Abstract
Adult neurogenesis, the generation of new neurons in the adult brain, occurs in the hippocampal dentate gyrus (DG) and the olfactory bulb (OB) of all mammals, but the functions of these new neurons are not entirely clear. Originally, adult-born neurons were considered to have excitatory effects on the DG network, but recent studies suggest a net inhibitory effect. Therefore, we hypothesized that selective removal of newborn neurons would lead to increased susceptibility to the effects of a convulsant. This hypothesis was tested by evaluating the response to the chemoconvulsant kainic acid (KA) in mice with reduced adult neurogenesis, produced either by focal X-irradiation of the DG, or by pharmacogenetic deletion of dividing radial glial precursors. In the first 4 hrs after KA administration, when mice have the most robust seizures, mice with reduced adult neurogenesis had more severe convulsive seizures, exhibited either as a decreased latency to the first convulsive seizure, greater number of convulsive seizures, or longer convulsive seizures. Nonconvulsive seizures did not appear to change or they decreased. Four-21 hrs after KA injection, mice with reduced adult neurogenesis showed more interictal spikes (IIS) and delayed seizures than controls. Effects were greater when the anticonvulsant ethosuximide was injected 30 min prior to KA administration; ethosuximide allows forebrain seizure activity to be more easily examined in mice by suppressing seizures dominated by the brainstem. These data support the hypothesis that reduction of adult-born neurons increases the susceptibility of the brain to effects of KA.
Keywords: hippocampus, subgranular zone (SGZ), convulsive seizures, electroencephalography (EEG), interictal spikes (IIS), dentate gyrus, epilepsy, inhibition
INTRODUCTION
Adult-born neurons are generated in the hippocampal dentate gyrus (DG) and the subventricular zone (SVZ) throughout the lifetime of mammals (Seki et al., 2013; Belzung and Wigmore, 2013). In the DG, newborn neurons become granule cells (GCs) - the primary cell type. Although they constitute a relatively small fraction of the total GC population (Cameron and McKay, 2001), removal of adult-born neurons compromises normal functions of the DG such as pattern separation (Clelland et al., 2009; Sahay et al., 2011a).
Recordings from immature GCs have shown that they exhibit increased excitability and long-term potentiation (LTP) compared to GCs born in early development (Schmidt-Hieber et al., 2004; Esposito et al., 2005; Markwardt and Overstreet-Wadiche, 2008; Ge et al., 2007), which has led to the idea that young adult-born GCs increase excitability of the adult DG network (Marin-Burgin et al., 2012; Song et al., 2012). However, adult-born neurons may also play a role in DG function by a net inhibitory effect on the DG-CA3 network. In support of this idea, mature GCs innervate diverse GABAergic interneurons; this has led some to propose that a net inhibitory effect in area CA3 is normal (Acsády et al., 1998). When extracellular recordings were made in the GC layer of the DG in mice in which adult DG neurogenesis had been selectively deleted, GCs discharged in bursts that were greater than those in controls (Lacefield et al., 2012). Another study revealed that following tasks that required specific cognitive demands, animals with reduced adult neurogenesis exhibited greater expression of the immediate early gene Arc in the GC layer (indicative of greater GC activity) compared to controls (Burghardt et al., 2012). Using voltage-sensitive dye imaging, a recent study showed that a reduction in adult neurogenesis in the hippocampus increased excitability in the GC layer, and that increasing adult hippocampal neurogenesis had the opposite effect (Ikrar et al., 2013).
If adult-born neurons have a net inhibitory effect, they could contribute to one of the proposed functions of the DG - to act as a ‘gate’ to protect the hippocampus from seizures arising in neocortex (Heinemann et al., 1992; Lothman et al., 1992; Hsu, 2007). Therefore, we asked whether reducing adult-born neurons in a normal animal would influence seizures. We used C57Bl6/J mice because they have high rates of adult neurogenesis (Kempermann et al., 1997), and examined the response of mice to the convulsant kainic acid (KA). Because acute seizures are an important clinical issue, and C57Bl6/J mice are resistant to epilepsy (McKhann et al., 2003; Schawecker, 2011) we focused only on the hours immediately following KA administration (i.e., 24 hours). Two methods were used to reduce adult neurogenesis: focal, low-dose X-irradiation (Santarelli et al., 2003), and mice with herpes simplex virus thymidine kinase (hsv-TK) in glial fibrillary acidic protein (GFAP) - expressing cells (GFAP-TK mice; Sofroniew et al., 1999; Schloesser et al., 2009).
Pilot studies showed that seizures elicited by KA injection in control mice often involved running and bouncing, indicative of brainstem activation (Gale, 1992), which can mask forebrain seizures in rodents (Gale, 1992; Eells et al., 2004). Pretreatment with the anticonvulsant ethosuximide inhibits seizure activity in the brainstem (Mares et al., 1994), which is critical for the generation of the severe convulsive behaviors such as running and bouncing (Browning et al., 1981; Fromm, 1985). Therefore, we used ethosuximide pretreatment to optimize conditions for a study where forebrain networks were of interest – i.e., the location of adult-born neurons. We found that a decrease in adult born neurons in the hippocampus increases convulsive seizures, with no effect or a decrease in nonconvulsive seizures. Our results support the hypothesis that reduction of adult-born neurons increases susceptibility to effects of KA.
MATERIALS AND METHODS
I. General information
All chemicals were purchased from Sigma-Aldrich (St. Louis, MO) unless otherwise stated. Mice were housed in standard mouse cages, with a 12 hr: 12 hr light/dark cycle, and food (Purina 5001; W.F. Fisher, Somerville, NJ) and water ad libitum.
II. Methods to suppress adult neurogenesis
For focal, low-dose X-irradiation, male 6–8 week-old C57BL6/J mice (Jackson Laboratories) were anesthetized with sodium pentobarbital (6 mg/kg), placed in a stereotaxic frame, and exposed to cranial irradiation using a XRAD320 system (Precision Xray Inc., North Branford, CT) operated at 300 kV and 12 mA. Animals were protected with a lead shield that covered the entire body, except for a 4 × 14 mm treatment field above the hippocampus. X-rays were filtered using a 2 mm aluminum filter with a 36 mm source-to-skin distance. Three 2.5 Gy doses were delivered over approximately 2 min 45 sec, with 3 days between doses, and a 7.5 Gy cumulative dose.
As an alternative method to suppress adult neurogenesis, we used mice with the herpes simplex virus – thymidine kinase (hsv-TK) transgene under the control of the mouse glial fibrillary acid protein promoter (GFAP-TK+) or mice without the transgene (GFAP-TK−). Mice were backcrossed onto C57BL6/J mice (Jackson Laboratories, Bar Harbor, ME) for at least 6 generations. Starting 6 weeks of age, they were fed valganciclovir (VGCV; 165 mg/kg chow; Custom Animal Diets) 5 days/week and normal chow on other days. It is important to note that injection of GCV i.p. has adverse effects on the gastrointestinal tract but treatment with VCGV in the chow does not (Schloesser et al., 2009). Presumably this is due to a direct exposure of the intestines during i.p. injection vs. indirect exposure during oral administration. We confirmed that the intestines were normal in appearance in 3 mice that were euthanized immediately after the 6 week-long treatment with chow containing VGCV. The appearance (skin, fur) was similar to control mice. We also measured body weight before and after VCGV treatment and found that it was not significantly different (GFAP-TK−: 23.8 ± 3.4 g, n = 36; GFAP-TK+: 24.4 ± 2.3 g; n = 39; t-test, p = 0.211), suggesting that food intake was unaffected by the transgene.
II. EEG recordings of KA-induced seizures
A. Implantation of electrodes for EEG
Mice were anesthetized with chloral hydrate (55 mg/kg i.p.) and implanted stereotaxically with an epidural electrode (0.10”diameter screws; # 8209; Pinnacle Technologies; Lawrence, KS) over the left frontal cortex (FC; anterior-posterior coordinate relative to Bregma, AP =−0.5 mm; mediolateral coordinate relative to the midline, ML = 1.5 mm) and another electrode overlying right occipital cortex (OC; AP = −3.5 mm; ML= 2.0 mm). Stainless steel 75 µm-diameter lacquer-coated wire (#787000; A-M Systems, Sequim, WA) was twisted to make bipolar electrodes that were placed into each dorsal hippocampus (AP = −2.5 mm, ML = 2.5 mm, depth below skull surface = 2.5 mm). Electrodes were soldered to an 8-pin connector (#8400-SE4, Pinnacle) and cemented (Grip cement; Caulk Dentsply). The location of electrodes in hippocampus was confirmed in 60 of 61 mice using cresyl violet staining. Video-EEG was acquired (500 Hz; Pinnacle) and analyzed offline (Acqknowledge; Biopac Systems, Inc.).
B. Seizure induction
KA (16–25 mg/kg s.c.; Milestone Pharmatech) was dissolved in phosphate buffered saline (PBS); ethosuximide (150 mg/kg; i.p.) was dissolved in 20% ethanol in PBS. Between 10:30 a.m. and 2:00 p.m., animals were removed from their home cage and placed in a new cage where their pin connector was attached to a cable with a commutator (Pinnacle) to allow freedom of movement. Baseline recordings were made for >10 min, and the animal was injected s.c. between the shoulder blades with KA using a 29 gauge needle and low-volume (0.33 cc) syringe to optimize the accuracy of each dose, which we estimate (from the accuracy of this syringe) was ±1.2 mg/kg. For this reason, doses between 20 and 25 mg/kg were not tested (see Results). KA was made as a concentrated stock solution (12 mg/ml in PBS) and was stored at 4°C for up to 1 month. For a subset of GFAP-TK mice, the investigator was blinded to the genotype during KA administration. This was not possible for X-irradiated animals because X-irradiation led to a band of hair where irradiation occurred that lacked pigmentation.
C. Quantification of EEG
A seizure was defined as rapid, rhythmic (>3 Hz) deflections >2x the standard deviation of noise, lasting >3 sec (the duration of the shortest seizure in the current study was 11 sec). Seizures were rated as convulsive if they were accompanied by stage 3–5 behaviors (Racine, 1972). In three mice, the interrater reliability of convulsive vs. nonconvulsive seizures was tested for 38 seizures by two investigators and there was 100% agreement. The two investigators measured the latency to the first convulsive seizure in these animals (25.0 ± 0.9 min vs. 25.2 ± 0.9 min; t-test, p = 0.855).
The latency to onset of a seizure was defined as the time of KA injection to the time when the peak-to-peak amplitude of the EEG exceeded 1.25x of the baseline mean in all electrodes. To validate interrater reliability, the onset of the first seizure was examined in three mice by two investigators; there was 100% agreement. The end of a seizure was defined as the time when the EEG returned to the baseline mean or (in the case of postictal depression) a value lower than the baseline mean. Duration was defined as the time between seizure onset and the end of a seizure (Supplementary Fig. 1).
Interictal spikes (IIS) were defined as brief (<200 msec) deflections in all leads that were >2x the standard deviation of noise. Digital detection of IIS was validated in 3 animals for the first 10 min of the 4th hour after KA; the mean number of IIS was 311 (manual) vs. 316 (digital).
As shown in Fig. 1, animals developed seizures soon after KA administration, and they became progressively more frequent. After approximately 2–4 hrs, the EEG returned to baseline and there was intermittent spiking (i.e. IIS), which lasted up to 24 hrs after KA injection. The end of the acute period was the time when spiking on all leads had transitioned from a period of high frequency (>1 Hz) to low frequency (≤1 Hz). In addition to analysis of this ~2–4 hrs after KA, we analyzed the first 45 min following KA injection, because we hypothesized that during this time, when seizures are not typically severe, an effect of a very small part of the brain (adult neurogenesis in the DG or olfactory bulb) might be more readily detected (Supplementary Fig. 2).
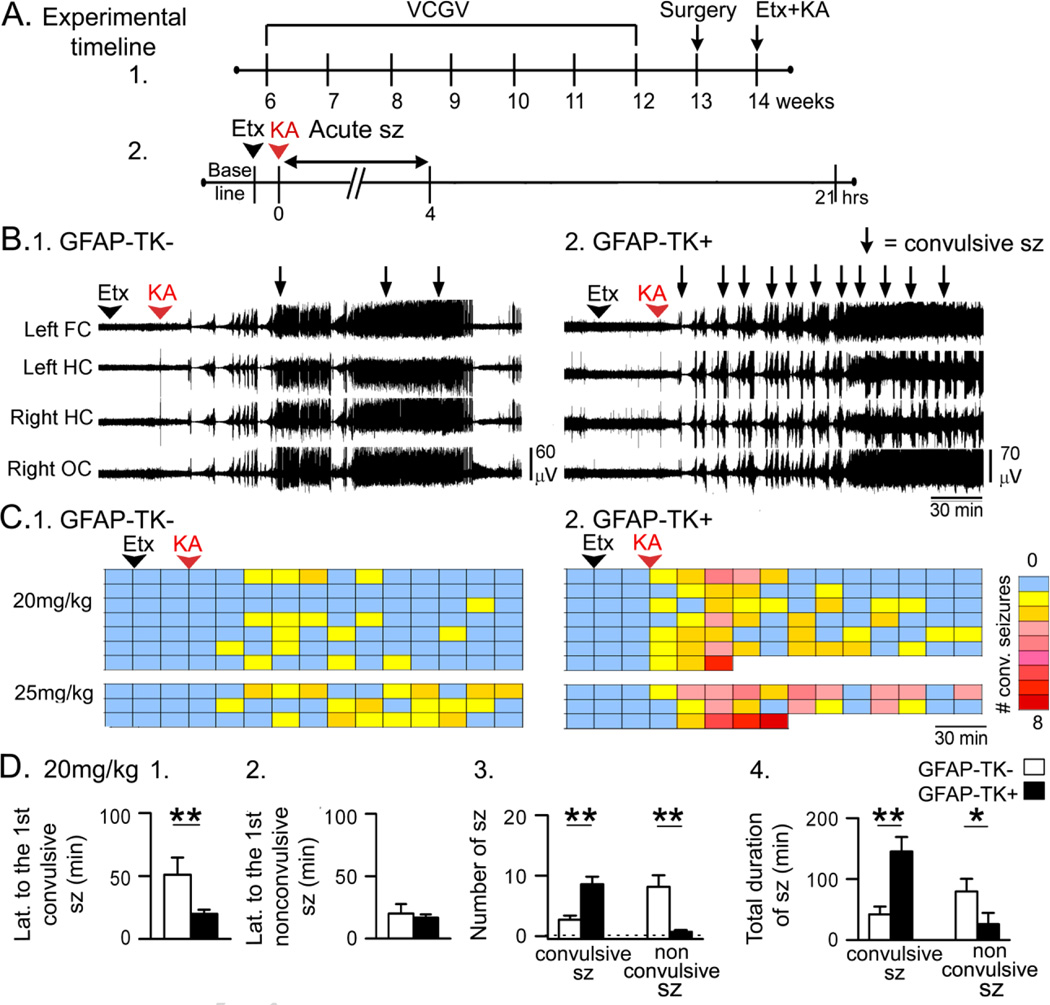
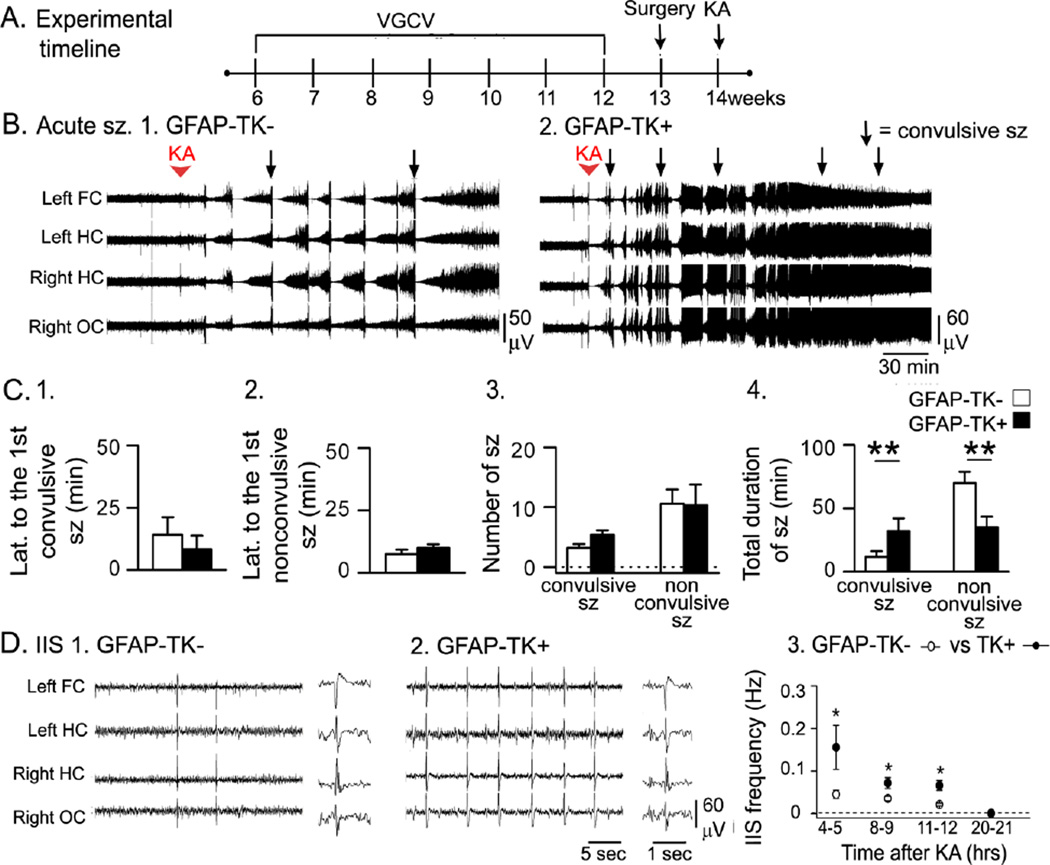
Figure 1. Greater acute effects of KA in GFAP-TK+ mice compared to GFAP-TK− mice.
A. The experimental timeline is shown. Valganciclovir (VGCV)-treated chow was used for 6 weeks. One week later, mice were implanted with electrodes, and approximately a week later, ethosuximide (Etx) was injected, followed by KA after 30 min. Sample sizes are as follows: 20 mg/kg, n = 7/group; 25 mg/kg, n = 3/group. In this figure and all others, sz = seizures.
B. Representative traces from a GFAP-TK− and a GFAP-TK+ mouse (20 mg/kg KA) show that convulsive seizures (arrows) occurred sooner and more often in the GFAP-TK+ mouse.
C. Dividing the 3 hrs after KA into 15 min-long bins shows that GFAP-TK+ mice had more convulsive seizures (stages 3–5, Racine, 1972). Each row is for a different mouse. Right: Colors are used to indicate the number of convulsive seizures/bin, ranging from 0 (blue) to 8 (red). After an animal died there are no more bins shown for that animal.
D. 1–2. GFAP-TK+ mice that were administered 20 mg/kg KA had a shorter latency to the first convulsive seizure but not the first nonconvulsive seizure. 3. GFAP-TK+ mice had a greater number of convulsive seizures and fewer nonconvulsive seizures. 4. When all convulsive seizure durations were summed, the total duration was greater in GFAP-TK+ mice. The total duration of nonconvulsive seizures was shorter in GFAP-TK+ mice. For this figure and all other figures, two asterisks reflect a main effect of reducing adult neurogenesis by two-way ANOVA and significance by post-hoc test (p<0.05). A single asterisk indicates there was a significant main effect of suppressing adult neurogenesis but the post-hoc comparison did not reach significance.
III. Anatomical procedures
A. General procedures
Mice were transcardially perfused with 4% paraformaldehyde after deep urethane anesthesia (2.5 g/kg, i.p.). The brain was post-fixed overnight and 50 µm-thick sections were cut (TPI- 3000, Vibratome Co., St. Louis, MO). Figures were composed using Adobe Photoshop 7.0 (Adobe Systems, Redmond, WA).
B. Immunohistochemistry
Detailed methods are provided in the Supplementary Material. In brief, sections were incubated in Triton-X 100 (0.25%) in 1 M TRIS buffer (“TRIS A”; pH 7.6). After washing in TRIS A for 10 min, sections were blocked with serum dissolved in TRIS A, washed, and incubated overnight in primary antibody diluted in TRIS containing 0.005% bovine serum albumin at room temperature. The next day, sections were washed, transferred to secondary antibody containing 0.005% bovine serum albumin diluted in TRIS A for 45 min, washed, and transferred to avidin-biotin complex solution (Standard ABC kit; Vector Laboratories; Burlingame, CA) at the dilution suggested by the manufacturer. After washing, sections were reacted in 0.5 mg/ml diaminobenzidine (DAB), 20 nM glucose oxidase, 7.5 mM ammonium chloride, and 11 mM D-glucose. For doublecortin (DCX), 5 mM NiCl2 was added to intensify staining. Sections were washed, mounted on subbed slides and dehydrated in increasing concentrations of ethanol the next day, followed by clearing in Xylene. Slides were coverslipped with Permount (Fisher Scientific, Pittsburgh, PA). Sections were viewed with a brightfield microscope (BX51; Olympus of America, Center Valley, PA). Photographs were taken with a digital camera (RET 2000R-F-CLR-12, Q-Imaging, Surrey, BC, Canada) using Q-capture software (Q-Imaging).
An antibody to DCX (goat polyclonal, 1:500; Jackson ImmunoResearch Inc., West Grove, PA) was used to stain immature neurons (Brown et al., 2003; Couillard-Despres et al., 2005). Sections were blocked in 5% normal donkey serum, incubated in primary antibody (goat polyclonal, 1:3,000; #SC-8066; Santa Cruz Biotechnology Inc., Santa Cruz, CA) and then a biotinylated secondary antibody (donkey anti-goat; 1:500; Jackson ImmunoResearch Inc. West Grove, PA). To detect radial glia and mature astrocytes, an antibody against glial fibrillary acidic protein (GFAP) was used (mouse monoclonal; 1:1,000, EMD Millipore, Billerica, MA). Blocking serum was 5% normal horse serum (Vector) and a biotinylated secondary antibody was diluted 1:400 (horse anti-mouse; Vector). To detect TK expression, an antibody against hsv-TK was diluted 1:4,000 (a kind gift from Dr. Michael Sofroniew; rabbit polyclonal; Imura et. al 2003). Normal goat serum (5%; Vector) and a goat anti-rabbit secondary antibody (1:400; Vector) were used.
C. Immunofluorescence
To determine whether suppression of adult neurogenesis affected microglia, we used a rabbit polyclonal antibody against Iba-1, a marker of microglia (Lalancette-Hebert et al., 2007; Zattoni et al., 2011). Procedures for immunofluorescence are described in detail elsewhere (Duffy et al., 2011). In brief, free-floating sections were washed in 0.1 M phosphate buffer (PB), blocked in 5% goat serum for 1 hr and incubated in primary antibody (Iba-1, 1:500, #019-19741, Wako USA Inc., Richmond, VA) overnight at room temperature. Sections were then washed with PB, incubated in Alexa Fluor® 488 goat anti-rabbit IgG (1:400; Life Technologies; Grand Island, NY) for 2 hrs and washed with PB. Sections were mounted and coverslipped with Vectashield (#H-1000; Vector Laboratories, Burlingame, CA) and viewed with a confocal microscope (LSM 510 Meta, Zeiss, Carl Zeiss Microimaging, Thornwood, NY). For both immunohistochemistry and immunofluorescence, sections that were stained with a given antibody were photographed using the same settings.
D. Quantification of immunolabeled sections
Immunoreactivity (ir) was quantified by calculating the area within a region of interest (ROI) that exceeded a threshold (Lee et al., 2012; Duffy et al., 2011). First, the ROI was outlined at 10x magnification (Image Analysis software; Bioquant; Nashville, TN; Duffy et al., 2011). In the DG, the ROI was drawn to outline an area in the DG where immature neurons were normally present, and could be drawn consistently across sections (based on landmarks in background staining such as the border of the GCL and inner molecular layer and the lateral tips of the upper and lower blades; as shown in Fig. 7). The threshold was set so that the soma and dendrites of an immunoreactive cell was above threshold, but the area around the cell where ir was negligible was below threshold (Fig. 7). The area of suprathreshold ir within the ROI was expressed as a percent of the total area of the ROI. The mean was determined for each animal based on 3 coronal sections in the dorsal DG, spaced approximately 300 µm apart. For all analyses, the investigator was blinded.
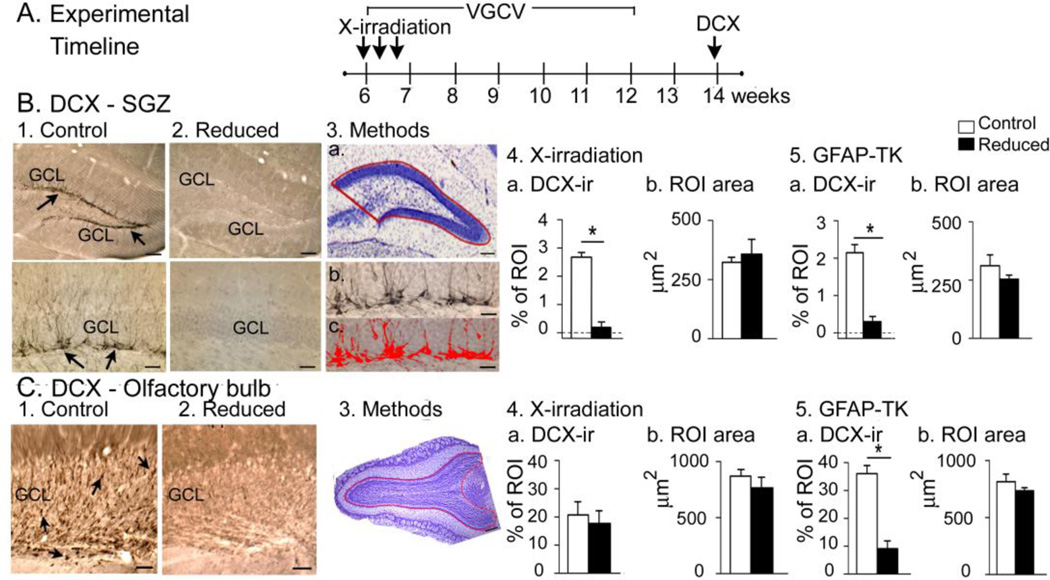
Figure 7. Confirmation of suppression of adult neurogenesis.
A. The experimental timeline used to confirm that adult neurogenesis was suppressed by X-irradiation.
B. 1–2. DCX-ir in a sham (1a–b) and X-irradiated mouse (2a–b) shows strong DCX-ir (arrows) only in the sham. GCL, granule cell layer. Calibrations, 75 µm (1a, 2a); 30 µm (1b, 2b). 3.a. The ROI used to quantify DCX-ir. b-c. DCX-ir before (b) and after thresholding DCX-ir in red (c). Calibration, 75 µm (a); 30 µm (b, c).
4. DCX-ir in X-irradiated mice was negligible compared to sham (Mann-Whitney U-test, p = 0.045; n = 3/group) and there was no difference in ROI area (t-test, p = 0.646).
5. DCX-ir in GFAP-TK+ mice was negligible compared to GFAP-TK− mice (Mann-Whitney U-test, p<0.001; n = 3/group) and there was no difference in ROI area (t-test, p = 0.257.
C. 1–2. DCX-ir (arrows) in the OB was robust in the GFAP-TK− (left) but not GFAP-TK+ mice (right). G, granule cell layer. Calibration, 100 µm.
3. The OB ROI is shown in a cresyl violet-stained section.
4. X-irradiated mice were not significantly different from sham mice in DCX-ir or ROI area (t-tests, p = 0.481, p = 0.694 respectively, n = 3/group).
5. GFAP-TK+ mice had a negligible DCX-ir relative to GFAP-TK− mice but no differences in ROI area (t-tests, p<0.001, p = 0.299 respectively, n = 3/group).
DCX-ir cells in C57BL6/J mice usually were overlapping (Fig. 7B), so we confirmed our DCX analysis with 129SvEv mice – a strain with fewer DCX-ir cells that rarely were overlapping. This analysis (n = 3/group; Supplementary Fig. 3) confirmed the result that was obtained using digital thresholding for C57Bl6 mice shown in Fig. 7.
IV. Statistics
Data are presented as mean ± standard error of the mean and p<0.05. Analysis of Variance (ANOVA; two-way and repeated measures ANOVA; RMANOVA), post-hoc Fisher’s PLSD tests, Student’s t-tests (two-tailed), Mann-Whitney U tests, and Fisher’s Exact tests used Statview (SAS Institute, Cary, NC). Bartlett’s test was used to assess homoscedasticity of variance; where there was departure from homoscedasticity, it was minimized by log transformation.
Two-way ANOVAs were conducted using condition (intact or reduced adult neurogenesis) and method (GFAP-TK or X-irradiation) as main factors. Note that the Results do not report interaction effects for the main factors in the ANOVAs because they were not significant.
RESULTS
I. Effects of KA in ethosuximide-pretreated mice
A. Acute effects of KA
1. GFAP-TK+ mice
Fig. 1A shows the timeline when GFAP-TK mice were administered VGCV, implanted with EEG electrodes and administered ethosuximide and KA. On the day of KA administration, baseline EEG was collected for at least 10 min, ethosuximide was injected, and 30 min later, KA was administered (Fig. 1A). Examples of the EEG are presented in Fig. 1B, and convulsive seizures during that time are presented in Fig. 1C.
GFAP-TK+ mice with reduced neurogenesis had a greater response to KA during the first 4 hrs compared to GFAP-TK− mice (20 mg/kg, n = 7/group; 25 mg/kg, n = 3/group; Fig. 1B–C). For both 20 mg/kg and the 25 mg/kg KA groups, there were deaths from respiratory arrest during tonic-clonic seizures, and only in GFAP-TK+ mice (Fig. 1C). These data suggest greater seizure severity in mice with reduced adult neurogenesis.
The seizures and mortality in the GFAP-TK+ mice were severe so additional animals were not examined, and quantification of seizures was conducted for the mice that were administered 20 mg/kg KA.
For the latency to the first convulsive seizure, two-way ANOVA was conducted with data for the GFAP-TK mice (Fig. 1D1) and X-irradiated/Sham-irradiated mice (Fig. 2D1). There was a main effect of reduced neurogenesis (F (1, 24)12.75; p=0.001) with GFAP-TK+ mice exhibiting a significantly shorter latency than GFAP-TK− mice (post-hoc test, p = 0.019, Fig. 1D1). There was also a main effect of the method used to suppress adult neurogenesis (F(1,24)10.62, p= 0.003), which could be due to the fact that there was a more robust effect in GFAP-TK+ mice compared to X-irradiation (compare Fig. 1D1 and 2D1).
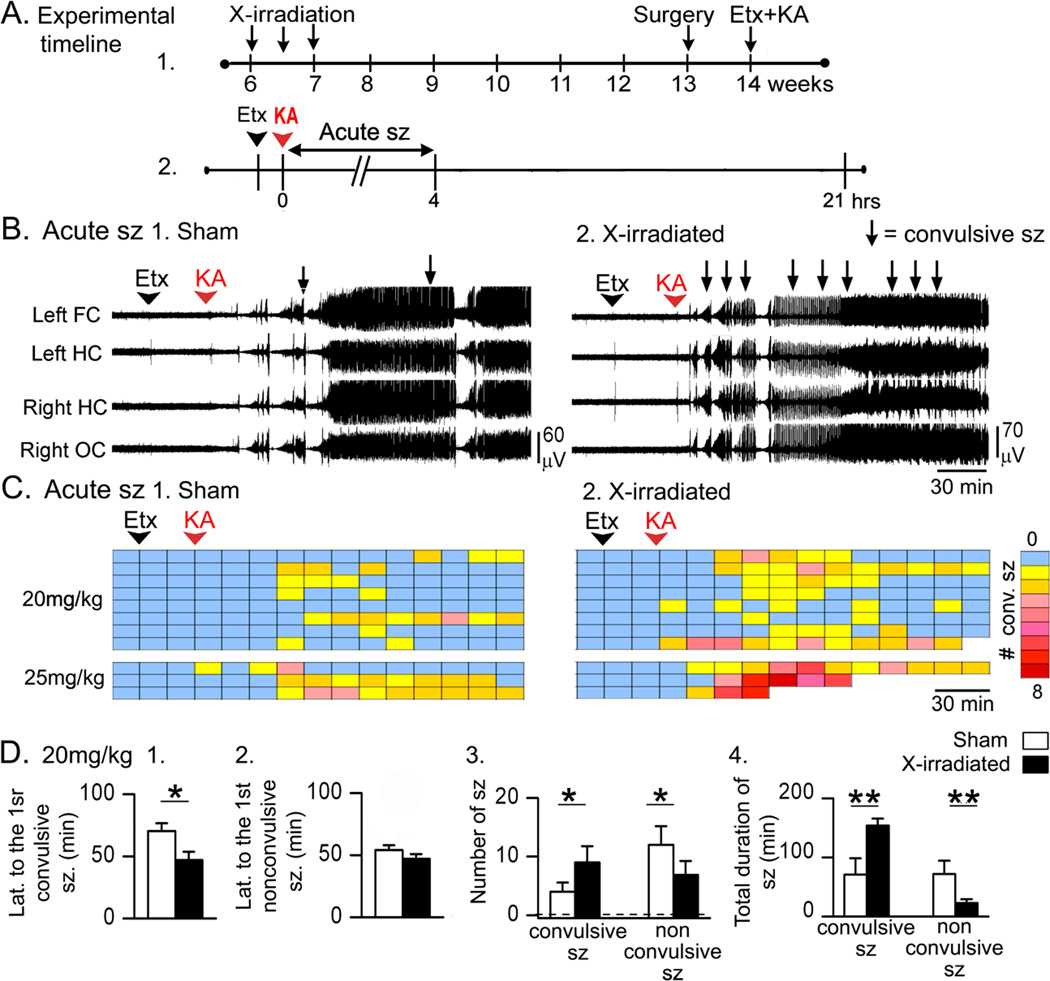
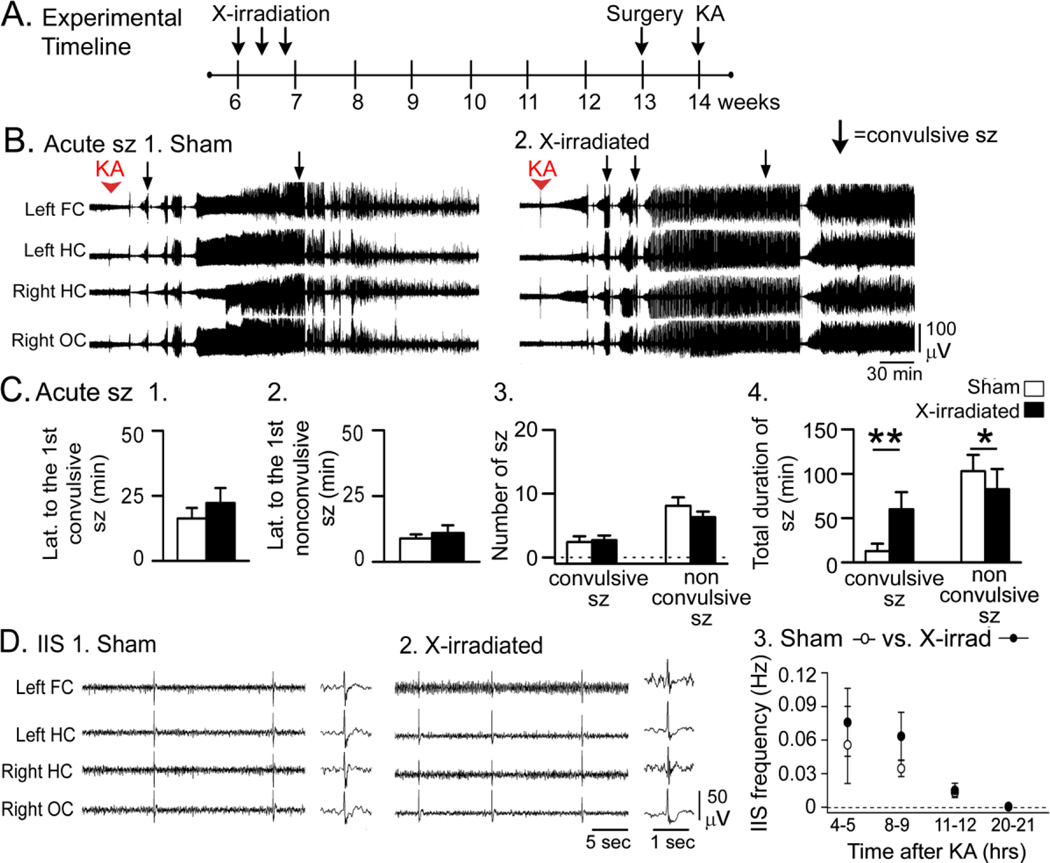
Figure 2. Acute effects of KA were greater in X-irradiated mice compared to sham controls.
A. The experimental timeline is shown. Six weeks after sham-treatment or X-irradiation, electrodes were implanted (surgery). One week later, mice were pretreated with Etx and injected with KA after 30 min. Sample sizes are as follows: 20 mg/kg, n = 8/group; 25 mg/kg, n = 3/group.
B. 1–2. Representative traces show that convulsive seizures (arrows) occurred sooner and were greater in number in X-irradiated mice compared to sham controls.
C. Dividing the 3 hrs after KA into 15 min-long bins shows that X-irradiated mice had more convulsive seizures (stages 3–5, Racine, 1972). Each row is for a different mouse. Right: Colors are used to indicate the number of convulsive seizures/bin, ranging from 0 (blue) to 8 (red).
D. 1–2. X-irradiated mice that were administered 20 mg/kg KA had a shorter latency to the first convulsive seizure, but not the first nonconvulsive seizure. 3. X-irradiated mice had no significant differences from Sham-irradiated mice in the numbers of seizures. 4. Like GFAP-TK+ mice (Fig. 1D4), there was a longer total convulsive seizure duration and shorter nonconvulsive seizure duration in X-irradiated mice compared to Sham-irradiated mice.
In contrast to the latency to the first convulsive seizure, there was no significant effect of reduced neurogenesis on the latency to the first nonconvulsive seizure (two-way ANOVA, F (1, 19)0.20, p = 0.658; Fig. 1D2).
Next, the number of convulsive seizures in the first 4 hrs after KA injection was analyzed. There was a significant main effect of suppressed neurogenesis (F (1, 24)9.46, p = 0.005) with GFAP-TK+ mice exhibiting a greater number of convulsive seizures than GFAP-TK− mice (post-hoc test, p = 0.002; Fig. 1D3). A main effect of method was not detected (F (1, 24)0.005, p = 0.946). There was also a significant effect of reduced neurogenesis on the number of nonconvulsive seizures (F (1, 19)13.37, p = 0.002) with fewer nonconvulsive seizures in GFAP-TK+ mice than GFAP-TK− mice (post-hoc test, p = 0.031; Fig. 1D3). There was a main effect of method (F (1, 19)9.85, p = 0.005), which could be related to the greater effect observed in GFAP-TK+ mice relative to X-irradiated mice (compare Fig. 1D3 and Fig. 2D3).
Interestingly, the total number of seizures (the sum of all convulsive and nonconvulsive seizures) in GFAP-TK+ and GFAP-TK− mice was not significantly different (10.9 ± 2.6, 9.3 ± 1.5, respectively; t-test, p = 0.607). Because the total number was similar but the convulsive seizures increased and nonconvulsive seizures decreased, the results suggest that the seizures which normally would have been nonconvulsive were more severe (i.e., convulsive) when adult neurogenesis was reduced.
There was a main effect of reduced adult neurogenesis on the total duration of convulsive seizures (F (1, 24)11.65, p = 0.002; Fig. 1D4) without an effect of the method used to reduce adult neurogenesis (F (1, 24)1.76, p = 0.197). The total duration of convulsive seizures was longer in GFAP-TK+ mice compared to GFAP-TK− mice (post-hoc test, p=0.002; Fig. 1D4).
There also was a main effect of reduced neurogenesis on the total duration of nonconvulsive seizures (F (1, 19)17.69, p < 0.001) without a main effect of the method to suppress adult neurogenesis (F (1, 19)1.02, p = 0.326). However, despite the fact that GFAP-TK+ mice tended to exhibit shorter durations than GFAP-TK− mice (Fig. 1D4), the post-hoc test did not reach significance (p = 0.090).
Taken together, these results suggest that seizures were more severe (i.e., convulsive) in mice with reduced adult neurogenesis because there was a shorter latency to the first convulsive seizure, a greater number of convulsive seizures, and a longer total duration of convulsive seizures.
2. X-irradiated mice
The experimental timeline for X-irradiation and KA administration are shown in Fig. 2A. Mice were administered 20 mg/kg KA (8/group) or 25 mg/kg KA (3/group; Fig. 2B–C). X-irradiated mice had a greater response to KA than sham controls; examples of the EEG are shown in Fig. 2B and convulsive seizures during that time are presented in Fig. 2C. There were 3 deaths during convulsive seizures, and they only occurred in X-irradiated mice (Fig. 2C). Like GFAP-TK+ mice, death occurred during tonic-clonic seizures and appeared to be caused by respiratory arrest, suggesting a greater seizure severity in mice with reduced adult neurogenesis. Like the GFAP-TK+ mice, there were very severe seizures and mortality in the X-irradiated mice that were administered 25 mg/kg KA (2/3 X-irradiated mice compared to 0/3 sham-treated mice). In the group administered 20 mg/kg KA, 1/8 X-irradiated mice died in response to KA (compared to 0/8 sham-treated mice). Because of mortality in the 25 mg/kg KA group, the quantification below is for 20 mg/kg KA (Fig. 2D).
Quantification of the data for the 20 mg/kg dose was conducted with the GFAP-TK mice by two-way ANOVAs as described above. Two-way ANOVA statistics are provided above and post-hoc tests for X-irradiated mice are reported below.
Regarding the latency to the first convulsive seizure, where there was a main effect of reduced adult neurogenesis, X-irradiated mice generally showed shorter latencies but were not significantly different from Sham-irradiated mice (post-hoc test, p = 0.075; Fig. 2D1). As described above, there was no detectable main effect of reduced adult neurogenesis on the latency to the first nonconvulsive seizure (Fig. 2D2). Regarding the number of convulsive seizures, two-way ANOVA showed a significant main effect of reduced neurogenesis but the differences between X-irradiated mice and Sham-irradiated mice did not reach significance (p = 0.080; Fig. 2D3). Likewise, there was a significant main effect of reduced adult neurogenesis on the number of nonconvulsive seizures by two-way ANOVA and generally X-irradiated mice showed fewer nonconvulsive seizures than Sham-irradiated mice, but the post-hoc test showed that differences between X-irradiated mice and Sham-irradiated mice were not significant (p= 0.054; Fig. 2D3). These results suggest that reduced neurogenesis had a robust effect in GFAP-TK mice and less of an effect in X-irradiated mice; but the direction of the effect was the same, so that when all mice with reduced neurogenesis were analyzed together, there were significant main effects of reduced adult neurogenesis by two-way ANOVA.
Regarding total duration of convulsive seizures, where there was a main effect of reduced adult neurogenesis, total duration was longer in X-irradiated mice compared to Sham-irradiated mice (post-hoc test, p = 0.015; Fig 2D4). There was a shorter duration of nonconvulsive seizures in X-irradiated mice compared to Sham-irradiated mice (post-hoc test, p = 0.011; Fig. 2D4). Taken together, data from X-irradiated mice showed similar effects as GFAP-TK mice but they were not always as robust.
B. Delayed effects of KA
1. GFAP-TK+ mice
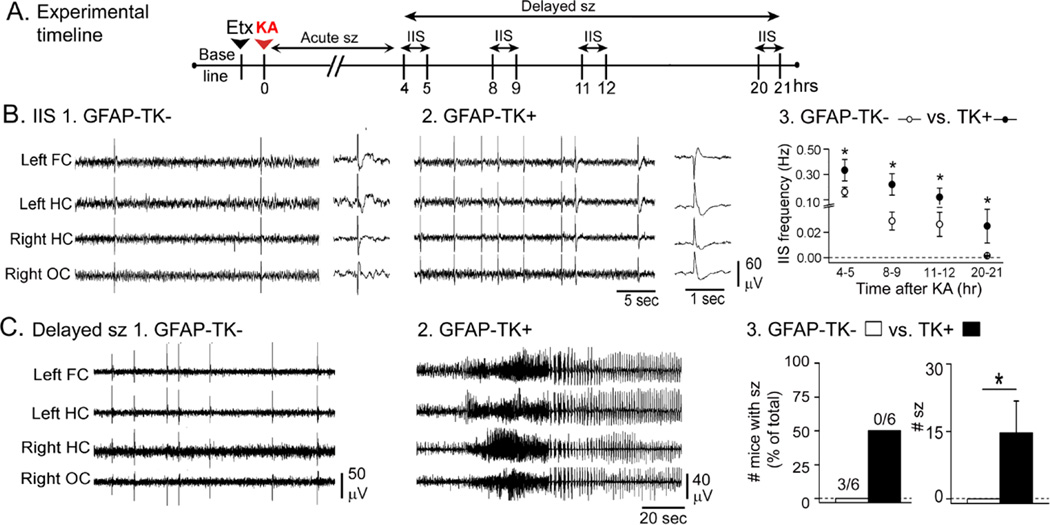
Once the acute period of intense seizures had passed, ~2–4 hrs after KA injection, most mice exhibited IIS and some mice exhibited delayed seizures as well. Fig. 3A shows the times when IIS and delayed seizures were analyzed.
Figure 3. Greater delayed effects of KA in GFAP-TK+ mice compared to GFAP-TK−mice.
A. The experimental timeline is shown. Animals were treated with ethosuximide and 20 mg/kg KA. IIS were analyzed during the 4–21 hrs after KA as shown. Sample sizes are as follows: GFAP-TK−: n = 7; GFAP-TK+: n = 6.
B. Representative examples of IIS from a GFAP-TK− (1) and GFAP-TK+ (2) mouse. For 1–2, the trace to the right shows an IIS at higher temporal resolution. Qualitatively, IIS had similar morphologies. 3. There was a higher IIS frequency in GFAP-TK+ mice compared to GFAP-TK− mice at all the time points tested.
C. 1–2. Representative examples of records from a GFAP-TK− and a GFAP-TK+ mouse at similar delays (approximately 7.5 hrs) after KA.
3. The number of mice with delayed seizures was not significantly greater in GFAP-TK+ mice compared to TK− mice, but the mean number of seizures per mouse was greater in GFAP-TK+ mice.
IIS after acute seizures lasted well into the overnight hours after KA; in mice with suppressed adult neurogenesis, they lasted the longest – as long as 24 hrs after KA injection, which is when recordings were terminated. IIS morphology was qualitatively similar in different experimental groups (Fig. 3B, insets), but IIS frequency was higher in GFAP-TK+ mice (Fig. 3B1–3). This difference was statistically significant overall and at each individual time point (RMANOVA, F(3,27)8.177; p = 0.042, post-hoc comparisons at 4–5, 8–9, 12–13, and 20–21 hrs after KA, all p<0.05; Fig. 3B3).
Regarding delayed seizures, all of the mice with delayed seizures were GFAP-TK+ mice. When the number of mice that had delayed seizures was compared, there was no statistical difference between GFAP-TK− (0/6) and GFAP-TK+ mice (3/6; Fisher’s Exact Test; p = 0.181), but GFAP-TK+ mice had a significantly greater number of delayed seizures compared to GFAP-TK− mice (t-test, p = 0.049; Fig. 3C3).
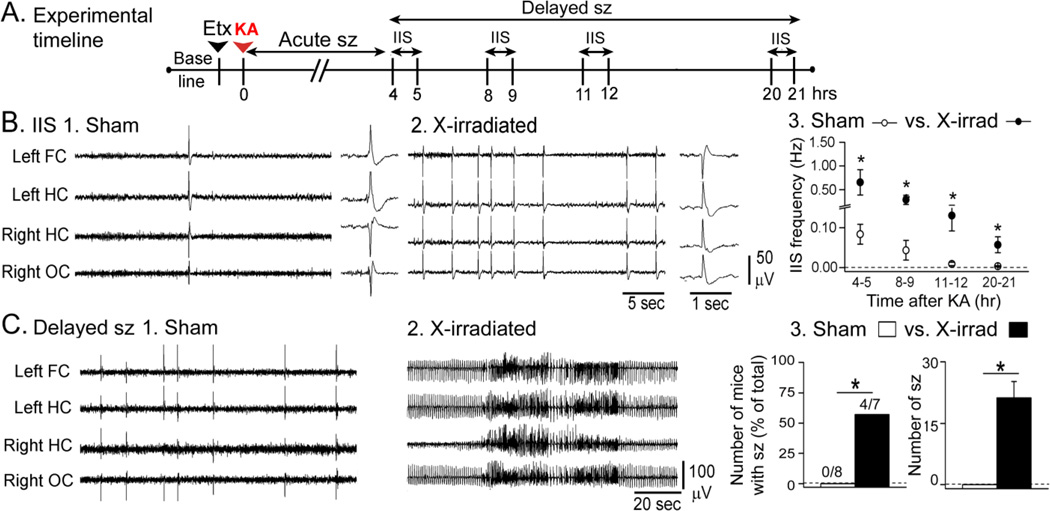
2. X-irradiated mice
IIS and delayed seizures were evaluated in sham and X-irradiated mice as shown in Fig. 4A. IIS were qualitatively similar (Fig. 4B, insets), but were higher in frequency and lasted longer in X-irradiated mice compared to sham-treated mice. The higher IIS frequency was significant overall and at each individual time point (RMANOVA; F(3,42)4.928; p = 0.005; post-hoc comparisons at 4–5, 8–9,12–13 and 20–21 hrs after KA, all p<0.05; Fig. 4B3).
Figure 4. Greater delayed effects of KA in X-irradiated mice compared to sham controls.
A. The experimental timeline is shown. Animals were treated with ethosuximide and 20 mg/kg KA. IIS were analyzed during the 4–21 hrs after KA. Sample sizes are as follows: GFAP-TK−: n = 8; GFAP-TK+: n = 7.
B. Representative examples of IIS from a sham-treated (1) and X-irradiated (2) mouse. For 1–2, the trace to the right shows an IIS at higher temporal resolution. Qualitatively, IIS were similar. 3. There was a higher IIS frequency in X-irradiated mice compared to sham-treated mice at all the time points tested.
C. 1–2. Representative examples of records from a X-irradiated and a sham-treated mouse at similar delays (approximately 7.5 hrs) after KA.
3. There were significantly more X-irradiated mice that exhibited delayed seizures compared to sham-treated mice. The mean number of seizures per mouse was greater in X-irradiated mice also.
Some mice had delayed seizures, and all of these were X-irradiated mice. Thus, 4/7 X-irradiated mice had delayed seizures, compared to 0/8 sham-treated mice (Fisher’s Exact test; p = 0.025). In addition, the mean number of delayed seizures was significantly greater in X-irradiated mice (t-test, p = 0.021; Fig. 4C3). Thus, for both methods to suppress adult neurogenesis, delayed effects of KA were greater when adult neurogenesis was reduced.
II. Effects of KA without pretreatment with ethosuximide
A. KA without ethosuximide pretreatment: GFAP-TK+ mice
1. Acute effects
KA (without ethosuximide) was injected as shown by the timeline in Fig. 5A. Sixteen mg/kg KA was injected in GFAP-TK− (n = 13) and GFAP-TK+ mice (n = 14). This lower dose was used because initial studies showed high mortality when 20 mg/kg was administered. One explanation is that mice administered 20 mg/kg KA had less mortality when ethosuximide was used (Fig. 1–4) because of a protective effect of ethosuximide on mortality by decreasing brainstem-associated seizures (Browning et al., 1981; Fromm, 1985; Mares et al., 1994).
Figure 5. In mice that were not pretreated with ethosuximide, GFAP-TK+ mice had a greater response to KA than GFAP-TK− mice.
A. The experimental timeline is shown for treatment of GFAP-TK mice with 16 mg/kg KA (GFAP-TK−, n = 13; GFAP-TK+, n = 14).
B. Representative examples of the response to KA in a GFAP-TK− and GFAP-TK+ mouse show a greater response in the GFAP-TK+ mouse.
C. 1–2. GFAP-TK+ mice had similar latencies to GFAP-TK+ mice for the first convulsive and first nonconvulsive seizure. 3. There were more convulsive seizures in GFAP-TK+ mice compared to GFAP-TK− mice. 4. The total duration of convulsive seizures was greater and total duration of nonconvulsive seizures was shorter in GFAP-TK+ mice compared to GFAP-TK− mice.
D. 1–2. Representative IIS in a GFAP-TK− and GFAP-TK+ mouse.
3. GFAP-TK+ mice had a higher IIS frequency than GFAP-TK− mice.
Fig. 5B shows representative EEG traces and the timing of convulsive seizures from a GFAP-TK− and GFAP-TK+ mouse that were administered 16 mg/kg KA. Two-way ANOVA was conducted with data from Fig. 5 for GFAP-TK mice and Fig. 6 for X-irradiated/Sham-irradiated mice. There was no significant main effect of suppressed neurogenesis on the latency to the first convulsive seizure (F (1, 34)0.005, p = 0.945) or first nonconvulsive seizure (F (1, 39)0.671, p = 0.416; Fig. 5C1–2).
Figure 6. Comparison of the response of sham and X-irradiated mice to KA without pretreatment with ethosuximide.
A. The experimental timeline is shown for treatment of Sham- and X-irradiated mice with 16 mg/kg KA (n = 10/group).
B. Example of the response to KA in a Sham-treated and X-irradiated mouse.
C. 1–2. There were no significant differences in the latency to the first seizure. 3. There no significant differences in the numbers of seizures. 4. The total duration of convulsive seizures was greater in X-irradiated mice compared to Sham-irradiated mice.
D. 1–3. There were no significant differences in IIS frequency between Sham-treated and X-irradiated mice 4–21 hrs after KA administration.
Reduced adult neurogenesis almost met p criterion for significance for the number of convulsive seizures, but did not (F (1, 34)3.94, p = 0.055; Fig. 5C3). The trend towards significance is probably because of an effect in GFAP-TK+ mice to increase the number of seizures, which was significant compared to GFAP-TK− mice (p = 0.036; Fig. 5C3). There was no effect of method (F (1, 34)1.43, p = 0.240). Regarding the number of nonconvulsive seizures, there was no effect of reduced adult neurogenesis (F (1, 39)0.716, p = 0.403; Fig. 5C3).
For total duration of convulsive seizures, there was a main effect of suppressed neurogenesis (F (1, 34)12.13, p = 0.001) without an effect of method (F (1, 34)0.69, p = 0.412; Fig. 5C4). Compared to GFAP-TK− mice, GFAP-TK+ mice had a significantly greater total duration of convulsive seizures (post-hoc test, p = 0.009; Fig. 5C4). There was a significant main effect of reduced neurogenesis on the total duration of nonconvulsive seizures (F (1, 39)3.89, p = 0.049; Fig. 5C4), without a main effect of method (F (1, 39)1.19, p = 0.283). There were shorter durations in GFAP-TK+ mice compared to GFAP-TK− mice (post-hoc test, p= 0.030; Fig. 5C4).
There were 5 deaths during convulsive seizures, all in GFAP-TK+ mice. Again, the deaths were due to respiratory arrest during tonic-clonic seizures. These data support the results described above using ethosuximide pretreatment, showing that mice with reduced adult neurogenesis exhibit greater seizure severity in response to KA.
2. Delayed effects
GFAP-TK+ mice exhibited a higher IIS frequency than GFAP-TK− mice (RMANOVA, F (1, 39)15.118; p<0.001; post-hoc tests at 4–5 hrs, p = 0.042; 8–9 hrs, p = 0.004; 11–12 hrs, p = 0.001; Fig. 5D). There were no delayed seizures in mice of either genotype.
B. KA without ethosuximide pretreatment: X-irradiated mice
1. Acute effects
KA was injected in Sham and X-irradiated mice (n = 10 each) as shown in Fig. 6A using 16 mg/kg KA. Fig. 6B shows representative EEG traces and the timing of convulsive seizures from a sham and a X-irradiated mouse. There were 2 deaths in response to KA: one sham-treated, and one X-irradiated mouse.
As described above, reduced neurogenesis did not influence the latency to the first convulsive or nonconvulsive seizure (Fig. 6C1–2). X-irradiated and Sham-irradiated mice were not different by post-hoc test (all p>0.05) when the numbers of seizures were analyzed (Fig. 6C3). Regarding the total duration of convulsive seizures, where there was a main effect of reduced adult neurogenesis, X-irradiated mice had a longer mean duration of convulsive seizures (post-hoc test, p = 0.035; Fig. 6C4) like GFAP-TK+ mice. However, the differences between X-irradiated and Sham-irradiated mice in the total durations of nonconvulsive seizures were not significant (p = 0.312; Fig. 6C4).
These data suggest an effect of suppressed adult neurogenesis in X-irradiated mice but a very small effect. Therefore it is notable that other data also supported the hypothesis that X-irradiated mice had more severe seizures than Sham-irradiated mice. We analyzed the first 45 min after KA administration to examine the interseizure interval. The time after the first 45 min was not examined because after this time, interseizure intervals usually were short in all animals – presumably because seizures become more severe approximately 45 min after KA injection. First we established that, like the studies of the first 4 hrs of seizures, that the total duration of convulsive seizures during the first 45 min was longer in X-irradiated than Sham-irradiated mice (Supplementary Fig. 2). Then we examined the interseizure interval, and found it was significantly shorter in X-irradiated mice (Supplementary Fig. 2). These data are consistent with the compressed records shown in Figures 1, 2, 5 and 6, where the animals with reduced adult neurogenesis often had seizures that with a relatively brief interseizure interval. The data are also consistent with the hypothesis that X-irradiated mice had a susceptibility to longer convulsive seizures because of a shorter postictal period after each convulsive seizure.
We then asked whether these small differences between X-irradiated and Sham-irradiated mice could be shown in another strain of mice, which would strengthen the case that they exist, even if they were small. We found that in the 129SvEv strain similar differences between X-irradiated and Sham-irradiated mice were present: X-irradiated mice had a longer total duration of convulsive seizures in the first 45 min after KA administration (t-test, p = 0.015) and the mean duration of convulsive seizures for the first 45 min was also longer (p = 0.009; Supplementary Fig. 4). In summary, differences between X-irradiated mice and Sham-irradiated mice in the acute response to KA were reproducible, although they were small in the absence of ethosuximide pretreatment.
2. Delayed effects
There were no differences between Sham and X-irradiated mice in IIS frequency, possibly because it was very low in frequency in both groups (RMANOVA, F (1, 37)0.576; p = 0.452; Fig. 6D). There were no delayed seizures. One reason for the lack of difference in the groups may be unrelated to adult neurogenesis: the seizures in the acute period were simply not very severe, and when that occurred, IIS and delayed seizures were rare.
C. Effects of ethosuximide vs. vehicle pretreatment
Ethosuximide was used to suppress seizures dominated by brainstem activity. To determine whether there was an effect of vehicle, we compared vehicle-pretreated mice with mice treated with KA alone. These experiments were conducted in an additional cohort of age-matched naive mice (n = 5/group). There were no differences between the groups in any of the parameters for convulsive or nonconvulsive seizures in the first 4 hrs after KA administration (Supplementary Fig. 5).
Next, we determined if there was an effect of ethosuximide on location of seizure onset. Consistent with the idea that ethosuximide increases seizures that involve forebrain structures, e.g., hippocampus, we observed that a higher proportion of seizures involved hippocampal electrodes when ethosuximide was administered compared to vehicle treatment (Supplementary Fig. 6).
III. Focal seizures
The results described above pertain to seizures with EEG manifestations in all leads. However, there were seizures that involved only a subset of leads, i.e. focal seizures (Supplementary Fig. 7). We refer to a structure as ‘involved’ when an electrode showed seizure activity. Mice were pooled (Control: sham and GFAP-TK−; Reduced adult neurogenesis: X-irradiated and GFAP-TK+) for analysis because focal seizures were rare. In mice that were administered ethosuximide and KA, no differences were detected between groups in the number of mice that had focal seizures (p = 1.000; Supplementary Fig. 7). There was also no difference in the number of focal seizures per mouse, (p = 0.813; Supplementary Fig. 7). Next we asked if there were differences in the degree focal seizures involved the hippocampus. Significantly more focal seizures involved the hippocampus in mice with suppressed adult neurogenesis compared to controls (p = 0.047), and significantly fewer seizures involved the cortex in mice with suppressed adult neurogenesis (p = 0.005; Supplementary Fig. 7).
There were no differences in focal seizures when KA was administered alone (i.e., without ethosuximide; Supplementary Fig. 7). These results support the view that ethosuximide pretreatment optimized the evaluation of seizure activity in the forebrain.
IV. Additional analyses of seizures
Since parameters related to convulsive seizures were most affected in mice with reduced adult neurogenesis, we asked whether the evolution of an electrographic seizure to a convulsive seizure would be faster in these mice. We called this parameter the ‘convulsive index’ (Supplementary Fig. 8). GFAP-TK+ mice that were administered ethosuximide and 20 mg/kg KA showed a shorter convulsive index (p = 0.046) compared to GFAP-TK− mice. There were no significant differences in this parameter between sham-treated and X-irradiated mice pretreated with ethosuximide (p = 0.298). GFAP-TK+ mice that were administered 16 mg/kg KA showed a shorter convulsive index than GFAP-TK− mice (p = 0.038). However sham and X-irradiated mice that were administered 16 mg/kg KA were not significantly different (p = 0.245; Supplementary Fig. 8).
We also hypothesized that the time between convulsive seizures (interseizure interval or ISI; Supplementary Fig. 9) would be shorter when adult neurogenesis was reduced. We defined the ISI as the time from the end of one seizure to the onset of the next (Supplementary Fig. 9). GFAP-TK+ mice that were administered ethosuximide and 20 mg/kg KA showed a shorter convulsive ISI (p = 0.047) without any differences in nonconvulsive ISI (p = 0.191). X-irradiated mice administered ethosuximide and 20 mg/kg KA had a shorter convulsive ISI compared to sham-treated mice (p = 0.035) and no differences in nonconvulsive ISI (p = 0.189). When GFAP-TK mice were administered KA alone, GFAP-TK+ mice had a shorter convulsive ISI compared to GFAP-TK− mice (p = 0.028) and no differences in nonconvulsive ISI (p = 0.761). Similarly, X-irradiated mice that were administered 16 mg/kg KA showed a shorter convulsive ISI (p = 0.005) compared to sham-treatment, and no differences between the groups in nonconvulsive ISI (p = 0.637; Supplementary Fig. 9).
V. Confirmation of suppression of adult neurogenesis
Mice were perfusion-fixed at ages when KA would have been injected (n = 3/group; Fig. 7A). DCX-ir confirmed a dramatic reduction of adult neurogenesis in X-irradiated and GFAP-TK+ mice (Fig. 7B). The mean areas of the regions of interest (ROIs; see Methods) were not different (Fig. 7B4–5), indicating that shrinkage – a potential consequence of progenitor cell death – was not detectable.
DCX-ir was also examined in the OB, where it was dramatically reduced in GFAP-TK+ mice (Fig. 7C1–2) with no differences in ROI areas (Fig. 7C3–5). The results confirmed that adult neurogenesis was suppressed in the DG of X-irradiated mice, with no detectable effect in the OB, whereas both the DG and OB were affected in GFAP-TK+ mice as would be expected.
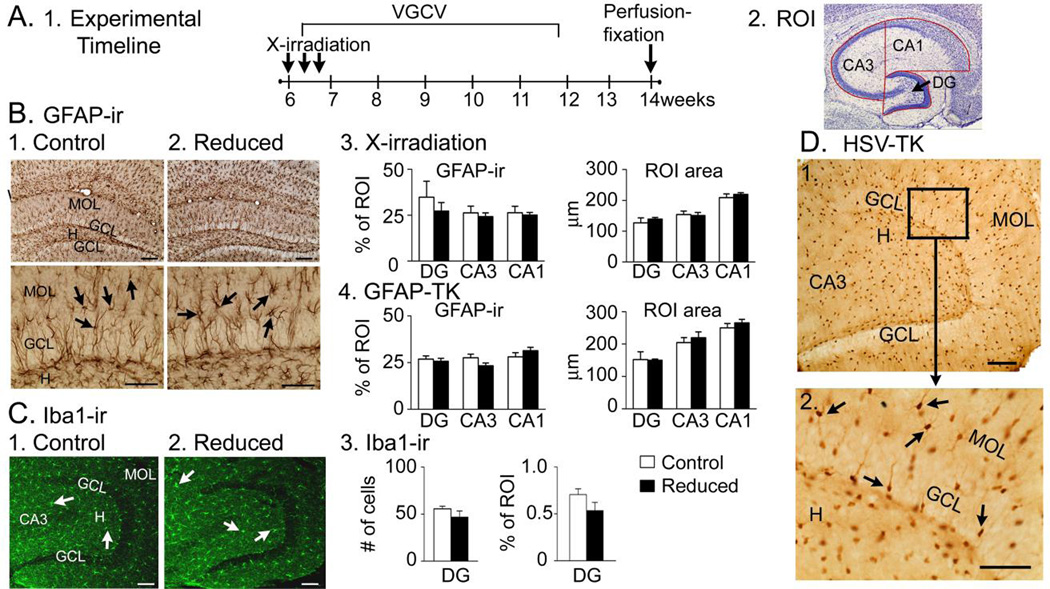
At the time when KA would have been injected, GFAP-ir in the DG of control mice could not be distinguished from mice with suppressed adult neurogenesis (Fig. 8A–B), suggesting that reduction of adult neurogenesis did not affect mature astrocytes at this time. Comparison of Iba1, a microglial marker, showed no significant differences across groups for the DG (Fig. 8C) or CA3 (all p>0.05, data not shown), suggesting microglia were not affected. As an additional control, an antibody to hsv-TK was used to show that expression of hsv-TK in GFAP-TK mice was specific to cells with the morphology of astrocytes (Fig. 8D).
Figure 8. Specificity of GFAP-TK+ mice.
A. 1. The experimental timeline is shown.
2. The ROIs used to examine the DG, CA1 and CA3 are shown.
B. 1–2. GFAP-ir was similar in mice with normal and reduced adult neurogenesis. Arrows point to GFAP-ir cells. Calibration (Top), 100 µm; (Bottom), 75 µm.
3. There were no differences in GFAP-ir in sham- and X-irradiated mice (DG, CA1, CA3: t-tests, p = 0.488, 0.652, 0.750 respectively, n = 3/group) or ROI area (t-tests, p = 0.519, 0.841, 0.521, n = 3/group).
4. Differences in GFAP-ir were not detected in comparisons of GFAP-TK− and GFAP-TK+ mice (t-tests, p = 0.633, 0.0848, 0.2603 respectively, n = 3/group) or ROI area (t-tests, p = 0.623, 0.545, 0.380 respectively, n = 3/group).
C. 1–2. Iba 1-ir (arrows) was similar in GFAP-TK− (1) and GFAP-TK+ (2) mice. Calibrations, 100 µm.
3. Mice with reduced adult neurogenesis were not different from controls in Iba 1-ir in the DG (t-test, p = 0.194, n = 3/group) or the DG ROI area (t-test, p = 0.763, n = 3/group).
D. Top: A representative section from a GFAP-TK+ mouse stained with an antibody to hsv-TK shows numerous cells in the DG with the morphology of astrocytes (arrows). Calibration, 100 µm.
Bottom: The area surrounded by the box is expanded. Calibration, 100 µm.
DISCUSSION
I. Summary
We demonstrated a surprising effect of reducing adult-born neurons on seizures using two independent methods of reducing adult neurogenesis. We found that mice with reduced adult neurogenesis were more susceptible to the effects of KA. When ethosuximide was not used, the results were less striking but still robust in the GFAP-TK+ mice; this is discussed further below.
II. Reducing adult neurogenesis increases the response to KA
There were several effects of reducing adult neurogenesis that are consistent with a more severe response to KA. In the first 4 hrs after KA administration, mice with reduced adult neurogenesis had a decrease in the latency to the first convulsive seizure, a greater number and longer convulsive seizures, and a decrease (or no change) in parameters for nonconvulsive seizures. Many mice with reduced adult neurogenesis showed greater mortality due to convulsive seizures, higher IIS frequency and delayed seizures after KA administration.
GFAP-TK+ mice exhibited a greater effect of reduced adult neurogenesis than X-irradiated mice, which is interesting because both sites of adult neurogenesis (the DG and OB) are affected in the GFAP-TK+ mice but only the DG in X-irradiated mice. Therefore, we speculate that there is a facilitatory effect of reduced OB adult neurogenesis on KA-induced seizures. A role of the OB is consistent with the fact that the target of the OB, the piriform cortex, plays a major role in seizure generation in rodents (Loscher and Ebert, 1996; McIntyre and Gilby, 2008; Laufs, 2012). For example, removal of the OB facilitates kindled seizures evoked by piriform cortex (Cain and Corcoran, 1978).
In some ways, the results suggest a ‘switch’ in seizures from nonconvulsive to convulsive in mice with reduced adult neurogenesis. Thus, in many animals with reduced adult neurogenesis, there was a reduction in nonconvulsive seizures with increased convulsive seizures in the first 4 hrs after KA administration, and no change in the total number of seizures (i.e., all seizures irrespective of whether they were convulsive or nonconvulsive). Animals that did not show this effect, but had reduced adult neurogenesis, were the animals that were administered KA without ethosuximide and X-irradiated, suggesting that ethosuximide enhances this effect and that reduced OB neurogenesis plays a role. The idea that adult neurogenesis regulates the likelihood that a seizure will become convulsive has clinical implications (discussed further in Sections V and VI).
III. DG as a ‘gate’ for excitatory activity entering the hippocampus
The idea that the DG could act as a ‘gate’ to seizures by restricting the amplification or reverberation of seizures has been previously proposed (Heinemann et al., 1992; Lothman et al., 1992; Hsu, 2007). Newborn granule cells could facilitate this function, possibly supporting inhibition (Sahay et al., 2011a; Sahay et al., 2011b; Ikrar et al., 2013). In disease, reduced adult-born neurons in the chronic phase of epilepsy (Hattiangady et al., 2004), or abnormalities in adult-born neurons (Scharfman, 2004; Danzer, 2008; Parent and Murphy, 2008; Shapiro et al., 2008; Cameron et al., 2011; Koyama et al., 2012; Ribak et al., 2012; Fournier et al., 2013) may prevent this function, contributing to seizures and cognitive dysfunction (for further discussion, see below).
How adult-born GCs inhibit the network in the DG is not clear, although in the OB it is known that adult-born GCs are inhibitory interneurons. In the DG, inhibition of mature GCs by adult-born GCs could be indirect, by projections to local interneurons that in turn inhibit mature GCs (Toni et al., 2008). Electrophysiological evidence for direct inhibition of mature GCs by adult-born GCs has been recently presented (Drew et al., 2013).
IV. Caveats
Ethosuximide could have had effects other than those that involve brainstem. For example, by blocking thalamocortical oscillations, ethosuximide could theoretically increase seizure activity elsewhere (Cassidy and Gale, 1998). Ethosuximide may have influenced mature GCs, because T-type Ca2+ channels are expressed in the GC layer (Talley et al., 1999). However, it is unclear how antagonism of T-type Ca2+ channels in this location would facilitate seizures – one would predict it would reduce them.
The similarity of results with two methods to reduce adult neurogenesis suggests that the results are not related to the method to reduce adult-born neurons. However, we cannot exclude a potential effect on adult glia or microglia, because there could theoretically be functional effects that are not evident by protein expression.
Another caveat is that there are potential explanations for the results besides the hypothesis that adult-born neurons are normally inhibitory. As mentioned above, new data using optogenetics to acutely activate nestin-expressing progenitors does suggest that adult-born neurons in the dentate gyrus exert an inhibitory effect on mature granule cells (Drew et al., 2013). However, a compensatory change in the brain following reduction of adult neurogenesis could contribute to the findings of the present study, because a substantial delay occurred between the start of suppressed adult neurogenesis and KA administration. During this delay there could have been changes in the dentate gyrus (or elsewhere) that compensate for the loss of adult-born neurons, which has been proposed (Singer et al., 2011). One study of brain radiation, however, argues against this hypothesis (Raedt et al., 2007). In this study, low dose brain radiation suppressed proliferation and adult neurogenesis during the days after radiation, assessed either by BrdU-labeling or DCX staining. Kindling was conducted in the days immediately after radiation, and the authors found that afterdischarge threshold was lower in radiated animals and the severity of convulsive seizures was greater in radiated animals. However, radiation affected the entire brain in this study, and kindling was conducted with a short delay after radiation, allowing very little time for the acute effects of radiation on processes besides adult neurogenesis to subside.
Regarding IIS, it is important to point out why we interpret increased IIS in mice with reduced adult neurogenesis to reflect a more severe response to KA, because in epilepsy, it is not clear that IIS are excitatory or inhibitory (Staley and Dudek, 2006; de Curtis and Avanzini, 2001). We associate the IIS with increased severity of seizures because only the animals with convulsive seizures in the 4 hrs after KA had IIS afterwards (4–24 hrs after KA). In animals that had a brief response to KA, and no or few convulsive seizures, IIS were not present after the brief response or they were extremely rare. Also, in pilot studies, IIS occurred after high doses of KA (16 mg/kg) relative to low doses (8–12 mg/kg). Furthermore, when IIS occurred in the overnight hours after KA administration, they were most frequent when there were seizures interspersed between IIS. When there were no seizures, IIS were infrequent.
V. Implications for seizures
Although seizures are often considered only in the context of epilepsy, seizures also occur in individuals without epilepsy, and are remarkably common. Thus, approximately 4% of the population will have a single unprovoked seizure by the age of 80, and 30–40% of those individuals will develop a second seizure (which leads to the diagnosis of epilepsy; Herman, 2004). There are many reasons why seizures are common, and one is that many factors in the lifetime of an individual can lead to a seizure, such as illness, infection, a cerebrovascular event, traumatic injury, or stroke (England et al., 2012). Seizures also occur in alcoholism during the withdrawal period, and are an important clinical concern (Hillborn et al., 2003; Rogawski, 2005). In these individuals, our data would suggest that the seizure would be more likely to be severe (i.e., more likely to be convulsive) if there was reduced adult neurogenesis. Importantly, individuals who have a heart attack or stroke, i.e., the elderly, have reduced adult neurogenesis because there is a substantial decline in adult neurogenesis with age (Couillard-Despres, 2013; Hamilton et al., 2013). In animal models of alcoholism, adult neurogenesis also declines (Crews et al., 2006; Nixon et al., 2010). Therefore, our results suggest that adult neurogenesis could be a potential contributing factor in seizures.
VI. Implications for temporal lobe epilepsy (TLE)
A. Chronic epilepsy
The results are potentially relevant to TLE because the dentate gyrus has been implicated (Margerison and Corsellis, 1966; Heinemann et al 1992; Lothman et al 1992; Sloviter, 1994; Masukawa et al., 1999; Santhakumar et al., 2001; Dudek and Sutula, 2007). The results of the present study suggest that adult neurogenesis may be one of the factors that influences seizure susceptibility in TLE. Our data suggest that reducing adult neurogenesis would worsen seizures by increasing the number of convulsive seizures and their duration. Regarding nonconvulsive seizures, which decreased after reducing adult neurogenesis, the decrease in nonconvulsive seizures was usually accompanied by an increase in convulsive seizures, so we view the decrease in nonconvulsive seizures as negative also.
Adult-born neurons may also contribute to TLE in its chronic phase, when the neurogenic niche appears to be reduced (Blümcke et al., 2001; Hattiangady et al., 2004, but see Jessberger et al., 2005). During this late phase, it has been suggested that epilepsy worsens or progresses (Nearing et al., 2007; Bernhardt et al., 2013). In animals, it has also been reported that seizures during the weeks and months after SE increase progressively (Williams et al., 2009). Our data suggest that few adult born neurons could contribute to “progression.”
B. Early stages of epileptogenesis
In contrast to the chronic period, adult neurogenesis is increased when one examines the hippocampus early in the process of epileptogenesis in animal models. For example, after systemic pilocarpine- or KA-induced SE, there is a dramatic rise in adult-born neurons in the subsequent days and weeks (Parent et al., 1997, Gray and Sundstrom, 1998; Covolan et al., 2000; Nakagawa et al., 2000; Scharfman et al., 2000). Electrically-induced SE (Mohapel et al., 2004) and kindling (Parent et al., 1998; Scott et al., 1998; Nakagawa et al., 2000; Ferland et al., 2002) are similar. However, if SE is extremely severe there appears to be toxicity to progenitors (Mohapel et al., 2004) although not always (Yang et al., 2008; Hung et al., 2012). If KA is injected into the hippocampus rather than administered systemically, the neurogenic niche is adversely affected (Bouilleret et al., 1999; Kralic et al., 2005). In young animals, there is limited capacity to increase adult neurogenesis after SE (Bender et al., 2003; Porter, 2008, Lauren et al., 2013), possibly because the rate is already high.
In the animal models where adult neurogenesis increases, the surviving newborn neurons would be likely to decrease epileptogenesis in light of the findings presented here. Indeed, the GCs born after SE which migrate correctly and integrate properly appear to be quiescent or inhibitory (Jakubs et al., 2006). However, diverse characteristics have been reported for adult GCs after SE, which could be due to variability in pathology (Wood et al., 2011). Many newborn neurons become abnormal and could increase excitability rather than decrease it. For example, some GCs born in the days or weeks after SE migrate into the hilus instead of the granule cell layer and establish an aberrant ectopic population of granule cells (hilar ectopic granule cells or hEGCs; Parent et al., 1997; Scharfman et al., 2000; Jessberger et al., 2007; Jiao and Nadler, 2007; Yang et al., 2008; Fournier et al., 2010; Zhang et al., 2012; Koyama et al., 2012; Hester and Danzer, 2013) that creates a potential epileptic focus (Scharfman, 2004; Scharfman and Gray, 2009; Cameron et al., 2011; Pierce et al., 2011). HEGCs appear to have this pro-epileptic effect in the febrile seizure model also (Koyama et al., 2012).
Other abnormalities in maturation of newborn GCs also occur, such as abnormal dendrites that extend into the hilus rather than the molecular layer, called “hilar basal dendrites.” (Dashtipour et al., 2003; Ribak et al., 2012). These dendrites have more excitatory input (Thind et al., 2008), so they may promote excitability (Ribak et al., 2000; Shapiro et al., 2005; Ribak et al., 2012, but see Becker et al., 2012). There are other abnormalities that can develop also, such as hypertrophy (Pun et al., 2012) or malalignment (Murphy et al., 2012). Hypertrophy appears to have a robust pro- epileptic effect (Pun et al., 2012).
In light of these studies, adult neurogenesis early in epileptogenesis may promote seizures by increasing aberrant neurogenesis. In fact, several studies support this idea (Jung et al., 2004, Jung et al., 2006; Cho et al., 2013) but not if seizure susceptibility is tested immediately after reducing adult-born cells (Pekcec et al., 2008; Pekcec et al., 2011).
C. Depression and TLE
Depression is a common comorbidity in TLE (Kanner, 2008; Kanner and Hesdorffer, 2012). Our data support the idea that adult neurogenesis may be a contributing factor in this comorbidity, because reduced adult neurogenesis in the DG is considered to be a potential cause of depression based on animal models (Santarelli et al., 2003; Sahay et al., 2007). Our findings also suggest an explanation for the observation that some antidepressants, which increase adult neurogenesis like fluoxetine (Santarelli et al., 2003), can reduce seizures (Thome-Souza et al., 2007; Hamid and Kanner, 2013).
VII. Conclusions
Our results suggest that reduction of adult neurogenesis exacerbates effects of KA. These data suggest a novel effect of reducing adult-born neurons on KA-induced seizures.
Supplementary Material
Highlights.
Reduction of adult neurogenesis increases susceptibility to the effects of KA.
Convulsive seizures and interictal spikes (IIS) were affected primarily.
Results were similar with two methods to reduce adult neurogenesis.
Effects on convulsive seizures and IIS were similar with two methods to produce seizures.
Ethosuximide enhanced effects of reduced adult neurogenesis on KA-induced seizures.
Acknowledgments
Supported by NIH grants NS-081203, MH-090606, NS-081203, NYSTEM 026430, CURE and the New York State Office of Mental Health. We thank Dr. Michael Sofroniew for the antibody to herpes simplex virus-thymidine kinase, and Drs. Neil MacLusky and Karen Gale for discussion.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Acsády L, Kamondi A, Sík A, Freund T, Buzsáki G. GABAergic cells are the major postsynaptic targets of mossy fibers in the rat hippocampus. J Neurosci. 1998;18:3386–3403. doi: 10.1523/JNEUROSCI.18-09-03386.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker D, Willems LM, Vnencak M, Zahn N, Schuldt G, Jedlicka P, Maggio N, Deller T, Vlachos A. Functional and structural properties of dentate granule cells with hilar basal dendrites in mouse entorhino-hippocampal slice cultures. PLoS One. 2012;11:e48500. doi: 10.1371/journal.pone.0048500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belzung C, Wigmore P. Neurogenesis and neural plasticity. Berlin: Springer-Verlag; 2013. [Google Scholar]
- Bender RA1, Dubé C, Gonzalez-Vega R, Mina EW, Baram TZ. Mossy fiber plasticity and enhanced hippocampal excitability, without hippocampal cell loss or altered neurogenesis, in an animal model of prolonged febrile seizures. Hippocampus. 2003;13:399–412. doi: 10.1002/hipo.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhardt BC, Kim H, Bernasconi N. Patterns of subregional mesiotemporal disease progression in temporal lobe epilepsy. Neurology. 2013;81:1840–1847. doi: 10.1212/01.wnl.0000436069.20513.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blümcke I, Schewe JC, Normann S, Brustle O, Schramm J, Elger CE, Wiestler OD. Increase of nestin-immunoreactive neural precursor cells in the dentate gyrus of pediatric patients with early-onset temporal lobe epilepsy. Hippocampus. 2001;11:311–321. doi: 10.1002/hipo.1045. [DOI] [PubMed] [Google Scholar]
- Bouilleret V, Ridoux V, Depaulis A, Marescaux C, Nehlig A, Le Gal La Salle G. Recurrent seizures and hippocampal sclerosis following intrahippocampal kainate injection in adult mice: electroencephalography, histopathology and synaptic reorganization similar to mesial temporal lobe epilepsy. Neuroscience. 1999;89:717–729. doi: 10.1016/s0306-4522(98)00401-1. [DOI] [PubMed] [Google Scholar]
- Brown JP, Couillard-Despres S, Cooper-Kuhn CM, Winkler J, Aigner L, Kuhn HG. Transient expression of doublecortin during adult neurogenesis. J Comp Neurol. 2003;467:1–10. doi: 10.1002/cne.10874. [DOI] [PubMed] [Google Scholar]
- Browning RA, Turner FJ, Simonton RL, Bundman MC. Effect of midbrain and pontine tegmental lesions on the maximal electroshock seizure pattern in rats. Epilepsia. 1981;22:583–594. doi: 10.1111/j.1528-1157.1981.tb04130.x. [DOI] [PubMed] [Google Scholar]
- Burghardt NS, Park EH, Hen R, Fenton AA. Adult-born hippocampal neurons promote cognitive flexibility in mice. Hippocampus. 2012;22:1795–1808. doi: 10.1002/hipo.22013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cain DP, Corcoran ME. Kindling in olfactory-lesioned rats. Behav Biol. 1978;22:264–268. doi: 10.1016/s0091-6773(78)92320-9. [DOI] [PubMed] [Google Scholar]
- Cameron HA, McKay RD. Adult neurogenesis produces a large pool of new granule cells in the dentate gyrus. J Comp Neurol. 2001;435:406–417. doi: 10.1002/cne.1040. [DOI] [PubMed] [Google Scholar]
- Cameron MC, Zhan RZ, Nadler JV. Morphologic integration of hilar ectopic granule cells into dentate gyrus circuitry in the pilocarpine model of temporal lobe epilepsy. J Comp Neurol. 2011;519:2175–2192. doi: 10.1002/cne.22623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassidy RM, Gale K. Mediodorsal thalamus plays a critical role in the development of limbic motor seizures. J Neurosci. 1998;18:9002–9009. doi: 10.1523/JNEUROSCI.18-21-09002.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clelland CD, Choi M, Romberg C, Clemenson GD, Jr, Fragniere A, Tyers P, Jessberger S, Saksida LM, Barker RA, Gage FH, Bussey TJ. A functional role for adult hippocampal neurogenesis in spatial pattern separation. Science. 2009;325:210–213. doi: 10.1126/science.1173215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K, Ito N, Lybrand Z, Good L, Birnbaum S, Kernie SG, Scharfman HE, Eisch AJ, Hsieh J. The role of adult-generated granule neurons in epileptogenesis and cognitive impairment. Society for Neuroscience Abs. 2013;248:16. [Google Scholar]
- Couillard-Despres S, Winner B, Schaubeck S, Aigner R, Vroemen M, Weidner N, Bogdahn U, Winkler J, Kuhn HG, Aigner L. Doublecortin expression levels in adult brain reflect neurogenesis. Eur J Neurosci. 2005;21:1–14. doi: 10.1111/j.1460-9568.2004.03813.x. [DOI] [PubMed] [Google Scholar]
- Covolan L, Ribeiro LT, Longo BM, Mello LE. Cell damage and neurogenesis in the dentate granule cell layer of adult rats after pilocarpine- or kainate-induced status epilepticus. Hippocampus. 2000;10:169–180. doi: 10.1002/(SICI)1098-1063(2000)10:2<169::AID-HIPO6>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Couillard-Després S. Hippocampal neurogenesis and ageing. Curr Top Behav Neurosci. 2013;15:343–355. doi: 10.1007/7854_2012_232. [DOI] [PubMed] [Google Scholar]
- Crews FT, Mdzinarishvili A, Kim D, He J, Nixon K. Neurogenesis in adolescent brain is potently inhibited by ethanol. Neurosci. 2006;137:437–445. doi: 10.1016/j.neuroscience.2005.08.090. [DOI] [PubMed] [Google Scholar]
- Danzer SC. Postnatal and adult neurogenesis in the development of human disease. Neuroscientist. 2008;14:446–458. doi: 10.1177/1073858408317008. [DOI] [PubMed] [Google Scholar]
- Danzer SC. Depression, stress, epilepsy and adult neurogenesis. Exp Neurol. 2012;233:22–32. doi: 10.1016/j.expneurol.2011.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dashtipour K, Wong AM, Obenaus A, Spigelman I, Ribak CE. Temporal profile of hilar basal dendrite formation on dentate granule cells after status epilepticus. Epilepsy Res. 2003;54:141–151. doi: 10.1016/s0920-1211(03)00082-2. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Avanzini G. Interictal spikes in focal epileptogenesis. Prog Neurobiol. 2001;63:541–567. doi: 10.1016/s0301-0082(00)00026-5. [DOI] [PubMed] [Google Scholar]
- Drew LJ, Khierbek MA, Scharfman HE, Hen R. Recruitment of local inhibitory neurons by adult-born granule cells. Soc Neurosci Abs 770.01. 2013 [Google Scholar]
- Dudek FE, Sutula TP. Epileptogenesis in the dentate gyrus: a critical perspective. Prog Brain Res. 2007;163:755–773. doi: 10.1016/S0079-6123(07)63041-6. [DOI] [PubMed] [Google Scholar]
- Duffy AM, Schaner MJ, Wu SH, Staniszewski A, Kumar A, Arévalo JC, Arancio O, Chao MV, Scharfman HE. A selective role for ARMS/Kidins220 scaffold protein in spatial memory and trophic support of entorhinal and frontal cortical neurons. Exp Neurol. 2011;229:409–420. doi: 10.1016/j.expneurol.2011.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eells JB, Clough RW, Browning RA, Jobe PC. Comparative fos immunoreactivity in the brain after forebrain, brainstem, or combined seizures induced by electroshock, pentylenetetrazol, focally induced and audiogenic seizures in rats. Neuroscience. 2004;123:279–292. doi: 10.1016/j.neuroscience.2003.08.015. [DOI] [PubMed] [Google Scholar]
- England MJ, Liverman CT, Schultz AM, Strawbridge LM. Epilepsy across the spectrum: promoting health and understanding. Epilepsy Behav. 2012;25:266–276. doi: 10.1016/j.yebeh.2012.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espósito MS, Piatti VC, Laplagne DA, Morgenstern NA, Ferrari CC, Pitossi FJ, Schinder AF. Neuronal differentiation in the adult hippocampus recapitulates embryonic development. J Neurosci. 2005;25:10074–10086. doi: 10.1523/JNEUROSCI.3114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferland RJ, Gross RA, Applegate CD. Increased mitotic activity in the dentate gyrus of the hippocampus of adult C57BL/6J mice exposed to the flurothyl kindling model of epileptogenesis. Neuroscience. 2002;115:669–683. doi: 10.1016/s0306-4522(02)00514-6. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Andersen DR, Botterill JJ, Sterner EY, Lussier AL, Caruncho HJ, Kalynchuk LE. The effect of amygdala kindling on hippocampal neurogenesis coincides with decreased reelin and DISC1 expression in the adult dentate gyrus. Hippocampus. 2010;20:659–671. doi: 10.1002/hipo.20653. [DOI] [PubMed] [Google Scholar]
- Fournier NM, Botterill JJ, Marks WN, Guskjolen AJ, Kalynchuk LE. Impaired recruitment of seizure-generated neurons into functional memory networks of the adult dentate gyrus following long-term amygdala kindling. Exp Neurol. 2013;244:96–104. doi: 10.1016/j.expneurol.2012.11.031. [DOI] [PubMed] [Google Scholar]
- Fromm GH. Effects of different classes of antiepileptic drugs on brain-stem pathways. Fed Proc. 1985;44:2432–2435. [PubMed] [Google Scholar]
- Gale K. Subcortical structures and pathways involved in convulsive seizure generation. J Clin Neurophysiol. 1992;9:264–277. doi: 10.1097/00004691-199204010-00007. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, ming GL, Song H. A critical period for enhanced synaptic plasticity in newly generated neurons of the adult brain. Neuron. 2007;54:559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WP, Sundstrom LE. Kainic acid increases the proliferation of granule cell progenitors in the dentate gyrus of the adult rat. Brain Res. 1998;790:52–59. doi: 10.1016/s0006-8993(98)00030-4. [DOI] [PubMed] [Google Scholar]
- Hamid H, Kanner AM. Should antidepressant drugs of the selective serotonin reuptake inhibitor family be tested as antiepileptic drugs? Epilepsy Behav. 2013;26:261–265. doi: 10.1016/j.yebeh.2012.10.009. [DOI] [PubMed] [Google Scholar]
- Hamilton LK, Joppé SEM, Cochard L, Fernandes KJ. Aging and neurogenesis in the adult forebrain: what we have learned and where we should go from here. Eur J Neurosci. 2013;37:1978–1986. doi: 10.1111/ejn.12207. [DOI] [PubMed] [Google Scholar]
- Hattiangady B, Rao MS, Shetty AK. Chronic temporal lobe epilepsy is associated with severely declined dentate neurogenesis in the adult hippocampus. Neurobiol Dis. 2004;17:473–490. doi: 10.1016/j.nbd.2004.08.008. [DOI] [PubMed] [Google Scholar]
- Hauser WA, Beghi E. First seizure definitions and worldwide incidence and mortality. Epilepsia. 2008;49(Suppl 1):8–12. doi: 10.1111/j.1528-1167.2008.01443.x. [DOI] [PubMed] [Google Scholar]
- Heinemann U, Beck H, Dreier JP, Ficker E, Stabel J, Zhang CL. The dentate gyrus as a regulated gate for the propagation of epileptiform activity. Epilepsy Res. 1992;(Suppl 7):273–280. [PubMed] [Google Scholar]
- Herman ST. Single Unprovoked Seizures. Curr Treat Options Neurol. 2004;6:243–255. doi: 10.1007/s11940-004-0016-5. [DOI] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Accumulation of abnormal adult-generated hippocampal granule cells predicts seizure frequency and severity. J Neurosci. 2013;33:8926–8936. doi: 10.1523/JNEUROSCI.5161-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hester MS, Danzer SC. Hippocampal granule cell pathology in epilepsy - A possible structural basis for comorbidities of epilepsy? Epilepsy Behav. 2014 doi: 10.1016/j.yebeh.2013.12.022. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillbom M, Pieninkeroinen I, Leone M. Seizures in alcohol-dependent patients: epidemiology, pathophysiology and management. CNS Drugs. 2003;17:1013–1030. doi: 10.2165/00023210-200317140-00002. [DOI] [PubMed] [Google Scholar]
- Hsu D. The dentate gyrus as a filter or gate: A look back and a look ahead. Prog Brain Res. 2007;163:601–613. doi: 10.1016/S0079-6123(07)63032-5. [DOI] [PubMed] [Google Scholar]
- Hung YW, Yang DI, Huang PY, Lee TS, Kuo TB, Yiu CH, Shih YH, Lin YY. The duration of sustained convulsive seizures determines the pattern of hippocampal neurogenesis and the development of spontaneous epilepsy in rats. Epilepsy Res. 2011;98:206–215. doi: 10.1016/j.eplepsyres.2011.09.015. [DOI] [PubMed] [Google Scholar]
- Ikrar T, Guo N, He K, Besnard A, Levinson S, Hill A, Lee HK, Hen R, Xu X, Sahay A. Adult neurogenesis modifies excitability of the dentate gyrus. Front Neural Circuits. 2013;7:204–235. doi: 10.3389/fncir.2013.00204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imura T, Kornblum HI, Sofroniew MV. The predominant neural stem cell isolated from postnatal and adult forebrain but not early embryonic forebrain expresses GFAP. J Neurosci. 2003;23:2824–2832. doi: 10.1523/JNEUROSCI.23-07-02824.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakubs K, Nanobashvili A, Bonde S, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Environment matters: synaptic properties of neurons born in the epileptic adult brain develop to reduce excitability. Neuron. 2006;52:1047–1059. doi: 10.1016/j.neuron.2006.11.004. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Römer B, Babu H, Kempermann G. Seizures induce proliferation and dispersion of doublecortin-positive hippocampal progenitor cells. Exp Neurol. 2005;196:342–351. doi: 10.1016/j.expneurol.2005.08.010. [DOI] [PubMed] [Google Scholar]
- Jessberger S, Zhao C, Toni N, Clemenson GD, Jr, Li Y, Gage FH. Seizure-associated, aberrant neurogenesis in adult rats characterized with retrovirus-mediated cell labeling. J Neurosci. 2007;27:9400–9407. doi: 10.1523/JNEUROSCI.2002-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Nadler JV. Stereological analysis of GluR2-immunoreactive hilar neurons in the pilocarpine model of temporal lobe epilepsy: correlation of cell loss with mossy fiber sprouting. Exp Neurol. 2007;205:569–582. doi: 10.1016/j.expneurol.2007.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung KH, Chu K, Kim M, Jeong SW, Song YM, Lee ST, Kim JY, Lee SK, Roh JK. Continuous cytosine-b-d-arabinofuranoside infusion reduces ectopic granule cells in adult rat hippocampus with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Eur J Neurosci. 2004;19:3219–3226. doi: 10.1111/j.0953-816X.2004.03412.x. [DOI] [PubMed] [Google Scholar]
- Jung KH, Chu K, Lee ST, Kim J, Sinn DI, Kim JM, Park DK, Lee JJ, Kim SU, Kim M, Lee SK, Roh JK. Cyclooxygenase-2 inhibitor, celecoxib, inhibits the altered hippocampal neurogenesis with attenuation of spontaneous recurrent seizures following pilocarpine-induced status epilepticus. Neurobiol Dis. 2006;2:237–246. doi: 10.1016/j.nbd.2006.02.016. [DOI] [PubMed] [Google Scholar]
- Kanner AM. Mood disorder and epilepsy: A neurobiologic perspective of their relationship. Dialogues Clin Neurosci. 2008;10:39–45. doi: 10.31887/DCNS.2008.10.1/amkanner. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanner AM, Hesdorffer DC. Neuropsychiatric complications of epilepsy. Handb Clin Neurol. 2012;107:461–482. doi: 10.1016/B978-0-444-52898-8.00037-9. [DOI] [PubMed] [Google Scholar]
- Kempermann G, Kuhn HG, Gage FH. Genetic influence on neurogenesis in the dentate gyrus of adult mice. Proc Natl Acad Sci U S A. 1997;94:10409–10414. doi: 10.1073/pnas.94.19.10409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klempin F, Kempermann G. Adult hippocampal neurogenesis and aging. Eur Arch Psychiatry Clin Neurosci. 2007;257:271–280. doi: 10.1007/s00406-007-0731-5. [DOI] [PubMed] [Google Scholar]
- Koyama R, Tao K, Sasaki T, Ichikawa J, Miyamoto D, Muramatsu R, Matsuki N, Ikegaya Y. GABAergic excitation after febrile seizures induces ectopic granule cells and adult epilepsy. Nat Med. 2012;18:1271–1278. doi: 10.1038/nm.2850. [DOI] [PubMed] [Google Scholar]
- Kralic JE, Ledergerber DA, Fritschy JM. Disruption of the neurogenic potential of the dentate gyrus in a mouse model of temporal lobe epilepsy with focal seizures. Eur J Neurosci. 2005;22:1916–1927. doi: 10.1111/j.1460-9568.2005.04386.x. [DOI] [PubMed] [Google Scholar]
- Lacefield CO, Itskov V, Reardon T, Hen R, Gordon JA. Effects of adult-generated granule cells on coordinated network activity in the dentate gyrus. Hippocampus. 2012;22:106–116. doi: 10.1002/hipo.20860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalancette-Hebert M, Gowing G, Simard A, Weng YC, Kriz J. Selective ablation of proliferating microglial cells exacerbates ischemic injury in the brain. J Neurosci. 2007;27:2596–2605. doi: 10.1523/JNEUROSCI.5360-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laufs H. Functional imaging of seizures and epilepsy: Evolution from zones to networks. Curr Opin Neurol. 2012;25:194–200. doi: 10.1097/WCO.0b013e3283515db9. [DOI] [PubMed] [Google Scholar]
- Laurén HB, Ruohonen S, Kukko-Lukjanov TK, Virta JE, Grönman M, Lopez-Picon FR, Järvelä JT, Holopainen IE. Status epilepticus alters neurogenesis and decreases the number of GABAergic neurons in the septal dentate gyrus of 9-day-old rats at the early phase of epileptogenesis. Brain Res. 2013;1516:33–44. doi: 10.1016/j.brainres.2013.04.028. [DOI] [PubMed] [Google Scholar]
- Ledergerber D, Fritschy JM, Kralic JE. Impairment of dentate gyrus neuronal progenitor cell differentiation in a mouse model of temporal lobe epilepsy. Exp Neurol. 2006;199:130–142. doi: 10.1016/j.expneurol.2006.02.010. [DOI] [PubMed] [Google Scholar]
- Lee SW, Clemenson GD, Gage FH. New neurons in an aged brain. Behav Brain Res. 2012;227:497–507. doi: 10.1016/j.bbr.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemmens EM, Schijns OE, Beuls EA, Hoogland G. Cytogenesis in the dentate gyrus after neonatal hyperthermia-induced seizures: what becomes of surviving cells? Epilepsia. 2008;49:853–860. doi: 10.1111/j.1528-1167.2007.01476.x. [DOI] [PubMed] [Google Scholar]
- Liu YB, Lio PA, Pasternak JF, Trommer BL. Developmental changes in membrane properties and postsynaptic currents of granule cells in rat dentate gyrus. J Neurophysiol. 1996;76:1074–1088. doi: 10.1152/jn.1996.76.2.1074. [DOI] [PubMed] [Google Scholar]
- Löscher W, Ebert U. The role of the piriform cortex in kindling. Prog Neurobiol. 1996;50:427–481. doi: 10.1016/s0301-0082(96)00036-6. [DOI] [PubMed] [Google Scholar]
- Lothman EW, Stringer JL, Bertram EH. The dentate gyrus as a control point for seizures in the hippocampus and beyond. Epilepsy Res. 1992;(Suppl 7):301–313. [PubMed] [Google Scholar]
- Mares P, Pohl M, Kubova H, Zelizko M. Is the site of action of ethosuximide in the hindbrain? Physiol Res. 1994;43:51–56. [PubMed] [Google Scholar]
- Margerison JH, Corsellis JA. Epilepsy and the temporal lobes. A clinical, electroencephalographic and neuropathological study of the brain in epilepsy, with particular reference to the temporal lobes. Brain. 1996;89:499–530. doi: 10.1093/brain/89.3.499. [DOI] [PubMed] [Google Scholar]
- Marin-Burgin A, Mongiat LA, Pardi MB, Schinder AF. Unique processing during a period of high excitation/inhibition balance in adult-born neurons. Science. 2012;335:1238–1242. doi: 10.1126/science.1214956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwardt S, Overstreet-Wadiche L. GABAergic signalling to adult-generated neurons. J Physiol. 2008;586:3745–3749. doi: 10.1113/jphysiol.2008.155713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masukawa LM, Burdette LJ, McGonigle P, Wang H, O'Connor W, Sperling MR, O'Connor MJ, Uruno K. Physiological and anatomical correlates of the human dentate gyrus: consequences or causes of epilepsy. Adv Neurol. 1999;79:781–794. [PubMed] [Google Scholar]
- McIntyre DC, Gilby KL. Mapping seizure pathways in the temporal lobe. Epilepsia. 2008;49(Suppl 3):23–30. doi: 10.1111/j.1528-1167.2008.01507.x. [DOI] [PubMed] [Google Scholar]
- McKhann GM, Wenzel HJ, Robbins CA, Sosunov AA, Schwartzkroin PA. Mouse strain differences in kainic acid sensitivity, seizure behavior, mortality, and hippocampal pathology. Neuroscience. 2003;122:551–561. doi: 10.1016/s0306-4522(03)00562-1. [DOI] [PubMed] [Google Scholar]
- McCloskey DP, Hintz TM, Pierce JP, Scharfman HE. Stereological methods reveal the robust size and stability of ectopic hilar granule cells after pilocarpine-induced status epilepticus in the adult rat. Eur J Neurosci. 2006;24:2203–2210. doi: 10.1111/j.1460-9568.2006.05101.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohapel P, Ekdahl CT, Lindvall O. Status epilepticus severity influences the long-term outcome of neurogenesis in the adult dentate gyrus. Neurobiol Dis. 2004;15:196–205. doi: 10.1016/j.nbd.2003.11.010. [DOI] [PubMed] [Google Scholar]
- Murphy BL, Hofacer RD, Faulkner CN, Loepke AW, Danzer SC. Abnormalities of granule cell dendritic structure are a prominent feature of the intrahippocampal kainic acid model of epilepsy despite reduced postinjury neurogenesis. Epilepsia. 2012;53:908–921. doi: 10.1111/j.1528-1167.2012.03463.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakagawa E, Aimi Y, Yasuhara O, Tooyama I, Shimada M, McGeer PL, Kimura H. Enhancement of progenitor cell division in the dentate gyrus triggered by initial limbic seizures in rat models of epilepsy. Epilepsia. 2000;41:10–18. doi: 10.1111/j.1528-1157.2000.tb01498.x. [DOI] [PubMed] [Google Scholar]
- Nearing K, Madhavan D, Devinsky O. Temporal lobe epilepsy: A progressive disorder? Rev Neurol Dis. 2007;4:122–127. [PubMed] [Google Scholar]
- Nixon K, Morris SA, Liput DJ, Kelso ML. Roles of neural stem cells and adult neurogenesis in adolescent alcohol use disorders. Alcohol. 2010;44:39–56. doi: 10.1016/j.alcohol.2009.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Yu TW, Leibowitz RT, Geschwind DH, Sloviter RS, Lowenstein DH. Dentate granule cell neurogenesis is increased by seizures and contributes to aberrant network reorganization in the adult rat hippocampus. J Neurosci. 1997;17:3727–3738. doi: 10.1523/JNEUROSCI.17-10-03727.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parent JM, Janumpalli S, McNamara JO, Lowenstein DH. Increased dentate granule cell neurogenesis following amygdala kindling in the adult rat. Neurosci Lett. 1998;247:9–12. doi: 10.1016/s0304-3940(98)00269-9. [DOI] [PubMed] [Google Scholar]
- Parent JM, Murphy GG. Mechanisms and functional significance of aberrant seizure-induced hippocampal neurogenesis. Epilepsia. 2008;49(Suppl 5):19–25. doi: 10.1111/j.1528-1167.2008.01634.x. [DOI] [PubMed] [Google Scholar]
- Pekcec A, Fuest C, Mühlenhoff M, Gerardy-Schahn R, Potschka H. Targeting epileptogenesis-associated induction of neurogenesis by enzymatic depolysialylation of NCAM counteracts spatial learning dysfunction but fails to impact epilepsy development. J Neurochem. 2008;105:389–400. doi: 10.1111/j.1471-4159.2007.05172.x. [DOI] [PubMed] [Google Scholar]
- Pekcec A1, Lüpke M, Baumann R, Seifert H, Potschka H. Modulation of neurogenesis by targeted hippocampal irradiation fails to affect kindling progression. Hippocampus. 2011;8:866–876. doi: 10.1002/hipo.20802. [DOI] [PubMed] [Google Scholar]
- Pierce JP, McCloskey DP, Scharfman HE. Morphometry of hilar ectopic granule cells in the rat. J Comp Neurol. 2011;519:1196–1218. doi: 10.1002/cne.22568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter BE. Neurogenesis and epilepsy in the developing brain. Epilepsia. 2008;5:50–54. doi: 10.1111/j.1528-1167.2008.01637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pun RY, Rolle IJ, Lasarge CL, Hosford BE, Rosen JM, Uhl JD, Schmeltzer SN, Faulkner C, Bronson SL, Murphy BL, Richards DA, Holland KD, Danzer SC. Excessive activation of mTOR in postnatally generated granule cells is sufficient to cause epilepsy. Neuron. 2012;6:1022–1034. doi: 10.1016/j.neuron.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racine RJ. Modification of seizure activity by electrical stimulation. II. Motor seizure. Electroencephalogr Clin Neurophysiol. 1972;32:281–294. doi: 10.1016/0013-4694(72)90177-0. [DOI] [PubMed] [Google Scholar]
- Raedt R, Boon P, Persson A, Alborn AM, Boterberg T, Van Dycke A, Linder B, De Smedt T, Wadman WJ, Ben-Menachem E, Eriksson PS. Radiation of the rat brain suppresses seizure-induced neurogenesis and transiently enhances excitability during kindling acquisition. Epilepsia. 2007;10:1952–1963. doi: 10.1111/j.1528-1167.2007.01146.x. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Tran PH, Spigelman I, Okazaki MM, Nadler JV. Status epilepticus-induced hilar basal dendrites on rodent granule cells contribute to recurrent excitatory circuitry. J Comp Neurol. 2000;428:240–253. doi: 10.1002/1096-9861(20001211)428:2<240::aid-cne4>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Ribak CE, Shapiro LA, Yan XX, Dashtipour K, Nadler JV, Obenaus A, Spigelman I, Buckmaster PS. Seizure-induced formation of basal dendrites on granule cells of the rodent dentate gyrus. In: Noebels JL, Avoli M, Rogawski MA, Olsen RW, Delgado-Escueta AV, editors. Jasper's basic mechanisms of the epilepsies. 4th. Bethesda MD: 2012. [PubMed] [Google Scholar]
- Rogawski MA. Update on the neurobiology of alcohol withdrawal seizures. Epilepsy Curr. 2005;5:225–30. doi: 10.1111/j.1535-7511.2005.00071.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Drew MR, Hen R. Dentate gyrus neurogenesis and depression. Prog Brain Res. 2007;163:697–722. doi: 10.1016/S0079-6123(07)63038-6. [DOI] [PubMed] [Google Scholar]
- Sahay A, Scobie KN, Hill AS, O'Carroll CM, Kheirbek MA, Burghardt NS, Fenton AA, Dranovsky A, Hen R. Increasing adult hippocampal neurogenesis is sufficient to improve pattern separation. Nature. 2011a;472:466–470. doi: 10.1038/nature09817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahay A, Wilson DA, Hen R. Pattern separation: A common function for new neurons in hippocampus and olfactory bulb. Neuron. 2011b;70:582–528. doi: 10.1016/j.neuron.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez RM, Ribak CE, Shapiro LA. Synaptic connections of hilar basal dendrites of dentate granule cells in a neonatal hypoxia model of epilepsy. Epilepsia. 2012;53(Suppl 1):98–108. doi: 10.1111/j.1528-1167.2012.03481.x. [DOI] [PubMed] [Google Scholar]
- Santarelli L, Saxe M, Gross C, Surget A, Battaglia F, Dulawa S, Weisstaub N, Lee J, Duman R, Arancio O, Belzung C, Hen R. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science. 2003;301:805–809. doi: 10.1126/science.1083328. [DOI] [PubMed] [Google Scholar]
- Santhakumar V, Ratzliff AD, Jeng J, Toth Z, Soltesz I. Long-term hyperexcitability in the hippocampus after experimental head trauma. Ann Neurol. 2001;50:708–717. doi: 10.1002/ana.1230. [DOI] [PubMed] [Google Scholar]
- Saxe MD, Battaglia F, Wang JW, Malleret G, David DJ, Monckton JE, Garcia AD, Sofroniew MV, Kandel ER, Santarelli L, Hen R, Drew MR. Ablation of hippocampal neurogenesis impairs contextual fear conditioning and synaptic plasticity in the dentate gyrus. Proc Natl Acad Sci U S A. 2006;103:17501–17506. doi: 10.1073/pnas.0607207103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Goodman JH, Sollas AL. Granule-like neurons at the hilar/CA3 border after status epilepticus and their synchrony with area CA3 pyramidal cells: Functional implications of seizure-induced neurogenesis. J Neurosci. 2000;20:6144–6158. doi: 10.1523/JNEUROSCI.20-16-06144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Sollas AL, Smith KL, Goodman JH, Jackson MB. Structural and functional asymmetry in the normal and epileptic rat dentate gyrus. J Comp Neurol. 2002;454:424–439. doi: 10.1002/cne.10449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE. Functional implications of seizure-induced neurogenesis. Adv Exp Med Biol. 2004;548:192–212. doi: 10.1007/978-1-4757-6376-8_14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scharfman HE, Pierce JP. New insights into the role of hilar ectopic granule cells in the dentate gyrus based on quantitative anatomic analysis and three-dimensional reconstruction. Epilepsia. 2012;53(Suppl 1):109–115. doi: 10.1111/j.1528-1167.2012.03480.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schawecker PS. The relevance of individual genetic background and its role in animal models of epilepsy. Epilepsy Res. 2011;97:1–11. doi: 10.1016/j.eplepsyres.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schloesser RJ, Manji HK, Martinowich K. Suppression of adult neurogenesis leads to an increased hypothalamo-pituitary-adrenal axis response. Neuroreport. 2009;20:553–557. doi: 10.1097/WNR.0b013e3283293e59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429:184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Scott BW, Wang S, Burnham WM, De Boni U, Wojtowicz JM. Kindling-induced neurogenesis in the dentate gyrus of the rat. Neurosci Lett. 1998;248:73–76. doi: 10.1016/s0304-3940(98)00355-3. [DOI] [PubMed] [Google Scholar]
- Seki T, Sawamoto K, Parent JM, Alvarez-Buylla A. I. Neurobiology. Tokyo: Springer; 2011. Neurogenesis in the adult brain. [Google Scholar]
- Shapiro LA, Korn MJ, Ribak CE. Newly generated dentate granule cells from epileptic rats exhibit elongated hilar basal dendrites that align along GFAP-immunolabeled processes. Neuroscience. 2005;136:823–31. doi: 10.1016/j.neuroscience.2005.03.059. [DOI] [PubMed] [Google Scholar]
- Shapiro LA, Ribak CE, Jessberger S. Structural changes for adult-born dentate granule cells after status epilepticus. Epilepsia. 2008;49(Suppl 5):13–18. doi: 10.1111/j.1528-1167.2008.01633.x. [DOI] [PubMed] [Google Scholar]
- Singer BH, Gamelli AE, Fuller CL, Temme SJ, Parent JM, Murphy GG. Compensatory network changes in the dentate gyrus restore long-term potentiation following ablation of neurogenesis in young-adult mice. Proc Natl Acad Sci U S A. 2011;108:5437–5442. doi: 10.1073/pnas.1015425108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloviter RS. The functional organization of the hippocampal dentate gyrus and its relevance to the pathogenesis of temporal lobe epilepsy. Ann Neurol. 1994;35:640–654. doi: 10.1002/ana.410350604. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV, Bush TG, Blumauer N, Lawrence K, Mucke L, Johnson MH. Genetically-targeted and conditionally-regulated ablation of astroglial cells in the central, enteric and peripheral nervous systems in adult transgenic mice. Brain Res. 1999;835:91–95. doi: 10.1016/s0006-8993(99)01639-x. [DOI] [PubMed] [Google Scholar]
- Song J, Christian KM, ming GL, Song H. Modification of hippocampal circuitry by adult neurogenesis. Dev Neurobiol. 2012;72:1032–1043. doi: 10.1002/dneu.22014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staley KJ, Dudek FE. Interictal spikes and epileptogenesis. Epilepsy Curr. 2006;6:199–202. doi: 10.1111/j.1535-7511.2006.00145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talley EM, Cribbs LL, Lee JH, Daud A, Perez-Reyes E, Bayliss DA. Differential distribution of three members of a gene family encoding low voltage-activated (T-type) calcium channels. J Neurosci. 1999;19:1895–1911. doi: 10.1523/JNEUROSCI.19-06-01895.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thind KK, Ribak CE, Buckmaster PS. Synaptic input to dentate granule cell basal dendrites in a rat model of temporal lobe epilepsy. J Comp Neurol. 2008;509:190–202. doi: 10.1002/cne.21745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thome-Souza MS, Kuczynski E, Valente KD. Sertraline and fluoxetine: Safe treatments for children and adolescents with epilepsy and depression. Epilepsy Behav. 2007;10:417–425. doi: 10.1016/j.yebeh.2007.01.004. [DOI] [PubMed] [Google Scholar]
- Toni N, Laplagne DA, Zhao C, Lombardi G, Ribak CE, Gage FH, Schinder AF. Neurons born in the adult dentate gyrus form functional synapses with target cells. Nat Neurosci. 2008;11:901–907. doi: 10.1038/nn.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams PA, White AM, Clark S, Ferraro DJ, Swiercz W, Staley KJ, Dudek FE. Development of spontaneous recurrent seizures after kainate-induced status epilepticus. J Neurosci. 2009;29:2103–2012. doi: 10.1523/JNEUROSCI.0980-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood JC, Jackson JS, Jakubs K, Chapman KZ, Ekdahl CT, Kokaia Z, Kokaia M, Lindvall O. Functional integration of new hippocampal neurons following insults to the adult brain is determined by characteristics of pathological environment. Exp Neurol. 2011;2:484–493. doi: 10.1016/j.expneurol.2011.03.019. [DOI] [PubMed] [Google Scholar]
- Yang F, Wang JC, Han JL, Zhao G, Jiang W. Different effects of mild and severe seizures on hippocampal neurogenesis in adult rats. Hippocampus. 2008;18(5):460–468. doi: 10.1002/hipo.20409. [DOI] [PubMed] [Google Scholar]
- Zhang W, Huguenard JR, Buckmaster PS. Increased excitatory synaptic input to granule cells from hilar and CA3 regions in a rat model of temporal lobe epilepsy. J Neurosci. 2012;32:1183–1196. doi: 10.1523/JNEUROSCI.5342-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Nadler JV. Enhanced tonic GABA current in normotopic and hilar ectopic dentate granule cells after pilocarpine-induced status epilepticus. J Neurophysiol. 2009;102:670–681. doi: 10.1152/jn.00147.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan RZ, Timofeeva O, Nadler JV. High ratio of synaptic excitation to synaptic inhibition in hilar ectopic granule cells of pilocarpine-treated rats. J Neurophysiol. 2010;104:3293–3304. doi: 10.1152/jn.00663.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zattoni M, Mura ML, Deprez F, Schwendener RA, Engelhardt B, Frei K, Fritschy JM. Brain infiltration of leukocytes contributes to the pathophysiology of temporal lobe epilepsy. J Neurosci. 2011;31:4037–4050. doi: 10.1523/JNEUROSCI.6210-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.