Abstract
Background and Aims:
Transversus abdominis plane (TAP) block has proven to be an effective component of multimodal analgesic regimens for a variety of abdominal procedures. Magnesium sulphate (MgSO4) N-methyl-D-aspartate receptor antagonist has the potential to be an ideal adjuvant in TAP block. We studied the efficacy of MgSO4 as an adjuvant to bupivacaine in TAP block in patients scheduled for total abdominal hysterectomy (TAH) under subarachnoid block (SAB).
Methods:
Sixty-five women belonging to American Society of Anesthesiologists physical status 1 or 2, aged between 35 and 70 years, scheduled for TAH under SAB were recruited. Patients in Group B (n = 32) received 18 mL 0.25% bupivacaine (45 mg) with 2 mL normal saline (NS), whereas those in Group BM (n = 33) received 18 mL 0.25% bupivacaine (45 mg) with 1.5 mL (150 mg) MgSO4 and 0.5 mL NS in the ultrasound (USG)-guided TAP block performed on each side after the completion of the surgery under SAB. They were evaluated for pain at 0, 2, 4, 6, 12 and 24 h, time to first rescue analgesic and duration of postoperative analgesia were noted.
Results:
The post-operative visual analogue scale (VAS) scores were lower in Group BM at 4, 6 and 12 h (P < 0.05). Mean duration of analgesia was significantly prolonged in Group BM with lesser requirement of rescue analgesic (P < 0.05) up to 12 h.
Conclusion:
MgSO4 (150 mg) as an adjuvant to bupivacaine in USG-guided TAP block reduces post-operative pain scores, prolongs the duration of analgesia and decreases demands for rescue analgesics.
Keywords: Adjuvants, anaesthesia, analgesia, magnesium sulphate, pain, postoperative.
INTRODUCTION
Patients undergoing gynaecological surgery utilising transverse lower abdominal incisions experience severe pain during the first 48 h postoperatively, thus requiring a well-planned analgesia regimen in the post-operative period. Total abdominal hysterectomy (TAH) is a commonly performed major surgical procedure where the postoperative pain incidence approaches almost 32%.[1]
The multifactorial origin of pain following abdominal hysterectomy includes incisional pain, pain from deeper (visceral) structures and dynamic pain such as during coughing or mobilising but significant post-operative pain mostly occurs from the abdominal wall incision. Therefore, a multimodal approach to post-operative analgesia after TAH is required so as to block nociceptive transmission from both the abdominal wall incision, visceral sites. There has been a preference for opioids as a part of multimodal regimen despite the fact that the use of opioids can result in significant adverse effects including sedation, nausea, vomiting and respiratory depression, thus delaying early mobilisation of patients.
In this regard, the transversus abdominis plane (TAP) block seems to be an ideal approach in alleviating post-operative pain in patients undergoing lower abdominal gynaecological surgeries, especially when used as part of multimodal analgesia regimen. The technical simplicity, reliable analgesia makes TAP block, a preferred option for lower abdominal surgeries as has been reported by the American Society of Regional Anesthesia.[2]
The duration of TAP block is limited to the effect of administered local anaesthetics (LAs). However, recently adjuvants such as epinephrine, ketamine and clonidine are added to LA solution in concentrations advocated for other peripheral blocks to prolong the effect of TAP block with promising results. Evidence supporting the presence of N-methyl-D-aspartate (NMDA) receptors in skin and muscles[3] have led to the use of magnesium sulphate (MgSO4) (NMDA antagonist) via different routes for brachial plexus block[4] and via neuraxial route.[5] Until date, no study has been done to evaluate the role of MgSO4 as an adjuvant in TAP block. Therefore, we intended this study to evaluate the role of MgSO4 as an adjuvant to bupivacaine in ultrasound (USG)-guided TAP block in for post-operative analgesia in patients scheduled for TAH under subarachnoid block (SAB).
METHODS
After approval by the Institutional Ethics Committee, this study was carried out in 65 adult female patients (35–70 years age group) belonging to American Society of Anesthesiologists physical status 1 or 2 scheduled for TAH under SAB over a period of 18 months. The exclusion criteria included patient's refusal to block, having bleeding disorders, local infection at the site where needle for block was to be inserted, history of seizures, respiratory or cardiac diseases, patients on calcium channel blockers. Randomisation was achieved by computer-generated random number table. Random group assigned was enclosed in a sealed opaque envelope to ensure concealment of allocation sequence. Sealed envelope was opened by an anaesthesiologist not involved in the study to prepare the drug solution according to randomisation. The observer who collected the peri-operative data as well as the patients were blinded to the drug solution administered.
During preanaesthetic visit, the patients were explained about the study purpose, advantages and risks of procedure and instructed to demand analgesia as per requirement and informed written consent was obtained. Patients were educated about the 10 cm visual analogue scale (VAS) during the preoperative assessment. All the patients were kept nil orally for 8 h before surgery and no premedication was given. In the operation theatre, after securing 18-gauge intravenous (IV) cannula, 0.9% sodium chloride (normal saline [NS]) infusion was commenced. After establishing standard anaesthesia monitoring, baseline measurements such as heart rate (HR), non-invasive blood pressure and peripheral oxygen saturation were recorded.
All patients undergoing TAH were given SAB under all aseptic conditions in the right lateral position using 26-gauge Quincke spinal needle at L3–L4 interspace and 15 mg of 0.5% hyperbaric bupivacaine was injected after confirming free flow of CSF. After confirmation of adequate level (T4), surgery was started. After the surgery was over and the SAB sensory level regressed to T8 dermatome, USG-guided TAP block (using SonoSite™ Micromax machine, linear high-frequency probe, 6–13 MHz) was performed under all aseptic precautions with respective drug solutions. After draping the abdominal part between the twelfth rib bone and iliac crest with umbilicus at the centre-external oblique muscle, internal oblique muscle, transversus abdominis muscle, and their fascia were identified beneath the skin and the subcutaneous tissue. A 23-gauge spinal needle was advanced by a USG-guided in-plane technique at the anterior axillary line and the exact location of the needle tip checked by USG. After checking the exact location of the needle tip, 1 mL of NS was injected to open the plane and after confirmation of hypoechoic area on USG image, the study solution of 20 mL was injected. Equal amount of the same solution was also injected on the opposite side using identical technique. The patients in Group B (n = 32) received 18 mL 0.25% bupivacaine (45 mg) with 2 mL NS on either side, whereas the ones in Group BM (n = 33) received 18 mL 0.25% bupivacaine (45 mg) with 1.5 mL (150 mg) of MgSO4 and 0.5 mL NS on either side.
Postoperatively, the patients were evaluated for pain, nausea or vomiting in the post-anaesthesia care unit at time 0 (time of completion of TAP block), 2, 4, 6, 12 and 24 h by an investigator blinded to the group assignment. Whenever the VAS >4, diclofenac 1 mg/kg was administered intramuscularly as a rescue analgesic and if the pain persisted, tramadol 2 mg/kg IV was administered.
Patients were asked to rate average pain they experience postoperatively on a 10 cm VAS: No pain 0 to very severe pain 10. Patients were asked to rate the severity of nausea, vomiting on a four-point scale: None (1), mild (2), moderate (3) and severe (4). Twenty-four hours after the surgery, the patients were asked to rate on a 3-point scale regarding their satisfaction with pain management: Highly satisfied (1), satisfied (2) or dissatisfied (3).
The primary outcome measure in this study was the post-operative VAS score. The secondary outcome measures included the number of supplemental analgesic requirements, duration of post-operative analgesia that is time to first analgesic request from the time of giving block, nausea vomiting score and patient's satisfaction. All the patients were monitored in the peri-operative period for haemodynamic stability and any side effects.
Data were collected and entered in MS Excel 2010. Statistical analysis was performed using SPSS software 17 (SPSS Inc., Chicago, IL, USA). The one-sample Kolmogorov–Smirnov test was employed to determine whether data sets differed from a normal distribution. Normally distributed data were analysed using a repeat-measures general linear model analysis of variance (ANOVA) for time-related variables, whereas non-normally distributed data were analysed using the Mann–Whitney U-test and categorical data were analysed using the Chi-square test. P < 0.05 was considered statistically significant.
Sample size was estimated using pain scores as the primary variable. Assuming a standard deviation (SD) of 10 mm, the minimum needed sample size to detect a difference of 10 mm on the VAS of 10 cm, with type I error of 0.05 and power of 80% was 54. Hence, each group required at least 27 patients. We included 65 patients in our study to account for probable block failures and drop outs.
RESULTS
A total number of patients enroled during the study period were 65 in both groups being 32 and 33. Five patients were excluded 2 in Group B, 3 in Group BM [Figure 1] because of intra-operative requirement of rescue analgesia in the form of injection fentanyl 1 µg/kg. After excluding these patients, the total number of patients taken for the study was thirty in each group. They were comparable with each other with respect to age, weight and duration of surgery and ASA status [Table 1].
Figure 1.
Flow chart of patients recruited and analysed in two groups
Table 1.
Demographic data of patients in two groups
The difference in mean VAS at 0 and 2 h (VAS-0, VAS-2 h) was found to be statistically insignificant (mean ± SD: 1.87 ± 1.04, 1.87 ± 1.74 vs. 2.07 ± 1.11, 1.73 ± 1.36) in Groups B and BM, respectively (P = 0.902). However, there was statistically significant decrease in VAS scores at 4 and 6 h (Group BM: 1.40 ± 1.70, 2.40 ± 1.33 vs. Group B: 2.40 ± 1.43, 4.53 ± 2.62; [P 0.032 and P 0.002]). Higher VAS was recorded in Group B (3.80 ± 1.77) and Group BM (3.13 ± 1.55), respectively (P = 0.042) at 12 h [Figure 2].
Figure 2.
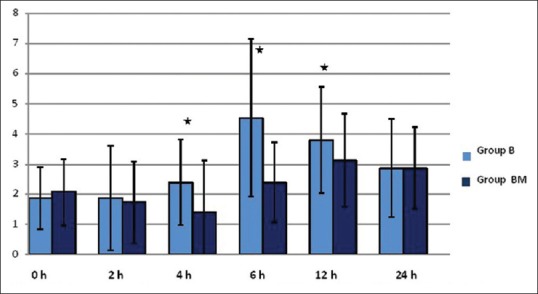
Mean visual analogue score over 24 h postoperatively. Values expressed as mean ± standard deviation
Mean duration of analgesia was significantly prolonged in Group BM compared to Group B (968.00 ± 161.06 min vs. 397.67 ± 92.84 min; P 0.000) [Figure 3].
Figure 3.
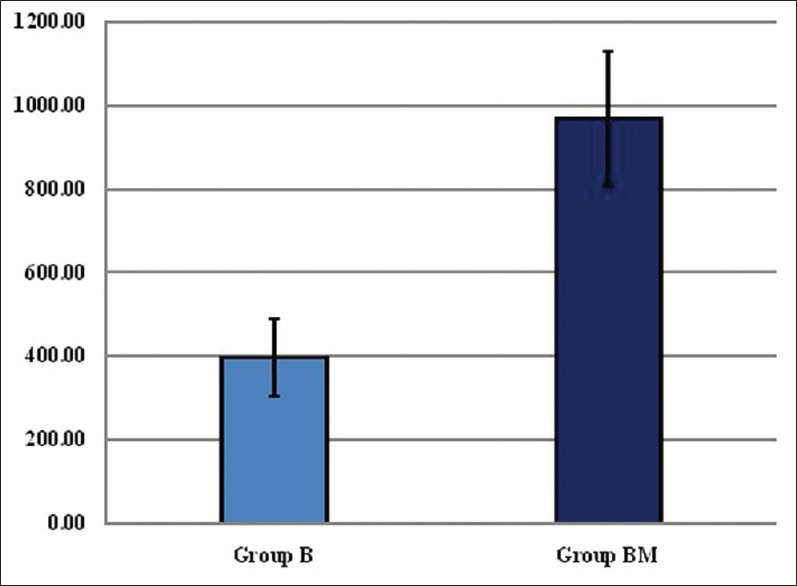
Mean duration of analgesia (mins). Values expressed as mean ± standard deviation
In the first 4 h, there were two demands for rescue analgesic in Group B (6.6%) and none in Group BM. Between 4 and 6 h, eight patients in Group B (26.6%) demanded rescue analgesia with none in Group BM. Between 6 and 12 h, 38 patients in Group B (93.3%) and two in Group BM (6.6%) demanded rescue analgesic. Between 12 and 24 h, eight patients in Group B (26.6%), and 29 in Group BM (96.6%) demanded rescue analgesia. The number of requirement of rescue analgesic was more in Group B as compared to BM in the first 12 h, and patients in BM group required analgesia after 12 h [Figure 4].
Figure 4.
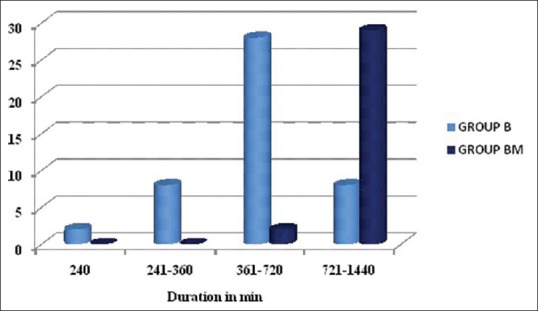
Number of analgesic demands at different time intervals
Higher mean post-operative nausea and vomiting score was recorded in Group B compared to Group BM (1.17 ± 0.38 vs. 1.07 ± 0.25, P = 0.488). Satisfaction score was better in Group BM compared to Group B (1.33 ± 0.48 vs. 1.77 ± 0.68, P = 0.021) [Figure 5].
Figure 5.
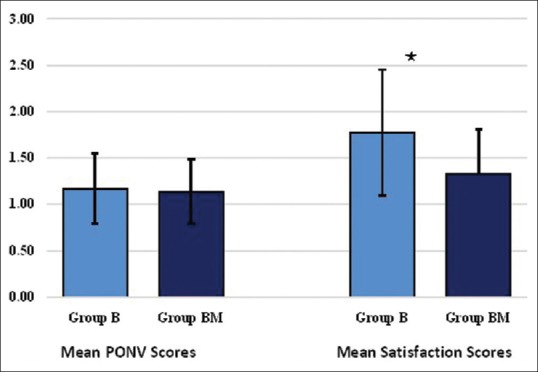
Mean postoperative nausea and vomiting and satisfaction score. Mean ± standard deviation
DISCUSSION
TAP block has proved beneficial in various abdominal surgeries as a part of a multimodal regimen for post-operative analgesia by virtue of its simplicity and effectiveness in providing analgesia, appropriateness for surgical procedures where parietal pain is a significant component of post-operative pain, lower pain scores, and reduction in opioid-related side effects.
The analgesic benefit of USG-guided TAP block in TAH has already been established.[6] Increased interest in TAP blocks is likely attributable to the advent of USG guidance since the benefits are related to enhanced accuracy of LA deposition. The analgesic efficacy of bupivacaine in TAP block has been studied[7] and our study shows similar analgesic benefits. The reduction in VAS scores for initial 2 h in our study may be the result of residual effect of SAB because the authors of the other study have given the block during general anaesthesia.
Regarding the number of rescue analgesics in TAP block,[8] maximum number of demand boluses were observed between 4 and 24 h with plain bupivacaine, while our study shows maximum demands between 6 and 12 h. One reason for initial discrepancy can be the difference in anaesthesia technique that is general anaesthesia versus SAB (present study). The bupivacaine group required rescue analgesic from 4 to 12 h and magnesium group demanded rescue analgesic after 12 h postoperatively indicating shorter pain-free period and more requirement of analgesia in the bupivacaine group. Therefore, use of magnesium in TAP block has a beneficial effect in reducing the number of systemic analgesic requirement.
With regards to total duration of analgesia, most of the authors have claimed a mean duration of analgesia as 4–6 h with the use of plain bupivacaine in TAP block and our study also shows similar results. The prolonged duration with bupivacaine in TAP block has also been attributed to the poor vascularity of TAP as demonstrated in the study using ropivacaine in TAP block.[9]
Unfortunately, duration of TAP block is still limited by the efficacy of LA administered and the dose used, which is again dependant on the maximum permitted dose for that agent. This has led to the use of adjuvants such as clonidine, dexmedetomidine to prolong the effect of LA in TAP block. The realisation of analgesic potency of magnesium by virtue of its NMDA receptor antagonist has led to its use via various routes for providing pre-emptive analgesia and to prolong post-operative analgesia. Therefore, we intended to use MgSO4 150 mg in USG-guided TAP block as an adjuvant to bupivacaine in patients scheduled for TAH under SAB.
The dose of magnesium used in this study was based on the data from a study[10] concluding that the addition of MgSO4 in a dose of 150 mg provided a pronounced prolongation of the duration of sensory and motor blocks compared with adding magnesium 100 mg, without systemic or neurotoxicity. In another study,[11] MgSO4 was used in 200 mg and 100 mg dose as sole agent for post-operative analgesia in axillary block and the duration of pain relief in MgSO4 groups were better than control group, whereas the duration of pain relief with 200 mg MgSO4 was better than 100 mg MgSO4 and also morphine consumption was less in both study drugs than control group. None of the patients showed any neurological deficits in the form of sensory or motor blockade with perineural MgSO4.
In our study, the addition of MgSO4 to bupivacaine in a dose of 150 mg has led to lower VAS pain scores, prolongation of analgesia and less requirement of rescue analgesia. Our study shows significantly lower VAS scores with the use of magnesium at 4, 6 and 12 h after the block. The results are comparable to the studies,[12,13] as the authors also found significant reductions in post-operative VAS scores with the use of MgSO4 in intra articular blocks and femoral nerve blocks, respectively. The duration of analgesia was prolonged with the use of MgSO4 150 mg (968.00 ± 161.06 vs. 397.67 ± 92.84 min, P = 0.0000). In our study, the nausea vomiting score was comparable in both groups and no other complications such as injury to viscera or any local complications were seen. Statistically significant difference was also found in terms of satisfaction score being better in magnesium group. Our data support specific action of magnesium on peripheral nerves leading to better pain scores and decrease in post-operative analgesic requirement. However, as TAP block has no effect on the visceral component of pain; therefore, it is more beneficial if supplemented along with systemic analgesics as a component of multimodal regimen for post-operative pain management.
CONCLUSION
Addition of MgSO4 in a dose of 150 mg to bupivacaine in TAP block decreases VAS scores postoperatively, prolongs the duration of analgesia and decreases the number of demands for rescue analgesia. Further studies are, however, required to establish efficacy of magnesium in TAP block.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Brandsborg B, Nikolajsen L, Kehlet H, Jensen TS. Chronic pain after hysterectomy. Acta Anaesthesiol Scand. 2008;52:327–31. doi: 10.1111/j.1399-6576.2007.01552.x. [DOI] [PubMed] [Google Scholar]
- 2.Sharkey A, Borglum J, Blanco R, McDonnell J. TAP block: Past, present, and future. Am Soc Reg Anesth Pain Med. 2014;12:15–7. [Google Scholar]
- 3.Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, et al. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003;90:2098–105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- 4.Lee AR, Yi HW, Chung IS, Ko JS, Ahn HJ, Gwak MS, et al. Magnesium added to bupivacaine prolongs the duration of analgesia after interscalene nerve block. Can J Anaesth. 2012;59:21–7. doi: 10.1007/s12630-011-9604-5. [DOI] [PubMed] [Google Scholar]
- 5.Ghatak T, Chandra G, Malik A, Singh D, Bhatia VK. Evaluation of the effect of magnesium sulphate vs. clonidine as adjunct to epidural bupivacaine. Indian J Anaesth. 2010;54:308–13. doi: 10.4103/0019-5049.68373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Atim A, Bilgin F, Kilickaya O, Purtuloglu T, Alanbay I, Orhan ME, et al. The efficacy of ultrasound-guided transversus abdominis plane block in patients undergoing hysterectomy. Anaesth Intensive Care. 2011;39:630–4. doi: 10.1177/0310057X1103900415. [DOI] [PubMed] [Google Scholar]
- 7.Amany AS, Khaled MM. Effect of adding dexamethasone to bupivacaine on transversus abdominis plane block for abdominal hysterectomy: A prospective randomized controlled trial. Saudi J Anaesth. 2012;6:229–33. doi: 10.4103/1658-354X.101213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Almarakbi WA, Kaki AM. Dexmedetomidine plus bupivacaine in TAP block. Saudi J Anaesth. 2014;8:161–6. doi: 10.4103/1658-354X.130683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McDonnell JG, Curley G, Carney J, Benton A, Costello J, Maharaj CH. The analgesic efficacy of transversus abdominis plane block after caesarean delivery: A randomized controlled trial. Anesth Analg. 2008;106:186–91. doi: 10.1213/01.ane.0000290294.64090.f3. [DOI] [PubMed] [Google Scholar]
- 10.Gunduz A, Bilir A, Gulec S. Magnesium added to prilocaine prolongs the duration of axillary plexus block. Reg Anesth Pain Med. 2006;31:233–6. doi: 10.1016/j.rapm.2006.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Goyal P, Jaiswal R, Hooda S, Goyal R, Lal J. Role of magnesium sulphate for brachial plexus analgesia. Internet J Anesthesiol 2008 [serial on the internet] 2009 Available at http://www.ispub.com/journal/the_internet_journal_of_anesthesiology/volume_21_number_1/article/role of magnesium-sulphate-for-brachial-plexus-analgesiahtml . [Google Scholar]
- 12.Bondok RS, Abd El-Hady AM. Intra-articular magnesium is effective for postoperative analgesia in arthroscopic knee surgery. Br J Anaesth. 2006;97:389–92. doi: 10.1093/bja/ael176. [DOI] [PubMed] [Google Scholar]
- 13.El Shamaa HA, Ibrahim M, Eldesuky H. Magnesium sulfate in femoral nerve block-does postoperative analgesia differ?. A comparative study. Egypt J Anaesth. 2014;30:169–73. [Google Scholar]