Abstract
Background
Readmission within 30 days after hospitalization for heart failure is a major public health problem.
Objective
To examine whether timing and type of post-discharge follow-up impacts risk of 30-day readmission in adults hospitalized for heart failure.
Design
Nested matched case-control study (January 1, 2006 to June 30, 2013).
Setting
A large, integrated healthcare delivery system in Northern California.
Participants
Hospitalized adults with a primary diagnosis of heart failure discharged to home without hospice care.
Measurements
Outpatient visits and telephone calls with cardiology and general medicine providers in non-emergency department and non-urgent care settings were counted as follow-up care. Statistical adjustments were made for differences in patient sociodemographic and clinical characteristics, acute severity of illness, hospitalization characteristics and post-discharge medication changes and laboratory testing.
Results
Among 11,985 eligible adults, early initial outpatient contact within 7 days after discharge was associated with lower odds of readmission (adjusted odds ratio [OR] 0.81, 95% CI: 0.70–0.94), whereas later outpatient contact between 8 and 30 days after hospital discharge was not significantly associated with readmission (adjusted OR 0.99, 95% CI: 0.82–1.19). Initial contact by telephone was associated with lower adjusted odds of 30-day readmission (adjusted OR 0.85, 95% CI 0.69–1.06) but was not statistically significant.
Conclusions
In adults discharged to home after hospitalization for heart failure, outpatient follow-up with a cardiology or general medicine provider within 7 days was associated with a lower chance of 30-day readmission.
Keywords: heart failure readmission, 30-day readmission, post-discharge care
Heart failure (HF) poses a substantial health and economic burden nationally and is expected to grow significantly in the next two decades in the United States.1, 2 Improving the efficiency and effectiveness of HF care after hospitalization has been a major focus among payers and policy makers to reduce HF-related costs and morbidity. Among Medicare patients hospitalized for HF, more than 20% are readmitted within 30 days and the most common reason for re-hospitalization is HF.3, 4 A significant fraction of readmissions after HF hospitalizations are seen as preventable given substantial hospital-level variation in readmission rates that are not explained by differences in patient characteristics.5 In 2013, the Centers for Medicare and Medicaid Services (CMS) began to penalize hospitals with higher than expected risk-adjusted readmission rates for HF through reduced Medicare payments.6
Multiple, small randomized studies of post-discharge interventions after hospitalization for HF have reported mixed results, with two meta-analyses suggesting possible benefit with various types of interventions, including home visits, clinic visits, and telephone calls.7–12 A larger randomized trial in high-risk patients in Canada showed no benefit from a combination of interventions (home visits, clinic appointments, and telephone calls), but the interventions were fragmented from the patients’ usual care providers.13 On the other hand, in an observational study relying on clinical registry data linked to administrative claims from hospitals of fee-for-service Medicare beneficiaries, hospitals in the lowest quartile of one-week post-discharge follow-up rates after HF admissions had the highest 30-day readmission rates.14
The implications for health systems of implementing a policy focusing on early follow-up strategies are substantial with regards to resource utilization and workflow. To address this, we examined a large, contemporary cohort of adults hospitalized with HF who were carefully characterized longitudinally through a comprehensive electronic health record within an integrated healthcare delivery system to explore the association between different post-discharge outpatient HF management strategies and the risks of readmission at 30 days.
METHODS
Study Participants
The study was conducted within Kaiser Permanente Northern California, a large, integrated healthcare delivery system that provides comprehensive care to more than 3.7 million members within the San Francisco Bay Area. Its membership is highly representative of the local surrounding and statewide population, except for slightly lower representation at the extremes of age and income.15, 16 We included subjects hospitalized from January 1, 2006 through June 30, 2013 with a primary discharge diagnosis of HF (International Classification of Diseases, Ninth Edition [ICD-9] codes 398.91, 402.01, 402.11, 402.91, 428.0, 428.1, or 428.9) which has been shown to have a positive predictive value of >95% for clinical heart failure.17 We excluded patients who died during their index hospitalization or who were discharged to a location other than their home (e.g., skilled nursing facility or hospice facility). Patients on chronic dialysis or discharged to home hospice were also excluded, because these groups had structured, pre-determined outpatient contact schedules.
Post-Discharge Follow-up Patterns
The main exposure of interest was the frequency, timing and type of follow-up within the first 30 days post-discharge. Relevant post-discharge follow-up was defined as outpatient, non-emergency department telephone calls or clinic visits with internal medicine, family medicine, or cardiology providers. Visits to providers outside of these specialties were excluded because they were unlikely to have been directly involved in HF management. Urgent care and emergency department visits were also excluded because these contacts were likely due to clinical deterioration instead of planned follow-up after hospitalization.
The initial patient contact after hospital discharge was used to categorize follow-up as either in clinic or by telephone. Providers conducting telephone calls were trained to monitor patient symptoms, vital signs and weights recorded at home; initiate, discontinue or change medications; request laboratory and other diagnostic testing, and schedule in-person clinic visits. There was no ability to see the patient or conduct a physical examination through a telephone visit. Non-physician providers conducting telephone calls included nurses and pharmacists who were trained to follow an outpatient HF treatment protocol. Non-physician providers may or may not have previously known the patient prior to hospital discharge. Internal medicine and cardiology physicians following up with patients generally were familiar with the patient before hospital discharge. Outpatient contacts that only involved diagnostic testing, such as electrocardiograms, echocardiograms, or radiological studies, were also excluded. Finally, visits that only occurred for a specific procedure, such as a vaccination or an infusion, were also excluded.
The timing of the first post-discharge follow-up, the number of post-discharge telephone calls and clinic visits within 30 days, and clinic vs. telephone contact were used to characterize the type of post-discharge follow-up each patient received. The timing of the first post-discharge follow-up was categorized as within 1–7 days, 8–30 days, or no follow-up within 30 days after discharge. Follow-up within 7 days was selected because a prior study suggested that follow-up in this early time period could be beneficial for reducing readmissions.14 The number of post-discharge contacts was calculated by counting the number of outpatient contacts meeting the above criteria.
Outcomes
The primary outcome of interest was all-cause readmission within 30 days of index hospital discharge. All-cause readmissions at health plan-owned and outside facilities were defined as any inpatient admission after discharge from the index hospitalization, excluding emergency department visits not resulting in admission, and were identified from comprehensive hospital discharge and billing claims databases. The health plan billing claims databases are highly reliable for detecting the small fraction of hospitalizations outside of plan-owned facilities. Censoring due to death or disenrollment from the health plan was also ascertained. Deaths were comprehensively identified from Kaiser Permanente hospitalization files, administrative records (e.g., proxy reporting), California state death certificate files18 and Social Security Administrative vital status files.19
Covariates
Age, gender, self-reported race/ethnicity, and census-based estimates of graduation from high school and of annual household income above $35,000 were identified from health plan databases. We also controlled for the calendar year of index hospitalization for HF. We ascertained information on coexisting illnesses based on diagnoses or procedures using ICD-9 codes, laboratory results, or specific therapies from health plan hospitalization discharge, ambulatory visit, laboratory, and pharmacy databases; diabetes mellitus registry;20 and regional cancer registry.21 The baseline diagnoses were determined at the time of admission for the index HF hospitalization, which included acute myocardial infarction, unstable angina, stroke, prior coronary bypass graft surgery, prior percutaneous coronary intervention, ventricular tachycardia or fibrillation, atrial fibrillation or flutter, implantable cardioverter defibrillator placement, permanent pacemaker implantation, diabetes mellitus, hypertension, systemic cancer, chronic liver disease, chronic lung disease, mitral or aortic valvular disease, dementia, and depression. Left ventricular (LV) systolic function was determined from review of relevant diagnostic test results found in the electronic medical record surrounding the index date. LV systolic function was classified into one of four categories: normal or hyperdynamic (LV ejection fraction ≥ 50%), mild LV systolic dysfunction (LV ejection fraction 40–49%), moderate LV systolic dysfunction (LV ejection fraction 30–39%), or severe LV systolic dysfunction (LV ejection fraction < 30%).
Baseline and time-updated laboratory measurements of estimated glomerular filtration rate, serum potassium level, and hemoglobin level were ascertained. Estimated glomerular filtration rate (eGFR, ml/min/1.73 m2) was calculated using the CKD-EPI equation22 based on ambulatory, non-emergency department serum creatinine measurements. The outpatient, non-emergency department laboratory values closest in time before the index HF hospitalizations were used to determine baseline laboratory values. Time-varying values of laboratory results were ascertained throughout the follow-up period using health plan laboratory databases. A laboratory test was defined as any outpatient test for serum creatinine level, serum potassium level, hemoglobin, or B-type natriuretic peptide.
We also controlled for outpatient receipt of β-blockers, angiotensin-converting enzyme inhibitors (ACEI), angiotensin II receptor blockers (ARB), hydralazine, nitrates, loop diuretics, thiazide diuretics, potassium supplementation, digoxin, and calcium channel blockers based on dispensing found in ambulatory pharmacy databases. Baseline medication use was determined at the time of admission for the index HF hospitalization. As described previously,23 longitudinal medication use was estimated from drug refill patterns using the calculated day supply for each prescription. We also adjusted for the number of changes in HF medication (defined above) and laboratory tests completed during the follow-up period, as these processes of care may be markers of illness severity. A change in HF medication was defined as addition, discontinuation, or change in dose.
To account for the severity of illness during the index hospitalization, we also adjusted for the hospital length of stay and a validated laboratory-based acute physiology (LAP) score.24 Statistical models that used the combination of the LAP score and a comorbidity score to estimate inpatient mortality and 30-day mortality after admission were validated in a cohort of 409,305 hospitalizations within KPNC from 2002 to 2005.24 The c statistic for the model estimating inpatient mortality was 0.88, while that for 30-day mortality was 0.86. The LAP score was calculated from serum albumin; anion gap; arterial pH, PaCO2, and PaO2; bicarbonate; total serum bilirubin; blood urea nitrogen; serum creatinine; serum glucose; serum sodium; serum troponin I; hematocrit; and total white blood cell count. Eligible laboratory test results were those obtained in the 24-hour time frame preceding hospitalization.
Statistical Analysis
Analyses were performed using SAS software, version 9.3 (Cary, N.C.). Baseline characteristics at the time of admission for the index HF hospitalization are presented as means with standard deviations and frequencies with percentages. Crude readmission rates within 30 days were calculated for each category of post-discharge outpatient follow-up. Each type of follow-up was compared to no follow-up within 30 days using Student’s t-test.
We conducted a nested matched case control study with conditional logistic regression to examine the independent association between post-discharge follow-up variables and the outcome of all-cause readmission after appropriately addressing potential survivor bias through individual-level matching on duration of available follow-up time among cases and controls to ensure equal opportunity to receive follow-up care. This is critical as patients can receive more follow-up solely due to surviving and not being readmitted to the hospital. For each case, we randomly selected five eligible controls that were matched by amount of follow-up time. For example, a patient readmitted 10 days after hospital discharge would be considered a case. All patients who had not been readmitted by the 10th day of follow-up would then be eligible to be a control for this particular case. Cases and their controls matched by follow-up time from hospital discharge would have equal opportunity to receive follow-up care. In addition, for patients with a hospital readmission, only eligible outpatient contacts before the first readmission were analyzed in the regression models for readmission as an outcome. Because death precludes readmission, there is the possibility that higher rates of death could lead to lower rates of readmission. Therefore, patients who died within 30 days of discharge without a readmission were excluded from the all-cause readmission analysis to address this possible effect of competing risk between death and readmission. Finally, missing laboratory and LVEF data were assigned to a separate “missing” category.
Three separate conditional logistic regression models were created, one for each exposure of interest: frequency, timing and type of follow-up. These exposures were not included in the same model because there was significant correlation between them. For each regression model, all the covariates described previously were used to adjust for residual differences between patients.
RESULTS
Study Sample and Patient Characteristics
We identified 11,985 eligible adults who were hospitalized for HF between January 1, 2006 and June 30, 2013, who were not receiving chronic dialysis, and who were discharged to home without hospice care. From this eligible study population, we identified 1,587 cases who were readmitted to the hospital within the first 30 days post-discharge. These cases were then matched with 7,935 eligible controls individually matched on having the same follow-up time as the corresponding case. Case patients were significantly older and had more comorbidities than control patients, including being more likely to have a heart failure hospitalization in the prior year, a history of myocardial infarction, atrial fibrillation or flutter, valvular disease, diabetes, depression, dementia, lung disease, cancer, chronic kidney disease and anemia (Table 1).
TABLE 1.
Characteristics of cases readmitted within 30 days and matched control patients following a hospitalization for acute heart failure.
| Characteristic | Case (N=1587) |
Control (N=7935) |
P-Value |
|---|---|---|---|
| Mean (SD) age, yrs | 76.4 (12.0) | 74.4 (13.2) | <0.001 |
| Women | 784 (49.4%) | 3934 (49.6%) | 0.90 |
| Race/Ethnicity | 0.07 | ||
| White | 1213 (76.4%) | 5811 (73.2%) | |
| Black/African American | 187 (11.8%) | 1015 (12.8%) | |
| Asian/Pacific Islander | 143 (9.0%) | 814 (10.3%) | |
| Native American | 7 (0.4%) | 37 (0.5%) | |
| Other/Unknown | 37 (2.3%) | 258 (3.3%) | |
| Annual household income ≥ $35,000 (USD) | 1463 (92.4%) | 7234 (91.3%) | 0.17 |
| Graduation from high school | 1285 (81.1%) | 7793 (82.0%) | 0.34 |
| Medical History | |||
| Hospitalization for heart failure within prior year | 214 (13.5%) | 650 (8.2%) | <0.001 |
| Myocardial infarction | 207 (13.0%) | 788 (9.9%) | <0.001 |
| Unstable angina | 54 (3.4%) | 293 (3.7%) | 0.57 |
| Coronary artery bypass graft surgery | 41 (2.6%) | 195 (2.5%) | 0.77 |
| Percutaneous coronary intervention | 77 (4.9%) | 357 (4.5%) | 0.54 |
| Atrial fibrillation or flutter | 693 (43.7%) | 3163 (39.9%) | <0.01 |
| Ventricular fibrillation or tachycardia | 37 (2.3%) | 139 (1.8%) | 0.12 |
| Mitral or aortic valvular disease | 407 (25.6%) | 1499 (18.9%) | <0.001 |
| Stroke or transient ischemic attack | 87 (5.5%) | 445 (5.6%) | 0.84 |
| Hypertension | 1360 (85.7%) | 6731 (84.8%) | 0.38 |
| Diabetes mellitus | 694 (43.7%) | 3250 (41.0%) | <0.05 |
| Depression | 363 (22.9%) | 1561 (19.7%) | <0.01 |
| Dementia | 114 (7.2%) | 424 (5.3%) | <0.01 |
| Chronic lung disease | 664 (41.8%) | 2810 (35.4%) | <0.001 |
| Chronic liver disease | 50 (3.2%) | 221 (2.8%) | 0.42 |
| Cancer | 361 (22.7%) | 1409 (17.8%) | <0.001 |
| Permanent pacemaker | 63 (4.0%) | 207 (2.6%) | <0.01 |
| Implantable cardioverter defibrillator | 29 (1.8%) | 80 (1.0%) | <0.01 |
| Left ventricular ejection fraction | 0.45 | ||
| Normal (≥ 50%) | 585 (36.9%) | 2766 (34.9%) | |
| Mildly reduced (40–49%) | 161 (10.1%) | 758 (9.6%) | |
| Moderately reduced (30–39%) | 158 (10.0%) | 845 (10.6%) | |
| Severely reduced (< 30%) | 149 (9.4%) | 785 (9.9%) | |
| Unknown, not retrievable from records | 534 (33.6%) | 2781 (35.0%) | |
| Estimated glomerular filtration rate, mL/min/1.73 m2 | <0.001 | ||
| ≥ 60 | 488 (30.7%) | 2829 (35.7%) | |
| 45 to < 60 | 339 (21.4%) | 1670 (21.0%) | |
| 30 to < 45 | 341 (21.5%) | 1461 (18.4%) | |
| < 30 | 225 (14.2%) | 740 (9.3%) | |
| Unknown | 194 (12.2%) | 1235 (15.6%) | |
| Potassium, mEq/L | <0.001 | ||
| < 4.0 | 273 (17.2%) | 1222 (15.4%) | |
| 4.0–4.9 | 986 (62.2%) | 4758 (60.0%) | |
| ≥ 5.0 | 184 (11.6%) | 820 (10.3%) | |
| Unknown | 194 (12.2%) | 1235 (15.6%) | |
| Hemoglobin, g/dL | <0.001 | ||
| < 10.0 | 154 (9.7%) | 620 (7.8%) | |
| 10.0–11.9 | 493 (31.1%) | 1935 (24.4%) | |
| ≥ 12.0 | 749 (47.2%) | 3930 (49.5%) | |
| Unknown | 191 (12.0%) | 1450 (18.3%) | |
| Baseline Medications | |||
| Angiotensin-converting enzyme inhibitor | 741 (46.7%) | 3392 (42.7%) | <0.01 |
| Angiotensin II receptor blocker | 283 (17.8%) | 1365 (17.2%) | 0.54 |
| Beta-blocker | 1048 (66.0%) | 5179 (65.3%) | 0.56 |
| Loop diuretic | 930 (58.6%) | 4036 (50.9%) | <0.001 |
| Thiazide diuretic | 401 (25.3%) | 1788 (22.5%) | <0.05 |
| Potassium supplementation | 423 (26.7%) | 1615 (20.4%) | <0.001 |
| Calcium channel blocker | 463 (29.2%) | 2215 (27.9%) | 0.31 |
| Digoxin | 248 (15.6%) | 971 (12.2%) | <0.001 |
| Nitrates | 335 (21.1%) | 1274 (16.1%) | <0.001 |
| Hydralazine | 192 (12.1%) | 867 (10.9%) | 0.18 |
Index Hospitalization Characteristics and Post-Discharge Follow-up
Median length of stay for the index hospitalization was 3.0 days (interquartile range 2 to 5 days). The median laboratory-based acute physiology (LAP) score was 19 (interquartile range 9 to 31), which is associated with a predicted in-hospital mortality risk of between 1% and 5%.25 The timing, number and types of post-discharge follow-up contacts with a general medicine or cardiology provider are described for cases and controls in Table 2. Overall, about 70% of patients had either a clinic visit or a telephone call within 30 days of hospital discharge, with about 50% having contact within the first 7 days. The majority (84%) of the follow-up contacts were clinic visits, as opposed to telephone calls (16%). Almost all (94%) clinic visits were with physicians, while 45% of the telephone calls were made by non-physician providers. There was no significant difference in 30-day follow-up between cases and controls (70% vs. 72%, P=0.10). However, cases were less likely to have follow-up within 7 days compared with controls (50% vs. 53%, P=0.01)
TABLE 2.
Post-discharge outpatient follow-up characteristics of cases readmitted within 30 days and matched controls after a hospitalization for heart failure
| Case (N=1587) |
Control (N=7935) |
P-Value | |
|---|---|---|---|
| Time to First Contact | <0.05 | ||
| 1 to 7 days | 793 (50.0%) | 4241 (53.4%) | |
| 8 to 30 days | 324 (20.4%) | 1502 (18.9%) | |
| None within 30 days | 470 (29.6%) | 2192 (27.6%) | |
| Number of Contacts Within 30 days | <0.001 | ||
| >2 | 272 (17.1%) | 1134 (14.3%) | |
| 1 or 2 | 845 (53.2%) | 4609 (58.1%) | |
| 0 | 470 (29.6%) | 2192 (27.6%) | |
| Type of First Contact | 0.25 | ||
| Clinic visit | 942 (59.4%) | 4812 (60.6%) | |
| Telephone contact | 175 (11.0%) | 931 (11.7%) | |
| None within 30 days | 470 (29.6%) | 2192 (27.6%) |
Crude Readmission Rates
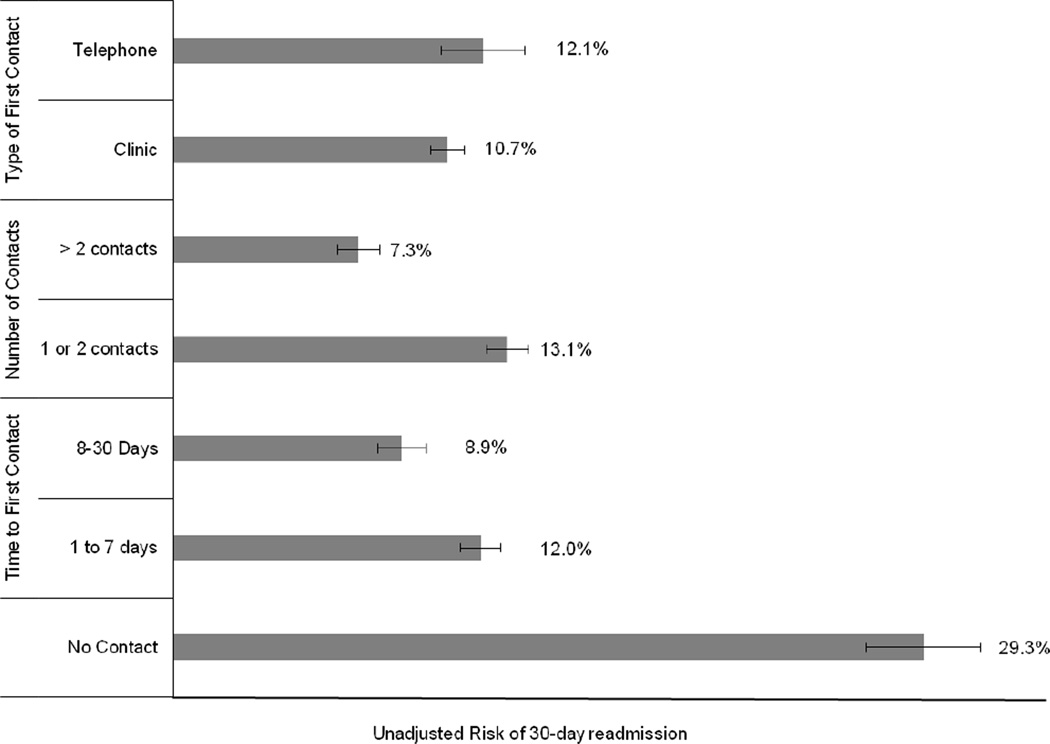
The overall 30-day readmission rate for the study cohort was 13.2%, with an overall 30-day mortality rate of 3.0%. Crude rates of 30-day readmission stratified by follow-up timing, number, and type are shown in Figure 1. Any follow-up, regardless of timing, number, and type, was associated with a significantly lower crude risk of readmission within 30 days of discharge (P<0.01).
Figure 1.
Unadjusted Risk of 30-Day Readmission by Post-Discharge Follow-up Contact
Associations of Follow-up Characteristics and 30-day Readmission
In matched case-control analyses, follow-up within 7 days post-discharge was associated with 19% (95% CI: 6% to 30%) lower adjusted odds of readmission (Table 3) after extensive adjustment for comorbidities, hospitalization characteristics, severity of acute illness, laboratory values, baseline HF medication use, and the number of laboratory checks and HF medication changes. Initial contact after 7 days post-discharge was not significantly associated with lower adjusted odds of readmission. Initial contact by clinic visit was associated with a 15% (95% CI: 2% to 27%) lower adjusted odds of readmission. Initial contact by telephone—frequently conducted by non-physicians—was associated with lower adjusted odds of 30-day readmission (adjusted OR 0.85, 95% CI 0.69–1.06) but was not statistically significant. The adjusted odds of readmission within 30 days of discharge was lower for 1 to 2 follow-up contacts compared to no follow-up. Greater than 2 contacts trended toward lower odds of readmission with a similar point estimate, but did not meet statistical significance. The detailed regression model results are available for viewing on-line (Tables, Supplemental Digital Content 1, 2, and 3).
TABLE 3.
Post-discharge follow-up characteristics and odds of 30-day readmission after a hospitalization for heart failure
| Crude Odds Ratio (95% Confidence Interval) |
Adjusted Odds Ratioa (95% Confidence Interval) |
|
|---|---|---|
| Time to first contact | ||
| None within 30 days | Reference | Reference |
| 1 to 7 days | 0.87 (0.76–0.99) | 0.81 (0.70–0.94) |
| 8 to 30 days | 1.00 (0.84–1.18) | 0.99 (0.82–1.19) |
| Number of contacts within 30 days | ||
| None within 30 days | Reference | Reference |
| >2 | 1.12 (0.93–1.35) | 0.87 (0.70–1.07) |
| 1 or 2 | 0.85 (0.75–0.97) | 0.85 (0.73–0.98) |
| Type of first contact | ||
| None within 30 days | Reference | Reference |
| Clinic visit | 0.90 (0.79–1.02) | 0.85 (0.73–0.98) |
| Telephone contact | 0.87 (0.71–1.05) | 0.85 (0.69–1.06) |
Covariates included age, gender, race/ethnicity, annual household income, graduation from high school, hypertension, diabetes, myocardial infarction, unstable angina, coronary artery bypass graft surgery, percutaneous coronary intervention, atrial fibrillation/flutter, ventricular tachycardia/fibrillation, mitral/aortic valvular disease, depression, dementia, chronic lung disease, chronic liver disease, cancer, permanent pacemaker, implantable cardioverter defibrillator, hospitalization for stroke or transient ischemic attack, hospitalization for heart failure within the prior year, left ventricular ejection fraction category, eGFR, serum potassium, hemoglobin, cardiovascular medication use (ACE inhibitor, ARB, β-blocker, loop diuretic, thiazide diuretic, potassium supplementation, calcium channel blocker, digoxin, nitrates, and hydralazine), and the number of laboratory checks and heart failure medication changes during follow-up.
DISCUSSION
Within a large, diverse integrated healthcare delivery system, we found that the timing of initial follow-up was a significant factor affecting the odds of readmission in a contemporary sample of mostly older adults discharged following hospitalization for acute heart failure. Follow-up within one week was independently associated with 19% lower odds of readmission, whereas initial follow-up after one week was not significantly associated with readmission. Compared to no follow-up, 1 or 2 total contacts within 30 days of hospital discharge and initial contact by clinic visit were independently associated with lower odds of readmission. Early telephone call follow-up was associated with a trend toward lower 30-day readmission rates.
We systematically explored how various aspects of follow-up (timing, type, and total number) were associated with 30-day readmission. Our study findings extend existing limited evidence that early follow-up within 7 days of hospital discharge is beneficial for preventing readmission in patients with heart failure.14 There was no benefit observed with initial contact between 8 to 30 days after discharge compared to no follow-up at all (adjusted OR 0.99, 95% CI: 0.82–1.19). One novel finding that may warrant further study is that early initial contact by telephone—which was frequently (45%) conducted by non-physician providers (e.g., nurses or pharmacists)—had a trend toward decreased odds of 30-day readmission (adjusted OR 0.85, 95% CI 0.69–1.06). The point estimate of the odds ratio for initial contact by telephone was the same as for initial contact through a clinic visit. However, the confidence interval was wider, because only 12% of patients had initial contact by telephone, as compared to 60% of patients with initial contact in clinic.
Outside of initial contact 8 to 30 days after hospital discharge which showed no beneficial association, we observed similar strengths of association between various types of post-discharge follow-up and lower odds of readmission (odds ratios 0.81 to 0.87) compared to a previous observational study.14 However, our observations were different from various other studies that showed no benefit for post-discharge follow-up.13, 26–29 Post-discharge follow-up as observed in our study may have had a greater impact on reducing 30-day readmission compared to these other studies for multiple reasons. Our study was conducted in a fully integrated health care delivery system with a single electronic medical record, compared with other more fragmented systems of care.13 Additionally, only follow-up contacts with internal medicine, family medicine, or cardiology providers were included, as opposed to any type of physician follow-up.14 General medicine and cardiology providers are more likely to address HF-specific care and post-hospitalization issues that could affect readmission. In our study, patients who were discharged to hospice care or to a nursing facility or receiving chronic dialysis were also excluded, because they were effectively placed in a different system of care that either had different goals of care or did not involve short-term outpatient follow-up. By excluding patients who are predictably near death and placed in hospice care and those discharged to nursing homes, the follow-up interventions advocated by current HF guidelines30 were likely to be more effective in patients who would be most likely to benefit from them.
Systematic follow-up after hospital discharge for HF is generally regarded as likely to reduce readmission and is actively being employed in many hospital systems.31 However, what is not fully known is how specific aspects of follow-up care, including timing, provider type (i.e., nurse, pharmacist, physician), method of contact (i.e., telephone, video, clinic, in-home), and extent of coordination with other providers, affects the effectiveness of interventions. A systematic review of 21 randomized trials of transitional care, not specifically for HF patients, implied that higher intensity follow-up, specifically with home visits, reduced readmission.32 However, a recent, significantly larger randomized trial showed that intensive follow-up with combination of home visits, telephone calls, and clinic visits did not show reduction in readmission in high risk patients.13 Some major reasons for the lack of benefit, as cited by authors, were lack of coordination with primary care physicians and incompatibility of electronic medical records. These study data support that a high degree of care coordination in addition to greater intensity of follow-up may be important for reducing readmission.
With the advent of accountable care organizations and the mandate for meaningful use of the electronic medical record, healthcare delivery systems in the United States are becoming more integrated and transparent between networks of providers.33, 34 Our results imply that clinicians may be able to leverage this increasing integration to improve the effectiveness of transitional care and reduce hospital readmissions in high-risk populations. While an initial in-person clinic visit was associated with reduced odds of 30-day readmission, an early initial contact by telephone with a physician or non-physician provider may be more practical to implement by many healthcare delivery systems compared with clinic visits for all patients. Although we did not show a definitive benefit for initial contact by telephone, this specific finding warrants further investigation.
In an observational study, we cannot completely exclude the possibility of unmeasured confounders and residual treatment selection bias, despite our ability to adjust for a large number of individual-level patient and hospitalization characteristics and use of individual-level matching to account for differential follow-up time among patients. Differences in disease severity or other patient characteristics could have led to a higher or lower likelihood of both early follow-up and readmission. Indeed, we observed that cases tended to be more ill than the controls, as measured by age and comorbid conditions, and that adjustment for these differences strengthened the association between early follow-up and lower odds of readmission. Also, the study was conducted within an integrated healthcare system with a comprehensive electronic medical record, which could have potentiated the observed benefit of outpatient follow-up. Our findings may not be fully generalizable to other practice settings with less transparency and coordination of care between hospital and outpatient locations. Data regarding LV systolic function was unavailable for approximately a third of the study cohort, so we were unable to evaluate potential effect modification of follow-up patterns and LV systolic function on 30-day outcomes.
In conclusion, early in-person clinic follow-up with general medicine or cardiology providers within 7 days after a HF hospitalization was independently associated with lower odds of readmission within 30 days. Initial contact with telephone calls—nearly half being conducted by non-physicians—showed a trend towards lower odds of readmission. Our study provides strong evidence that systematic, early in-person clinic follow-up after HF hospitalization could decrease 30-day readmission. Early telephone follow-up, which is easier for patients and health care providers, may also offer similar benefit as an in-person clinic visit.
Supplementary Material
Acknowledgments
Supported by research grants from the Kaiser Permanente Northern California Community Benefit Fund and grants RC1HL099395 and U19HL091179 from the National Heart, Lung and Blood Institute of the National Institutes of Health, U.S. Department of Health and Human Services.
Footnotes
The authors report no conflict of interest.
References
- 1.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics C, Stroke Statistics S. Heart disease and stroke statistics--2012 update: A report from the american heart association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG on behalf of the American Heart Association Advocacy Coordinating Committee CoAT, Vascular Biology CoCR, Intervention CoCCCoE, Pr. Forecasting the impact of heart failure in the united states: A policy statement from the american heart association. Circulation. Heart failure. 2013 doi: 10.1161/HHF.0b013e318291329a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the medicare fee-for-service program. The New England journal of medicine. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 4.Dharmarajan K, Hsieh AF, Lin Z, Bueno H, Ross JS, Horwitz LI, Barreto-Filho JA, Kim N, Bernheim SM, Suter LG, Drye EE, Krumholz HM. Diagnoses and timing of 30-day readmissions after hospitalization for heart failure, acute myocardial infarction, or pneumonia. JAMA. 2013;309:355–363. doi: 10.1001/jama.2012.216476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Keenan PS, Normand SL, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Wang Y, Krumholz HM. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circulation. Cardiovascular quality and outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 6.Patient Protection and Affordable Care Act PLN-, §2702, 124, Stat. 119.
- 7.Jaarsma T, Halfens R, Huijer Abu-Saad H, Dracup K, Gorgels T, van Ree J, Stappers J. Effects of education and support on self-care and resource utilization in patients with heart failure. European heart journal. 1999;20:673–682. doi: 10.1053/euhj.1998.1341. [DOI] [PubMed] [Google Scholar]
- 8.Rainville EC. Impact of pharmacist interventions on hospital readmissions for heart failure. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 1999;56:1339–1342. [PubMed] [Google Scholar]
- 9.McDonald K, Ledwidge M, Cahill J, Kelly J, Quigley P, Maurer B, Begley F, Ryder M, Travers B, Timmons L, Burke T. Elimination of early rehospitalization in a randomized, controlled trial of multidisciplinary care in a high-risk, elderly heart failure population: The potential contributions of specialist care, clinical stability and optimal angiotensin-converting enzyme inhibitor dose at discharge. European journal of heart failure. 2001;3:209–215. doi: 10.1016/s1388-9842(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 10.Phillips CO, Wright SM, Kern DE, Singa RM, Shepperd S, Rubin HR. Comprehensive discharge planning with postdischarge support for older patients with congestive heart failure: A meta-analysis. JAMA. 2004;291:1358–1367. doi: 10.1001/jama.291.11.1358. [DOI] [PubMed] [Google Scholar]
- 11.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: A systematic review. Annals of internal medicine. 2011;155:520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 12.Leppin AL, Gionfriddo MR, Kessler M, Brito JP, Mair FS, Gallacher K, Wang Z, Erwin PJ, Sylvester T, Boehmer K, Ting HH, Murad MH, Shippee ND, Montori VM. Preventing 30-day hospital readmissions: A systematic review and meta-analysis of randomized trials. JAMA internal medicine. 2014;174:1095–1107. doi: 10.1001/jamainternmed.2014.1608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dhalla IA, O'Brien T, Morra D, Thorpe KE, Wong BM, Mehta R, Frost DW, Abrams H, Ko F, Van Rooyen P, Bell CM, Gruneir A, Lewis GH, Daub S, Anderson GM, Hawker GA, Rochon PA, Laupacis A. Effect of a postdischarge virtual ward on readmission or death for high-risk patients: A randomized clinical trial. JAMA. 2014;312:1305–1312. doi: 10.1001/jama.2014.11492. [DOI] [PubMed] [Google Scholar]
- 14.Hernandez AF, Greiner MA, Fonarow GC, Hammill BG, Heidenreich PA, Yancy CW, Peterson ED, Curtis LH. Relationship between early physician follow-up and 30-day readmission among medicare beneficiaries hospitalized for heart failure. JAMA. 2010;303:1716–1722. doi: 10.1001/jama.2010.533. [DOI] [PubMed] [Google Scholar]
- 15.Krieger N. Overcoming the absence of socioeconomic data in medical records: Validation and application of a census-based methodology. American journal of public health. 1992;82:703–710. doi: 10.2105/ajph.82.5.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gordon NP. Characteristics of adult health plan members in kaiser permanente’s northern california region, as estimated from the 2011 member health survey. Division of Research, Kaiser Permanente Medical Care Program, Oakland, CA. 2013 [Google Scholar]
- 17.Ruo B, Capra AM, Jensvold NG, Go AS. Racial variation in the prevalence of atrial fibrillation among patients with heart failure: The epidemiology, practice, outcomes, and costs of heart failure (epoch) study. J Am Coll Cardiol. 2004;43:429–435. doi: 10.1016/j.jacc.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Arellano MG, Petersen GR, Petitti DB, Smith RE. The california automated mortality linkage system (camlis) Am J Public Health. 1984;74:1324–1330. doi: 10.2105/ajph.74.12.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lash TL, Silliman RA. A comparison of the national death index and social security administration databases to ascertain vital status. Epidemiology. 2001;12:259–261. doi: 10.1097/00001648-200103000-00021. [DOI] [PubMed] [Google Scholar]
- 20.Selby JV, Ray GT, Zhang D, Colby CJ. Excess costs of medical care for patients with diabetes in a managed care population. Diabetes Care. 1997;20:1396–1402. doi: 10.2337/diacare.20.9.1396. [DOI] [PubMed] [Google Scholar]
- 21.Fireman BH, Fehrenbacher L, Gruskin EP, Ray GT. Cost of care for patients in cancer clinical trials. J Natl Cancer Inst. 2000;92:136–142. doi: 10.1093/jnci/92.2.136. [DOI] [PubMed] [Google Scholar]
- 22.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Go AS, Yang J, Ackerson LM, Lepper K, Robbins S, Massie BM, Shlipak MG. Hemoglobin level, chronic kidney disease, and the risks of death and hospitalization in adults with chronic heart failure: The anemia in chronic heart failure: Outcomes and resource utilization (anchor) study. Circulation. 2006;113:2713–2723. doi: 10.1161/CIRCULATIONAHA.105.577577. [DOI] [PubMed] [Google Scholar]
- 24.Escobar GJ, Greene JD, Scheirer P, Gardner MN, Draper D, Kipnis P. Risk-adjusting hospital inpatient mortality using automated inpatient, outpatient, and laboratory databases. Medical care. 2008;46:232–239. doi: 10.1097/MLR.0b013e3181589bb6. [DOI] [PubMed] [Google Scholar]
- 25.Liu V, Kipnis P, Gould MK, Escobar GJ. Length of stay predictions: Improvements through the use of automated laboratory and comorbidity variables. Medical care. 2010;48:739–744. doi: 10.1097/MLR.0b013e3181e359f3. [DOI] [PubMed] [Google Scholar]
- 26.Harrison JD, Auerbach AD, Quinn K, Kynoch E, Mourad M. Assessing the impact of nurse post-discharge telephone calls on 30-day hospital readmission rates. Journal of general internal medicine. 2014;29:1519–1525. doi: 10.1007/s11606-014-2954-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Linden A, Butterworth S. A comprehensive hospital-based intervention to reduce readmissions for chronically ill patients: A randomized controlled trial. The American journal of managed care. 2014;20:783–792. [PubMed] [Google Scholar]
- 28.Keyhani S, Myers LJ, Cheng E, Hebert P, Williams LS, Bravata DM. Effect of clinical and social risk factors on hospital profiling for stroke readmission: A cohort study. Annals of internal medicine. 2014;161:775–784. doi: 10.7326/M14-0361. [DOI] [PubMed] [Google Scholar]
- 29.Stranges PM, Marshall VD, Walker PC, Hall KE, Griffith DK, Remington T. A multidisciplinary intervention for reducing readmissions among older adults in a patient-centered medical home. The American journal of managed care. 2015;21:106–113. [PubMed] [Google Scholar]
- 30.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: Accf/aha guidelines for the diagnosis and management of heart failure in adults: A report of the american college of cardiology foundation/american heart association task force on practice guidelines: Developed in collaboration with the international society for heart and lung transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 31.Bradley EH, Curry L, Horwitz LI, Sipsma H, Wang Y, Walsh MN, Goldmann D, White N, Pina IL, Krumholz HM. Hospital strategies associated with 30-day readmission rates for patients with heart failure. Circulation. Cardiovascular quality and outcomes. 2013;6:444–450. doi: 10.1161/CIRCOUTCOMES.111.000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Naylor MD, Aiken LH, Kurtzman ET, Olds DM, Hirschman KB. The care span: The importance of transitional care in achieving health reform. Health affairs. 2011;30:746–754. doi: 10.1377/hlthaff.2011.0041. [DOI] [PubMed] [Google Scholar]
- 33.Auerbach DI, Liu H, Hussey PS, Lau C, Mehrotra A. Accountable care organization formation is associated with integrated systems but not high medical spending. Health affairs. 2013;32:1781–1788. doi: 10.1377/hlthaff.2013.0372. [DOI] [PubMed] [Google Scholar]
- 34.Furukawa MF, Patel V, Charles D, Swain M, Mostashari F. Hospital electronic health information exchange grew substantially in 2008–12. Health affairs. 2013;32:1346–1354. doi: 10.1377/hlthaff.2013.0010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.