Abstract
Episodic memory impairment due to aging has been linked to hippocampal dysfunction. Evidence exists for alterations in specific circuits within the hippocampal system that are closely coupled to individual differences in the presence and severity of such memory loss. Here, we used the newly developed Diversity Outbred (DO) mouse that was designed to model the genetic diversity in human populations. Young and aged DO mice were tested in a hippocampal-dependent water maze task. Young mice showed higher proficiency and more robust memory compared to the overall performance of aged mice. A substantial number of the older mice, however, performed on par with the normative performance of the younger mice. Stereological quantification of somatostatin-immunoreactive neurons in the dentate hilus showed that high-performing young and unimpaired aged mice had similar numbers of somatostatin-positive interneurons, while aged mice that were impaired in the spatial task had significantly fewer such neurons. These data in the DO model tie loss of hilar inhibitory network integrity to age-related memory impairment, paralleling data in other rodent models.
Keywords: dentate, hippocampus, memory, somatostatin, water maze
Introduction
Failure of episodic memory, linked to hippocampal dysfunction, is a common but not inevitable feature of aging. Such age-related impairment can further worsen to an extent greater than expected for a person's age, leading to a diagnosis of amnestic mild cognitive impairment (aMCI), a condition that increases risk for further progression to Alzheimer's dementia (Petersen et al., 1999; Petersen, 2004). Recent evidence from animal models and human clinical studies has demonstrated that diminished pattern separation, a computational function mediated by hippocampal circuits involving the dentate gyrus, contributes to these age-related conditions of memory loss (Wilson et al., 2005; Yassa et al., 2011). Encoding deficits indicative of diminished pattern separation are associated with weakened input to CA3/dentate gyrus originating from layer II entorhinal neurons and reduced integrity of inhibitory interneuron networks in the hilus of the dentate gyrus (Smith et al., 2000; Scheff et al., 2006; Gallagher and Koh, 2012; Spiegel et al., 2013).
Here, we used the newly developed Diversity Outbred (DO) mouse to study individual differences in neurocognitive aging. The DO mouse population was designed to model the genetic diversity found in human populations, maximizing allelic variation throughout the genome while maintaining normal levels of hetereozygosity (Churchill et al., 2012; Svenson et al., 2012). The increased genetic diversity in the DO mouse model produces high levels of phenotypic variation rendering it a more powerful system to investigate individual differences in behavior than common laboratory inbred mouse strains (Logan et al., 2013), which have limited genetic diversity and are prone to early and late-life recessive allelic effects.
As part of our extended effort to use the DO mouse model to identify the genetics that contribute to preserved and impaired memory during aging with potential relevance to the human population, we behaviorally characterized hippocampal-dependent memory performance in young and aged male DO mice in a water maze protocol. The protocol, with sparse training and interpolated probe tests, was optimized for sensitivity to detect individual differences in cognitive aging apart from confounds due to physical disability or pathological conditions (Gallagher et al., 1993). The overall performance of healthy aged DO mice was found to be less proficient than the young mice. A substantial number of older DO mice, however, performed on a par with the normative performance of the younger adults.
Recent evidence has linked differences in cognitive aging to changes in the inhibitory interneuron network in the hilus that maintains sparse encoding and pattern separation in the dentate gyrus (Andrews-Zwilling et al., 2010, 2012; Spiegel et al., 2013). We therefore examined the integrity of those inhibitory interneurons in behaviorally characterized young and aged DO mice. We stereologically quantified somatostatin-immunoreactive interneuron number in the dentate hilus of the hippocampus, which have been shown to be most susceptible to the effects of aging with hippocampal-dependent memory impairment (Spiegel et al., 2013). The vulnerability of those interneurons, given their anatomical and functional attributes, could contribute to impoverished pattern separation as a basis for episodic memory failure in the aged brain.
Materials and Methods
Subjects
Male DO mice from different litters were obtained between 3 and 6 weeks old from The Jackson Laboratory (Bar Harbor, Maine). The mice were housed in cohorts of 4–5 per cage at 25°C and maintained on a 12-hr light/dark cycle. Mice that showed aggression towards cage mates were singly housed. All cages were lined with corncob bedding and equipped with a short polyvinylchloride pipe and a nestlet for environment enrichment and nest building. Food (Purina autoclave laboratory rodent diet) and water were provided ad libitum. The mice were checked for health on a weekly basis but otherwise left undisturbed until behavioral characterization at 4–8 months old (young) or 18–24 months old (aged). Preliminary data indicated that DO mice have a median lifespan of ∼22–23 months of age (G. Churchill and D. Harrison, personal communication). All procedures in the current investigations were approved by the Institutional Animal Care and Committee in accordance with the National Institutes of Health directive.
Apparatus
The water maze is a circular tank with a diameter of 135 cm and a depth of 45 cm. It was filled with tepid water (24 ± 1°C) made opaque by the addition of white tempera paint. A retractable escape platform with a diameter of 12 cm was located 1 cm below the water surface at the center of one of the four quadrants of the maze. The maze was surrounded by curtains with patterns affixed to provide a configuration of spatial cues. For all trials in the water maze, mice were tracked live with HVS Image analyzing software and videotaped through a camera mounted directly above the maze.
Behavioral Procedures
Mice received spatial memory assessments in the water maze using a protocol involved sparse training designed to maximize sensitivity for memory impairment in aging (Gallagher et al., 1993). Mice were trained for 10 days (three trials per day) to locate a camouflaged escape platform that remained at the same location throughout training in the water maze. At the start of each trial, mice were placed in the water at the perimeter of the pool, with the starting locations varied across trials. Individual trials lasted for 60 s or until the mouse successfully located the platform. If the mouse did not locate the escape platform, the experimenter guided and placed the mouse on the platform where it remained for 10 s. The mouse was then removed from the platform and placed in a holding cage until the next trial. Every sixth trial consisted of a probe trial (free swim with no escape platform) that served to assess the development of a spatially localized search for the escape platform. After 30 s of the probe trial, the platform was raised and the mouse was permitted to escape.
Cued test (six trials in two blocks; 30 s each trial) with a visible escape platform (1 cm above the water surface) were given after training to assess sensory, motoric, and motivational factors that could influence task performance independent of spatial learning. The visible platform raised above the water surface is moved randomly to different locations in the maze at the beginning of each trial. An exclusion criterion was stipulated prior to behavioral assessment to eliminate mice that failed the cued test. Mice that were unable to locate the visible platform on three of the six cued trials or recorded an average of more than 20 s over six cued trials were excluded from the study.
Perfusion and Tissue Preparation
At the end of all behavioral characterization, a subset of young mice, aged mice with intact memory performance, and aged mice with impaired memory performance (n = 7/group; see results for the performance of these mice) were selected for somatostatin immunohistochemistry. The subsets of mice were selected based on their performance in the spatial learning task. Aged mice that required five of more probe trials to reach a performance criterion (see results) were designated as ‘aged impaired’ mice, and those that reached the performance criterion in less than five probe trials, as almost all the young mice did, were designated as ‘aged unimpaired’ mice. The mice were deeply anesthetized with isoflurane and perfused transcardially with sterile saline. The brains were extracted and a hemi-brain was post-fixed in 4% paraformaldehyde at 4°C for 48 hr, and transferred to 16% sucrose in 4% paraformaldehyde at 4°C for 24 hr. The other hemi-brain was dissected and preserved for another study. The brains in the present study were frozen with powdered dry ice and stored at −80°C. Just prior to immunohistological processing, the brains were sectioned in the coronal plan at 40 μm thickness.
Immunohistochemistry
Brain sections were immunostained for somatostatin (Santa Cruz Biotechnology, Santa Cruz, CA; catalog number SC7819) as described in Spiegel et al. (2013). Briefly, free-floating sections were washed in 0.1 M phosphate-buffered saline (PBS), and then endogenous peroxidases were quenched in 0.3% hydrogen peroxide in PBS. After additional PBS washes, sections were blocked in 5% normal horse serum in PBS with 0.3% Triton. Sections were then incubated with primary antibody at a dilution of 1:1,600 in PBS containing 0.15% Triton and 3% normal serum for 72 hr at 4°C with agitation. Following primary antibody incubation, sections were washed in PBS and reacted with biotinylated secondary antibody horse antigoat IgG (Vector Laboratories, Burlingame, CA) diluted in PBS with 0.15% Triton and 5% normal horse serum for 45 min. The secondary antibody was detected with avidin-biotin complex (ABC Elite; Vector Laboratories) and the avidin-biotin complex was visualized with nickel-enhanced diaminobenzadine (Vector Laboratories). Tissue sections were mounted onto gelatin coated slides and dried, dehydrated with increasing ethanol concentrations, cleared in xylene, and coverslipped using DPX mounting media.
Unbiased Stereology
Somatostatin-positive neurons were quantified using a Zeiss Axioplan 2 microscope equipped with a motorized stage under the control of MBF Stereo Investigator software (Version 9.10.4, Bioscience MicroBright Field, Williston, VT). The optical fractionator method was applied (West et al., 1991; Rapp and Gallagher, 1996), and all analyses were conducted blind with regards to animal age and cognitive status. Counts were performed throughout the rostrocaudal extent of the hilar region of the dentate gyrus, and were derived unilaterally (hemi-brain), from a minimum of 9 histological sections, and spaced at 400 μm. Regions of interest were defined according to the Paxinos and Watson rat brain atlas (1997) and digitized under a 5× objective lens. Counts were taken with a 100×/ 1.4 numerical aperture oil immersion objective and surveyed at evenly spaced X–Y intervals of 40 μm × 40 μm. The dimensions of the unbiased counting frame were set to 40 μm × 40 μm. Counting was further confined to no greater than 9 μm centered within the thickness, or z-axis, of the histological sections and guard zones of 2–3 μm were used to eliminate potential biases introduced by the cutting process (appropriate sampling parameters were derived from preliminary test-tissue analysis). Section thickness was recorded at each site. The sampling scheme was established to obtain an estimate of neuron number in the hilus with estimated coefficients of error at or below 0.10. Stereo Investigator software was used to calculate total neuron number and the corresponding coefficient of error. Because the somatostatin stains obscured the nucleus, the most superficial point at which the cell soma was visibly filled with the diaminobenzadine reaction product was identified and used as the counting unit. As changes in somatostatin-immunoreactive neuron number were proportional along the dorsoventral axis, the data were collapsed across sections through the dorsal and ventral hippocampus. Digital photomicrographs were acquired using Stereo Investigator software and adjusted for brightness and contrast in Adobe Photoshop (Adobe Systems, San Jose, CA, USA).
Results
Behavioral Assessment
Young (4–8 months old; n = 75) and aged (18–24 months old; n = 96) DO mice were trained and tested in a water maze using a protocol sensitive to memory impairment in aging. During training, young and aged mice showed improved performance in locating the hidden platform, with the aged mice showing less proficiency than the young mice in the escape task as measured with cumulative search error (Fig. 1). This measure represents deviations from an optimal search for the escape platform that is largely unconfounded by swim speed (Gallagher et al., 1993), and has been shown to be highly sensitive for detection of group differences (Maei et al., 2009). While the cumulative search errors between the groups were comparable on the first trial, t(169) = 0.87, P = 0.387, the aged mice showed higher search error than the young mice, F(1, 169) = 8.73, P = 0.004 (repeated measures ANOVA). The mice in both groups improved over training, F(4, 676) = 327.77, P = 0.001, with no difference in group by trial interaction, F(4, 676) = 0.94, P = 0.439. The same pattern of results was observed with escape latency as the dependent measure. The young and aged mice showed no difference in escape latency on the first training trial, t(169) = 1.63, P = 0.105. Thereafter, they showed improved latency in locating the hidden platform, F(9, 1521) = 129.88, P = 0.001, with the aged mice showing less proficiency than the young mice in the escape task, F(1, 169) = 20.85, P = 0.001. There was no age by trial interaction, F(9, 1521) = 1.11, P = 0.353, indicating parallel improvement between the groups over trials.
Figure 1.
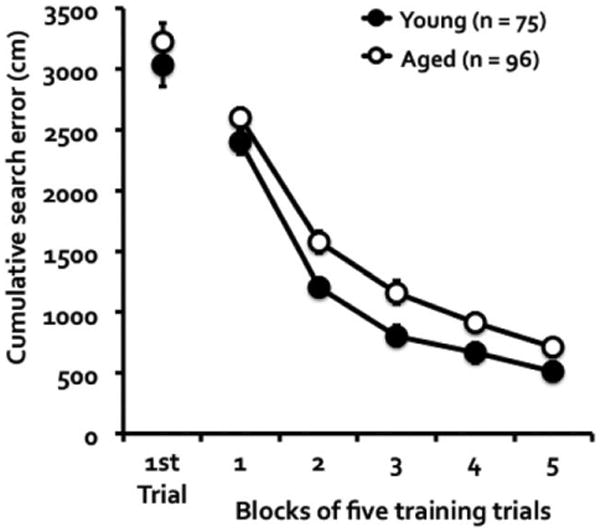
Cumulative search error of young and aged DO mice during water maze training. The measure refers to the deviation from a direct path to the escape platform. The age groups did not differ on the first training trial, but the young mice were more proficient than the aged mice in learning to escape over the course of training.
Probe trials interpolated between blocks of training trials were given to examine the development of a spatially localized search for the escape platform. Overall, the aged DO mice developed a weaker spatial bias for the target location than the young mice (Fig. 2). A repeated measures ANOVA of the percent time spent in target quadrant (Fig. 2A) showed a significant interaction of age groups by probe trials, F(4, 676) = 2.63, P = 0.034, a main effect of age groups, F(1, 169) = 6.19, P = 0.014, and a main effect of probe trials, F(4, 676) = 92.64, P = 0.001. The same analysis of the number of crossings in the target annulus (Fig. 2B) also showed a significant interaction of age by probe trials, F(4, 676) = 3.06, P = 0.016, a main effect of groups, F(1, 169) = 23.61, P = 0.001, and a main effect of probe trials, F(4, 676) = 91.61, P = 0.001. Taken together, these data indicated that aged DO mice developed a less robust hippocampal-dependent memory than their younger counterparts.
Figure 2.
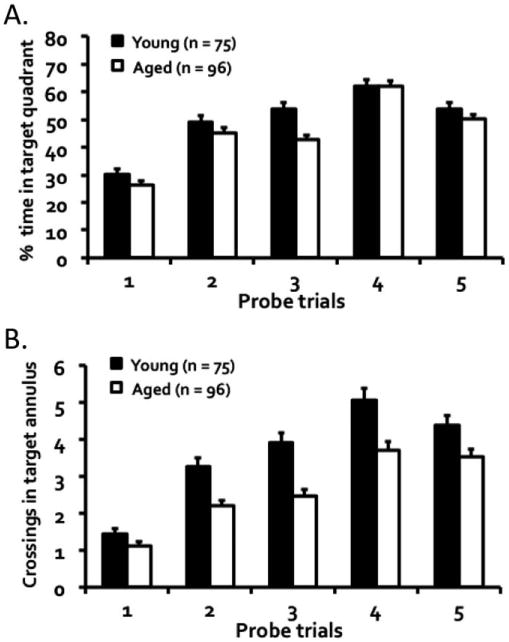
Performance during memory probe trials as measured by (A) percent time spent searching in the target quadrant and (B) the number of crossings in the target annulus twice the size of the escape platform. Both young and aged mice progressively improved over trials, but the aged mice overall showed a weaker spatial bias for the target location than the young mice.
A performance criterion, similar to that adopted in rodent studies of neurocognitive aging using the water maze (Jiang et al., 1989; Gallagher et al., 1989, 1993), was used here to assess acquisition proficiency of DO mice in locating the escape platform. Proficiency was reached on a probe trial when 35% of the duration of the trial was traversed in the target quadrant and at least three crossings occurred in the target annulus. As shown in Figure 3, 84% of young mice reached criterion by the third probe trial, whereas only about half of aged mice achieved that proficiency at that point; Kolmogorov–Smirnov test showed a significant age-related impairment in reaching acquisition proficiency, D = 0.32, P = 0.001.
Figure 3.
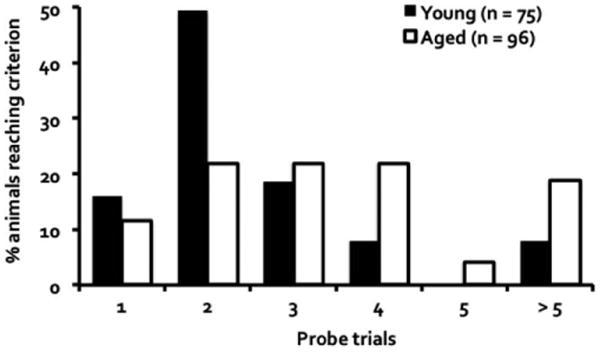
Percentage of mice that initially reached an acquisition criterion at each probe trial. The criterion was reached when 35% of the duration of the probe trial was spent in the target quadrant and at least three target annulus crossings occurred.
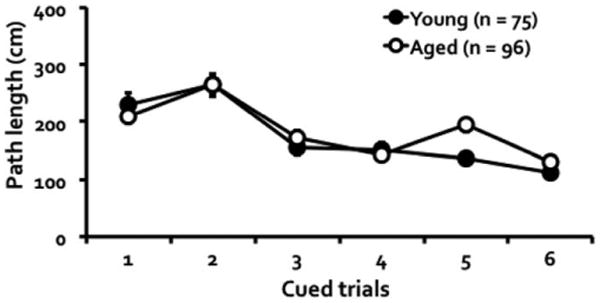
Mice that failed the cued test as described in the method section were excluded from the analyses reported above. Of the 80 young mice tested at the start, 5 (6.25%) failed to meet this criterion and in the older cohort consisting of 116 mice, 20 (17.24%) were excluded on that basis. We adopted a more stringent exclusion criterion in this study so that any deficit seen in the spatial task could not be attributed to suboptimal motivational or sensorimotor function. Of the mice included in the spatial learning task, performance during cued testing showed a significant age by trial interaction, F(5, 845) = 2.39, P = 0.036, a main effect of trial, F(5, 845) = 35.21, P = 0.001, but no main effect of age, F(1, 169) = 1.06, P = 0.305 (Fig. 4).
Figure 4. Young and aged mice did not differ in their performance during hippocampal-independent cued trials in which the escape platform was visible.
Immunohistochemical Assessment
To examine whether aged mice with divergent spatial cognitive status differed in the integrity of their hilar interneuron network, the brains of a subset of DO mice representing intact or impaired cognition were immunostained for somatostatin expression. Aged mice that showed lower proficiency in acquiring the behavioral task (five or more probe trials to reach the performance criterion; ‘aged impaired’; n = 7) were compared to age-matched cohorts that demonstrated higher proficiency in the task (less than five probe trials to reach the criterion; ‘aged unimpaired’; n = 7). Young mice, which almost all performed on par with the aged unimpaired mice, were also included as comparison (n = 7). Figure 5 showed the performance of these subsets of mice in the behavioral characterization as measured by the percent time in target quadrant (Fig. 5A), F(8, 72) = 2.20, P = 0.037 for group by trial interaction, F(2, 18) = 36.84, P = 0.001 for the main effect of groups, and F(4, 72) = 18.43, P = 0.001 for the main effect of trials, and the number of crossings (Fig. 5B), F(8, 72) = 3.55, P = 0.002 for group by trial interaction, F(2, 18) = 36.62, P = 0.001 for the main effect of group, and F(4, 72) = 10.51, P = 0.001 for the main effect of trial. Importantly, further analyses showed that both young and aged unimpaired mice had a significantly stronger memory than the aged impaired mice, ps = 0.001 (main group effects of young or aged unimpaired versus aged impaired). The mice in these groups however did not differ in their cued performance (Fig. 5C), F(10, 90) = 1.72, P = 0.087 for group by cued trial interaction, F(2, 18) = 0.54, P = 0.590 for the main effect of group, but did show improvement in cued performance over trials, F(5, 90) = 9.52, P = 0.001.
Figure 5.
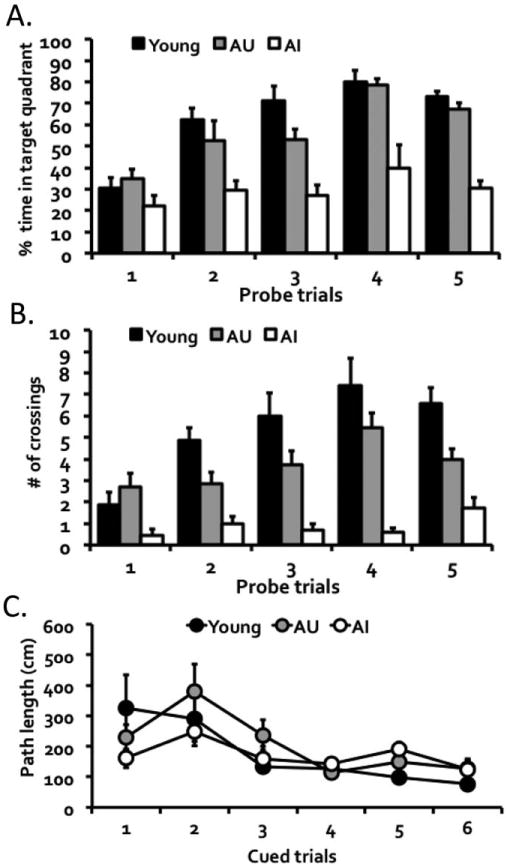
Behavioral performances of mice selected for the somatostatin study. Mice (n = 7 per group) were selected based on an acquisition criterion, which requires the demonstration of learning proficiency based on both (A) percent time spent searching in the target quadrant and (B) the number of crossings in the target annulus. Young and aged memory-unimpaired (AU) mice showed stronger hippocampal-dependent memory performance than aged memory-impaired mice (AI). (C) The mice in all three groups, however, did not differ in hippocampal-independent cued performance.
Stereological quantification of somatostatin-expressing neurons in the dentate hilus showed a significant difference among the groups, H = 6.06, P = 0.048 (Kruskal–Wallis test; W = 0.91, P = 0.044, Shapiro–Wilk test of normality; Figs. 6 and 7A). In particular, the aged impaired mice had significantly fewer somatostatin-positive neurons than young, H = 4.44, P = 0.035, and aged unimpaired mice, H = 4.44, P = 0.035. The young and aged unimpaired mice however did not differ in their hilar somatostatin expression, H = 0.01, P = 0.949. Furthermore, the somatostatin expression was significantly correlated with memory performance (percent time in target quadrant on the final probe test in the original water maze assessment), r = 0.51, P = 0.018 (n = 21; Fig. 7B). The same analysis restricted to the aged mice similarly showed a strong correlation, r = 0.59, P = 0.026 (n = 14).
Figure 6.
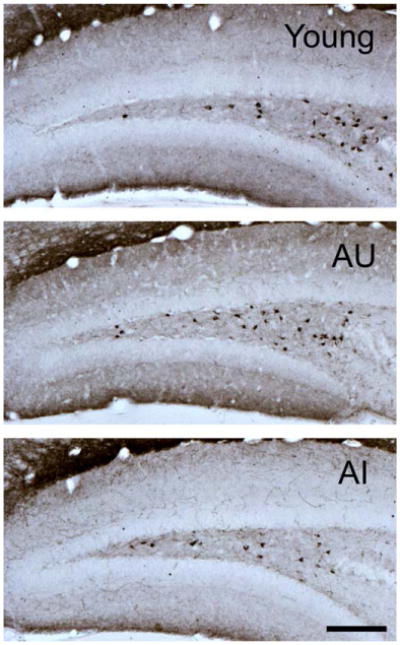
Representative photomicrographs of somatostatin-immunoreactive neurons in the hilus of young (top panel), aged memory-unimpaired (AU; middle panel), and aged memory-impaired (AI; bottom panel) mice. Scale bar = 150 μm. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 7.
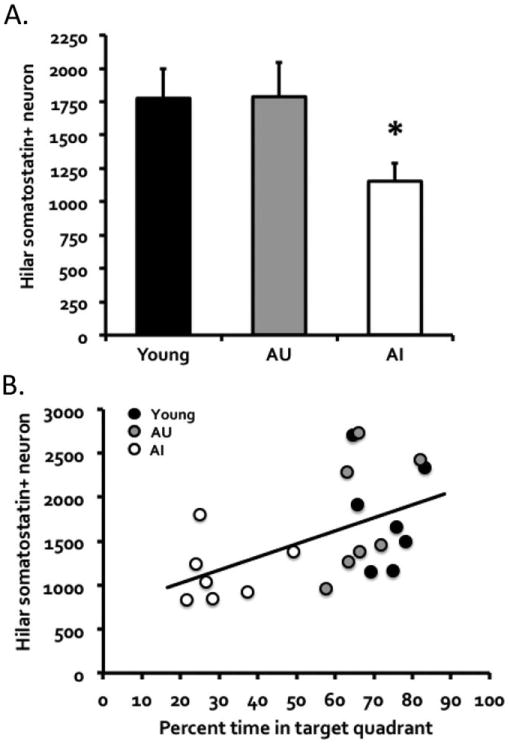
(A) Somatostatin-positive interneuron counts in the dentate hilus of young, aged memory-unimpaired (AU), and aged memory-impaired (AI) mice (n = 7 per group). Note that the numbers represent neuron counts for one hemisphere of the brain. Young and AU mice had comparable numbers of somatostatin-positive interneurons, whereas AI mice had significantly fewer such interneurons. (B) Furthermore, the somatostatin interneuron counts were significantly correlated with memory performance as measured by percent time in target quadrant on the final probe test in the water maze. The same analysis restricted to the aged mice similarly showed a significant positive correlation.
Discussion
Young DO mice exhibited greater proficiency in learning to locate a hidden escape platform during training and stronger spatial memory for the escape location during probe trials compared to the overall performance of healthy, aged DO mice. A substantial number of older DO mice, however, performed on a par with the normative performance of younger adults. Stereological quantification for somatostatin-positive neurons showed that high-performing young and unimpaired aged DO mice had comparable numbers of somatostatin-positive hilar interneurons, while aged mice that were impaired in the spatial task had significantly fewer such neurons. Across the full spectrum of individual differences, the number of hilar somatostatin interneurons was strongly associated with spatial memory such that mice with the lowest numbers of somatostatin-positive neurons exhibited the worst performance. These data in the DO model extend findings that tie loss of somatostatin-positive interneuron integrity to age-related memory impairment, as reported for individual differences in outbred Long-Evans rats (Spiegel et al., 2013), and in models of ApoE4 expression as mice exhibit augmentation of an effect of aging on that specific interneuron population (Andrews-Zwilling et al., 2010).
The newly developed DO mouse population is derived from the same eight founder strains as the Collaborative Cross recombinant inbred strains which were designed specifically for the investigation of complex traits and disorders including cognitive dysfunction and neurodegenerative diseases (The Complex Trait Consortium, 2004; Churchill et al., 2012). The randomized outbreeding strategy used to establish the DO population creates high genetic and phenotypic diversity such that each DO mouse is a unique individual; this stands in contrast to the limited genetic context and narrow behavioral variation of conventional laboratory inbred mouse strains. The DO is thus designed as a more powerful tool to model individual differences in age-associated cognitive impairment. Overall, the aged DO mice showed weaker hippocampal-dependent memory than the younger DO mice. However, consistent with findings in aged outbred rats, rhesus monkeys, and humans (for review, see Rapp and Amaral, 1992), many of the aged DO mice showed little or no impairment; they learned the task as readily as the younger mice. Those individual variations allow for the study of neuro-biochemical alterations that are tightly coupled to age-associated memory dysfunction and its preservation independent of chronological age.
The decreased number of somatostatin-positive neurons in the hilus of cognitively impaired aged DO mice reported here is consistent with findings in other rodent models of aging. Andrews-Zwilling et al. (2010) reported an age-dependent reduction selective for the somatostatin-positive interneurons in the hilus of mice, which was augmented in ApoE4 knock-in mice. In that report, assessment of spatial cognition revealed a strong association between loss of hilar interneuron integrity and behavioral impairment similar to the findings we observed in DO mice. Spiegel et al. (2013) recently reported that the hilar somatostatin-positive interneurons exhibit an age-dependent change in outbred Long-Evans rats, with significantly reduced number in the subpopulation of aged rats with behavioral impairment while unimpaired aged rats had somatostatin interneuron numbers that did not differ from young adults. Other evidence in the outbred rat model suggests that reduced somatostatin protein expression rather than frank neuron loss accounts for the stereological data because quantification of total interneuron numbers showed no neuron loss in the hilus (Spiegel et al., 2013). The absence of frank neurode-generation in that report is consistent with neuron preservation throughout the hippocampus and parahippocampal cortical regions in aged rats, independent of cognitive status (Rapp and Gallagher, 1996; Rapp et al., 2002).
Somatostatin-positive neurons in the hilus colocalize with glutamic acid decarboxylase (Freund and Buzsaki, 1996) and define a subtype of hilar inhibitory interneurons that controls entorhinal cortical input at the termination zone of the perforant path (Freund and Buzsaki, 1996). A loss of function affecting this subpopulation could diminish the sparse encoding mediated by the granule cells of the dentate gyrus (also see Andrews-Zwilling et al., 2012). Such sparse encoding, whereby input from cortex activates a very limited subset of granule cells, is thought to subserve the ability to distinctively represent similar experiences, a property referred to as pattern separation in computational models of hippocampal function (McClelland and Goddard, 1996). Mounting evidence supports a loss of pattern separation in aging as a contributing basis for memory impairment (Wilson et al., 2005; Yassa et al., 2010, 2011; Palmer and Good, 2011). Interestingly, postmortem studies of Alzheimer's patients have shown reduced expression of somatosatin in the hippocampus and cortical areas (Davies et al., 1980; Beal et al., 1985, 1986; Dournaud et al., 1994), and the decreased expression was correlated with severity of cognitive deficits (Tamminga et al., 1987; Dournaud et al., 1995) and neuropathology (Dawbarn et al., 1986). The current findings in aged DO mice with memory impairment are therefore consistent with other evidence in animal models and clinical studies for a loss of hilar integrity underlying such age-related memory impairment.
The concept that decreased inhibitory function in the hippocampal memory network contributes to age-related memory dysfunction, as suggested by the present findings, is consistent with evidence that a condition of overactivity affects the dentate gyrus/CA3 subregions in both animal models and humans with age-related memory impairment (Wilson et al., 2005; Andrews-Zwilling et al., 2010; Yassa et al., 2010; Bakker et al., 2012). Moreover, targeting that overactivity has been found to improve hippocampal-dependent memory function (Andrews-Zwilling et al., 2010; Bakker et al., 2012; Koh et al., 2013). With respect to loss of inhibitory function, such treatments have included ones to potentiate inhibitory GABAergic tone with pentobarbital or GABAA α5 receptor positive allosteric modulators, which were found to rescue impairment in apoE4 knock-in mice and in aged impaired rats, respectively (Andrews-Zwilling et al., 2010; Koh et al., 2013). Thus the DO model, with evidence for a similar condition contributing to memory loss in aging could provide a useful model for further basic research and translational studies.
Acknowledgments
We thank Daniel Burruss and Robert McMahan for careful management of the DO colony and excellent behavioral testing of the mice at Johns Hopkins University, and Dr. Gary Churchill at The Jackson Laboratory for advice on the DO population. This research was supported by a Senior Scholar Award, AG-SS-206408, from the Ellison Medical Foundation and by a grant, P01-AG-09973, from the National Institute on Aging to M.G. The authors have no actual or potential conflicts of interest within the last three years that could inappropriately influence this work.
Grant sponsor: Ellison Medical Foundation; Grant number: AG-SS-206408; Grant sponsor: National Institute on Aging; Grant number: P01-AG-09973.
References
- Andrews-Zwilling Y, Bien-Ly N, Xu Q, Li G, Bernardo A, Yoon SY, Zwilling D, Yan TX, Chen L, Huang Y. Apolipoprotein E4 causes age- and Tau-dependent impairment of GABAergic interneurons, leading to learning and memory deficits in mice. J Neurosci. 2010;30:13707–13717. doi: 10.1523/JNEUROSCI.4040-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews-Zwilling Y, Gillespie AK, Kravitz AV, Nelson AB, Devidze N, Lo I, Yoon SY, Bien-Ly N, Ring K, Zwilling D, Potter GB, Rubenstein JL, Kreitzer AC, Huang Y. Hilar GABAergic interneuron activity controls spatial learning and memory retrieval. PLoS One. 2012;7:e40555. doi: 10.1371/journal.pone.0040555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Krauss GL, Albert MS, Speck CL, Jones LR, Stark CE, Yassa MA, Bassett SS, Shelton AL, Gallagher M. Reduction of hippocampal hyperactivity improves cognition in amnestic mild cognitive impairment. Neuron. 2012;74:467–474. doi: 10.1016/j.neuron.2012.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beal MF, Mazurek MF, Tran VT, Chattha G, Bird ED, Martin JB. Reduced numbers of somatostatin receptors in the cerebral cortex in Alzheimer's disease. Science. 1985;229:289–291. doi: 10.1126/science.2861661. [DOI] [PubMed] [Google Scholar]
- Beal MF, Mazurek MF, Svendsen CN, Bird ED, Martin JB. Widespread reduction of somatostatin-like immunoreactivity in the cerebral cortex in Alzheimer's disease. Ann Neurol. 1986;20:489–495. doi: 10.1002/ana.410200408. [DOI] [PubMed] [Google Scholar]
- Churchill GA, Gatti DM, Munger SC, Svenson KL. The diversity outbred mouse population. Mamm Genome. 2012;23:713–718. doi: 10.1007/s00335-012-9414-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies P, Katzman R, Terry RD. Reduced somatostatin-like immunoreactivity in cerebral cortex from cases of Alzheimer disease and Alzheimer senile dementia. Nature. 1980;288:279–280. doi: 10.1038/288279a0. [DOI] [PubMed] [Google Scholar]
- Dawbarn D, Rossor MN, Mountjoy CQ, Roth M, Emson PC. Decreased somatostatin immunoreactivity but not neuropeptide Y immunoreactivity in cerebral cortex in senile dementia of Alzheimer type. Neurosci Lett. 1986;70:154–159. doi: 10.1016/0304-3940(86)90455-6. [DOI] [PubMed] [Google Scholar]
- Dournaud P, Cervera-Pierot P, Hirsch E, Javoy-Agid F, Kordon C, Agid Y, Epelbaum J. Somatostatin messenger RNA-containing neurons in Alzheimer's disease: An in situ hybridization study in hippocampus, parahippocampal cortex and frontal cortex. Neuroscience. 1994;61:755–764. doi: 10.1016/0306-4522(94)90399-9. [DOI] [PubMed] [Google Scholar]
- Dournaud P, Delaere P, Hauw JJ, Epelbaum J. Differential correlation between neurochemical deficits, neuropathology, and cognitive status in Alzheimer's disease. Neurobiol Aging. 1995;16:817–823. doi: 10.1016/0197-4580(95)00086-t. [DOI] [PubMed] [Google Scholar]
- Freund TF, Buzsáki G. Interneurons of the hippocampus. Hippocampus. 1996;6:347–470. doi: 10.1002/(SICI)1098-1063(1996)6:4<347::AID-HIPO1>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell RD. Relationship of age-related decline across several behavioral domains. Neurobiol Aging. 1989;10:691–708. doi: 10.1016/0197-4580(89)90006-7. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging: Development of a learning index for performance in the Morris water maze. Behav Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Koh MT. Episodic memory on the path to Alzheimer's disease. Curr Opin Neurobiol. 2011;21:929–934. doi: 10.1016/j.conb.2011.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang HK, Owyang VV, Hong JS, Gallagher M. Elevated dynorphin in the hippocampal formation of aged rats: Relation to cognitive impairment on a spatial learning task. Proc Natl Acad Sci USA. 1989;86:2948–2951. doi: 10.1073/pnas.86.8.2948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koh MT, Rosenzweig-Lipson S, Gallagher M. Selective GABAA α5 positive allosteric modulators improve cognitive function in aged rats with memory impairment. Neuropharmacology. 2013;64:145–152. doi: 10.1016/j.neuropharm.2012.06.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan RW, Robledo RF, Recla JM, Philip VM, Bubier JA, Jay JJ, Harwood C, Wilcox T, Gatti DM, Bult CJ, Churchill GA, Chesler EJ. High-precision genetic mapping of behavioral traits in the diversity outbred mouse population. Genes Brain Behav. 2013;12:424–437. doi: 10.1111/gbb.12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maei HR, Zaslavsky K, Teixeira CM, Frankland PW. What is the most sensitive measure of water maze probe test performance? Front Integr Neurosci. 2009;3:4. doi: 10.3389/neuro.07.004.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Goddard NH. Considerations arising from a complementary learning systems perspective on hippocampus and neocortex. Hippocampus. 1996;6:654–665. doi: 10.1002/(SICI)1098-1063(1996)6:6<654::AID-HIPO8>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Palmer A, Good M. Hippocampal synaptic activity, pattern separation and episodic-like memory: Implications for mouse models of Alzheimer's disease pathology. Biochem Soc Trans. 2011;39:902–909. doi: 10.1042/BST0390902. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 3rd. San Diego: Academic Press; 1997. [Google Scholar]
- Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangalos EG, Kokmen E. Mild cognitive impairment: Clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- Petersen RC. Mild cognitive impairment as a diagnostic entity. J Intern Med. 2004;256:183–194. doi: 10.1111/j.1365-2796.2004.01388.x. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Amaral DG. Individual differences in the cognitive and neurobiological consequences of normal aging. Trends Neurosci. 1992;15:340–345. doi: 10.1016/0166-2236(92)90051-9. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc Natl Acad Sci USA. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Deroche PS, Mao Y, Burwell RD. Neuron number in the parahippocampal region is preserved in aged rats with spatial learning deficits. Cereb Cortex. 2002;12:1171–1179. doi: 10.1093/cercor/12.11.1171. [DOI] [PubMed] [Google Scholar]
- Scheff SW, Price DA, Schmitt FA, Mufson EJ. Hippocampal synaptic loss in early Alzheimer's disease and mild cognitive impairment. Neurobiol Aging. 2006;27:1372–1384. doi: 10.1016/j.neurobiolaging.2005.09.012. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immuno-reactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegel AM, Koh MT, Vogt NM, Rapp PR, Gallagher M. Hilar interneuron vulnerability distinguishes aged rats with memory impairment. J Comp Neurol. 2013;521:3508–3523. doi: 10.1002/cne.23367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svenson K, Gatti DM, Valdar W, Welsh CE, Cheng R, Chesler EJ, Palmer AA, McMillan L, Churchill GA. High-resolution genetic mapping using the mouse Diversity Outbred populations. Genetics. 2012;190:437–447. doi: 10.1534/genetics.111.132597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- The Complex Trait Consortium. The Collaborative Cross, a community resource for the genetic analysis of complex traits. Nat Genetics. 2004;36:1133–1137. doi: 10.1038/ng1104-1133. [DOI] [PubMed] [Google Scholar]
- West MJ, Slomianka L, Gundersen HJ. Unbiased stereological estimation of the total number of neurons in the subdivisions of the rat hippocampus using the optical fractionator. Anat Rec. 1991;231:482–497. doi: 10.1002/ar.1092310411. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Stark SM, Bakker A, Albert MS, Gallagher M, Stark CE. High-resolution structural and functional MRI of hippocampal CA3 and dentate gyrus in patients with amnestic mild cognitive impairment. Neuroimage. 2010;51:1242–1252. doi: 10.1016/j.neuroimage.2010.03.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yassa MA, Lacy JW, Stark SM, Albert MS, Gallagher M, Stark CE. Pattern separation deficits associated with increased hippocampal CA3 and dentate gyrus activity in nondemented older adults. Hippocampus. 2011;21:968–979. doi: 10.1002/hipo.20808. [DOI] [PMC free article] [PubMed] [Google Scholar]


