Abstract
Objectives:
To prospectively examine the association between human papilloma virus (HPV) colonization of the colonic mucosa and the development of colorectal polyps (CRPs), and colorectal cancer (CRC) in Saudi Arabia.
Methods:
A case control study was performed between January 2013 and December 2014. All eligible patients underwent standard diagnostic colonoscopy. Patients with polyps or colorectal cancer were considered cases, while those with any other endoscopic findings were controls. Biopsy samples from polyps and tumors, and/or from normal colonic mucosa were acquired. Human papilloma virus colonization was detected using a hybrid capture technique of samples taken from both normal tissue, and CRPs and CRC. The association between HPV and CRPs/CRC was evaluated.
Results:
A total of 132 patients were recruited. The mean age was 53 (±15.9) years. Sixty patients had endoscopically detectable CRPs/CRC, and 72 had either inflammation or normal endoscopic evaluations. Only 4 (0.8%) of the 132 samples that were collected and analyzed were positive for the HPV gene. Statistical analysis did not identify any significant association between HPV colonization and the presence of CRPs/CRC. The only significant predictor of detecting CRPs/CRC on colonoscopy was symptomatic presentation (odds ratio=11.072, 95% confidence interval 4.7-26.2, p<0.001).
Conclusion:
Human papilloma virus colonic colonization is rare in Saudi Arabia. An association between HPV colonization and CRP/CRC development could not be identified in this cohort of patients.
Colorectal cancer (CRC) is one of the most common malignancies worldwide,1 and one of the leading cancers in the Saudi population.2-6 Human papillomavirus (HPV) infection has been associated with benign (warts)7 and malignant (pre-cancer and cancer) genitals,8 perianal,9 and oral lesions10 as well as with rectal cancer through large cohort and case-control studies.11-15 Preventive measures such as mass vaccination campaigns have reduced the incidence of HPV-related genital lesions.16,17 However, the role of HPV in the pathogenesis of colorectal polyps (CRPs) and adenomas is still undetermined despite some evidence that suggest an association between HPV and CRC exists.18,19 Based on the well-recognized etiologic role of HPV in cervical, ano-genital, and oro-pharyngeal carcinogenesis, a possible role of HPV 16/18 in the pathogenesis of colon cancers and polyps has been proposed, and western publications have suggested that HPV colonization in the colonic mucosa may contribute to the development of CRC.20,21 Other studies have contradicted this hypothesis.22 Such an association if proven may largely influence the development of preventive strategies against CRC, such as mass vaccinations and patient education campaigns. The aim of this study is to prospectively examine the association between HPV colonization of the colonic mucosa and the development of CRP and cancer in the Kingdom of Saudi Arabia.
Methods
We performed a prospective case control study involving 132 adult patients, who were recruited between January 2013 and December 2014. Included patients were those referred to the Endoscopy Unit, King Abdulaziz University Hospital (KAUH), Jeddah, Saudi Arabia for standard diagnostic flexible sigmoidoscopy or ileo-colonoscopy to investigate lower gastrointestinal symptoms or to undergo screening colonoscopy for primary or secondary prevention of CRC. Diagnosis of CRP or CRC was based on typical histological criteria. The only inclusion criteria was age from 18-85 years and the only exclusion criteria was patient refusal to consent to enrollment in the study. All patients were investigated with either colonoscopy or flexible sigmoidoscopy and patients unable to perform the diagnostic procedure were excluded.
Biopsy samples were collected from each patient recruited at the time of the endoscopic evaluation. Samples were taken from any polyp or mass found in any colonic segment and/or a single sample was retrieved from normal colonic mucosa if no abnormalities were detected. The board certified gastroenterologists and colorectal surgeons who performed the endoscopic evaluations for study participants were blinded to the results of HPV detection. Similarly, the laboratory personnel who received the colonic samples were blinded to the endoscopic reports. Clinical and demographic data were collected prior to the procedure.
Methods of HPV determination
In the Virology laboratory, the Digene procedure was performed to extract DNA and detect HPV in tissue. The Hybrid Capture 2 (HC2) assay was performed according to the manufacturer (Digene Corporation, Gaithersburg, MD, USA). The HPV DNA in biopsies was screened using Digene (HC2) technology that detects RNA:DNA hybrids using a signal-amplified, chemiluminescent signal. Hybrid capture 2 delivers the accuracy and flexibility necessary for routine detection of HPV DNA in biopsies, that can differentiate between 2 HPV DNA groups; the low-risk HPV types (6, 11, 42, 43, 44) and the high/intermediate risk HPV types (16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 68). Reports were given as either positive or negative for HPV (Figure 1).
Figure 1.
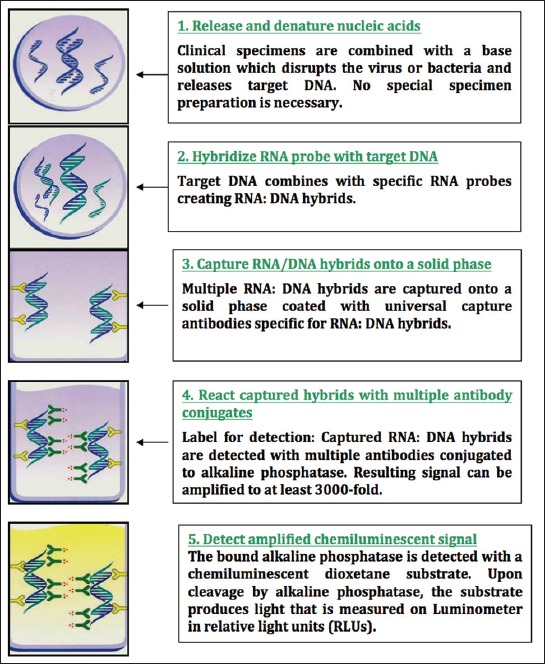
The basic steps of the hybrid capture assay. DNA - deoxyribonucleic acid, RNA - ribonucleic acid
Outcomes
The primary outcome of this study was the detection of HPV colonization in normal colonic mucosa and CRP/CRC lesions.
Sample size calculation
For sample size calculation, we hypothesized that the incidence of HPV detection in patients with CRPs or CRC is twice as high as the baseline incidence rate of HPV in patients with normal colonic mucosa (40% versus 20%). Assuming a type 1 error of 0.05 and 80% power to detect HPV, we estimated that 132 samples would be needed to detect an odds ratio (OR) of at least 3 (2-sided).
Statistical analysis
Data was collected and entered into a standard data entry sheet. Subsequently, data was cleaned and prepared for analysis. Descriptive statistics were expressed as mean±SD for continuous variables and as proportions for categorical variables. Student t-test or Mann-Whitney U test was used to compare means, and Chi-square or Fishers exact test was used for comparisons between frequencies, where appropriate. These tests were carried out with the assumption of normal distribution. Otherwise, Welch’s t-test for 2 group means was used as an alternative. The presence of CRPs/CRC lesions was identified as the dependent study variable and defined as a binary outcome. Patients presenting with symptoms were considered cases, while asymptomatic patients undergoing screening were controls. A Binary Logistic Regression Model (BLRM), with Backward Conditional Elimination with Enter Criteria=0.05 and Elimination=0.10 was used to determine the significant predictors of CRPs/CRC with 95% confidence intervals (95% CI). We used IBM SPSS statistical software, version 22.0 (IBMCorp, Armonk, NY, USA) for statistical analysis with a 2-sided 5% significance level that were used for all statistical inferences and precision of estimates measured using the 95% CI.
Ethical considerations
The King Abdulaziz University Research Ethics Board for research involving human subjects approved this clinical study, and all recruited patients provided written informed consent for participation. The study was conducted in accordance with the principles of the Helsinki Declaration.
Results
Patients characteristics
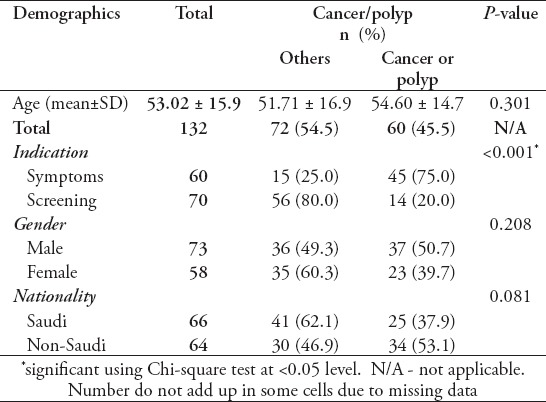
Baseline characteristics of the study population are summarized in Table 1. One hundred and thirty-two samples were collected. Clinical data was missing for 2 patients. Only 15 (11%) patients had inflammatory bowel disease (IBD).
Table 1.
Baseline demographics and clinical characteristics of patients undergoing standard diagnostic colonoscopy (N=130).
Primary end points
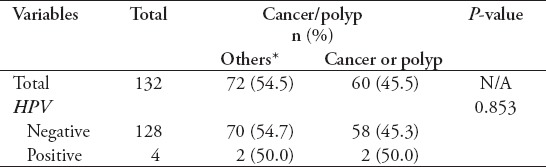
A total of 132 colorectal biopsies specimens were received in the Virology Laboratory from the Endoscopy Unit. A total of 4 (3%) specimens tested positive for the HPV Gene. Twenty-six patients were found to have polyps (20%) and 34 (26%) had CRC, of which only 2 tested positive for HPV (Table 2).
Table 2.
Results of human papillomavirus (HPV) gene detection in colonic samples.
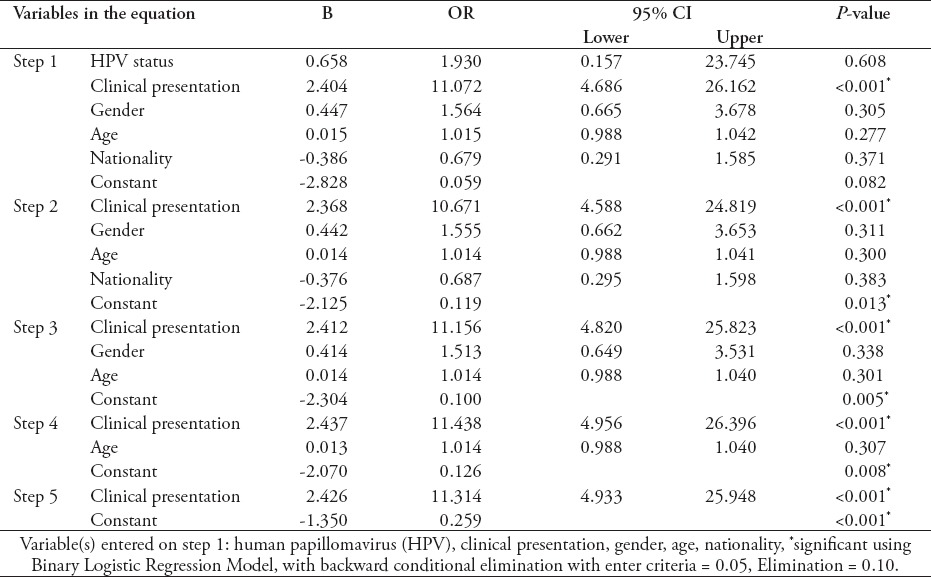
Statistical analysis did not identify any significant association between HPV colonization of the colon and the presence of CRPs or CRC (Table 3). The only significant predictor of CRPs/CRC was symptomatology.
Table 3.
Results of the binary logistic regression model for predictors of colorectal polyps and cancer.
Discussion
Human papillomavirus infection of epidermal or mucosal epithelial cells causes benign and sometimes malignant neoplasms. Certain types of HPVs such as HPV 16, 18, 31, and 45 have been detected in ano-genital cancers, particularly cancers of the cervix,17 and anus7,9,11 and are considered high-risk (H-R) genotypes or oncogenic sub-types of HPV. Integration of the viral genome into the cancer cell genome is a characteristic of the infection by these HPVs.23,24 Other types of HPV, such as low-risk (L-R) or non-oncogenic HPV6 and HPV11, induce benign ano-genital warts and are rarely found in ano-genital malignancies.25 The HPV DNA has been detected in tumor tissues of head and neck cancers,26 oral cancers,27 esophageal cancers,28 and some skin cancers,29 as well as lung cancers.30 Even though HPV DNA has been previously detected in CRC tissues by in situ hybridization21,31 and PCR,18,32,33 it was not detectable by regular PCR in another study by Snietura et al34 and a survey of HPV16 virus-like particle antibodies in patients with epithelial cancers also failed to provide an association between HPV and CRC.35 Similarly, our results show no association between HPV and CRPs/CRC detection.
Colorectal cancer is largely preventable through intensive, mass screening programs to remove premalignant colonic polyps as proposed by many cohort studies.36-38 Since CRC mostly arises from adenomas, recognized as CRPs, removing such lesions reduces the overall risk. While CRC in advanced and incurable stages often produce clinical findings, premalignant adenomatous polyps and early, highly curable, CRC are often asymptomatic. Cappell39 suggested also that the persistently high incidence and mortality is largely due to ineffective implementation of established screening protocols due to patient fears regarding screening tests, physician under-referral for screening, and test costs. Cappell,39,40 also reported a screening protocol where adenomas or early cancers are difficult to be detected by clinical presentation and provides the rationale for mass screening of asymptomatic adults over 50 years for early detection and prevention of CRC by colonoscopy, which is considered the primary screening test worldwide. All polyps identified during colonoscopy should be removed by endoscopic polypectomy. Endoscopic mucosal resection (EMR) is required for deeply penetrating non-cancerous polyps. According to the American Society guidelines,41 colonoscopy is repeated every 10 years if the index colonoscopy revealed no lesions, but is repeated more frequently if adenomatous polyps were identified at the first colonoscopy due to an increased risk of subsequent CRPs or CRC. Identifying an association between CRC and a potentially preventable infectious agent, such as HPV, may theoretically reduce the incidence of pre-malignant lesions and ultimately the incidence of CRC. However, our results do not support the presence of such an association, at least in patients of this specific cultural background.
Our study is limited by its small sample size and case control design, which has many inherent biases such as selection bias. Future large randomized control studies are needed to further elucidate this association.
In conclusion, HPV colonic colonization appears to be rare (<1%) in Saudi Arabia, which might be influenced by the cultural background. No association between HPV colonization and CRP/CRC could be depicted in this cohort of patients. Based on these results, HPV colonization is not considered a risk factor for CRP/CRC in Saudi Arabia and in countries of similar cultural background.
Acknowledgment
We would like to acknowledge and thank all the nurses and hospital staff who assisted in acquiring the study samples and the Virology Laboratory technical team for their much appreciated professional work that helped us accomplish this project.
Footnotes
Related Articles.
Nabi G. Knowledge of Saudi female university students regarding cervical cancer and acceptance of the human papilloma virus. Saudi Med J 2015; 36: 254.
Al-Shaikh GK, Almussaed EM, Fayed AA, Khan FH, Syed SB, Al-Tamimi TN, et al. Knowledge of Saudi female university students regarding cervical cancer and acceptance of the human papilloma virus vaccine. Saudi Med J 2014; 35: 1223-1230.
Altinbas SK, Tapisiz OL. Human papillomavirus, vaccines, and protection from cervical cancer. Saudi Med J 2012; 33: 1270-1277.
References
- 1.Sankaranarayanan R, Swaminathan R, Brenner H, Chen K, Chia KS, Chen JG, et al. Cancer survival in Africa, Asia, and Central America: a population-based study. Lancet Oncology. 2010;11:165–173. doi: 10.1016/S1470-2045(09)70335-3. [DOI] [PubMed] [Google Scholar]
- 2.Ibrahim EM, Zeeneldin AA, El-Khodary TR, Al-Gahmi AM, Bin Sadiq BM. Past present and future of colorectal cancer in the Kingdom of Saudi Arabia. Saudi J Gastroenterol. 2008;14:178–182. doi: 10.4103/1319-3767.43275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Isbister WH, Murad M, Habib Z. Rectal cancer in the Kingdom of Saudi Arabia: the King Faisal Specialist Hospital experience. Aust N Z J Surg. 2000;70:269–274. doi: 10.1046/j.1440-1622.2000.01805.x. [DOI] [PubMed] [Google Scholar]
- 4.Mansoor I, Zahrani IH, Abdul Aziz S. Colorectal cancers in Saudi Arabia. Saudi Med J. 2002;23:322–327. [PubMed] [Google Scholar]
- 5.Mosli MH, Al-Ahwal MS. Colorectal cancer in the Kingdom of Saudi Arabia: need for screening. Asian Pac J Cancer Prev. 2012;13:3809–3813. doi: 10.7314/apjcp.2012.13.8.3809. [DOI] [PubMed] [Google Scholar]
- 6.Mosli MH, Al-Ahwal MS. Does the increasing trend of colorectal cancer incidence in jeddah reflect a rise in the Kingdom of Saudi Arabia? Asian Pac J Cancer Prev. 2012;13:6285–6288. doi: 10.7314/apjcp.2012.13.12.6285. [DOI] [PubMed] [Google Scholar]
- 7.Kraut AA, Schink T, Schulze-Rath R, Mikolajczyk RT, Garbe E. Incidence of anogenital warts in Germany: a population-based cohort study. BMC Infect Dis. 2010;10:360. doi: 10.1186/1471-2334-10-360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hemminki K, Li X, Vaittinen P. Time trends in the incidence of cervical and other genital squamous cell carcinomas and adenocarcinomas in Sweden, 1958-1996. Eur J Obstet Gynecol Reprod Biol. 2002;101:64–69. doi: 10.1016/s0301-2115(01)00508-5. [DOI] [PubMed] [Google Scholar]
- 9.D'Ambrogio A, Yerly S, Sahli R, Bouzourene H, Demartines N, Cotton M, Givel JC. Human papilloma virus type and recurrence rate after surgical clearance of anal condylomata acuminata. Sex Transm Dis. 2009;36:536–540. doi: 10.1097/OLQ.0b013e3181a866a3. [DOI] [PubMed] [Google Scholar]
- 10.Tinoco JA, Silva AF, Oliveira CA, Rapoport A, Fava AS, Souza RP. Human papillomavirus (HPV) infection and its relation with squamous cell carcinoma of the mouth and oropharynx. Rev Assoc Med Bras. 2004;50:252–256. doi: 10.1590/s0104-42302004000300029. [DOI] [PubMed] [Google Scholar]
- 11.Orlando G, Beretta R, Fasolo MM, Amendola A, Bianchi S, Mazza F, et al. Anal HPV genotypes and related displasic lesions in Italian and foreign born high-risk males. Vaccine. 2009;27(Suppl 1):A24–A29. doi: 10.1016/j.vaccine.2008.12.026. [DOI] [PubMed] [Google Scholar]
- 12.Wilkinson JR, Morris EJ, Downing A, Finan PJ, Aravani A, Thomas JD, et al. The rising incidence of anal cancer in England 1990-2010: a population-based study. Colorectal dis. 2014;16:O234–O239. doi: 10.1111/codi.12553. [DOI] [PubMed] [Google Scholar]
- 13.Petersen I, Klein F. HPV in non-gynecological tumors. Pathologe. 2008;29:118–122. doi: 10.1007/s00292-008-1051-x. German. [DOI] [PubMed] [Google Scholar]
- 14.Erol D, Bulut Y, Yuce H, Ozercan IH. Investigation of the presence of human papillomavirus DNA in various gastrointestinal carcinoma samples. Mikrobiyol Bul. 2009;43:259–268. Turkish. [PubMed] [Google Scholar]
- 15.Mlynarczyk B, Malejczyk M, Muszynski J, Majewski S. The occurrence of human papillomavirus--HPV in the biopsies from colon polyps and cancer. Med Dosw Mikrobiol. 2009;61:191–196. Polish. [PubMed] [Google Scholar]
- 16.Bonanni P, Bechini A, Donato R, Capei R, Sacco C, Levi M, et al. Human papilloma virus vaccination: impact and recommendations across the world. Ther Adv Vaccines. 2015;3:3–12. doi: 10.1177/2051013614557476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mesher D, Cuschieri K, Hibbitts S, Jamison J, Sargent A, Pollock KG, et al. Type-specific HPV prevalence in invasive cervical cancer in the UK prior to national HPV immunisation programme: baseline for monitoring the effects of immunisation. J Clin Pathol. 2015;68:135–140. doi: 10.1136/jclinpath-2014-202681. [DOI] [PubMed] [Google Scholar]
- 18.Chen TH, Huang CC, Yeh KT, Chang SH, Chang SW, Sung WW, et al. Human papilloma virus 16 E6 oncoprotein associated with p53 inactivation in colorectal cancer. World J Gastroenterol. 2012;18:4051–4058. doi: 10.3748/wjg.v18.i30.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bodaghi S, Yamanegi K, Xiao SY, Da Costa M, Palefsky JM, Zheng ZM. Colorectal papillomavirus infection in patients with colorectal cancer. Clin Cancer Res. 2005;11:2862–2867. doi: 10.1158/1078-0432.CCR-04-1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirgan D, Manalo P, Hall M, McGregor B. Association of human papillomavirus and colon neoplasms. Arch Surg. 1990;125:862–865. doi: 10.1001/archsurg.1990.01410190060009. [DOI] [PubMed] [Google Scholar]
- 21.Kirgan D, Manalo P, McGregor B. Immunohistochemical demonstration of human papilloma virus antigen in human colon neoplasms. J Surg Res. 1990;48:397–402. doi: 10.1016/0022-4804(90)90002-j. [DOI] [PubMed] [Google Scholar]
- 22.Gornick MC, Castellsague X, Sanchez G, Giordano TJ, Vinco M, Greenson JK, et al. Human papillomavirus is not associated with colorectal cancer in a large international study. Cancer Causes Control. 2010;21:737–743. doi: 10.1007/s10552-010-9502-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zubillaga-Guerrero MI, Illades-Aguiar B, Leyva-Vazquez MA, Flores-Alfaro E, Castaneda-Saucedo E, Munoz-Valle JF, et al. The integration of HR-HPV increases the expression of cyclins A and E in cytologies with and without low-grade lesions. J Cytol. 2013;30:1–7. doi: 10.4103/0970-9371.107504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chand V, John R, Jaiswal N, Johar SS, Nag A. High-risk HPV16E6 stimulates hADA3 degradation by enhancing its SUMOylation. Carcinogenesis. 2014;35:1830–1839. doi: 10.1093/carcin/bgu104. [DOI] [PubMed] [Google Scholar]
- 25.Liang LY, Du H, Wang C, Zhang W, Chen Y, Qu XF, et al. Crosssectional survey of human papilloma virus subtype distribution and cervical intraepithelial neoplasia in Shenzhen. Beijing Da Xue Xue Bao. 2013;45:114–118. Chinese. [PubMed] [Google Scholar]
- 26.Jelihovschi I, Bidescu AC, Tucaliuc SE, Iancu LS. Detection of Human Papilloma Virus in Head and Neck Squamous Cell Carcinomas: A Literature Review. Rev Med Chir Soc Med Nat Iasi. 2015;119:502–509. [PubMed] [Google Scholar]
- 27.Gupta S, Gupta S. Role of human papillomavirus in oral squamous cell carcinoma and oral potentially malignant disorders: A review of the literature. Indian J Dent. 2015;6:91–98. doi: 10.4103/0975-962X.155877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mohiuddin MK, Chava S, Upendrum P, Latha M, Zubeda S, Kumar A, et al. Role of Human papilloma virus infection and altered methylation of specific genes in esophageal cancer. Asian Pac J Cancer Prev. 2013;14:4187–4193. doi: 10.7314/apjcp.2013.14.7.4187. [DOI] [PubMed] [Google Scholar]
- 29.Escutia B, Ledesma E, Serra-Guillen C, Gimeno C, Vilata JJ, Guillen C, et al. Detection of human papilloma virus in normal skin and in superficial and nodular basal cell carcinomas in immunocompetent subjects. J Eur Acad Dermatol Venereol. 2011;25:832–838. doi: 10.1111/j.1468-3083.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 30.Sagerup CM, Nymoen DA, Halvorsen AR, Lund-Iversen M, Helland A, Brustugun OT. Human papilloma virus detection and typing in 334 lung cancer patients. Acta Oncol. 2014;53:952–957. doi: 10.3109/0284186X.2013.879608. [DOI] [PubMed] [Google Scholar]
- 31.Salepci T, Yazici H, Dane F, Topuz E, Dalay N, Onat H, et al. Detection of human papillomavirus DNA by polymerase chain reaction and southern blot hybridization in colorectal cancer patients. J BUON. 2009;14:495–499. [PubMed] [Google Scholar]
- 32.Cheng JY, Sheu LF, Meng CL, Lee WH, Lin JC. Detection of human papillomavirus DNA in colorectal carcinomas by polymerase chain reaction. Gut. 1995;37:87–90. doi: 10.1136/gut.37.1.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Damin DC, Caetano MB, Rosito MA, Schwartsmann G, Damin AS, Frazzon AP, et al. Evidence for an association of human papillomavirus infection and colorectal cancer. Eur J Surg Oncol. 2007;33:569–574. doi: 10.1016/j.ejso.2007.01.014. [DOI] [PubMed] [Google Scholar]
- 34.Snietura M, Waniczek D, Nowakowska-Zajdel E, Piglowski W, Kopec A, Muc-Wierzgon M. Does human papilloma virus participate in colorectal carcinogenesis? J Biol Regul Homeost Agents. 2012;26:757–762. [PubMed] [Google Scholar]
- 35.Audeau A, Han HW, Johnston MJ, Whitehead MW, Frizelle FA. Does human papilloma virus have a role in squamous cell carcinoma of the colon and upper rectum? Eur J Surg Oncol. 2002;28:657–660. doi: 10.1053/ejso.2002.1304. [DOI] [PubMed] [Google Scholar]
- 36.Xirasagar S, Li YJ, Hurley TG, Tsai MH, Hardin JW, Hurley DM, et al. Colorectal cancer prevention by an optimized colonoscopy protocol in routine practice. Int J Cancer. 2015;136:E731–E42. doi: 10.1002/ijc.29228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loberg M, Kalager M, Holme O, Hoff G, Adami HO, Bretthauer M. Long-term colorectal-cancer mortality after adenoma removal. N Engl J Med. 2014;371:799–807. doi: 10.1056/NEJMoa1315870. [DOI] [PubMed] [Google Scholar]
- 38.Nishihara R, Wu K, Lochhead P, Morikawa T, Liao X, Qian ZR, et al. Long-term colorectal-cancer incidence and mortality after lower endoscopy. N Engl J Med. 2013;369:1095–105. doi: 10.1056/NEJMoa1301969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Cappell MS. From colonic polyps to colon cancer: pathophysiology, clinical presentation, screening and colonoscopic therapy. Minerva Gastroenterol Dietol. 2007;53:351–373. [PubMed] [Google Scholar]
- 40.Cappell MS. Reducing the incidence and mortality of colon cancer: mass screening and colonoscopic polypectomy. Gastroenterol Clin North Am. 2008;37:129–160. doi: 10.1016/j.gtc.2007.12.003. vii-viii. [DOI] [PubMed] [Google Scholar]
- 41.Smith RA, Manassaram-Baptiste D, Brooks D, Doroshenk M, Fedewa S, Saslow D, et al. Cancer screening in the United States 2015: a review of current American cancer society guidelines and current issues in cancer screening. CA Cancer J Clin. 2015;65:30–54. doi: 10.3322/caac.21261. [DOI] [PubMed] [Google Scholar]


