phelan, Email: Herb.Phelan@UTSouthwestern.edu.
Peter Muskat, Email: muskatp@UCMAIL.UC.EDU.
Mohammad Rahbar, Email: Mohammad.H.Rahbar@uth.tmc.edu.
References
- 1.Cotton BA, Dossett LA, Au BK, Nunez TC, Robertson AM, Young PP. Room for (performance) improvement: provider-related factors associated with poor outcomes in massive transfusion. The Journal of trauma. 2009;67:1004–1012. doi: 10.1097/TA.0b013e3181bcb2a8. [DOI] [PubMed] [Google Scholar]
- 2.Cotton BA, Au BK, Nunez TC, Gunter OL, Robertson AM, Young PP. Predefined massive transfusion protocols are associated with a reduction in organ failure and postinjury complications. The Journal of trauma. 2009;66:41–48. doi: 10.1097/TA.0b013e31819313bb. discussion 8–9. [DOI] [PubMed] [Google Scholar]
- 3.Tan JN, Burke PA, Agarwal SK, Mantilla-Rey N, Quillen K. A massive transfusion protocol incorporating a higher FFP/RBC ratio is associated with decreased use of recombinant activated factor VII in trauma patients. American journal of clinical pathology. 2012;137:566–571. doi: 10.1309/AJCPQZNCHM5PIK8O. [DOI] [PubMed] [Google Scholar]
- 4.Khan S, Allard S, Weaver A, Barber C, Davenport R, Brohi K. A major haemorrhage protocol improves the delivery of blood component therapy and reduces waste in trauma massive transfusion. Injury. 2013;44:587–592. doi: 10.1016/j.injury.2012.09.029. [DOI] [PubMed] [Google Scholar]
- 5.O'Keeffe T, Refaai M, Tchorz K, Forestner JE, Sarode R. A massive transfusion protocol to decrease blood component use and costs. Archives of surgery. 2008;143:686–690. doi: 10.1001/archsurg.143.7.686. discussion 90–1. [DOI] [PubMed] [Google Scholar]
- 6.McDaniel LM, Neal MD, Sperry JL, Alarcon LH, Forsythe RM, Triulzi D, et al. Use of a massive transfusion protocol in nontrauma patients: activate away. Journal of the American College of Surgeons. 2013;216:1103–1109. doi: 10.1016/j.jamcollsurg.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 7.Riskin DJ, Tsai TC, Riskin L, Hernandez-Boussard T, Purtill M, Maggio PM, et al. Massive transfusion protocols: the role of aggressive resuscitation versus product ratio in mortality reduction. Journal of the American College of Surgeons. 2009;209:198–205. doi: 10.1016/j.jamcollsurg.2009.04.016. [DOI] [PubMed] [Google Scholar]
- 8.Koffka K. Principles of gestalt psychology. New York: Harcourt, Brace and Company; 1935. [Google Scholar]
- 9.Bolte A, Goschke T. Intuition in the context of object perception: intuitive gestalt judgments rest on the unconscious activation of semantic representations. Cognition. 2008;108:608–616. doi: 10.1016/j.cognition.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 10.Pelli DG, Majaj NJ, Raizman N, Christian CJ, Kim E, Palomares MC. Grouping in object recognition: the role of a Gestalt law in letter identification. Cognitive neuropsychology. 2009;26:36–49. doi: 10.1080/13546800802550134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ali N, Peebles D. The effect of Gestalt laws of perceptual organization on the comprehension of three-variable bar and line graphs. Human factors. 2013;55:183–203. doi: 10.1177/0018720812452592. [DOI] [PubMed] [Google Scholar]
- 12.Kabrhel C, Camargo CA, Jr, Goldhaber SZ. Clinical gestalt and the diagnosis of pulmonary embolism: does experience matter? Chest. 2005;127:1627–1630. doi: 10.1378/chest.127.5.1627. [DOI] [PubMed] [Google Scholar]
- 13.Cook C. Is clinical gestalt good enough? The Journal of manual & manipulative therapy. 2009;17:6–7. doi: 10.1179/106698109790818223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Koontz NA, Gunderman RB. Gestalt theory: implications for radiology education. AJR American journal of roentgenology. 2008;190:1156–1160. doi: 10.2214/AJR.07.3268. [DOI] [PubMed] [Google Scholar]
- 15.Klein JG. Five pitfalls in decisions about diagnosis and prescribing. Bmj. 2005;330:781–783. doi: 10.1136/bmj.330.7494.781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dubose JJ, Scalea TM, Holcomb JB, Shrestha B, Okoye O, Inaba K, et al. Open abdominal management after damage-control laparotomy for trauma: a prospective observational American Association for the Surgery of Trauma multicenter study. The journal of trauma and acute care surgery. 2013;74:113–120. doi: 10.1097/TA.0b013e31827891ce. discussion 1120–2. [DOI] [PubMed] [Google Scholar]
- 17.Rahbar MH, Fox EE, del Junco DJ, Cotton BA, Podbielski JM, Matijevic N, et al. Coordination and management of multicenter clinical studies in trauma: Experience from the PRospective Observational Multicenter Major Trauma Transfusion (PROMMTT) Study. Resuscitation. 2012;83:459–464. doi: 10.1016/j.resuscitation.2011.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yucel N, Lefering R, Maegele M, Vorweg M, Tjardes T, Ruchholtz S, et al. Trauma Associated Severe Hemorrhage (TASH)-Score: probability of mass transfusion as surrogate for life threatening hemorrhage after multiple trauma. The Journal of trauma. 2006;60:1228–1236. doi: 10.1097/01.ta.0000220386.84012.bf. discussion 36–7. [DOI] [PubMed] [Google Scholar]
- 19.McLaughlin DF, Niles SE, Salinas J, Perkins JG, Cox ED, Wade CE, et al. A predictive model for massive transfusion in combat casualty patients. The Journal of trauma. 2008;64:S57–S63. doi: 10.1097/TA.0b013e318160a566. discussion S. [DOI] [PubMed] [Google Scholar]
- 20.Schreiber MA, Perkins J, Kiraly L, Underwood S, Wade C, Holcomb JB. Early predictors of massive transfusion in combat casualties. Journal of the American College of Surgeons. 2007;205:541–545. doi: 10.1016/j.jamcollsurg.2007.05.007. [DOI] [PubMed] [Google Scholar]
- 21.Nunez TC, Voskresensky IV, Dossett LA, Shinall R, Dutton WD, Cotton BA. Early prediction of massive transfusion in trauma: simple as ABC (assessment of blood consumption)? The Journal of trauma. 2009;66:346–352. doi: 10.1097/TA.0b013e3181961c35. [DOI] [PubMed] [Google Scholar]
- 22.Cotton BA, Dossett LA, Haut ER, Shafi S, Nunez TC, Au BK, et al. Multicenter validation of a simplified score to predict massive transfusion in trauma. The Journal of trauma. 2010;69(Suppl 1):S33–S39. doi: 10.1097/TA.0b013e3181e42411. [DOI] [PubMed] [Google Scholar]
- 23.Penaloza A, Verschuren F, Meyer G, Quentin-Georget S, Soulie C, Thys F, et al. Comparison of the unstructured clinician gestalt, the wells score, and the revised geneva score to estimate pretest probability for suspected pulmonary embolism. Annals of emergency medicine. 2013;62:117–124. e2. doi: 10.1016/j.annemergmed.2012.11.002. [DOI] [PubMed] [Google Scholar]
- 24.Mitra B, Rainer TH, Cameron PA. Predicting massive blood transfusion using clinical scores post-trauma. Vox sanguinis. 2012;102:324–330. doi: 10.1111/j.1423-0410.2011.01564.x. [DOI] [PubMed] [Google Scholar]
- 25.Sears BW, Luchette FA, Esposito TJ, Dickson EL, Grant M, Santaniello JM, et al. Old fashion clinical judgment in the era of protocols: is mandatory chest X-ray necessary in injured patients? The Journal of trauma. 2005;59:324–330. doi: 10.1097/01.ta.0000179450.01434.90. discussion 30–2. [DOI] [PubMed] [Google Scholar]
- 26.Dillard E, Luchette FA, Sears BW, Norton J, Schermer CR, Reed RL, 2nd, et al. Clinician vs mathematical statistical models: which is better at predicting an abnormal chest radiograph finding in injured patients? The American journal of emergency medicine. 2007;25:823–830. doi: 10.1016/j.ajem.2006.12.009. [DOI] [PubMed] [Google Scholar]
- 27.Goettler CE, Waibel BH, Goodwin J, Watkins F, Toschlog EA, Sagraves SG, et al. Trauma intensive care unit survival: how good is an educated guess? The Journal of trauma. 2010;68:1279–1287. doi: 10.1097/TA.0b013e3181de3b99. discussion 87–8. [DOI] [PubMed] [Google Scholar]
- 28.Nunez TC, Dutton WD, May AK, Holcomb JB, Young PP, Cotton BA. Emergency department blood transfusion predicts early massive transfusion and early blood component requirement. Transfusion. 2010;50:1914–1920. doi: 10.1111/j.1537-2995.2010.02682.x. [DOI] [PubMed] [Google Scholar]
- 29.Savage SA, Zarzaur BL, Croce MA, Fabian TC. Redefining massive transfusion when every second counts. The journal of trauma and acute care surgery. 2013;74:396–400. doi: 10.1097/TA.0b013e31827a3639. discussion -2. [DOI] [PubMed] [Google Scholar]