Abstract
Background
There are limited data on mortality outcomes associated with use of amiodarone in atrial fibrillation and flutter (AF).
Methods
We evaluated the association of amiodarone use with mortality in patients with newly-diagnosed AF using complete data from the Department of Veterans Affairs (VA) national health care system. We included patients seen in an outpatient setting within 90 days of a new diagnosis for non-valvular AF between VA fiscal years 2004–2008. Multivariate and propensity-matched Cox proportional hazards were used to evaluate the association of amiodarone use to death.
Results
Of 122,465 patients (353,168 person-years of follow-up; age 72.1±10.3 years; 98.4% males), amiodarone was prescribed in 11,655 (9.5%). Cumulative, unadjusted mortality rates were higher for amiodarone recipients than for non-recipients (87 vs. 73 per 1000 person-years, P<0.001). However, in multivariate and propensity matched survival analysis, there was no significant difference in mortality (multivariate HR: 1.01, 95%CI: 0.97–1.05, P=0.51; propensity-matched HR: 1.02, 95%CI: 0.97–1.07, P=0.45). The hazard of death was not modified by age, sex, heart failure, kidney function, beta blocker use, or warfarin use but there was evidence of effect modification among patients diagnosed with AF as an inpatient versus outpatient.
Conclusion
In a national health care system population of newly-diagnosed AF, overall use of amiodarone as an early treatment strategy was not associated with mortality.
Keywords: atrial fibrillation, amiodarone, mortality, safety
INTRODUCTION
Clinical practice guidelines currently endorse amiodarone for maintenance of sinus rhythm1,2 and it remains one of the most widely prescribed anti-arrhythmic drugs (AADs) for atrial fibrillation and atrial flutter (AF, collectively).3 Used in a variety of clinical profiles, amiodarone can be prescribed in patients with drug refractory AF or those with multiple, complex comorbidities that preclude use of other AADs. For example, unlike flecainide and propafenone, amiodarone is not contraindicated in coronary or structural heart disease4–6, nor is it renally excreted as are sotalol and dofetilide6–8. However, extended use of amiodarone may predispose patients to long-term pulmonary, thyroid, or liver toxicity.9,10
Despite its widespread use, particularly in complex clinical cases, there is limited and conflicting data on clinical outcomes in patients with AF that receive amiodarone.11–15 We therefore investigated the association of amiodarone therapy with mortality in a large cohort of patients with newly-diagnosed AF from a national health care system. We also evaluated the association of amiodarone with death in chronic kidney disease and end-stage renal disease.
METHODS
The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study is a retrospective cohort study of patients with newly-diagnosed AF treated in the Department of Veterans Affairs (VA) national health care system. Methods for cohort selection have been previously described in detail.16,17 Since amiodarone could be prescribed later or at more advanced stages of AF compared to other AF therapies, we used a new disease cohort design to minimize survival bias and immortal person-time bias. Datasets used in this study represent the claims data and electronic health records covering the full denominator of VA users. These include data from the VA National Patient Care Database (NPCD)18; the VA Decision Support System (DSS) national pharmacy extract19; the VA Fee Basis Inpatient and Outpatient datasets19; the VA Laboratory DSS extract20; and the VA Vital Status File21,22.
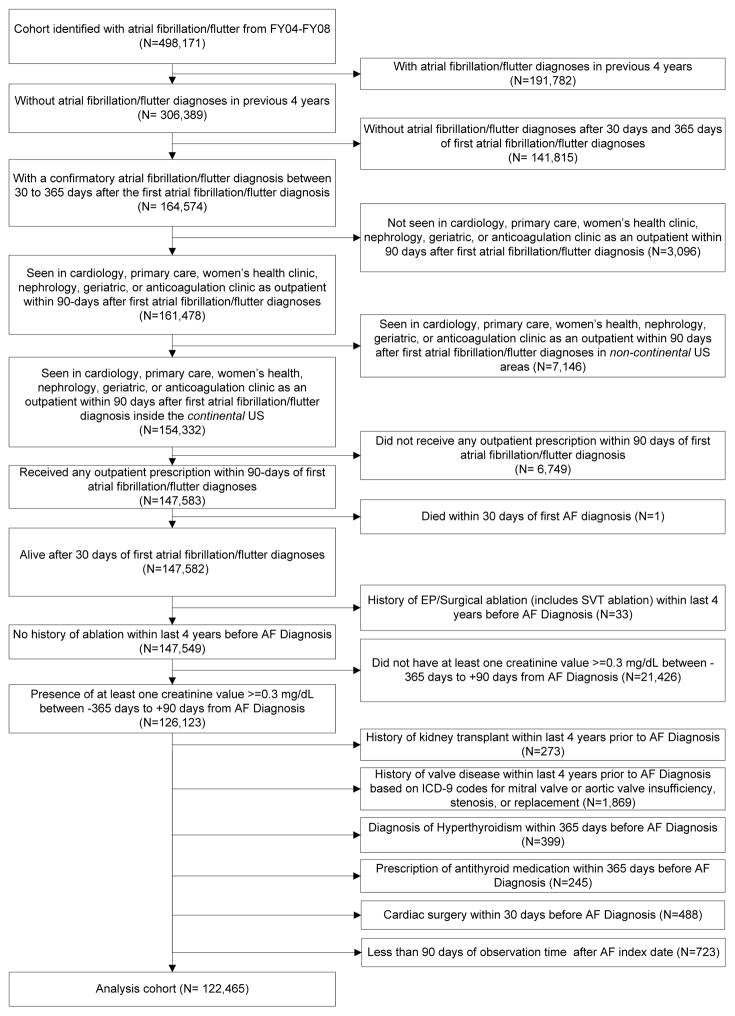
Figure 1 illustrates our cohort inclusion criteria. All patients were required to have a primary or secondary diagnosis of atrial fibrillation or atrial flutter (International Classification of Diseases, 9th Revision [ICD-9] 427.31 or 427.32) associated with an inpatient or outpatient VA encounter between October 1, 2003 and September 30, 2008 (VA Fiscal Years 2004–2008). All patients were also required a second confirmatory diagnosis between 30 and 365 days after the date of the index AF diagnosis, at least one outpatient visit in the first 90 days after index AF diagnosis, and any outpatient drug prescription in the first 90 days.
Figure 1. Consort Diagram.
Shows detailed inclusion and exclusion criteria used to select the cohort of 122,465 patients studied in this analysis.
The exclusion criteria were history of: AF diagnosis in the four years prior to the index AF date, defined by AF ICD-9 codes or CPT codes for catheter or surgical ablation; valve disease, repair, or replacement; thyroid disease; kidney transplant; or cardiac surgery within 30 days of the index AF date.
Primary Exposure Variables and Outcomes
The primary exposure was receipt of outpatient amiodarone within the first 90 days after index AF diagnosis. The primary outcome was time to death, which was determined at any point after the 90-day exposure ascertainment window. The Vital Status file used to determine death has been previously shown to have 97.6% agreement and 98.3% sensitivity for detection of deaths identified by the National Death Index.22 Patients with no record of death were assumed to be alive until September 30th, 2011, the last date for which vital status records from all sources was fully ascertained.
Clinical covariates
We identified baseline patient comorbidities using comorbidity-specific ICD-9 codes up to two years prior to the index AF date using algorithms based on the Agency for Healthcare Research and Quality (AHRQ) Clinical Classification System.23 The Charlson comorbidity score and the CHADS2 score were calculated using diagnostic algorithms previously validated in VA data.15,24 Receipt of concomitant drug therapies was ascertained using the same methods as for the primary exposure.
We also assessed kidney function using the CKD-EPI formula to approximate the estimated glomerular filtration rate (eGFR) for each patient, using the most recent outpatient serum creatinine from 365 days before to 90 days after the index AF diagnosis, as previously described.17 We also stratified patients by eGFR (in mL/min/1.73 m2): ≥ 90; <90 to ≥ 60; <60 to ≥ 45; <45 to ≥ 30; <30 to ≥ 15; and <15. A separate eGFR group for dialysis-dependent CKD was identified using ICD-9 and Common Procedural Terminology (CPT-4) codes for dialysis-related procedures or diagnoses.
Statistical Analysis
We compared differences in baseline characteristics between amiodarone-treated and untreated patients using chi-squared tests for categorical variables and t-tests for continuous variables. We performed Cox regression to estimate the hazard of death associated with receipt of amiodarone, using an “intention-to-treat” model based on drug receipt and adjusting for age, sex, race, hypertension, prior stroke, heart failure, diabetes, Charlson comorbidity score, CHADS2 score, cardiovascular medications, and eGFR stratum. To account for amiodarone exposure based on duration and intensity of drug therapy, we also performed an adherence-adjusted analysis by calculating patient-level medication possession ratio (MPR) of amiodarone and performing Cox regression with MPR as a time-varying covariate. The MPR was calculated as the proportion of total outpatient days’ supply of amiodarone divided by the total number of days from index AF diagnosis to date of death or censoring, truncated at an upper bound of 1.0. For all Cox models, the assumption of proportional hazards was found to be valid by examining Schoenfeld residuals.
Propensity Matching
Since amiodarone-treated patients may differ in baseline characteristics compared to patients not treated with amiodarone, we attempted to minimize confounding by indication using propensity score matching. We calculated the propensity to be treated with amiodarone using all patient baseline characteristics as independent variables in multivariate logistic regression. We tested pairwise interactions of covariates and retained the terms that significantly improved model fit. Propensity score balance and overlap were qualitatively assessed using propensity score distributions and standardized differences in observed characteristics. Cox regression was performed in a 1:1 matched cohort of amiodarone recipients and non-recipients, using nearest-neighbor matching without replacement. Hosmer-Lemeshow goodness-of-fit test and the C-statistic were used to determine model fit. We additionally estimated cumulative incidence of death using the Kaplan-Meier method and log-rank tests to assess differences between treated and untreated groups.
Stratified and Subgroup Analyses
We performed subgroup analyses, stratifying patients dichotomously based on the following characteristics: sex, age <65, heart failure, prior myocardial infarction, outpatient versus inpatient index AF diagnosis, new amiodarone treatment versus prior amiodarone exposure, daily dose (>200, ≤ 200 mg), use of loading dose, and treatment concomitant warfarin or beta blocker therapy. For each variable, we tested for effect modification of the associations between receipt of amiodarone and mortality. An interaction term P-value of <0.05 was considered significant for effect modification.
Role of funding source
The study was funded by the American Heart Association, Veterans Health Administration, and the Gilead Sciences Cardiovascular Scholars Program. No funding agencies were involved with study design, data assembly and analysis, or manuscript preparation. The study was approved by the local Institutional Review Board. All analyses were performed using SAS, version 9.1 (Cary, NC) and STATA, version 11.0 (College Station, TX).
RESULTS
We identified 122,465 patients with newly-diagnosed AF (Figure 1). The mean age was 72.1 ± 10.3 years, 98.4% of the population was male, and 36.8% had eGFR <60 mL/min/1.73m2 or were on dialysis (Table 1). A total of 11,655 (9.5%) received amiodarone during the first 90 days after initial AF diagnosis. There were 4,811 patients who received amiodarone prior to the first (index) AF diagnosis. During the index prescription of amiodarone, 2,400 patients received a loading or tapering dosage. Of remaining patients, the median and mode dose was 200 mg.
Table 1.
Baseline Characteristics.
|
|
|||
|---|---|---|---|
| Amiodarone prescribed within 90 days after AF diagnosis
| |||
| Yes (N=11,655) | No (N=110,810) | P | |
| Index AF diagnosis | |||
| Inpatient Diagnosis | 2,635 (22.6%) | 12,472 (11.3%) | <0.001 |
| Outpatient Diagnosis | 9,020 (77.4%) | 98,338 (88.7%) | <0.001 |
|
| |||
| Age, mean ± SD, years | 70.9 ± 9.9 | 72.2 ± 10.3 | <0.001 |
|
| |||
| Female, no. (%) | 121 (1.0%) | 1,859 (1.7%) | <0.001 |
|
| |||
| Race, White, no. (%) | 9,937 (85.3%) | 94,513 (85.3%) | 0.92 |
|
| |||
| Charlson Comorbidity Index, mean ± SD | 1.2 ± 1.2 | 0.92± 1.1 | <0.001 |
|
| |||
| CHADS2 score, mean ± SD | 1.7 ± 1.2 | 1.7 ± 1.2 | <0.001 |
|
| |||
| CHADS2 score group | <0.001 | ||
| CHADS2 0–1, no. (%) | 5,238 (44.9%) | 52,539 (47.4%) | <0.001 |
| CHADS2 2–3, no. (%) | 5,564 (47.7%) | 50,406 (45.5%) | <0.001 |
| CHADS2 4–6, no. (%) | 853 (7.3%) | 7,865 (7.1%) | 0.38 |
|
| |||
| Congestive Heart Failure, no. (%) | 2,638 (22.6%) | 16,679 (15.1%) | <0.001 |
|
| |||
| Hypertension, no. (%) | 7,564 (64.9%) | 69,937 (63.1%) | <0.001 |
|
| |||
| Age ≥ 75 years, no. (%) | 4,885 (41.9%) | 52,974 (47.8%) | <0.001 |
|
| |||
| Diabetes, no. (%) | 3,563(30.6%) | 31,739 (28.6%) | <0.001 |
|
| |||
| Prior Stroke/TIA, no. (%) | 681 (5.8%) | 6,879 (6.2%) | 0.12 |
|
| |||
| Prior MI, no. (%) | 768 (6.6%) | 4,751 (4.3%) | <0.001 |
|
| |||
| eGFR group, mL/min/1.73m2 | <0.001 | ||
| eGFR ≥ 90, no. (%) | 1,199 (10.3%) | 14,256 (12.9%) | <0.001 |
| eGFR 60–89, no. (%) | 5,108 (43.8%) | 56,802 (51.3%) | <0.001 |
| eGFR 45–59, no. (%) | 2,838 (24.4%) | 23,938 (21.6%) | <0.001 |
| eGFR 30–44, no. (%) | 1,723 (14.8%) | 11,560 (10.4%) | <0.001 |
| eGFR 15–29, no. (%) | 579 (5.0%) | 3,159 (2.9%) | <0.001 |
| eGFR <15, no. (%) | 103 (0.88%) | 567 (0.51%) | <0.001 |
| Dialysis, no. (%) | 105 (0.90%) | 528 (0.48%) | <0.001 |
|
| |||
| eGFR, mean ± SD, mL/min/1.73m2 | 62.4 ± 21.3 | 67.3 ± 20.3 | <0.001 |
|
| |||
| Cardiovascular Medications | |||
| Aspirin, no. (%) | 2,332 (20.0%) | 16,379 (14.8%) | <0.001 |
| Clopidogrel, no. (%) | 1,001 (8.6%) | 5,439 (4.9%) | <0.001 |
| Aspirin + Clopidogrel, no. (%) | 520 (4.5%) | 2,215 (2.0%) | <0.001 |
| ACE Inhibitor or Angiotensin Receptor Blockers, no. (%) | 7,048 (60.5%) | 57,556 (51.9%) | <0.001 |
| Alpha blockers, no. (%) | 274 (2.4%) | 2,138 (1.9%) | 0.002 |
| Diuretics, no. (%) | 6,827 (58.6%) | 50,220 (45.3%) | <0.001 |
| Niacin or Fibrates, no. (%) | 934 (8.0%) | 7,708 (7.0%) | <0.001 |
| Statins, no. (%) | 7,212 (61.9%) | 57,586 (52.0%) | <0.001 |
| Warfarin, no. (%) | 6,618 (56.8%) | 62,270 (56.2%) | 0.22 |
|
| |||
| Antiarrhythmic Drugs | <0.001 | ||
| All Class I, no. (%) | 132 (1.1%) | 2,484 (2.2%) | <0.001 |
| Class III (Sotalol/ Dofetilide), no. (%) | 86 (0.74%) | 4,088 (3.7%) | <0.001 |
|
| |||
| Rate-controlling drugs | <0.001 | ||
| All beta-blockers, no. (%) | 7,590 (65.1%) | 63,721 (57.5%) | <0.001 |
| Metoprolol, no. (%) | 5,240 (45.0%) | 41,567 (37.5%) | <0.001 |
| Carvedilol, no. (%) | 1,486 (12.8%) | 6,220 (5.6%) | <0.001 |
| Atenolol, no. (%) | 792 (6.8%) | 14,728 (13.3%) | <0.001 |
| Other, no. (%) | 72 (0.62%) | 1,206 (1.1%) | <0.001 |
| All calcium-channel blockers, no. (%) | 2,881 (24.7%) | 34,201 (30.9%) | <0.001 |
| Diltiazem, no. (%) | 1,238 (10.6%) | 16,559 (14.9%) | <0.001 |
| Verapamil, no. (%) | 145 (1.2%) | 2,561 (2.3%) | <0.001 |
| Other, no. (%) | 1,498 (12.9%) | 15,081 (13.6%) | 0.02 |
|
| |||
| Digoxin, no. (%) | 2,849 (24.4%) | 25,830 (23.3%) | 0.01 |
Compared to non-recipients in the full cohort, amiodarone recipients had significantly higher cardiovascular and overall comorbidity burden including greater prevalence of heart failure, prior myocardial infarction, diabetes, and advanced chronic kidney disease. Amiodarone recipients also had higher prevalence of concomitant drug therapies such as aspirin, beta blockers, angiotensin-receptor blockers, antiplatelet therapy, and diuretics. However, there were no differences in mean CHADS2 score or in prevalence of prior stroke or warfarin use.
Supplemental Figure 1 shows the propensity distributions and overlap for recipients and non-recipients of amiodarone in the full cohort. The Hosmer-Lemeshow goodness of fit test was P=0.90 and the C-statistic was 0.69. There was strong balance of propensity scores between recipients and non-recipients, with no standardized differences of covariates for matched patients >0.1025; the highest standardized difference was 0.052 (Supplemental Table 1).
Outcomes
The total follow-up time was 353,168 patient-years (Table 2); 28,723 (23.5%) patients died during the observation period. In unadjusted analysis, amiodarone recipients had a slightly higher risk of death compared to non-recipients in the full cohort (87 vs 73 deaths per 1,000 person-years; P<0.001) but not in the propensity matched cohort (89 vs 87 per 1,000 person-years; P=0.36) (Table 2). In the full cohort, after adjustment for baseline covariates, amiodarone was not significantly associated with mortality (HR: 1.01, 95%CI: 0.97–1.05, P=0.51) (Table 3). After adjusting for amiodarone adherence as a time-varying covariate, amiodarone was associated with lower hazard of death in the full cohort (HR: 0.90, 95%CI: 0.85–0.94, P<0.001). In the propensity-matched cohort, amiodarone treatment was not significantly associated with mortality, with or without adherence adjustment.
Table 2.
Unadjusted Mortality Incidence Rates.
| Treatment | Person-Time (Yrs) | Deaths | Mortality Rate (per 1,000 Person-Years), unadjusted | Incidence Rate Ratio (95% CI), unadjusted | P |
|---|---|---|---|---|---|
| Full Cohort | |||||
|
| |||||
| No Amiodarone | 318,935 | 25,646 | 73 | – | – |
| Amiodarone | 34,233 | 3,077 | 87 | 1.19 (1.15–1.24) | <0.001 |
|
| |||||
| Overall | 353,168 | 28,723 | 81 | – | – |
|
| |||||
| Propensity-Matched Cohorts | |||||
|
| |||||
| No Amiodarone | 30,770 | 2,788 | 87 | – | – |
| Amiodarone | 32,134 | 2,977 | 89 | 1.02 (0.98–1.08) | 0.36 |
|
| |||||
| Overall | 62,904 | 5,765 | 88 | – | – |
Table 3.
Multivariate and Propensity-Matched Cox Regression Results.
| Model | ||||
|---|---|---|---|---|
|
| ||||
| Amiodarone
| ||||
| Full Cohort | Propensity-Matched Cohort | |||
|
| ||||
| HR (95% CI) N=122,465 |
P | HR (95% CI) N=21,716 |
P | |
| Unadjusted | 1.11 (1.07–1.16) | <0.001 | — | — |
| Age, sex, race | 1.18 (1.14–1.23) | <0.001 | — | — |
|
| ||||
| Full model* | ||||
| All patients | 1.01 (0.97–1.05) | 0.51 | 1.02 (0.97–1.07) | 0.45 |
| Adherence (MPR)-adjusted† | 0.90 (0.85–0.94) | <0.001 | 1.12 (0.97–1.31) | 0.13 |
|
| ||||
| eGFR group, mL/min/1.73m2 | ||||
| eGFR ≥ 90 | 1.00 (0.86–1.17) | 0.97 | 1.10 (0.90–1.36) | 0.35 |
| eGFR 60–89 | 1.00 (0.94–1.07) | 0.96 | 1.03 (0.95–1.13) | 0.46 |
| eGFR 45–59 | 0.97 (0.90–1.05) | 0.50 | 0.95 (0.85–1.05) | 0.28 |
| eGFR 30–44 | 0.98 (0.90–1.07) | 0.68 | 1.05 (0.94–1.18) | 0.36 |
| eGFR 15–29 | 1.01 (0.89–1.15) | 0.87 | 0.98 (0.83–1.16) | 0.82 |
| eGFR <15 | 0.80 (0.58–1.09) | 0.16 | 0.93 (0.63–1.35) | 0.69 |
| Dialysis | 1.37 (1.02–1.86) | 0.04 | 1.23 (0.84–1.80) | 0.29 |
For full cohort, full models adjusted for age, sex, race, hypertension, stroke, heart failure, diabetes mellitus, CHADS2 score, Charlson comorbidity score, digoxin, beta blockers, diuretics, anti-platelet agents, warfarin, statins, niacin/fibrates, ACE inhibitors/angiotensin receptor blockers, and eGFR group.
Full adherence (MPR)-adjusted model was created by adjusting for amiodarone medication possession ratio (MPR) from date of first AF diagnosis to date of death or censoring, as a time-varying exposure. Also adjusted for age, sex, race, hypertension, stroke, heart failure, diabetes mellitus, CHADS2 score, Charlson comorbidity score, digoxin, beta blockers, diuretics, anti-platelet agents, warfarin, statins, niacin/fibrates, ACE inhibitors/angiotensin receptor blockers, and eGFR group.
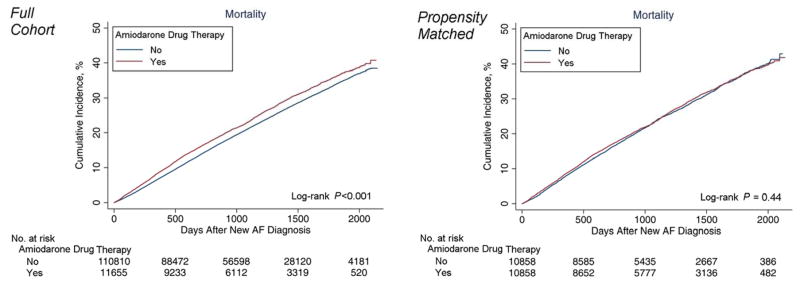
Figure 2 shows the cumulative incidence of death in both the full and propensity-matched cohorts. The cumulative incidence of death was significantly higher in amiodarone recipients in the full cohort (log-rank P<0.001), but not in the propensity matched cohort (log-rank P=0.44).
Figure 2. Cumulative Incidence of Death in Full and Propensity-Matched Cohorts.
Shows cumulative incidence of death, comparing treated and untreated patients for amiodarone, with curves estimated using the Kaplan-Meier method. Differences in treated and untreated groups were assessed using the log-rank test.
Relationship to kidney function
With multivariate adjustment and propensity matching, amiodarone was not associated with an increased hazard of death in any stratum of kidney function as determined by the estimated GFR (Table 3). However in patients on dialysis, the point estimate for amiodarone and mortality just crossed significance in the full cohort multivariate model (HR: 1.37, 95%CI: 1.02–1.86, P=0.04), but not in the propensity-matched analysis. There was no evidence of effect modification present across strata of kidney function.
Additional subgroup analysis
Multivariate and propensity-matched analyses are shown for 18 clinically relevant subgroups in Table 4. In patients with age <65, there was evidence of effect modification in the multivariate regression for amiodarone (P<0.001) but not after propensity matching. Consistent with the overall result, there was no heterogeneity in treatment effect among new versus prior amiodarone users, daily prescribed dose (>200 versus ≤ 200 mg), or prescription or non-prescription of a loading dose.
Table 4.
Subgroup Analysis: Association of Amiodarone with Mortality.
| Subgroup |
|
|||
|---|---|---|---|---|
| Amiodarone
| ||||
| Full Cohort* | Propensity-Matched Cohort† | |||
|
| ||||
| HR (95% CI) | P | HR (95% CI) | P | |
| Male | 1.01 (0.97–1.05) | 0.51 | 1.04 (0.99–1.10) | 0.10 |
| Female | 0.93 (0.61–1.42) | 0.73 | 0.95 (0.54–1.65) | 0.85 |
|
| ||||
| Age ≥ 65 | 0.95 (0.91–0.99) | 0.02** | 0.99 (0.93–1.05) | 0.69 |
| Age <65 | 1.16 (1.05–1.28) | 0.003** | 0.98 (0.93–1.03) | 0.40 |
|
| ||||
| Previous diagnosis of heart failure | 1.01 (0.94–1.08) | 0.88 | 0.99 (0.90–1.08) | 0.80 |
| No previous diagnosis of heart failure | 1.01 (0.96–1.06) | 0.72 | 1.01 (0.95–1.07) | 0.85 |
|
| ||||
| Previous diagnosis of myocardial infarction | 1.06 (0.92–1.23) | 0.40 | 1.14 (0.94–1.38) | 0.18 |
| No previous diagnosis of myocardial infarction | 1.01 (0.97–1.05) | 0.62 | 1.01 (0.95–1.06) | 0.79 |
|
| ||||
| Treated with Warfarin | 1.02 (0.96–1.07) | 0.53 | 1.04 (0.97–1.12) | 0.27 |
| Treated without Warfarin | 1.02 (0.96–1.08) | 0.59 | 1.00 (0.93–1.08) | 0.96 |
|
| ||||
| Treated with beta blocker | 1.01 (0.96–1.06) | 0.61 | 1.02 (0.96–1.09) | 0.48 |
| Not treated with beta blocker | 1.05 (0.99–1.12) | 0.13 | 1.04 (0.95–1.13) | 0.38 |
|
| ||||
| Inpatient index AF diagnosis | 0.86 (0.79–0.93) | <0.001** | 0.77 (0.69–0.87) | <0.001** |
| Outpatient index AF diagnosis | 1.05 (1.01–1.10) | 0.02** | 1.07 (1.01–1.13) | 0.02** |
|
| ||||
| New user of amiodarone | 1.01 (0.97–1.05) | 0.58 | 0.96 (0.91–1.02) | 0.17 |
| Received amiodarone at any time in the 2 years prior to index prescription | 1.03 (0.84–1.26) | 0.77 | 1.19 (0.94–1.50) | 0.15 |
|
| ||||
| New user of amiodarone (Outpatient AF diagnosis subgroup) | 1.06 (1.01–1.10) | 0.02 | 0.99 (0.93–1.06) | 0.76 |
| Received amiodarone at any time in the 2 years prior to index prescription (Outpatient AF diagnosis subgroup) | 0.99 (0.79–1.24) | 0.90 | 1.26 (0.98–1.63) | 0.08 |
|
| ||||
| >200 mg daily dose of index prescription | 1.10 (0.92–1.33) | 0.29 | 1.01 (0.94–1.08) | 0.78 |
| ≤200 mg daily dose of index prescription | 1.00 (0.97–1.05) | 0.68 | 1.02 (0.97–1.09) | 0.42 |
|
| ||||
| Loading dose | 0.95 (0.88–1.03) | 0.25 | 0.98 (0.90–1.08) | 0.71 |
| No loading dose | 1.03 (0.99–1.07) | 0.18 | 1.03 (0.97–1.09) | 0.30 |
Adjusted for age, sex, race, hypertension, stroke, heart failure, diabetes, CHADS2 score, Charlson comorbidity score, digoxin, beta blockers, diuretics, anti-platelet agents, warfarin, statins, niacin/fibrates, ACE inhibitors/angiotensin receptor blockers, and eGFR group.
P-value for interaction was significant (P ≤ 0.05)
Covariates retained for propensity-adjusted subgroup analysis are identical to those listed on Supplemental Table 1 for the propensity-matched cohort. Potential covariates analyzed include: age, sex, race, Charlson Comorbidity Index, CHADS2 0–1, CHADS2 2–3, CHADS2 4–6, mean CHADS2 score, heart failure, hypertension, diabetes, prior stroke/TIA, eGFR ≥ 90, eGFR 60–89, eGFR 45–59, eGFR 30–44, eGFR 15–29, eGFR <15, dialysis, diuretics, niacin or fibrates, statins, warfarin, all beta-blockers, anti-platelet agents, ACE inhibitors/angiotensin receptor blockers, or digoxin. A relevant covariate was removed from the model when that variable defined the subgroup being analyzed. No P-values for interaction were significant (P ≤ 0.05) in propensity-matched analyses.
There was evidence of effect modification and divergence of treatment effect based on site of index AF diagnosis. Among patients with an inpatient index diagnosis, amiodarone appeared to be associated with reduced mortality in the adjusted and propensity models, while among those diagnosed as an outpatient, there was a weakly significant increase in risk of death. However, among outpatient-diagnosed new users of amiodarone, there was no treatment heterogeneity observed.
DISCUSSION
In this cohort of 122,465 patients with newly-diagnosed AF, we found that amiodarone was prescribed as an initial therapy in higher risk patients, compared to non-recipients of the drug. The principal finding is that amiodarone was not associated with increased hazard of death in multivariate and propensity-matched analyses. These results were consistent across all strata of kidney function and even in patients with coronary disease or heart failure. In patients with age <65 years and those on dialysis in the full cohort, amiodarone was associated with a small but significant increase in hazard of death. However, this association was not found in either subgroup in the propensity-matched analysis. These findings suggest that amiodarone may not cause excess harm (or benefit) with respect to survival when used as an initial or early treatment strategy.
Clinical trials examining amiodarone in AF
Information on safety and effectiveness of amiodarone largely exist in older clinical trial data. In a secondary analysis of the AFFIRM trial, amiodarone was not associated with cardiovascular mortality, however it significantly increased hazard of non-cardiovascular death over rate control (HR: 1.11, 95%CI: 1.01–1.24).26 In the SAFE-T trial, participants were predominantly male Veterans with similar age and prevalence of cardiovascular comorbidities as in our cohort.27 Although amiodarone was superior to both sotalol and placebo in maintaining sinus rhythm, SAFE-T was underpowered to assess differences in mortality risk with only 665 subjects.27 Other older clinical trials examining amiodarone use in AF have enrolled even fewer subjects. When data from AFFIRM, SAFE-T, and ten other clinical trials were pooled in a meta-analysis by Doyle et al.9, amiodarone was more effective in converting persistent AF to sinus rhythm compared to placebo and was not significantly associated with all-cause death (pooled RR: 0.95, 95%CI: 0.81–1.11; I2=0%).
More recently, a secondary analysis of the ROCKET-AF trial showed that 1,132 amiodarone users with prevalent AF and high stroke risk did not experience an increase in risk of death compared to non-users, after adjusting across treatment assignment (rivaroxaban or warfarin).11 Although a minority of patients in ROCKET-AF was treated with an anti-arrhythmic drug at baseline in the trial, amiodarone was the most common AAD prescribed and patients treated with amiodarone were among the highest risk subjects, consistent with our findings in the present study.
Observational data on amiodarone
Outside of clinical trials, observational data on amiodarone in AF remain sparse. Prior to our analysis, the largest cohort study on amiodarone and mortality retrospectively examined 141,500 patients that were admitted for AF in Denmark between 1995 and 2004.14 The study found no significant increase in risk of death with amiodarone use14, although the cohort was derived from a national registry that included only inpatient hospitalizations. The comorbidity burden in the Danish cohort was considerably lower than in our study. Fewer subjects received background warfarin (18% vs 56% in our study) or beta blocker (18% vs 58%) therapies, which could also explain the observed survival benefit found among users of flecainide, propafenone, and sotalol in the cohort, respectively.
Our findings extend prior observations on amiodarone by examining its use across the spectrum of kidney function, as other antiarrhythmic drugs are generally contraindicated in moderate to severe CKD. Amiodarone itself may decrease renal blood flow and raise creatinine levels28, which can raise safety concerns in patients with kidney disease. In dialysis patients, we observed discordance in results between the multivariate analysis and the propensity-matched analysis which may be due to a greater degree of residual confounding in the full cohort. However, despite no evidence of a mortality association in the propensity-matched analysis, amiodarone should continue to be used cautiously in patients with dialysis due to the limited number of patients in this subgroup.
Amiodarone in concomitant AF and heart failure
There was no evidence of treatment heterogeneity in our analysis, including in patients with heart failure. Previously, the COMET trial found an increased risk of death from circulatory failure with amiodarone use in patients with acute or chronic heart failure, regardless of NYHA functional class.29 In our cohort, amiodarone was not associated with increased mortality in patients with a previous heart failure diagnosis at baseline. To our knowledge, this observational cohort includes the largest population of patients to date with concomitant heart failure and AF that received amiodarone.
Treatment heterogeneity based on site of index AF diagnosis
The divergence in results among patients with an index diagnosis as an inpatient versus outpatient (Table 4) could be due to a number of factors. First, differential confounding by indication could explain the observed differences. Second, survival biases associated with surviving a hospitalization in order to be included in the denominator could bias results toward better survival (or conversely greater harm among those diagnosed at outpatients). When accounting for new amiodarone use, treatment effects attenuated in the outpatient diagnosis subgroup and effect modification was no longer present. These data are hypothesis generating.
Study limitations
There are important limitations to our study. Confounding by indication is of greatest concern since amiodarone-treated patients may be unsuitable for other drugs. The reduced hazard of death observed after accounting for adherence could indicate endogeneity, a form of confounding, since health status may itself inform adherence. Our cohort predominantly consists of male Veterans, which limits generalizability of our overall findings for female patients and non-Veteran populations. Despite the large cohort, analysis in small and highly morbid subgroups, such as patients on dialysis, could be underpowered. Additionally, knowledge of concomitant therapies or other anti-arrhythmic drugs prescribed prior to amiodarone are based on pharmacy claims, therefore we cannot confirm whether patients consumed all therapies that were dispensed to them, or if other anti-arrhythmic drugs were taken concomitantly with amiodarone. However, only 83 patients (0.7% of amiodarone recipients) received an outpatient or inpatient prescription for another oral anti-arrhythmic drug in the 90-day window of ascertainment of amiodarone exposure.
Next, due to limitations in our dataset, we could not determine ejection fraction or NYHA functional classification. Likewise, we could not establish a precise mechanism of death for users and non-users of amiodarone as our primary outcome was time to all-cause death.
We applied an intention-to-treat (ITT) design based on receipt of amiodarone within the first 90 days of index AF diagnosis. By excluding patients with prevalent AF disease, the new disease cohort minimizes survival and immortal person-time bias (i.e. patients who were “well enough” to survive longer to be given amiodarone). It is possible that patients in the control arm of the study experienced crossover of therapy to amiodarone after the initial 90 days after index AF diagnosis, which could bias results, likely toward the null. The ITT approach is therefore suited to evaluate amiodarone as an initial treatment strategy and results may not be generalizable to situations in which amiodarone is used later in the course of AF. Although adherence adjusted analyses does allow some adjustment for treatment adherence, a medication possession ratio of less than 1.0 may underestimate true amiodarone exposure owing to the long elimination time of amiodarone. In contrast, observational studies which use a time-dependent definition of exposure, may be subject to bias by informative censoring in situations where underlying health status can impact treatment discontinuation.30
CONCLUSIONS
In patients with newly-diagnosed AF, receipt of amiodarone as an early treatment strategy was not associated with mortality, regardless of age, sex, kidney function, heart failure status, concomitant therapies, or drug adherence.
Supplementary Material
Acknowledgments
All authors contributed materially to study concept and design, acquisition and interpretation of data, or drafting and critical revision of the manuscript for important intellectual content. Dr. Turakhia is supported by a Veterans Health Services Research & Development Career Development Award (CDA09027-1), an American Heart Association National Scientist Development Grant (09SDG2250647), and a VA Health Services and Development MERIT Award (IIR 09-092).
Footnotes
Disclosures: None. All authors have approved the final article.
Online Appendix: Supplemental Table 1 and Supplemental Figure 1 are found in the Online Appendix.
References
- 1.Camm AJ, Lip GY, De Caterina R, et al. 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J. 2012;33(21):2719–47. doi: 10.1093/eurheartj/ehs253. [DOI] [PubMed] [Google Scholar]
- 2.January CT, Wann LS, Alpert JS, et al. 2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: Executive Summary: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2014 doi: 10.1161/CIR.0000000000000040. [DOI] [PubMed] [Google Scholar]
- 3.Zimetbaum P, Ho KK, Olshansky B, et al. Variation in the utilization of antiarrhythmic drugs in patients with new-onset atrial fibrillation. Am J Cardiol. 2003;91(1):81–3. doi: 10.1016/s0002-9149(02)03004-7. [DOI] [PubMed] [Google Scholar]
- 4.Echt DS, Liebson PR, Mitchell LB, et al. Mortality and morbidity in patients receiving encainide, flecainide, or placebo. The Cardiac Arrhythmia Suppression Trial. N Engl J Med. 1991;324(12):781–8. doi: 10.1056/NEJM199103213241201. [DOI] [PubMed] [Google Scholar]
- 5.Aliot E, Capucci A, Crijns HJ, et al. Twenty-five years in the making: flecainide is safe and effective for the management of atrial fibrillation. Europace. 2011;13(2):161–73. doi: 10.1093/europace/euq382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zimetbaum P. Antiarrhythmic drug therapy for atrial fibrillation. Circulation. 2012;125(2):381–9. doi: 10.1161/CIRCULATIONAHA.111.019927. [DOI] [PubMed] [Google Scholar]
- 7.Nabar A, Rodriguez LM, Timmermans C, et al. Class IC antiarrhythmic drug induced atrial flutter: electrocardiographic and electrophysiological findings and their importance for long term outcome after right atrial isthmus ablation. Heart. 2001;85(4):424–9. doi: 10.1136/heart.85.4.424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Torp-Pedersen C, Brendorp B, Kober L. Dofetilide: a class III anti-arrhythmic drug for the treatment of atrial fibrillation. Expert Opin Investig Drugs. 2000;9(11):2695–704. doi: 10.1517/13543784.9.11.2695. [DOI] [PubMed] [Google Scholar]
- 9.Doyle JF, Ho KM. Benefits and risks of long-term amiodarone therapy for persistent atrial fibrillation: a meta-analysis. Mayo Clin Proc. 2009;84(3):234–42. doi: 10.4065/84.3.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wolkove N, Baltzan M. Amiodarone pulmonary toxicity. Can Respir J. 2009;16(2):43–8. doi: 10.1155/2009/282540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Steinberg BA, Hellkamp AS, Lokhnygina Y, et al. Use and outcomes of antiarrhythmic therapy in patients with atrial fibrillation receiving oral anticoagulation: Results from the ROCKET AF trial. Heart Rhythm. 2014;11(6):925–32. doi: 10.1016/j.hrthm.2014.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steinberg JS, Sadaniantz A, Kron J, et al. Analysis of cause-specific mortality in the Atrial Fibrillation Follow-up Investigation of Rhythm Management (AFFIRM) study. Circulation. 2004;109(16):1973–80. doi: 10.1161/01.CIR.0000118472.77237.FA. [DOI] [PubMed] [Google Scholar]
- 13.Lafuente-Lafuente C, Longas-Tejero MA, Bergmann JF, et al. Antiarrhythmics for maintaining sinus rhythm after cardioversion of atrial fibrillation. Cochrane Database Syst Rev. 2012;5:CD005049. doi: 10.1002/14651858.CD005049.pub3. [DOI] [PubMed] [Google Scholar]
- 14.Andersen SS, Hansen ML, Gislason GH, et al. Antiarrhythmic therapy and risk of death in patients with atrial fibrillation: a nationwide study. Europace. 2009;11(7):886–91. doi: 10.1093/europace/eup119. [DOI] [PubMed] [Google Scholar]
- 15.Gage BF, Waterman AD, Shannon W, et al. Validation of clinical classification schemes for predicting stroke: results from the National Registry of Atrial Fibrillation. JAMA. 2001;285(22):2864–70. doi: 10.1001/jama.285.22.2864. [DOI] [PubMed] [Google Scholar]
- 16.Turakhia M, Hoang DD, Xu X, et al. Differences and trends in stroke prevention anticoagulation in primary care vs cardiology specialty management of new atrial fibrillation: The Retrospective Evaluation and Assessment of Therapies in AF (TREAT-AF) study. Am Heart J. 2013;165(1):93–101. e1. doi: 10.1016/j.ahj.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 17.Turakhia MP, Santangeli P, Winkelmayer WC, et al. Increased mortality associated with digoxin in contemporary patients with atrial fibrillation: findings from the TREAT-AF study. J Am Coll Cardiol. 2014;64(7):660–8. doi: 10.1016/j.jacc.2014.03.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cowper DC, Hynes DM, Kubal JD, et al. Using administrative databases for outcomes research: select examples from VA Health Services Research and Development. J Med Syst. 1999;23(3):249–59. doi: 10.1023/a:1020579806511. [DOI] [PubMed] [Google Scholar]
- 19.Smith MW, Joseph GJ. Pharmacy data in the VA health care system. Med Care Res Rev. 2003;60(3 Suppl):92S–123S. doi: 10.1177/1077558703256726. [DOI] [PubMed] [Google Scholar]
- 20.VA Information Resource Center. VIReC Research User Guide: VHA Decision Support System Clinical National Data Extracts. 2. Hines, IL: U.S. Dept. of Veterans Affairs, Health Services Research and Development Service, VA Information Resource Center; 2009. [Google Scholar]
- 21.Arnold N, Sohn M-W, Maynard C, et al. VIReC Technical Report 2: VANDI Mortality Data Merge Project. Hines, IL: VA Information Resource Center; 2006. [Google Scholar]
- 22.Sohn MW, Arnold N, Maynard C, et al. Accuracy and completeness of mortality data in the Department of Veterans Affairs. Popul Health Metr. 2006;4:2. doi: 10.1186/1478-7954-4-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Healthcare Cost and Utilization Project (HCUP) Clinical Classification Software (CCS) Agency for Healthcare Research and Quality; Rockville, MD: 2012. [Google Scholar]
- 24.Frayne SM, Halanych JH, Miller DR, et al. Disparities in diabetes care: impact of mental illness. Arch Intern Med. 2005;165(22):2631–2638. doi: 10.1001/archinte.165.22.2631. [DOI] [PubMed] [Google Scholar]
- 25.Cohen J. The t test for means. In: Cohen J, editor. Statistial Power Analysis for the Behavioral Sciences. Toronto, Canada: Academic Press Inc; 1977. [Google Scholar]
- 26.Saksena S, Slee A, Waldo AL, et al. Cardiovascular outcomes in the AFFIRM Trial (Atrial Fibrillation Follow-Up Investigation of Rhythm Management). An assessment of individual antiarrhythmic drug therapies compared with rate control with propensity score-matched analyses. J Am Coll Cardiol. 2011;58(19):1975–85. doi: 10.1016/j.jacc.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Singh BN, Singh SN, Reda DJ, et al. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med. 2005;352(18):1861–72. doi: 10.1056/NEJMoa041705. [DOI] [PubMed] [Google Scholar]
- 28.Pollak PT, Sharma AD, Carruthers SG. Creatinine elevation in patients receiving amiodarone correlates with serum amiodarone concentration. Br J Clin Pharmacol. 1993;36(2):125–7. doi: 10.1111/j.1365-2125.1993.tb04207.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Torp-Pedersen C, Metra M, Spark P, et al. The safety of amiodarone in patients with heart failure. J Card Fail. 2007;13(5):340–5. doi: 10.1016/j.cardfail.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 30.Rao SR, Schoenfeld DA. Survival methods. Circulation. 2007;115(1):109–13. doi: 10.1161/CIRCULATIONAHA.106.614859. [DOI] [PubMed] [Google Scholar]
- 31.Slain D, Kincaid SE, Dunsworth TS. Discrepancies between home medications listed at hospital admission and reported medical conditions. Am J Geriatr Pharmacother. 2008;6(3):161–6. doi: 10.1016/j.amjopharm.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 32.Cornish PL, Knowles SR, Marchesano R, et al. Unintended medication discrepancies at the time of hospital admission. Arch Intern Med. 2005;165(4):424–9. doi: 10.1001/archinte.165.4.424. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.