Abstract
Background
Gender differences exist in anterior cruciate ligament (ACL) cross-sectional area and lateral tibial slope. Biomechanical principles suggest that the direction of these gender differences should induce larger peak ACL strains in females under dynamic loading.
Hypothesis
Peak ACL relative strain during a simulated pivot landing is significantly greater in female ACLs than male ACLs.
Study Design
Controlled laboratory study.
Methods
Twenty cadaveric knees from height- and weight-matched male and female cadavers were subjected to impulsive 3-dimensional test loads of 2 times body weight in compression, flexion, and internal tibial torque starting at 15° of flexion. Load cells measured the 3-dimensional forces and moments applied to the knee, and forces in the pretensioned quadriceps, hamstring, and gastrocnemius muscle equivalents. A novel, gender-specific, nonlinear spring simulated short-range and longer range quadriceps muscle tensile stiffness. Peak relative strain in the anteromedial bundle of the ACL (AM-ACL) was measured using a differential variable reluctance transducer, while ACL cross-sectional area and lateral tibial slope were measured using magnetic resonance imaging. A repeated-measures Mann-Whitney signed-rank test was used to test the hypothesis.
Results
Female knees exhibited 95% greater peak AM-ACL relative strain than male knees (6.37% [22.53%] vs 3.26% [11.89%]; P = .004). Anterior cruciate ligament cross-sectional area and lateral tibial slope were significant predictors of peak AM-ACL relative strain (R2 = .59; P = .001).
Conclusion
Peak AM-ACL relative strain was significantly greater in female than male knees from donors of the same height and weight. This gender difference is attributed to a smaller female ACL cross-sectional area and a greater lateral tibial slope.
Clinical Relevance
Since female ACLs are systematically exposed to greater strain than their male counterparts, training and injury prevention programs should take this fact into consideration.
Keywords: anterior cruciate ligament, gender, strain, cross-sectional area, lateral tibial slope
More than 350 000 anterior cruciate ligament (ACL) injuries occur in the United States each year.19 These injuries, which typically occur during jump and pivot landings7,40 in sports like basketball, volleyball, and soccer,1,3,34,45 are of considerable public health concern because they significantly increase the risk for developing knee osteoarthritis later in life.31
It is unclear why a disproportionate number of these injuries occur in women.1,3,25,34,36,45 Possible explanations include hormonal effects,48,58 differences in knee alignment,16,24 and knee joint laxity,48,51 as well as anatomic differences in intercondylar notch,2,13,51 posterior tibial slope,8,22 and medial tibial depth (MTD).22 The female ACL has a cross-sectional area (CSA) that is 20% to 30% smaller than the male ACL,2,11,13,37 along with fewer collagen fibrils per unit area23 and a slightly smaller tensile elastic modulus.11 Furthermore, smaller female muscles provide significantly less resistance to shear and torque at the knee.57,59 However, there is no evidence that any of the above factors result in greater ACL strain in the female knee during a pivot landing.
From basic biomechanical principles, the strain in a ligament is proportional to the tensile force on the ligament, and inversely proportional to its CSA and tensile modulus of elasticity. For a given tensile force, the smaller the CSA and/or modulus, the greater the strain on the ligament; this places a smaller ACL at a greater risk of rupture than a larger ACL for a given ultimate tensile stress or strain. The question addressed in this study is whether the smaller female ACL experiences greater strains than the larger male ACL in a pivot landing. If so, the clinical significance is that women would be at increased risk for an earlier ACL rupture because of the well-known inverse relationship between the permissible cyclic stress in the ligament and the number of cycles that it can survive before material fatigue failure.47,50 From biomechanical principles, a greater tibial plateau slope would be expected to further increase the stress placed on the ligament by increasing tibiofemoral translation under the dynamic compressive load associated with landing. This effect would further reduce the number of loading cycles an ACL might sustain before rupture. Indeed, increased lateral tibial slope (LTS) and decreased MTD have previously been shown to greatly increase the risk of ACL injury in women.22
The objectives of this study, therefore, were to determine (1) whether a gender difference exists in ACL strain, and (2) whether this gender difference in ACL strain can be explained by differences in knee anatomy as exemplified by ACL CSA and LTS. An established cadaveric knee construct capable of delivering a 2-times body weight (2 × BW) impulsive compound load (compression, flexion, and internal tibial torque) was used to measure the peak strain in the anteromedial (AM) bundle of the ACL (AM-ACL).39,54–56 A new feature was the addition of a custom spring to represent the gender-specific nonlinear quadriceps response to stretch to better model both the known gender difference in quadriceps stiffness18 and the bilinear stiffness of a whole muscle responding to rapid stretch in vivo.14,15,20,30,32 Our primary null hypothesis was that there is no difference in peak AM-ACL relative strain between height- and weight-matched female and male knees equipped with their gender-specific quadriceps muscle. Our secondary hypothesis was that a smaller ACL CSA (at 30% length from the tibial insertion), a greater LTS, and a shorter MTD, determined from MRI scans before impact testing, would correlate with increased peak AM-ACL relative strain.
MATERIALS AND METHODS
Specimen Procurement and Preparation
Unembalmed cadaver limbs were acquired from the University of Michigan Anatomical Donations Program and were visually examined for indications of surgery and deformities before specimen harvesting. The age, height, and weight of the 10 male and 10 female donors are shown in Table 1. The donors were height- and weight-matched to ensure each specimen was within 10% of our target weight of 73 kg and height of 175 cm. After the limbs were harvested, they were dissected, keeping the knee ligamentous structures intact, along with the tendons of the quadriceps (rectus femoris), medial hamstrings (gracilis, sartorius, semimembranosus, semitendinosus), lateral hamstrings (biceps femoris), and the medial and lateral gastrocnemius. The distal tibia and proximal femur were cut 20 cm from the knee joint line. The distal tibia and fibula and proximal femur was potted in a polyvinyl chloride cylinder (6 cm tall, 10 cm in diameter) filled with polymethylmethacrylate (PMMA). The specimens were stored frozen at −20°C, and thawed at room temperature for 12 hours before impact testing.
TABLE 1.
Descriptive Statistics of the Study Donorsa
| Gender | Age, y | Height, cm | Weight, kg |
|---|---|---|---|
| Female (n = 10) | 65.7 ± 18.4 | 171.5 ± 3.4 | 68.8 ± 5.0 |
| Male (n = 10) | 60.8 ± 17.2 | 174.5 ± 9.7 | 71.8 ± 5.3 |
Data presented as mean ± standard deviation.
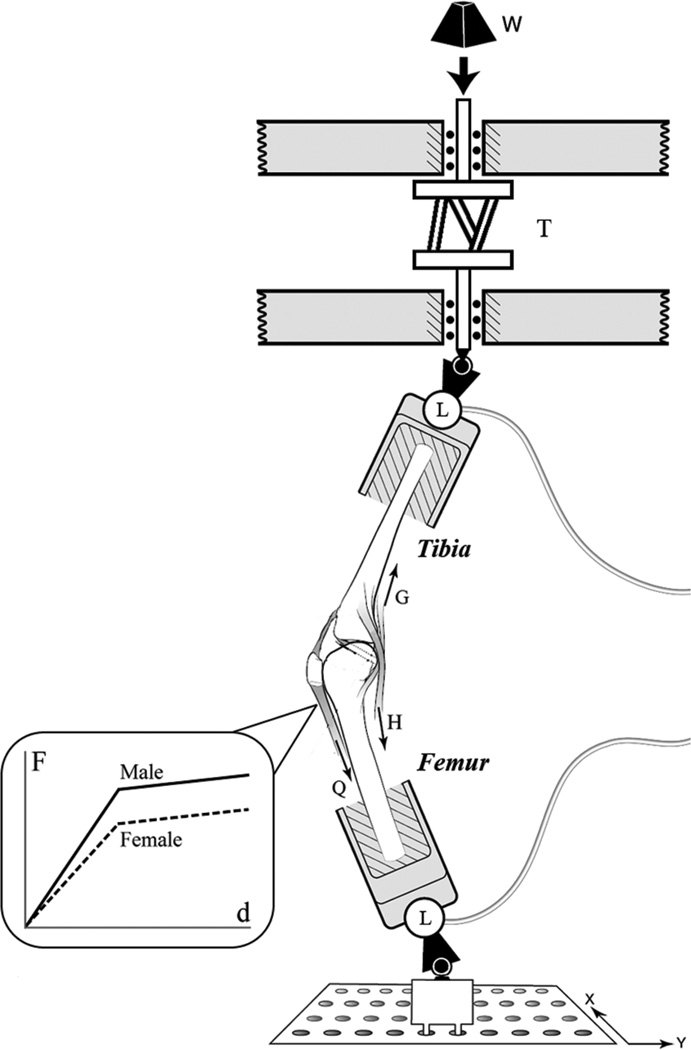
Testing Apparatus
A single-legged pivot landing was simulated with a modified Withrow testing apparatus54–56 (Figure 1). To commence each trial, a crossbow hair-trigger release mechanism was tripped to abruptly release a mass (W, Figure 1) to fall 7 cm and strike an impact shaft. The impact shaft was located in series with an axial rotational device39 (T, Figure 1), delivering an impulsive compression force and axial torque to a universal joint connected in series with a 6-axis load cell (MC3A-1000, AMTI, Watertown, Massachusetts) attached to the distal tibia (L, Figure 1). The axial rotation device consisted of 2 circular aluminum plates spaced by 3 equi-inclined struts. By adjusting the strut angle, the desired gain between applied compressive force and internal tibial torque can be produced. If only a compressive force was desired (ie, the baseline trials), an axial cylindrical rod was keyed between the 2 circular plates to prevent an internal tibial torque from being applied. The impulsive force delivered to the specimen was equivalent to 2 × BW of each specimen and peaked at 60 milliseconds. This system has been previously shown to be reproducible and a good representation of the knee loading during a jump or pivot landing.39,54–56 The initial knee flexion angle was 15°, consistent with ACL injury video analysis,40 and the impulsive force was delivered 3 cm posterior to the knee joint. The 3-dimensional (3D) action forces and moments on the distal tibia were measured at 2 kHz with the 6-axis load cell. As a double-check on the loads applied to the knee, a second 6-axis load cell identical to the first recorded the 3D reaction forces and moments at the proximal femur.
Figure 1.
Diagram of the modified Withrow testing apparatus. Inset: A novel bilinear stiffness quadriceps spring was developed for this experiment to model the in vivo female quadriceps muscle response to stretch. Additional springs were added in parallel to achieve the 20% greater quadriceps stiffness in males.
Knee kinematics were recorded with an Optotrak Certus camera system (Northern Digital, Inc, Waterloo, Ontario, Canada) at 400 Hz with 2 sets of 3 infrared emitting diodes (IREDs) attached to a plate affixed to the 6-axis load cells, which were rigidly attached to the tibia and femur by securing the polymethylmethacrylate-potted bones in an aluminum cylinder in series with the load cell. Before impact testing, the IRED triads affixed to the sagittal plane of the 6-axis load cell were aligned to be parallel with the sagittal plane of the specimen. The knee construct was digitized to relate IRED location to anatomic landmarks on the knee. This setup allows the absolute and relative 3D translations and rotations of each condyle, relative to the tibial plateau, to be calculated throughout the entire impact trial.
The relative strain was measured with a differential variable reluctance transducer (DVRT) placed at the distal third of the anteromedial region of the ACL (3-mm stroke length, MicroStrain, Inc, Burlington, Vermont). Absolute AM-ACL strain cannot be measured because the length of the ligament in a zero-strain state is unknown. Cryoclamps were used to grip the tendons of the rectus femoris, lateral and medial hamstrings, and lateral and medial gastrocnemius. Muscle tension was recorded with series load cells attached to the cryoclamps (TLL-1K and TLL-500, Transducer Techniques, Inc, Temecula, California). The quadriceps was attached to a 4.5-kN load cell, while the hamstrings and gastrocnemius muscles were attached to a 2.25-kN load cell (see Q, H, and G in Figure 1). Each muscle tendon and its corresponding load cell were attached in series with a spring acting as a muscle equivalent to model each muscle’s stretch response to the simulated pivot landing. These muscle equivalents were monitored before each trial to ensure the muscle forces were balanced and the knee maintained an initial knee flexion angle of 15°. The 5 muscle tendons were attached to winches on the distal tibia and proximal femur that could be ratcheted to increase or decrease muscle force prior to the impact trial.
The medial and lateral hamstrings and medial and lateral gastrocnemius were pretensioned to 70 N using woven nylon cord (stiffness, 200 N/mm). A new addition to the modified Withrow testing apparatus for this study was the use of a nonlinear spring to represent the short-range and longer range quadriceps bilinear elastic and viscous resistance to rapid stretch (Figure 1, inset) during the first 100 milliseconds of the impulsive loading. This stretch response is similar to the response of in vivo muscle, as seen in muscle fibers14,15,30,32 and whole muscle20 near optimal sarcomere and fiber length. The female spring (initial stiffness, 155 N/mm; final stiffness, 27 N/mm) was assigned systematically 20% less muscle stiffness than the male spring (initial stiffness, 193 N/mm; final stiffness, 46 N/mm), reflecting in vivo data on gender difference in muscle properties18 attributable to muscle mass representing a smaller percentage of body mass in the female.29 The nonlinear quadriceps stiffness values were determined experimentally with a linear best fit to the spring’s short-range and long-range force-length curve. The preimpact quadriceps muscle tension was 180 N.
Testing Protocol
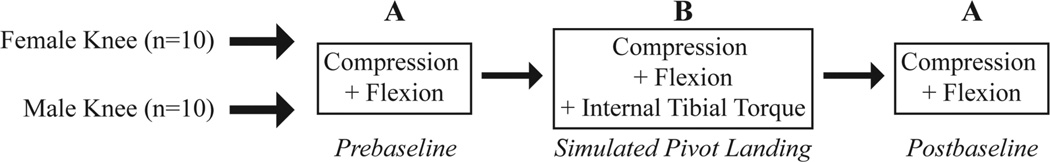
A cross-sectional repeated measures design (A-B-A, Figure 2) was used to measure peak AM-ACL relative strain in height-and weight-matched male and female cadaver limbs. The impact mass was dropped from the same 7-cm drop height to deliver the same energy input across all 3 testing blocks. The A testing blocks represent the prebaseline and postbaseline trials of a 2 × BW compression force with a nominal 41-N·m flexion moment. The B testing block simulated a pivot landing using a 2 × BW compression force combined with a 17.5-N·m internal tibial torque and 37-N·m knee flexion moment. Since these are orthogonal moments, the same resultant moment (41 N·m) as testing blocks A will occur, but the resultant moment will have a different direction. This loading combination has been shown to induce large dynamic ACL strains.39 A comparison of the prebaseline and postbaseline trials allows for a final check on the integrity of the ACL response to the standardized baseline loading after the simulated pivot-landing trials. Each specimen was preconditioned with 5 trials at the beginning of the experiment to reduce data variability. The specimen underwent 5 impact trials for each testing block in the A-B-A design, with 1 preconditioning trial between each testing block to remove hysteresis effects. A total of 22 impact trials were applied to each specimen. This is less than half the number of trials used in the original experiment of Withrow et al56 to minimize the risk of injuring the ACL.
Figure 2.
Flowchart for the repeated-measures experimental design.
Magnetic Resonance Imaging Scans
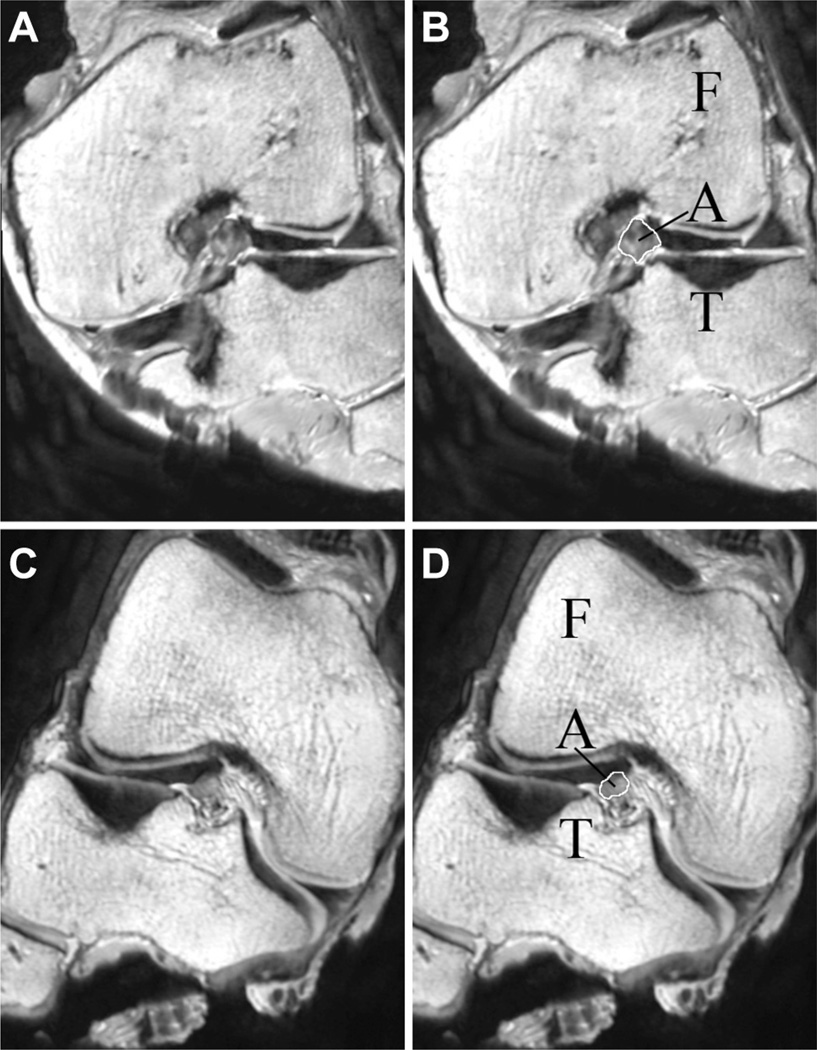
T2-weighted 3D sagittal MRI scans were acquired from 9 male and 9 female thawed cadaver limbs before impact testing (Philips Healthcare 3T scanner [Best, the Netherlands], 3D proton-density sequence; repetition time/echo time, 1000/35 milliseconds; slice thickness, 0.7 mm; pixel spacing, 0.35 mm × 0.35 mm; field of view, 160 mm) and examined by a blinded observer using OsiriX (version 3.7.1, open source, www.osirix-viewer.com). The MRI scans were reconstructed to measure the ACL CSA from an oblique-axial view of the ligament13 (Figure 3). The length of the ligament was measured using an oblique-coronal view, and the ACL CSA was measured at 30% of the ligament’s length from the tibial insertion. The measurement location is consistent with the DVRT’s placement on the distal third of the AM-ACL during impact testing and the known location of the minimum CSA of the ligament.21 The ACL CSA was outlined with a pencil tool and OsiriX calculated the area within the pencil border. The LTS was measured as described by Hudek et al.27 The longitudinal axis of the tibia was defined by a line connecting the centers of 2 circles fit within the proximal tibial head on the central sagittal image. The center of articulation of the lateral tibial compartment was selected from an axial view, and a line was connected between the most anterior and posterior points of the lateral tibial plateau using the sagittal view. The angle between this line and a line perpendicular to the longitudinal axis was defined as the LTS. To determine medial tibial slope (MTS) and MTD, the tibial center of articulation of the medial tibial plateau was defined. The MTS was measured as the angle between the line perpendicular to the longitudinal axis and a line connecting the superior-anterior and posterior points on the medial tibial plateau. Medial tibial depth was measured by drawing a line along the subchondral bone margin from the most anterior to the most posterior medial tibial plateau. With use of a custom algorithm, MTD was calculated as the peak distance between the subchondral bone line and a line connecting the most superior points on the anterior and posterior portions of the medial tibial plateau. The intraclass correlation coefficient for these MRI measurements was 0.88 for ACL CSA, 0.93 for LTS and MTS, and 0.88 for MTD. Finally, femoral bicondylar width was measured at the level of the popliteal groove using a coronal view to determine the relative knee size of the age-, height-, and weight-matched donors, as described by Vrooijink et al.52
Figure 3.
(A) Oblique-axial view of the left knee of male specimen #33400 at 30% of the ligament’s length from the tibial insertion. (B) The femur (F), tibia (T), and anterior cruciate ligament (ACL, A) are labeled on male specimen #33400. The cross-sectional area of the ACL is outlined in white. (C) Oblique-axial view of the right knee of female specimen #33272 at 30% of the ligament’s length from the tibial insertion. (D) The femur (F), tibia (T), and ACL (A) are labeled on female specimen #33272. The cross-sectional area of the ACL is outlined in white.
Statistical Analyses
The raw kinematic and 6-axis load cell data were analyzed using a custom algorithm in MATLAB (2009b, The MathWorks, Natick, Massachusetts). For each specimen, the mean peak AM-ACL relative strain over 5 impact trials was recorded for the prebaseline, simulated pivot landing, and postbaseline testing blocks. The primary and secondary hypotheses tested were as follows: H1, there would be no significant gender difference in peak AM-ACL relative strain under prebaseline, simulated pivot landing, or postbaseline testing (ie, blocks A-B-A, Figure 2); and H2, there would not be a significant linear correlation between peak AM-ACL relative strain during the simulated pivot landing and ACL CSA, LTS, MTS, and MTD. In addition, a tertiary hypothesis (H3) that there was no significant difference in peak AM-ACL relative strain between the prebaseline and postbaseline testing blocks by gender was tested as a check of ACL integrity. All statistical analyses were performed with PASW Statistics 18 (SPSS Inc, Chicago, Illinois). Hypothesis 1 was tested with a nonparametric Mann-Whitney signed-rank test with exact significance. Hypothesis 2 was tested with a stepwise multiple linear regression between peak AM-ACL relative strain and the independent variables (gender, ACL CSA, LTS, and MTD). Hypothesis 3 was tested with a Wilcoxon signed-rank test with exact significance. Independent t tests were used to test if gender differences existed in ACL CSA, LTS, MTS, MTD, and femoral bicondylar width, along with exploring the effect of gender on peak AM-ACL strain rate. A simulated pivot landing with an internal tibial torque was selected for our ACL strain measurement because it results in greater ACL strain within our knee construct than valgus loading.39,54 All statistical tests assumed significance at P<.05. Assuming an alpha level of .05 and a power of 80%, the sample size was determined from a 2-tailed, independent power analysis using pilot data on the peak AM-ACL relative strain under the simulated pivot landing for each gender. The effect size was found to be 1.38, requiring 10 male and 10 female knees to reach 80% power. A post hoc power analysis showed 84% power was achieved. Finally, a hyperelastic representation of ACL response to uniaxial elongation17 was used to calculate the theoretical effect on peak ACL strain of known gender differences in ACL CSAs and moduli of elasticity11 (see Appendix A, available in the online version of this article at http://ajs.sagepub.com/supplemental/).
RESULTS
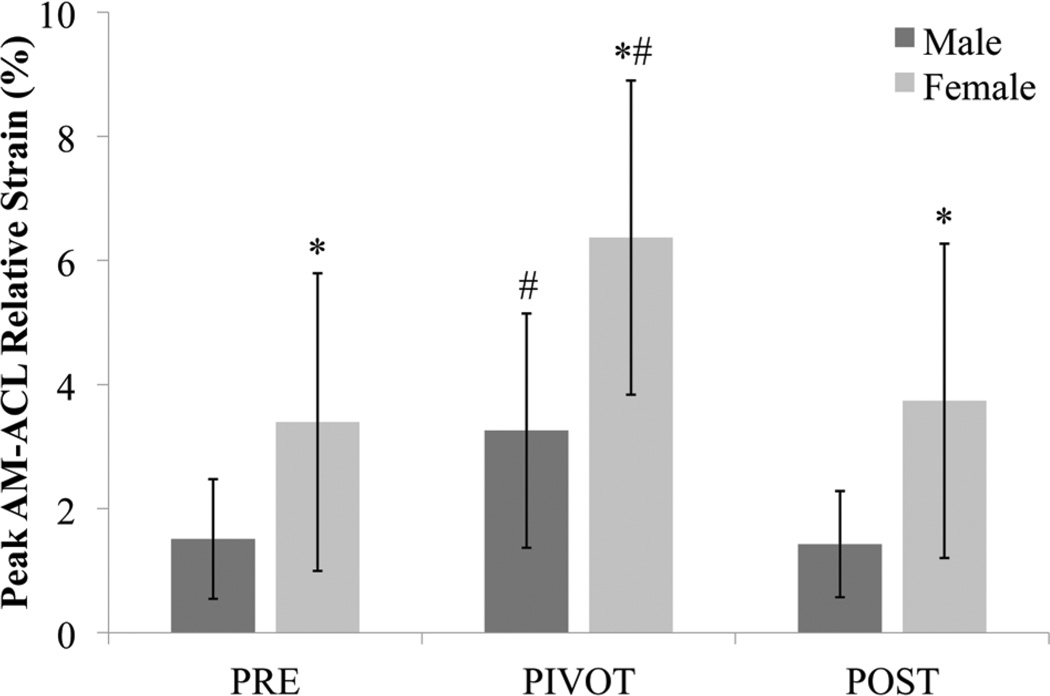
The simulated pivot landing test condition (ie, block B, Figure 2) resulted in significantly greater peak AM-ACL relative strain than the prebaseline and postbaseline test conditions in male (P = .005 and P = .005, respectively) and female (P = .005 and P = .005, respectively) knees. The primary hypothesis, H1, was rejected in that the peak AM-ACL relative strain was significantly different between male and female cadaver knees under the simulated pivot-landing test condition (P = .004, Figure 4), along with both the prebaseline and postbaseline test conditions (P = .023 and P = .011, respectively). As a check on ACL integrity after the simulated pivot-landing testing block, we failed to reject H3 in that no significant difference in peak AM-ACL relative strain was found between the prebaseline and postbaseline conditions in male (P = .508) or female (P = .139) knees. There is a trend (P = .068) toward a difference in peak AM-ACL strain rate between female knees (232.4 ± 90.6%/second) and male knees (156.2 ± 84.5%/second) under the simulated pivot-landing testing block.
Figure 4.
The mean peak AM-ACL (anteromedial bundle of the anterior cruciate ligament) relative strain (error bars = 1 standard deviation) for male (dark gray) and female (light gray) height- and weight-matched cadaver knees over the 3 loading trial blocks: prebaseline (PRE), simulated pivot landing (PIVOT), and postbaseline (POST). *Denotes significant gender difference within testing block (P < .05); #Significant difference between PIVOT testing block and the corresponding PRE and POST testing blocks by gender (P < .05).
Gender differences in the loads applied to the knee and the resultant kinematics and muscle loads are examined in Table 2. There was a trend toward female knees being loaded under a lower peak impulsive compressive force across all 3 testing conditions, along with a smaller peak internal tibial torque during the simulated pivot landing. Since the knees were matched for height and weight and received the same energy input, the trend toward lower peak compressive force and internal tibial torque was likely attributable to the female knees having lower input impedance because of their lower quadriceps stiffness, leading to their significantly greater knee flexion (as seen in Table 2). The resultant peak quadriceps force was 5% less in the female knees in spite of the larger change in knee flexion angle. Lastly, the female knees exhibited a significantly larger increase in internal tibial rotation than the male knees.
TABLE 2.
Input Forces and Torques and Outcome Forces and Kinematics Under the 3 Loading Conditions by Gendera
| Prebaseline | Simulated Pivot Landing | Postbaseline | ||||
|---|---|---|---|---|---|---|
| Female | Male | Female | Male | Female | Male | |
| Inputs | ||||||
| Compressive force, N | 1196 ± 189 | 1416 ± 84 | 688 ± 95 | 814 ± 119 | 1183 ± 207 | 1361 ± 139 |
| Internal tibial torque, N·m | 1.0 ± 1.0 | 1.0 ± 0.5 | 17.6 ± 4.9 | 19.5 ± 4.9 | 0.9 ± 0.9 | 1.2 ± 0.9 |
| Outcomes | ||||||
| Quadriceps force, N | 774 ± 106 | 806 ± 118 | 888 ± 128 | 928 ± 115 | 772 ± 91 | 783 ± 163 |
| Knee flexion angle, deg | 7.1 ± 2.1b | 4.6 ± 0.7 | 8.9 ± 2.3 | 6.5 ± 1.0 | 7.1 ± 2.2 | 4.7 ± 0.9 |
| Anterior tibial translation, mm | 2.5 ± 0.9 | 1.7 ± 0.8 | 4.0 ± 1.1 | 3.4 ± 1.7 | 2.4 ± 1.1 | 1.7 ± 0.7 |
| Internal tibial rotation, deg | 3.5 ± 1.7 | 2.9 ± 1.1 | 16.3 ± 3.2b | 11.6 ± 2.3 | 3.6 ± 1.9 | 2.9 ± 0.9 |
Data presented as mean ± standard deviation.
Using the Bonferroni correction, P < .0028 is significant for the gender comparisons in this table.
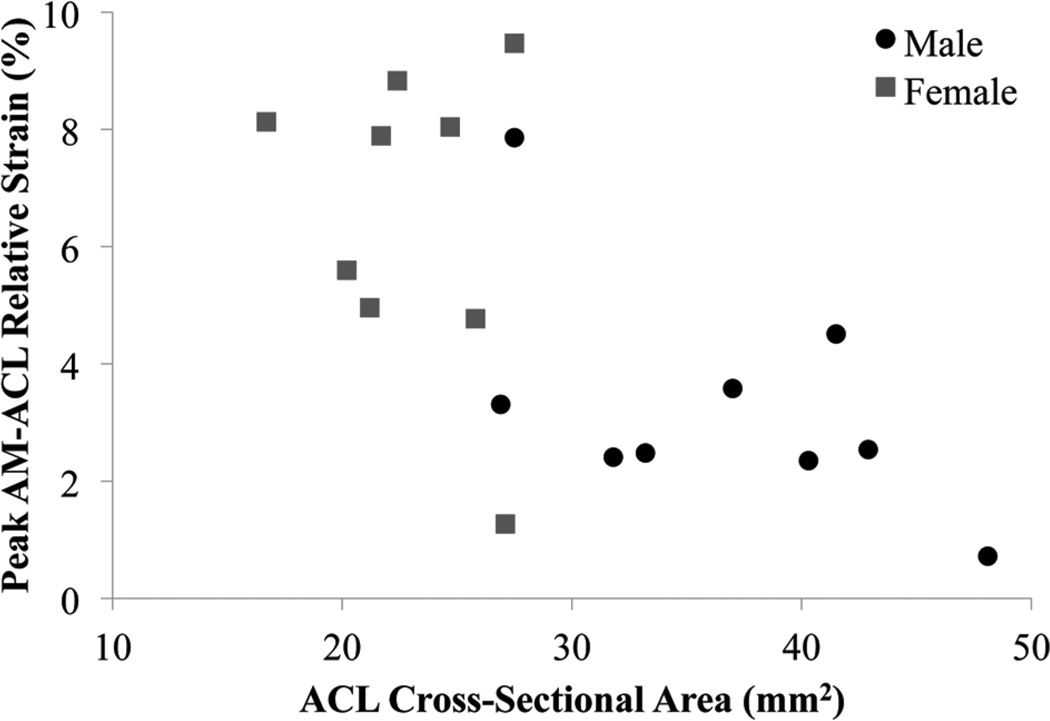
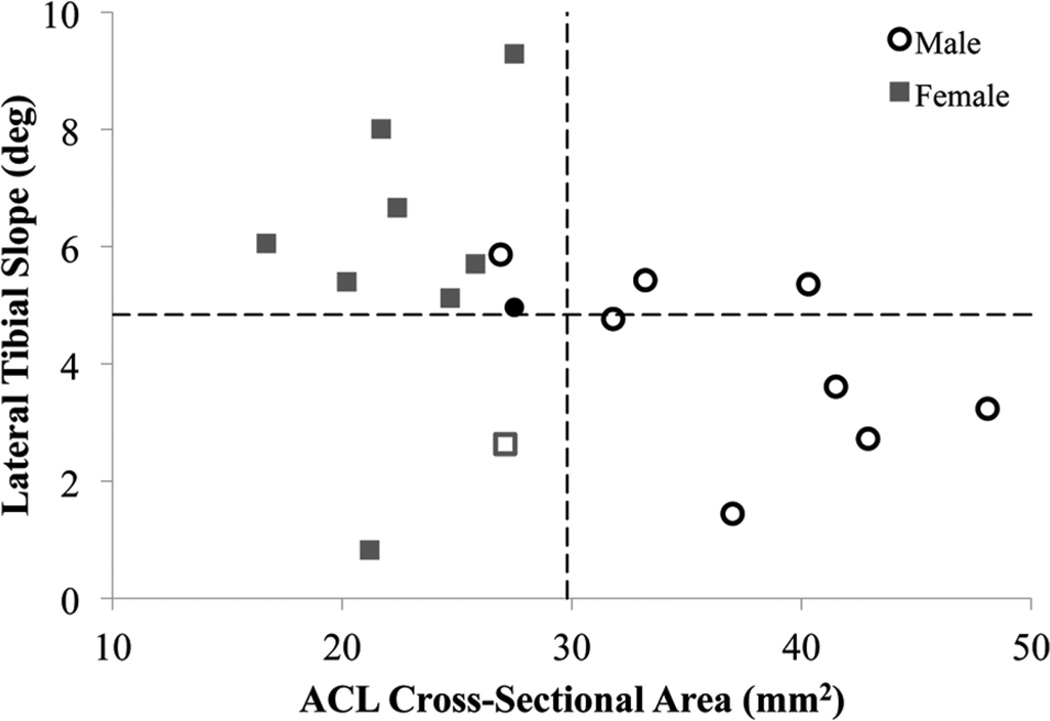
The secondary hypothesis H2 was rejected because ACL CSA (standardized β = –0.480, P = .015; Figure 5) and LTS (β = 0.459, P = .016) were significant coefficients within the linear regression with peak AM-ACL relative strain (R2 = .59, P = .001). Knees with increased peak AM-ACL relative strain had increased LTS and decreased ACL CSA, relative to the mean values of the entire subset of knees tested (Figure 6). The stepwise regression eliminated gender and MTD as significant coefficients. There were significant gender differences in ACL CSA, MTD, and bicondylar width, while LTS and MTS did not reach the level of significance (Table 3).
Figure 5.
A scatter plot of peak AM-ACL (anteromedial bundle of the anterior cruciate ligament) relative strain versus ACL cross-sectional area for 18 knees under the simulated pivot-landing testing block B. Male knees are represented by black circles and female knees are represented by gray squares.
Figure 6.
A scatter plot of peak AM-ACL (anteromedial bundle of the anterior cruciate ligament) relative strain versus ACL cross-sectional area for 18 knees under the simulated pivot-landing testing block B. Dashed lines indicate the mean lateral tibial slope and ACL cross-sectional area for the entire group. Male knees are represented by circles and female knees are represented by squares. A filled marker indicates a peak AM-ACL relative strain greater than the median strain, and an unfilled marker indicates a peak AM-ACL relative strain less than the median strain.
TABLE 3.
Descriptive Statistics for the Knee Morphologic Parametersa
| Female | Male | |
|---|---|---|
| ACL cross-sectional area, mm2 | 24.2 ± 8.4b | 39.6 ± 11.7 |
| Lateral tibial slope, deg | 5.3 ± 2.5 | 4.3 ± 1.7 |
| Medial tibial slope, deg | 4.8 ± 1.9 | 4.2 ± 2.8 |
| Medial tibial depth, mm | 1.6 ± 0.6c | 2.5 ± 0.8 |
| Femoral bicondylar width, mm | 67.8 ± 4.3c | 73.8 ± 3.9 |
Data presented as mean ± standard deviation. ACL, anterior cruciate ligament.
P < .001 between genders.
P < .05 between genders.
DISCUSSION
This study demonstrates a gender difference in peak AM-ACL relative strain in knees from age-, height-, and weight-matched male and female cadavers placed under 3D standardized impulsive loads simulating a unipedal pivot landing. Our results support the primary hypothesis that female knees equipped with realistic female muscle equivalents will develop greater peak AM-ACL relative strain than male knees equipped with male muscle equivalents. It is noteworthy that the female knees demonstrated greater peak AM-ACL relative strain with and without the addition of a 17.5-N·m internal tibial torque to the testing protocol.
This study utilized a novel nonlinear quadriceps spring in vitro to model the known in vivo force-length behavior of a whole muscle placed under rapid stretch,20 since the quadriceps muscle will rapidly stretch during the first 100 milliseconds of a jump landing.28 Whole muscle exhibits both elastic and viscous (time-dependent) behavior under sudden stretch49: its short-range stiffness is dominated by its strongly bound sarcomeres and its longer range behavior is determined by reflex, elastic, and viscous behaviors. These properties combine to yield the nonlinear behavior that has been shown in frog muscle fibrils,14,15,30,49 feline muscle fibers,32 and rabbit whole muscle20 under rapid stretch. Because the quadriceps stretch phase only lasts 80 milliseconds, this interval is too short for any reflex38 or volitional changes in muscle activation to substantially affect quadriceps force, so the nonlinear spring representation of muscle stretch behavior is reasonable.
Muscles are the primary active joint stabilizers helping to protect knee ligaments from injury. A third novel feature of this study is the experimental design: male knees were equipped with male muscle equivalents and female knees with female muscle equivalents. This design was based on the knowledge that women have lower whole-body muscle mass than men,29 leading to female knee muscles developing less shear57 and torsional26,41,46,59 resistance at the knee. Because of lower muscle mass, women develop less active muscle stiffness in their quadriceps18 and hamstrings4,5,18 than do men. Hence, the female quadriceps spring device was designed to have 20% less quadriceps stiffness18 because women have approximately 20% less knee muscle extensor strength42–44,57 (the maximal tensile stiffness of a muscle being proportional to its muscle strength6).
The effect of gender on peak ACL strain has only been examined in 2 prior studies. Mizuno et al35 compared gender-specific models of ACL strain using both a computer simulation and modest quasistatic loading of cadaver knees in the absence of muscle forces. Their models exhibited a 65% greater strain in females under a 10-N·m internal tibial torque combined with a 10-N·m valgus moment when the knee was in 15° of flexion. Our study corroborates and extends the work of Mizuno et al by representing a pivot landing under realistic athletic ground reactions (2 × BW impulsive compressive load and 17.5-N·m internal tibial torque, along with realistic muscle forces). Weinhold et al53 also demonstrated greater AM-ACL relative strain in knees of unspecified gender with quasistatic female muscle loads. Our study also extends the findings of Weinhold et al by dynamically modeling the muscle response to a pivot landing, rather than using a quasistatic approach.
The gender difference in ACL strain appears to be partially caused by gender differences in ACL CSA and the modulus of elasticity. A hyperelastic model predicts a 1.67-fold difference in ACL strain between males and females based on the gender difference in ACL CSAs reported in this study and in the published moduli of elasticity11 (Appendix A, available online). Hence, the gender difference in ACL CSA and modulus of elasticity theoretically explains about two-thirds of the observed 95% gender difference in peak ACL strain. Lateral tibial slope, as demonstrated in our regression model, explains some of the remaining gender difference in ACL strain. Our theoretical analysis in the Appendix assumed that the ACL primarily exhibits hyperelastic behavior9,10,60; we assume that viscous effects are small because hysteresis was minimal after several loading cycles. Although there was a trend toward a gender difference in peak AM-ACL strain rate, the 30% difference in strain rate would have little effect on ACL behavior.61
The ACL CSAs in the present study are smaller than those reported, for example, by Chandrashekar et al11 and Dienst et al.13 Our measurements differ from the Chandrashekar et al study because a different imaging modality (3D scanner) was used, and their study did not control for the height and weight of the specimens; these studies used larger specimens than in the present study. We utilized a similar method for measuring ACL CSA as Dienst et al; they measured ACL CSA at midsubstance. We measured the ACL CSA at 30% ligament length from the tibial insertion because it corresponds with the location of the DVRT during testing and is the minimum ACL CSA.21
The present study finds a significant inverse linear relationship between peak AM-ACL relative strain and ACL CSA (Figure 5). Although Chandrashekar et al11 examined the relationship between ACL size and strain using tensile testing of the femur-ACL-tibia complex, they failed to demonstrate any relationship between the ACL strain at failure and the minimum area of the ligament. Our results agree with recent work showing that ACL-injured subjects have a smaller ACL volume in the contralateral knee when compared with matched controls, with gender being an insignificant regression covariate.12 Lateral tibial slope has been identified as an important risk factor in ACL injuries,8,22 but we are unaware of a previous study that has demonstrated a strong linear relationship between LTS and ACL strain. It is plausible that a greater LTS will increase the anterior tibial translation and internal rotation of the tibia during a pivot landing, thus leading to an increase in ACL strain.
The strengths of this study include the repeated-measures design; the use of age-, height-, and weight-matched cadavers; the inclusion of muscle forces including a nonlinear representation of quadriceps behavior; and a blinded observer to measure the morphologic parameters on the MRI scans. The study has limitations unlikely to affect the overall findings. First, the strain was only measured within the anteromedial region of the ACL and may not represent the overall strain on the ligament. However, Markolf et al33 found a strong relationship between AM-ACL strain and overall ACL force. In addition, we were only able to record relative strain, rather than absolute strain. The absolute strain on the ACL is likely larger than the relative strain because the pretensioned muscle forces place a preload on the ligament before impact. Although the cadaver donors were age-, height-, and weight-matched, the female knees were smaller than the male knees, indicated by a gender difference in bicondylar width. While ACL CSA and LTS are important determinants of ACL strain in a pivot landing, they explain only 60% of the variance in the strain data. Other potential sources of variance include the ligament’s modulus of elasticity, indicated by the hyperelastic model, and other morphologic parameters including MTD, MTS, and femoral notch size. Increasing the sample size would likely increase the number of significant morphologic parameters within our regression model, including MTD. Although currently insignificant within our regression model, MTD was significantly smaller in the female knees in our study and previous work has indicated that a 1-mm reduction in MTD will triple the odds of an ACL injury occurring.22
We conclude that during a pivot landing, height- and weight-matched female knees experience systematically greater ACL strain than male knees because of a smaller ACL CSA. In addition, the trend toward a greater LTS in females proved to be a significant predictor of ACL strain. Future training and ACL injury prevention programs might usefully benefit from considering these morphologic factors.
ACKNOWLEDGMENT
We thank Ms Ashley Brower for aiding in the procurement and preparation of the specimens and Mr Charles Roehm for machining the housing for the spring device. We thank the donors and their families, and Dr Catherine Brandon and Mrs Suzan Lowe for helping with the magnetic resonance images.
source of funding: Funding was provided by PHS Grant R01 ARO54821 and an NDSEG Fellowship (to D.B.L.).
Footnotes
Presented as a poster at the 37th annual meeting of the AOSSM, San Diego, California, July 2011.
One or more of the authors has declared the following potential conflict of interest
REFERENCES
- 1.Agel J, Arendt EA, Bershadsky B. Anterior cruciate ligament injury in National Collegiate Athletic Association basketball and soccer: a 13-year review. Am J Sports Med. 2005;33(4):524–530. doi: 10.1177/0363546504269937. [DOI] [PubMed] [Google Scholar]
- 2.Anderson AF, Dome DC, Gautam S, Awh MH, Rennirt GW. Correlation of anthropometric measurements, strength, anterior cruciate ligament size, and intercondylar notch characteristics to sex differences in anterior cruciate ligament tear rates. Am J Sports Med. 2001;29(1):58–66. doi: 10.1177/03635465010290011501. [DOI] [PubMed] [Google Scholar]
- 3.Arendt E, Dick R. Knee injury patterns among men and women in collegiate basketball and soccer: NCAA data and review of literature. Am J Sports Med. 1995;23(6):694–701. doi: 10.1177/036354659502300611. [DOI] [PubMed] [Google Scholar]
- 4.Blackburn JT, Bell DR, Norcross MF, Hudson JD, Kimsey MH. Sex comparison of hamstring structural and material properties. Clin Biomech (Bristol, Avon) 2009;24(1):65–70. doi: 10.1016/j.clinbiomech.2008.10.001. [DOI] [PubMed] [Google Scholar]
- 5.Blackburn JT, Padua DA, Riemann BL, Guskiewicz KM. The relationships between active extensibility, and passive and active stiffness of the knee flexors. J Electromyogr Kinesiol. 2004;14(6):683–691. doi: 10.1016/j.jelekin.2004.04.001. [DOI] [PubMed] [Google Scholar]
- 6.Blanpied P, Smidt GL. The difference in stiffness of the active plantar-flexors between young and elderly human females. J Gerontol. 1993;48(2):M58–M63. doi: 10.1093/geronj/48.2.m58. [DOI] [PubMed] [Google Scholar]
- 7.Boden BP, Dean GS, Feagin JA, Jr, Garrett WE., Jr Mechanisms of anterior cruciate ligament injury. Orthopedics. 2000;23(6):573–578. doi: 10.3928/0147-7447-20000601-15. [DOI] [PubMed] [Google Scholar]
- 8.Brandon ML, Haynes PT, Bonamo JR, Flynn MI, Barrett GR, Sherman MF. The association between posterior-inferior tibial slope and anterior cruciate ligament insufficiency. Arthroscopy. 2006;22(8):894–899. doi: 10.1016/j.arthro.2006.04.098. [DOI] [PubMed] [Google Scholar]
- 9.Butler DL, Guan Y, Kay MD, Cummings JF, Feder SM, Levy MS. Location-dependent variations in the material properties of the anterior cruciate ligament. J Biomech. 1992;25(5):511–518. doi: 10.1016/0021-9290(92)90091-e. [DOI] [PubMed] [Google Scholar]
- 10.Butler DL, Kay MD, Stouffer DC. Comparison of material properties in fascicle-bone units from human patellar tendon and knee ligaments. J Biomech. 1986;19(6):425–432. doi: 10.1016/0021-9290(86)90019-9. [DOI] [PubMed] [Google Scholar]
- 11.Chandrashekar N, Mansouri H, Slauterbeck J, Hashemi J. Sex-based differences in the tensile properties of the human anterior cruciate ligament. J Biomech. 2006;39(16):2943–2950. doi: 10.1016/j.jbiomech.2005.10.031. [DOI] [PubMed] [Google Scholar]
- 12.Chaudhari AM, Zelman EA, Flanigan DC, Kaeding CC, Nagaraja HN. Anterior cruciate ligament-injured subjects have smaller anterior cruciate ligaments than matched controls: a magnetic resonance imaging study. Am J Sports Med. 2009;37(7):1282–1287. doi: 10.1177/0363546509332256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dienst M, Schneider G, Altmeyer K, et al. Correlation of intercondylar notch cross sections to the ACL size: a high resolution MR tomographic in vivo analysis. Arch Orthop Trauma Surg. 2007;127(4):253–260. doi: 10.1007/s00402-006-0177-7. [DOI] [PubMed] [Google Scholar]
- 14.Edman KA, Elzinga G, Noble MI. Enhancement of mechanical performance by stretch during tetanic contractions of vertebrate skeletal muscle fibres. J Physiol. 1978;281:139–155. doi: 10.1113/jphysiol.1978.sp012413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Edman KA, Elzinga G, Noble MI. Residual force enhancement after stretch of contracting frog single muscle fibers. J Gen Physiol. 1982;80(5):769–784. doi: 10.1085/jgp.80.5.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford KR, Myer GD, Toms HE, Hewett TE. Gender differences in the kinematics of unanticipated cutting in young athletes. Med Sci Sports Exerc. 2005;37(1):124–129. [PubMed] [Google Scholar]
- 17.Fung YC. Elasticity of soft tissues in simple elongation. Am J Physiol. 1967;213(6):1532–1544. doi: 10.1152/ajplegacy.1967.213.6.1532. [DOI] [PubMed] [Google Scholar]
- 18.Granata KP, Wilson SE, Padua DA. Gender differences in active musculoskeletal stiffness, part I: quantification in controlled measurements of knee joint dynamics. J Electromyogr Kinesiol. 2002;12(2):119–126. doi: 10.1016/s1050-6411(02)00002-0. [DOI] [PubMed] [Google Scholar]
- 19.Griffin LY, Albohm MJ, Arendt EA, et al. Understanding and preventing noncontact anterior cruciate ligament injuries: a review of the Hunt Valley II meeting, January 2005. Am J Sports Med. 2006;34(9):1512–1532. doi: 10.1177/0363546506286866. [DOI] [PubMed] [Google Scholar]
- 20.Grover JP, Corr DT, Toumi H, et al. The effect of stretch rate and activation state on skeletal muscle force in the anatomical range. Clin Biomech (Bristol, Avon) 2007;22(3):360–368. doi: 10.1016/j.clinbiomech.2006.10.009. [DOI] [PubMed] [Google Scholar]
- 21.Hashemi J, Chandrashekar N, Cowden C, Slauterbeck J. An alternative method of anthropometry of anterior cruciate ligament through 3-D digital image reconstruction. J Biomech. 2005;38(3):551–555. doi: 10.1016/j.jbiomech.2004.04.010. [DOI] [PubMed] [Google Scholar]
- 22.Hashemi J, Chandrashekar N, Mansouri H, et al. Shallow medial tibial plateau and steep medial and lateral tibial slopes: new risk factors for anterior cruciate ligament injuries. Am J Sports Med. 2010;38(1):54–62. doi: 10.1177/0363546509349055. [DOI] [PubMed] [Google Scholar]
- 23.Hashemi J, Chandrashekar N, Mansouri H, Slauterbeck JR, Hardy DM. The human anterior cruciate ligament: sex differences in ultrastructure and correlation with biomechanical properties. J Orthop. Res. 2008;26(7):945–950. doi: 10.1002/jor.20621. [DOI] [PubMed] [Google Scholar]
- 24.Hewett TE, Myer GD, Ford KR, et al. Biomechanical measures of neuromuscular control and valgus loading of the knee predict anterior cruciate ligament injury risk in female athletes: a prospective study. Am J Sports Med. 2005;33(4):492–501. doi: 10.1177/0363546504269591. [DOI] [PubMed] [Google Scholar]
- 25.Hootman JM, Dick R, Agel J. Epidemiology of collegiate injuries for 15 sports: summary and recommendations for injury prevention initiatives. J Athl Train. 2007;42(2):311–319. [PMC free article] [PubMed] [Google Scholar]
- 26.Hsu WH, Fisk JA, Yamamoto Y, Debski RE, Woo SL. Differences in torsional joint stiffness of the knee between genders: a human cadaveric study. Am J Sports Med. 2006;34(5):765–770. doi: 10.1177/0363546505282623. [DOI] [PubMed] [Google Scholar]
- 27.Hudek R, Schmutz S, Regenfelder F, Fuchs B, Koch PP. Novel measurement technique of the tibial slope on conventional MRI. Clin Orthop Relat Res. 2009;467(8):2066–2072. doi: 10.1007/s11999-009-0711-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishikawa M, Niemela E, Komi PV. Interaction between fascicle and tendinous tissues in short-contact stretch-shortening cycle exercise with varying eccentric intensities. J Appl Physiol. 2005;99(1):217–223. doi: 10.1152/japplphysiol.01352.2004. [DOI] [PubMed] [Google Scholar]
- 29.Janssen I, Heymsfield SB, Wang ZM, Ross R. Skeletal muscle mass and distribution in 468 men and women aged 18–88 yr. J Appl Physiol. 2000;89(1):81–88. doi: 10.1152/jappl.2000.89.1.81. [DOI] [PubMed] [Google Scholar]
- 30.Julian FJ, Morgan DL. The effect on tension of non-uniform distribution of length changes applied to frog muscle fibres. J Physiol. 1979;293:379–392. doi: 10.1113/jphysiol.1979.sp012895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lohmander LS, Englund PM, Dahl LL, Roos EM. The long-term consequence of anterior cruciate ligament and meniscus injuries: osteoarthritis. Am J Sports Med. 2007;35(10):1756–1769. doi: 10.1177/0363546507307396. [DOI] [PubMed] [Google Scholar]
- 32.Malamud JG, Godt RE, Nichols TR. Relationship between short-range stiffness and yielding in type-identified, chemically skinned muscle fibers from the cat triceps surae muscles. J Neurophysiol. 1996;76(4):2280–2289. doi: 10.1152/jn.1996.76.4.2280. [DOI] [PubMed] [Google Scholar]
- 33.Markolf KL, Gorek JF, Kabo JM, Shapiro MS. Direct measurement of resultant forces in the anterior cruciate ligament. An in vitro study performed with a new experimental technique. J Bone Joint Surg Am. 1990;72(4):557–567. [PubMed] [Google Scholar]
- 34.Mihata LC, Beutler AI, Boden BP. Comparing the incidence of anterior cruciate ligament injury in collegiate lacrosse, soccer, and basketball players: implications for anterior cruciate ligament mechanism and prevention. Am J Sports Med. 2006;34(6):899–904. doi: 10.1177/0363546505285582. [DOI] [PubMed] [Google Scholar]
- 35.Mizuno K, Andrish JT, van den Bogert AJ, McLean SG. Gender dimorphic ACL strain in response to combined dynamic 3D knee joint loading: implications for ACL injury risk. Knee. 2009;16(6):432–440. doi: 10.1016/j.knee.2009.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mountcastle SB, Posner M, Kragh JF, Jr, Taylor DC. Gender differences in anterior cruciate ligament injury vary with activity: epidemiology of anterior cruciate ligament injuries in a young, athletic population. Am J Sports Med. 2007;35(10):1635–1642. doi: 10.1177/0363546507302917. [DOI] [PubMed] [Google Scholar]
- 37.Muneta T, Takakuda K, Yamamoto H. Intercondylar notch width and its relation to the configuration and cross-sectional area of the anterior cruciate ligament: a cadaveric knee study. Am J Sports Med. 1997;25(1):69–72. doi: 10.1177/036354659702500113. [DOI] [PubMed] [Google Scholar]
- 38.Nichols TR, Houk JC. Improvement in linearity and regulation of stiffness that results from actions of stretch reflex. J Neurophysiol. 1976;39(1):119–142. doi: 10.1152/jn.1976.39.1.119. [DOI] [PubMed] [Google Scholar]
- 39.Oh YK, Kreinbrink JL, Ashton-Miller JA, Wojtys EM. Effect of ACL transection on internal tibial rotation in an in vitro simulated pivot landing. J Bone Joint Surg Am. 2011;93(4):372–380. doi: 10.2106/JBJS.J.00262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Olsen OE, Myklebust G, Engebretsen L, Bahr R. Injury mechanisms for anterior cruciate ligament injuries in team handball: a systematic video analysis. Am J Sports Med. 2004;32(4):1002–1012. doi: 10.1177/0363546503261724. [DOI] [PubMed] [Google Scholar]
- 41.Park HS, Wilson NA, Zhang LQ. Gender differences in passive knee biomechanical properties in tibial rotation. J Orthop Res. 2008;26(7):937–944. doi: 10.1002/jor.20576. [DOI] [PubMed] [Google Scholar]
- 42.Pincivero DM, Dixon PT, Coelho AJ. Knee extensor torque, work, and EMG during subjectively graded dynamic contractions. Muscle Nerve. 2003;28(1):54–61. doi: 10.1002/mus.10393. [DOI] [PubMed] [Google Scholar]
- 43.Pincivero DM, Gandaio CM, Ito Y. Gender-specific knee extensor torque, flexor torque, and muscle fatigue responses during maximal effort contractions. Eur J Appl Physiol. 2003;89(2):134–141. doi: 10.1007/s00421-002-0739-5. [DOI] [PubMed] [Google Scholar]
- 44.Pincivero DM, Salfetnikov Y, Campy RM, Coelho AJ. Angle- and gender- specific quadriceps femoris muscle recruitment and knee extensor torque. J Biomech. 2004;37(11):1689–1697. doi: 10.1016/j.jbiomech.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 45.Prodromos CC, Han Y, Rogowski J, Joyce B, Shi K. A meta-analysis of the incidence of anterior cruciate ligament tears as a function of gender, sport, and a knee injury-reduction regimen. Arthroscopy. 2007;23(12):1320–1325. e6. doi: 10.1016/j.arthro.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 46.Schmitz RJ, Ficklin TK, Shimokochi Y, et al. Varus/valgus and internal/ external torsional knee joint stiffness differs between sexes. Am J Sports Med. 2008;36(7):1380–1388. doi: 10.1177/0363546508317411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shigley JE, Mitchell LD. Mechanical Engineering Design. 4th ed. New York, NY: McGraw-Hill; 1983. [Google Scholar]
- 48.Shultz SJ, Schmitz RJ, Beynnon BD. Variations in varus/valgus and internal/external rotational knee laxity and stiffness across the menstrual cycle. J Orthop Res. 2011;29(3):318–325. doi: 10.1002/jor.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Taylor DC, Dalton JD, Jr, Seaber AV, Garrett WE., Jr Viscoelastic properties of muscle-tendon units: the biomechanical effects of stretching. Am J Sports Med. 1990;18(3):300–309. doi: 10.1177/036354659001800314. [DOI] [PubMed] [Google Scholar]
- 50.Thornton GM, Schwab TD, Oxland TR. Cyclic loading causes faster rupture and strain rate than static loading in medial collateral ligament at high stress. Clin Biomech (Bristol, Avon) 2007;22(8):932–940. doi: 10.1016/j.clinbiomech.2007.05.004. [DOI] [PubMed] [Google Scholar]
- 51.Uhorchak JM, Scoville CR, Williams GN, Arciero RA, St Pierre P, Taylor DC. Risk factors associated with noncontact injury of the anterior cruciate ligament: a prospective four-year evaluation of 859 West Point cadets. Am J Sports Med. 2003;31(6):831–842. doi: 10.1177/03635465030310061801. [DOI] [PubMed] [Google Scholar]
- 52.Vrooijink SH, Wolters F, Van Eck CF, Fu FH. Measurements of knee morphometrics using MRI and arthroscopy: a comparative study between ACL-injured and non-injured subjects [published online ahead of print April 7, 2011] Knee Surg Sports Traumatol Arthrosc. doi: 10.1007/s00167-011-1502-4. [DOI] [PubMed] [Google Scholar]
- 53.Weinhold PS, Stewart JD, Liu HY, Lin CF, Garrett WE, Yu B. The influence of gender-specific loading patterns of the stop-jump task on anterior cruciate ligament strain. Injury. 2007;38(8):973–978. doi: 10.1016/j.injury.2006.12.024. [DOI] [PubMed] [Google Scholar]
- 54.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The effect of an impulsive knee valgus moment on in vitro relative ACL strain during a simulated jump landing. Clin Biomech (Bristol, Avon) 2006;21(9):977–983. doi: 10.1016/j.clinbiomech.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 55.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. Effect of varying hamstring tension on anterior cruciate ligament strain during in vitro impulsive knee flexion and compression loading. J Bone Joint Surg Am. 2008;90(4):815–823. doi: 10.2106/JBJS.F.01352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Withrow TJ, Huston LJ, Wojtys EM, Ashton-Miller JA. The relationship between quadriceps muscle force, knee flexion, and anterior cruciate ligament strain in an in vitro simulated jump landing. Am J Sports Med. 2006;34(2):269–274. doi: 10.1177/0363546505280906. [DOI] [PubMed] [Google Scholar]
- 57.Wojtys EM, Ashton-Miller JA, Huston LJ. A gender-related difference in the contribution of the knee musculature to sagittal-plane shear stiffness in subjects with similar knee laxity. J Bone Joint Surg Am. 2002;84(1):10–16. doi: 10.2106/00004623-200201000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Wojtys EM, Huston LJ, Boynton MD, Spindler KP, Lindenfeld TN. The effect of the menstrual cycle on anterior cruciate ligament injuries in women as determined by hormone levels. Am J Sports Med. 2002;30(2):182–188. doi: 10.1177/03635465020300020601. [DOI] [PubMed] [Google Scholar]
- 59.Wojtys EM, Huston LJ, Schock HJ, Boylan JP, Ashton-Miller JA. Gender differences in muscular protection of the knee in torsion in size-matched athletes. J Bone Joint Surg Am. 2003;85(5):782–789. doi: 10.2106/00004623-200305000-00002. [DOI] [PubMed] [Google Scholar]
- 60.Woo SL, Newton PO, MacKenna DA, Lyon RM. A comparative evaluation of the mechanical properties of the rabbit medial collateral and anterior cruciate ligaments. J Biomech. 1992;25(4):377–386. doi: 10.1016/0021-9290(92)90257-2. [DOI] [PubMed] [Google Scholar]
- 61.Woo SL, Peterson RH, Ohland KJ, Sites TJ, Danto MI. The effects of strain rate on the properties of the medial collateral ligament in skeletally immature and mature rabbits: a biomechanical and histological study. J Orthop Res. 1990;8(5):712–721. doi: 10.1002/jor.1100080513. [DOI] [PubMed] [Google Scholar]