Abstract
In recent years, there has been a considerable interest regarding the concept of lamellar keratoplasty (LK), which contributed in spreading the use of this procedure in the treatment of keratoconus. This is a new frontier in corneal surgery that minimizes trauma on the recipient patient since it works on a “closed bulb”. The LK surgery, in fact, aims to selectively replace diseased corneal stroma, leaving the healthy endothelium. The main advantage of LK is to avoid major causes of failure of penetrating keratoplasty as immunological rejection, and the late mismatch in the transplanted cornea, thus increasing the life of transplantation. In the last decade, several techniques of LK have been proposed, depending on how the anterior portion of the recipient cornea is removed. This article, through a literary research reviews the various emerging techniques of anterior lamellar surgery for the management of keratoconus, analyzing their indications, visual outcomes, and rate of complications.
Keratoplasty has developed rapidly in the past 10 years and penetrating keratoplasty (PK), a procedure consisting of full-thickness replacement of the cornea, has been the dominant procedure for more than half a century.1-3 However, in recent years, cases in which corneal disease does not involve the endothelium, lamellar technique has rapidly replaced penetrating grafts with better clinical results.4-5 This technique aims to selectively replace diseased corneal stroma in a way to minimize unnecessary replacement of the unaffected healthy endothelial layer. Thus, by retaining the patient’s own endothelium, the risk of endothelial rejection, a major cause of graft failure in PK is almost eliminated, and endothelial cell density is preserved.4-6 Consequently, there is no need for long-term immunosuppressive therapy with corticosteroids, decreasing the risk of cataract, glaucoma, and infections. Fasolo et al7 in their corneal transplant epidemiologic study have reported the first results on corneal graft survival in Italy. They estimate the graft survival to be 95% for PK, and 93% for anterior lamellar keratoplasty (LK) after one year, showing a decrease in the survival rate along the considered period. Indeed, in the LK group, they observed a stable 3-year survival rate of 93%, whereas patients who underwent PK showed a decrease of the graft survival rate after one year. From the results of a recent meta-analysis conducted by Liu et al8 on the efficacy and safety of LK versus PK, it appears that in terms of spherical equivalent, central corneal thickness, and astigmatism there are no significant differences between the 2 procedures. Nevertheless, fewer complications occur in the LK group, in which the corneal endothelial density is higher than in the PK group.8 An additional advantage of LK compared with PK is that sutures can be removed earlier, and visual recovery occurs sooner. Furthermore, since LK is an extra-ocular procedure, it lacks the risk associated with open eye surgery, such as expulsive hemorrhage, endophthalmitis, and iris/lens damage.9 The purpose of this article is to analyze the current techniques of LK surgery in the treatment of keratoconus, reviewing updated available literature.
Lamellar keratoplastyamellar with augmented thickness
Recent years have brought on a sea of change in the field of corneal transplantation with PK being phased into newer anterior LK techniques. In keratoconus, the aim of surgery is to augment a thin and steep cornea, and this can be achieved using a thick donor lamellar of normal curvature, thus tectonically strengthening the cornea, which reduces irregular astigmatism and subsequent ectasia, and reducing corneal steepness, and concomitant high myopia.
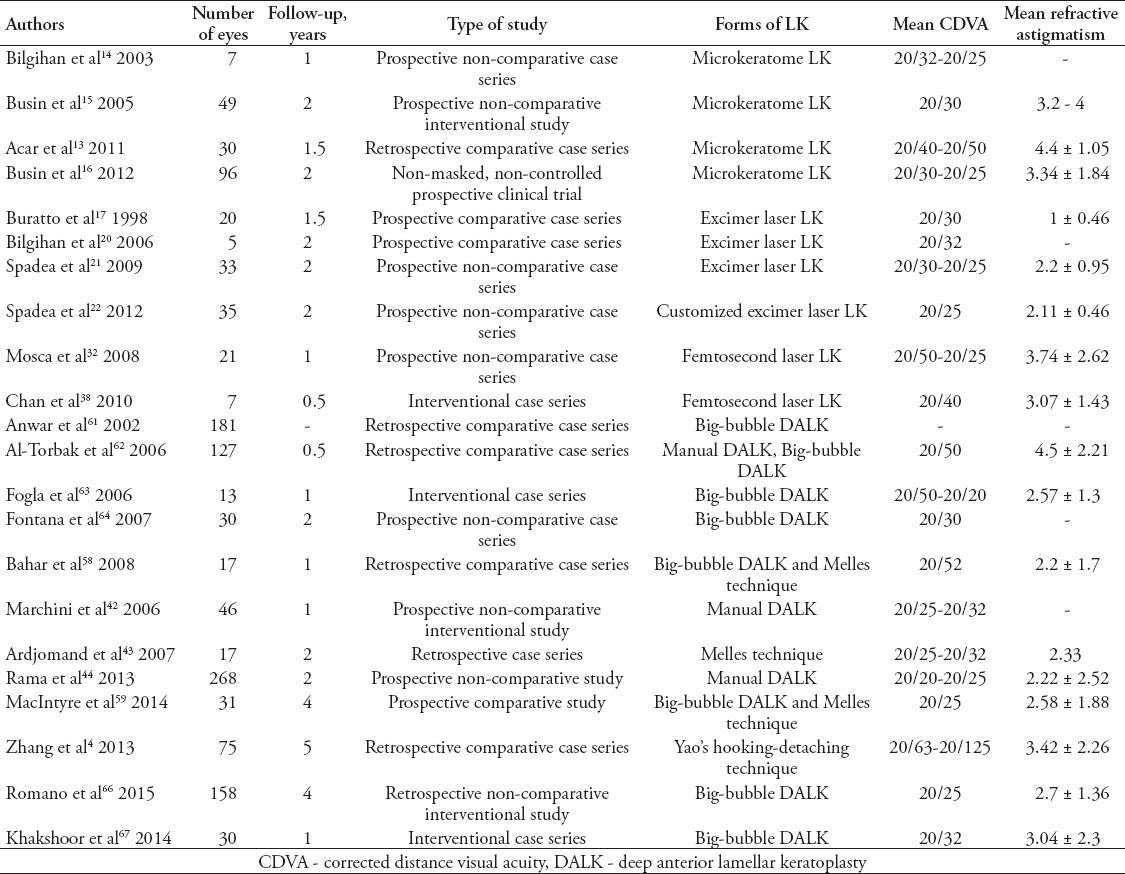
Thanks to the advent of new surgical devices, such as advanced microkeratome instrumentation, Excimer laser, and femtosecond laser, the results of lamellar techniques have been encouraging, with rapid visual rehabilitation and reduced risk of immune-mediated transplant rejection (Figure 1).10 With the increased availability of automated microkeratome for refractive procedures, such as Excimer laser in situ keratomileusis (LASIK), the use of microkeratome has increasingly been applied for dissection in LK. In the past, several authors11-13 have ventured in the LK surgery experiencing different techniques with the aid of microkeratome. Bilgihan et al14 described the “stromal sandwich technique”, consisting in the transplantation of a stromal button from a donor cornea under a corneal flap created in the host cornea. Busin et al15 described a more complex procedures suturing in the host bed a thicker and smaller lamella under tension, thus flattening the cone, restoring a normal corneal shape. Their surgery was not aimed at simply removing the central diseased corneal tissue and exchanging it with a healthy graft, but even at the remodeling of the ectatic cornea. Busin et al16 in 2012, trying to achieve a final corneal shape as similar as possible to the physiologic curvature of the donor cornea, introduced a modification of the microkeratome-assisted LK technique, including a full-thickness trephination of the residual bed before suturing the donor graft in place. They postulated that the recipient’s residual stroma can preserve a “keratoconus memory”, so through the disruption of the recipient’s architecture, they achieved a better postoperative refractive error, and spectacle-corrected visual acuity.
Figure 1.
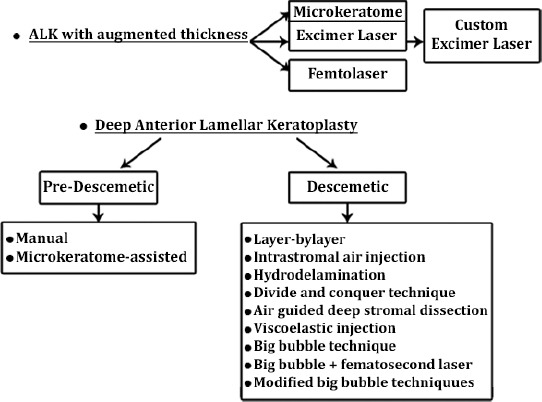
Forms of anterior lamellar keratoplasty (ALK) procedures available for keratoconus.
To simplify and standardize LK, Excimer laser ablation has been used to prepare the recipient bed, with encouraging results. Excimer laser lamellar keratoplasty (ELLK) of augmented thickness is a procedure in which a deep plano excimer laser ablation is performed on the host cornea and a donor lamellar button, with or without an excimer laser refractive ablation on the posterior surface, is sutured into the recipient bed.17 According to Serdarevic et al18 the overriding advantage of using an ELLK is the laser’s ability to remove tissue with a microscopic precision that is unattainable with other procedures. They assert that the laser does not interfere with wound-healing processes, including cell migration and proliferation, and production of new tissue. In 1992, Kubota et al19 examined the depth of ablation of the recipient bed with different counts of oscillations of an excimer laser beam to determine the correlation between planned and real depth. Their results showed that an excimer laser achieved a precise cut in terms of diameter, site, and in particular thickness, indicating its utility in reproducible corneal photo ablation in LK. Buratto et al17 in their keratoconic eyes series, treated with laser LK of augmented thickness, reported better results compared with those treated with PK after 18 months. They found that this technique accelerated epithelialization, facilitated suturing the donor button to the recipient cornea, and produced a considerable flattening effect. Bilgihan et al20 obtained similar results after treating 5 keratoconus patients with ELLK. Excimer laser-assisted dissection was found to be a reproducible technique that requires short surgical time in a subsequent study of 2009, in which anatomical and functional results were evaluated for 41 patients with keratoconus after ELLK. The procedure consisted of a mechanical deepithelialization and a phototherapeutic keratectomy (PTK) using an excimer laser with a 7.0 mm round stainless steel mask placed on the cornea to create a vertical and regular edge of the ablation. The goal of a minimum estimated residual corneal bed was 200 µm. A 2.5 mm stromal pocket was created around the circumference of the ablation floor in order to receive the donor lamella, obtained by means of a microkeratome, and then secured in the recipient bed with 16 interrupted 10-0 nylon sutures. The uncorrected distance visual acuity (UDVA) and the corrected distance visual acuity (CDVA) were significantly better during all follow-up examinations than preoperatively, thus showing that ELLK is as efficacious as PK for the surgical treatment of moderate to advanced keratoconus.21 However, in some cases the mechanical effects of the recipient’s original keratoconus persisted, especially in eyes with advanced and decentered ectasia.20 The introduction of a new-generation excimer laser with a comprehensive surgical planning application specific for laser lamellar transplantations, allowed the surgeon to create custom ablations for both the receiving bed and the lamella. In this way it is possible to plan different ablation depths in the same cornea as a function of corneal thickness differentials. Studies demonstrated that the custom technique provided a satisfactory increase in corneal thickness, restoring structural and optical integrity to the tissue (Figure 2).22
Figure 2.
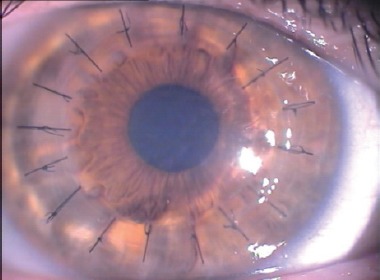
Biomicroscopic examination one month after custom excimer laser-assisted lamellar keratoplasty in a 31-year-old keratoconus patient. Sixteen interrupted 10-0 nylon sutures and a clear cornea are visible.
To reduce postoperative refractive errors in patients who had previously undergone LK for keratoconus, various techniques have been proposed, like excimer laser photorefractive keratectomy (PRK) and LASIK. However, a case of corneal ectasia was reported, following both excimer laser PRK and prior excimer laser-assisted LK for keratoconus, which was then successfully treated by corneal collagen crosslinking (CXL). This technique appeared to stabilize and partially reverse keratectasia in the 2-year postoperative follow-up period.23 Recently, the results of using combined treatment of customized PRK and prophylactic CXL in a group of patients with high ametropia and irregular astigmatism after ELLK have been reported. The mean correction of the spherical equivalent refractive error was greatly reduced, with an improvement in UDVA in all patients. Thus, demonstrating that the combination of these 2 treatments was safe and effective.24
The femtosecond laser provides a novel approach for corneal transplantation due to its accuracy and predictability. It uses a type of near infrared light with a wavelength of 1053 nm, which yields the shortest pulse obtainable and can focus on quite a limited area, smaller than a hair’s breadth.25,26 Thanks to these features, femtosecond laser cutting yields a smooth stromal interface, increasing the safety and precision of corneal transplantation.27 Both the donor graft and the recipient corneal lenticule are created using femtosecond laser, the host lenticule smaller in diameter than the donor and sutured with 10/0 nylon.28 Compared with mechanical microkeratome, the femtosecond laser presents a cutting accuracy twice as high, with a standard deviation of depth of cutting to be 12-18 µm, against the 20-60 µm with the microkeratome.29-31 Femtosecond laser LK can be performed with or without sutures, allowing for early visual rehabilitation inducing less astigmatism and avoiding other suture-related complications.32-36 With the aim to provide a more stable grafted cornea it is possible to increase the touch area, facilitating the cicatrisation between the donor and recipient cornea by increasing the side cut angle.36 If the graft was not sutured to the recipient cornea a bandage soft contact lens for 3-12 days was used, with no case of graft dehiscence.37 However, Chan et al38 noted the variability in stromal thickness in eyes with advanced keratoconus may limit the ability of the femtosecond laser to produce a uniform lamellar plane while leaving a minimal amount of residual corneal tissue. As well, given the potential risk of creating a descemet membrane perforation, they found it more safe to perform a manual dissection of the posterior lamella.
Deep anterior lamellar keratoplasty (DALK)
The author who first introduced the concept of “deep anterior lamellar keratoplasty” as a dissection of host tissue close to the descemet’s membrane (DM) was Anwar in 1972.39 He noted that this procedure led to the formation of a smooth and transparent recipient bed, with a functional outcome similar to that of PK. He also explained the importance of removing the layer endothelium-descemet from the donor edge to avoid an inflammatory reaction and an irregular interface.
Over the years, several surgical techniques have been studied and performed in order to obtain DM baring (Figure 1). When eliminating as much recipient tissue as possible, it is unclear if full stromal removal provides better results than cases, in which a small portion of the posterior stroma is left in place. However, despite the confused nomenclature, some surgeons refer to pre-descemetic DALK when LK enables the removal of 3 quarters or more of stroma to the deeper layers. This denotes that at least some posterior stromal layers are retained, and baring of DM is not achieved. According to Sarnicola et al,40 this could represent an advantage during the procedure of dissection of the host tissue, because he found that reaching a pre-descemetic level, the residual amount of stroma left in place prevented the microperforations from becoming macroperforations.
Pre-descemetic DALK
Current pre-descemetic DALK surgical techniques involve manual or microkeratome-assisted dissection near the DM. In 1997, Sugita and Kondo41 showed that there are no differences in visual acuity using a manual dissection technique that leaves a small amount of stroma, in place of the complete stromal dissection. Marchini et al42 intentionally left a minimal stromal thickness of 50 µm in order to reduce the risk of DM rupture. They used a vivo confocal microscopy to evaluate interface parameters (depth and reflectivity) of keratocyte and endothelial cell density over a 12-month follow-up, confirming Sugita and Kondo’s observation. Ardjomand et al43 compared visual function after DALK and after PK in 32 eyes with keratoconus and correlated it with corneal thickness. They demonstrated that eyes with a recipient corneal bed thickness of less than 20 µm had visual acuity similar to eyes with a PK, whereas those with a recipient thickness of greater than 80 µm had a significantly reduced visual acuity. Rama et al44 used a manual dissection technique guided by a calibrated knife incision based on ultrasonic pachymetry values. They treated 288 eyes and, according to Ardjomand et al,43 showed that eyes with lower values of recipient residue thickness are associated with better visual acuity. This could be explained by the fact that the residual bed thickness can determine the stiffness and corresponding resistance against the compressive forces of the donor graft. So it’s been hypothesized that thick residual recipient bed, together with a steep preoperative cornea and greater axial length, contribute to postoperative myopia in DALK.45 These results are consistent with several clinical studies46-48 available in literature comparing the visual outcome of descemetic and pre-descemetic DALK. However, pre-descemetic dissection is also indicated in cases of previous hydrops due to the risk that the DM will rupture at the hydrops scar. Chew et al49 describe a case of a boy with keratoconus and resolved hydrops who underwent bilateral manual DALK without baring of the DM. It demonstrated that good spectacle-corrected visual acuity can be achieved despite leaving a thin residual layer of the stroma unexcised. Consistent with this result, Anwar50 demonstrates the efficacy of a planned near-descemet dissection of the DALK in 22 patients with post-hydrops corneal scarring irregularity or corneal thinning because any surface irregularities are likely to be translated onto the final lamellar bed.50-51
Descemetic DALK
In 1972 Anwar39 first introduced his layer-by-layer manual dissection technique and despite have gone more than 40 years, it is still performed in some cases, such as pre-existing corneal perforation, strong stroma to DM adhesion, or inadequate visualization. It consists of performing a partial trephination of 70-80% of corneal thickness, followed by a limbal paracentesis incision used to evacuate the aqueous, or to inject air and fluid inside the anterior chamber. The corneal stroma is removed in layer using a bevel-up crescent knife, but the dissection of deeper layers becomes more difficult as DM is approached.52 Subsequently in 1984, Archila53 introduced a new technique of dissection with intrastromal air injection, which is considered the predecessor of other techniques of maximum depth dissection. It provided the injection of air into the corneal stroma until it becomes opaque in such a way to create a deep plane of dissection. After a partial trephination, the wound is deepened with a sharp crescent or a blunt spatula down to the DM, which appears as a clear dark area. The manual dissection of the stroma can be repeated as long as micro bubbles are visible to be sure that there is still a layer of stroma that protects the DM against the perforation. A full-thickness donor button including the DM and endothelium is then positioned in the recipient bed with interrupted sutures. Corneal emphysema provides good contrast, but the baring of DM is still a problem.53
In 1997 Sugita and Kondo41 used a technique combining air and fluid injection. A saline solution is injected in a small depression, which is created in the deeper stroma after a partial trephination. This procedure helps to achieve a cleavage plane over DM so that the loosened tissue can be removed in thin layers with forceps and scissors. The DM is recognized from its shiny and smooth appearance. This was later supported by Panda and Singh,54 who compared the efficacy of 3 adjunctive agents to facilitate recipient bed intralamellar dissection (air, hydroxypropylmethylcellulose, and balanced salt solution) demonstrating that the hydro delamination with saline solution is the easiest technique to perform.
In the development of DALK, a research line other than the techniques described up to now was disclosed by Tsubota et al55 in 1998. His “divide-and-conquer technique” differs from the others because the DM’s deep dissection is not performed by injecting any substances, and it is practiced completely manually. It originally derives from the application of cataract phacoemulsification technique to the deep LK. The corneal stroma is divided into 4 quadrants to facilitate lamellar dissection at approximately 70% deep, until the DM is exposed in the central area. This way it increases the repeatability and the standardization of the procedure.55 Manche et al56 described a technique, in which viscoelastic was forced into a previously made stromal pocket using a 25-gauge cannula, hoping the viscous material would dissect the DM from the overlying stroma. In the same year, Melles et al57 proposed a variation of this technique by adding the injection of air into the anterior chamber before the stromal dissection with viscoelastic to optimally highlight the cannula’s position, which contained the substance in the pre-descemetic plan. Over the years, this technique was performed, and is still proposed, by several authors43,58-60 with encouraging results.
After his first attempt of baring the DM in 2002, Anwar39 described the “big-bubble technique,” as a faster and more reliable way of separating the stroma from the DM. In this surgical technique, the cornea is trephined approximately 60-80% deep using a suction trephine and a 27 or 30-gauge needle. Attached to an air-filled syringe, it is inserted into the deep stroma through the bottom of the trephination groove. The separation of DM from the corneal stroma, characterized by a circular area with a dense white border, is caused by forceful injection of air. A keratectomy, anterior to the big-bubble, is carefully performed so as not to accidentally breech the bubble. Then, the bubble is pierced near the center of the cornea, and an opening in the anterior wall of the air-pocket is formed. The residual layers of stroma are firstly lifted with an iris spatula, then severed with a blade, and excised with scissors (Figures 3 & 4).61 Similar results have been reported from other case series62-67 of the big-bubble DALK technique for keratoconus. Ghanem et al68 pointed out that bubble formation is the key to decrease the risk of perforation in DALK, especially when a pachymetry-guided intrastromal air injection (pachy-bubble) is performed. However, Yao69 asserting that the many procedures developed for performing DALK are time-consuming and technically difficult, introduced their technique of stromal hooking with viscoelastic detaching process. It consists of creating a pocket in the recipient bed by means of a 3-quarter trephination, followed by a peeling of the remnant stroma to approach DM along the trephined margin in the area between 11 o’clock and one o’clock by the aid of a golf-shaped knife. In this area, the residual stromal fibers are hooked and lifted by forceps with a tip of concaved teeth for the consequent exposure of the DM. A 27-gauge cannula connected to a syringe containing viscoelastic is inserted in the pocket between the stroma and the DM, and the injection of viscoelastic material allows to get the detachment process. In cases where the primary exposure has not exactly reached the layer of the DM, a secondary hooking-detaching procedure can be performed.69 The same procedure was subsequently performed on 75 keratoconus eyes, which showed fewer postoperative complications compared with those who underwent PK.4
Figure 3.
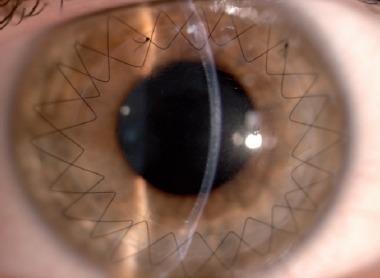
Biomicroscopic examination showed a clear and well integrated lamellar graft in a 35-year-old keratoconus patient who had descemetic deep anterior lamellar keratoplasty (big-bubble technique) 6 months previously. Two double-running 12 bites sutures are present.
Figure 4.
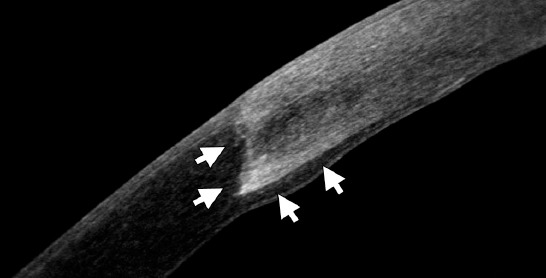
Anterior segment Fourier-domain optical coherence tomography image 2 years after deep anterior lamellar keratoplasty in a 22-year-old keratoconus patient. A healthy epithelium, the edge of the graft and the interface between donor and recipient cornea (arrows) are notable.
Recently, a distinct layer of corneal collagen, the Dua’s layer has been described, beyond the last row of keratocytes, which is thin but tough, and seems to provide a cleavage plane during the DM baring procedure. This observation suggests that the big-bubble cleaves off a distinct layer at the posterior surface of the corneal stroma, which is not residual stroma.70 Farid and Steinert71 in 2009, described a new approach combining big-bubble DALK with the femtosecond laser zigzag incision, in which the matching and interlocking donor-host wound increases surface area, and create a customized donor-host match. In a zigzag incision, the laser creates an angled posterior cut, a lamellar ring cut in midstroma, and an angled anterior cut, each of which intersects. According to Farid and Steinert,71 this type of wound allows rapid healing and good biomechanical stability. Furthermore, the laser cut allows an exact cut depth within 70 µm of the DM, allowing more precise placement of the air needle. Buzzonetti et al72,73 confirmed that the femtosecond laser could standardize the big-bubble technique in DALK, reducing the risk of intraoperative complications and allowing good refractive outcomes. Hoping to achieve higher success in attaining the big-bubble and lower rates of DM perforation, Tan and Mehta74 further modified Anwar’s original technique. Their innovation was to precede the formation of the bubble by the manual removal of the anterior half of the stroma after partial trephination. This way, they had the advantage of reducing the risk of perforation. When immediately entering with the needle into the deeper layers of the stroma, they introduced the needle more centrally, thus allowing for a more centralized bubble.
Discussion
As with all surgeries, even anterior LK is not a complication-free procedure, and compared with PK, it has a steep surgical learning curve. Regarding microkeratome-assisted LK, reasons for failure include lack of precision in flap diameter obtained in both recipient and donor dissection (because flap diameter is in part dependent on the variable suction pressures), delays or defects in epithelialization, epithelial ingrowths in the interface, fibrosis, and even vascularization (Table 1). The ELLK seems to be a safer and more standardized procedure since no complications were observed in the series we reviewed, except a case of altered reepithelialization, which required the replacement of the donor lamella 20 days after surgery.21 Complications, such as mild interface haze, interface inflammation, recurrence of pathology, haze after adjunctive PRK, dry eye, epithelial ingrowths, and suspicious ectasia, are common in femtosecond-assisted LK series.
Table 1.
Outcomes following lamellar keratoplasty (LK) surgeries for keratoconus in various reported series in literature.
The many DALK techniques developed share some disadvantages: they are technically difficult and time-consuming. The most feared complication in this case is the inadvertent DM perforation, which has a rate of incidence between 4.0-39.2%,41,42,58 and from 2.3-3.5% of cases necessitate conversion to PK.65,70-75 The big-bubble technique is the most popular in the current scenario of DALK, but it can be technically challenging when the air bubble is not formed. The main reason for failure in achieving the bubble is that the needle has not advanced deep enough into the posterior stromal layer with the consequent formation of a double chamber. Usually, folds in the DM following DALK are transient and improve over time; moreover, they are often located peripherally and have no impact on vision.
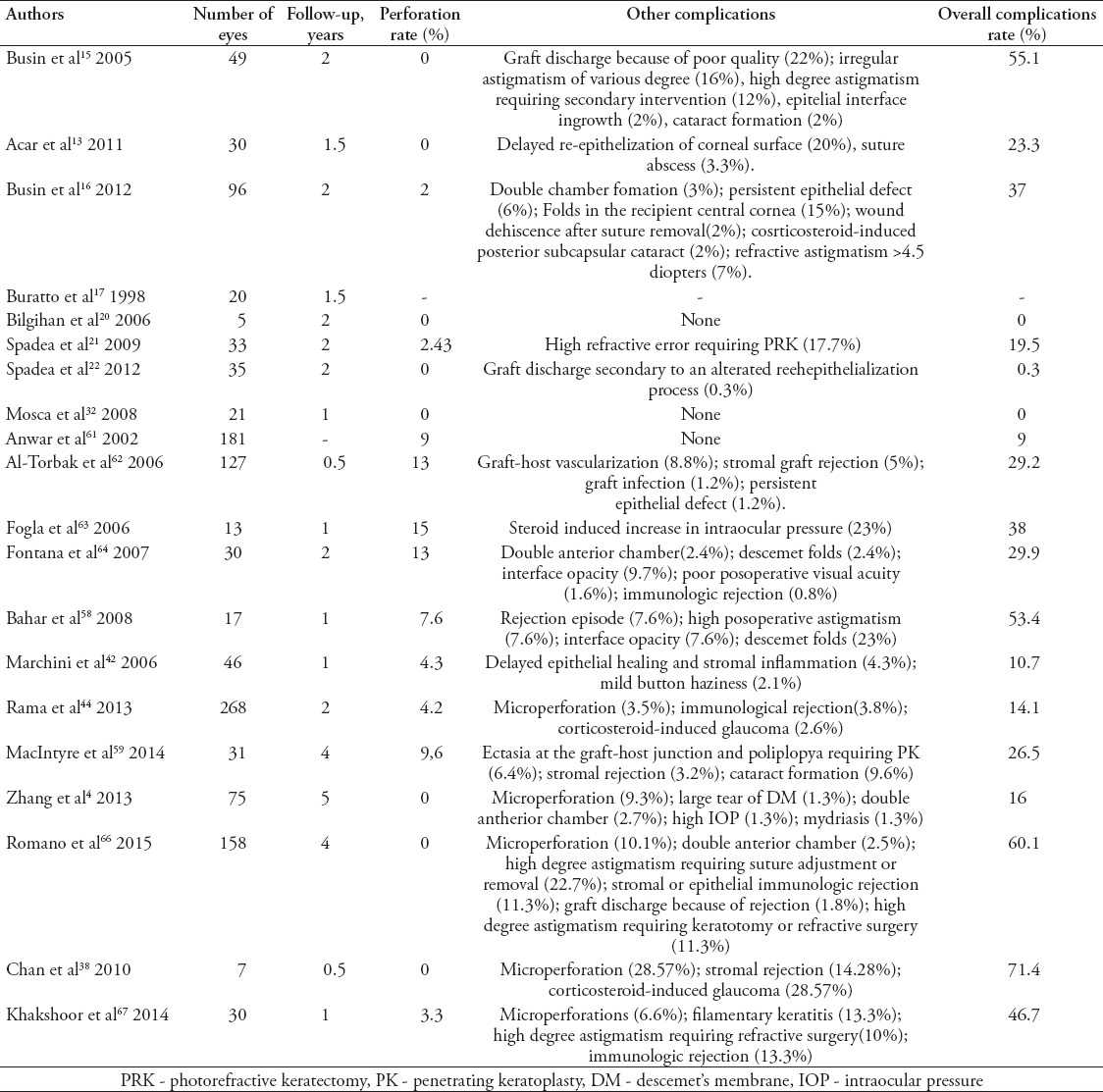
The visual acuity could be reduced by central folds, probably due to a raised level of higher-order aberrations. The responsible for folds in the DM is a mismatch between the recipient bed size and the donor button. A mismatch between the donor button and the recipient bed size is responsible for folds in the DM. Hence, over sizing the donor button by 0.25-0.5 mm is recommended to prevent this complication. Other complications, such as papillary blockage from mismanagement of the air bubble in anterior chamber, or a fixed dilated pupil (Urrets-Zavalia Syndrome) may occur when an inexperienced surgeon performs LK (Table 2).76
Table 2.
Complications rate following lamellar keratoplasty (LK) surgeries for keratoconus in various reported series in literature.
In conclusion, we have seen large developments in the field of corneal transplant surgery in the last decade. The surgical treatment of keratoconus has evolved from the replacement of the entire cornea to only replacing the layer of the cornea that is affected, leaving the undamaged corneal tissue untouched. Recent improvements in surgical techniques and advances in instrumentation have contributed to improve the visual outcomes of LK, with higher graft survival rates from lower rates of allograft rejection and late endothelial failure.
Acknowledgment
The authors thank Enzo Maria Vingolo, MD, for the critical revision and general supervision of the manuscript.
Footnotes
References.
-
*
References should be primary source and numbered in the order in which they appear in the text. At the end of the article the full list of references should follow the Vancouver style.
-
*
Unpublished data and personal communications should be cited only in
the text, not as a formal reference.
-
*
The author is responsible for the accuracy and completeness of references
and for their correct textual citation.
-
*
When a citation is referred to in the text by name, the accompanying
reference must be from the original source.
-
*
Upon acceptance of a paper all authors must be able to provide the full paper for each reference cited upon request at any time up to publication.
-
*
Only 1-2 up to date references should be used for each particular point in the text.
Sample references are available from:
References
- 1.Jhanji V, Sharma N, Vajpayee RB. Management of keratoconus: current scenario. Br J Ophthalmol. 2011;95:1044–1050. doi: 10.1136/bjo.2010.185868. [DOI] [PubMed] [Google Scholar]
- 2.Spadea L, Bianco G, Mastrofini MC, Balestrazzi E. Penetrating keratoplasty with donor and recipient corneas of the same diameter. Ophthalmic Surg Lasers. 1996;27:425–430. [PubMed] [Google Scholar]
- 3.Arenas E, Esquenazi S, Anwar M, Terry M. Lamellar corneal transplantation. Surv Ophthalmol. 2012;57:510–529. doi: 10.1016/j.survophthal.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 4.Zhang YM, Wu SQ, Yao YF. Long-term comparison of full-bed deep anterior lamellar keratoplasty and penetrating keratoplasty in treating keratoconus. J Zhejiang Univ Sci B. 2013;14:438–450. doi: 10.1631/jzus.B1200272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coster DJ, Lowe MT, Keane MC, Williams KA. Australian Corneal Graft Registry Contributors. A comparison of lamellar and penetrating keratoplasty outcomes: a registry study. Ophthalmology. 2014;121:978–987. doi: 10.1016/j.ophtha.2013.12.017. [DOI] [PubMed] [Google Scholar]
- 6.Reinhart WJ, Musch DC, Jacobs DS, Lee WB, Kaufman S, Shtein RM. Deep anterior lamellar keratoplasty as an alternative to penetrating keratoplasty a report by the american academy of ophthalmology. Ophthalmology. 2011;118:209–218. doi: 10.1016/j.ophtha.2010.11.002. [DOI] [PubMed] [Google Scholar]
- 7.Fasolo A, Frigo AC, Böhm E, Genisi C, Rama P, Spadea L, et al. The CORTES study: corneal transplant indications and graft survival in an Italian cohort of patients. Cornea. 2006;25:507–515. doi: 10.1097/01.ico.0000214211.60317.1f. [DOI] [PubMed] [Google Scholar]
- 8.Liu H, Chen Y, Wang P, Li B, Wang W, Su Y, et al. Efficacy and safety of deep anterior lamellar keratoplasty vs. penetrating keratoplasty for keratoconus: a meta-analysis. PLoS One. 2015;10:e0113332. doi: 10.1371/journal.pone.0113332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Abdelkader A. Influence of different keratoplasty techniques on the biomechanical properties of the cornea. Acta Ophthalmol. 2013;91:e567–e572. doi: 10.1111/aos.12136. [DOI] [PubMed] [Google Scholar]
- 10.Tan DT, Ang LP. Automated lamellar therapeutic keratoplasty for post-PRK corneal scarring and thinning. Am J Ophthalmol. 2004;138:1067–1069. doi: 10.1016/j.ajo.2004.06.062. [DOI] [PubMed] [Google Scholar]
- 11.Jiménez-Alfaro I, Pérez-Santonja JJ, Gómez Tellería G, Bueno Palacín JL, Puy P. Therapeutic lamellar keratoplasty with an automated microkeratome. J Cataract Refract Surg. 2001;27:1161–1165. doi: 10.1016/s0886-3350(00)00889-0. [DOI] [PubMed] [Google Scholar]
- 12.Tan DTH, Ang LP. Modified automated lamellar therapeutic keratoplasty for keratoconus: a new technique. Cornea. 2006;25:1217–1219. doi: 10.1097/01.ico.0000248388.39767.42. [DOI] [PubMed] [Google Scholar]
- 13.Acar BT, Arslan OS, Buttanri IB, Sevim MS, Acar S. Comparing deep anterior lamellar keratoplasty and automated lamellar therapeutic keratoplasty in patients with keratoconus. Jpn J Ophthalmol. 2011;55:327–332. doi: 10.1007/s10384-011-0044-0. [DOI] [PubMed] [Google Scholar]
- 14.Bilgihan K, Ozdek SC, Sari A, Hasanreisoglu B. Microkeratome-assisted lamellar keratoplasty for keratoconus: stromal sandwich. J Cataract Refract Surg. 2003;29:1267–1272. doi: 10.1016/s0886-3350(02)02055-2. [DOI] [PubMed] [Google Scholar]
- 15.Busin M, Zambianchi L, Arffa RC. Microkeratome-assisted lamellar keratoplasty for the surgical treatment of keratoconus. Ophthalmology. 2005;112:987–997. doi: 10.1016/j.ophtha.2005.01.024. [DOI] [PubMed] [Google Scholar]
- 16.Busin M, Scorcia V, Zambianchi L, Ponzin D. Outcomes from a modified microkeratome-assisted lamellar keratoplasty for keratoconus. Arch Ophthalmol. 2012;130:776–782. doi: 10.1001/archophthalmol.2011.1546. [DOI] [PubMed] [Google Scholar]
- 17.Buratto L, Belloni S, Valeri R. Excimer laser lamellar keratoplasty of augmented thickness for keratoconus. J Refract Surg. 1998;14:517–525. doi: 10.3928/1081-597X-19980901-09. [DOI] [PubMed] [Google Scholar]
- 18.Serdarevic ON, Hanna K, Gribomont AC, Savoldelli M, Renard G, Pouliquen Y. Excimer laser trephination in penetrating keratoplasty. Morphologic features and wound healing. Ophthalmology. 1988;95:493–505. doi: 10.1016/s0161-6420(88)33160-x. [DOI] [PubMed] [Google Scholar]
- 19.Kubota T, Seitz B, Tetsumoto K, Naumann GO. Lamellar excimer laser keratoplasty: reproducible photoablation of corneal tissue. A laboratory study. Doc Ophthalmol. 1992;82:193–200. doi: 10.1007/BF00160765. [DOI] [PubMed] [Google Scholar]
- 20.Bilgihan K, Ozdek SC, Sari A, Hasanreisoğlu B. Excimer laser-assisted anterior lamellar keratoplasty for keratoconus, corneal problems after laser in situ keratomileusis, and corneal stromal opacities. J Cataract Refract Surg. 2006;32:1264–1269. doi: 10.1016/j.jcrs.2006.02.056. [DOI] [PubMed] [Google Scholar]
- 21.Spadea L, Giammaria D, Fiasca A, Verrecchia V. Excimer laser-assisted lamellar keratoplasty for the surgical treatment of keratoconus. J Cataract Refract Surg. 2009;35:105–112. doi: 10.1016/j.jcrs.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Spadea L, Gizzi R, Evangelista Conocchia N, Urbano S. Optical pachymetry-guided custom excimer laser-assisted lamellar keratoplasty for the surgical treatment of keratoconus. J Cataract Refract Surg. 2012;38:1559–1567. doi: 10.1016/j.jcrs.2012.05.029. [DOI] [PubMed] [Google Scholar]
- 23.Spadea L. Collagen crosslinking for ectasia following PRK performed in excimer laser-assisted keratoplasty for keratoconus. Eur J Ophthalmol. 2012;22:274–277. doi: 10.5301/ejo.5000019. [DOI] [PubMed] [Google Scholar]
- 24.Spadea L, Paroli M. Simultaneous topography-guided PRK followed by corneal collagen cross-linking after lamellar keratoplasty for keratoconus. Clin Ophthalmol. 2012;6:1793–1800. doi: 10.2147/OPTH.S37280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hou P, Lu Y, Ye F, Lan W, Huang Z. Application of femtosecond laser in ocular surgery. Eye Sci. 2013;28:103–107. [PubMed] [Google Scholar]
- 26.Farid M, Steinert RF. Femtosecond laser-assisted corneal surgery. Curr Opin Ophtahalmol. 2010;21:288–292. doi: 10.1097/ICU.0b013e32833a8dbc. [DOI] [PubMed] [Google Scholar]
- 27.Jones YJ, Goins KM, Sutphin JE, Mullins R, Skeie JM. Comparison of the femtosecond laser (IntraLase) versus manual microkeratome (Moria ALTK) in dissection of the donor in endothelial keratoplasty: initial study in eye bank eyes. Cornea. 2008;27:88–93. doi: 10.1097/ICO.0b013e31815771f5. [DOI] [PubMed] [Google Scholar]
- 28.Almousa R, Samaras KE, Khan S, Lake DB, Daya SM. Femtosecond laser-assisted lamellar keratoplasty (FSLK) for anterior corneal stromal diseases. Int Ophthalmol. 2014;34:49–58. doi: 10.1007/s10792-013-9794-7. [DOI] [PubMed] [Google Scholar]
- 29.Flanagan GW, Binder PS. Precision of flap measurements for laser in situ keratomileusis in 4428 eyes. J Refract Surg. 2003;19:113–123. doi: 10.3928/1081-597X-20030301-05. [DOI] [PubMed] [Google Scholar]
- 30.Kezirian GM, Stonecipher KG. Comparison of the IntraLase femtosecond laser and mechanical keratomes for laser in situ keratomileusis. J Cataract Refract Surg. 2004;30:804–811. doi: 10.1016/j.jcrs.2003.10.026. [DOI] [PubMed] [Google Scholar]
- 31.Binder PS. Flap dimensions created with the IntraLase FS laser. J Cataract Refract Surg. 2004;30:26–32. doi: 10.1016/S0886-3350(03)00578-9. [DOI] [PubMed] [Google Scholar]
- 32.Mosca L, Fasciani R, Tamburelli C, Buzzonetti L, Guccione L, Mandarà E, et al. Femtosecond laser-assisted lamellar keratoplasty: early results. Cornea. 2008;27:668–672. doi: 10.1097/ICO.0b013e31816736b1. [DOI] [PubMed] [Google Scholar]
- 33.Baradaran-Rafii A, Eslani M. Femtosecond laser assisted corneal transplantation. Br J Ophthalmol. 2013;97:675–676. doi: 10.1136/bjophthalmol-2012-302196. [DOI] [PubMed] [Google Scholar]
- 34.Hoffart L, Proust H, Matonti F, Catanèse M, Conrath J, Ridings B. [Femtosecond-assisted anterior lamellar keratoplasty] J Fr Ophthalmol. 2007;30:689–694. doi: 10.1016/s0181-5512(07)91356-x. French. [DOI] [PubMed] [Google Scholar]
- 35.Hou P, Lu Y, Ye F, Lan W, Huang Z. Application of femtosecond laser in ocular surgery. Eye Sci. 2013;28:103–107. [PubMed] [Google Scholar]
- 36.Shousha MA, Yoo SH, Kymionis GD, Ide T, Feuer W, Karp CL, et al. Long-term results of femtosecond laser-assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2011;118:315–323. doi: 10.1016/j.ophtha.2010.06.037. [DOI] [PubMed] [Google Scholar]
- 37.Yoo SH, Kymionis GD, Koreishi A, Ide T, Goldman D, Karp CL, et al. Femtosecond laser–assisted sutureless anterior lamellar keratoplasty. Ophthalmology. 2008;115:1303–1307. doi: 10.1016/j.ophtha.2007.10.037. 1307.e1. [DOI] [PubMed] [Google Scholar]
- 38.Chan CC, Ritenour RJ, Kumar NL, Sansanayudh W, Rootman DS. Femtosecond laser-assisted mushroom configuration deep anterior lamellar keratoplasty. Cornea. 2010;29:290–295. doi: 10.1097/ICO.0b013e3181b77873. [DOI] [PubMed] [Google Scholar]
- 39.Anwar M. Dissection technique in lamellar keratoplasty. Br J Ophthalmol. 1972;56:711–713. doi: 10.1136/bjo.56.9.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sarnicola V, Toro P, Sarnicola C, Sarnicola E, Ruggiero A. Long-term graft survival in deep anterior lamellar keratoplasty. Cornea. 2012;31:621–626. doi: 10.1097/ICO.0b013e31823d0412. [DOI] [PubMed] [Google Scholar]
- 41.Sugita J, Kondo J. Deep lamellar keratoplasty with complete removal of pathological stroma for vision improvement. Br J Ophthalmol. 1997;81:184–188. doi: 10.1136/bjo.81.3.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marchini G, Mastropasqua L, Pedrotti E, Nubile M, Ciancaglini M, et al. Deep lamellar keratoplasty by intracorneal dissection: a prospective clinical and confocal microscopic study. Ophthalmology. 2006;113:1289–1300. doi: 10.1016/j.ophtha.2006.01.071. [DOI] [PubMed] [Google Scholar]
- 43.Ardjomand N, Hau S, McAlister JC, Bunce C, Galaretta D, Tuft SJ, et al. Quality of vision and graft thickness in deep anterior lamellar and penetrating corneal allografts. Am J Ophthalmol. 2007;143:228–235. doi: 10.1016/j.ajo.2006.10.043. [DOI] [PubMed] [Google Scholar]
- 44.Rama P, Knutsson KA, Razzoli G, Matuska S, Viganò M, Paganoni G. Deep anterior lamellar keratoplasty using an original manual technique. Br J Ophthalmol. 2013;97:23–27. doi: 10.1136/bjophthalmol-2011-301168. [DOI] [PubMed] [Google Scholar]
- 45.Oh BL, Kim MK, Wee WR. Comparison of clinical outcomes of same-size grafting between deep anterior lamellar keratoplasty and penetrating keratoplasty for keratoconus. Korean J Ophthalmol. 2013;27:322–330. doi: 10.3341/kjo.2013.27.5.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sarnicola V, Toro P, Gentile D, Hannush SB. Descemetic DALK and predescemetic DALK: outcomes in 236 cases of keratoconus. Cornea. 2010;29:53–59. doi: 10.1097/ICO.0b013e3181a31aea. [DOI] [PubMed] [Google Scholar]
- 47.Fontana L, Parente G, Sincich A, Tassinari G. Influence of graft-host interface on the quality of vision after deep anterior lamellar keratoplasty in patients with keratoconus. Cornea. 2011;30:497–502. doi: 10.1097/ico.0b013e3181d25e4d. [DOI] [PubMed] [Google Scholar]
- 48.Schiano-Lomoriello D, Colabelli-Gisoldi RA, Nubile M, Oddone F, Ducoli G, Villani CM, et al. Descemetic and predescemetic DALK in keratoconus patients: a clinical and confocal perspective study. Biomed Res Int. 2014;2014:123156. doi: 10.1155/2014/123156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Chew AC, Mehta JS, Tan DT. Deep lamellar keratoplasty after resolution of hydrops in keratoconus. Cornea. 2011;30:454–459. doi: 10.1097/ICO.0b013e3181f0b1f3. [DOI] [PubMed] [Google Scholar]
- 50.Anwar HM, Anwar M. Predescemetic dissection for healed hydrops--judicious use of air and fluid. Cornea. 2011;30:1502–1509. doi: 10.1097/ICO.0b013e31822018b9. [DOI] [PubMed] [Google Scholar]
- 51.Tan DT, Dart JK, Holland EJ, Kinoshita S. Corneal transplantation. Lancet. 2012;379:1749–1761. doi: 10.1016/S0140-6736(12)60437-1. [DOI] [PubMed] [Google Scholar]
- 52.Tan DT, Anshu A. Anterior lamellar keratoplasty: ‘Back to the Future’- a review. Clin Experiment Ophthalmol. 2010;38:118–127. doi: 10.1111/j.1442-9071.2009.02180.x. [DOI] [PubMed] [Google Scholar]
- 53.Archila EA. Deep lamellar keratoplasty dissection of host tissue with intrastromal air injection. Cornea. 1984-1985;3:217–218. [PubMed] [Google Scholar]
- 54.Panda A, Singh R. Intralamellar dissection techniques in lamellar keratoplasty. Cornea. 2000;19:22–25. doi: 10.1097/00003226-200001000-00005. [DOI] [PubMed] [Google Scholar]
- 55.Tsubota K, Kaido M, Monden Y, Satake Y, Bissen-Miyajima H, Shimazaki J. A new surgical technique for deep lamellar keratoplasty with single running suture adjustment. Am J Ophthalmol. 1998;126:1–8. doi: 10.1016/s0002-9394(98)00067-1. [DOI] [PubMed] [Google Scholar]
- 56.Manche EE, Holland GN, Maloney RK. Deep lamellar keratoplasty using viscoelastic dissection. Arch Ophthalmol. 1999;117:1561–1565. doi: 10.1001/archopht.117.11.1561. [DOI] [PubMed] [Google Scholar]
- 57.Melles GR, Remeijer L, Geerards AJ, Beekhuis WH. A quick surgical technique for deep, anterior lamellar keratoplasty using visco-dissection. Cornea. 2000;19:427–432. doi: 10.1097/00003226-200007000-00004. [DOI] [PubMed] [Google Scholar]
- 58.Bahar I, Kaiserman I, Srinivasan S, Ya-Ping J, Slomovic AR, Rootman DS. Comparison of three different techniques of corneal transplantation for keratoconus. Am J Ophthalmol. 2008;146:905–912.e1. doi: 10.1016/j.ajo.2008.06.034. [DOI] [PubMed] [Google Scholar]
- 59.MacIntyre R, Chow SP, Chan E, Poon A. Long-term outcomes of deep anterior lamellar keratoplasty versus penetrating keratoplasty in Australian keratoconus patients. Cornea. 2014;33:6–9. doi: 10.1097/ICO.0b013e3182a9fbfd. [DOI] [PubMed] [Google Scholar]
- 60.Cano-Ortiz A, Villarrubia A. [Corneal transplantation in keratoconus: penetrating keratoplasty versus deep anterior lamellar keratoplasty with Melles technique] Arch Soc Esp Oftalmol. 2015;90:4–8. doi: 10.1016/j.oftal.2014.07.011. Spanish. [DOI] [PubMed] [Google Scholar]
- 61.Anwar M, Teichmann KD. Big-bubble technique to bare Descemet’s membrane in anterior lamellar keratoplasty. J Cataract Refract Surg. 2002;28:398–403. doi: 10.1016/s0886-3350(01)01181-6. [DOI] [PubMed] [Google Scholar]
- 62.Al-Torbak AA, Al-Motowa S, Al-Assiri A, Al-Kharashi S, Al-Shahwan S, Al-Mezaine H, et al. Deep anterior lamellar keratoplasty for keratoconus. Cornea. 2006;25:408–412. doi: 10.1097/01.ico.0000220777.70981.46. [DOI] [PubMed] [Google Scholar]
- 63.Fogla R, Padmanabhan P. Results of deep lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2006;141:254–259. doi: 10.1016/j.ajo.2005.08.064. [DOI] [PubMed] [Google Scholar]
- 64.Fontana L, Parente G, Tassinari G. Clinical outcomes after deep anterior lamellar keratoplasty using the big-bubble technique in patients with keratoconus. Am J Ophthalmol. 2007;143:117–124. doi: 10.1016/j.ajo.2006.09.025. [DOI] [PubMed] [Google Scholar]
- 65.Feizi S, Javadi MA, Jamali H, Mirbabaee F. Deep anterior lamellar keratoplasty in patients with keratoconus: big-bubble technique. Cornea. 2010;29:177–182. doi: 10.1097/ICO.0b013e3181af25b7. [DOI] [PubMed] [Google Scholar]
- 66.Romano V, Iovieno A, Parente G, Soldani AM, Fontana L. Long-term clinical outcomes of deep anterior lamellar keratoplasty in patients with keratoconus. Am J Ophthalmol. 2015;159:505–511. doi: 10.1016/j.ajo.2014.11.033. [DOI] [PubMed] [Google Scholar]
- 67.Khakshoor H, Eslampoor A, Rad SS, Vejdani A. Modified deep anterior lamellar keratoplasty for the treatment of advanced keratoconus with steep corneal curvature to help in eliminating the wrinkles in the Descemet’s membrane. Indian J Ophthalmol. 2014;62:392–395. doi: 10.4103/0301-4738.121108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ghanem RC, Bogoni A, Ghanem VC. Pachymetry-guided intrastromal air injection (“pachy-bubble”) for deep anterior lamellar keratoplasty: results of the first 110 cases. Cornea. 2015;34:625–631. doi: 10.1097/ICO.0000000000000413. [DOI] [PubMed] [Google Scholar]
- 69.Yao YF. A novel technique for performing full-bed deep lamellar keratoplasty. Cornea. 2008;27(Suppl 1):S19–S24. doi: 10.1097/ICO.0b013e31817f445f. [DOI] [PubMed] [Google Scholar]
- 70.Dua HS, Faraj LA, Said DG, Gray T, Lowe J. Human corneal anatomy redefined: a novel pre-Descemet’s layer (Dua’s layer) Ophthalmology. 2013;120:1778–1785. doi: 10.1016/j.ophtha.2013.01.018. [DOI] [PubMed] [Google Scholar]
- 71.Farid M, Steinert RF. Deep anterior lamellar keratoplasty performed with the femtosecond laser zigzag incision for the treatment of stromal corneal pathology and ectatic disease. J Cataract Refract Surg. 2009;35:809–813. doi: 10.1016/j.jcrs.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 72.Buzzonetti L, Laborante A, Petrocelli G. Standardized big-bubble technique in deep anterior lamellar keratoplasty assisted by the femtosecond laser. J Cataract Refract Surg. 2010;36:1631–1636. doi: 10.1016/j.jcrs.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 73.Buzzonetti L, Petrocelli G, Valente P. Femtosecond laser and big-bubble deep anterior lamellar keratoplasty: a new chance. J Ophthalmol. 2012;2012:264590. doi: 10.1155/2012/264590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tan DTH, Mehta JS. Future directions in lamellar corneal transplantation. Cornea. 2007;26(9 Suppl 1):S21–S28. doi: 10.1097/ICO.0b013e31812f685c. [DOI] [PubMed] [Google Scholar]
- 75.Sarnicola V, Toro P. Blunt cannula for descemetic deep anterior lamellar keratoplasty. Cornea. 2011;30:985–988. doi: 10.1097/ICO.0b013e3181e848c3. [DOI] [PubMed] [Google Scholar]
- 76.Urrets-Zavalia A. Fixed dilated pupil, iris atrophy and secondary glaucoma: a distinct clinical entity following penetrating keratoplasty for keratoconus. Am J Ophthalmol. 1963;56:257–265. doi: 10.1016/0002-9394(63)91861-0. [DOI] [PubMed] [Google Scholar]


