Abstract
Background
Severe primary graft dysfunction (PGD) is a major cause of early morbidity and mortality in patients after lung transplantation. The etiology and pathophysiology of PGD is not fully characterized and whether intraoperative fluid administration increases the risk for PGD remains unclear from previous studies. We therefore tested the hypothesis that increased total intraoperative fluid volume during lung transplantation is associated with the development of grade-3 PGD.
Methods
This retrospective cohort analysis included patients who had lung transplantation at the Cleveland Clinic between January 2009 and June 2013. We used multivariable logistical regression with adjustment for donor, recipient, and perioperative confounding factors to examine the association between total intraoperative fluid administration and development of grade-3 PGD in the initial 72 postoperative hours. Secondary outcomes included time to initial extubation and intensive care unit length of stay.
Results
Grade-3 PGD occurred in 123 of 494 patients (25%) who had lung transplantation. Patients with grade-3 PGD received a larger volume of intraoperative fluid (median 5.0 [3.8, 7.5] L) than those without grade-3 PGD (3.9 [2.8, 5.2] L). Each intraoperative liter of fluid increased the odds of grade-3 PGD by approximately 22% (adjusted odds ratio [95%CI] of 1.22 (1.12,1.34), P <0.001). The volume of transfused red blood cell concentrate was associated with grade-3 PGD (1.1 [0.0, 1.8] L for PGD-3 versus 0.4 [0.0, 1.1 for non-grade-3 PGD] L; adjusted OR CI 95% 1.7 (1.08, 2.7); P = 0.002). Increased fluid administration was associated with longer intensive care unit stay (adjusted hazard ratio (97.5% CI): 0.92 [0.88, 0.97], P < 0.001), but not time to initial tracheal extubation (hazard ratio [97.5% CI]: 0.97[0.93,1.02], P =0.17).
Conclusions
Increased intraoperative fluid volume is associated with the most severe form of PGD after lung transplant surgery. Limiting fluid administration may reduce the risk for development of grade-3 PGD and thus improve early postoperative morbidity and mortality after lung transplantation.
Introduction
Lung transplantation is a complex, life-saving treatment for patients with end-stage pulmonary disease. Although early survival has improved in the past decade, primary graft dysfunction (PGD), a form of acute lung injury that develops in the initial 72 hours after surgery, remains an important cause of early postoperative morbidity and mortality.1 PGD is categorized into grades of severity by the International Society of Heart and Lung Transplantation consensus definitions.2,3 Grade-3 PGD, the most severe form, occurs in up to 25% of patients and is associated with prolonged mechanical ventilation, early mortality of 16–36%,4,5 and development of chronic lung allograft dysfunction, a serious complication which worsens the long-term survival after lung transplantation.6
Because a severe form of PGD is associated with substantial mortality and morbidity, identifying risk factors for its development may provide opportunities to improve outcomes of patients having lung transplantation. Donor risk factors for PGD include advanced age, smoking history and prolonged lung ischemic time.7,8,9,10 Variables associated with the recipient, such as the underlying transplant diagnosis and pulmonary hypertension, also increase the risk for PGD.11,12,13 Most of these factors, however, are not amenable to modification.
Few studies focus on the intraoperative anesthetic management of patients undergoing lung transplantation and particularly on the relationship between intraoperative fluid management and subsequent development of PGD. Several exploratory analyses suggest that elevated intraoperative cardiac filling pressures and large volumes of intraoperative colloid solutions are associated with hypoxemia and prolonged mechanical ventilation,14,15 but these studies are limited by small sample size, potential residual confounding, and varying definitions of PGD. Further, they combined all grades of PGD into a single category resulting in significant heterogeneity in severity of illness and associated mortality, thus limiting the ability to identify important risk factors. Although published guidelines for lung transplantation recommend restrictive perioperative fluid administration,16,17 conclusive evidence supporting this recommendation is lacking.
Our objective was thus to examine the association between the total volume and type of fluids given during lung transplant surgery and development of grade-3 PGD. Specifically, we tested the primary hypothesis that total intraoperative fluid volume is positively associated with development of grade-3 PGD after lung transplantation. We also tested our secondary hypothesis that individual components of fluid therapy have a positive association with the development of grade-3 PGD.
Methods
The study was approved by the IRB who waived the requirement for written informed patient consent. We examined records of patients who had lung transplantation at the Cleveland Clinic between January 2009 and June 2013. Prospective evaluation for PGD was recorded in 98% of patients having lung transplantation after 2009. Patient data were obtained from the Electronic Data Interface of Transplantation at the Cleveland Clinic. Postoperatively, patients were managed by a dedicated team of lung transplant pulmonologists who evaluated graft function to determine the presence and severity of PGD in the first 72 hours after surgery. The assessment of the severity of PGD was performed at 0, 24, 48 and 72 hours after transplantation and the highest grade of PGD during the initial 72 hours after lung reperfusion was recorded. Intraoperative data were retrieved from the Cleveland Clinic Perioperative Health Documentation System, which contains the electronic anesthesia record. Preoperative diagnosis, indication for transplant, and the cause of end-stage lung disease were determined from International Classification of Disease 9 (ICD-9) codes.
We included adults who had single or bilateral lung transplants as a primary or reoperative procedure, performed with or without the use of cardiopulmonary bypass (CPB). Lung transplantation with concomitant cardiac operation (e.g., coronary artery bypass) was also included. Donors were either brain dead or donation after circulatory death. Patients having multiorgan transplants were excluded.
Intraoperative monitoring of the lung transplant recipient included standard ASA monitors supplemented with arterial and pulmonary artery catheters. A transesophageal echocardiogram was used to assess the volume status and myocardial function. The decision to use CPB was made on an individual basis depending on the recipient’s apparent cardiopulmonary reserve. Donor assessment, lung procurement, and preservation followed standard procedures as previously described.18,19
Intraoperative fluid management consisted of crystalloid (normal saline or lactate Ringer’s), colloid solution (5% albumin), salvaged and washed red blood cells, and/or allogeneic blood products. The decision to transfuse blood products was made jointly by the attending surgeon and anesthesiologist. The target hemoglobin typically was ≥ 8 g/dL and blood component therapy was given in the setting of persistent microvascular bleeding. Hemodynamic targets included serum lactate levels, mixed venous oxygen saturation and respiratory systolic pressure variability. Options for pharmacologic hemodynamic support included vasoconstrictor therapy with phenylephrine, norepinephrine and vasopressin. For inotropic support we used epinephrine and milrinone to target a cardiac index above 2.0L/min/m2. Inhaled epoprostenol or nitric oxide was used as necessary for profound hypoxemia or right ventricular dysfunction due to pulmonary hypertension.
We recorded the total ischemia time, which included the time from the donor’s cardiac arrest until harvest (warm ischemia time 1), graft preservation (cold ischemia time) and placement of the graft in the recipient until reperfusion of the graft (warm ischemia time 2).20 We designated reperfusion of the second lung as the overall reperfusion time for bilateral lungs. We captured central venous pressure (CVP), pulmonary artery pressure, and cardiac index after pulmonary artery clamp release. Initial mechanical ventilation of the transplanted lung consisted of pressure-controlled ventilation to target a tidal volume of 6 ml/kg ideal body weight, inspired oxygen 30–50% as tolerated, and positive end-expiratory pressure 10 cmH2O. Patients were weaned from mechanical ventilation as soon as possible postoperatively with the use of a standardized postoperative algorithm for weaning and tracheal extubation.
Study Outcomes
Our primary outcome was grade-3 PGD diagnosed prospectively by lung transplant pulmonologists during the initial 72 post-transplant hours using the consensus International Society of Heart and Lung Transplant refined definitions (Table 1).21 Grade-3 PGD is defined by arterial oxygen partial pressure/fraction of inspired oxygen (PaO2/FiO2) ratio < 200 mmHg and bilateral infiltrates on chest radiograph. Grade-3 PGD was also defined by the use of extracorporeal life support within the initial 72 postoperative hours or use of inhaled pulmonary vasodilators for more than 48 hours during mechanical ventilation. The reference comparison group included patients with grade-0 (no PGD), grade-1 or grade-2 PGD. The secondary outcomes included time to initial intensive care unit (ICU) tracheal extubation (days) and length of ICU stay (days).
Table 1.
International Society for Heart and Lung Transplantation Primary Graft Dysfunction Definition and Grading.
| Grade | PaO2/FiO2 | Radiographic Infiltrates |
|---|---|---|
| 0 | > 300 | Absent |
| 1 | > 300 | Present |
| 2 | 200–300 | Present |
| 3 | < 200 | Present |
- Presence of extracorporeal life support post lung transplantation is considered grade-3
- FiO2 > 50% and use of inhaled nitric oxide or inhaled epoprostenol while on mechanical ventilation > 48 hours is considered grade-3.
- Any patient who is extubated on nasal cannula with FiO2 < 30% are graded as 0 or 1 depending on chest radiograph findings.
- Absence of infiltrates on chest radiograph is graded as 0 even if PaO2/FiO2 ratio < 300
Statistical analysis
Statistical analysis was performed with SAS 9.3 statistical software. We used a multivariable logistic regression model to assess the association between the primary outcome grade-3 PGD and the total volume of intraoperative fluids via a backward variable selection procedure (with stay criterion P < 0.15), starting with all variables from the univariable analysis which had a P value < 0.25 (Table 2). We further assessed the association between grade-3 PGD and the volume of each type of fluid given including crystalloid and colloid solutions as well as packed red blood cells, fresh frozen plasma, platelet concentrate and cryoprecipitate transfusions. We considered nonlinear effects of total intraoperative fluid volume on the primary outcome using a restricted cubic spline of fluids in the multivariable logistic regression. The Pearson correlation coefficient and variance inflation factor were performed for multi-collinearity diagnostics among predictors, and the logistic model fit was checked by Hosmer and Lemeshow’s goodness-of-fit test.
Table 2.
Univariable logistic regression analysis with baseline and perioperative characteristics of the lung transplant cohort (N = 494)
| Characteristics | PGD Grade ≥ 3 (N = 123) | PGD Grade 0–2 (N = 371) | OR (95% CI)* | P value* |
|---|---|---|---|---|
| Donor | ||||
| Age (years) | 40±15 | 38±16 | 1.09 (0.95, 1.24) a | 0.22 |
| History of smoking, N % | 15 (12) | 58 (16) | 0.75 (0.41, 1.38) | 0.35 |
| Donation after cardiac death, N % | 7 (6) | 21 (6) | 1.01 (0.42, 2.43) | 0.99 |
| Cause of brain death of the donor, N % | 0.49 | |||
| Anoxia | 13 (11) | 48 (13) | 0.79 (0.31, 2.02) | |
| Cerebrovascular accident | 56 (46) | 140 (38) | 1.16 (0.53, 2.54) | |
| Trauma | 44 (36) | 154 (42) | 0.83 (0.37, 1.83) | |
| Other | 10 (8) | 29 (8) | Ref = 1 | |
| Ratio of PaO2/FiO2 | 422 [356, 478] | 431 [373, 487] | 0.99 (0.97, 1.01) a | 0.33 |
| Recipient | ||||
| Female, N % | 47 (38) | 125 (34) | 1.22 (0.80, 1.86) | 0.36 |
| Caucasian, N % | 107 (87) | 338 (91) | 0.65 (0.35, 1.23) | 0.19 |
| Age (years) | 56 ± 11 | 58 ± 12 | 0.88 (0.75, 1.04) a | 0.12 |
| Weight | 75 ± 15 | 76 ± 16 | 0.94 (0.83, 1.07) | 0.35 |
| Extracorporeal life support, N % | 3 (2) | 9 (2) | 1.01 (0.27, 3.77) | 0.99 |
| Mechanical ventilation, N % | 6 (5) | 14 (4) | 1.31 (0.49, 3.48) | 0.59 |
| Cause of End Stage Lung Disease, N % | ||||
| Idiopathic pulmonary hypertension | 9 (7) | 8 (2) | 3.58 (1.35, 9.50) | 0.01 |
| Chronic obstructive pulmonary disease | 23 (19) | 87 (23) | 0.75 (0.45, 1.25) | 0.27 |
| Interstitial lung disease | 60 (49) | 191 (51) | 0.90 (0.60, 1.35) | 0.60 |
| Cystic fibrosis | 9 (7) | 39 (11) | 0.67 (0.32, 1.43) | 0.30 |
| Sarcoidosis | 5 (4) | 10 (3) | 1.53 (0.51, 4.57) | 0.45 |
| Intraoperative factors | ||||
| Ischemia time (hours) | 5.9 [4.8, 6.8] | 5.1 [4.4, 5.9] | 1.44 (1.23, 1.68) | <0.001 |
| Surgical time (hours) | 7.4 ± 2.0 | 6.4 ± 1.9 | 1.32 (1.17, 1.49) | <0.001 |
| Double transplant, N % | 80 (65) | 207 (56) | 1.47 (0.97, 2.25) | 0.073 |
| Use of cardiopulmonary bypass, N % | 98 (80) | 241 (65) | 2.11 (1.30, 3.45) | 0.003 |
| Total dose of norepinephrine (mcg) | 550 [121, 1267] | 338 [83, 734] | 1.02 (0.99, 1.05) b | 0.23 |
| Total dose of epinephrine (mcg) | 149 [0, 513] | 30 [0, 382] | 1.7 (1.16, 2.49) b | 0.006 |
| Use of vasopressin , N % | 72 (59) | 167 (45) | 1.72 (1.14, 2.61) | 0.01 |
| Use of milrinone , N % | 21 (17) | 32 (9) | 2.18 (1.21, 3.95) | 0.01 |
| Average CVP postreperfusion (mmHg) | 15.2 ± 4.2 | 14.4 ± 3.9 | 1.68 (1.01, 2.79) a | 0.048 |
| Average mean PAP post-reperfusion (mmHg) | 29.7 ± 6.6 | 28.1 ± 5.9 | 1.55 (1.10, 2.18) a | 0.012 |
| Cardiac index (L/min/m2) | 2.6 ± 0.7 | 2.6 ± 0.6 | 0.50 (0.02, 13.6) a | 0.68 |
| Average PEEP post reperfusion (cmH2O) | 9.7 ± 2.6 | 9.2 ± 2.3 | 2.12 (0.90, 4.97) a | 0.085 |
| Average FiO2 postreperfusion (%) | 67 ± 20 | 60 ± 19 | 1.22 (1.10, 1.36) a | <0.001 |
| Average tidal volume/kg (ml/kg) postreperfusion | 6.4 ± 1.7 | 6.2 ± 1.9 | 1.53 (0.49, 4.74) a | 0.46 |
| Surgeon | 0.10 | |||
| A | 40 (33) | 117 (32) | Ref =1 | |
| B | 10 (8) | 42 (11) | 0.7 (0.32, 1.52) | |
| C | 21 (17) | 79 (21) | 0.78 (0.43, 1.42) | |
| D | 20 (16) | 75 (20) | 0.78 (0.42, 1.44) | |
| E | 32 (26) | 58 (16) | 1.61 (0.92, 2.83) | |
| Year of transplant | 0.82 (0.70, 0.96) | 0.014 | ||
| 2009 | 43 (35) | 97 (23) | ||
| 2010 | 28 (26) | 90 (27) | ||
| 2011 | 27 (25) | 73 (22) | ||
| 2012 | 13 (12) | 84 (25) | ||
| 2013 | 15 (14) | 44 (13) | ||
ASD - absolute standardized difference; COPD - chronic obstructive pulmonary disease; CVP - central venous pressure; FiO2 - fraction of inspired oxygen; PaO2 - partial arterial pressure of oxygen; PAP - pulmonary artery pressure; PEEP - positive end expiratory pressure; OR - odds ratio
odds ratio (95% CI) and P values from the univariable logistic regression; odds ratio for a one-unit increase in the continuous predictors unless
10-unit and
1000-unit increases
The significance level for the association between grade-3 PGD and the total volume of intraoperative fluids was P < 0.05. The significance level for each individual component of intraoperative fluids was P < 0.008 (i.e., 0.05/6, Bonferroni correction), corresponding to 99.2% confidence intervals (CI). Significance criterion for the 2 secondary outcomes was P <0.025 (Bonferroni correction, 0.05/2); thus 97.5% CIs were reported.
The association between total volume of intraoperative fluids and time to tracheal extubation and time to ICU discharge alive (length of ICU stay) was assessed with Cox proportional hazard models. Patients who died before ICU discharge were considered failures and assigned a length of stay 1 day longer than any ICU patients discharged alive. Confounding variable selection was also performed by using the univariable screen with P < 0.25 and following a backward elimination variable selection with stay criterion P < 0.15.
Power consideration
With an average historical incidence of 25% for grade-3 PGD, we estimated a sample size of 458 to detect a clinically important odds ratio of 1.2 between grade-3 PGD and total amount of fluids with 85% power, assuming a normal distribution for total amount of fluids with mean ± SD of 4.3 ± 1.9 (liters) at 0.05 significance level.
Results
Among 562 patients who were screened, 14 were excluded from the analysis because they had combined heart-lung transplantation. After reviewing the remaining 548 records, 54 patients were excluded because of a missing diagnosis of PGD (n=36) or incomplete donor information or recipient intraoperative records (n=18). Results from 494 patients were thus available for the final analysis (Figure 1). Baseline characteristics of the donor, recipient and intraoperative variables and their unadjusted OR (CI 95%) from the univariable analysis are shown in Table 2.
Figure 1.
Consolidated Standards of Reporting Trials Flow Diagram. PGD = Primary graft dysfunction.
Primary outcome
One hundred twenty-three patients (25%) developed grade-3 PGD after lung transplantation. Among these, 109 (88%) were diagnosed in the first 24 hours (defined as early PGD) and 14 (12%) developed PGD between 24–72 hours (late PGD). The 30-day mortality for grades 0–2 PGD was 2% (7/371 patients), whereas it was 10% (12/123 patients) for grade-3 PGD. Among patients with grade-3 PGD, 7 of 123 patients required extracorporeal life support in the form of extracorporeal membrane oxygenation in the first 72 hours after transplantation. Of these, 6 patients had veno-venous support and 1 patient had veno-arterial support.
The median [Q1,Q3] total volume of intraoperative IV fluid was 5.0 [3.8, 7.5] L in patients with grade-3 PGD and 3.8 [2.9, 5.2] L in patients with grade 0–2 PGD. The multivariable analysis showed a significant association between total volume of intraoperative fluids and development of grade 3 PGD, after adjusting for idiopathic pulmonary hypertension, ischemia time, double lung transplant, use of CPB, average mean pulmonary artery pressure post-reperfusion and average FiO2 post-reperfusion (Appendix 1). The odds for development of grade-3 PGD increased by approximately 22% for each additional liter of intraoperative fluid administered (adjusted OR [95%CI] 1.22 [1.12, 1.34]; P < 0.001) (Table 3). The restricted cubic spline of the total volume of intraoperative fluids in multivariable logistic models showed that the nonlinear effect on grade 3 PGD was not significant (All nonlinear effect P values > 0.05 with knots = 3, 4, and 5 in spline function, respectively); there was thus no need to include a quadratic or cubic term in the model. The Hosmer and Lemeshow's goodness-of-fit test indicated the model was correctly specified (P = 0.49).
Table 3.
Multivariable Association between Grade-3 Primary Graft Dysfunction (PGD) and total volume of intraoperative fluids and its components (N =494)
| Variables | PGD Grade ≥ 3 (N = 123) | PGD Grade 0–2 (N = 371) | Adjusted OR (95% CI)* | P value* |
|---|---|---|---|---|
| Total volume of intraoperative fluids (L) | 5.0 [3.8,7.5] | 3.9 [2.9,5.2] | 1.22 (1.12, 1.34) | <0.001 |
| Components of intraoperative fluids | 99.2% CI | |||
| Volume of crystalloid solution (L) | 3.0 [2.2, 4.0] | 2.7 [2.1, 3.5] | 1.0 (0.73, 1.37) | 0.99 |
| Volume of colloid solution (L) | 0.3[0, 0.5] | 0.3 [0, 0.5] | 1.61(0.73, 3.60) | 0.11 |
| Volume of red blood cells (L) a | 1.1 [0, 1.8] | 0.4 [0, 1.1] | 1.7 (1.08, 2.7) | 0.002 |
| Fresh frozen plasma (N of patients received, %) | 54 (44) | 84 (23) | 0.99 (0.38, 2.53) | 0.97 |
| Platelet concentrate (N of patients received, %) | 58 (47) | 86 (23) | 0.98 (0.39, 2.48) | 0.96 |
| Cryoprecipitate (N of patients received, %) | 24 (20) | 12 (3) | 2.54 (0.68, 9.54) | 0.06 |
Data are presented as mean ± SD, N (%), or median [25th, 75th percentiles].
CI – confidence interval; OR - odds ratio adjusted for the history of Idiopathic pulmonary hypertension, ischemia time, double lung transplant, use of cardiopulmonary bypass, average mean pulmonary artery pressure post-reperfusion, and average fraction of inspired oxygen post-reperfusion; N – number of patients Significance criterion for the total volume of intraoperative fluids was P < 0.05. As for the individual components of intraoperative fluids, P < 0.008 (Bonferroni correction, 0.05/6) was required to claim significance.
259 (54%) patients received red blood transfusion.
Furthermore, the volume of red blood cell concentrate was significantly larger in patients who developed a severe form of PGD, than those who did not (1.1 [0.0, 1.8] L versus 0.4 [0.0, 1.1] L; adjusted OR CI 95% 1.7 (1.08, 2.7); multivariable P = 0.002), Fifty-four percent of patients in the cohort received red blood cell concentrate transfusion intraoperatively. No other fluid component was significantly associated with grade-3 PGD after applying Bonferroni correction. When we adjusted the fluid volume to a patient’s weight, we found that the volume of total intraoperative fluids administered per kilogram body weight, had a positive association with grade-3 PGD, adjusted OR (95% CI) of 1.13 (1.06, 1.21), multivariable P <0.001.
Secondary outcomes
The median [Q1,Q3] time to tracheal extubation in the cohort was 1 [0, 3] day, and the ICU stay was 5 [3, 13] days. Patients were 8% more likely to have longer ICU stay for each liter increase in the volume of total intraoperative fluids [multivariable hazard ratio (97.5% CI): 0.92 [0.88, 0.97], P < 0.001], adjusted for mechanical ventilation, idiopathic pulmonary hypertension and sarcoidosis, use of CPB, average mean pulmonary artery pressure post-reperfusion, average positive end-expiratory pressure post-reperfusion, and transplant year. We did not find an association between the volume of total intraoperative fluids and the time to tracheal extubation [multivariable hazard ratio (97.5% CI): 0.97 [0.93,1.02]; P = 0.17], (Figure 2) adjusting for donor age, recipient gender, age, mechanical ventilation, chronic obstructive pulmonary disease, surgical time, use of CPB, use of intraoperative milrinone, average mean pulmonary artery pressure post-reperfusion, and average positive end expiratory pressure post-reperfusion.
Figure 2.
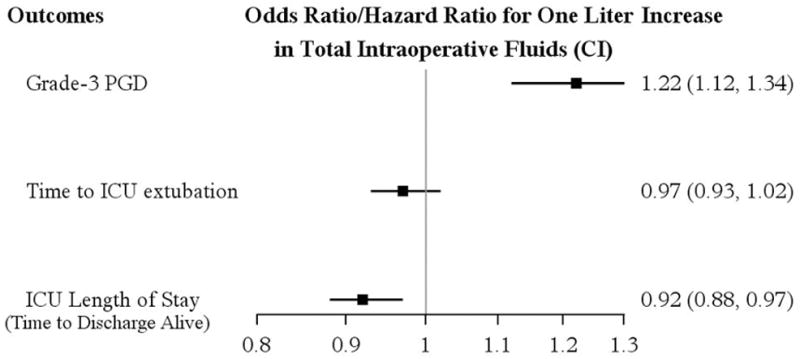
Multivariable associations between the total volume of intraoperative fluids and primary outcome (Grade-3 PGD) and secondary outcomes adjusting for covariates. The odds for grade-3 PGD increased by 22% (OR 1.22 (1.12, 1.34)) for each additional liter of intraoperative fluids administered (P < 0.001). The estimated hazard ratio < 1 for time to extubation and ICU length of stay, indicates that patients are less likely to be extubated and less likely to be discharged from the ICU, with increasing amount of intraoperative fluids.
CI – confidence interval; OR – odds ratio; ICU – intensive care unit; PGD – primary graft dysfunction
Patients with grade-3 PGD had longer time to tracheal extubation and lCU stay. Grade-3 PGD patients had a median [Q1, Q3] time to tracheal extubation of 3 [1, 12] days and ICU stay of 14 [5, 53] days, while non-grade-3 PGD patients had 1 [0, 2] and 3 [1, 12] days, accordingly.
Discussion
Our investigation demonstrated that intraoperative fluid volume was associated with grade-3 PGD. Each additional liter of fluid given during surgery increased the odds for grade-3 PGD by approximately 22%. After adjustment for a patient’s body weight, the odds for development of grade-3 PGD increased by 13% for each additional liter of fluid. Similar to other reports,22,23 25% of our lung transplant patients developed grade-3 PGD.
Our results are consistent with the current knowledge of the complex pathophysiology of lung graft dysfunction. PGD is a manifestation of ischemia-reperfusion injury, a process that starts in the donor after brain death due to the sympathetic surge and systemic inflammation,24,25,26 and peaks shortly after reperfusion of the transplanted lungs. A decrease in alveolar fluid clearance,27,28,29 because of disruption of lymphatic drainage during lung procurement30 further aggravates this process. These mechanisms may explain why transplanted lungs are particularly sensitive to large volume fluid administration.
Transfusion of red blood cell concentrate in our study was positively associated with severe PGD. Fifty-four percent of patients in the cohort received red blood cell transfusion during lung transplantation. Patients with grade-3 PGD were given a significantly higher volume of red blood cells than patients with lower grades of PGD. Allogenic blood transfusion may augment lung injury and predispose to PGD by causing fluid overload, increased hydrostatic forces in the pulmonary vessels or transfusion-related acute lung injury. Consistent with this theory, the Lung Transplant Outcomes Group recently reported a significant association between blood transfusions and PGD development,31 with transfusing more than a liter of blood being associated with a 2-fold increase in the incidence of PGD. A large meta-analysis similarly found that transfusion of red blood cells and plasma was associated with PGD development.32
Although we found a positive association between the total amount of fluids administered and development of grade-3 PGD, there was no such association between the non-blood components of fluid therapy (crystalloids and colloids) and grade-3 PGD. Our results contrast with one report15 that found that colloid solution administration was associated with prolonged mechanical ventilation and lower postoperative oxygenation. The authors speculated that increased capillary permeability along with the impaired lymphatic drainage of the transplanted lungs caused sequestration of the colloid molecules in the extravascular lung space. However, they gave a colloid with a smaller molecular weight (Gelofusine®, B. Braun, Australia, molecular weight 30,000 Dalton), rather than 5% human albumin (molecular weight 65,000 Dalton), which is our usual colloid therapy. Given the difference in the molecular weight, their findings cannot be generalized to other colloid solutions.
Fluid administration may also contribute to PGD by increasing cardiac filling, which in turn increases pulmonary blood flow and aggravates ischemia-reperfusion injury.33 An increased CVP, defined as CVP > 7 mmHg after lung reperfusion, has been associated with prolonged mechanical ventilation, longer ICU stay and increased mortality.14 These authors used CVP as a surrogate for increased fluid administration and concluded that low cardiac filling pressures may improve outcomes in lung transplant patients. In our study, overall post-reperfusion CVP did not differ significantly between patients who developed grade-3 PGD and those who did not, suggesting that the amount of fluid administration, rather than the CVP per se, is associated with graft dysfunction. We note that CVP is a poor marker of intravascular volume status and cardiac loading conditions, because it not only reflects the intravascular volume but also venous tone and right ventricular function.34
Although high-quality evidence of the influence of fluid administration on lung graft function is lacking, the current clinical recommendations include judicious fluid administration in patients undergoing lung transplantation.35 Consistent with these recommendations, implementation of hemodynamic and fluid administration guidelines for management of post lung transplant patients resulted in less positive postoperative fluid balance and reduced postoperative PGD severity.36
Our study differs from previous analyses in several ways. First, we used the current International Society of Heart and Lung Transplantation classification to define PGD.2,21 Second, the diagnosis was made prospectively by pulmonologists with expertise in the management of patients having lung transplantation. This contrasts with previous studies, which identified PGD retrospectively from the patients’ records using PaO2/FiO2 ratio alone or in combination with radiographic findings.14,15,23 This approach is problematic since the PaO2/FiO2 ratio and radiographic findings markers are nonspecific and may be altered in other conditions leading to pulmonary edema such as left ventricular failure, hyperacute rejection, lung infection, or obstruction of pulmonary venous flow. Third, we examined only patients with the most severe form of PGD, because it causes the worse clinical outcomes. While most patients develop a mild form of graft dysfunction in the first 6–12 hours after surgery, that appears to have little clinical importance. In contrast, grade-3 PGD substantially increases 30-day and overall mortality rate.4
Previous studies examining the effect of perioperative fluid administration on lung graft function included patients with less stringent definitions of graft dysfunction and varying degrees of severity.23,37 In contrast, we focused specifically on prospectively identified and well-documented grade-3 PGD. Finally, as a high volume lung transplant center, we included a large number of patients accrued over a short period, thus reducing the potential effects of time-dependent practice changes, although we nonetheless adjusted for year of surgery.
Our study is retrospective, and has limitations inherent with this study design. Although our analysis adjusted for numerous potential confounding factors, we cannot exclude unobserved confounding. More importantly, fluid intake and balance in the early postoperative period were not recorded; however, about 90% of our patients developed grade 3-PGD shortly after reperfusion and within 24 hours. We believe that the major contributor to overall fluid volume is the intraoperative course due to the hemodynamic changes with reperfusion and during surgical hemostasis. This theory is supported by a landmark study21 showing that PaO2/FiO2 at 6 hours after lung reperfusion is the most important predictor of duration of mechanical ventilation. Furthermore, there is substantial variance in the oxygenation index in the first 12 hours after reperfusion, which stabilizes thereafter — emphasizing the vulnerability of the transplanted lungs during the initial reperfusion hours.
In summary, many factors have been linked to postoperative graft dysfunction. Our analysis suggests that an increase in the total amount of intraoperative fluid is associated with an increased risk of developing severe early pulmonary graft dysfunction. Our results are consistent with current therapeutic strategies, which suggest restricting perioperative fluid administration may be beneficial for patients having lung transplantation.
Acknowledgments
Funding: The study used internal funds only. Dr. Andra Duncan receives salary support from NIH K23 HL093065
Appendix 1. Associations between baseline and intraoperative factors and grade-3 primary graft dysfunction (PGD)
| Factors | units | Unadjusted OR (95% CI) | Adjusted OR (95% CI) |
|---|---|---|---|
| Fluids (Liter) | 1 | 1.25 (1.16, 1.36) | 1.22 (1.12, 1.34) |
| Idiopathic Pulmonary Hypertension | Yes vs. no | 3.58 (1.35, 9.50) | 3.95 (1.39, 11.2) |
| Ischemic time (hour) | 1 | 1.44 (1.23, 1.68) | 1.32 (1.11, 1.56) |
| Double lung transplant | Yes vs. no | 1.47 (0.97, 2.25) | 0.61 (0.34, 1.1) |
| Use of cardiopulmonary bypass | Yes vs. no | 2.11 (1.30, 3.45) | 1.97 (1.06, 3.66) |
| Average mean PAP post-reperfusion (mmHg) | 1 | 1.55 (1.10, 2.18) | 1.05 ( 1.1, 1.09) |
| Average FiO2 post-reperfusion (%) | 10 | 1.22 (1.10, 1.36) | 1.28 (1.14, 1.44) |
For the final multivariable logistic regression, a backward-elimination variable selection method with the stay criterion of P < 0.15 was performed, starting with all the variables from the univariable logistic regression models in table 2 that had a P value < 0.25.
PAP – pulmonary artery pressure; FiO2 – fraction of inspired oxygen; OR – odds ratio
Footnotes
Reprints will not be available from the authors.
The authors declare no conflicts of interest.
Disclosures
Name: Mariya A. Geube, MD*
Contribution: This author participated in the study design, data collection, conduct of the study, manuscript preparation.
Attestation: This author attests to have approved the final manuscript and to have reviewed the original study data and data analysis.
Name: Silvia E. Perez-Protto, MD*
Contribution: This author participated in the study design, data collection, conduct of the study, manuscript preparation.
Attestation: This author attests to have approved the final manuscript and to have reviewed the original study data and data analysis.
Name: Tory L. McGrath, MD
Contribution: This author participated in the study design, conduct of the study, manuscript preparation.
Attestation: This author attests to have approved the final manuscript.
Name: Dongsheng Yang, MS
Contribution: This author participated in the study design, data collection, data analysis, conduct of the study, manuscript preparation. Dongsheng Yang is the archival author responsible for maintaining the study records.
Attestation: This author attests to the integrity of the original data and the analysis reported in the manuscript, to have reviewed the original study data and data analysis, and to have approved the final manuscript.
Name: Daniel I. Sessler, MD
Contribution: This author participated in the study design and manuscript preparation.
Attestation: This author attests to have approved the final manuscript.
Name: Marie M. Budev, DO
Contribution: This author helped with data collection and manuscript preparation.
Attestation: This author attests to have approved the final manuscript.
Name: Andrea Kurz, MD
Contribution: This author helped with study design and manuscript preparation.
Attestation: This author attests to have approved the final manuscript.
Name: Kenneth R. McCurry, MD
Contribution: This author helped with study design and manuscript preparation.
Attestation: This author attests to have approved the final manuscript.
Name: Andra E. Duncan, MD, MS
Contribution: This author helped with study design, conduct of the study and manuscript preparation.
Attestation: This author attests to have approved the final manuscript.
This manuscript was handled by: Avery Tung, MD
Contributor Information
Mariya A. Geube, Department of Cardiothoracic Anesthesia, Cleveland Clinic, Cleveland, Ohio.
Silvia E. Perez-Protto, Department of Anesthesiology and Critical Care, Cleveland Clinic, Cleveland, Ohio.
Tory L. McGrath, Department of Cardiothoracic Anesthesia, Cleveland Clinic, Cleveland, Ohio.
Dongsheng Yang, Departments of Quantitative Health Sciences and Outcomes Research, Cleveland Clinic, Cleveland, Ohio.
Daniel I. Sessler, Department of Outcomes Research, Cleveland Clinic, Cleveland, Ohio.
Marie M. Budev, Transplantation Center, Department of Pulmonology, Allergy and Critical Care, Cleveland Clinic, Cleveland, Ohio.
Andrea Kurz, Department of Outcomes Research, Department of General Anesthesiology, Cleveland Clinic, Cleveland, Ohio.
Kenneth R. McCurry, Transplantation Center, Department of Thoracic and Cardiovascular Surgery, Department of Pathobiology, Cleveland Clinic, Cleveland, Ohio.
Andra E. Duncan, Department of Cardiothoracic Anesthesia and Department of Outcomes Research, Cleveland Clinic, Cleveland Clinic, Cleveland, Ohio.
References
- 1.Christie J, Sager J, Kimmel S, Vivek A, Gaughan C, Blumental N, Kotloff R. Impact of primary graft failure on outcomes following lung transplantation. Chest. 2005;127:161–165. doi: 10.1378/chest.127.1.161. [DOI] [PubMed] [Google Scholar]
- 2.Christie JD, Carby M, Bag R, Corris P, Hertz M, Weill D. Report of the ISHLT working group on primary lung graft dysfunction: Part II Definition. J Heart Lung Transplant. 2005;24:1454–1459. doi: 10.1016/j.healun.2004.11.049. [DOI] [PubMed] [Google Scholar]
- 3.Prekker M, Nath D, Walker A, Johnson A, Hertz M, Herrington C, Radosevich D, Dahlberg P. Validation of the Proposed International Society for Heart and Lung Transplantation Grading System for Primary Graft Dysfunction After Lung Transplantation. The J Heart and Lung Transplant. 2006;25(4):371–78. doi: 10.1016/j.healun.2005.11.436. [DOI] [PubMed] [Google Scholar]
- 4.Christie J, Bellamy S, Ware L, Lederer D, Hadjiliadis D, Lee J, Robinson N, Localio A, Wille K, Lama V, Palmer S, Orens J, Weinacher A, Crespo M, Demissie E, Kimmel S, Kawut S. Construct validity of the primary graft dysfunction after lung transplantation. J Heart Lung Transplant. 2010;29:1231–9. doi: 10.1016/j.healun.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whitson B, Prekker M, Herrington C, Whelan T, Radosevich D, Hertz M, Dahlberg P. Primary graft dysfunction and long-term pulmonary function after lung transplantation. J Heart Lung Transplant. 2007;26:1004–11. doi: 10.1016/j.healun.2007.07.018. [DOI] [PubMed] [Google Scholar]
- 6.Daud SA, Yusen RD, Meyers BF, Chakinala MM, Walter MJ, Aloush AA, Patterson GA, Trulock EP, Hachem RR. Impact of immediate primary lung allograft dysfunction on bronchiolitis obliterance syndrome. Am J Respir Crit Care Med. 2007;175:507–13. doi: 10.1164/rccm.200608-1079OC. [DOI] [PubMed] [Google Scholar]
- 7.Christie J, Kotloff R, Pochettino A, Arcasoy S, Rosengard B, Landis R, Kimmel S. Clinical risk factors for primary graft failure following lung transplantation. Chest. 2003;124:1232–1241. doi: 10.1378/chest.124.4.1232. [DOI] [PubMed] [Google Scholar]
- 8.Thabut G, Mal H, Cerrina J, Dartevelle P, Dromer C, Velly J, Stern M, Loirat P, Leseche G, Bertocchi M, Mornex J, Haloun A, Despins P, Pison C, Blin D, Reynaud-Gaubert M. Graft ischemic time and outcome of lung transplantation: a multicenter analysis. Am J Resp Crit Care Med. 2005;171(7):786–91. doi: 10.1164/rccm.200409-1248OC. [DOI] [PubMed] [Google Scholar]
- 9.Oto T, Griffiths A, Levvey B, Pilcher D, Whitford H, Kotsimbos T, Rabinov M, Esmore D, Williams T, Snell G. A donor history of smoking affects early but not late outcome in lung transplantation. Transplantation. 2004;78:599–606. doi: 10.1097/01.tp.0000131975.98323.13. [DOI] [PubMed] [Google Scholar]
- 10.Perrot M, Bonser R, Dark J, Kelly R, McGiffin D, Menza R, Pajaro O, Schueler S, Verleden G ISHLT Working Group on Primary Lung Graft Dysfunction. Report of the ISHLT working group on primary lung graft dysfunction part III: Donor-related risk factors and markers. J Heart Lung Transplant. 2005;24:1460–1467. doi: 10.1016/j.healun.2005.02.017. [DOI] [PubMed] [Google Scholar]
- 11.Fang A, Stunder S, Kawut S, Ahya V, Lee J, Wille K, Lama V, Ware L, Orens J, Weinacker A, Palmer S, Creaspo M, Lederer D, Deutchman C, Kohl B, Bellamy S, Demissie E, Christie J Lung Transplant Outcome Group. Elevated pulmonary artery pressure is a risk factor for primary graft dysfunction following lung transplantation for idiopathic pulmonary fibrosis. Chest. 2011;139(4):782–787. doi: 10.1378/chest.09-2806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Whitson BA, Nath DS, Johnson AC, Walker AR, Prekker ME, Radosevitch DM, Herrington CS, Dahlberg PS. Risk factors for primary graft dysfunction after lung transplantation. J Thorac Cardiovasc Surg. 2006;131:73–80. doi: 10.1016/j.jtcvs.2005.08.039. [DOI] [PubMed] [Google Scholar]
- 13.Kuntz C, Hadjiliadis D, Ahya V, Kotloff R, Pochettino A, Lewis J, Christie J. Risk factors for early Primary Graft Dysfunction After Lung Transplantation: a registry study. Clin Transplant. 2009;23:819–830. doi: 10.1111/j.1399-0012.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 14.Pilcher D, Scheinkestel C, Snell G, Davey-Quinn A, Bailey M, Williams T. High central venous pressure is associated with prolonged mechanical ventilation and increased mortality after lung transplantation. J Thorac Cardiovasc Surg. 2005;129:912–8. doi: 10.1016/j.jtcvs.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15.McIlroy D, Pilcher D, Snell G. Does anesthetic management affect early outcomes after lung transplant? An exploratory analysis. Br J Anesth. 2009;102:506–14. doi: 10.1093/bja/aep008. [DOI] [PubMed] [Google Scholar]
- 16.Shargall Y, Guenther G, Vivek A, Ardehali A, Singhal A, Keshavjee S. Report of the ISHLT working group on primary graft dysfunction part VI: treatment. J Heart Lung Transplant. 2005;24:1489–1500. doi: 10.1016/j.healun.2005.03.011. [DOI] [PubMed] [Google Scholar]
- 17.Snell G, Klepetko W. Perioperative lung transplant management. Eur Respir Mon. 2003;26:130–142. [Google Scholar]
- 18.Pettersson G, Yun J, Norgaard M. Bronchial artery revascularization in lung transplantation : technique, experience and outcomes. Curr Opin Organ Transplant. 2010;15:572–577. doi: 10.1097/MOT.0b013e32833e16fc. [DOI] [PubMed] [Google Scholar]
- 19.Mason D, Brown C, Murthy S, Vakil N, Lyon C, Budev M, Pettersson G. Growing single-center experience with lung transplantation using donation after cardiac death. Ann Thorac Surg. 2012;94:406–12. doi: 10.1016/j.athoracsur.2012.03.059. [DOI] [PubMed] [Google Scholar]
- 20.Halazun K, Al-Mukhtar A, Willis S, Ahmad N. Warm ischemia in transplantation: search for a consensus definition. Transplantation proceedings. 2007;39:1329–1331. doi: 10.1016/j.transproceed.2007.02.061. [DOI] [PubMed] [Google Scholar]
- 21.Oto T, Levvy B, Snell G. Potential refinements of the International Society for Heart and Lung Transplantation Primary Graft Dysfunction Grading System. J Hear Lung Transplant. 2007;26:431–6. doi: 10.1016/j.healun.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 22.Lee J, Christie J. Primary graft dysfunction. Clin Chest Med. 2011;32:279–293. doi: 10.1016/j.ccm.2011.02.007. [DOI] [PubMed] [Google Scholar]
- 23.Kriesel D, Krupnick A, Puri V, Guthrie T, Trulock E, Meyers B, Patterson G. Short-and long-term outcomes of 1000 adult lung transplant recipients at a single center. J Thorac Cardiovasc Surg. 2011;141:215–22. doi: 10.1016/j.jtcvs.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 24.Lee J, Christie J, Keshavjee S. Primary Graft Dysfunction: Definition, Risk Factors, Short- and Long Term Outcomes. Semin Respir Crit Care Med. 2010;31:161–171. doi: 10.1055/s-0030-1249111. [DOI] [PubMed] [Google Scholar]
- 25.Weissa S, Kotschb K, Francuskia M, Reutzel-Selke A, Mantouvalou L, Klemz R, Kuecuek O, Jonas S, Wesslau C, Ulrich F, Pascher A, Volk H, Tullins S, Neuhaus P, Pratschke J. Brain death activates donor organs and is associated with worse ischemia reperfusion injury after liver transplantation. American J Transplant. 2007;7:1584–1593. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 26.Avlonitis V, Wigfield C, Golledge H, Kirby J, Dark J. Early hemodynamic injury during donor brain death determines the severity of primary graft dysfunction after lung transplantation. American J Transplant. 2007;7:83–90. doi: 10.1111/j.1600-6143.2006.01593.x. [DOI] [PubMed] [Google Scholar]
- 27.Ware L, Golden S, Finkenbeiner W, Matthay M. Alveolar epithelial fluid transport capacity in reperfusion lung injury after lung transplantation. Am J Respir Crit Care Med. 1999;159:980–8. doi: 10.1164/ajrccm.159.3.9802105. [DOI] [PubMed] [Google Scholar]
- 28.Ware L, Fang X, Wang Y, Sakuma T, Hall T, Matthay M. Selected contribution: mechanisms that may stimulate the resolution of alveolar edema in the transplanted lung. J Appl Physiol. 2002;93:1869–1874. doi: 10.1152/japplphysiol.00252.2002. [DOI] [PubMed] [Google Scholar]
- 29.Feltracco P, Falasco G, Barbieri S, Milevoj M, Serra E, Ori C. Anesthetic considerations for nontransplant procedures in lung transplant patients. J Clinic Anesthes. 2011;23:508–516. doi: 10.1016/j.jclinane.2011.05.002. [DOI] [PubMed] [Google Scholar]
- 30.Sugita M, Ferraro P, Dagenais A, Clermont M, Barbry P, Michel R, Berthiaume J. Alveolar liquid clearance and sodium channel expression are decreased in transplanted canine lung. Am J Respir Crit Care Med. 2003;167:1440–50. doi: 10.1164/rccm.200204-312OC. [DOI] [PubMed] [Google Scholar]
- 31.Diamond J, Lee J, Kawut S, Shah R, Localio A, Bellamy S, Lederer D, Cantu E, Kohl B, Lama V, Bhorade S, Crespo M, Demissie E, Sonett J, Wille K, Orens J, Shah A, Weinacher A, Arcasoy S, Shah P, Wilkes D, Ware L, Palmer S, Christie J Lung Transplant Outcomes Group. Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation. Am J Respir Crit Care Med. 2013;187:527–34. doi: 10.1164/rccm.201210-1865OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Liu Y, Liu Y, Su L, Jiang S-j. Recipient-Related Clinical Risk Factors for Primary Graft Dysfunction after Lung Transplantation: A Systematic Review and Meta-Analysis. PLoS ONE. 2014;9(3):e92773. doi: 10.1371/journal.pone.0092773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeLima N, Binns O, Buchanan S, Mauney M, Cope J, Shockey K, Tribble C, Kron I. Eurocollins solution exacerbates lung injury in the setting of high-flow reperfusion. J Thorac Cardiovasc Surg. 1996 doi: 10.1016/s0022-5223(96)70184-8. 112-111-16. [DOI] [PubMed] [Google Scholar]
- 34.Marik PE, Baram M, Vahid B. Does central venous pressure predict fluid responsiveness? A systematic review of the literature and the tale of seven mares. Chest. 2008;134(1):172–8. doi: 10.1378/chest.07-2331. [DOI] [PubMed] [Google Scholar]
- 35.Schuurmans M, Benden C, Inci I. Practical approach to early postoperative management of lung transplant recipients. Swiss Med Wkly. 2013;143:w13773. doi: 10.4414/smw.2013.13773. [DOI] [PubMed] [Google Scholar]
- 36.Currey J, Pilcher D, Davies A, Scheinkestel C, Botti M, Bailey M, Snell G. Implementation of a management guideline aimed at minimizing the severity of primary graft dysfunction after lung transplant. J Thorac Cardiovasc Surg. 2010;139:154–61. doi: 10.1016/j.jtcvs.2009.08.031. [DOI] [PubMed] [Google Scholar]
- 37.Myles P, Weeks A, Buckland M, Silvers A, Bujor M, Langley M. Anesthesia for bilateral sequential lung transplantation: Experience with 64 cases. J Cardiothoracic Vascular Anesth. 1997;11(2):177–183. doi: 10.1016/s1053-0770(97)90210-x. [DOI] [PubMed] [Google Scholar]


