Abstract
Objective:
This review focuses on the current knowledge on the implication and significance of beta 2 microglobulin (β2M), a conservative immune molecule in vertebrate.
Data Sources:
The data used in this review were obtained from PubMed up to October 2015. Terms of β2M, immune response, and infection were used in the search.
Study Selections:
Articles related to β2M were retrieved and reviewed. Articles focusing on the characteristic and function of β2M were selected. The exclusion criteria of articles were that the studies on β2M-related molecules.
Results:
β2M is critical for the immune surveillance and modulation in vertebrate animals. The dysregulation of β2M is associated with multiple diseases, including endogenous and infectious diseases. β2M could directly participate in the development of cancer cells, and the level of β2M is deemed as a prognostic marker for several malignancies. It also involves in forming major histocompatibility complex (MHC class I or MHC I) or like heterodimers, covering from antigen presentation to immune homeostasis.
Conclusions:
Based on the characteristic of β2M, it or its signaling pathway has been targeted as biomedical or therapeutic tools. Moreover, β2M is highly conserved among different species, and overall structures are virtually identical, implying the versatility of β2M on applications.
Keywords: Beta 2 Microglobulin, Immune Dysregulation, Major Histocompatibility Complex Class I or Like Molecules
INTRODUCTION
Beta 2 microglobulin (β2M) is a small protein (11,800 Dalton), presenting in nearly all nucleated cells and most biological fluids, including serum, urine, and synovial fluid.[1,2] No genetic variant of β2M is known in human.[3] The human β2M shows 70% amino acid sequence similarity to the murine protein and both of them locate on the syntenic chromosomes.[1,4] The secondary structure of β2M consists of seven β-strands which are organized into two β-sheets linked by a single disulfide bridge, presenting a classical β-sandwich typical of the immunoglobulin (Ig) domain.[5,6,7] β2M has no transmembrane region and contains a distinctive molecular structure called a constant-1 Ig superfamily domain, sharing with other adaptive immune molecules including major histocompatibility complex (MHC) class I and class II.[8] Two evolutionary conserved tryptophan (Trp) residues are important for correct structural fold and function of β2M.[3,9] Trp60 is exposed to the solvent at the apex of a protein loop and is critical for promoting the association of β2M in MHC I. The mutation of Trp60 increases the stabilization of β2M, inhibits β2 amyloidogenic propensity, and weakens the interaction with the heavy chain of MHC I. Trp95 is buried in the β2M core, and the mutation of Trp95 destabilizes the protein, yielding nonfibrillar β2M aggregates. Both Trp residues play differential and complementary roles in the structure of β2M, distinctly affecting β2M toward self-aggregation into amyloid fibrils. Once the aspartate residue is replaced by asparagine residue at position 76, β2M becomes thermodynamically unstable and remarkably fibrillogenic in vitro under physiological conditions.[10]
Normally, β2M is noncovalently linked with the other polypeptide chain (α chain) to form MHC I or like structures, including MHC I, neonatal Fc receptor (FcRn), a cluster of differentiation 1 (CD1), human hemochromatosis protein (HFE), Qa, and so on. β2M makes extensive contacts with all three domains of the α chain.[11] Thus, the conformation of α chain is highly dependent on the presence of β2M. Although α1 and α2 domains differ among molecules, α3 domain and β2M are relatively conserved, where the intermolecular interaction occurs.[12] A number of residues at the points of contact with β2M are shared among MHC I or like molecules.[13] Furthermore, interactions with α1 and α2 domains are important for the paired association of α3 domain and β2M in the presence of native antigens.[14] β2M could dissociate from such molecules and shed into the serum, where it is transported to the kidneys to be degraded and excreted. An 88-kD protein (calnexin) associates rapidly and quantitatively with newly synthesized murine MHC I molecules within the endoplasmic reticulum.[15] Both β2M and peptide are required for efficient calnexin dissociation and subsequent MHC I transport.[16]
Not only β2M is to interact with and stabilize the tertiary structure of the MHC I or like molecules, but also it is extensively involved in the functional regulation of survival, proliferation, apoptosis, and even metastasis in cancer cells.[17] As well as a cancer prognostic marker, β2M is also a promising cancer therapeutic target. Although β2M acts as both a positive and negative growth factor in different cancer cells, the application of anti-β2M antibodies induces cancer cell apoptosis and do not block the down-regulation effect of β2M in myeloma cells.[18] Moreover, systemic β2M accumulation in aging blood promoted age-related cognitive dysfunction and impairs neurogenesis, suggesting that β2M may be targeted therapeutically in old age.[19] Thus, targeting β2M will shed light on the modulatory activity in the immune system and provide new pathways on cancer or aging-related therapeutics. This review will only focus on the characteristic and function of β2M under present knowledge. For MHC I or like molecules, they have been well reviewed previously and are not the scope of this study.
REGULATION AND MODULATION OF BETA 2 MICROGLOBULIN
β2M expresses at a constant level in many cells, however, the formation of β2M would be enhanced in the presence of IFN-α.[17] β2M could induce the expression of interleukin 6 (IL-6), 8 and 10 in several cell types, regulate the expression of hormone/growth factor, and coordinate the interaction between cytokines and their receptors.[20,21,22]
Like a prototypical oncogenic factor, β2M is able to stimulate growth and progression of various cancers.[23,24,25] In cancer bone metastasis, β2M allows cancer cells to continue to synthesize and deposit bone-like proteins. The growth and migration of mesenchymal stem cells would be promoted by exogenous overexpression of β2M through enhanced phosphorylation of cAMP response element-binding protein and upregulation of IL-6 and vascular endothelial growth factor.[26,27] β2M could support lethal bone and soft tissue metastasis via activating epithelial to mesenchymal transition.[28,29] β2M also acts as an apoptosis-inducing factor in several leukemic, lymphoma, and myeloma cell lines.[18,30,31] Inhibition of β2M enhanced the radiation sensitivity by induction of iron overload in prostate cancer cells.[32]
In patients suffering from long-term hemodialysis, a high concentration of serum β2M leads that β2M deposits in skeletal joints and forms amyloid plaques. Among the fibrils, full-length β2M is the major component, although other derivatives of β2M are also present.[33] Furthermore, the H51A point mutation of β2M exhibits a 2-fold increase in the lag-time of fibril formation.[34]
β2M also induces a dose- and time-dependent, cell-mediated calcium efflux from neonatal mouse calvariae that involves osteoclast stimulation, which is mediated by IL-1β partly.[35] The expression and covalent association of tapasin, assisting MHC I to load antigenic peptides, were enhanced by the presence of β2M.[36]
INDICATIONS FROM THE LEVEL OF FREE BETA 2 MICROGLOBULIN
The abnormal level of β2M in blood or urea is associated with multiple diseases, such as some acute and chronic inflammations, liver or renal dysfunctions, some viral infections, and several malignancies.[2,17] Furthermore, amyloidosis associated to hemodialysis is related with persistently high β2M serum levels.[37,38,39] In rare cases, a cerebrospinal fluid β2M level is used to assess a disease involved with the central nervous system.[40,41]
Serum and plasma β2M values reflect the activation of the cellular immune system, as well as a tumor marker in certain hematologic malignancies.[42,43,44,45,46,47] For the inflammatory bowel disease, β2M was suggested to be used as an activity parameter.[48] β2M levels also rise during infection with some viruses, including cytomegalovirus and human immunodeficiency virus (HIV).[49] Strong evidence showed cytomegalovirus could directly bind β2M via two envelope proteins.[50] Recently, soluble β2M was proposed as a possible serologic marker of neurologic disease during the infection of human T-cell leukemia virus.[51] On the other hand, abnormality of urine β2M values indicates renal filtration or reabsorption disorders. The small size of β2M allows it pass through the glomerular membrane, however, it can be reabsorbed in the proximal tubules by specific receptors. The disorder of kidney's glomeruli would cause increased β2M in blood and decreased β2M in urea, in contrast, the disorder of kidney's tubules would cause increased β2M in urea and decreased β2M in blood.[2] In lupus nephritis and neonates, the index of serum β2M/cystatin C is suggested to indicate the renal function.[52,53] Moreover, serum β2M levels at discharge would predict the long-term mortality and graft loss in kidney transplantation recipients.[54] A large nationally representative cohort exhibited serum β2M concentration was associated with a significantly increased risk of cardiovascular and all-cause mortality.[55] Recently, the concentration of β2M was also deemed as a marker of frailty in older people.[56] Thus, the β2M test could indicate how advanced the disease is and the likely prognosis for the patient at the time of diagnosis.
OUTCOMES DUE TO BETA 2 MICROGLOBULIN DEFICIENCY
β2M deficient mutant has been derived in different models and changes of cellular and humoral responses are evaluated in these β2M deficient animals.[57] The mechanism of MHC I presenting peptides to CD8+ T-cell was shown in Figure 1. Due to the lack of β2M determined MHC I molecules, the number of CD8+ T-cells significantly decreases in β2M deficient mice.[58,59,60,61,62] Therefore, the deficient mice are susceptible to intracellular pathogens, including Listeria monocytogenes, Mycobacterium tuberculosis, influenza virus, and so on. However, β2M deficient mice could generate CD4+ MHC II-restricted cytotoxic T-cells (CTL) following infection with Sendai virus or lymphocytic choriomeningitis virus.[63,64] Furthermore, MHC I-restricted CTL activity could be activated during infections by some specific pathogens even in β2M deficient mice.[65] In β2M deficient mice, natural killer cells are shown with increased sensitivity to MHC I heavy chain mediated inhibition.[66] Other cellular responses are relatively stable, including gamma delta+ T-cells and CD4+ T-cells.[67] Hemochromatosis is evident in β2M deficient mice, presenting iron overload.[62] The iron overload increases the sensitivity to the infection of M. tuberculosis.[61] The catabolism of IgG and albumin increased in β2M deficient mice due to low expression of FcRn.[68,69] However, the level of mucosal IgA was significantly increased during enteric infection of β2M deficient mice, indicating different roles of β2M in Ig catabolism. On the other hand, autoantibody-mediated inflammations or immune diseases are prevented or relieved in β2M deficient mice.[70,71] The disruption of β2M significantly reduced the expression of MHC I in human embryonic stem cells, presenting hypoimmunogenic and favoring transplantation therapies.[72] Furthermore, the loss of β2M contributes the immune evasion in cancer cells.[73]
Figure 1.
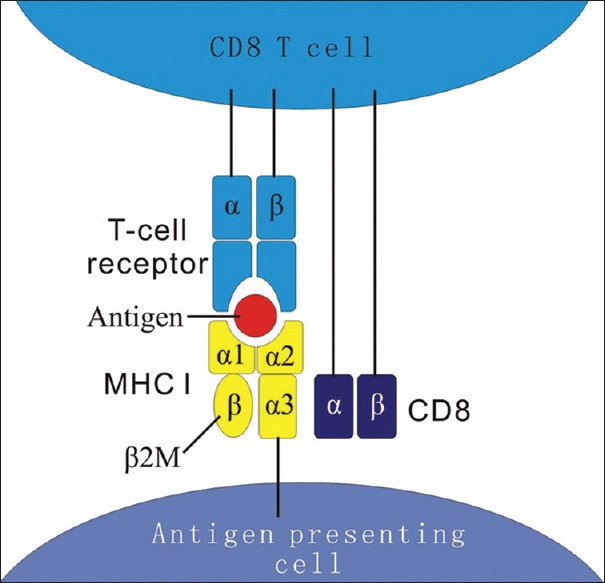
Schematic representation of MHC I antigen presentation to CD8 T-cell. MHC I consists of two polypeptide chains, α (α1, α2, α3) and β2M, which are noncovalently linked between α3 and β2M. The peptides (antigens) generated from cytosolic proteins bind the polymorphic groove between α1 and α2 and are displayed to CD8 T-cell receptors. Meanwhile, the CD8 co-receptor of CD8 T-cell would interact with α3. β2M: Beta 2 microglobulin; MHC I: Major histocompatibility complex class I; CD8: Cluster of differentiation 8.
SPECIFIC ROLES OF BETA 2 MICROGLOBULIN WITHIN HETERODIMERS
The main function of MHC I or MHC I-like molecules is related with molecular presentations or uptakes, depending on the structural-like grooves between α1 and α2 domains. Comparing with MHC I groove, the CD1 groove is relatively narrow, deep, and highly hydrophobic forming two deep binding pockets.[74,75] This hydrophobic channel is specific for binding hydrocarbon alkyl chains. Unlike MHC I, CD1 molecules are targeted to distinct endocytic compartments by cytoplasmic tails. On the other hand, the FcRn groove is collapsed, demonstrating a relatively flat groove. Although these structures are highly similar, different functions are presented with these molecules relating to site mutagenesis on specific binding sites. The most striking difference of FcRn is the closing of the groove that binds peptides in classical MHC I proteins, due to a kink in the α2 helix introduced by proline (Pro)-162.[13] Moreover, the pocket of FcRn is blocked by the positively charged side chain of arginine (Arg)-164. β2M stabilizes the tertiary structure of such heterodimers and also participates in the selections of MHC I-like restricted T-cells. For instance, the selection of invariant Vα19-Jα33+ cells is dependent on β2M.[76]
Major histocompatibility complex class I
MHC I molecules are found on nearly every nucleated cell of the body. Their function is to present short endogenous or exogenous peptides from within the cell to CTLs. β2M is crucial to stabilize cell surface MHC I, keep native structure of MHC I heavy chain, facilitate the binding of antigenic peptides, and generate additional high-affinity peptide-bindings.[77] However, some cells express a considerable number of surface MHC I heavy chain molecules not associated with β2M.[14,78,79] The superficial nonpeptide-associated heavy chains can associate with exogenously provided β2M and synthetic peptide antigens.[80] Moreover, normal β2M-sufficient cells grown in serum-free media devoid of β2M also require an exogenous β2M to efficiently bind synthetic peptide. By using this characteristic, β2M was developed to vaccine adjuvant for CTL activation.[81] Using the immunization protocol with human β2M, CTL responses were strongly primed with peptides from OVA, S. virus, and vesicular stomatitis virus in mice. The versatility of β2M in different species confirms the conservative evolutionary lineage of this small protein. ESAT-6, an abundantly secreted protein of M. tuberculosis, could directly interact with human β2M to inhibit the expression of MHC I, resulting in down-regulation of class I-mediated antigen presentation.[82]
Neonatal Fc receptor
FcRn is a heterodimer of a nonclassical MHC I alpha chain and β2M. It efficiently binds the two most abundant serum proteins, IgG, and albumin. Both proteins are protected by FcRn from lysosomal degradation and extend the catabolic half-lives.[69] Beside the protection role with FcRn heterodimer, β2M seems to have another way to protect the degradation of IgG and albumin.[83] Furthermore, FcRn is critically involved in the transport of IgG across cells, thus helping antigen delivery via transcytosis.[84]
β2M is critical for surface expression of FcRn, facilitating FcRn to exit the endoplasmic reticulum.[85] Furthermore, β2M is important for efficient pH-dependent binding of IgG by FcRn. Strong evidence show β2M could directly contact IgG ligand.[86] Not only overexpression of FcRn enhances the transcytosis of immune complexes and increases the number of antigen-specific IgM or IgG-producing B cells,[87] but also the expression of β2M increases the transcytosis of IgG between the basolateral and apical directions of epithelial cells.[88] Moreover, the application of β2M as adjuvant requires the temporal proximity with antigens, confirming that β2M facilitates the uptake of antigens.[81]
Cluster of differentiation 1
CD1 is a family of glycoproteins expressed on the surface of various antigen-presenting cells, and CD1-like genes have been found in many vertebrate genomes. They are closely related to the MHC I and are involved in the presentation of lipid antigens to restricted T-cells. According to protein sequence homologies, the members of the CD1 family are mainly divided into two groups.[89] Group 1 CD1 includes CD1a, -b, and -c and human, mouse, rat, and rabbit CD1d form group II. Furthermore, CD1e is proposed to form a third group due to its intracellular chaperone function.[90,91] The size and shape of the antigen-binding groove vary among different CD1 isoforms and decide the nature of the binding lipid molecules.
The excretion of different CD1 isoforms differs in the presence of β2M. For instance, heavy chains of CD1b are detained in the ER in β2M-deficient cells,[92] however, a portion of CD1d heavy chains can exit the ER and reach the cell surface independent onβ2M.[93,94,95] Interestingly, the non-β2M formed CD1d only presents at the apical site of intestinal epithelial cells.[96] Like the adjuvant application of β2M on CTL, β2M seems like a potential adjuvant to prime CD1d specific immune responses.
Human hemochromatosis protein
As an MHC I-like molecule, HFE is a ligand for the transferrin receptor, regulating the uptake of iron-bound transferrin. Hepcidin regulated by HFE degrades the iron transporter ferroportin on the cytoplasmic membrane of enterocytes and macrophages, resulting in decreased iron uptake from food and iron release from recycled red blood cells. Therefore, mutations of HFE or absence of β2M result in iron excess and hemochromatosis.[59,60,61] The mechanism of iron accumulation in the β2M deficient mouse may be more complex than only involving HFE.[62] In contrast to HFE α chain-deficient mice, β2M-deficient mice display increased levels of iron transporters and iron overload, suggesting that (an) additional β2M interacting protein(s) could be involved in controlling iron homeostasis. The interaction with β2M is crucial for surface expression of HFE.[97,98] Unlike other MHC I-like molecules, HFE does not bind any antigen.[99]
OTHER MAJOR HISTOCOMPATIBILITY COMPLEX I-LIKE MOLECULES
Qa-1 (HLA-E, human functional counterpart) is designated as nonclassical histocompatibility Ags, eliciting strong CTL responses.[100] β2M is required for an initial folding of Qa-1, however, transporter associated with antigen processing (TAP) is not necessary for processing of Qa-1 molecules. Furthermore, the presence of CD8α/α TCRα/β cells in intestinal intraepithelial lymphocytes is highly restricted by Qa-2 (HLA-G, human functional counterpart).[101]
Histocompatibility 2, M region locus 3 (H2-M3) associates with β2M to form MHC I-like structure. The expression of H2-M3 is confined to murid species, which are highly conserved. H2-M3 mainly presents N-formylated peptides, and its surficial expression is dependent on ligand binding. Once H2-M3 binds peptides in the endoplasmic reticulum, they transit rapidly to the cell surface, where they stimulate CD8+ αβ T-cells in a TAP-dependent manner.[102,103]
Human MR1 encoded on chromosome 1 is highly conserved among mammals and is more closely related to classical class I molecules than are other nonclassical class I family members.[104,105] The MR1 is responsible for activation of mucosal-associated invariant T-cells expressing semi-invariant T-cell receptors in the presence of bacteria. Moreover, the MR1 messenger RNA is ubiquitously expressed in different tissues or cell lines104, and the surficial expression of MR1 requires the presence of β2M.[106] The lack of β2M or MR1 increases the susceptibility to infection by Klebsiella pneumoniae.[107] MR1 is ideally suited to bind ligands originating from vitamin metabolites.[108]
MILL (MHC class I-like located near the leukocyte receptor complex) is a family of MHC I-like molecules, which are glycophosphatidylinositol-anchored glycoproteins associated with β2M. Surface expression of MILL does not require functional TAP molecules and is not related with the presentation of peptides.[109]
EVOLUTIONARY RELATIONSHIP AMONG MAJOR HISTOCOMPATIBILITY COMPLEX I OR LIKE MOLECULES
Given the structural similarities, it is believed that all these MHC I or MHC I-like molecules have evolutionary lineage with a common ancestor.[13] The MHC locus has been found in all jawed vertebrates, however, the proto-MHC could trace back to the cephalochordate (amphioxus) and jawless vertebrate lineages.[110,111,112] MHC II genes were firstly derived from proto-MHC by exon shuffling, combining an Ig-like C domain with a peptide binding region.[113] Subsequently, another peptide binding region exon was added to MHC II β chain to form the MHC I heavy chain, which happened at approximately 500 million years ago. The emergence of CD1 occurred in the reptile form lineage after the amphibian–reptile split roughly between 365 and 385 million years ago. The MR1 is highly conserved and seems to be unique to mammals.[114] Contrasting to positive selection on the ligand-binding site of MHC I, the conservative ligand-binding site of MR1 evolved under strong negative selection. H2-M3 is also highly conserved, and its expression is confined to murid species. The emergence of H2-M3 occurred 50–65 million years ago.[114] FcRn is supposed to share an ancestor with the MHCs that it does not with the CD1s.[13] Further evidence shows, FcRn diverged from the MHC near the most recent common ancestor of lizards and mammals.
β2M is believed to arise in a basal jawed vertebrate (gnathostome).[8] The close proximity of MHC I, MHC II, and β2M implies that they were derived from a common ancestor by tandem (cis) duplication.[8] β2M protein sequences are highly conserved among species, and overall structures are virtually identical. Ten residues are identical in all species, including the two characteristically spaced cysteine residues which form the disulfide bridge.[12]
CONCLUSION AND PERSPECTIVES
The structure and function are highly conserved not only in β2M but also in its related molecules. It indicates that β2M is irreplaceable in animals, especially in vertebrate. β2M involves in the network of cytokines, modulating the development of several cell lines. Furthermore, hormone, growth factors, and cognate receptors are also regulated by β2M. As a prognostic marker of various diseases, the level of β2M reflects the progress of the disease and the likely prognosis for the patient. The special role of β2M in regulating the survival, proliferation, apoptosis, and even metastasis of cancer cells makes itself being targeted for cancer therapeutics [Table 1].
Table 1.
Examples of multifunction of β2M
| Year | Author | Targeting | Mechanism | Application |
|---|---|---|---|---|
| 2007 | Yang et al.[22] | IL-6 and IGF-I receptors and signaling pathways | Anti-β2M mAbs redistribute or block IL-6 and IGF-I receptors or signaling pathways | Apoptosis of myeloma cells |
| 1995 | Rowley et al.[25] | Antagonistic activity to transforming growth factor beta 1 | Hormone/growth factor receptors | Immune regulation and cell proliferation |
| 2008 | Zhu and Shi[27] | Mesenchymal stem cells | Growth stimulator | Prognostic marker and therapeutic target of cancers |
| 1992 | Moe and Sprague[35] | Osteoblast | Mitogen | Therapeutic target |
| 2006 | Huang et al.[23] | Prostate cancer bone metastasis | Signaling and growth promoting factor | Therapeutic target |
| 2006 | Nomura et al.[24] | Human renal cell carcinoma | Growth stimulator via the β2M-protein kinase A-CREB-VEGF signaling pathway | Therapeutic target |
| 2002 | Min et al.[18] | Myeloma cells | Negative growth regulator, induce cell apoptosis | Therapeutic strategy |
| 2001 | Mori et al.[30] | Leukemic cell-bearing mice | Apoptosis-inducing activity via activation of caspase-3 and nuclear factor-kappa B | Therapy for leukemia |
| 2003 | Gordon et al.[31] | Human lymphoblastic leukemia cell line | Induce apoptosis via increasing reactive oxygen species | Therapy for leukemia |
| 1993 | Rock et al.[81] | MHC I reconstruction | MHC I or like molecules stabilizer | Vaccine adjuvant |
MHC I: Major histocompatibility complex class I; IL: Interleukin; CREB: cAMP response element-binding; VEGF: Vascular endothelial growth factor; β2M: Beta 2 microglobulin; IGF-I: Insulin-like growth factor-I; cAMP: Cyclic adenosine monophosphate.
As a key component of MHC I or like molecules, β2M is critical for CTL response. The CTL immune response is obligatory for prevention against intracellular pathogens. By use of MHC I α chain, β2M has been successfully employed as an adjuvant for augmented CTL immune responses. Due to the surface expression of other MHC I-like α chains (e.g., CD1d α chain), it can be deduced the application of β2M on additive immune responses. Since MHC I and CD1 could present different antigens from M. tuberculosis to CD8+ T-cells; the adjuvant effect of β2M may have dual applications on prevention of tuberculosis.
Though several β2M-related molecules have been identified, there are still some unknown β2M-related molecules. Besides FcRn and HFE, other β2M-related molecules involve in the catabolism of IgG and the homeostasis of iron. With the sequences of the whole genomes, more putative β2M-related molecules would be revealed.[115]
Financial support and sponsorship
This study was funded by the grant from the National Nature Science Foundation of China (No. 31201662).
Conflicts of interest
There are no conflicts of interest.
Footnotes
Edited by: Peng Lyu
REFERENCES
- 1.Güssow D, Rein R, Ginjaar I, Hochstenbach F, Seemann G, Kottman A, et al. The human beta 2-microglobulin gene. Primary structure and definition of the transcriptional unit. J Immunol. 1987;139:3132–8. [PubMed] [Google Scholar]
- 2.Drüeke TB, Massy ZA. Beta2-microglobulin. Semin Dial. 2009;22:378–80. doi: 10.1111/j.1525-139X.2009.00584.x. doi: 10.1111/j.1525-139X.2009.00584.x. [DOI] [PubMed] [Google Scholar]
- 3.Esposito G, Ricagno S, Corazza A, Rennella E, Gümral D, Mimmi MC, et al. The controlling roles of Trp60 and Trp95 in beta2-microglobulin function, folding and amyloid aggregation properties. J Mol Biol. 2008;378:887–97. doi: 10.1016/j.jmb.2008.03.002. doi: 10.1016/j.jmb.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 4.Cox DR, Sawicki JA, Yee D, Appella E, Epstein CJ. Assignment of the gene for beta 2-microglobulin (B2m) to mouse chromosome 2. Proc Natl Acad Sci U S A. 1982;79:1930–4. doi: 10.1073/pnas.79.6.1930. doi: 10.1073/pnas.79.6.1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Verdone G, Corazza A, Viglino P, Pettirossi F, Giorgetti S, Mangione P, et al. The solution structure of human beta2-microglobulin reveals the prodromes of its amyloid transition. Protein Sci. 2002;11:487–99. doi: 10.1110/ps.29002. doi: 10.1110/Ps.29002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Saper MA, Bjorkman PJ, Wiley DC. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991;219:277–319. doi: 10.1016/0022-2836(91)90567-p. doi: 10.1016/0022-2836(91)90567-P. [DOI] [PubMed] [Google Scholar]
- 7.Becker JW, Reeke GN., Jr Three-dimensional structure of beta 2-microglobulin. Proc Natl Acad Sci U S A. 1985;82:4225–9. doi: 10.1073/pnas.82.12.4225. doi: 10.1073/pnas.82.12.4225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ohta Y, Shiina T, Lohr RL, Hosomichi K, Pollin TI, Heist EJ, et al. Primordial linkage of ß2-microglobulin to the MHC. J Immunol. 2011;186:3563–71. doi: 10.4049/jimmunol.1003933. doi: 10.4049/jimmunol.1003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ricagno S, Colombo M, de Rosa M, Sangiovanni E, Giorgetti S, Raimondi S, et al. DE loop mutations affect beta2-microglobulin stability and amyloid aggregation. Biochem Biophys Res Commun. 2008;377:146–50. doi: 10.1016/j.bbrc.2008.09.108. doi: 10.1016/j.bbrc.2008.09.108. [DOI] [PubMed] [Google Scholar]
- 10.Valleix S, Gillmore JD, Bridoux F, Mangione PP, Dogan A, Nedelec B, et al. Hereditary systemic amyloidosis due to Asp76Asn variant ß2-microglobulin. N Engl J Med. 2012;366:2276–83. doi: 10.1056/NEJMoa1201356. doi: 10.1056/NEJMoa1201356. [DOI] [PubMed] [Google Scholar]
- 11.Bjorkman PJ, Saper MA, Samraoui B, Bennett WS, Strominger JL, Wiley DC. Structure of the human class I histocompatibility antigen, HLA-A2. Nature. 1987;329:506–12. doi: 10.1038/329506a0. doi: 10.1038/329506a0. [DOI] [PubMed] [Google Scholar]
- 12.Hee CS, Beerbaum M, Loll B, Ballaschk M, Schmieder P, Uchanska-Ziegler B, et al. Dynamics of free versus complexed ß2-microglobulin and the evolution of interfaces in MHC class I molecules. Immunogenetics. 2013;65:157–72. doi: 10.1007/s00251-012-0667-4. doi: 10.1007/s00251-012-0667-4. [DOI] [PubMed] [Google Scholar]
- 13.Simister NE, Ahouse JC. The structure and evolution of FcRn. Res Immunol. 1996;147:333–7. doi: 10.1016/0923-2494(96)89647-7. doi: 10.1016/0923-2494(96)89647-7. [DOI] [PubMed] [Google Scholar]
- 14.Allen H, Fraser J, Flyer D, Calvin S, Flavell R. Beta 2-microglobulin is not required for cell surface expression of the murine class I histocompatibility antigen H-2Db or of a truncated H-2Db. Proc Natl Acad Sci U S A. 1986;83:7447–51. doi: 10.1073/pnas.83.19.7447. doi: 10.1073/pnas.83.19.7447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Degen E, Williams DB. Participation of a novel 88-kD protein in the biogenesis of murine class I histocompatibility molecules. J Cell Biol. 1991;112:1099–115. doi: 10.1083/jcb.112.6.1099. doi: 10.1083/jcb.112.6.1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Degen E, Cohen-Doyle MF, Williams DB. Efficient dissociation of the p88 chaperone from major histocompatibility complex class I molecules requires both beta 2-microglobulin and peptide. J Exp Med. 1992;175:1653–61. doi: 10.1084/jem.175.6.1653. doi: 10.1084/jem.175.6.1653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shi C, Zhu Y, Su Y, Chung LW, Cheng T. Beta2-microglobulin: Emerging as a promising cancer therapeutic target. Drug Discov Today. 2009;14:25–30. doi: 10.1016/j.drudis.2008.11.001. doi: 10.1016/j.drudis.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 18.Min R, Li Z, Epstein J, Barlogie B, Yi Q. Beta (2)-microglobulin as a negative growth regulator of myeloma cells. Br J Haematol. 2002;118:495–505. doi: 10.1046/j.1365-2141.2002.03635.x. doi: 10.1046/j.1365-2141.2002.03635.x. [DOI] [PubMed] [Google Scholar]
- 19.Smith LK, He Y, Park JS, Bieri G, Snethlage CE, Lin K, et al. ß2-microglobulin is a systemic pro-aging factor that impairs cognitive function and neurogenesis. Nat Med. 2015;21:932–7. doi: 10.1038/nm.3898. doi: 10.1038/nm.3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Balint E, Sprague SM. Beta (2)-microglobulin and bone cell metabolism. Nephrol Dial Transplant. 2001;16:1108–11. doi: 10.1093/ndt/16.6.1108. doi: 10.1093/ndt/16.6.1108. [DOI] [PubMed] [Google Scholar]
- 21.Tsai CY, Wu TH, Yu CL, Lu JY, Tsai YY. Increased excretions of beta2-microglobulin, IL-6, and IL-8 and decreased excretion of Tamm-Horsfall glycoprotein in urine of patients with active lupus nephritis. Nephron. 2000;85:207–14. doi: 10.1159/000045663. doi: 10.1159/000045663. [DOI] [PubMed] [Google Scholar]
- 22.Yang J, Zhang X, Wang J, Qian J, Zhang L, Wang M, et al. Anti beta2-microglobulin monoclonal antibodies induce apoptosis in myeloma cells by recruiting MHC class I to and excluding growth and survival cytokine receptors from lipid rafts. Blood. 2007;110:3028–35. doi: 10.1182/blood-2007-06-094417. doi: 10.1182/blood-2007-06-094417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Huang WC, Wu D, Xie Z, Zhau HE, Nomura T, Zayzafoon M, et al. Beta2-microglobulin is a signaling and growth-promoting factor for human prostate cancer bone metastasis. Cancer Res. 2006;66:9108–16. doi: 10.1158/0008-5472.CAN-06-1996. doi: 10.1158/0008-5472.Can-06-1996. [DOI] [PubMed] [Google Scholar]
- 24.Nomura T, Huang WC, Zhau HE, Wu D, Xie Z, Mimata H, et al. Beta2-microglobulin promotes the growth of human renal cell carcinoma through the activation of the protein kinase A, cyclic AMP-responsive element-binding protein, and vascular endothelial growth factor axis. Clin Cancer Res. 2006;12:7294–305. doi: 10.1158/1078-0432.CCR-06-2060. doi: 10.1158/1078-0432.Ccr-06-2060. [DOI] [PubMed] [Google Scholar]
- 25.Rowley DR, Dang TD, McBride L, Gerdes MJ, Lu B, Larsen M. Beta-2 microglobulin is mitogenic to PC-3 prostatic carcinoma cells and antagonistic to transforming growth factor beta 1 action. Cancer Res. 1995;55:781–6. [PubMed] [Google Scholar]
- 26.Shi CM, Zhu Y, Huang WC, Zhau HE, Wang RX, Valerie OM, et al. Bi-directional interactions of bone marrow mesenchymal stem cells with human prostate cancer cells. J Urol. 2007;177:92. [Google Scholar]
- 27.Zhu Y, Shi CM. Beta 2-microglobulin, a novel factor for the expansion of mesenchymal stem cells. J Biotechnol. 2008;136:S177. doi: 10.1007/s10529-009-0027-0. doi: 10.1016/j.jbiotec.2008.07.391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Josson S, Nomura T, Lin JT, Huang WC, Wu D, Zhau HE, et al. ß2-microglobulin induces epithelial to mesenchymal transition and confers cancer lethality and bone metastasis in human cancer cells. Cancer Res. 2011;71:2600–10. doi: 10.1158/0008-5472.CAN-10-3382. doi: 10.1158/0008-5472.CAN-10-3382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang A, Wang B, Yang M, Shi H, Gan W. ß2-microglobulin induces epithelial-mesenchymal transition in human renal proximal tubule epithelial cells in vitro. BMC Nephrol. 2015;16:60. doi: 10.1186/s12882-015-0057-x. doi: 10.1186/s12882-015-0057-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mori M, Terui Y, Tanaka M, Tomizuka H, Mishima Y, Ikeda M, et al. Antitumor effect of beta2-microglobulin in leukemic cell-bearing mice via apoptosis-inducing activity: Activation of caspase-3 and nuclear factor-kappaB. Cancer Res. 2001;61:4414–7. doi: 10.1002/Ijc.10828. [PubMed] [Google Scholar]
- 31.Gordon J, Wu CH, Rastegar M, Safa AR. Beta2-microglobulin induces caspase-dependent apoptosis in the CCRF-HSB-2 human leukemia cell line independently of the caspase-3, -8 and -9 pathways but through increased reactive oxygen species. Int J Cancer. 2003;103:316–27. doi: 10.1002/ijc.10828. doi: 10.1002/Ijc.10828. [DOI] [PubMed] [Google Scholar]
- 32.Josson S, Matsuoka Y, Gururajan M, Nomura T, Huang WC, Yang X, et al. Inhibition of ß2-microglobulin/hemochromatosis enhances radiation sensitivity by induction of iron overload in prostate cancer cells. PLoS One. 2013;8:e68366. doi: 10.1371/journal.pone.0068366. doi: 10.1371/journal.pone.0068366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Esposito G, Michelutti R, Verdone G, Viglino P, Hernández H, Robinson CV, et al. Removal of the N-terminal hexapeptide from human beta2-microglobulin facilitates protein aggregation and fibril formation. Protein Sci. 2000;9:831–45. doi: 10.1110/ps.9.5.831. doi: 10.1110/ps.9.5.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leney AC, Pashley CL, Scarff CA, Radford SE, Ashcroft AE. Insights into the role of the beta-2 microglobulin D-strand in amyloid propensity revealed by mass spectrometry. Mol Biosyst. 2014;10:412–20. doi: 10.1039/c3mb70420c. doi: 10.1039/c3mb70420c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Moe SM, Sprague SM. Beta 2-microglobulin induces calcium efflux from cultured neonatal mouse calvariae. Am J Physiol. 1992;263(3 Pt 2):F540–5. doi: 10.1152/ajprenal.1992.263.3.F540. [DOI] [PubMed] [Google Scholar]
- 36.Simone LC, Smith BL, Solheim JC. Impact of beta 2-microglobulin on tapasin expression and covalent association. Cell Immunol. 2012;279:66–9. doi: 10.1016/j.cellimm.2012.09.010. doi: 10.1016/j.cellimm.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gejyo F, Yamada T, Odani S, Nakagawa Y, Arakawa M, Kunitomo T, et al. A new form of amyloid protein associated with chronic hemodialysis was identified as beta 2-microglobulin. Biochem Biophys Res Commun. 1985;129:701–6. doi: 10.1016/0006-291x(85)91948-5. doi: 10.1016/0006-291x(85)91948-5. [DOI] [PubMed] [Google Scholar]
- 38.Liabeuf S, Lenglet A, Desjardins L, Neirynck N, Glorieux G, Lemke HD, et al. Plasma beta-2 microglobulin is associated with cardiovascular disease in uremic patients. Kidney Int. 2012;82:1297–303. doi: 10.1038/ki.2012.301. doi: 10.1038/ki.2012.301. [DOI] [PubMed] [Google Scholar]
- 39.Jadoul M, Drüeke TB. ß2 microglobulin amyloidosis: An update 30 years later. Nephrol Dial Transplant. 2015 doi: 10.1093/ndt/gfv318. pii: gfv318. doi: 10.1093/ndt/gfv318. [DOI] [PubMed] [Google Scholar]
- 40.Muñana KR, Saito M, Hoshi F. Beta-2-microglobulin levels in the cerebrospinal fluid of normal dogs and dogs with neurological disease. Vet Clin Pathol. 2007;36:173–8. doi: 10.1111/j.1939-165x.2007.tb00204.x. doi: 10.1111/j.1939-165x.2007.tb00204.x. [DOI] [PubMed] [Google Scholar]
- 41.Svatonová J, Borecká K, Adam P, Lánská V. Beta2-microglobulin as a diagnostic marker in cerebrospinal fluid: A follow-up study. Dis Markers 2014. 2014 doi: 10.1155/2014/495402. 495402. doi: 10.1155/2014/495402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bethea M, Forman DT. Beta 2-microglobulin: Its significance and clinical usefulness. Ann Clin Lab Sci. 1990;20:163–8. [PubMed] [Google Scholar]
- 43.Yoo C, Yoon DH, Kim S, Huh J, Park CS, Park CJ, et al. Serum beta-2 microglobulin as a prognostic biomarker in patients with mantle cell lymphoma. Hematol Oncol. 2015 doi: 10.1002/hon.2188. doi: 10.1002/hon.2188. [DOI] [PubMed] [Google Scholar]
- 44.Seo S, Yoon DH, Yoo C, Jeong Y, Park JH, Kim S, et al. Prognostic significance of beta-2 microglobulin in patients with diffuse large B cell lymphoma in rituximab era. Blood. 2014;124:2982. [Google Scholar]
- 45.Muta T, Iida S, Matsue K, Sunami K, Isoda J, Harada N, et al. Predictive significance of serum beta 2-microglobulin levels and M-protein velocity for symptomatic progression of smoldering multiple myeloma. Blood. 2014;124:3379. [Google Scholar]
- 46.Wu L, Wang T, Gui W, Lin H, Xie K, Wang H, et al. Prognostic significance of serum beta-2 microglobulin in patients with non-Hodgkin lymphoma. Oncology. 2014;87:40–7. doi: 10.1159/000362670. doi: 10.1159/000362670. [DOI] [PubMed] [Google Scholar]
- 47.Yoo C, Yoon DH, Suh C. Serum beta-2 microglobulin in malignant lymphomas: An old but powerful prognostic factor. Blood Res. 2014;49:148–53. doi: 10.5045/br.2014.49.3.148. doi: 10.5045/br.2014.49.3.148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Yilmaz B, Köklü S, Yüksel O, Arslan S. Serum beta 2-microglobulin as a biomarker in inflammatory bowel disease. World J Gastroenterol. 2014;20:10916–20. doi: 10.3748/wjg.v20.i31.10916. doi: 10.3748/wjg.v20.i31.10916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chitra P, Bakthavatsalam B, Palvannan T. Beta-2 microglobulin as an immunological marker to assess the progression of human immunodeficiency virus infected patients on highly active antiretroviral therapy. Clin Chim Acta. 2011;412:1151–4. doi: 10.1016/j.cca.2011.01.037. doi: 10.1016/j.cca.2011.01.037. [DOI] [PubMed] [Google Scholar]
- 50.Grundy JE, McKeating JA, Griffiths PD. Cytomegalovirus strain AD169 binds beta 2 microglobulin in vitro after release from cells. J Gen Virol. 1987;68(Pt 3):777–84. doi: 10.1099/0022-1317-68-3-777. doi: 10.1099/0022-1317-68-3-777. [DOI] [PubMed] [Google Scholar]
- 51.Toledo-Cornell C, Santos S, Orge G, Glesby MJ, Carvalho EM. Soluble IL-2 receptor and beta-2 microglobulin as possible serologic markers of neurologic disease in HTLV-1 infection. J Med Virol. 2014;86:315–21. doi: 10.1002/jmv.23711. doi: 10.1002/jmv.23711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Madureira Silva MV, Moscoso-Solorzano GT, Nishida SK, Mastroianni-Kirsztajn G. Serum Beta 2-microglobulin/cystatin C index: A useful biomarker in lupus nephritis? Nephron Extra. 2012;2:169–76. doi: 10.1159/000339643. doi: 10.1159/000339643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.El-Frargy MS, El-Refaey AM, Eid R, Hussien MA. Serum cystatin-C and BETA 2-microglobulin as accurate markers in the early diagnosis of kidney injury in neonates: A single center study. Saudi J Kidney Dis Transpl. 2015;26:712–7. doi: 10.4103/1319-2442.160151. [DOI] [PubMed] [Google Scholar]
- 54.Astor BC, Muth B, Kaufman DB, Pirsch JD, Michael Hofmann R, Djamali A. Serum ß2-microglobulin at discharge predicts mortality and graft loss following kidney transplantation. Kidney Int. 2013;84:810–7. doi: 10.1038/ki.2013.172. doi: 10.1038/ki.2013.172. [DOI] [PubMed] [Google Scholar]
- 55.Cheung CL, Lam KS, Cheung BM. Serum beta-2 microglobulin concentration predicts cardiovascular and all-cause mortality. Int J Cardiol. 2013;168:4811–3. doi: 10.1016/j.ijcard.2013.07.014. doi: 10.1016/j.ijcard.2013.07.014. [DOI] [PubMed] [Google Scholar]
- 56.Annweiler C, Bataille R, Ferrière N, Douillet D, Fantino B, Beauchet O. Plasma beta-2 microglobulin as a marker of frailty in older adults: A pilot study. J Gerontol A Biol Sci Med Sci. 2011;66:1077–9. doi: 10.1093/gerona/glr104. [DOI] [PubMed] [Google Scholar]
- 57.Ardeniz Ö, Unger S, Onay H, Ammann S, Keck C, Cianga C, et al. ß2-Microglobulin deficiency causes a complex immunodeficiency of the innate and adaptive immune system. J Allergy Clin Immunol. 2015;136:392–401. doi: 10.1016/j.jaci.2014.12.1937. [DOI] [PubMed] [Google Scholar]
- 58.Roberts AD, Ordway DJ, Orme IM. Listeria monocytogenes infection in beta 2 microglobulin-deficient mice. Infect Immun. 1993;61:1113–6. doi: 10.1128/iai.61.3.1113-1116.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Ehrlich R, Lemonnier FA. HFE – A novel nonclassical class I molecule that is involved in iron metabolism. Immunity. 2000;13:585–8. doi: 10.1016/s1074-7613(00)00058-3. doi: 10.1016/S1074-7613(00)00058-3. [DOI] [PubMed] [Google Scholar]
- 60.Enns CA. Pumping iron: The strange partnership of the hemochromatosis protein, a class I MHC homolog, with the transferrin receptor. Traffic. 2001;2:167–74. doi: 10.1034/j.1600-0854.2001.020303.x. doi: 10.1034/j.1600-0854.2001.020303.x. [DOI] [PubMed] [Google Scholar]
- 61.Schaible UE, Collins HL, Priem F, Kaufmann SH. Correction of the iron overload defect in beta-2-microglobulin knockout mice by lactoferrin abolishes their increased susceptibility to tuberculosis. J Exp Med. 2002;196:1507–13. doi: 10.1084/jem.20020897. doi: 10.1084/jem.20020897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Muckenthaler MU, Rodrigues P, Macedo MG, Minana B, Brennan K, Cardoso EM, et al. Molecular analysis of iron overload in beta2-microglobulin-deficient mice. Blood Cells Mol Dis. 2004;33:125–31. doi: 10.1016/j.bcmd.2004.05.003. doi: 10.1016/j.bcmd.2004.05.003. [DOI] [PubMed] [Google Scholar]
- 63.Hou S, Doherty PC, Zijlstra M, Jaenisch R, Katz JM. Delayed clearance of Sendai virus in mice lacking class I MHC-restricted CD8+ T cells. J Immunol. 1992;149:1319–25. [PubMed] [Google Scholar]
- 64.Muller D, Koller BH, Whitton JL, LaPan KE, Brigman KK, Frelinger JA. LCMV-specific, class II-restricted cytotoxic T cells in beta 2-microglobulin-deficient mice. Science. 1992;255:1576–8. doi: 10.1126/science.1347959. doi: 10.1126/science.1347959. [DOI] [PubMed] [Google Scholar]
- 65.Quinn DG, Zajac AJ, Hioe CE, Frelinger JA. Virus-specific, CD8+ major histocompatibility complex class I-restricted cytotoxic T lymphocytes in lymphocytic choriomeningitis virus-infected beta2-microglobulin-deficient mice. J Virol. 1997;71:8392–6. doi: 10.1128/jvi.71.11.8392-8396.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Höglund P, Glas R, Ménard C, Kåse A, Johansson MH, Franksson L, et al. Beta2-microglobulin-deficient NK cells show increased sensitivity to MHC class I-mediated inhibition, but self tolerance does not depend upon target cell expression of H-2Kb and Db heavy chains. Eur J Immunol. 1998;28:370–8. doi: 10.1002/(SICI)1521-4141(199801)28:01<370::AID-IMMU370>3.0.CO;2-W. doi: 10.1002/(SICI)1521-4141(199801)28:01<370:AID-IMMU370>3.0.CO; 2-W. [DOI] [PubMed] [Google Scholar]
- 67.Correa I, Bix M, Liao NS, Zijlstra M, Jaenisch R, Raulet D. Most gamma delta T cells develop normally in beta 2-microglobulin-deficient mice. Proc Natl Acad Sci U S A. 1992;89:653–7. doi: 10.1073/pnas.89.2.653. doi: 10.1073/pnas.89.2.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Christianson GJ, Brooks W, Vekasi S, Manolfi EA, Niles J, Roopenian SL, et al. Beta 2-microglobulin-deficient mice are protected from hypergammaglobulinemia and have defective antibody responses because of increased IgG catabolism. J Immunol. 1997;159:4781–92. [PubMed] [Google Scholar]
- 69.Wani MA, Haynes LD, Kim J, Bronson CL, Chaudhury C, Mohanty S, et al. Familial hypercatabolic hypoproteinemia caused by deficiency of the neonatal Fc receptor, FcRn, due to a mutant beta2-microglobulin gene. Proc Natl Acad Sci U S A. 2006;103:5084–9. doi: 10.1073/pnas.0600548103. doi: 10.1073/pnas.0600548103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu Z, Roopenian DC, Zhou X, Christianson GJ, Diaz LA, Sedmak DD, et al. Beta2-microglobulin-deficient mice are resistant to bullous pemphigoid. J Exp Med. 1997;186:777–83. doi: 10.1084/jem.186.5.777. doi: 10.1084/jem.186.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Denic A, Pirko I, Wootla B, Bieber A, Macura S, Rodriguez M. Deletion of beta-2-microglobulin ameliorates spinal cord lesion load and promotes recovery of brainstem NAA levels in a murine model of multiple sclerosis. Brain Pathol. 2012;22:698–708. doi: 10.1111/j.1750-3639.2012.00576.x. doi: 10.1111/j.1750-3639.2012.00576.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lu PF, Chen JJ, He LX, Ren JT, Chen HD, Rao LJ, et al. Generating hypoimmunogenic human embryonic stem cells by the disruption of beta 2-microglobulin. Stem Cell Rev Rep. 2013;9:806–13. doi: 10.1007/s12015-013-9457-0. doi: 10.1007/s12015-013-9457-0. [DOI] [PubMed] [Google Scholar]
- 73.del Campo AB, Kyte JA, Carretero J, Zinchencko S, Méndez R, González-Aseguinolaza G, et al. Immune escape of cancer cells with beta2-microglobulin loss over the course of metastatic melanoma. Int J Cancer. 2014;134:102–13. doi: 10.1002/ijc.28338. doi: 10.1002/ijc.28338. [DOI] [PubMed] [Google Scholar]
- 74.Zeng Z, Castaño AR, Segelke BW, Stura EA, Peterson PA, Wilson IA. Crystal structure of mouse CD1: An MHC-like fold with a large hydrophobic binding groove. Science. 1997;277:339–45. doi: 10.1126/science.277.5324.339. doi: 10.1126/science.277.5324.339. [DOI] [PubMed] [Google Scholar]
- 75.Sidobre S, Kronenberg M. CD1 tetramers: A powerful tool for the analysis of glycolipid-reactive T cells. J Immunol Methods. 2002;268:107–21. doi: 10.1016/s0022-1759(02)00204-1. doi: 10.1016/s0022-1759(02)00204-1. [DOI] [PubMed] [Google Scholar]
- 76.Tilloy F, Treiner E, Park SH, Garcia C, Lemonnier F, de la Salle H, et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J Exp Med. 1999;189:1907–21. doi: 10.1084/jem.189.12.1907. doi: 10.1084/jem.189.12.1907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Ribaudo RK, Margulies DH. Independent and synergistic effects of disulfide bond formation, beta 2-microglobulin, and peptides on class I MHC folding and assembly in an in vitro translation system. J Immunol. 1992;149:2935–44. [PubMed] [Google Scholar]
- 78.Schnabl E, Stockinger H, Majdic O, Gaugitsch H, Lindley IJ, Maurer D, et al. Activated human T lymphocytes express MHC class I heavy chains not associated with beta 2-microglobulin. J Exp Med. 1990;171:1431–42. doi: 10.1084/jem.171.5.1431. doi: 10.1084/jem.171.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Bix M, Raulet D. Functionally conformed free class I heavy chains exist on the surface of beta 2 microglobulin negative cells. J Exp Med. 1992;176:829–34. doi: 10.1084/jem.176.3.829. doi: 10.1084/jem.176.3.829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vitiello A, Potter TA, Sherman LA. The role of beta 2-microglobulin in peptide binding by class I molecules. Science. 1990;250:1423–6. doi: 10.1126/science.2124002. doi: 10.1126/science.2124002. [DOI] [PubMed] [Google Scholar]
- 81.Rock KL, Fleischacker C, Gamble S. Peptide-priming of cytolytic T cell immunity in vivo using beta 2-microglobulin as an adjuvant. J Immunol. 1993;150:1244–52. [PubMed] [Google Scholar]
- 82.Sreejit G, Ahmed A, Parveen N, Jha V, Valluri VL, Ghosh S, et al. The ESAT-6 protein of Mycobacterium tuberculosis interacts with beta-2-microglobulin (ß2M) affecting antigen presentation function of macrophage. PLoS Pathog. 2014;10:e1004446. doi: 10.1371/journal.ppat.1004446. doi: 10.1371/journal.ppat.1004446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kim J, Bronson CL, Wani MA, Oberyszyn TM, Mohanty S, Chaudhury C, et al. Beta 2-microglobulin deficient mice catabolize IgG more rapidly than FcRn- alpha-chain deficient mice. Exp Biol Med (Maywood) 2008;233:603–9. doi: 10.3181/0710-RM-270. doi: 10.3181/0710-Rm-270. [DOI] [PubMed] [Google Scholar]
- 84.Baker K, Qiao SW, Kuo T, Kobayashi K, Yoshida M, Lencer WI, et al. Immune and non-immune functions of the (not so) neonatal Fc receptor, FcRn. Semin Immunopathol. 2009;31:223–36. doi: 10.1007/s00281-009-0160-9. doi: 10.1007/s00281-009-0160-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Praetor A, Hunziker W. Beta (2)-microglobulin is important for cell surface expression and pH-dependent IgG binding of human FcRn. J Cell Sci. 2002;115(Pt 11):2389–97. doi: 10.1242/jcs.115.11.2389. [DOI] [PubMed] [Google Scholar]
- 86.Raghavan M, Chen MY, Gastinel LN, Bjorkman PJ. Investigation of the interaction between the class I MHC-related Fc receptor and its immunoglobulin G ligand. Immunity. 1994;1:303–15. doi: 10.1016/1074-7613(94)90082-5. doi: 10.1016/1074-7613(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 87.Cervenak J, Bender B, Schneider Z, Magna M, Carstea BV, Liliom K, et al. Neonatal FcR overexpression boosts humoral immune response in transgenic mice. J Immunol. 2011;186:959–68. doi: 10.4049/jimmunol.1000353. doi: 10.4049/jimmunol.1000353. [DOI] [PubMed] [Google Scholar]
- 88.Claypool SM, Dickinson BL, Yoshida M, Lencer WI, Blumberg RS. Functional reconstitution of human FcRn in Madin-Darby canine kidney cells requires co-expressed human beta 2-microglobulin. J Biol Chem. 2002;277:28038–50. doi: 10.1074/jbc.M202367200. doi: 10.1074/jbc.M202367200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Calabi F, Milstein C. The molecular biology of CD1. Semin Immunol. 2000;12:503–9. doi: 10.1006/smim.2000.0271. doi: 10.1006/smim.2000.0271. [DOI] [PubMed] [Google Scholar]
- 90.de la Salle H, Mariotti S, Angenieux C, Gilleron M, Garcia-Alles LF, Malm D, et al. Assistance of microbial glycolipid antigen processing by CD1e. Science. 2005;310:1321–4. doi: 10.1126/science.1115301. doi: 10.1126/science.1115301. [DOI] [PubMed] [Google Scholar]
- 91.Angenieux C, Salamero J, Fricker D, Cazenave JP, Goud B, Hanau D, et al. Characterization of CD1e, a third type of CD1 molecule expressed in dendritic cells. J Biol Chem. 2000;275:37757–64. doi: 10.1074/jbc.M007082200. doi: 10.1074/jbc.M007082200. [DOI] [PubMed] [Google Scholar]
- 92.Sugita M, Porcelli SA, Brenner MB. Assembly and retention of CD1b heavy chains in the endoplasmic reticulum. J Immunol. 1997;159:2358–65. [PubMed] [Google Scholar]
- 93.Balk SP, Burke S, Polischuk JE, Frantz ME, Yang L, Porcelli S, et al. Beta 2-microglobulin-independent MHC class Ib molecule expressed by human intestinal epithelium. Science. 1994;265:259–62. doi: 10.1126/science.7517575. doi: 10.1126/science.7517575. [DOI] [PubMed] [Google Scholar]
- 94.Kim HS, Garcia J, Exley M, Johnson KW, Balk SP, Blumberg RS. Biochemical characterization of CD1d expression in the absence of beta2-microglobulin. J Biol Chem. 1999;274:9289–95. doi: 10.1074/jbc.274.14.9289. doi: 10.1074/jbc.274.14.9289. [DOI] [PubMed] [Google Scholar]
- 95.Amano M, Baumgarth N, Dick MD, Brossay L, Kronenberg M, Herzenberg LA, et al. CD1 expression defines subsets of follicular and marginal zone B cells in the spleen: Deta 2-microglobulin-dependent and independent forms. J Immunol. 1998;161:1710–7. [PubMed] [Google Scholar]
- 96.Somnay-Wadgaonkar K, Nusrat A, Kim HS, Canchis WP, Balk SP, Colgan SP, et al. Immunolocalization of CD1d in human intestinal epithelial cells and identification of a beta2-microglobulin-associated form. Int Immunol. 1999;11:383–92. doi: 10.1093/intimm/11.3.383. doi: 10.1093/intimm/11.3.383. [DOI] [PubMed] [Google Scholar]
- 97.Feder JN, Tsuchihashi Z, Irrinki A, Lee VK, Mapa FA, Morikang E, et al. The hemochromatosis founder mutation in HLA-H disrupts beta2-microglobulin interaction and cell surface expression. J Biol Chem. 1997;272:14025–8. doi: 10.1074/jbc.272.22.14025. doi: 10.1074/jbc.272.22.14025. [DOI] [PubMed] [Google Scholar]
- 98.Waheed A, Parkkila S, Zhou XY, Tomatsu S, Tsuchihashi Z, Feder JN, et al. Hereditary hemochromatosis: Effects of C282Y and H63D mutations on association with beta2-microglobulin, intracellular processing, and cell surface expression of the HFE protein in COS-7 cells. Proc Natl Acad Sci U S A. 1997;94:12384–9. doi: 10.1073/pnas.94.23.12384. doi: 10.1073/pnas.94.23.12384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lebrón JA, Bennett MJ, Vaughn DE, Chirino AJ, Snow PM, Mintier GA, et al. Crystal structure of the hemochromatosis protein HFE and characterization of its interaction with transferrin receptor. Cell. 1998;93:111–23. doi: 10.1016/s0092-8674(00)81151-4. doi: 10.1016/s0092-8674(00)81151-4. [DOI] [PubMed] [Google Scholar]
- 100.Robinson PJ, Travers PJ, Stackpoole A, Flaherty L, Djaballah H. Maturation of Qa-1b class I molecules requires beta 2-microglobulin but is TAP independent. J Immunol. 1998;160:3217–24. [PubMed] [Google Scholar]
- 101.Das G, Gould DS, Augustine MM, Fragoso G, Sciutto E, Stroynowski I, et al. Qa-2-dependent selection of CD8alpha/alpha T cell receptor alpha/beta(+) cells in murine intestinal intraepithelial lymphocytes. J Exp Med. 2000;192:1521–8. doi: 10.1084/jem.192.10.1521. doi: 10.1084/jem.192.10.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Shawar SM, Rodgers JR, Cook RG, Rich RR. Specialized function of the nonclassical MHC class I molecule Hmt: A specific receptor for N-formylated peptides. Immunol Res. 1991;10:365–75. doi: 10.1007/BF02919723. doi: 10.1007/bf02919723. [DOI] [PubMed] [Google Scholar]
- 103.Attaya M, Jameson S, Martinez CK, Hermel E, Aldrich C, Forman J, et al. Ham-2 corrects the class I antigen-processing defect in RMA-S cells. Nature. 1992;355:647–9. doi: 10.1038/355647a0. doi: 10.1038/355647a0. [DOI] [PubMed] [Google Scholar]
- 104.Riegert P, Wanner V, Bahram S. Genomics, isoforms, expression, and phylogeny of the MHC class I-related MR1 gene. J Immunol. 1998;161:4066–77. [PubMed] [Google Scholar]
- 105.Yamaguchi H, Hirai M, Kurosawa Y, Hashimoto K. A highly conserved major histocompatibility complex class I-related gene in mammals. Biochem Biophys Res Commun. 1997;238:697–702. doi: 10.1006/bbrc.1997.7379. doi: 10.1006/bbrc.1997.7379. [DOI] [PubMed] [Google Scholar]
- 106.Miley MJ, Truscott SM, Yu YY, Gilfillan S, Fremont DH, Hansen TH, et al. Biochemical features of the MHC-related protein 1 consistent with an immunological function. J Immunol. 2003;170:6090–8. doi: 10.4049/jimmunol.170.12.6090. doi: 10.4049/jimmunol.170.12.6090. [DOI] [PubMed] [Google Scholar]
- 107.Georgel P, Radosavljevic M, Macquin C, Bahram S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol Immunol. 2011;48:769–75. doi: 10.1016/j.molimm.2010.12.002. doi: 10.1016/j.molimm.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 108.Kjer-Nielsen L, Patel O, Corbett AJ, Le Nours J, Meehan B, Liu L, et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature. 2012;491:717–23. doi: 10.1038/nature11605. doi: 10.1038/Nature11605. [DOI] [PubMed] [Google Scholar]
- 109.Kajikawa M, Baba T, Tomaru U, Watanabe Y, Koganei S, Tsuji-Kawahara S, et al. MHC class I-like MILL molecules are beta2-microglobulin-associated, GPI-anchored glycoproteins that do not require TAP for cell surface expression. J Immunol. 2006;177:3108–15. doi: 10.4049/jimmunol.177.5.3108. doi: 10.4049/jimmunol.177.5.3108. [DOI] [PubMed] [Google Scholar]
- 110.Wang Y, Gu X. Evolutionary patterns of gene families generated in the early stage of vertebrates. J Mol Evol. 2000;51:88–96. doi: 10.1007/s002390010069. doi: 10.1007/s002390010069. [DOI] [PubMed] [Google Scholar]
- 111.Abi-Rached L, Gilles A, Shiina T, Pontarotti P, Inoko H. Evidence of en bloc duplication in vertebrate genomes. Nat Genet. 2002;31:100–5. doi: 10.1038/ng855. doi: 10.1038/Ng855. [DOI] [PubMed] [Google Scholar]
- 112.Blomme T, Vandepoele K, De Bodt S, Simillion C, Maere S, Van de Peer Y. The gain and loss of genes during 600 million years of vertebrate evolution. Genome Biol. 2006;7:R43. doi: 10.1186/gb-2006-7-5-r43. doi: 10.1186/Gb-2006-7-5-R43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Klein J, O’hUigin C. Composite origin of major histocompatibility complex genes. Curr Opin Genet Dev. 1993;3:923–30. doi: 10.1016/0959-437x(93)90015-h. doi: 10.1016/0959-437x(93)90015-h. [DOI] [PubMed] [Google Scholar]
- 114.Hansen TH, Huang S, Arnold PL, Fremont DH. Patterns of nonclassical MHC antigen presentation. Nat Immunol. 2007;8:563–8. doi: 10.1038/ni1475. doi: 10.1038/ni1475. [DOI] [PubMed] [Google Scholar]
- 115.Radosavljevic M, Cuillerier B, Wilson MJ, Clément O, Wicker S, Gilfillan S, et al. A cluster of ten novel MHC class I related genes on human chromosome 6q24.2.q25.3. Genomics. 2002;79:114–23. doi: 10.1006/geno.2001.6673. doi: 10.1006/geno.2001.6673. [DOI] [PubMed] [Google Scholar]


