Abstract
Background:
Chronic kidney disease (CKD) has become a public health problem. New interventions to slow or prevent disease progression are urgently needed. In this setting, cell therapies associated with regenerative effects are attracting increasing interest. We evaluated the effect of embryonic stem cells (ESCs) on the progression of CKD.
Methods:
Adult male Sprague–Dawley rats were subjected to 5/6 nephrectomy. We used pedicled greater omentum flaps packing ESC-loaded gelatin microcryogels (GMs) on the 5/6 nephrectomized kidney. The viability of ESCs within the GMs was detected using in vitro two-photon fluorescence confocal imaging. Rats were sacrificed after 12 weeks. Renal injury was evaluated using serum creatinine, urea nitrogen, 24 h protein, renal pathology, and tubular injury score results. Structural damage was evaluated by periodic acid-Schiff and Masson trichrome staining.
Results:
In vitro, ESCs could be automatically loaded into the GMs. Uniform cell distribution, good cell attachment, and viability were achieved from day 1 to 7 in vitro. After 12 weeks, in the pedicled greater omentum flaps packing ESC-loaded GMs on 5/6 nephrectomized rats group, the plasma urea nitrogen levels were 26% lower than in the right nephrectomy group, glomerulosclerosis index was 62% lower and tubular injury index was 40% lower than in the 5/6 nephrectomized rats group without GMs.
Conclusions:
In a rat model of established CKD, we demonstrated that the pedicled greater omentum flaps packing ESC-loaded GMs on the 5/6 nephrectomized kidney have a long-lasting therapeutic rescue function, as shown by the decreased progression of CKD and reduced glomerular injury.
Keywords: Chronic Renal Insufficiency, Embryonic Stem Cells, Gelatin Microcryogels, Regenerative Medicine
INTRODUCTION
Chronic kidney disease (CKD), which affects individuals worldwide,[1,2] is now recognized as a major public health problem. Interstitial fibrosis is regarded as the main pathway for CKD progression, which culminates in end-stage renal failure. Despite the great effort invested in identifying therapies for CKD, the number of patients requiring dialysis and kidney replacement continues to rise.[3] In addition, current treatment modalities and donor kidney availability are insufficient, further increasing the demand for newly available approaches to treat chronic nephropathy. Over the last decade, stem cells have become a promising therapeutic tool for the treatment of kidney diseases.[4,5,6,7]
Stem cells are undifferentiated cells characterized by their self-renewal ability and the capacity for multiline age differentiation.[8] Stem cell-based therapies are potentially effective in many human diseases, including kidney disease, and they have been proven to be safe and effective in a wide range of immunomediated diseases.[9,10] However, aside from hematopoietic stem cell transplantation for the treatment of hematological disorders and some dermal and corneal indications, all other approaches based on stem cells remain experimental. In spite of this, desperate patients who have no hope of a cure for their disease may be willing to risk stem cell-based therapies.[11] The emerging field of regenerative medicine is progressing rapidly and is supported by many studies, including animal preclinical experiments. Stem cell-based therapy may have the potential to be developed into a novel therapeutic approach to treating CKD.
Embryonic stem cells (ESCs) were initially derived from the inner cell mass of blastocysts of mouse embryos.[12] These cells have the ability to differentiate into several cell types of the mesodermal, endodermal, and ectodermal lineages. Therefore, they have the potential to be used as an effective tool for kidney regenerative therapy. It has been shown that mouse Wnt4 transfected-ESCs can differentiate into tubule-like structures that express aquaporin-2 both in vitro and in vivo and the expression of aquaporin-2 was enhanced in the presence of HGF and activin A.[13] Steenhard et al.[14] injected ESCs into day 12–13 embryonic metanephroi and then placed them into a Trans well organ culture. These ESCs differentiated into renal epithelial structures that resembled tubules with an efficiency approaching 50%. Overall, ESCs are a valuable cellular source for investigating the mechanism of kidney regeneration, but there are still many limitations to regeneration therapy in the clinic.
Three-dimensional (3D) microscale cellular niches (microniches) are optimal cell delivery and therapy vehicles based on biocompatible and biodegradable gelatin microcryogels (GMs). GMs can be degraded in the presence of trypsin/ethylenediaminetetraacetic acid (EDTA) solution within 70 min. In addition, GMs cannot be detected 28 days after being injected a tan intramuscular site, suggesting good biocompatibility and biodegradation in vivo. The 3D microniches can be constituted by priming the seeded cells in the GMs in vitro for accumulation of deposited ECM proteins, as well as for enhancing cell-cell interactions. The 3D cellular microniches result in microscale tissue-like ensembles, representing an optimal delivery strategy to facilitate cell protection from mechanical insults during injection and in vivo cell retention, engraftment and survival, and the ultimate therapeutic function at the lesion site.[15]
In this study, we overcame the difficulty inherent in using GMs by allowing the omentum to wrap up ESCs-loaded GMs fusing to a 5/6 nephrectomized kidney (a model of CKD in rats). This maneuver allowed us to permanently lodge ESCs in the injured kidney. We reported that by doing so, the progression of CKD was slowed, which was likely due to the presence of ESCs and their secretory paracrine factors in the vicinity of the injured kidney.
METHODS
Embryonic stem cells engraftment and culture
Murine pluripotent ESCs (C57Bl6/ERFP, Life Technologies, Carlsbad, CA, USA) have been well-characterized, and red fluorescent protein allowed tracking by fluorescence microscopy. These cells were maintained on gelatin-coated dishes in feeder-free (mouse embryonic fibroblasts, mitotically inactivated using gamma-rays), serum-free medium that contained 50% neurobasal supplemented with N2/50% DMEM/F12 supplemented with B27, 10% BSA, 2 mmol/L glutamine, and 1% penicillin-streptomycin (penicillin at 100 U/ml and streptomycin at 100 μg/ml, PS) (Life Technologies, Rockville, MD, USA), 1% leukemia inhibitory factor, and β-mercaptoethanol. To prepare the cells for use, the undifferentiated ESCs were dissociated using trypsin (0.05% trypsin/EDTA). Then, they were suspended in ESC growth medium. All cultures in this study were maintained at 37°C in 5% CO2.
Experimental animals and procedures
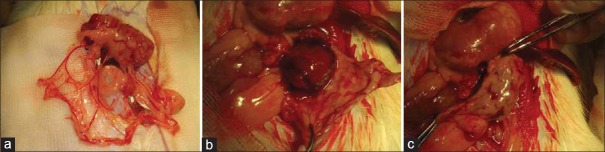
Male (Sprague–Dawley [SD]) rats (220–250 g) were obtained from the Experimental Animal Center of the Academy of Military Medical Sciences. The rats were housed at a constant room temperature with a 12-h light/dark cycle. Standard rodent chow and water were provided ad libitum. The animals were acclimated for 7 days before initiating the experiment. All animal protocols were approved by the Animal Ethics Committee of the Chinese PLA General Hospital and Military Medical College. Briefly, SD rats (body weight 220–250 g) were laparotomized under general anesthesia and underwent right nephrectomy (NPX) and surgical excision of the upper and lower poles of the left kidney (5/6 NPX). This is a well-established model in rats that induces progressive CKD without causing systemic hypertension.[16,17] We designed the pedicled greater omentum flap packing GMs or cells on remnant renal tissue to facilitate its fusion to the injured kidney [Figure 1a–1c].
Figure 1.
(a) The greater omentum is mainly composed of loose connective tissue, which has an abundant blood supply. (b and c) The pedicled great omentum wrapped the 5/6 nephrectomized renal tissue.
The following groups were evaluated: Sham, only removal of the renal capsule; NPX, 5/6 NPX and simultaneous complete omentectomy to prevent omentum from fusing to the injured kidney; NPX + OM (omentum), 5/6 NPX and pedicled greater omental direct packing on the remnant renal tissue; NPX + OM + ESCs, 5/6 NPX and pedicled greater omental packing of free ESCs on remnant renal tissue; and NPX + OM + GMs + ESCs, 5/6 NPX and pedicled greater omentum packing of ESC-loaded GMs on remnant renal tissue. Each experimental group comprised 8 rats. Rats were euthanized at 12 weeks after inducing CKD. After euthanasia, depending on the experiment, the remnant kidney was either cleared of the fused omentum before further biochemical processing or the attached omentum was left intact with the kidney tissue for histologic examination.
Cell autoloading into gelatin microcryogels and cell viability assessment
The harvested GMs were from the Department of Biomedical Engineering, School of Medicine, Tsinghua University. Before loading the cells, the harvested and dried GMs in the dish were sterilized by an ethylene oxide sterilization system that performed a 12-h degassing step under vacuum after 12 h of gas exposure (AN74j/Anprolene; Anderson Sterilization). ESCs were isolated and cultured in the ESC growth medium according to a previously reported protocol. A 60 µl ESC suspension was subsequently pipetted onto 600 tightly packed GMs and automatically absorbed to hydrate the porous structures and then maintained in a humidified chamber and incubated at 37°C for 2 h to allow cell attachment. Then, the culture medium was added for long-term culture. After 2 d of culture, we used the pedicled greater omentum flap packing ESC-loaded GMs on the 5/6 nephrectomized renal section tissue. The viability and fluorescence of ESCs-loaded GMs were assessed and imaged using a Leica two-photon fluorescence confocal imaging TCS SP5 System (Leica Microsystems, Mannheim, Germany) from day 1 to day 7 in vitro.
Measurement of renal function and chronic kidney disease evaluation
The rats were weighed weekly. On week 2, 4, 6, and 12, 24 h urine and blood samples were collected. To collect 24 h urine, the rats were placed in metabolism cages without food but with free access to water for 24 h. Urine was collected in an antibiotic/antimycotic solution (Sigma, St. Louis, MO, USA; A5955) and stored at −80°C. Blood samples were collected from the tail vein. Plasma creatinine and urea nitrogen were determined using commercial kits (sarcosine oxidase-peroxidase-antiperoxidase; Zixing, Shanghai, China). Urine protein was measured with Coomassie Blue.
Histopathological examination
All rats were sacrificed at week 12 after collecting blood and urine specimens. Harvested kidneys were fixed in 4% formalin for 24 h and underwent routine dehydration and paraffin embedding. Renal tissues were sectioned at 3-μm thickness and stained with hematoxylin and eosin, periodic acid-Schiff (PAS), and Masson trichrome staining using standard methods. Histological examinations were performed in a blinded fashion.
Assessment of glomerulosclerosis and tubular injury was performed using the semiquantitative scale described by Cao et al.[18] In brief, glomeruli in each kidney tissue were visualized at a magnification of ×400 and graded as follows: 0, normal; 1+, mesangial expansion and slight glomerular damage involving <25% of the glomerulus; 2+, mild sclerosis involving 25–50% of the glomerulus; 3+, moderate sclerosis involving 50–75% of the glomerulus; and 4+, severe sclerosis involving >75% of the glomerulus. Tubular injury was visualized at a magnification of ×200 and graded as follows: 0, normal; 1+, area of interstitial inflammation, fibrosis, and tubular dilation with cast formation (lesion) involving <25% of the field; 2+, lesion involving 25–50% of the field; 3+, lesion involving 50% –75% of the field; and 4+, lesion involving >75% of the field. The overall glomerulosclerosis index and tubular injury score (mean ± standard error of the mean [SEM]) were computed from the individual means of all of the rats in the group.
Statistical analyses
Measurement data are presented as mean ± SEM. Statistical analyses were performed using SPSS 17.0 statistical software (SPSS Inc., Chicago, IL, USA). Multiple comparisons of parametric data were performed using one-way analysis of variance, followed by Student–Newman–Keuls post-hoc tests. Student's t-test was used to compare differences between means;A P < 0.05 indicated statistically significant difference.
RESULTS
Embryonic stem cells could easily proliferate on the gelatin microcryogels
We examined the biocompatibility of GMs for microscale 3D cell culture. ESCs could be automatically loaded into GMs by simply pipetting the cells onto the surface of the collected GMs. Uniform cell distribution, good cell attachment, and viability were achieved from day 1 to 7 in vitro [Figure 2a–2c].
Figure 2.
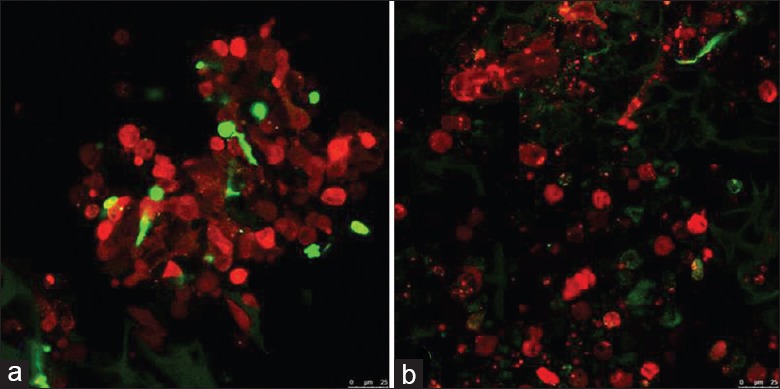
Images of gelatin microcryogels autoloaded with embryonic stem cells. (a) Embryonic stem cells persisted in gelatin microcryogels after 24 h. (b) Embryonic stem cells persisted in gelatin microcryogels after 7 d. Red fluorescent protein-labelled embryonic stem cells (red) and gelatin microcryogels (green) were detected in vitro using two-photon fluorescence confocal microscopy (original magnification, ×200).
Pedicled great omentum packing embryonic stem cells-loaded gelatin microcryogels on the injured kidney attenuated progression of chronic kidney disease
Except for the Sham and NPX groups, we observed fusion between the omentum and the two poles of the remnant left kidney [Figure 3a–3c]. Compared with the NPX rats, the NPX + OM + GMs + ESCs rats showed plasma creatinine levels that were 40% (74.01 ± 1.56 versus 122.98 ± 23.30) lower at week 4 and 33% (83.86 ± 8.61 versus 125.12 ± 13.47) lower at week 12 [Figure 4a]. In addition, plasma urea nitrogen levels in the NPX + OM + GMs + ESCs rats were 20% (12.34 ± 3.16 versus 15.46 ± 5.08) lower than in the NPX group at week 4 and 26% (15.25 ± 2.09 versus 20.66 ± 6.35) lower at week 12 [Figure 4b]. Plasma serum creatinine or urea nitrogen levels were not significantly different in the NPX, NPX + OM and NPX + OM + ESCs groups. There was also no difference in average body weight, urine output, and urine proteinuria among other groups, except for the Sham group.
Figure 3.
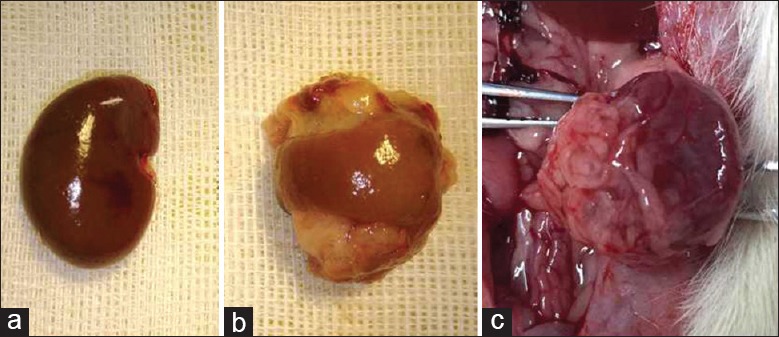
Fusion of omentum to the remnant kidney. (a) A normal adult rat kidney. (b) Omental attachment at the upper pole and lower pole of the remnant kidney 2 weeks after 5/6 nephrectomy. (c) The omentum remains tightly attached to the remnant kidney for up to 12 weeks.
Figure 4.
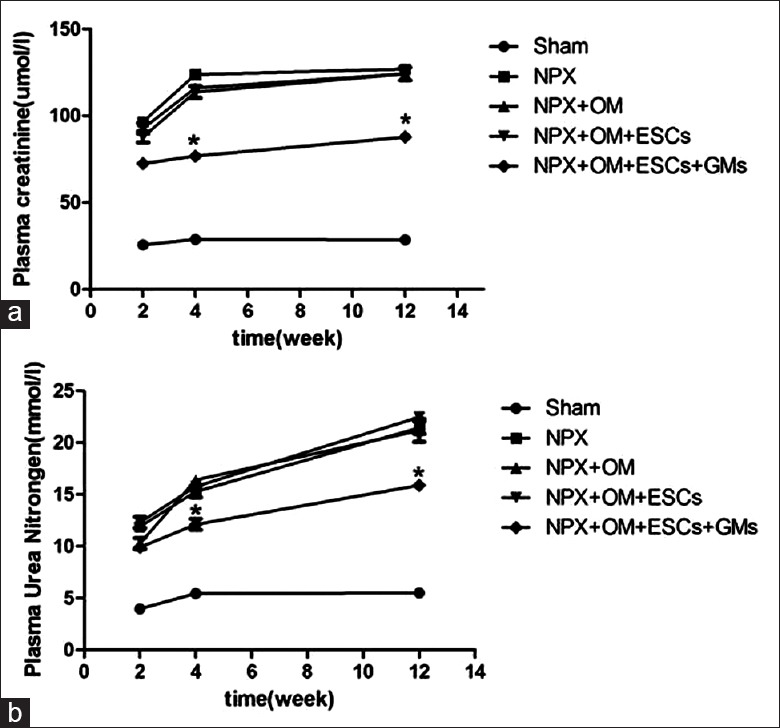
Renal functional parameters in each group. (a) Plasma creatinine levels are significantly lower in the nephrectomy+OM+gelatin microcryogels+embryonic stem cells rats (by 30–40%) at weeks 4 and 12 after inducing chronic kidney disease than in the nephrectomy rats. (b) Plasma urea nitrogen levels similarly are significantly lower in the nephrectomy+OM+gelatin microcryogels+embryonic stem cells rats (by 20–26%) at weeks 4 and 12 after inducing chronic kidney disease than in the nephrectomy rats. Data are expressed as the mean ± standard error of the mean. *P < 0.05 compared with nephrectomy at the respective time points. n = 8 in each group. NPX: Nephrectomy; OM: Omentum; ESCs: Embryonic stem cells; GMs: Gelatin microcryogels.
Histologic examination of the injured edge of kidney
Histologically, the injured edges of the NPX + OM + GMs + ESCs kidneys were demarcated by the omental tissue [PAS staining in Figure 5b and trichrome staining in Figure 6b]. Compared with the NPX kidneys that showed greater scarring of the glomeruli and tubules at the injured edges [Figures 5a and 6a], the NPX + OM + GMs + ESCs kidneys appeared to be better preserved [Figures 5b and 6b].
Figure 5.
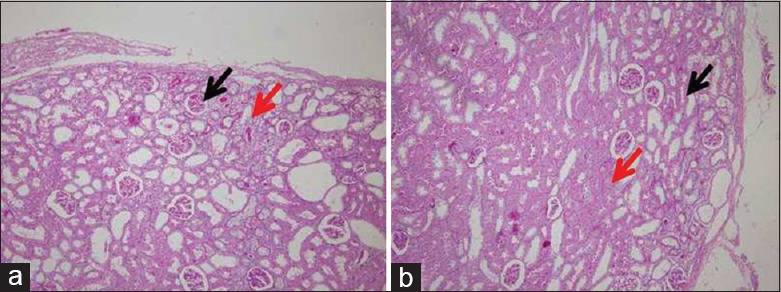
Injured edge of the kidney by periodic acid-Schiff staining 2 weeks after subtotal nephrectomy. (a) Nephrectomy kidneys show scarring of the tubules (red arrows), as well as the glomeruli (black arrows), at the injured edge. (b) In the nephrectomy+OM+gelatin microcryogels+embryonic stem cells kidneys, the injured edge, including the glomeruli and tubules, appears to be better preserved than the nephrectomy kidneys. OM: Omentum. (original magnification, × 100).
Figure 6.
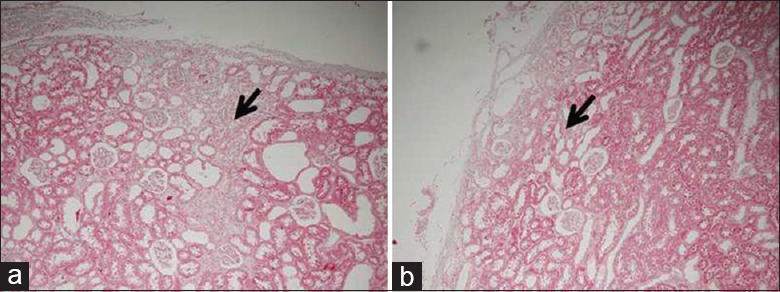
Injured edge of the kidney by trichrome staining to highlight fibrosis 2 weeks after subtotal nephrectomy. (a) Nephrectomy kidneys show extensive scarring (black color) at the injured edge. (b) By contrast, the nephrectomy+OM+gelatin microcryogels+embryonic stem cells kidneys showed much less scarring at the injured edge. OM: Omentum. (original magnification, × 100).
Glomerulosclerosis and tubular injury were reduced in the experimental rats
A gradient of glomerular as well as tubular injury was observed in the NPX + OM + GMs + ESCs kidneys. In the NPX + OM + GMs + ESCs group, the glomerulosclerosis index was 62% (1.29 ± 0.08 versus 3.42 ± 0.15) lower in the section than in the NPX group [Figure 7]. Similarly, the tubular injury index was 40% (0.68 ± 0.07 versus 1.15 ± 0.10) lower in the NPX + OM + GMs + ESCs kidneys; there were no significant changes between the NPX, NPX + OM and NPX + OM + ESCs groups [Figure 8].
Figure 7.
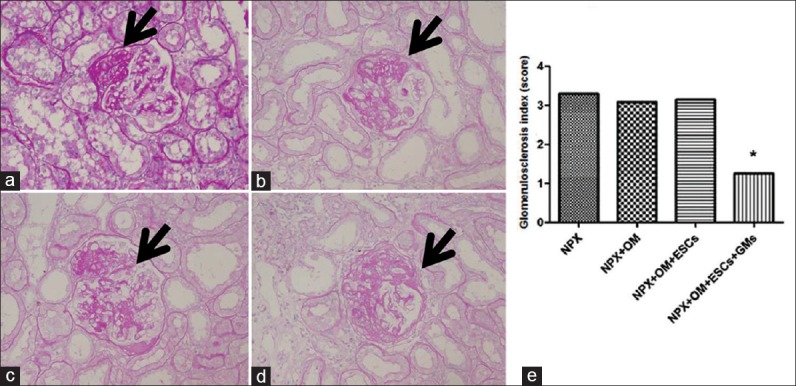
Kidney histology showing different grades of glomerular damage observed at week 12 after inducing chronic kidney disease in rats (periodic acid-Schiff staining). Arrows in the figure show damaged areas. (a) Glomerulus with a score of 1+showing mesangial expansion and slight glomerular damage involving <25% area of the glomerulus. (b) Glomerulus with a score of 2+showing mild sclerosis involving 25–50% area of the glomerulus. (c) Glomerulus with a score of 3+showing moderate sclerosis involving 50–75% area of the glomerulus. (d) Glomerulus with a score of 4+showing severe sclerosis involving >75% area of the glomerulus. (e) Glomerulosclerosis index at week 12 in the different groups of the remnant kidney. Compared with nephrectomy, nephrectomy+OM+gelatin microcryogels+embryonic stem cells rats showed 62% less glomerulosclerosis. Data are expressed as the mean ± standard error of the mean. *P < 0.05 compared with nephrectomy. n = 8 in each group. NPX: Nephrectomy; OM: Omentum; ESCs: Embryonic stem cells; GMs: Gelatin microcryogels. (original magnification, × 400).
Figure 8.
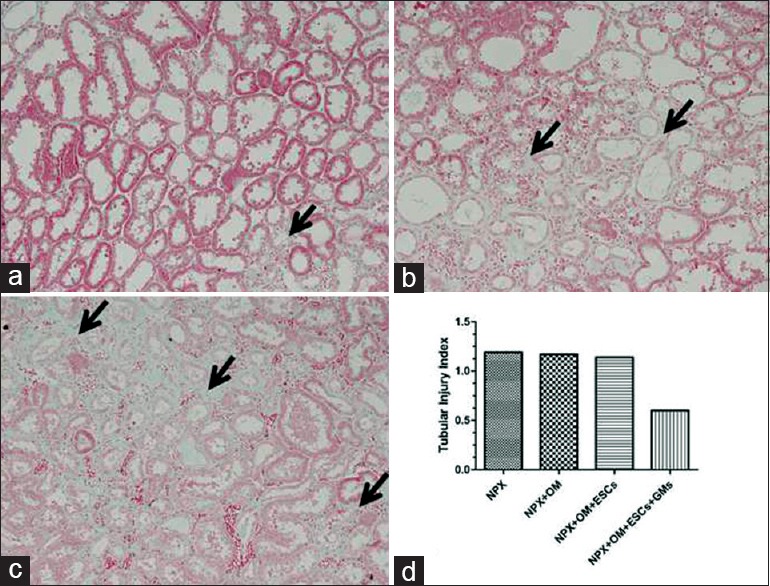
Kidney histology showing different grades of tubular injury at week 12 after inducing chronic kidney disease in rats (trichrome staining). Arrows in a–c show fields of interstitial expansion, fibrosis, and tubular dilation. (a) Representative tubulointerstitial area showing a histology score of 1+ (area of interstitial expansion, fibrosis, and tubular dilation involving <25% area of the total field). Arrows show areas of interstitial expansion. (b) Representative tubulointerstitial area showing a histology score of 2+ (lesion area between 25% and 50% of the total field). Left arrow shows interstitial fibrosis and the right arrow shows a dilated tubule. (c) Representative tubulointerstitial area showing a histology score of 3+ (lesions involving 50–75% of the total field). Arrows show interstitial fibrosis and dilated tubules. (d) Tubular injury index at week 12 in each group except the Sham group. Compared with nephrectomy, nephrectomy+OM+gelatin microcryogels+embryonic stem cells rats showed 40% less tubulointerstitial injury. However, none of these differences were statistically significant. Data are expressed as the mean ± standard error of the mean. n = 8 in each group. NPX: Nephrectomy; OM: Omentum; ESCs: Embryonic stem cells; GMs: Gelatin microcryogels. (original magnification, × 200).
DISCUSSION
Although adult stem cells have shown promise in treating AKI,[6,19,20,21] whether they can alleviate CKD has not been fully explored. One technical problem encountered in the experimental investigation of this issue is the fact that adult stem cells do not survive in the body for more than a few days after injection.[4,22,23] Therefore, stem cells will have to be injected every few days for several weeks to see an observable effect. GMs provide an optimal cell delivery and therapy platform that equips the cells for better therapeutic functions in vitro. The primed GMs constitute biomimetic 3D niches to accumulate ECMs, which can increase ESC viability, proliferation, and differentiation. In addition, the accumulated ECMs through cell priming within the in vitro microniches may increase the paracrine secretions of bioactive factors from the ESCs, which promote angiogenesis at the lesion sites. After cells are delivered to the lesion site, another challenge is preventing the leakage of dispersed cells into surrounding tissues and maintaining cell viability and function.[15] The greater omentum is mainly composed of loose connective tissue which has an abundant blood supply and lymph circulation.[24] Many collagen oblasts and capillaries can easily form ample collateral circulations and macrophages to eliminate inflammation and foreign substances, which allows a pedicled greater omentum flap to easily adhere to other tissues and may offer additional blood supply. In fact, the greater omentum is a natural patch that is widely used in many types of operations, such as plugging a lacerated wound in the liver, covering the wound surface after liver resection,[25] and wrapping the stoma in a pancreaticojejunostomy.[26] The pedicled greater omentum flap can transpose well-vascularized tissue with angiogenic and immunological properties to tissues without spontaneous healing capacities. We used the pedicled greater omentum flap to wrap the ESC-loaded GMs on the 5/6 nephrectomized kidney to prevent ESC leakage and allow the ESCs to survive in the body for longer periods of time.
We showed that ESCs could be automatically loaded into GMs by simply pipetting the cells onto the surface of the collected GMs. ESCs exhibited good cell attachment and viability in GMs in vitro. ESCs seeded within GMs resultedin tissue-like ensembles with enriched extracellular matrices and enhanced cell-cell interactions. Due to technical difficulties, we were unable to assess the survival of ESCs in vivo. We showed that pedicled omentum flaps packing ESC-loaded GMs on the 5/6 nephrectomized kidney improved residual renal function in the remnant kidney. After 4 weeks, 5/6 nephrectomized and omentectomized rats developed CKD, as demonstrated by elevated plasma creatinine/urea nitrogen levels. In contrast, the pedicled omentum flaps packing ESC-loaded GMs on the 5/6 nephrectomized kidney exhibited lower plasma creatinine/urea nitrogen levels. There was also reduced glomerulosclerosis and tubulointerstitial injury than in the specimens subjected to 5/6 NPX without GMs. In addition, the pedicled great omentum packing ESC-loaded GMs on the 5/6 nephrectomized kidneys showed fewer myofibroblastsin the interstitial area. Garcia-Gomez et al.[27] showed that 6 weeks after 5/6 NPX, fusing the polydextran gel particle activated omentum to the remnant kidney resulted in higher creatinine clearance and lower plasma creatinine/urea nitrogen levels. Our results showed that the pedicled omentum packing ESC-loaded GMs on the 5/6 nephrectomized kidney exhibited significantly lower plasma creatinine/urea nitrogen levels at 4 weeks, which was an earlier time frame. This was possible because the injured tissue in the fusion zone may be more responsive to the growth factors from ESC-loaded GMs.
To further understand the salutary effect of the pedicled omentum packing ESC-loaded GMs on the 5/6 nephrectomized kidney, we found that the fusion zone showed minimal fibrosis, good vascularization, and structural preservation of the parenchyma. In contrast, the injured edge of the 5/6 nephrectomized kidneys without GMs showed extensive fibrosis and dedifferentiation of tubules. At the same time, the pathological results also showed that not all nephrons could be preserved.
From our results regarding the differences in glomerular and tubular injury in the pedicled great omentum packing ESCs-loaded GMs on the 5/6 nephrectomized kidneys, it appears that the pedicled great omentum packing ESCs may have been instrumental in slowing the progression of CKD, mostly by rescuing the nephrons in the injured kidney. It is possible that this may be due to rapid revascularization and hemostasis of the injured edge kidneys by the omentum. The omentum brings about tissue repair by readily vascularizing the injured organs with which it fuses. We also suspected that ESCs may excrete high concentrations of paracrine growth factor. Chou et al.[28] suggest that rather than replacing the injured epithelial and endothelial cells, the major contribution of bone marrow-derived cells (BMDCs) to renal repair occurs through aparacrine mechanism. For example, in ischemic renal injury, BMDCs can initially ameliorate the injury either by directly inhibiting cellapoptosis or preventing inflammatory cell influx. During the repair phase, BMDCs secrete factors that promote tubular epithelial cell dedifferentiation andproliferation. On the basis of these results, we speculated that the recovery function in our model depended not only on the diffusible factors from ESCs but also on the proximity of the kidney parenchyma to the omentum.
ESCs are pluripotent stem cells that can give rise to all cell lineages of the body under appropriate culture conditions. In particular, labeled ESCs microinjected into developing metanephros in organ culture were exclusively located in the cortical nephrogenic zone and differentiated into epithelial cells resembling renal tubules and, occasionally, into glomerular tufts. In addition, when injected in vivo into mice, ESCs integrated into proximal tubules of newborn mice and were detectable for 7 months without teratoma formation.[29]
In summary, slowing the progression of CKD by the pedicled great omentum flaps packing ESC-loaded GMs on the 5/6 nephrectomized kidneys supports the concept that ESCs have the potential to treat CKD. Clearly, our study suggests that ESCs could be effective in treating CKD. In addition, the GMs-assisted cell therapy system holds great promise for achieving cell location, migration, and differentiation at the lesion site as a widely adopted platform technology. On the basis of our findings, we also believe that the omentum could help preserve the remnant kidney and that GMs offer a widely applicable cell delivery platform technology to boost the healing power of cell regenerative therapy. The present study offers a new perspective in stem cell therapy and regenerative medicine in CKD in which ESCs could play a beneficial therapeutic role.
Financial support and sponsorship
This study was granted by the National High Technology Research and Development Program of China (No. 2011AA020115), Major State Basic Research Development Program of China (No. 2014CBA02005), Chinese National Natural Sciences Foundation (No. 81470949 and 31170810), The Medical Technology Youth Training Project of PLA (No. 13QNP180) and Beijing NOVA Program (No. Z121107002512078), Hainan Province Medical Research Program (No. 13A110006).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Yi Cui
REFERENCES
- 1.Weiner DE, Tighiouart H, Stark PC, Amin MG, MacLeod B, Griffith JL, et al. Kidney disease as a risk factor for recurrent cardiovascular disease and mortality. Am J Kidney Dis. 2004;44:198–206. doi: 10.1053/j.ajkd.2004.04.024. doi: 10.1053/j.ajkd.2004.04.024. [DOI] [PubMed] [Google Scholar]
- 2.Chiurchiu C, Remuzzi G, Ruggenenti P. Angiotensin-converting enzyme inhibition and renal protection in nondiabetic patients: The data of the meta-analyses. J Am Soc Nephrol. 2005;16(Suppl 1):S58–63. doi: 10.1681/asn.2004110968. doi: 10.1681/ASN.2004110968. [DOI] [PubMed] [Google Scholar]
- 3.Flaquer M, Romagnani P, Cruzado JM. Growth factors and renal regeneration. Nefrologia. 2010;30:385–93. doi: 10.3265/Nefrologia.pre2010.Jun.10463. doi: 10.3265/Nefrologia. [DOI] [PubMed] [Google Scholar]
- 4.Bernardo ME, Pagliara D, Locatelli F. Mesenchymal stromal cell therapy: A revolution in regenerative medicine? Bone Marrow Transplant. 2012;47:164–71. doi: 10.1038/bmt.2011.81. doi: 10.1038/bmt.2011.81. [DOI] [PubMed] [Google Scholar]
- 5.Li M, Ikehara S. Bone-marrow-derived mesenchymal stem cells for organ repair. Stem Cells Int 2013. 2013 doi: 10.1155/2013/132642. 132642. doi: 10.1155/2013/132642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Biancone L, Bruno S, Deregibus MC, Tetta C, Camussi G. Therapeutic potential of mesenchymal stem cell-derived microvesicles. Nephrol Dial Transplant. 2012;27:3037–42. doi: 10.1093/ndt/gfs168. doi: 10.1093/ndt/gfs168. [DOI] [PubMed] [Google Scholar]
- 7.Hopkins C, Li J, Rae F, Little MH. Stem cell options for kidney disease. J Pathol. 2009;217:265–81. doi: 10.1002/path.2477. doi: 10.1002/path.2477. [DOI] [PubMed] [Google Scholar]
- 8.Feng Z, Ting J, Alfonso Z, Strem BM, Fraser JK, Rutenberg J, et al. Fresh and cryopreserved, uncultured adipose tissue-derived stem and regenerative cells ameliorate ischemia-reperfusion-induced acute kidney injury. Nephrol Dial Transplant. 2010;25:3874–84. doi: 10.1093/ndt/gfq603. doi: 10.1093/ndt/gfq603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Liang J, Zhang H, Hua B, Wang H, Lu L, Shi S, et al. Allogenic mesenchymal stem cells transplantation in refractory systemic lupus erythematosus: A pilot clinical study. Ann Rheum Dis. 2010;69:1423–9. doi: 10.1136/ard.2009.123463. doi: 10.1136/ard.2009.123463. [DOI] [PubMed] [Google Scholar]
- 10.Pistoia V, Raffaghello L. Potential of mesenchymal stem cells for the therapy of autoimmune diseases. Expert Rev Clin Immunol. 2010;6:211–8. doi: 10.1586/eci.09.86. doi: 10.1586/eci.09.86. [DOI] [PubMed] [Google Scholar]
- 11.Morigi M, Benigni A. Mesenchymal stem cells and kidney repair. Nephrol Dial Transplant. 2013;28:788–93. doi: 10.1093/ndt/gfs556. doi: 10.1093/ndt/gfs556. [DOI] [PubMed] [Google Scholar]
- 12.Leitch HG, McEwen KR, Turp A, Encheva V, Carroll T, Grabole N, et al. Naive pluripotency is associated with global DNA hypomethylation. Nat Struct Mol Biol. 2013;20:311–6. doi: 10.1038/nsmb.2510. doi: 10.1038/nsmb.2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mae S, Shono A, Shiota F, Yasuno T, Kajiwara M, Gotoda-Nishimura N, et al. Monitoring and robust induction of nephrogenic intermediate mesoderm from human pluripotent stem cells. Nat Commun. 2013;4:1367. doi: 10.1038/ncomms2378. doi: 10.1038/ncomms2378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Steenhard BM, Isom KS, Cazcarro P, Dunmore JH, Godwin AR, St John PL, et al. Integration of embryonic stem cells in metanephric kidney organ culture. J Am Soc Nephrol. 2005;16:1623–31. doi: 10.1681/ASN.2004070584. doi: 10.1681/ASN.2004070584. [DOI] [PubMed] [Google Scholar]
- 15.Li Y, Liu W, Liu F, Zeng Y, Zuo S, Feng S, et al. Primed 3D injectable microniches enabling low-dosage cell therapy for critical limb ischemia. Proc Natl Acad Sci U S A. 2014;111:13511–6. doi: 10.1073/pnas.1411295111. doi: 10.1073/pnas.1411295111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haltia O, Törmänen S, Eräranta A, Jokihaara J, Nordhausen K, Rysä J, et al. Vasopeptidase inhibition corrects the structure and function of the small arteries in experimental renal insufficiency. J Vasc Res. 2015;52:94–102. doi: 10.1159/000431368. doi: 10.1159/000431368. [DOI] [PubMed] [Google Scholar]
- 17.Schinner E, Wetzl V, Schlossmann J. Cyclic nucleotide signalling in kidney fibrosis. Int J Mol Sci. 2015;16:2320–51. doi: 10.3390/ijms16022320. doi: 10.3390/ijms16022320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cao Z, Cooper ME, Wu LL, Cox AJ, Jandeleit-Dahm K, Kelly DJ, et al. Blockade of the renin-angiotensin and endothelin systems on progressive renal injury. Hypertension. 2000;36:561–568. doi: 10.1161/01.hyp.36.4.561. doi: 10.1161/01.HYP.36.4.561. [DOI] [PubMed] [Google Scholar]
- 19.Tögel F, Hu Z, Weiss K, Isaac J, Lange C, Westenfelder C. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–42. doi: 10.1152/ajprenal.00007.2005. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 20.Schu S, Nosov M, O’Flynn L, Shaw G, Treacy O, Barry F, et al. Immunogenicity of allogeneic mesenchymal stem cells. J Cell Mol Med. 2012;16:2094–103. doi: 10.1111/j.1582-4934.2011.01509.x. doi: 10.1111/j.1582-4934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qian H, Yang H, Xu W, Yan Y, Chen Q, Zhu W, et al. Bone marrow mesenchymal stem cells ameliorate rat acute renal failure by differentiation into renal tubular epithelial-like cells. Int J Mol Med. 2008;22:325–32. doi: 10.3892/ijmm_00000026. [PubMed] [Google Scholar]
- 22.Ankrum J, Karp JM. Mesenchymal stem cell therapy: Two steps forward, one step back. Trends Mol Med. 2010;16:203–9. doi: 10.1016/j.molmed.2010.02.005. doi: 10.1016/j.molmed.2010.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mooney DJ, Vandenburgh H. Cell delivery mechanisms for tissue repair. Cell Stem Cell. 2008;2:205–13. doi: 10.1016/j.stem.2008.02.005. doi: 10.1016/j.stem.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 24.Casey K, Sabino J, Weiss JS, Kumar A, Valerio I. Limb salvage after vascular reconstruction followed by tissue transfer during the global war on terror. J Vasc Surg. 2015;61:734–40. doi: 10.1016/j.jvs.2014.10.039. doi: 10.1016/j.jvs.2014.10.039. [DOI] [PubMed] [Google Scholar]
- 25.Okano K, Asano E, Oshima M, Yamamoto N, Yachida S, Nishizawa Y, et al. Omental flap wrapping with fixation to the cut surface of the liver for reducing delayed gastric emptying after left-sided hepatectomy. Surg Today. 2013;43:1425–32. doi: 10.1007/s00595-012-0446-8. doi: 10.1007/s00595-012-0446-8. [DOI] [PubMed] [Google Scholar]
- 26.Maeda A, Ebata T, Kanemoto H, Matsunaga K, Bando E, Yamaguchi S, et al. Omental flap in pancreaticoduodenectomy for protection of splanchnic vessels. World J Surg. 2005;29:1122–6. doi: 10.1007/s00268-005-7900-3. doi: 10.1007/s00268-005-7900-3. [DOI] [PubMed] [Google Scholar]
- 27.Garcia-Gomez I, Pancholi N, Patel J, Gudehithlu KP, Sethupathi P, Hart P, et al. Activated omentum slows progression of CKD. J Am Soc Nephrol. 2014;25:1270–81. doi: 10.1681/ASN.2013040387. doi: 10.1681/ASN.2013040387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chou YH, Pan SY, Yang CH, Lin SL. Stem cells and kidney regeneration. J Formos Med Assoc. 2014;113:201–9. doi: 10.1016/j.jfma.2013.12.001. doi: 10.1016/j.jfma.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 29.Aggarwal S, Moggio A, Bussolati B. Concise review: Stem/progenitor cells for renal tissue repair: Current knowledge and perspectives. Stem Cells Transl Med. 2013;2:1011–9. doi: 10.5966/sctm.2013-0097. doi: 10.5966/sctm.2013-0097. [DOI] [PMC free article] [PubMed] [Google Scholar]