Abstract
It is unclear whether a concomitant kidney transplant grants survival benefit to liver transplant (LT) candidates with renal dysfunction (RD). We retrospectively studied LT candidates without RD (n = 714) and LT candidates with RD who underwent either liver transplant alone (RD-LTA; n = 103) or simultaneous liver–kidney transplant (RD-SLKT; n = 68). RD was defined as renal replacement therapy (RRT) requirement or modification of diet in renal disease (MDRD)-glomerular filtration rate (GFR) <25 mL/min/1.73 m2. RD-LTAs had worse one-yr post-transplant survival compared to RD-SLKTs (79.6% vs. 91.2%, p = 0.05). However, RD-LTA recipients more often had hepatitis C (60.2% vs. 41.2%, p = 0.004) and more severe liver disease (MELD 37.9 ± 8.1 vs. 32.7 ± 9.1, p = 0.0001). Twenty RD-LTA recipients died in the first post-transplant year. Evaluation of the cause and timing of death relative to native renal recovery revealed that only four RD-LTA recipients might have derived survival benefit from RD-SLKT. Overall, 87% of RD-LTA patients recovered renal function within one month of transplant. One yr after RD-LTA or RD-SLKT, serum creatinine (1.5 ± 1.2 mg/dL vs. 1.4 ± 0.5 mg/dL, p = 0.63) and prevalence of stage 4 or 5 chronic kidney disease (CKD; 5.9% vs. 6.8%, p = 0.11) were comparable. Our series provides little evidence that RD-SLKT would have yielded substantial short-term survival benefit to RD-LTA recipients.
Keywords: kidney, liver, outcomes, renal dysfunction, transplantation
Renal dysfunction (RD) is an important cause of morbidity and mortality following liver transplantation (LT) (1–5). Pre-transplant RD is a well-recognized predictor of the quality of post-transplant renal function as well as short- and long-term patient survival (2, 3, 6, 7). LT candidates develop RD for many different reasons. In addition to hepatorenal syndrome (HRS) (8–11), acute kidney injury (AKI) may result from bacterial infection/ sepsis or acute hemorrhage/hypovolemia. LT candidates may also harbor chronic RD from etiologies related or unrelated to their underlying liver disease. Finally, RD may also result from a combination of acute and chronic etiologies (8, 10, 12, 13). The frequently multifactorial nature of pre-transplant RD, in combination with the unpredictable intra-operative and post-transplant courses, has rendered accurate prediction of native renal recovery after LT difficult.
Since the incorporation of serum creatinine into liver allocation with the implementation of the model for end-stage liver disease (MELD) priority system in 2002, the prevalence of RD at LT has increased dramatically (14). Indeed, the annual number of simultaneous liver–kidney transplants (SLKTs) has nearly tripled (15). There has been vigorous debate as to the most appropriate circumstances that justify the diversion of a kidney away from end-stage renal disease (ESRD) patients. For ESRD patients, kidney transplantation, compared to ongoing dialysis, is known to bestow a 68% reduction in long-term mortality (16, 17). Moreover, the number of ESRD patients is ever increasing and their waits are ever lengthening. The higher rate of early post-transplant mortality for SLKT compared to ESRD recipients (15) exacerbates the sense that kidneys are unnecessarily lost. Finally, the debate has been further fueled by reports documenting “three functioning kidneys” after SLKT from several groups (18–20), including ours (21).
Several consensus conferences have been convened to standardize the evaluation algorithm and to define appropriate listing criteria for SLKT candidates, focusing primarily on glomerular filtration rate (GFR), duration of AKI/HRS, duration of renal replacement therapy (RRT) and, when available, renal biopsy results (22–24). Although some centers have reported that pre-transplant RRT or RD duration correlate with post-transplant renal function (3, 6, 7), these factors are clearly not specific determinants of permanent post-transplant chronic kidney disease (CKD) or ESRD. Indeed, determining if and when post-transplant native renal function recovers in SLKT recipients has been inconsistent, requiring nuclear medicine renal perfusion scans (18, 21, 23), which have typically not been done at standardized times after transplantation.
Arguably, the most important benefit of SLKT compared to liver transplant alone (LTA) may be to improve short-term (one yr) patient survival (23, 25, 26). In the absence of randomized trials of SLKT vs. LTA, there is grave concern that strong selection bias likely invalidates results of retrospective analyses. While there may be a subpopulation of LT recipients whose immediate post-operative survival depends on concomitant kidney transplantation, this subset has yet to be defined. Identification of such a subpopulation would provide the strongest justification for dual organ allocation, as survivors have the opportunity to pursue kidney after LT for long-term survival benefit.
We have therefore performed an extensive retrospective cohort analysis of LTAs and SLKTs performed at a single, high-volume center over a 10-yr period. Unlike previous studies, our focus was to dissect the cohort of RD-LTA recipients to delineate, in detail, the time course and quality of native renal recovery juxtaposed with causes of death within one yr of transplantation. Using these data, we aimed to determine whether LT candidates with RD would derive survival benefit from the addition of a kidney transplant.
Methods
Study cohort
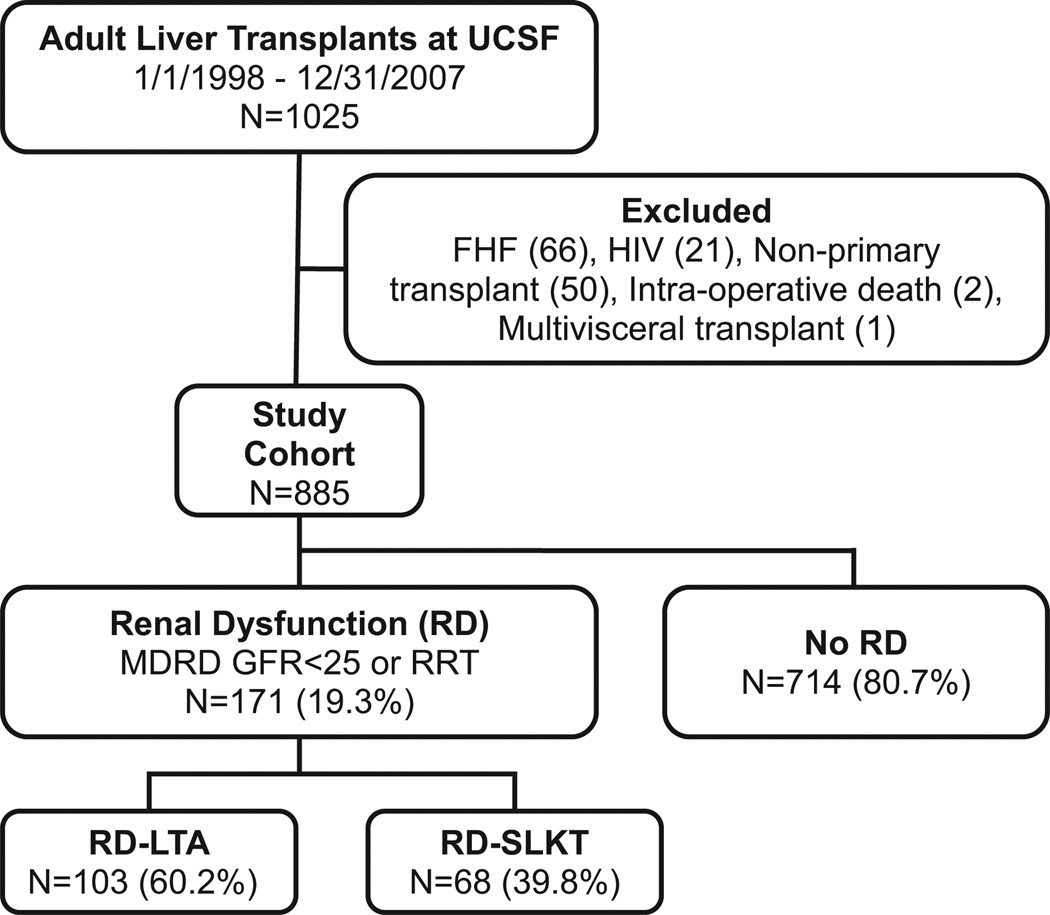
Records of all adults (≥18 yr) undergoing LT between January 1, 1998 and December 31, 2007, at the University of California San Francisco were retrospectively reviewed (n = 1025). Exclusion of 140 recipients resulted in a final study cohort of 817 LTA and 68 SLKT recipients (Fig. 1). Recipient and donor information was obtained from medical records and UNOS. Serum creatinine (Cr) was recorded longitudinally both prior to transplantation to determine the onset of RD and after transplantation (at 3, 12, and 24 months post-transplantation) to assess renal function. Criteria for SLKT were LT recipients on dialysis >2 wk, recipients with modification of diet in renal disease (MDRD)-GFR <25 mL/min/1.73 m2 for at least one month, or recipients with evidence of CKD in the setting of proteinuria and other metabolic conditions such as diabetes mellitus or hypertension. During the era of our study, there were no broadly accepted nor any published guidelines for SLKT listing. These were local decisions made by the transplant nephrologist consulting with the LT service and were not governed by set rules or protocols. In general, patients with RD at the time of transplant received LTA if MDRD-GFR <25 mL/ min/1.73 m2 for <1 month in patients without clinical evidence of CKD. SLKT recipients had longer pre-transplant RD and more RRT than RD-LTA recipients. Kidney biopsies were not performed in our patients with end-stage liver disease (ESLD) due to the risk of causing hemorrhagic complications. LTs were generally performed using caval replacement and duct-to-duct biliary anastomosis with the exception of recipients with primary sclerosing cholangitis, which underwent roux-en-Y hepaticojejunostomy. Venovenous bypass was not used during surgery for either LTA or SLKT recipients. Kidney transplants were performed immediately following completion of the biliary anastomosis and were routinely implanted in the right iliac fossa with vascular anastomoses to the external iliac vessels. Ureteral anastomoses were performed using the Lich-Gregoir extravesical ureteroneocystostomy method without ureteral stents. The immunosuppression protocol comprised an intravenous bolus of methylprednisolone (500 mg) at the time of liver reperfusion. Maintenance immunosuppression for both LTA and SLKT consisted of low-dose prednisone (5 mg/d), mycophenolate mofetil (MMF) (1 g twice daily), and tacrolimus. The goal for tacrolimus serum levels was 5–8 ng/mL for the first 3–6 months. For patients with RD, lower tacrolimus levels were often used (4–6 ng/mL). Cause of death was determined for all recipients with RD who died during the first post-transplant year. This study was approved by the University of California Institutional Review Board and conformed to ethical guidelines of the 1975 Declaration of Helsinki.
Fig. 1.
Study design and transplant cohorts. Records from all LTs performed at the University of California San Francisco between 1/1/1998 and 12/31/2007 were retrospectively evaluated and classified based on the presence or absence of pre-transplant RD. For those with pre-transplant RD, recipients were divided into RD-SLKT or RD-LTA. SLKT, simultaneous liver–kidney transplant; RD, renal dysfunction; LT, liver transplant; LTA, liver transplant alone.
Definition of pre-transplant RD and RD duration
The MDRD formula calculated using six variables (age, gender, African American race, serum Cr, urea, and albumin) was used to estimate GFR. RD was defined as MDRD-GFR <25 mL/min/1.73 m2 or RRT requirement as logistic regression analysis demonstrated the highest one-yr post-transplant mortality at this threshold. For candidates requiring RRT during the week preceding transplantation, values of Cr were assigned as 4.0 mg/dL as used in the MELD (27) and in previous studies (28), and values of GFR were assigned as 15 mL/ min/1.73 m2. The duration of MDRD-GFR <25 mL/min/1.73 m2 inclusive of RRT was defined as the duration of RD.
Definition of post-transplant RRT
Post-transplant RRT included any requirement for RRT initiated during the first post-transplant week. The interval between the transplant date and last RRT treatment was defined as duration of post-transplant RRT.
Statistical analysis
Descriptive statistics were compared according to pre-transplant RD status for the entire cohort and according to transplant type (RD-LTA vs. RD-SLKT) for the RD subset. Discrete and continuous variables were compared using Fisher’s exact and Mann–Whitney test, respectively. One-yr patient survival was determined using Kaplan–Meier analysis and compared using the log-rank test. All analyses were performed using SAS, version 9.1 (SAS Institute, Cary, NC). A p-value <0.05 was considered significant.
Results
Prevalence of RD and frequency of SLKT prior to and after MELD allocation
Our study period encompasses a decade (January 1, 1998–December 31, 2007) during which MELD allocation was instituted on February 27, 2002 (Table 1). As expected, the percentage of recipients with RD at the time of transplant increased significantly (p = 0.002) after MELD allocation from 13.5% (40 of 296) to 22.2% (131 of 589). In parallel, the volume of SLKTs similarly increased; 19 (6.4%) were performed prior to MELD allocation, while 62 (10.6%) were performed after MELD allocation. Notably, however, the frequency with which candidates with RD underwent SLKT remained constant before and after the institution of MELD allocation (38.0% [19 of 50] vs. 38.8% [62 of 160]).
Table 1.
Prevalence of RD and frequency of simultaneous liver–kidney transplant (SLKT) during the study period: January 1, 1998–December 31, 2007
| 1998 | 1999 | 2000 | 2001a | Pre-MELD Totals | 2002a | 2003 | 2004 | 2005 | 2006 | 2007 | Post-MELD Totals | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Number of Liver txs | 62 | 68 | 65 | 104 | 299 | 70 | 90 | 93 | 125 | 116 | 92 | 586 |
| Number of LTA | 58 | 64 | 61 | 97 | 280 | 63 | 81 | 79 | 115 | 102 | 84 | 524 |
| Number of RD Liver txs | 9 | 18 | 12 | 11 | 50 | 15 | 18 | 27 | 29 | 39 | 32 | 160 |
| Number of RD-LTA | 5 | 14 | 8 | 4 | 31 | 8 | 9 | 13 | 19 | 25 | 24 | 98 |
| Number of RD-SLKT | 4 | 4 | 4 | 7 | 19 | 7 | 9 | 14 | 10 | 14 | 8 | 62 |
LTA, liver transplant alone; SLKT, simultaneous liver–kidney transplant; RD, renal dysfunction; MELD, model for end-stage liver disease.
2001 ends on 2/26/2002; 2002 begins on 2/27/2002.
Baseline characteristics of LT candidates with and without RD
Of 885 LTs that comprise the study cohort, 171 recipients (19.3%) entered transplantation with RD (Fig. 1; Table 2). Several donor (age, gender, race, and BMI) and recipient (age, gender, and BMI) demographic characteristics were similar for the RD and no-RD cohorts. However, RD candidates were more commonly African American (9.4% vs. 5.5%; p = 0.027) and less likely to have hepatocellular carcinoma (HCC) (7.0% vs. 27.7%; p < 0.0001).
Table 2.
Characteristics of transplants according to the presence or absence of RD at time of transplantation
| No RD (n = 714)a | RD (n = 171) | p-Valueb | |
|---|---|---|---|
| Donor factors | |||
| Age (yr) | 41.4 ± 16.2 | 40.6 ± 15.2 | 0.60 |
| Male | 355 (49.7%) | 99 (57.9%) | 0.73 |
| Black | 39 (5.5%) | 8 (4.7%) | 0.79 |
| BMI | 26.2 ± 5.2 | 26.4 ± 5.6 | 0.68 |
| Recipient factors | |||
| Age (yr) | 53.6 ± 9.4 | 54.9 ± 8.7 | 0.11 |
| Male | 489 (68.5%) | 109 (63.7%) | 0.32 |
| Black | 39 (5.5%) | 16 (9.4%) | 0.027 |
| BMI | 27.2 ± 5.3 | 27.0 ± 6.0 | 0.41 |
| HCV | 359 (50.3%) | 87 (50.9%) | 0.88 |
| HCC | 198 (27.7%) | 12 (7.0%) | <0.0001 |
| MELD | 18.5 ± 8.2 | 37.4 ± 8.5 | <0.0001 |
| Pre-tx total bilirubin (mg/dL) | 6.8 ± 9.0 | 19.1 ± 15.6 | <0.0001 |
| Pre-tx INR | 1.8 ± 0.9 | 2.6 ± 1.7 | <0.0001 |
| Pre-tx Cr (mg/dL) | 1.1 ± 0.4 | 3.2 ± 1.3 | <0.0001 |
| MDRD-GFR (mL/min/1.73 m2) | 74.9 ± 30.0 | 16.3 ± 3.9 | <0.0001 |
| Transplant factors | |||
| Cold ischemia time (h) | 9.7 ± 2.9 | 9.2 ± 2.3 | 0.40 |
| Warm ischemia time (min) | 45.2 ± 10.0 | 44.4 ± 9.1 | 0.57 |
| Post-transplant factors | |||
| RRT requirement | 38 (5.3%) | 86 (50.3%) | <0.0001 |
| RRT duration | 9.4 ± 14.6 | 23.1 ± 63.9 1.4 ± 0.9 | 0.0044 |
| 12-mo Cr | 1.1 ± 0.4 | <0.0001 | |
| 24-mo Cr | 1.2 ± 0.5 | 1.4 ± 0.8 | <0.0001 |
| One-yr mortality | 45 (6.3%) | 27 (15.8%) | 0.0001 |
| Days to death | 723 ± 504 | 392 ± 591 | 0.0010 |
RRT, renal replacement therapy; RD, renal dysfunction; MELD, model for end-stage liver disease; MDRD, modification of diet in renal disease; GFR, glomerular filtration rate; INR, international normalized ratio.
Values shown are n (%) or means ± standard deviation
Bold type indicated statistical significance.
With respect to metrics of renal function, recipients with pre-transplant RD, compared to those without, had higher pre-transplant serum Cr (3.2 ± 1.3 vs. 1.1 ± 0.4 mg/dL; p < 0.0001) and lower MDRD-GFR (16.3 ± 3.9 vs. 74.9 ± 30.0 mL/min/1.73 m2; p < 0.0001). With respect to metrics of liver disease, recipients with pretransplant RD, compared to those without, had higher total bilirubin (19.1 ±15.6 vs. 6.8 ± 9.0 mg/dL; p < 0.0001) and international normalized ratio (INR) (2.6 ±1.7 vs. 1.8 ± 0.9; p < 0.0001), resulting in dramatically different MELD scores (37.4 ± 8.5 vs. 18.5 ± 8.2; p < 0.0001).
After transplantation (either LTA or SLKT), recipients with pre-transplant RD were more likely to require RRT (50.3% vs. 5.3%; p < 0.0001) and for longer duration (23.1 ± 63.9 vs. 9.4 ± 14.6 d; p = 0.004) than recipients without pre-transplant RD (Table 2). Moreover, post-transplant renal function was inferior in recipients with pre-transplant RD, as demonstrated by higher serum Cr at 12 months (1.4 ± 0.9 vs. 1.1 ± 0.4 mg/dL p < 0.0001) and at 24 months (1.4 ± 0.8 vs. 1.2 ± 0.5 mg/dL; p < 0.0001) after transplantation. All-cause mortality one yr following transplantation was higher in recipients with pre-transplant RD compared to those without RD (15.8% vs. 6.3%, p = 0.0001).
Baseline characteristics of RD-SLKT and RD-LTA candidates
Of the 171 candidates who entered transplantation with RD, 103 underwent LTA (RD-LTA) and 68 underwent SLKT (RD-SLKT). RD-LTA and RD-SLKT recipients were similar with respect to all donor and recipient demographic factors (Table 3). A larger proportion of RD-LTA recipients compared to RD-SLKT recipients was transplanted for hepatitis C virus (HCV) infection (60.2% vs. 41.2%; p = 0.004). Prior to transplantation, RD-LTA candidates had significantly higher total bilirubin (23.1 ± 14.6 vs. 13.1 ± 15.2 mg/dL; p < 0.0001), higher INR (2.8 ± 1.9 vs. 2.1 ± 1.0; p = 0.0017) and higher MELD scores (37.9 ±8.1 vs. 32.7 ±9.1; p = 0.0001) than RD-SLKT recipients. RD severity, as measured by serum Cr (3.3 ±1.4 vs. 2.8 ± 0.8 mg/dL, p = 0.35) and MDRD-GFR (16.4 ± 4.1 vs. 16.1 ± 3.6 mL/ min/1.73 m2, p = 0.51), was comparable between RD-LTA and RD-SLKT recipients. However, RD-SLKT recipients had longer duration of pretransplant RD (306.0 ± 505.0 vs. 23.8 ± 38.5 d; p < 0.0001) and required pre-transplant RRT more frequently (86.8% vs. 63.1%; p = 0.0008) and for a longer duration (188 ± 359 vs. 11.1 ± 9.5 d; p < 0.0001) than RD-LTA recipients.
Table 3.
Characteristics of transplants with RD according to LTA vs. simultaneous liver–kidney transplant
| RD – SLKT (n = 68)a |
RD – LTA (n = 103) |
p-Valueb | |
|---|---|---|---|
| Donor factors | |||
| Age (yr) | 39.4 ± 17.4 | 41.3 ± 13.6 | 0.42 |
| Male | 35 (51.5%) | 64 (62.1%) | 0.16 |
| Black | 2 (2.9%) | 6 (5.8%) | 0.88 |
| BMI | 26.3 ± 5.7 | 26.5 ± 5.6 | 0.67 |
| Recipient factors | |||
| Age (yr) | 55.1 ± 7.6 | 54.7 ± 9.4 | 0.87 |
| Male | 42 (61.8%) | 67 (65.1%) | 0.75 |
| Black | 16 (23.5%) | 8 (7.8%) | 0.46 |
| BMI | 26.7 ± 5.9 | 27.3 ± 6.1 | 0.55 |
| HCV | 28 (41.2%) | 62 (60.2%) | 0.0040 |
| HCC | 5 (7.4%) | 7 (6.8%) | 1.00 |
| MELD (mean) | 32.7 ± 9.1 | 37.9 ± 8.1 | 0.0001 |
| MELD (median, range) | 31.7 (15.9–50.3) | 38.1 (15.4–62.3) | <0.0001 |
| Pre-tx total bilirubin (mg/dL) | 13.1 ± 15.2 | 23.1 ± 14.6 | <0.0001 |
| Pre-tx INR | 2.1 ± 1.0 | 2.8 ± 1.9 | 0.0017 |
| Pre-tx Cr (mg/dL) | 2.8 ± 0.8 | 3.3 ± 1.4 | 0.35 |
| MDRD-GFR (mL/min/1.73 m2) | 16.1 ± 3.6 | 16.4 ± 4.1 | 0.51 |
| Pre-tx | 306 ± 505 | 23.8 ± 38.5 | <0.0001 |
| RD duration (d) | |||
| Pre-tx RRT | 59 (86.8%) | 65 (63.1%) | 0.0008 |
| Pre-tx | 188 ± 359 | 11.1 ±9.5 | <0.0001 |
| RRT duration (d) | |||
| Transplant factors | |||
| Cold ischemia time (h) | 9.2 ± 2.3 | 9.3 ± 2.3 | 0.96 |
| Warm ischemia time (min) | 43.2 ± 7.2 | 45.3 ± 10.3 | 0.14 |
| Post-transplant | |||
| RRT | 25 (36.8%) | 61 (59.2%) | 0.0049 |
| RRT duration (d) | 6.9 ± 7.7 | 29.7 ± 74.9 | 0.035 |
| RRT >14 d | 2 (3.3%) | 18 (17.5%) | 0.0005 |
| 12-mo Cr (mg/dL) | 1.4 ± 0.5 | 1.5 ± 1.2 | 0.63 |
| 12-mo | 50.7 ± 6.6 | 45.9 ± 5.4 | 0.38 |
| MDRD-GFR (mL/min/1.73 m2) | |||
| 24-mo Cr (mg/dL) | 1.5 ± 1.0 | 1.3 ± 0.4 | 0.64 |
| One-yr mortality | 6 (8.8%) | 20 (19.4%) | 0.060 |
| Days to death | 589 ± 567 | 293 ± 589 | 0.020 |
RRT, renal replacement therapy; RD, renal dysfunction; MELD, model for end-stage liver disease; MDRD, modification of diet in renal disease; LTA, liver transplant alone; GFR, glomerular filtration rate; INR, international normalized ratio.
Values shown are n (%) or means ± standard deviation
Bold type indicated statistical significance.
Short- and long-term renal outcomes for RD-LTA and RD-SLKT recipients
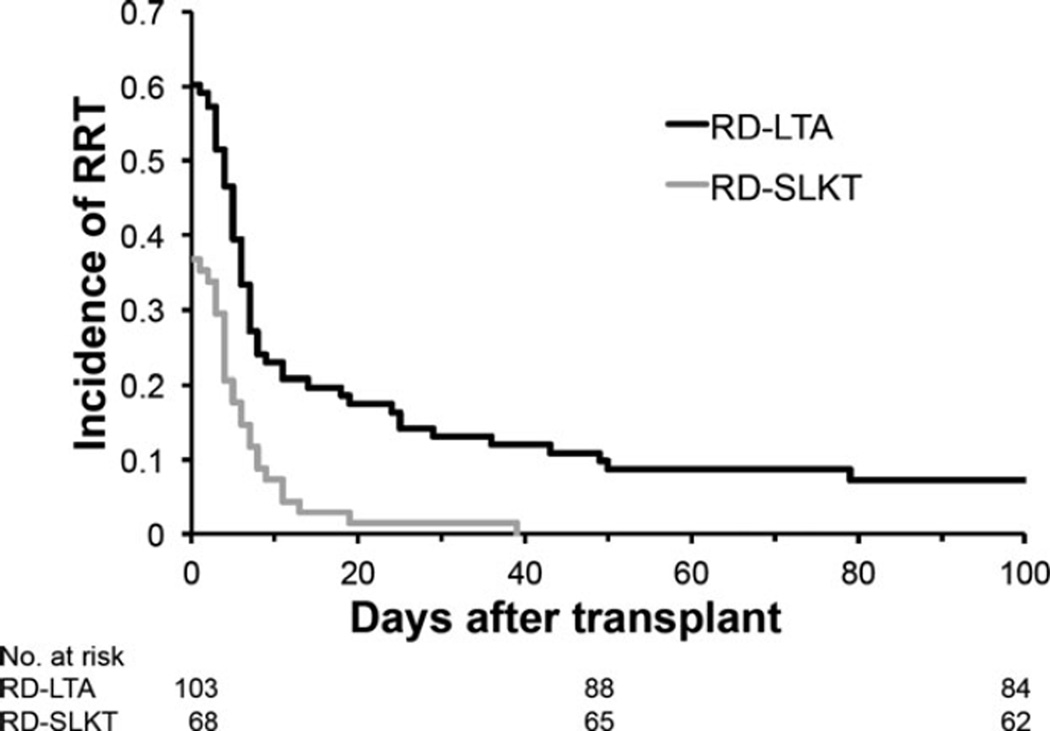
Immediately after transplantation, fewer RD-SLKT than RD-LTA recipients required post-transplant RRT (36.8% vs. 59.2%; p = 0.0049) and for a shorter duration (6.9 ± 7.7 vs. 29.7 ± 74.9 d, p = 0.035; Table 3). Of 103 RD-LTA recipients, 13 (12.6%) required RRT for >30 d and seven (6.8%) required RRT for >100 d post-transplantation. Only 1/68 SLKT recipients (1.5%) required RRT >3 wk post-transplant (Fig. 2). There were, however, no differences in serum Cr between RD-LTA and RD-SLKT survivors at either one yr (RD-LTA 1.5 ± 1.2 mg/dL vs. RD-SLKT 1.4 ± 0.5 mg/dL for SLKT recipients; p = 0.63) or two yr (RD-LTA 1.3 ± 0.4 mg/dL vs. RD-SLKT 1.5 ± 1.0 mg/dL; p = 0.64) after transplantation. At one yr post-transplantation, the prevalence of stage 4 or 5 CKD (MDRD-GFR <30 mL/min/1.73 m2 or RRT requirement; National Kidney Foundation Practice Guidelines [29]), was also comparable (6.8% RD-SLKT vs. 5.9% RD-LTA; p = 0.11).
Fig. 2.
Kaplan–Meier graph showing renal replacement therapy (RRT) requirement and duration following LT in patients with pre-transplant RD. Compared to RD-LTA recipients, RD-SLKT recipients recovered renal function more rapidly following LT (p < 0.0001). SLKT, simultaneous liver–kidney transplant; RD, renal dysfunction; LT, liver transplant; LTA, liver transplant alone.
RD duration prior to RD-LTA does not predict renal recovery following transplantation
Duration of pre-transplant RRT has been suggested as selection criterion for SLKT, with previous recommendations of RRT for ≥8 wk (23), ≥6 wk (22), or ≥4 wk (24) prior to transplantation. However, as the initiation of RRT in the setting of advanced liver disease is not standardized, we questioned whether the duration and severity of RD (with or without RRT) might be a better predictor of renal recovery following transplantation (3). The OPTN Kidney Transplantation Committee and the Liver and Intestinal Organ Transplantation Committee have proposed that sustained RD–estimated GFR ≤25 mL/min/1.73 m2 by 6-variable MDRD or direct measurement by iothalamate clearance, with or without RRT for ≥6 wk for SLKT listing (OPTN Proposed Policy 3.5.10) (30). Currently the recommendation for SLKT in the setting of hyperoxaluria is for GFR ≤25 for six wk or more for SLKT listing, Policy 3.6.4.5.5. Therefore, we separately analyzed outcomes for RD-LTA recipients who “violated” the six-wk rule to assess the rule’s discriminatory power.
Twelve candidates with RD for ≥6 wk underwent RD-LTA, rather than expected RD-SLKT. These 12 candidates had a median (range) pretransplant Cr of 4.0 (2.3–7.3) mg/dL, with corresponding MDRD-GFR of 13.0 (6.3–22.5) mL/ min/1.73 m2. Median (range) of RD duration for these 12 candidates was 58.5 (43–290) d. Five of the 12 candidates received RRT for a median (range) of 17 (3–45) d prior to transplant. After transplant, six of the 12 recipients with ≥6 wk of pre-transplant RD did not require any post-transplant RRT, four required 5, 5, 7, and 8 d of post-transplant RRT (5, 5, 7, and 8 d) while two required 50 and 403 d of post-transplant RRT. Three patients died within one yr of transplantation from graft-versus-host disease, metastatic cholangiocarcinoma, and metastatic hepatocellular carcinoma. One yr after transplantation, median (range) serum Cr and GFR were 1.4 (0.8–2.4) mg/ dL and 34.7 (20.1–61.0) mL/min/1.73 m2 for eight recipients (three deceased; one remaining on RRT).
One-yr patient survival and causes of death for RD-LTA recipients
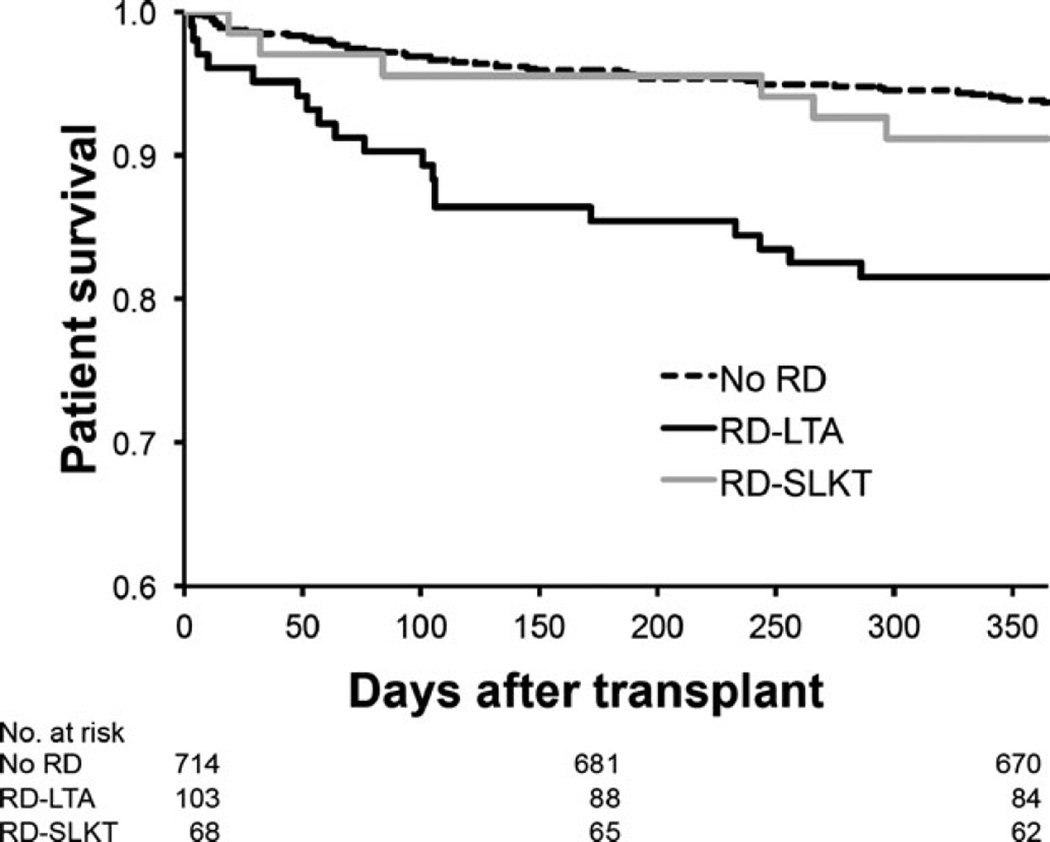
RD-SLKT recipients exhibited higher one-yr post-transplant survival rates than RD-LTA recipients (91.2% vs. 80.5%, p = 0.05) (Fig. 3). Notably, patient survival for RD-SLKT recipients was comparable to that of LTA recipients entering transplantation without RD (93.7% vs. 91.2%, p = 0.43).
Fig. 3.
Kaplan–Meier graph of one-yr post-transplant survival for recipients without RD (No RD) or with RD that received LTA (RD-LTA) or SLKT (RD-SLKT). One-yr survival was comparable for No RD vs. RD-SLKT (93.7% vs. 91.2%; p = 0.43) but differed significantly between No RD vs. RD-LTA (93.7% vs. 80.5%; p < 0.0001) and RD-LTA and RD-SLKT (91.2% vs. 80.5%; p = 0.05). SLKT, simultaneous liver–kidney transplant; RD, renal dysfunction; LTA, liver transplant alone.
Cause and timing of death along with duration of post-transplant RRT for the 20 of 103 RD-LTA recipients who died within one yr of transplant were evaluated to consider whether the absence of concomitant kidney transplantation might have reasonably contributed to death (Table 4). The 20 deaths occurred at a median (range) of 88.5 (3–286) d after transplant. Seven RD-LTA recipients died from severe liver dysfunction / liver failure: two from primary non-function (death on post-transplant days 4 and 10), one from inferior vena cava and hepatic artery thrombosis (death on post-transplant day 3), one from hepatic infarction (death on post-transplant day 243), and three from recurrent HCV or chronic rejection (death on post-transplant days 105, 172, and 233). The remaining 13 RD-LTA recipients died of cardiovascular causes (n = 3; death on post-transplant days 6, 29 and 184), neurologic catastrophe (n = 1; death on post-transplant day 48), malignancy (n = 3; death on post-transplant days 76, 101 and 286), and multisystem organ failure (MSOF) (n = 6; death on post-transplant days 52, 57, 64, 106, 106, and 256).
Table 4.
Deaths within one yr of RD-LTA
| Pt number | Diagnosis | Pre-Tx Cr (mg/dL) |
Pre-tx HD duration (d) |
Cause of death |
Post-tx HD duration (d) |
Days to death |
Interval between HD cessation and death (d) |
|---|---|---|---|---|---|---|---|
| 1 | Budd Chiari | HD | 8 | Livera | 3 | 3 | 0 |
| 2 | HBV | HD | 7 | Liver | 4 | 4 | 0 |
| 3 | CC | HD | 28 | Cardiovascular | 6 | 6 | 0 |
| 4 | HCV | HD | 23 | Liver | 10 | 10 | 0 |
| 5 | HCV | HD | 7 | MSOF | 52 | 52 | 0 |
| 6 | HCV | HD | 6 | MSOF | 57 | 57 | 0 |
| 7 | HCV | 5.2 | 0 | MSOF | 106 | 106 | 0 |
| 8 | HCV/ALD | HD | 2 | MSOF | 256 | 256 | 0 |
| 9 | HBV | HD | 8 | Cardiovascular | 4 | 29 | 25 |
| 10 | HCV | HD | 15 | Neurologic | 14 | 48 | 34 |
| 11 | HCV | 5.2 | 0 | MSOF | 0 | 64 | 64 |
| 12 | HCV | 2.3 | 0 | Malignancy | 0 | 76 | 76 |
| 13 | HCV | HD | 7 | Liver | 8 | 105 | 97 |
| 14 | ALD/AIH | 3.5 | 0 | Malignancy | 0 | 101 | 101 |
| 15 | PSC | 2.5 | 0 | MSOF | 1 | 106 | 105 |
| 16 | HCV | 1.6 | 0 | Liver | 0 | 172 | 172 |
| 17 | ALD/HCV | 3.4 | 0 | Cardiovascular | 6 | 184 | 178 |
| 18 | PBC | 2.8 | 0 | Liver | 0 | 233 | 233 |
| 19 | HCV | HD | 4 | Liver | 0 | 243 | 243 |
| 20 | HCV | HD | 17 | Malignancy | 8 | 286 | 278 |
HD, hemodialysis; CC, cryptogenic cirrhosis; HBV, hepatitis B cirrhosis; HCV, hepatitis C cirrhosis; ALD, alcoholic liver disease; AID, autoimmune liver disease; PBC, primary biliary cirrhosis; PSC, primary sclerosing cholangitis; MSOF, multisystem organ failure; LTA, liver transplant alone; RD, renal dysfunction; d, days.
Liver dysfunction or failure.
Post-transplant RRT duration relative to time of death was determined (Table 4). Among the 20 RD-LTA recipients who died, 12 recipients died after and eight recipients died before recovery of native renal function. Among the 12 recipients who resolved RD prior to death, six never required post-transplant RRT and died 64–253 d after transplantation (patients 11, 12, 14, 16, 18, and 19; Tables 4 and 5). These six RD-LTA recipients had nadir serum Cr <1.5 mg/dL (Table 5), indicative of full native renal recovery and excellent renal function. It is unlikely that concomitant kidney transplantation would have altered their outcomes. For the remaining six who died of cardiovascular (n = 2), hepatic, neurologic, malignant, and MSOF (n = 1 each) etiologies, RRT was required for a median (range) of 7 (1–14) d and discontinued 101 (25–278) d prior to death (patients 9, 10, 13, 15, 17, and 20; Tables 4 and 5). Similarly, five of these six RD-LTA recipients had nadir serum Cr <1.5 mg/dL, while the remaining recipient had a nadir of 1.7 mg/dL. The causes of death, the modest duration of RRT, the substantial interval between RRT cessation and death, the high quality of native renal recovery, and renal function together suggest that concomitant kidney transplantation would not have salvaged these recipients. Of the 68 patients that underwent SLKT, 27 (39.7%) required post-transplant RRT for an average of 8.0 ± 7.0 d, median of 5 d, range (1–39 d). During the follow-up period included in this study, no SLKT patients returned to hemodialysis (HD) after being off HD.
Table 5.
Post-transplant renal function for RD-LTA recipients who died within one yr following transplantation
| Serum Cr (mg/dL) |
||||||||
|---|---|---|---|---|---|---|---|---|
| Pt number | 1 wk | 2 wk | 3 wk | 4 wk | 2 months | 3 months | 6 months | Death/ Post-tx day |
| 9 | 2.1 | 1.7 | 2.1 | 2.3 | Died | Died | Died | 2.3/29 |
| 10 | HD | HD | 0.6 | 0.5 | Died | Died | Died | 0.4/48 |
| 11 | 1.9 | 1.5 | 1.3 | 1.5 | 1.1 | Died | Died | HD/64 |
| 12 | 1.6 | 1.6 | 2.0 | 2.2 | 1.3 | Died | Died | 1.5/76 |
| 13 | 1.6 | 1.2 | 0.8 | 0.9 | 0.8 | 1.1 | Died | 0.7/105 |
| 14 | HD | 1.1 | 1.0 | 1.0 | 1.0 | 2.5 | Died | 1.8/101 |
| 15 | 1.4 | 1.2 | 1.2 | 1.1 | 0.9 | 1.4 | Died | 3.2/106 |
| 16 | 1.3 | 1.6 | 0.9 | 1.1 | 0.6 | 0.7 | Died | HD/172 |
| 17 | HD | 1.7 | 2.1 | 1.6 | HD | 3.7 | 1.5 | HD/184 |
| 18 | 2.3 | 1.3 | 1.0 | 1.1 | 1.4 | 1.6 | 1.9 | 2.0/233 |
| 19 | 2.3 | 1.3 | 1.6 | 1.4 | 2.4 | 2.1 | 2.2 | 1.9/243 |
| 20 | HD | 1.5 | 0.8 | 1.5 | 0.8 | 1.1 | 1.3 | 3.5/286 |
HD, hemodialysis; LTA, liver transplant alone; RD, renal dysfunction.
Among the eight RD-LTA recipients who remained on RRT until death, four died early, within 10 d of transplantation, of acute liver (n = 3) or cardiac failure (n = 1). Transplantation of a kidney would unlikely have altered the outcome for these recipients from death to survival as full RRT would have been available during the perioperative period. Had these four patients not suffered early post-operative death, they may have hypothetically obtained survival benefit from a renal graft, but this is unable to be determined. The remaining four RD-LTA recipients died of MSOF 52–256 d after transplantation while still requiring RRT. Ongoing RD may well have contributed to MSOF and ultimately death for these recipients such that concomitant kidney transplantation may conceivably offered survival benefit.
Discussion
Given the prevalence and diversity of RD in LT candidates and the inherent constraints to maximize survival benefit for kidney transplant alone, LTA, and SLKT recipients, there remains an urgent need to refine and validate indications for SLKT. Although the UNOS/OPTN registry data are expansive, they lack the detail to delineate the etiology, severity and duration of pre-transplant RD, the duration of post-transplant RRT, the quality of post-transplant renal function, and, finally and importantly, the cause of death after transplantation. We have therefore performed an extensive retrospective cohort analysis focusing on RD-LTAs performed at a single, high-volume center over a 10-yr period using granular data to optimally assess the potential injury imposed by LTA vs. the potential benefit endowed by SLKT.
In our study, 19.3% of adult LTs had documented pre-transplant RD, as defined by MDRD-GFR <25 mL/min/1.73 m2 or RRT requirement. This prevalence is generally comparable to previous reports based on national OPTN or SRTR data of 15.5% (RD defined by Cr >2.0 mg/dL or RRT requirement [1]) to 29.9% (RD defined by Cr >1.5 mg/dL or RRT requirement [31]). As expected, the prevalence of RD increased after MELD allocation, from 13.5 to 22.2%. Candidates with RD underwent either RD-LTA or RD-SLKT. Our data clearly show that RD-LTA and RD-SLKT recipients differ in fundamental respects, with the former characterized by high liver disease severity and the latter by high renal disease severity. These fundamental differences epitomize the strong selection bias that imperils comparisons of survival outcomes after RD-LTA and RD-SLKT. We contend that these differences along with peri- and post-transplant events, rather than the choice of LTA vs. SLKT, likely account for the survival differential between RD-LTA and RD-SLKT recipients that we have observed at the single center level and that others have observed using national registry data (25, 26). As a result, we do not believe that it is valid to directly compare outcomes or to conclude that LTA recipients would have enjoyed superior results had they undergone SLKT (25, 26).
Instead, to assess whether simultaneous transplantation of a kidney might have provided survival benefit to RD-LTA recipients, we chose to determine the cause of all deaths within one yr of transplant along with the timing of death relative to the requirement for RRT. We contend that a simultaneous kidney transplant would not have altered the survival outcome if the post-transplant RRT was never required (6 of 20 RD-LTA recipients), if RRT was of short duration with recovery of excellent native renal function long before death (6 of 20 RD-LTA recipients), or if death was early after transplantation secondary to devastating complications of primary non-function (PNF) or cardiovascular complications (4 of 20 RD-LTA recipients). However, for four RD-LTA recipients who died of MSOF without native renal recovery, it is possible that SLKT would have improved renal function and thereby bestowed survival benefit. It is critical to remember, however, that this is a retrospective assessment. It would likely be impossible to identify these four (of 103) RD-LTA recipients prospectively.
Our examination of both survival and renal outcomes for the RD-LTA and RD-SLKT cohorts suggests that our single center decision-making process for selecting transplant type has been imperfect. There have been errors in both directions: A modest number of RD-LTA recipients might have benefited from RD-SLKT as discussed above while a modest number of RD-SLKTs were likely unnecessary as we have previously reported (21). In this study, RD-SLKT recipients with a pre-operative diagnosis of type 1 HRS (baseline GFR ≥30 mL/min/1.73 m2) underwent technetium-99 m-mercaptoacetyltriglycine nuclear scans to measure the native kidney contribution to the overall renal function. Only four of the 23 subjects (17.4%) demonstrated native renal function that consisted of a contribution ≥50% of total renal function (21). We would argue that there are and that there will always be insufficient data and knowledge for flawless decision making (32). Fundamentally, the liver and kidney transplantation communities will need to determine the criteria for AKI in LT candidates that merit diversion of a kidney away from ESRD candidates who derive clear survival benefit from kidney transplantation. The likelihood and magnitude of enhanced survival benefit for chronic liver disease candidates will need to be weighed against the demerits of producing RD-SLKT recipients with three functioning kidneys. Moreover, consideration could be given to providing a safety net of preferred access to deceased donor kidneys for those who meet criteria for RD-SLKT but undergo RD-LTA and fail to recover adequate native renal function (24). A provision for, in essence, “rescue allocation” of kidney after LT, may facilitate physicians and patients to choose RD-LTA in cases of ambiguity with respect to the reversibility of native renal function.
In considering the weaknesses of our data and their analyses, clearly, the primary issue is that it is a single center experience. As such, we are limited by modest numbers and a center-specific culture of transplantation practice that might limit generalizability. We however would extend that the granularity of available data is essential to answering the questions that we posed. Moreover, our center may be ideally suited to study LT candidates with pre-transplant RD for several considerations. First, we are located in a UNOS region of relative organ scarcity, resulting in high MELD scores at transplant (33). Second, our center has a representative prevalence of RD among LT candidates and a representative proportion of candidates undergoing SLKT (14, 15). Third, our center has a high volume of not only liver but also kidney transplantation. Moreover, our kidney transplant candidates experience protracted wait times upwards of eight yr for a deceased donor kidney. The nephrologists who consult on liver waitlisted candidates with RD and determine the appropriateness of RD-LTA vs. RD-SLKT are none other than our transplant nephrologists. Their dual roles almost certainly color their perspective, demanding a balanced consideration of the potential benefit of SLKT and its impact on ESRD candidates.
Thus, in summary, we have shown that pretransplant RD is clearly associated with inferior post-transplant survival and that this decrement is borne by RD-LTA recipients and not by RD-SLKT recipients. We contend that the superior one-yr outcomes of RD-SLKT relative to RD-LTA likely reflect inherent pre-transplant differences between these two cohorts that drove the choice of transplantation. Moreover, we contend that the absence of a kidney allograft does not account for the majority of deaths observed among RD-LTA recipients. Beyond survival considerations, the quality of renal function for RD-LTA and RD-SLKT recipients was comparable with a low prevalence of severe CKD. We believe that our data, along with those of others recently reported (18, 19, 34), can further inform discussions between the liver and kidney transplantation communities as to the optimal allocation and utilization of precious deceased donor kidneys to maximize survival benefit for both ESLD and ESRD patients.
Acknowledgments
This work was supported by grants from the American Society of Transplantation (T.V.B.) and the American Association for the Study of Liver Disease (T.V.B.).
Footnotes
Conflict of interest: The authors of this manuscript have no conflicts of interest to disclose as described by Clinical Transplantation.
Authors’ contributions
T.V.B.: Concept/design, data analysis/interpretation, drafting the article, and data collection; K.E.L.: Data analysis/interpretation and drafting the article; P.A.V.: Data analysis/interpretation and drafting the article; A.B.: Statistics; M.A.: data analysis; S.F.: Concept/design, data analysis/interpretation, and drafting the article.
References
- 1.Gonwa TA, McBride MA, Anderson K, Mai ML, Wadei H, Ahsan N. Continued influence of preoperative renal function on outcome of orthotopic liver transplant (OLTX) in the US: where will MELD lead us? Am J Transplant. 2006;6:2651. doi: 10.1111/j.1600-6143.2006.01526.x. [DOI] [PubMed] [Google Scholar]
- 2.Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic liver transplantation. Hepatology. 2002;35:1179. doi: 10.1053/jhep.2002.33160. [DOI] [PubMed] [Google Scholar]
- 3.Campbell MS, Kotlyar DS, Brensinger CM, et al. Renal function after orthotopic liver transplantation is predicted by duration of pretransplantation creatinine elevation. Liver Transpl. 2005;11:1048. doi: 10.1002/lt.20445. [DOI] [PubMed] [Google Scholar]
- 4.Pawarode A, Fine DM, Thuluvath PJ. Independent risk factors and natural history of renal dysfunction in liver transplant recipients. Liver Transpl. 2003;9:741. doi: 10.1053/jlts.2003.50113. [DOI] [PubMed] [Google Scholar]
- 5.Sanchez EQ, Gonwa TA, Levy MF, et al. Preoperative and perioperative predictors of the need for renal replacement therapy after orthotopic liver transplantation. Transplantation. 2004;78:1048. doi: 10.1097/01.tp.0000137176.95730.5b. [DOI] [PubMed] [Google Scholar]
- 6.Brown RS, Jr, Lombardero M, Lake JR. Outcome of patients with renal insufficiency undergoing liver or liver-kidney transplantation. Transplantation. 1996;62:1788. doi: 10.1097/00007890-199612270-00018. [DOI] [PubMed] [Google Scholar]
- 7.Northup PG, Argo CK, Bakhru MR, Schmitt TM, Berg CL, Rosner MH. Pretransplant predictors of recovery of renal function after liver transplantation. Liver Transpl. 2010;16:440. doi: 10.1002/lt.22008. [DOI] [PubMed] [Google Scholar]
- 8.Moreau R. Hepatorenal syndrome in patients with cirrhosis. J Gastroenterol Hepatol. 2002;17:739. doi: 10.1046/j.1440-1746.2002.02778.x. [DOI] [PubMed] [Google Scholar]
- 9.Moreau R, Lebrec D. Acute renal failure in patients with cirrhosis: perspectives in the age of MELD. Hepatology. 2003;37:233. doi: 10.1053/jhep.2003.50084. [DOI] [PubMed] [Google Scholar]
- 10.Davis CL, Gonwa TA, Wilkinson AH. Pathophysiology of renal disease associated with liver disorders: implications for liver transplantation. Part I. Liver Transpl. 2002;8:91. doi: 10.1053/jlts.2002.31516. [DOI] [PubMed] [Google Scholar]
- 11.Francoz C, Glotz D, Moreau R, Durand F. The evaluation of renal function and disease in patients with cirrhosis. J Hepatol. 2010;52:605. doi: 10.1016/j.jhep.2009.11.025. [DOI] [PubMed] [Google Scholar]
- 12.Carvalho GC, Regis Cde A, Kalil JR, et al. Causes of renal failure in patients with decompensated cirrhosis and its impact in hospital mortality. Ann Hepatol. 2012;11:90. [PubMed] [Google Scholar]
- 13.Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48:2064. doi: 10.1002/hep.22605. [DOI] [PubMed] [Google Scholar]
- 14.Sharma P, Schaubel DE, Guidinger MK, Goodrich NP, Ojo AO, Merion RM. Impact of MELD-based allocation on end-stage renal disease after liver transplantation. Am J Transplant. 2011;11:2372. doi: 10.1111/j.1600-6143.2011.03703.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Locke JE, Warren DS, Singer AL, et al. Declining outcomes in simultaneous liver-kidney transplantation in the MELD era: ineffective usage of renal allografts. Transplantation. 2008;85:935. doi: 10.1097/TP.0b013e318168476d. [DOI] [PubMed] [Google Scholar]
- 16.Merion RM, Ashby VB, Wolfe RA, et al. Deceased-donor characteristics and the survival benefit of kidney transplantation. JAMA. 2005;294:2726. doi: 10.1001/jama.294.21.2726. [DOI] [PubMed] [Google Scholar]
- 17.Wolfe RA, Ashby VB, Milford EL, et al. Comparison of mortality in all patients on dialysis, patients on dialysis awaiting transplantation, and recipients of a first cadaveric transplant. N Engl J Med. 1999;341:1725. doi: 10.1056/NEJM199912023412303. [DOI] [PubMed] [Google Scholar]
- 18.Francis JM, Palmer MR, Donohoe K, et al. Evaluation of native kidney recovery after simultaneous liver-kidney transplantation. Transplantation. 2012;93:530. doi: 10.1097/TP.0b013e3182449161. [DOI] [PubMed] [Google Scholar]
- 19.Levitsky J, Baker T, Ahya SN, et al. Outcomes and native renal recovery following simultaneous liver-kidney transplantation. Am J Transplant. 2012;12:2949. doi: 10.1111/j.1600-6143.2012.04182.x. [DOI] [PubMed] [Google Scholar]
- 20.Palmer MR, Donohoe KJ, Francis JM, Mandelbrot DA. Evaluation of relative renal function for patients who had undergone simultaneous liver-kidney transplants using Tc-99 m-MAG3 scintigraphy with attenuation correction from anatomical images and SPECT/CT. Nucl Med Commun. 2011;32:738. doi: 10.1097/MNM.0b013e328347e958. [DOI] [PubMed] [Google Scholar]
- 21.Vagefi PA, Qian JJ, Carlson DM, et al. Native renal function after combined liver-kidney transplant for type 1 hepatorenal syndrome: initial report on the use of postoperative Technetium-99 m-mercaptoacetyltriglycine scans. Transpl Int. 2013;26:471. doi: 10.1111/tri.12066. [DOI] [PubMed] [Google Scholar]
- 22.Davis CL, Feng S, Sung R, et al. Simultaneous liver-kidney transplantation: evaluation to decision making. Am J Transplant. 2007;7:1702. doi: 10.1111/j.1600-6143.2007.01856.x. [DOI] [PubMed] [Google Scholar]
- 23.Eason JD, Gonwa TA, Davis CL, Sung RS, Gerber D, Bloom RD. Proceedings of consensus conference on simultaneous liver kidney transplantation (SLK) Am J Transplant. 2008;8:2243. doi: 10.1111/j.1600-6143.2008.02416.x. [DOI] [PubMed] [Google Scholar]
- 24.Nadim MK, Sung RS, Davis CL, et al. Simultaneous liver-kidney transplantation summit: current state and future directions. Am J Transplant. 2012;12:2901. doi: 10.1111/j.1600-6143.2012.04190.x. [DOI] [PubMed] [Google Scholar]
- 25.Fong TL, Khemichian S, Shah T, Hutchinson IV, Cho YW. Combined liver-kidney transplantation is preferable to liver transplant alone for cirrhotic patients with renal failure. Transplantation. 2012;94:411. doi: 10.1097/TP.0b013e3182590d6b. [DOI] [PubMed] [Google Scholar]
- 26.Schmitt TM, Kumer SC, Al-Osaimi A, et al. Combined liver-kidney and liver transplantation in patients with renal failure outcomes in the MELD era. Transpl Int. 2009;22:876. doi: 10.1111/j.1432-2277.2009.00887.x. [DOI] [PubMed] [Google Scholar]
- 27.Kamath PS, Wiesner RH, Malinchoc M, et al. A model to predict survival in patients with end-stage liver disease. Hepatology. 2001;33:464. doi: 10.1053/jhep.2001.22172. [DOI] [PubMed] [Google Scholar]
- 28.Bahirwani R, Campbell MS, Siropaides T, et al. Transplantation: impact of pretransplant renal insufficiency. Liver Transpl. 2008;14:665. doi: 10.1002/lt.21367. [DOI] [PubMed] [Google Scholar]
- 29.Levey AS, Coresh J, Balk E, et al. National Kidney Foundation practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Ann Intern Med. 2003;139:137. doi: 10.7326/0003-4819-139-2-200307150-00013. [DOI] [PubMed] [Google Scholar]
- 30. http://optn.transplant.hrsa.gov/PublicComment/pubcom-mentPropSub_237.pdf.
- 31.Thuluvath PJ, Guidinger MK, Fung JJ, Johnson LB, Rayhill SC, Pelletier SJ. Liver transplantation in the United States, 1999–2008. Am J Transplant. 2010;10:1003. doi: 10.1111/j.1600-6143.2010.03037.x. [DOI] [PubMed] [Google Scholar]
- 32.Feng S, Trotter JF. Can we stop waiting for godot? Establishing selection criteria for simultaneous liver-kidney transplantation. Am J Transplant. 2012;12:2869. doi: 10.1111/j.1600-6143.2012.04295.x. [DOI] [PubMed] [Google Scholar]
- 33.Lai JC, Roberts JP, Vittinghoff E, Terrault NA, Feng S. Patient, center and geographic characteristics of nationally placed livers. Am J Transplant. 2012;12:947. doi: 10.1111/j.1600-6143.2011.03962.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hibi T, Sageshima J, Molina E, et al. Predisposing factors of diminished survival in simultaneous liver/kidney transplantation. Am J Transplant. 2012;12:2966. doi: 10.1111/j.1600-6143.2012.04121.x. [DOI] [PubMed] [Google Scholar]