Abstract
Objectives:
To evaluate electrophysiologic pattern of subclinical diabetic peripheral neuropathy (DPN) in children and adolescents with type 1 diabetes mellitus (T1DM) based on nerve conduction study.
Methods:
In this cross sectional study, 40 children and adolescents (62.5% female with mean age of 12.73 ± 0.43 years) with T1DM for at least 5 years attending the Pediatrics Clinics. Tabriz University of Medical Sciences, Tabriz, Iran, between 2014 and 2015 were recruited. Demographic and laboratory findings were recorded and all patients underwent clinical neurological examination and electrophysiologic studies.
Results:
According to electrophysiologic studies, DPN was found in 57.5% of patients including early stage of neuropathy (15%), mild sensory axonal neuropathy (25%), mild sensory motor axonal neuropathy (10%), and moderate sensory motor axonal neuropathy (7.5%). Age, duration of diabetes, fasting blood sugar, and glycosylated hemoglobin levels had no significant difference between patients with and without DPN. Reduced deep tendon reflexes were observed in the upper limb (30%) and lower limb (47.5%) of patients, which were both significantly higher in DPN patients (upper limb [p=0.03] and lower limb [p=0.04]). The most frequent electrophysiologic findings were unobtainable H-reflex, low amplitude sural, and median sensory responses.
Conclusion:
Subclinical DPN is a common complication found in children and adolescents with TIDM and peripheral sensory axonal neuropathy is the most frequent type. Nerve conduction study is recommended for early detection of DPN and prevention of its progress.
Type 1 diabetes mellitus (T1DM) is the most common chronic endocrine disease in childhood, which is usually diagnosed among children, adolescents, and young adults.1 Children and adolescents are at higher risk of long term complications due to the longer period of T1DM.2 Diabetic peripheral neuropathy (DPN) is a major complication of T1DM with significant morbidity and mortality in adulthood. The DPN involves impairment of the large and/or small nerve fibers, and can be diagnosed by various methods.2,3 Sensory, motor, or autonomic nerves can be involved, often coexisting, but it is usually sensory dominant with eventual involvement of te\hemotor nerve fibers.4,5 Abnormalities of nerve conduction are common findings in children with diabetes and the highest prevalence for DPN is found in children and adolescents with poor glycemic control and longer duration of diabetes.5,6 Once symptoms appear, there are few effective therapeutic strategies;7 thus, early identification of DPN, especially in children and adolescents is crucial to prepare appropriate measures to prevent its development.8,9 Nerve conduction studies (NCS) are the most common method for the diagnosis of DPN and its sequelae.10 It is also possible that patients have subclinical neuropathy long before occurrence of clinically evident neuropathy.11 However, few studies have evaluated the subclinical neuropathy in these patients, and also the prevalence of early stages of DPN among children and adolescents is not well-known. In this study, we evaluated the frequency of different degrees of subclinical DPN in children and adolescents with T1DM and its possible related factors among children visiting the Tabriz Children Hospital. This hospital is the main referral center for disease of children in the north-west of Iran and our results can be applied to all the population.
Methods
In this cross-sectional study, 40 children and adolescents with T1DM visiting the Pediatrics Clinics of Tabriz University of Medical Sciences. Tabriz, Iran between 2014 and 2015 were recruited. All patients under 18 years old with a diagnosis of T1DM for at least 5 years with no previous history of neuropathy due to other diseases, and no medications with possible complications of neuropathy were included. This study was conducted in compliance with the ethical principles of Helsinki Declaration. This study was approved by the ethics committee of Tabriz University of Medical Sciences, and informed consent was obtained from all study participants. Demographic and laboratory findings were recorded for all patients. All patients underwent electrodiagnostic studies. All subjects underwent detailed neurological examination and muscle force, vibration, temperature, pain, proprioception, and fine touch perceptions, as well as pinprick and deep tendon reflexes (DTR) were evaluated. Synergy electromyograph (EMG Medelec Synergy, Istanbul, Turkey) machine was used to perform electrophysiological tests for all patients. Standard motor and antidromic sensory NCS were performed in at least 4 motor nerves (median, ulnar, tibial, and peroneal), and 3 sensory nerves (median, ulnar, and sural). In the motor nerves, distal latency (DL), amplitude and duration of compound muscle action potential (CMAP), and nerve conduction velocity (NCV) were evaluated. The F-wave minimal latency was measured after supramaximal stimulation of motor nerves and identifying 10 F-waves. The H-reflex was recorded from the soleus after stimulation of the tibial nerve. Amplitude of sensory nerve action potential (SNAP), peak latency, and NCV was measured in sensory nerves.12,13 Findings were considered abnormal if the recorded values were beyond mean ± 2.5 standard deviation of our laboratory control. A physiatrist unaware of the patient’s information performed those electrophysiological tests. Peripheral nervous system involvement was indicated if there were at least 2 abnormal independent neurophysiological nerve parameters. According to these findings, subjects were divided into 2 groups: patients with DPN, and patients without DPN. Electrophysiologic findings were interpreted by a physician trained in clinical neurophysiology and regarding reference values. According to electrophysiologic changes patients were considered to have axonal sensory neuropathy if SNAP amplitude was reduced, sensory motor axonal neuropathy if SNAP and CMAP was reduced, and early stage of neuropathy if H-reflex was reduced or absent. Reduction in NCV more than 70% of the normal value was considered as demyelinating neuropathy.
All statistical tests were performed using the Statistical Package for Social Sciences for Windows version 17 (SPSS Inc., Chicago, IL, USA). Quantitative data were presented as mean ± standard deviation (SD), while qualitative data were demonstrated as frequency and percent (%). Independent t test for quantitative date and chi-square test or Fisher’s exact tests, as appropriate were used to compare data between groups of patients. A p<0.05 was considered statistically significant.
Results
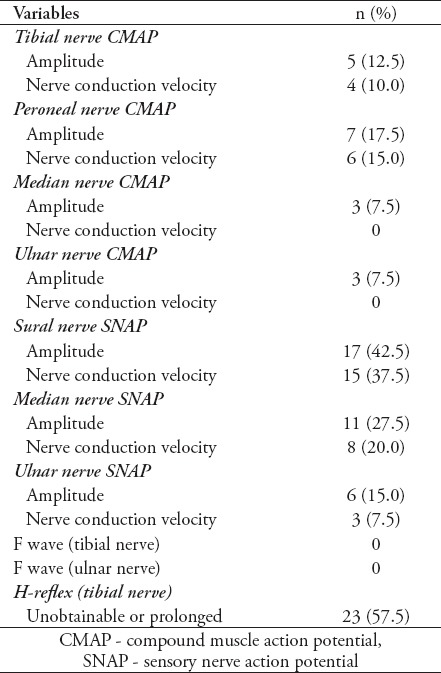
In this study, 40 T1DM patients including 15 (37.5%) males and 25 (62.5%) females with mean age of 12.73 ± 0.43 years were included. Mean duration of diabetes was 6.63 ± 0.25 years. Electrophysiologic studies showed neuropathy in 23 patients (57.5%) including early stage of neuropathy in 6 patients (15%), mild sensory axonal neuropathy in 10 patients (25%), mild sensory motor axonal neuropathy in 4 cases (10%), and moderate sensory motor axonal neuropathy in 3 cases (7.5%). Table 1 demonstrates demographic and laboratory findings between normal and neuropathic patients. Although patients with peripheral neuropathy had higher duration of diabetes, higher fasting blood sugar (FBS) and glycated hemoglobin (HbA1C) compared with patients without neuropathy, the difference was not significant. Due to the insignificant findings, none of them were included in the regression analysis. Signs and symptoms of neuropathy were evaluated in diabetic patients. Muscle force was 5/5 in both upper and lower limbs in both groups. There were no cases of numbness, muscle weakness, abnormalities of fine touch/pinprick, pain/temperature, and vibration/proprioception. Deep tendon reflexes were reduced in 12 patients (30%) in the upper limb and in 19 patients (47.5%) in the lower limb. Neuropathic group had significantly more cases of reduced DTR in both upper and lower limbs (Table 2). Electrodiagnostic findings in patients are demonstrated in Table 3. Unobtainable H-reflex and low amplitude sural and median sensory responses were most frequent electrophysiologic findings. Abnormal NCV was mostly observed in sensory sural and sensory median nerves.
Table 1.
Demographic and laboratory findings between normal and neuropathic patients.
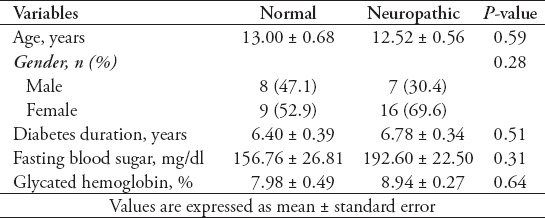
Table 2.
Deep tendon reflexes (DTR) in limbs of patients with and without diabetic peripheral neuropathy.
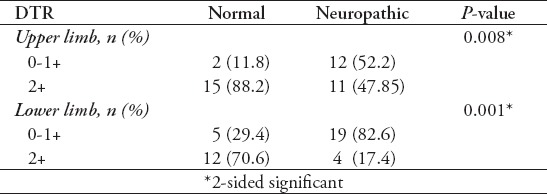
Table 3.
Abnormal electrodiagnostic findings in 40 patients with type I diabetes mellitus.
Discussion
Peripheral neuropathy is a common complication of both type I and type II diabetes that predisposes to patients to foot ulceration and amputation.4,14 Early diagnosis of DPN is essential to identify patients at risk for latent complications (especially ulcer and amputation). Previous studies have reported DPN between 25% and 66%.6,15 In this study, we evaluated the frequency of DPN in children and adolescents with T1DM and found subclinical peripheral nephropathy in 57.5% of patients. We did not found any factors predicting DPN. It was reported that it is possible that patients with DN have variable degrees of sensory loss in their physical examination,16 or even present with normal findings. Baba and Ozaki17 found polyneuropathy using neurologic examinations in 43% and using electrodiagnostic studies in 63% of children and adolescents without clinical neuropathy.
In our study, although electrophysiological studies were indicative of neuropathy, physical examination did not show any significant findings. Only reduced DTR was observed in less than 50% of cases. It could be regarded as subclinical neuropathy. Similar to our findings, Nelson et al18 found subclinical neuropathy based on nerve conduction findings in 57% of Canadian children and adolescents with at least 5 years duration of T1DM. The most prevalent abnormal electrodiagnostic findings were related to H-reflex and sural and median sensory nerves. Similar findings are reported in previous studies.19,20 Following an axonal injury, nerve fibers involved in action potential are reduced, which causes reduced CMAP or SNAP.
Sensory axonal neuropathies were the most common peripheral neuropathies among our patients. Sensory polyneuropathy is regarded as the most common presentation of neuropathy in diabetic patients.21 Symptoms and findings regarding sensory nerve damage occur earlier than motor nerve damage, possibly due to thinner and longer nerves in sensory nerves, which could be more vulnerable to metabolic alterations.3,7 Many different factors are introduced to have a role in developing DPN, such as age, male gender, duration of diabetes, and glycemic control. However, different studies have reported conflicting results in this regard. Diabetes duration is considered as the most important factor in developing DPN,5,22-24 while others did not confirm these findings.25-27 In our study, DPN patients had higher duration of diabetes but the difference was not significant. We also observed insignificantly higher FBS and HbA1C in diabetic patients with neuropathy. Similar to our findings, other studies found no association between DPN and HbA1C values,23,27 while others have indicated HbA1c as a risk factor for DPN.28 Although hyperglycemia is recommended as the primary risk factor for DPN,5,29 but it is also reported that neuropathy can occur in the absence of hyperglycemia.30,31
This study suffers from some limitations, such as small sample size and age matched control group; however, each value of nerve conduction was compared with age matched normal data reported by Parano et al.32
In conclusion, as subclinical peripheral neuropathy is frequent in diabetic children and adolescents, this complication can be diagnosed by exact evaluation of signs and symptoms of neuropathy, comprehensive neurologic examination, and proper electrodiagnostic studies. Overall duration of the disease, levels of FBS and HbA1c were increased in DPN, although not significant and considering the previous studies, it seems that better glycemic control in long time could well prevent diabetic neuropathy. However, due to poor cooperation of children for neurologic examination, NCS is needed for early detection of peripheral neuropathy with increase in duration of the disease. Further studies considering an age matched group and long-term follow up of these patients with NCS could yield newer information in this regard.
Footnotes
Case Reports.
Case reports will only be considered for unusual topics that add something new to the literature. All Case Reports should include at least one figure. Written informed consent for publication must accompany any photograph in which the subject can be identified. Figures should be submitted with a 300 dpi resolution when submitting electronically. The abstract should be unstructured, and the introductory section should always include the objective and reason why the author is presenting this particular case. References should be up to date, preferably not exceeding 15.
References
- 1.Bluestone JA, Herold K, Eisenbarth G. Genetics pathogenesis and clinical interventions in type 1 diabetes. Nature. 2010;464:1293–1300. doi: 10.1038/nature08933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Louraki M, Karayianni C, Kanaka-Gantenbein C, Katsalouli M, Karavanaki K. Peripheral neuropathy in children with type 1 diabetes. Diabetes Metab. 2012;38:281–289. doi: 10.1016/j.diabet.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Pasnoor M, Dimachkie MM, Kluding P, Barohn RJ. Diabetic neuropathy part1: overview and symmetric phenotypes. Neurol Clin. 2013;31:425–445. doi: 10.1016/j.ncl.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Spallone V, Morganti R, D'Amato C, Cacciotti L, Fedele T, Maiello MR, et al. Clinical correlates of painful diabetic neuropathy and relationship of neuropathic pain with sensorimotor and autonomic nerve function. Eur J Pain. 2011;15:153–160. doi: 10.1016/j.ejpain.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 5.Li G, Sun C, Wang Y, Liu Y, Gang X, Gao Y, et al. A clinical and neuropathological study of Chinese patients with diabetic peripheral neuropathy. PloS One. 2014;9:ne91772. doi: 10.1371/journal.pone.0091772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hasani N, Khosrawi S, Hashemipour M, Haghighatiyan M, Javdan Z, Taheri MH, et al. Prevalence and related risk-factors of peripheral neuropathy in children with insulin-dependent diabetes mellitus. J Res Med Sci. 2013;18:132–136. [PMC free article] [PubMed] [Google Scholar]
- 7.Won JC, Kim SS, Ko KS, Cha BY. Current status of diabetic peripheral neuropathy in Korea: report of a hospital-based study of type 2 diabetic patients in Korea by the diabetic neuropathy study group of the Korean diabetes association. Diabetes Metab J. 2014;38:25–31. doi: 10.4093/dmj.2014.38.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javadzadeh A, Ghorbanihaghjo A, Adl FH, Andalib D, Khojasteh-Jafari H, Ghabili K. Calcium dobesilate reduces endothelin-1 and high-sensitivity C-reactive protein serum levels in patients with diabetic retinopathy. Mol Vis. 2013;19:62–68. [PMC free article] [PubMed] [Google Scholar]
- 9.Samahy MH, Elbarbary NS, Elmorsi HM. Current status of diabetes management, glycemic control and complications in children and adolescents with diabetes in Egypt. Where do we stand now? And where do we go from here? Diabetes Res Clin Pract. 2015;107:370–376. doi: 10.1016/j.diabres.2015.01.004. [DOI] [PubMed] [Google Scholar]
- 10.Chiles NS, Phillips CL, Volpato S, Bandinelli S, Ferrucci L, Guralnik JM, et al. Diabetes, peripheral neuropathy, and lower-extremity function. J Diabetes Complications. 2014;28:91–95. doi: 10.1016/j.jdiacomp.2013.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albers JW, Herman WH, Pop-Busui R, Martin CL, Cleary P, Waberski B, et al. Subclinical neuropathy among Diabetes Control and Complications Trial participants without diagnosable neuropathy at trial completion: possible predictors of incident neuropathy? Diabetes Care. 2007;30:2613–2618. doi: 10.2337/dc07-0850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ayromlou H, Mohammad-Khanli H, Yazdchi-Marandi M, Rikhtegar R, Zarrintan S, Golzari SE, et al. Electrodiagnostic evaluation of peripheral nervous system changes in patients with multiple sclerosis. Malays J Med Sci. 2013;20:32–38. [PMC free article] [PubMed] [Google Scholar]
- 13.Oskouei AE, Talebi GA, Shakouri SK, Ghabili K. Effects of neuromobilization maneuver on clinical and electrophysiological measures of patients with carpal tunnel syndrome. J Phys Ther Sci. 2014;26:1017–1022. doi: 10.1589/jpts.26.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gill HK, Yadav SB, Ramesh V, Bhatia E. A prospective study of prevalence and association of peripheral neuropathy in Indian patients with newly diagnosed type 2 diabetes mellitus. J Postgrad Med. 2014;60:270–275. doi: 10.4103/0022-3859.138750. [DOI] [PubMed] [Google Scholar]
- 15.Dyck PJ, Kratz KM, Karnes JL, Litchy WJ, Klein R, Pach JM, et al. The prevalence by staged severity of various types of diabetic neuropathy, retinopathy, and nephropathy in a population-based cohort: The Rochester Diabetic Neuropathy Study. Neurology. 1993;43:817–824. doi: 10.1212/wnl.43.4.817. [DOI] [PubMed] [Google Scholar]
- 16.Deli G, Bosnyak E, Pusch G, Komoly S, Feher G. Diabetic neuropathies: diagnosis and management. Neuroendocrinology. 2013;98:267–280. doi: 10.1159/000358728. [DOI] [PubMed] [Google Scholar]
- 17.Baba M, Ozaki I. Electrophysiological changes in diabetic neuropathy: from subclinical alterations to disabling abnormalities. Arch Physiol Biochem. 2001;109:234–240. doi: 10.1076/apab.109.3.234.11595. [DOI] [PubMed] [Google Scholar]
- 18.Nelson D, Mah JK, Adams C, Hui S, Crawford S, Darwish H, et al. Comparison of conventional and non-invasive techniques for the early identification of diabetic neuropathy in children and adolescents with type 1 diabetes. Pediatr Diabetes. 2006;7:305–310. doi: 10.1111/j.1399-5448.2006.00208.x. [DOI] [PubMed] [Google Scholar]
- 19.Madani S, Larijani B, Erfani M, Heshmat R. Comparison of clinical evaluation with neurophysiologic findings of sural nerve in the diagnosis of diabetic neuropathy. Iranian Journal of Diabetes and Metabolism. 2004;3:89–98. [Google Scholar]
- 20.Soveid M, Ghavanini M, Shirdel E, Omrani G. Clinical and electroneurographic of neuropathy in Diabetic patients living in Shiraz. Iranian Journal of Diabetes and Metabolism. 2004;3:53–56. [Google Scholar]
- 21.Boulton AJ, Vinik AI, Arezzo JC, Bril V, Feldman EL, Freeman R, et al. Diabetic neuropathies: a statement by the American Diabetes Association. Diabetes Care. 2005;28:956–962. doi: 10.2337/diacare.28.4.956. [DOI] [PubMed] [Google Scholar]
- 22.Kiani J, Moghimbeigi A, Azizkhani H, Kosarifard S. The prevalence and associated risk factors of peripheral diabetic neuropathy in Hamedan, Iran. Arch Iran Med. 2013;16:17–19. [PubMed] [Google Scholar]
- 23.Lee SS, Han HS, Kim H. A 5-yr follow-up nerve conduction study for the detection of subclinical diabetic neuropathy in children with newly diagnosed insulin-dependent diabetes mellitus. Pediatr Diabetes. 2010;11:521–528. doi: 10.1111/j.1399-5448.2009.00636.x. [DOI] [PubMed] [Google Scholar]
- 24.Jaiswal M, Lauer A, Martin CL, Bell RA, Divers J, Dabelea D, et al. Peripheral neuropathy in adolescents and young adults with type 1 and type 2 diabetes from the SEARCH for Diabetes in Youth follow-up cohort: a pilot study. Diabetes Care. 2013;36:3903–3908. doi: 10.2337/dc13-1213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karsidag S, Morali S, Sargin M, Salman S, Karsidag K, Us O. The electrophysiological findings of subclinical neuropathy in patients with recently diagnosed type 1 diabetes mellitus. Diabetes Res Clin Pract. 2005;67:211–219. doi: 10.1016/j.diabres.2004.07.017. [DOI] [PubMed] [Google Scholar]
- 26.Meh D, Denislic M. Subclinical neuropathy in type I diabetic children. Electroencephalogr Clin Neurophysiol. 1998;109:274–280. doi: 10.1016/s0924-980x(98)00017-4. [DOI] [PubMed] [Google Scholar]
- 27.Karavanaki K, Baum JD. Prevalence of microvascular and neurologic abnormalities in a population of diabetic children. J Pediatr Endocrinol Metab. 1999;12:411–422. doi: 10.1515/jpem.1999.12.3.411. [DOI] [PubMed] [Google Scholar]
- 28.Bao XH, Wong V, Wang Q, Low LC. Prevalence of peripheral neuropathy with insulin-dependent diabetes mellitus. Pediatr Neurol. 1999;20:204–209. doi: 10.1016/s0887-8994(98)00141-6. [DOI] [PubMed] [Google Scholar]
- 29.Deshpande AD, Harris-Hayes M, Schootman M. Epidemiology of diabetes and diabetes-related complications. Phys Ther. 2008;88:1254–1264. doi: 10.2522/ptj.20080020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Smith AG, Ramachandran P, Tripp S, Singleton JR. Epidermal nerve innervation in impaired glucose tolerance and diabetes-associated neuropathy. Neurology. 2001;57:1701–1704. doi: 10.1212/wnl.57.9.1701. [DOI] [PubMed] [Google Scholar]
- 31.Kikkawa Y, Kuwabara S, Misawa S, Tamura N, Kitano Y, Ogawara K, et al. The acute effects of glycemic control on nerve conduction in human diabetics. Clin Neurophysiol. 2005;116:270–274. doi: 10.1016/j.clinph.2004.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Parano E, Uncini A, De Vivo DC, Lovelace RE. Electrophysiologic correlates of peripheral nervous system maturation in infancy and childhood. J Child Neurol. 1993;8:336–338. doi: 10.1177/088307389300800408. [DOI] [PubMed] [Google Scholar]


