Abstract
Van Hofwegen et al. demonstrated that Escherichia coli rapidly evolves the ability to use citrate when long selective periods are provided (D. J. Van Hofwegen, C. J. Hovde, and S. A. Minnich, J Bacteriol 198:1022–1034, 2016, http://dx.doi.org/10.1128/JB.00831-15). This contrasts with the extreme delay (15 years of daily transfers) seen in the long-term evolution experiments of Lenski and coworkers. Their idea of “historical contingency” may require reinterpretation. Rapid evolution seems to involve selection for duplications of the whole cit locus that are too unstable to contribute when selection is provided in short pulses.
TEXT
In this issue, Van Hofwegen, Hovde, and Minnich suggest that the long-term evolution experiments (LTEE) of Lenski and coworkers may need to be reinterpreted (1). In those experiments, 12 parallel cultures of Escherichia coli have been serially subcultured daily for over 25 years (2). The lines grow in minimal medium that contains citrate and a low level of glucose. Growth stops when glucose is exhausted because E. coli is characteristically unable to use citrate as an aerobic carbon and energy source (3). The essentially nongrowing cultures remain in stationary phase, under selection for citrate use, for the rest of the day and are then diluted 100-fold into fresh medium. After about 15 years (31,500 generations), 1 of the 12 lines gained the ability to use citrate (4, 5). It is remarkable that this change occurred only after many generations of growth and that the change altered a basic species characteristic of E. coli. Extensive analysis of the final citrate-using (Cit+) clone and cells from previous generations has suggested that citrate usage evolved by a multistep genetic process in which the critical event was a mutation that allowed aerobic expression of a preexisting gene encoding a citrate transporter (CitT). The CitT protein is an antiporter, which imports citrate in exchange for succinate, and its gene is normally expressed only anaerobically (6, 7). In the evolved clone, aerobic CitT expression is provided by fusing citT to a foreign promoter, usually at the join point of a gene duplication. To explain the long delay—15 years—in achieving this goal, Blount et al. (4, 5) have suggested that the adaptation process involves three steps—potentiation, actualization, and refinement. “Potentiation” involves initial neutral mutations that do not immediately improve growth but allow later improvement by the critical promoter fusion (“actualization”). The initial event is the “historical contingency” that allows the secondary change to be effective and delays growth improvement. Later “refinements” are achieved by increasing expression of the dctA gene, which encodes a transporter that helps recover the succinate lost during citrate import (8). Since this process alters a fundamental characteristic of E. coli as a species, acquisition of citrate use has been discussed as possibly exemplifying a process that might lead to speciation (5). These are the conclusions that may need to be reconsidered.
Van Hofwegen et al. demonstrated that typical (Cit−) E. coli strains can rapidly and repeatedly acquire the ability to use citrate in the presence of oxygen when selection is imposed continuously on a single batch culture (“direct selection”) without serial transfers or with intermittent nonselective growth (“modified direct selection”). Such rapid adaptation was previous reported by Hall (9). In a batch culture, any mutant lineage with even slightly improved growth on citrate can expand in the population and can acquire further improvements. More importantly, the authors report that the Cit+ variants appearing rapidly in batch conditions are generated by essentially the same series of events as those described by Blount et al. (potentiation, actualization, and refinement) (4, 5). Rapid adaptation can also occur during serial transfers if the selective period is extended to 7 days (see Fig. 1). The authors suggest that the delayed appearance of Cit+ clones in the LTEE is due to the use of a short (less than 1 day) selective period followed by 100-fold dilution and a period of nonselective growth. This “intermittent” or “pulsed” selection may make it difficult for early adaptive mutations to accumulate sufficiently to successfully traverse the dilution bottleneck from one period of selection into the next. Thus, the delay may not reflect the necessity of a nonselective potentiation event (the “historical contingency”) but may rather be due to selective events with a low probability of serial transfer. Remarkably, Van Hofwegen et al. showed that Cit+ mutants are selected rapidly and repeatedly without this delay when the population is exposed to continuous selection or when the selective period between transfers is extended from 1 to 7 days.
FIG 1.
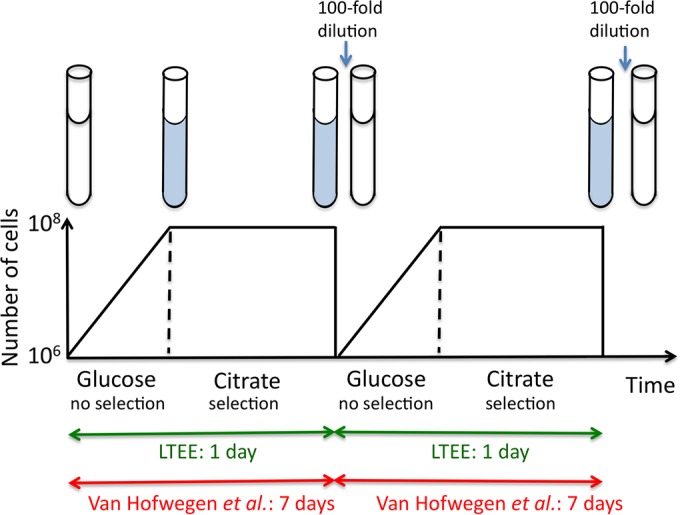
The work of Van Hofvegen et al. depicted here and the long-term evolution experiment (LTEE) of Lenski and colleagues involve serial transfers of E. coli cultures grown in minimal medium that contains citrate and a low level of glucose. Each lineage is diluted 1/100 into fresh medium, where it grows with no selection for citrate use. After the glucose supply is exhausted, growth stops and selection for the ability to use citrate is imposed.
The serial transfer used by Van Hofwegen et al. and in the LTEE is diagrammed in Fig. 1. Note that a period of growth on glucose (no selection for citrate use) is followed by a period in which citrate is the only carbon and energy source and most cells cannot grow. Thus, selection for citrate use is “intermittent” or “pulsed.” Rare mutants with slightly improved growth may divide during the selective stationary phase. However, to be retained by the lineage and acquire subsequent improvements, these cells must persist through the next period of unrestricted growth on glucose and successfully traverse the bottleneck imposed by dilution into fresh medium. To pass through this barrier, improving cells must retain their initial genetic change and compete well with the predominant unimproved population during the nonselective growth period. If the initial mutations are reversible copy number changes with fitness costs (as suggested by Van Hofwegen et al.), success may be rare and may thus explain the delayed evolution of fully Cit+ cells in LTEE.
Van Hofwegen et al. provide evidence that rapid evolution to the use of citrate proceeds by a model suggested several years ago to explain the appearance of Lac+ revertants in the Cairns experiment (10–12). In the Cairns experiment, 108 cells of a leaky lac mutant were plated on lactose medium, where revertant Lac+ colonies appeared and accumulated over a period of several days (13). This model proposed a multistep process in which the initial event is a simple duplication of the mutant lac allele, which may occur prior to selection. This dosage increase provides a modest growth improvement on lactose medium. As the duplication mutant grows under conditions of selection (or replicates its lac region), growth can be improved by further amplification of the parental mutant lac allele. (This could be viewed as potentiation, but it involves selection). Ultimately, sufficient lac alleles are replicated (more gene copies/cell and more cells/clone) to allow realization (i.e., actualization) of a rare point mutation restoring a Lac+ allele. This rare event is made more likely by the preceding amplification of the target sequence (i.e., potentiation). Further growth improvement (i.e., refinement) results from loss of nonrevertant copies of the lac region and consequent stabilization of a single revertant lac+ allele. In this model, the entire reversion process occurs under conditions of constant selection.
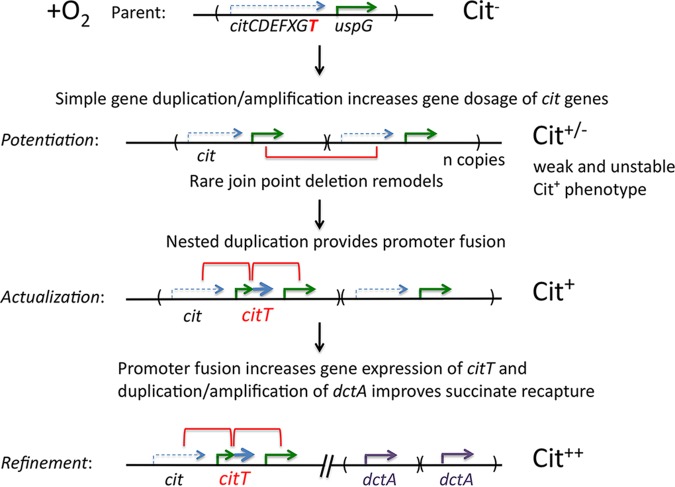
Figure 2 depicts this model as it might be used to explain the evolution of citrate use in both the batch culture and serial transfer experiments. Notice that the “silent” cit operon is expressed only anaerobically in E. coli (7). However, dense cell cultures are likely to have low oxygen levels. This makes it likely that the citrate utilization genes may be expressed at least slightly in stationary phase and that citrate usage could be improved by increasing the dosage of the entire cit operon, including the critical citT gene. Van Hofwegen et al. demonstrated a duplication of the citT region in their selection experiments. Duplication of the cit region could improve growth slightly without requiring a citT promoter fusion. Amplification of this region would also provide multiple substrates for a deletion event that fuses an active promoter to the “silent” citT gene. This rare event could also reflect a de novo duplication event within one of the initial repeats. This fusion event is rare but would become more likely as the initial citT duplication amplifies and cell numbers increase. Creation of a promoter fusion at a duplication junction is the actualization step. This possibility does not contradict recent evidence of the occurrence of point mutations that affect acetate metabolism (14), since selected duplication and amplification could be the primary event. Once the fusion event has occurred, further improvement (refinement) can result from amplification of original large duplication with its nested promoter fusion or by simple amplification of the small promoter fusion duplication. Growth on citrate is also improved by amplification or increased transcription of the dctA gene encoding an aerobic C4-dicarboxylate/H+ transporter (DctA). Indeed, DctA enhances utilization of citrate by recapturing succinate, which is excreted during citrate uptake by CitT as shown by Quandt et al. (8) and by Van Hofwegen et al. These steps are easy to visualize when selection is imposed in a single long-term batch culture or during serial transfer with extended citrate selection. The process is more difficult with daily serial dilutions.
FIG 2.
The model of Van Hofvegen et al. proposes adaptation to aerobic utilization of citrate by a multistep process. The E. coli cit operon (citCDEFXGT) encodes a citrate lyase and the citrate permease CitT—a citrate/succinate antiporter. The cit genes are expressed only anaerobically, leaving cells unable to grow aerobically on citrate. Restored expression of the CitT transporter allows E. coli to use citrate as a carbon and energy source (6). During the potentiation step, duplication and amplification of the cit region are proposed to slightly improve growth and amplification of the region provides enough target sequence to permit the rare deletion event that provides a promoter expressing CitT and generates a new shorter nested duplication. This remodeling is the actualization step, which fuses the citT coding sequence to an aerobically expressed gene (such as uspG), allowing aerobic growth on citrate. During the refinement step, growth on citrate may be improved by amplification or increased transcription of the dctA gene encoding an aerobic C4-dicarboxylate transporter (DctA). This transporter improves growth by recapturing the succinate which is lost during uptake of citrate.
During a serial-dilution LTEE, intermittent short periods of strong selection are interspersed with periods of unrestricted growth (Fig. 1). The potentiating large duplications probably occur at high frequency but are likely to be very unstable (15). Such amplifications may improve growth during selection for citrate use but may be subject to rapid loss during periods of unselected growth on glucose. In general, duplication loss rates are higher than formation rates, leading to steady-state frequencies that are typically in the order of 10−3 to 10−4 for most sites in the Salmonella chromosome (16). The high loss rate (10−2/cell/generation) makes it likely that potentiating duplications and amplifications are lost during nonselective growth period. This problem is made worse because unselected duplications can impose a substantial fitness cost (16). Amplifications that enhance growth on citrate are likely to retard growth on glucose. An additional problem noted in the paper is that cells form clumps and filaments when growing on limiting citrate. This reduces the number of “infective centers” and makes it less likely that a slightly improved cell type will get through the bottleneck imposed by dilution and persist to the next selection period. The transitory nature of simple amplifications may explain why the posited simple duplications were not apparent in sequences of cells from early tubes from the LTEE—they may have been lost during nonselective growth prior to sequencing. Many aspects of the pure selection model have been supported by the experiments of Van Hofwegen et al. Other aspects are eminently testable.
We suggest that the primary message of the paper by Van Hofwegen et al. is that the series of events used to explain adaptation in the short-transfer LTEE (and in speciation) might need to be revised. The amplification model supported by this article has already be proposed as a means of evolving new genes (17) and has been experimentally shown to be capable of generating a novel genetic function within as few as 3,000 cell generations (18). Both of these processes are arguably more difficult to achieve than the ability to activate a single silent gene. It would appear that the delay in the LTEE may not reflect need for a neutral potentiation step but the difficulty of intermittent selection acting on frequent copy number variants. The bottleneck in serial dilutions is hard to traverse when initial improvements are due to an unstable copy number variant that is counterselected during the intervening rapid growth period.
ACKNOWLEDGMENT
Our laboratory is supported in part by a National Institutes of Health grant (GM27068 to J.R.R.).
The views expressed in this Commentary do not necessarily reflect the views of the journal or of ASM.
REFERENCES
- 1.Van Hofwegen DJ, Hovde CJ, Minnich SA. 2016. Rapid evolution of citrate utilization by Escherichia coli by direct selection requires citT and dctA. J Bacteriol 198:1022–1034. doi: 10.1128/JB.00831-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lenski RE, Rose MR, Simpson SC, Tadler SC. 1991. Long-term experimental evolution in Escherichia coli. I. Adaptation and divergence during 2,000 generations. Am Nat 138:1315–1341. doi: 10.1086/285289. [DOI] [Google Scholar]
- 3.Dimroth P. 2004. Molecular basis for bacterial growth on citrate or malonate. EcoSal Plus 2004. doi: 10.1128/ecosalplus.3.4.6. [DOI] [PubMed] [Google Scholar]
- 4.Blount ZD, Barrick JE, Davidson CJ, Lenski RE. 2012. Genomic analysis of a key innovation in an experimental Escherichia coli population. Nature 489:513–518. doi: 10.1038/nature11514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Blount ZD, Borland CZ, Lenski RE. 2008. Historical contingency and the evolution of a key innovation in an experimental population of Escherichia coli. Proc Natl Acad Sci U S A 105:7899–7906. doi: 10.1073/pnas.0803151105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pos KM, Dimroth P, Bott M. 1998. The Escherichia coli citrate carrier CitT: a member of a novel eubacterial transporter family related to the 2-oxoglutarate/malate translocator from spinach chloroplasts. J Bacteriol 180:4160–4165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yamamoto K, Matsumoto F, Oshima T, Fujita N, Ogasawara N, Ishihama A. 2008. Anaerobic regulation of citrate fermentation by CitAB in Escherichia coli. Biosci Biotechnol Biochem 72:3011–3014. doi: 10.1271/bbb.80301. [DOI] [PubMed] [Google Scholar]
- 8.Quandt EM, Deatherage DE, Ellington AD, Georgiou G, Barrick JE. 2014. Recursive genomewide recombination and sequencing reveals a key refinement step in the evolution of a metabolic innovation in Escherichia coli. Proc Natl Acad Sci U S A 111:2217–2222. doi: 10.1073/pnas.1314561111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall BG. 1982. Chromosomal mutation for citrate utilization by Escherichia coli K-12. J Bacteriol 151:269–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kugelberg E, Kofoid E, Andersson DI, Lu Y, Mellor J, Roth FP, Roth JR. 2010. The tandem inversion duplication in Salmonella enterica: selection drives unstable precursors to final mutation types. Genetics 185:65–80. doi: 10.1534/genetics.110.114074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kugelberg E, Kofoid E, Reams AB, Andersson DI, Roth JR. 2006. Multiple pathways of selected gene amplification during adaptive mutation. Proc Natl Acad Sci U S A 103:17319–17324. doi: 10.1073/pnas.0608309103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Maisnier-Patin S, Roth JR. 2015. The origin of mutants under selection: how natural selection mimics mutagenesis (adaptive mutation). Cold Spring Harb Perspect Biol 7:a018176. doi: 10.1101/cshperspect.a018176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cairns J, Foster PL. 1991. Adaptive reversion of a frameshift mutation in Escherichia coli. Genetics 128:695–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quandt EM, Gollihar J, Blount ZD, Ellington AD, Georgiou G, Barrick JE. 2015. Fine-tuning citrate synthase flux potentiates and refines metabolic innovation in the Lenski evolution experiment. Elife 4:e09696. doi: 10.7554/eLife.09696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Reams AB, Roth JR. 2015. Mechanisms of gene duplication and amplification. Cold Spring Harb Perspect Biol 7:a016592. doi: 10.1101/cshperspect.a016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reams AB, Kofoid E, Savageau M, Roth JR. 2010. Duplication frequency in a population of Salmonella enterica rapidly approaches steady state with or without recombination. Genetics 184:1077–1094. doi: 10.1534/genetics.109.111963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bergthorsson U, Andersson DI, Roth JR. 2007. Ohno's dilemma: evolution of new genes under continuous selection. Proc Natl Acad Sci U S A 104:17004–17009. doi: 10.1073/pnas.0707158104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Näsvall J, Sun L, Roth JR, Andersson DI. 2012. Real-time evolution of new genes by innovation, amplification, and divergence. Science 338:384–387. doi: 10.1126/science.1226521. [DOI] [PMC free article] [PubMed] [Google Scholar]