Abstract
In this review, we summarized published reports that investigated the role of Nigella sativa (NS) and its active constituent, thymoquinone (TQ) in oral health and disease management. The literature studies were preliminary and scanty, but the results revealed that black seed plants have a potential therapeutic effect for oral and dental diseases. Such results are encouraging for the incorporation of these plants in dental therapeutics and hygiene products. However, further detailed preclinical and clinical studies at the cellular and molecular levels are required to investigate the mechanisms of action of NS and its constituents, particularly TQ.
Currently there is a worldwide growing interest on the use of medicinal herbs or plants in the treatment of various diseases due to their promising results and fewer side effects.1 According to the World Health Organization (WHO), 60-80% of the world’s population particularly in developing countries, depends on herbal remedies or traditional medicine for their primary health care and treatment. Moreover, the WHO has encouraged developing countries to use their medicinal plants as a resource to generate effective health care programs.2,3 One of the top ranked evidence-based herbal medicines, which has been described as the “miracle herb of the century is Nigella sativa (NS).3,4 Nigella sativa is an annual flowering plant in the family Ranunculaceae (Table 1) also called black cumin, black seed, or Habbatul Barakah is native to the south and southwest Asia, and is cultivated in several countries in the Mediterranean region, South Europe, Syria, Turkey, and Saudi Arabia.5,6 Historically, ancient Egyptian and Greek physicians used NS seeds in the management of several diseases, such as headache and toothache, congestion of the nose, intestinal worms, promotion of menstruation, and production of milk.7,8 The black seed has a spiritual and religious impact in many Muslim populations, as the prophet Mohammad said “use the black seed regularly because it is a cure for every disease, except death,” which could explain its extensive use by millions of Muslims all over the world.9
Table 1.
Scientific classification of Nigella sativa.8

Researchers have attributed the health promoting benefits of the black seed to its active components and high nutritional content.3 The seeds are composed of 28-36% fixed oils, proteins, alkaloids and saponins, and 0.4-2.5% essential oils. Many pharmacologically active compounds have been isolated from black seeds, but the most reported active constituents are thymoquinone (TQ), dithymoquinone, thymol, and thymohydroquinone. Several nutritional components are found in the seeds, such as carbohydrates, fats, essential amino acids and vitamins. The seeds are also considered to be a source of potassium, calcium, and iron.4,8 Several authors have extensively reviewed NS and its active component, TQ, and found them to have many pharmacological properties, such as: antimicrobial (antibacterial, anthelmintic, antifungal, and antiviral); anti-inflammatory; analgesic; histamine release inhibitor; antihypertensive; hypoglycemic; anticarcinogenic; antioxidant; and hepatoprotective among others.3,5,6 Oral health and the integrity of the oral cavity are crucial to the general health of human beings. Many oral diseases are preventable, and for many years, the use of plants, oral health care, and therapeutic practices have been closely related.10-12 A literature review revealed that studies relating NS to oral health are scant, and as of this date, no published report summarizes their findings. The aim of this report is to summarize the published studies on the role of NS and its active component, TQ, in oral health.
PubMed and Google Scholar were used for data search, and up-to-date papers in English were selected using the following key words: Nigella sativa, thymoquinone, oral health, dental health, periodontal diseases, caries, pulp treatment, oral cancer, and oral squamous carcinoma. The collected data are presented in subheadings related to oral health issues.
Dental caries
Dental caries or tooth decay is a progressive, irreversible microbial disease that is characterized by bacterial fermentation of carbohydrates resulting in acid production, and the subsequent destruction of the hard tissues of the tooth. Streptococcus mutans (S. mutans) and Streptococcus sobrinus are the major suspected pathogens in the initiation and progression of dental caries.13-15 According to a WHO report, dental caries affect nearly all adults at some point in their life.16 It remains a health problem and a challenging disease inspite of the worldwide public health strategies that have focused on preventive methods, such as water fluoridation, and application of topical fluorides.13 Recently, some authors investigated the preventive effect of natural products on dental caries.17,18 Although many authors believed that NS and TQ might have a role in caries prevention because of their antibacterial properties, there have been few published studies on this topic. Only one study13 was found with a rat experimental model, its aim was to investigate the effect of NS on caries initiation through the inhibition of microbiome formation. In that study,13 the animals were challenged with S. mutans and fed a diet rich of sucrose. The results showed that the rats’ treatment with 10 mg of TQ per body weight (in kg) in oral gel or drinking water statistically reduced the caries score (p=0.02) and plaque index (p=0.01) in comparison with the control groups. The authors attributed the decrease in dental caries to the microbial biofilm inhibition properties of TQ.13
Streptococcus mutans remain the major pathogen in dental caries. Its pathogenicity is due to the production of extracellular polysaccharides and its ability to bind dental tissues through a biofilm and subsequent plaque formation.15,19 The microbiome is composed of salivary glycoproteins, bacteria (cocci, bacilli, and filamentous forms) and their metabolic end products arranged in a matrix of extracellular material. Dental microbiome accumulates on and adheres to the teeth, restorations and prosthetic appliances in the mouth, and plays an important role in oral and dental diseases.20 Natural products have recently been investigated as promising alternatives to synthetic antimicrobial drugs, including herbal products, such as NS and TQ.1,12,13,18,21-23 Harzallah et al18 carried out a research to assess the anticariogenic activity of the Tunisian NS essential oil and its TQ. They used the method of broth microdilution to evaluate the minimum inhibitory concentration (MIC) of the NS essential oil and its TQ for oral cariogenic pathogens. Surprisingly, they found that 2.43 mg/disc essential oil contained only 3.35 µg/ml TQ, and showed more antimicrobial activity than the pure TQ compound (150 µg/disc). The latter was active against all of the studied strains, especially S. mutans, which showed inhibition zones of 24.5 ± 0.71 mm, and Streptococcus mitis (S. mitis) with 22 ± 1.41 mm. They explained the antibacterial effect of NS essential oil, even with a minimal amount of TQ, as being caused by its other more potent bactericidal components, such as p-cymene, limonene, and apinene.18 The black seed oil extracts were not only found to have a bactericidal effect against S. mutans but also to have an effect on inhibiting the adherence of S. mutans to the surface of the teeth.
Abd-Awn et al1 conducted a research using an agar diffusion test followed by a minimum bactericidal concentration (MBC) determination to test the NS oil extract’s sensitivity to S. mutans and its ability to inhibit bacterial adherence to the dental plaque compared with chlorhexidine gluconate. The results showed that the black seed oil extract has 10% MBC against S. mutans.1 However, the authors recommended that further studies with different types of extracts (including those from different regions) be evaluated for the antibacterial activity of the black seed. In addition, they recommended an analysis of the extracts for their active ingredients, which are responsible for their antibacterial activity. They also cited the urgent need for finding a standard method for extract preparation.1 The last point was found to be valid, as Mohammed24 evaluated 2 different extracts of NS against 2 cariogenic bacteria, S. mutans and S. mitis. The results demonstrated that the inhibition zone for the ethanol extract was 12.7 mm against S. mutans, and 10.4 mm against S. mitis, while the inhibition zone for the ether extract was 6.3 mm against S. mutans, and 5.1 mm against S. mitis. This means that methanol extraction had a stronger effect against the bacteria than did the ether extract.24
Periodontal and gingival diseases
Periodontitis is microbiome-related dental inflammation caused by Gram negative bacteria, resulting in connective tissue destruction beyond the gingiva around the teeth.25,26 Almost 200 systemic diseases are connected to oral health according to the American Dental Association.27 Periodontitis alone was found to have an established association with several systemic conditions, such as diabetes mellitus, cardiovascular and pulmonary diseases, low birth weight, and other conditions.28,29 As for dental caries, proper oral hygiene and microbiome control are also very important strategies for periodontal disease prevention. However, most of the population does not perform proper mechanical microbiome control due to lack of motivation or dexterity.30 The regimen of periodontal treatment often includes the use of antibacterial agents, which can reach diseased sites systemically, or topically. A recent systematic review showed that mouthwashes containing chlorhexidine present high antiplaque (mean reduction of 40%), and antigingivitis efficacy (mean reduction of 28%), however, their adverse effects (such as, teeth and soft tissue staining) limit their use.31 Many herbal products have been used as alternatives to these synthetic drugs.20 Because of the antibacterial, anti-inflammatory, and antioxidative properties of NS and TQ, the authors believe that these products could have a role in periodontal disease.32 In the rat model proposed by Al-Wafi et al,13 besides the anti-caries assessment, an evaluation of the potential preventive role of TQ on gingival inflammation was conducted. The results revealed that rats treated with TQ in drinking water or an oral gel had statistically significant lower periodontal indices and subgingival bacterial counts in comparison with both the negative and positive control groups. Additionally, their mandibular tissues, which were taken for histological examination to determine the degree of inflammation, demonstrated no signs of inflammation compared with the controls. Another in vivo study,32 also in a rat periodontitis model, investigated the preventive role of TQ in periodontal inflammation initiation and progression. In that study,32 the gingival margins of the rats’ molars were ligated with a 4/0 silk suture to induce periodontitis. The experiment included 24 rats that were randomly and equally distributed into 3 groups; nonligated (NL), ligature only (LO), and ligature plus daily treatment (TQ at 10 mg/kg). On day 11, the animals were sacrificed and in each group the alveolar bone levels of molars were clinically measured and examined histopathologically. The results revealed that there were statistically significant higher bone losses in the LO group in comparison with the NL and the TQ groups. The same was reported regarding the ratio of inflammatory cell infiltration to osteoclasts. However, the reverse was true regarding the osteoblasts, which showed significant lower activity in the LO group than in the NL and TQ groups. Thus, it was concluded that the oral administration of TQ helped in periodontal disease prevention as it diminished alveolar bone resorption.32 Additionally, a clinical randomized single-blind split-mouth trial26 was carried out to evaluate the effectiveness of a TQ-impregnated periodontal chip in chronic periodontitis. The study included the periodontal pockets of 12 male patients with chronic periodontitis. They were distributed into 3 groups: control (no treatment), plain chitosan chips, and TQ chips.26 Interestingly, the results showed significant gains in the clinical attachments in the TQ groups compared with the other groups. Al-Bayaty et al26 advised the use of TQ chips in chronic periodontitis patients as an adjunctive treatment during the scaling and root planing, or for maintenance visits.
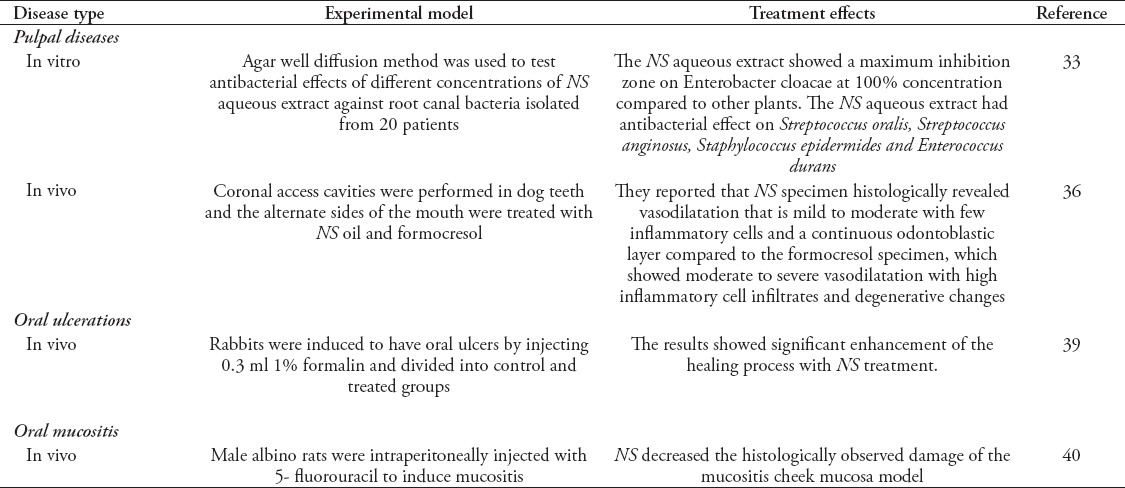
Pulpal diseases
Intracanal antisepsis is considered to be one of the fundamental steps in root canal therapy. Antimicrobial irrigating solutions and other locally used disinfecting agents and medicaments play a key role in the eradication of microbes.33 An ideal root canal irrigant should have high efficacy against microorganisms in biofilms while being systemically nontoxic and noncaustic to periodontal tissues.34 Although current irrigation regimens using sodium hypochlorite (NaOCl) exhibit excellent antimicrobial activity, caustic, and toxic effects to vital tissues are often noted. Therefore, there is a need for agents that are both antibacterial and that exert minimal tissue irritation. Plant-derived products represent a rich source of antimicrobial compounds, and some have been incorporated into oral hygiene products. However, their application in endodontics is less well documented.35 Only 2 studies33,36 have been found to evaluate the possible use of NS or TQ in endodontics. One study33 evaluated the antibacterial action of NS with 5 different concentrations of aluminum potassium sulfate (alum), Salvadora persica (Siwak), and a combination of all these on root canal bacteria isolated from 20 patients. The authors33 found that all 3 tested herbs had bactericidal effects, and that the NS aqueous extract (100% concentration) showed maximum inhibition zones of 26 mm in diameter for Enterobacter cloacae, 22 mm in diameter for Streptococcus oralis, 21 mm in diameter for Streptococcus anginosus, 20 mm in diameter for Staphylococcus epidermides, and 16 mm in diameter for Enterobacter cloacae, Streptococcus oralis, Streptococcus anginosus, Staphylococcus epidermides and Enterococcus durans. Another study36 searched for a new capping medicament in pediatric dentistry to replace formocresol because of its reported side effects. The study compared the histopathological pulp response to NS oil and formocresol (FC) in dogs. The results showed that NS specimens histologically revealed mild to moderate vasodilatation with few inflammatory cells and a continuous odontoblastic layer. On the other hand, FC specimens showed advanced inflammation with severe vasodilatation and inflammatory cell infiltration and degeneration. Thus, application of NS maintained the vitality of the pulp, which makes it a good pulpotomy agent in clinical practice.36
Oral ulcerations, oral mucositis and wound healing
Oral ulcerations are common painful lesions that are related to various conditions ranging from minor local trauma to significant systemic conditions, such as hematological, gastroenterological, dermatological, and immunological diseases and malignancies.37,38 Topical NS oil was found to have a good therapeutic effect on the healing of chemically induced oral ulcers. Al-Douri and Al-Kazaz39 carried out an experiment on 12 rabbits. The authors created the ulcers with 0.3 ml of 1% formalin injections in the cheek mucosa of the rabbits followed by topical application of NS twice a day for 3 days. The animals were sacrificed on the fifth day, and their cheek mucosae were histologically examined. The results showed a significant healing process enhancement with NS treatment, and a marked anti-inflammatory activity and differences in the rate of epithelization between the NS and control groups. The authors justified the results by asserting that the NS oil accelerated the healing of ulcers, because it inhibited the growth of pathogenic organisms at the site of the ulcers, which could retard the healing process. Moreover, the TQ flavonoids and the other components in the NS oil (vitamins and minerals) make it an excellent promoter of ulcer healing.39
Oral mucositis due to chemotherapy and radiotherapy represents a problematic side effect in cancer treatment. A study in a rat model evaluated the capacity of NS to decrease the severity of chemotherapy-induced mucositis.40 In that study,40 25 male albino rats were divided randomly into 3 groups: control, untreated, and treated. An intraperitoneal injection of 5 fluorouracil (5FU) was used to induce mucositis in rats, and their cheek pouches on the right side were scratched with a wire brush to induce mucositis. Rats in the untreated group received oral physiologic saline, and those in the treated group were gavaged daily with 1 ml of NS seed extract (400 mg/kg). Rats were sacrificed on day 14, and the cheek pouch areas were excised and prepared for histological and immunohistochemical analysis using Bcl2 and PCNA immune labelling. The results showed that in comparison with controls and 5FU untreated rats, NS decreased the histologically observed damage of the cheek mucosa in the mucositis model. The results suggested NS as a promising prophylactic adjunct to conventional chemotherapy for reducing the severity of oral mucositis.40
Despite reports on the accelerated healing process and scarless wounds in the oral cavity, in contrast to wounds on the skin, healing oral wounds remains a challenge due to the continuous saliva flow, and the presence of microorganisms that tend to interrupt and slow down the healing process. An in vitro study41 investigated the wound healing promoting properties of 4 aqueous extracts of Asian traditional medicinal plants: NS, Melastoma malabathricum, Pluchea indica, and Piper sarmentosum. The in vitro scratch assay was used to assess the effect on wound healing activity, while the extracts’ effect on collagen synthesis by human gingival fibroblasts (HGF) was determined by hydroxyproline levels. The NS extract (NSE) showed a significant enhancement of HGF proliferation compared with other extracts and accelerated wound closure despite its lack of significant effect on collagen synthesis.41
Bone healing/socket and implants
Recently, it has been reported that black seed induced bone healing in extracted teeth sockets and manifested faster bone trabeculae formation.42 This was an animal study,42 in which the therapeutic effect of both powder and oil forms of NS on socket healing were investigated. The sample included 24 male New Zealand rabbits. The researchers extracted the rabbits’ incisors, filled the extraction sites with NS powder or oil material, and compared the healing with the other side of the mouth (control group).42 The extraction sites were examined radiographically and histologically after one, and 6 weeks. The results showed an early detection of osteoid formation at the first week and bone trabeculae formation at the 6th week in sockets treated with NS (powder and oil). However, in the histological study, it was found that at the extraction sites, NS powder was more effective than the NS oil in terms of bone formation.42 They attributed this result to the complete absorption of the powder compared with the oil, which was applied with a piece of cotton for only 5 minutes and then removed.42
Natural plants were proposed as a coating agent because the biological molecules improve osseointegration in dental implants.43 However, the literature concerning NS and bone healing is contradictory. A disappointing study was conducted by El-Sweify et al44 to identify the effect of calcium hydroxide powder mixed with NS oil compared with bioglass on the healing of mandibular bone defects in rats. The experiment included 40 albino rats that were subjected to surgical procedures to induce a mandibular surgical defect and were distributed into 4 groups. Group I was the control group, where the bony defects were left empty; Group II had bony defects that were filled with calcium hydroxide powder mixed with saline; Group III had bony defects that were filled with calcium hydroxide mixed with NS oil; and Group IV had bony defects that were filled with ready made bioactive glass particulates. After 3 weeks, one half of the animals were sacrificed, while the other half was sacrificed after 6 weeks. The results revealed that all of the used materials were biocompatible with the animal model, but the bioglass seemed to have the highest osteoconductive and osteostimulatory effects among the materials used. There was no bone formation in Groups I, II, and III after 3 and 6 weeks. Group IV after 3 weeks showed only minute amounts of osteoid tissues; however, after 6 weeks, more fibrous tissues were seen, and the borders of the defects were of normal thickness, and were smooth and lined by a well-defined osteoblastic layer.44 On the other hand, an in vivo study43 was conducted to investigate the black seed oil extract’s effect on the bone implant interface’s mechanical and histological properties. In the experiment, 12 New Zealand rabbits received 4 screw-shaped (84 CpTi) implants in the tibiae bone. The implants were divided into 2 groups; coated, and uncoated with black seed oil extract. The results showed that over different periods of time, the coated implants had significantly better torque resistance than the uncoated implants, and histologically revealed early osseointegration with an osteophilic surface, as well as no adverse tissue reaction.43
Microbial diseases. A) Antibacterial activity
There have been many studies that have investigated the antimicrobial effect of NS, but they are not in the scope of this review, although a few are mentioned. Currently, there is an urgent need for new antibacterial drugs to overcome the emergence of oral bacterial resistance. Interestingly, an in vitro study22 was carried out to assess the TQ’s inhibitory and resistance-modifying ability against a panel of pathogenic bacteria, including oral isolates. The results revealed that TQ demonstrated a selective antimicrobial property; it was effective against Gram positive bacteria, but Gram negative bacteria were resistant to it.22 However, there was a 4-fold potential effect of tetracycline and benzalkonium chloride (BC) against the tested pathogens. Thus, TQ can be used in multidrug resistant infections.22
More recently, a laboratory study45 was conducted to compare the antibacterial effect of NS oil extract with nanosilver and amoxicillin using the disk diffusion method on oral S. mutans and S. sanguis. The results showed that the NS oil at a concentration of 330 mg/mL, demonstrated an inhibitory zone of 22 mm for S. mutans, and 9.75 mm for S. sanguis. Meanwhile, the bacterial inhibitory zone of each of the nanosilver concentrations at 3500 ppm and the standard amoxicillin disc (25 µg) was 33.5-19.75 mm for S. mutans and 11-21.75 mm for S. sanguis.45 Thus, nanosilver had the highest antibacterial activity against both bacterial strains, while NS and amoxicillin had a similar effect on S. mutans and NS was more effective than amoxicillin against S. sanguis.45
B) Antifungal activity
Oral candidiasis is a common infection in the oral cavity caused by fungi, mostly Candida albicans.46 Amphotericin B and triazoles, such as fluconazole and itraconazole are the most common antifungal agents that have been used systemically, or topically for fungal infections. Unfortunately, with a prolonged course of therapy, fungal resistance erupts as a challenge. Again, the medicinal plants have been attractive to scientists as possible alternative fungal treatments.47-50
In Hail province, a study50 assessed the in vitro inhibitory activity of ethanol extracts from 6 plants against oral candidal isolates. The latter were 65 Candida isolates collected from 175 patients attending private dental clinics. The experimental plants, which were purchased from local Saudi markets, included the following: Zingiber officinale (ginger); Cinnamomum (cinnamon); NS (black cumin); Syzygium aromaticum (clove); Piper nigrum (black pepper), and chamomile. It has been found that the highest inhibitory effects were reached with a 100 µg/ml concentration of cinnamon (34.6 mm) and clove (31.5 mm), while the same concentration of black cumin and chamomile recorded the smallest inhibition zone.50 However, the mean values of the inhibition zone of both the black cumin and chamomile were not significantly different from that of Amphotericin B.5 Another study,51 claimed by the authors to be the first of its kind investigated the in vitro antifungal properties of Amphotericin B, ketoconazole, and TQ against Candida albicans (yeasts and biofilm) in their nanoparticle forms in comparison with their conventional forms. The results showed that the nanosized drugs’ fo-rmulation increased the drug’s efficacy 24 times against Candida albicans in both yeast and biofilm forms. Thus, this finding represents a major innovation in the medical field, as biofilm growth is more protected from host defenses and has enhanced resistance to antimicrobial agents.51
Oral cancer
Oral cancer is considered to be a multistep disease of accumulated genetic and epigenetic alterations in epithelial cells, which generally precedes histological changes. These changes accumulate throughout malignant transformation from benign to precancer lesions to the invasive cancer state.52,53 Oral cancer is ranked as the sixth most frequent cancer worldwide. Unfortunately, the 5-year survival for oral cancer has remained at 50% for a long time. This occurrence is due to several factors, such as late diagnosis, disease relapse, resistance to chemotherapy, and metastasis.54,55 Premalignant lesions could be subclinical conditions with normal mucosa or clinically obvious lesions, such as leukoplakia, erythroplakia, and submucous fibrosis. Studies showed that premalignant lesions are reversible conditions, as epithelial tissues retain normal mucosal morphology. This is true if carcinogenic insults related to an unhealthy lifestyle habits (such as, alcohol consumption and tobacco smoking) were adjusted. However, changing unhealthy habits might be hard for some, or undesirable for others. Instead, using pharmacological or natural products to prevent the progression of premalignant to cancerous lesions is a very attractive approach known as chemoprevention.54,56-58 Studies revealed that TQ restrains proliferation and promotes apoptosis in human cancer cell lines.59-61
In addition, TQ can be used as a chemosensitizing agent with drugs, or can be used with other chemotherapy drugs to achieve a synergistic effect. An example of this co-treatment is the combination of TQ and cisplatin, which was found to have a synergistic effect in preventing the proliferation and invasion of human lung cancer.62 Here, we focus on the effect of TQ in oral cancer treatment and prevention. Although only a few studies have been performed on the effect of TQ on oral cancer, it is still promising.60,63,64 A study by Chu et al60 revealed that the treatment of human oral cancer with TQ induces cell death by the 2 distinct mechanisms of apoptosis and autophagy. Apoptosis is programmed cell death. It is regulated by either the receptor-mediated death pathway, or by the mitochondrial pathway. Apoptosis causes changes to the cell that are characterized by cell shrinkage, bulge of the membrane, breakage of DNA, and caspase activation.65 Autophagy is an important physiological process that sustains cellular homeostasis under stress conditions. In cancer therapy studies, autophagy-inducing agents are used to stimulate nonapoptotic cell death.66 Experiments were performed in vitro and in vivo, which strongly supported the TQ’s antitumor activity. In vitro, treating SASVO3 oral cancer cells with TQ inhibited the growth of cells. These cells were arrested at the sub G1 phase (apoptotic cell population). Protein expression by western blots of Bcl2 (an inhibitor of apoptosis) and Bid were decreased in a dose-dependent fashion.
On the contrary, the protein expression of Bax (a promoter of apoptosis) and cleaved caspase 9 were also increased in a dose-dependent fashion. To verify the activation of apoptosis through caspase 9, the authors incubated SASVO3 cells with TQ and the caspase 9 inhibitor. Staining with annexinV/PI revealed a decrease in the TQ-induced apoptotic population after treatment with the inhibitors. Taken together, TQ induced apoptosis through the initiation of caspase 9. Autophagy-related proteins Beclim 1, class III PI3K complex, Rubicon, and Atg family proteins were expressed in SASVO3 cells treated with TQ. Additionally, as a protein indicator of autophagy, LC3II increased after TQ treatment and TQ induced autophagy. In vivo, nude mice were injected with luciferase expressing SASVO3 cells. Mice were treated with either TQ or olive oil (control). The TQ treatment reduced tumor weight by 3.4-fold by the 20th day. Protein expression of the TQ-treated tumor showed high levels of Bax and LC3II compared with the control animals. These results supported the in vitro results, where TQ treatment prompted apoptosis and autophagy in vivo. This study verified the need of multiple target therapy as an effective approach in anticancer treatment.60 Abdelfadil et al64 carried out a study on the treatment of oral cancer cells with TQ. Their results showed that TQ induced apoptosis. The mouse cell line used in that study represents chemically induced oral squamous cell carcinoma (T28). Treating T28 with TQ induced apoptosis through the blocking of the p38β MAPK pathway and increased expression of proapoptotic proteins Bid and Bad with the activation of p53. Apoptosis in T28 cells occurred through caspase 3 cleavage. The difference in the apoptosis pathway between this study and the previous one is probably due to the use of different cell lines (human versus animal). Interestingly, TQ treatment induced downregulation of COX2, which in turn, decreases cell survival. Abdelfadil et al64 stated that TQ has a promising potential in anticancer therapy by promoting apoptosis and reducing cell survival. The TQ not only can be used as a treatment for cancer but also as a chemopreventive. It prevents premalignant lesions to progress to cancer. Many studies have illustrated this concept regarding TQ in different cancer types.67,68 Only one oral cancer prevention study through the use of TQ has been published.63 They used 7,12 dimethyl benz (a) anthracene (DMBA) in an induced hamster buccal pouch carcinogenesis model to study TQ chemoprevention. The DMBA is known to induce multistep carcinogenesis. It starts with a sequence of hyperplasia, dysplasia and carcinoma, which is very similar to the development of oral cancer. For animals treated with TQ, the incidence of oral neoplasm and cytokeratin expression was significantly prevented.63 The NSE can be used in combination with chemotherapeutics, such as cisplatin, to enhance the anticancer activity of the drug and as a protective agent for organs from its cytotoxic effect. We verified the protective effect of NSE in a DMBA-induced hamster buccal pouch carcinogenesis model treated with cisplatin.69 The NSE protected the hamster kidney from nephrotoxic effects. Kidney function tests showed lower values in hamsters treated with TQ and cisplatin than hamsters treated with cisplatin alone. In addition, as found in this study, liver functions were better in the NSE-treated groups. In summary, NSE, and particularly TQ are promising products in the prevention and treatment of oral cancer.
Conclusion and recommendations
The few last decades have shown a rise in using herbal medicine investigations to replace conventional treatments for various diseases and conditions.42 Oral and dental diseases have not been an exception. The literature reported several primary studies1,13,18,24,26,32,33,36,39,40 that investigated the role of NS and its active constituent TQ in different areas of dentistry. Interestingly, the results were positive and promising (Tables 2 & 3). The NS and TQ demonstrated several potential therapeutic effects on different oral and dental diseases. The NS and TQ possess antiplaque activity, thus, they can help in preventing both caries and periodontal diseases. Several studies assessed the sensitivity of oral pathogens against NS extracts and/or TQ, and the results were equivalent to, synergistic with, or even better than the regularly used antibiotics, such as amoxicillin or tetracycline.22,25
Table 2.
The effects of Nigella sativa (NS) extracts and thymoquinone (TQ) in dental caries and periodontal diseases.
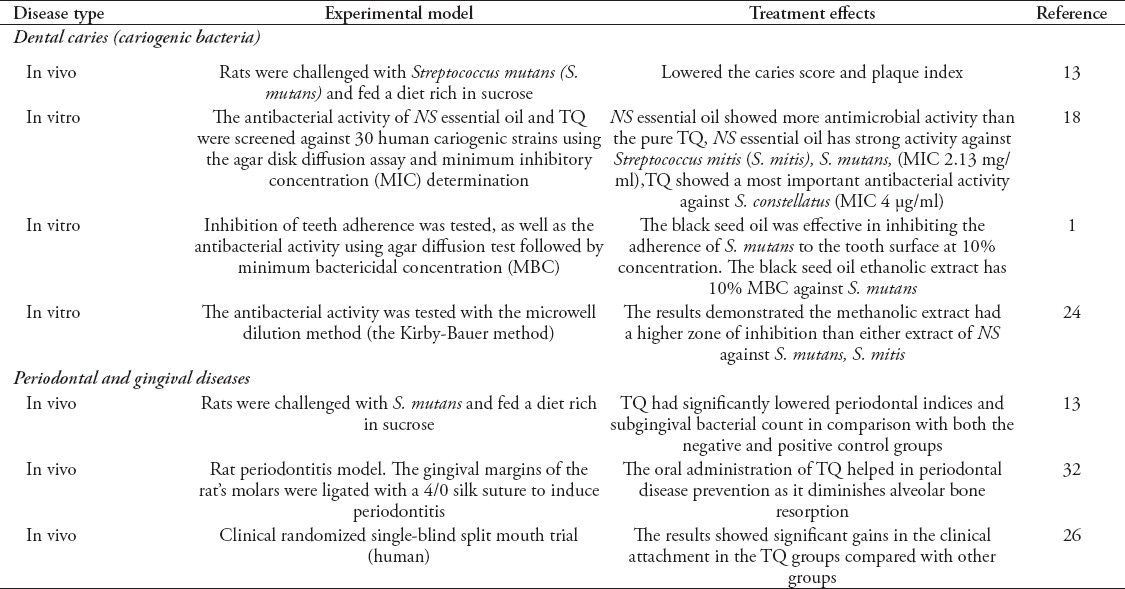
Table 3.
The effects of Nigella sativa (NS) extracts and thymoquinone (TQ) in common oral diseases.
In addition, the seed extracts have anticandidal activity, as well as wound and bone healing promoting activities.42,51 Such results are encouraging for the incorporation of these plants in dental therapeutics and hygiene products. However, there were few studies, and they were mainly conducted on animals. Therefore, more clinically controlled trials on humans are needed, especially because the plant has no toxicity or health hazards that have been reported by any researchers who have been working on related clinical trials for years.7,70 There are still several plant properties that are not well studied in relation to dental health or diseases, including the anti-inflammatory and analgesic effects.4,71 This could furnish the basis for a good natural analgesic for toothache treatment. Additionally, the immunomodulatory activity,72,73 makes the seed a good alternative for oral immune diseases, as well as its antioxidant and its antineoplastic activities, which have been extensively studied in relation to several cancers, including oral cancer.3,53-68 Moreover, further detailed preclinical and clinical studies are required at the cellular and molecular levels to investigate the mechanisms of action of NS and its constituents, particularly TQ. Overall, the studies that investigated the role of NS and its active component TQ are preliminary, but the results revealed that the plant has a potential therapeutic effect for oral and dental diseases.
Footnotes
References
- 1.Abd-Awn B, Al-Dhaher Z, Al-Dafaai R. The effect of black seed oil extracts on mutans streptococci in comparison to chlorhexidine gluconate (in vitro) Journal of Baghdad College of Dentistry. 2012;24:126–131. [Google Scholar]
- 2.World Health Organization. Traditional medicine strategy 2002-2005. Geneva (CH): WHO Publications; 2002. pp. 1–6. [Google Scholar]
- 3.Ahmad I, Tripathi J, Sharma M, Karchulli MS, Umer L. Nigella sativa - a medicinal herb with immense therapeutic potential (a systematic review) International Journal of Biological & Pharmaceutical Research. 2014;5:755–762. [Google Scholar]
- 4.Ahmad A, Husain A, Mujeeb M, Khan S, Najmi A, Siddique N, et al. A review on therapeutic potential of Nigella sativa: a miracle herb. Asian Pac J Trop Biomed. 2013;3:337–352. doi: 10.1016/S2221-1691(13)60075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tariq M. Nigella sativa feeds: folklore treatment in modern day medicine. Saudi J Gastroenterol. 2008;14:105–106. doi: 10.4103/1319-3767.41725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shrivastava R, Agrawal R, Parveen Z. A review on therapeutic applications of Nigella Sativa. Journal of Chemistry and Chemical Sciences. 2011;1:241–248. [Google Scholar]
- 7.Gaur S, Shrivastava B, Gaur S, Bhardwaj R, Khanchandani R. Medicinal and therapeutical potential of Nigella sativa. International Journal of Medical and Applied Sciences Research. 2014;1:32–39. [Google Scholar]
- 8.Tembhurne S, Feroz S, More B, Sakarkar D. A review on therapeutic potential of Nigella sativa (kalonji) seeds. Journal of Medicinal Plants Research. 2014;8:167–177. [Google Scholar]
- 9.Rahmani AH, Alzohairy MA, Khan MA, Aly SM. Nigella Sativa and its active constituents thymoquinone shows pivotal role in the diseases prevention and treatment. Asian Journal of Pharmaceutical and Clinical Research. 2015;8:48–53. [Google Scholar]
- 10.de Oliveira JR1, de Castro VC, das Graças Figueiredo Vilela P, Camargo SE, Carvalho CA, Jorge AO, et al. Cytotoxicity of Brazilian plant extracts against oral microorganisms of interest to dentistry. BMC Complement Altern Med. 2013;13:208. doi: 10.1186/1472-6882-13-208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ocheng F, Bwanga F, Joloba M, Borg-Karlson AK, Gustafsson A, Obua C. Antibacterial activities of extracts from Ugandan medicinal plants used for oral care. J Ethnopharmacol. 2014;155:852–855. doi: 10.1016/j.jep.2014.06.027. [DOI] [PubMed] [Google Scholar]
- 12.Haque M, Alsareii S. A review of the therapeutic effects of using miswak (Salvadora Persica) on oral health. Saudi Med J. 2015;36:530–543. doi: 10.15537/smj.2015.5.10785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Shaker A, Al-Wafi H. Benefits of thymoquinone, a Nigella Sativa extract in preventing dental caries initiation and improving gingival health. ProQuest LLC. 2014:72. [Google Scholar]
- 14.Gómez SI, Jaramillo LM, Moreno GC, Roa NS, Rodríguez A. Differential reactivity of salivary IgA and IgG against Streptococcus mutans proteins in humans with different caries experience. Acta Odontol Latinoam. 2015;28:3–12. doi: 10.1590/S1852-48342015000100001. [DOI] [PubMed] [Google Scholar]
- 15.Angius F, Madeddu MA, Pompei R. Nutritionally variant streptococci interfere with streptococcus mutans adhesion properties and biofilm formation. New Microbiol. 2015;38:259–266. [PubMed] [Google Scholar]
- 16.World Health Organization. Oral Health Fact Sheet N°318. Geneva (CH): World Health Organization; 2012. [Google Scholar]
- 17.Pai MR, Acharya LD, Udupa N. Evaluation of antiplaque activity of Azadirachta indica leaf extract gel a 6-week clinical study. J Ethnopharmacol. 2004;90:99–103. doi: 10.1016/j.jep.2003.09.035. [DOI] [PubMed] [Google Scholar]
- 18.Harzallah H, Kouidhi B, Flamini G, Bakhrouf A, Mahjoub T. Chemical composition, antimicrobial potential against cariogenic bacteria and cytotoxic activity of Tunisian Nigella sativa essential oil and thymoquinone. Food Chemistry. 2011;129:1469–1474. [Google Scholar]
- 19.Gupta P, Gupta N, Pawar AP, Birajdar SS, Natt AS, Singh HP. Role of sugar and sugar substitutes in dental caries: a review. ISRN Dent 2013. 2013:519421. doi: 10.1155/2013/519421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mazumdar M, Chatterjee A, Majumdar S, Chandrika M, Patki PS. Evaluation of the Safety and Efficacy of Complete Care Herbal Toothpaste in Controlling Dental Plaque, Gingival Bleeding and Periodontal Diseases. J Homeop Ayurv Med. 2013;2:124. [Google Scholar]
- 21.Khalid A, Rehman U, Sethi A, Khilji S, Urooj F, Khan M, et al. Antimicrobial activity analysis of extracts of Acacia modesta, Artimisia absinthium, Nigella sativa and Saussurea lappa against Gram positive and Gram negative microorganisms. African Journal of Biotechnology. 2011;10:4574–4580. [Google Scholar]
- 22.Kouidhi B, Zmantar T, Jrah H, Souiden Y, Chaieb K, Mahdouani K, et al. Antibacterial and resistance-modifying activities of thymoquinone against oral pathogens. Ann Clin Microbiol Antimicrob. 2011;10:29. doi: 10.1186/1476-0711-10-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bakathir HA, Abbas NA. Detection of the antibacterial effect of Nigella sativa ground seeds with water. Afr J Tradit Complement Altern Med. 2011;8:159–164. doi: 10.4314/ajtcam.v8i2.63203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mohammed NA. Effect of Nigella Sativa L extracts against streptococcus mutans and streptococcus mitis in vitro. Journal of Baghdad College of Dentistry. 2012;24:154–157. [Google Scholar]
- 25.Lucas VS, Roberts GJ. Oro-dental health in children with chronic renal failure and after renal transplantation: a clinical review. Pediatr Nephrol. 2005;20:1388–1394. doi: 10.1007/s00467-005-1929-2. [DOI] [PubMed] [Google Scholar]
- 26.Al-Bayaty F, Kamaruddin A, Ismail M, Abdulla M. Formulation and Evaluation of a New Biodegradable Periodontal Chip Containing Thymoquinone in a Chitosan Base for the Management of Chronic Periodontitis. Journal of Nanomaterials 2013. 2013:397308. [Google Scholar]
- 27.Loos BG. Systemic effects of periodontitis. Int J Dent Hyg. 2006;4:34–38. doi: 10.1111/j.1601-5037.2006.00200.x. [DOI] [PubMed] [Google Scholar]
- 28.Bokhari SA, Khan AA. Growing burden of noncommunicable diseases: the contributory role of oral diseases, Eastern Mediterranean Region perspective. East Mediterr Health J. 2009;15:1011–1020. [PubMed] [Google Scholar]
- 29.Wahid A, Chaudhry S, Ehsan A, Butt S, Khan AA. Bidirectional relationship between chronic kidney disease and periodontal disease. Pak J Med Sci. 2013;29:211–215. doi: 10.12669/pjms.291.2926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Haas AN, Pannuti CM, Andrade AKP, Escobar EC, Almeida ER, Costa FO, et al. Mouthwashes for the control of supragingival biofilm and gingivitis in orthodontic patients: evidence-based recommendations for clinicians. Braz Oral Res. 2014;28:1–8. doi: 10.1590/1807-3107bor-2014.vol28.0021. [DOI] [PubMed] [Google Scholar]
- 31.Van Leeuwen MP, Slot DE, Van der Weijden GA. Essential oils compared to chlorhexidine with respect to plaque and parameters of gingival inflammation: a systematic review. J Periodontol. 2011;82:174–194. doi: 10.1902/jop.2010.100266. [DOI] [PubMed] [Google Scholar]
- 32.Ozdemir H, Kara MI, Erciyas K, Ozer H, Ay S. Preventive effects of thymoquinone in a rat periodontitis model: a morphometric and histopathological study. J Periodontal Res. 2012;47:74–80. doi: 10.1111/j.1600-0765.2011.01406.x. [DOI] [PubMed] [Google Scholar]
- 33.Nader MI, Al-Thwaini AN, Abdul-Hassan IA, Ali AW. Effect of Nigella Sativa (black seed) Salvadora Persica (Siwak) and aluminum potassium sulphate (ALUM) aqueous extracts on isolated bacteria from teeth root canal. Iraqi J Biotech. 2010;9:99–104. [Google Scholar]
- 34.Senia ES, Marshall FJ, Rosen S. The solvent action of sodium hypochlorite on pulp tissue of extracted teeth. Oral Surg Oral Med Oral Pathol. 1971;31:96–103. doi: 10.1016/0030-4220(71)90040-5. [DOI] [PubMed] [Google Scholar]
- 35.Jhajharia K, Parolia A, Shetty KV, Mehta LK. Biofilm in endodontics: A review. J Int Soc Prev Community Dent. 2015;5:1–12. doi: 10.4103/2231-0762.151956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Omar OM, Khattab NM, Khater DS. Nigella Sativa oil as a pulp medicament for pulpotomized teeth: a histopathological evaluation. J Clin Pediatr Dent. 2012;36:335–342. doi: 10.17796/jcpd.36.4.n6674435856q86w8. [DOI] [PubMed] [Google Scholar]
- 37.Porter SR, Leao JC. Review article: oral ulcers and its relevance to systemic disorders. Aliment Pharmacol Ther. 2005;21:295–306. doi: 10.1111/j.1365-2036.2005.02333.x. [DOI] [PubMed] [Google Scholar]
- 38.Muñoz-Corcuera M, Esparza-Gómez G, González-Moles MA, Bascones-Martínez A. Oral ulcers: clinical aspects. A tool for dermatologists. Part I. Acute ulcers. Clin Exp Dermatol. 2009;34:289–294. doi: 10.1111/j.1365-2230.2009.03220.x. [DOI] [PubMed] [Google Scholar]
- 39.Al-Douri A, Al-Kazaz S. The effect of Nigella Sativa oil (black seed) on the healing of chemically induced oral ulcer in rabbit (experimental study) Al-Rafidain Dent J. 2010;10:151–157. [Google Scholar]
- 40.Lotfy AO, Zayed M. Immunohistochemical study of the effect of Nigella Sativa L extract on chemotherapy induced oral mucositis in Albino rats. Cairo Dental Journal. 2009;25:159–166. [Google Scholar]
- 41.Ab Rahman MR, Abdul Razak F, Mohd Bakri M. Evaluation of wound closure activity of Nigella sativa, Melastoma malabathricum, Pluchea indica, and Piper sarmentosum extracts on scratched monolayer of human gingival fibroblasts. Evid Based Complement Alternat Med 2014. 2014:190342. doi: 10.1155/2014/190342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Al-Hijazi AY, Mohammed HS. Evaluation of the effect of Nigella Sativa oil and powder on socket healing process. Journal of Natural Sciences Research. 2013;13:135–141. [Google Scholar]
- 43.Alnajar SSA, Mohammed SA. Mechanical and histological significance of Nigella Sativa oil extract on bone-implant interface. Journal of Baghdad College of Dentistry. 2009;21:39–43. [Google Scholar]
- 44.El-Sweify AA, Hassan MM, Ahmed IH, Basha AA. Comparative study between the effect of calcium hydroxide mixed with Nigella Sativa oil versus bioglass on healing of induced mandibular bone defects in rats. SCVMJ. 2008;13:449–459. [Google Scholar]
- 45.Laysar HA, Niakan M, Taghi GM, Jafarian Z, Mostafavizade M, Niakan S. Comparison of the antibacterial activity of various concentrations of Nigella Sativa and Nanosilver on the growth of S. sanguis and S. mutans. J Res Dent Sci. 2013;9:179–186. [Google Scholar]
- 46.Farah CS, Lynch N, McCullough MJ. Oral fungal infections: an update for the general practitioner. Aust Dent J. 2010;55(Suppl 1):48–54. doi: 10.1111/j.1834-7819.2010.01198.x. [DOI] [PubMed] [Google Scholar]
- 47.Fani MM, Kohanteb J, Dayaghi M. Inhibitory activity of garlic (Allium sativum) extract on multidrug-resistant Streptococcus mutans. J Indian Soc Pedod Prev Dent. 2007;25:164–168. doi: 10.4103/0970-4388.37011. [DOI] [PubMed] [Google Scholar]
- 48.Alsaidy D. Isolate, Diagnosis and Treatment of Yeast Candida albicans accompanying the human body. International Journal of Advanced Research. 2014;2:1081–1086. [Google Scholar]
- 49.Doudi M, Setorki M, Hoveyda L. Comparing the antifungal effects of five essential oils plants eucalyptus, cinnamon, wormwood, sagebrush and iranian rose damascena on three standard strains of candida albicans in vitro. International Journal of Biology, Pharmacy and Allied Sciences. 2014;3:490–500. [Google Scholar]
- 50.Fareid MA. In vitro: evaluation of inhibitory activity of some plant extracts against oral candidiasis. New York Science Journal. 2014;7:66–76. [Google Scholar]
- 51.Randhawa MA, Gondal M, Al-Zahrani A, Rashid SG, Ali A. Synthesis, morphology and antifungal activity of nano-particulated amphotericin-B, ketoconazole and thymoquinone against Candida albicans yeasts and Candida biofilm. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2015;50:119–124. doi: 10.1080/10934529.2015.975042. [DOI] [PubMed] [Google Scholar]
- 52.Califano J, van der Riet P, Westra W, Nawroz H, Clayman G, Piantadosi S, et al. Genetic progression model for head and neck cancer: implications for field cancerization. Cancer Res. 1996;56:2488–2492. [PubMed] [Google Scholar]
- 53.Jaiswal G, Jaiswal S, Kumar R, Sharma A. Field cancerization: concept and clinical implications in head and neck squamous cell carcinoma. J Exp Ther Oncol. 2013;10:209–214. [PubMed] [Google Scholar]
- 54.Arnaoutakis D, Bishop J, Westra W, Califano JA. Recurrence patterns and management of oral cavity premalignant lesions. Oral Oncol. 2013;49:814–817. doi: 10.1016/j.oraloncology.2013.04.008. [DOI] [PubMed] [Google Scholar]
- 55.Kuriakose MA, Sharan R. Oral cancer prevention. Oral Maxillofac Surg Clin North Am. 2006;18:493–511. doi: 10.1016/j.coms.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 56.Khan Z, Bisen PS. Oncoapoptotic signaling and deregulated target genes in cancers: special reference to oral cancer. Biochim Biophys Acta. 2013;1836:123–145. doi: 10.1016/j.bbcan.2013.04.002. [DOI] [PubMed] [Google Scholar]
- 57.Saba NF, Haigentz M, Jr, Vermorken JB, Strojan P, Bossi P, Rinaldo A, et al. Prevention of head and neck squamous cell carcinoma: removing the “chemo” from “chemoprevention”. Oral Oncol. 2015;51:112–118. doi: 10.1016/j.oraloncology.2014.11.002. [DOI] [PubMed] [Google Scholar]
- 58.Thompson L. World Health Organization classification of tumours: pathology and genetics of head and neck tumours. Ear Nose Throat J. 2006;85:74. [PubMed] [Google Scholar]
- 59.Brown RK, Wilson G, Tucci MA, Benghuzzi HA. The effects of thymoquinone and Doxorubicin on leukemia and cardiomyocyte cell lines. Biomed Sci Instrum. 2014;50:391–396. [PubMed] [Google Scholar]
- 60.Chu SC, Hsieh YS, Yu CC, Lai YY, Chen PN. Thymoquinone induces cell death in human squamous carcinoma cells via caspase activation-dependent apoptosis and LC3-II activation-dependent autophagy. PloS One. 2014;9:e101579. doi: 10.1371/journal.pone.0101579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sutton KM, Greenshields AL, Hoskin DW. Thymoquinone a bioactive component of black caraway seeds, causes G1 phase cell cycle arrest and apoptosis in triple-negative breast cancer cells with mutant p53. Nutr Cancer. 2014;66:408–418. doi: 10.1080/01635581.2013.878739. [DOI] [PubMed] [Google Scholar]
- 62.Jafri SH, Glass J, Shi R, Zhang S, Prince M, Kleiner-Hancock H. Thymoquinone and cisplatin as a therapeutic combination in lung cancer: in vitro and in vivo. J Exp Clin Cancer Res. 2010;29:87. doi: 10.1186/1756-9966-29-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rajkamal G, Suresh K, Sugunadevi G, Vijayaanand MA, Rajalingam K. Evaluation of chemopreventive effects of Thymoquinone on cell surface glycoconjugates and cytokeratin expression during DMBA induced hamster buccal pouch carcinogenesis. BMB Rep. 2010;43:664–669. doi: 10.5483/BMBRep.2010.43.10.664. [DOI] [PubMed] [Google Scholar]
- 64.Abdelfadil E, Cheng YH, Bau DT, Ting WJ, Chen LM, Hsu HH, et al. Thymoquinone induces apoptosis in oral cancer cells through p38beta inhibition. Am J Chin Med. 2013;41:683–696. doi: 10.1142/S0192415X1350047X. [DOI] [PubMed] [Google Scholar]
- 65.Speirs CK, Hwang M, Kim S, Li W, Chang S, Varki V, et al. Harnessing the cell death pathway for targeted cancer treatment. Am J Cancer Res. 2011;1:43–61. [PMC free article] [PubMed] [Google Scholar]
- 66.Gewirtz DA. Cytoprotective and nonprotective autophagy in cancer therapy. Autophagy. 2013;9:1263–1265. doi: 10.4161/auto.25233. [DOI] [PubMed] [Google Scholar]
- 67.Gali-Muhtasib HU, Abou Kheir WG, Kheir LA, Darwiche N, Crooks PA. Molecular pathway for thymoquinone-induced cell-cycle arrest and apoptosis in neoplastic keratinocytes. Anticancer Drugs. 2004;15:389–399. doi: 10.1097/00001813-200404000-00012. [DOI] [PubMed] [Google Scholar]
- 68.ElKhoely A, Hafez HF, Ashmawy AM, Badary O, Abdelaziz A, Mostafa A, et al. Chemopreventive and therapeutic potentials of thymoquinone in HepG2 cells: mechanistic perspectives. J Nat Med. 2015;69:313–323. doi: 10.1007/s11418-015-0895-7. [DOI] [PubMed] [Google Scholar]
- 69.Al-Attas SA, Munshi A, Noorwali A, Algrigri MA, Abohager EA, Zahran FM. Nigella sativa extract chemoprevention in oral cancer: in vivo study. Advances in Environmental Biology. 2015;9:75–90. [Google Scholar]
- 70.Al-Quorain AA. Nigella Sativa. Saudi J Med Med Sci. 2015;3:1. [Google Scholar]
- 71.Bashir MU, Qureshi HJ. Analgesic effect of Nigella sativa seeds extract on experimentally induced pain in albino mice. J Coll Physicians Surg Pak. 2010;20:464–467. [PubMed] [Google Scholar]
- 72.Osman MT, Hamza AJ, Omar E, Adnan A. The new miracle of Habbatus Sauda: its major component thymoquinone can be used in the management of autoimmune diseases. Procedia -Social and Behavioral Sciences. 2014;121:304–314. [Google Scholar]
- 73.Salem ML. Immunomodulatory and therapeutic properties of the Nigella sativa L. seed. Int Immunopharmacol. 2005;5:1749–1770. doi: 10.1016/j.intimp.2005.06.008. [DOI] [PubMed] [Google Scholar]


