Abstract
The maternal-fetal environment plays an important role in developmental programming of adult disease. Metabolic and hormonal dysfunction during human fetal development accompanies gestational diabetes as a common occurrence in polycystic ovary syndrome (PCOS) mothers, while human fetal androgen excess from congenital adrenal hyperplasia or virilizing tumors precedes PCOS-like symptoms after birth. To date, clinical studies of infant blood levels at term have yet to confirm that human fetal androgen excess promotes PCOS development after birth. Earlier in development, however, circulating androgen levels in the second trimester female human fetus can normally rise into the male range. Furthermore, midgestational amniotic testosterone levels are elevated in female fetuses of PCOS compared to normal mothers and might influence fetal development, since experimentally-induced fetal androgen excess in animals produces a PCOS-like phenotype with reproductive and metabolic dysfunction. Such alterations in the maternal-fetal environment likely program adult PCOS by epigenetic modifications of genetic susceptibility of the fetus to PCOS after birth. Understanding this phenomenon requires advanced fetal surveillance technologies and postnatal assessment of midgestational androgen exposure for new clinical strategies to improve reproduction in PCOS women, optimize long-term health of their offspring, and minimize susceptibility to acquiring PCOS in future generations.
Keywords: polycystic ovary syndrome (PCOS), hyperandrogenism, hyperinsulinemia, adiposity, developmental programming, fetal development
Introduction
Polycystic ovary syndrome (PCOS) is a heterogeneous syndrome in women characterized by luteinizing hormone (LH) hypersecretion, ovarian hyperandrogenism, oligo-anovulation and hyperinsulinemia from insulin resistance. The 1990 National Institutes of Health (NIH)-National Institute of Child Health and Human Development-Conference of PCOS in 1990 recommended that the diagnostic criteria should be hyperandrogenism and/or hyperandrogenemia with oligo-anovulation, excluding other endocrinopathies, including congenital adrenal hyperplasia (CAH), Cushing's syndrome, thyroid dysfunction, hyperprolactinemia, androgen-producing tumors and drug-induced androgen excess1. With a 6.6% estimated prevalence of NIH-defined PCOS in the United States, the annual economic burden of treating PCOS-related diabetes, menstrual dysfunction and anovulatory infertility was $4.4 billion in 2005, not considering inflation or pregnancy-related complications, including gestational diabetes, preeclampsia and miscarriage2.
In 2003, the Rotterdam consensus expanded the diagnostic criteria of PCOS to include at least two of the following three features: 1) clinical or biochemical hyperandrogenism, 2) oligo-anovulation, and 3) polycystic ovaries (PCO), excluding the previously described endocrinopathies3. These newer Rotterdam criteria for PCOS combine all patients defined by 1990 NIH criteria (i.e., classic PCOS) with additional women with either 1) clinical/biochemical hyperandrogenism and PCO (i.e., ovulatory PCOS) or 2) PCO with ovulatory dysfunction (but without signs of androgen excess). As a result, the 6–10% worldwide prevalence of PCOS by 1990 NIH criteria has increased to about twice that level using broader Rotterdam criteria due to the inclusion of multiple PCOS phenotypes4–6. As the most common PCOS phenotype, classic PCOS has the greatest reproductive and metabolic dysfunction7–8, while ovulatory PCOS patients have a lower body mass index (BMI) and lesser degrees of hyperinsulinemia and hyperandrogenism than classic PCOS patients. Women with combined PCO and oligo-anovulation (without androgen excess), who do not fulfill the diagnosis of PCOS by the Androgen Excess Society, appear least affected7–9.
Emerging data suggest that the phenotypic expression of adult PCOS may be influenced by the endocrine-metabolic status of the maternal-fetal environment5. This hypothesis agrees with the increased prevalence of PCOS in women with classical congenital adrenal hyperplasia (CAH) from 21 hydroxylase deficiency or congenital adrenal virilizing tumors10–13, and with the ability of discrete experimentally-induced prenatal testosterone excess to program a permanent PCOS-like phenotype in several species14.
This paper addresses the developmental origins of PCOS, whereby maternal maladaptations to pregnancy at a critical gestational age permanently alter fetal susceptibility to PCOS phenotypic expression after birth. This theory of developmental origins of PCOS is based upon the premise that alterations in the maternal-fetal environment permanently program adult disease by epigenetic modifications of genetic susceptibility of the fetus to disease after birth.
Endocrine antecedent to PCOS
Women with congenital adrenal virilizing tumors or classical CAH from 21 hydroxylase deficiency have an increased risk of developing a PCOS-like syndrome in adulthood10–13. Such gestational susceptibility to androgens implicates exposure of the female fetus to androgen excess as a modifier of PCOS phenotypic expression after birth. To date, however, a relationship between hyperandrogenism in utero and PCOS phenotypic expression in adulthood has been elusive. Women born from opposite sex-twins, for example, do not display an increased prevalence of PCOS-like features, although presumably they share a prenatal environment with a male co-twin that increases their exposure to testosterone15. Using relative finger length as an anatomical marker of in utero androgen exposure, some16–17, but not all18–19, PCOS women have altered length of the second finger relative to the fourth finger as a male characteristic that correlates with hyperandrogenism and ovarian volume (Table 1)18–20. Adult female rhesus monkeys with PCOS-like features induced by early-to-midgestation testosterone excess also exhibit alteration of the same finger length ratio, implying an association between PCOS and finger length mediated by prenatal androgen excess21. Elevated umbilical cord testosterone levels also occur in some22–23, but not all24–25, newborns of PCOS mothers, but such study findings are inconsistent, perhaps from differences in placental steroidogenesis24–25 or to cord blood collection at term22,24,26, a time point past the critical period of human ovarian differentiation27–29.
Table 1.
Reduced second to fourth digit (finger) ratio as a marker of androgen excess in utero in PCOS women. See references 16–19.
| Author | Participant | PCOS definition |
Findings |
|---|---|---|---|
| Cattrall 2005 | 70 PCOS 70 NL women |
NIH | Reduced 2D:4D ratio in right hand of PCOS vs. normal women |
| Lujan 2010 | 98 PCOS 51 NL women |
Rotterdam | No difference 2D:4D ratio 2D:4D ratio positively correlates with hirsutism, free androgen index and ovarian volume |
| Lujan 2010 | 96 PCOS 48 NL women 50 men |
Rotterdam | No difference 2D:4D ratio by computer analysis 2D:4D ratio positively correlates with hirsutism, testosterone and free androgen index |
| Palomba 2012 | 30 PCOS 545 NL women |
Rotterdam (All androgen excess) |
Increased 2D:4D ratio in 4–11 year old daughters of PCOS vs. normal mothers |
Perhaps more importantly, amniotic fluid testosterone levels in the second trimester of human development are normally higher in the male than the female fetus, allowing a wider range of testosterone levels to differentially affect fetal development compared to term umbilical vein blood testosterone concentrations that are similar between sexes30. Second trimester amniotic fluid testosterone levels also are elevated in female fetuses of PCOS compared to normal mothers (Table 2)17, suggesting that androgen overproduction can occur during human female fetal development under certain pregnancy conditions31. Given these findings, therefore, androgen action during the second trimester of human development may influence the developmental programming of the female fetus, assuming a critical time interval in a susceptible fetus when developmental programming occurs.
Table 2.
Second trimester amniotic fluid testosterone levels (nmol/L) from pregnant women with and without hyperandrogenic polycystic ovary syndrome. See reference 17.]
| N | Mean | SD | |
|---|---|---|---|
| Control mothers | |||
| Male fetus | 21 | 0.80* | 0.16 |
| Female fetus | 24 | 0.36 | 0.10 |
| PCOS mothers | |||
| Male fetus | 17 | 0.87* | 0.21 |
| Female fetus | 13 | 0.53¶ | 0.12 |
, P< 0.001 fetal sex difference
, P< 0.02 female fetus, control vs. PCOS mother
Ontogeny of the human fetal ovary
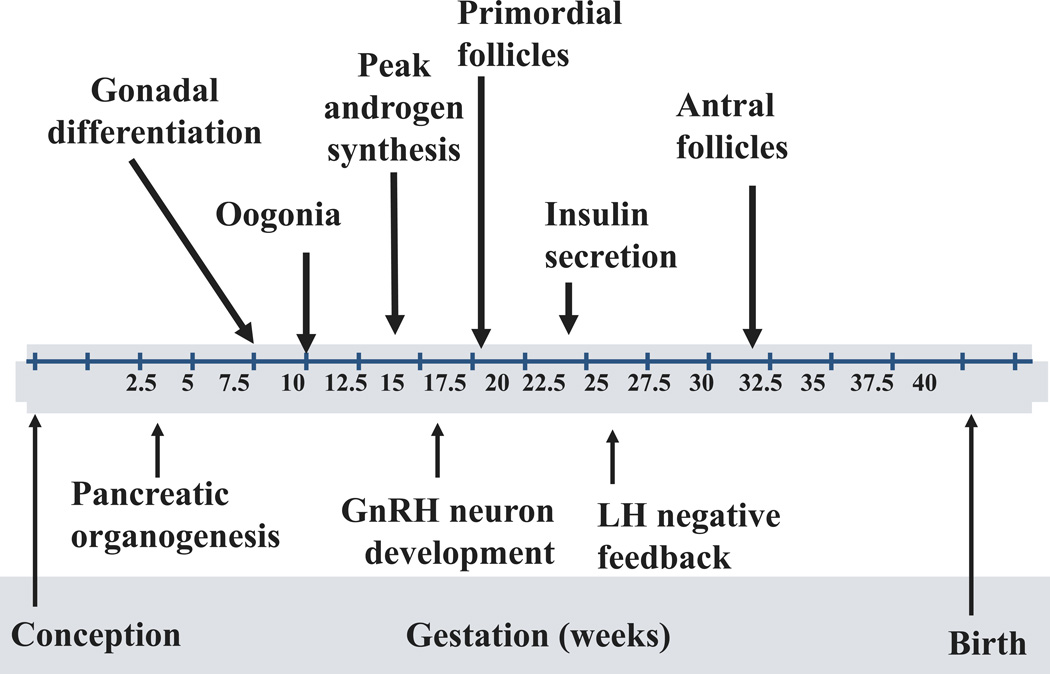
If true, a pivotal issue is the ability of the second trimester human fetal ovary to produce androgen during early folliculogenesis. Human ovarian folliculogenesis begins in early fetal development, when germ cells migrate to the gonadal ridge and multiply by mitosis until about 20 weeks of gestation, reaching a maximum number of about 7 million (Figure 1)32. Oogonial mitosis becomes superimposed with meiosis between 14 and 26 weeks of gestation, as ovigerous cords, packed with oogonia and oocytes, develop into abundant primordial and primary follicles, along with occasional secondary follicles27. Simultaneously, a loose network of primary interstitial cells, containing 17a hydroxylase-17, 20-lyase (P450c17), the major steroidogenic enzyme responsible for androgen production, develops within the stroma as it differentiates in response to local paracrine factors, including extracellular matrix proteins, or fibrillins27,33. Prominent between 15 and 19 weeks of gestation, these primary interstitial cells27 are replaced between 27 and 32 weeks gestation by other P450c17 immunoreactive cells, which collect around growing primary follicles to form the theca interna layer of emerging secondary and small Graafian follicles, joined shortly thereafter by additional P450c17 immunoreactive cells in the hilum. By midgestation, the human fetal ovary has the capacity to produce and detect sex steroids, including androgen and estrogen27,29,34–38, with estrogen believed to regulate folliculogenesis and oocyte development in utero39–41. Together, endocrine (i.e., gonadotropins) and paracrine facilitators of follicular growth (i.e., androgens, growth factors such as activin and insulin-like growth factors) interact with survival and atresia factors to establish the maximal germ cell endowment of the fetal ovary, which then diminishes to about 1–2 million at birth and 300,000 by menarche28,32.
Figure 1.
Ontogeny of human fetal development.
During the second trimester of human fetal development, a transient rise of pituitary gonadotropins increases androgen production by the testes compared to the ovary, temporarily elevating circulating androgen levels in the male compared to the female fetus28,42. This sexual dimorphism in androgen production by the human fetus disappears by birth28,42. At midgestation, human fetal ovaries also have several steroidogenic enzymes; genes encoding multiple steroid signaling pathways; and receptors to steroids, insulin, insulin-like growth factor (IGF)-I and IGF-II27,29,34,35. They do not, however, have functional LH-like receptors37. Nevertheless, cultured human fetal ovaries at this gestational age can metabolize pregnenolone sulfate to dehydroepiandrosterone (DHEA) and androstenedione36 and also can secrete in decreasing amounts DHEA, progesterone and estrone; with lesser amounts of androstenedione, estradiol and testosterone37.
Therefore, although lacking functional LH-like receptors, midgestational human fetal ovaries may produce androgens in vivo, particularly in response to insulin, which may contribute to wide variation in fetal androgen production, as evidenced by 40% of midgestational female fetuses having elevated serum androgen levels into the normal male range41,42. This hypothesis agrees with previous reports in diabetic women of elevated amniotic fluid testosterone levels43, along with findings of hirsutism, ovarian theca-lutein cysts and thecal cell hyperplasia in their female stillbirth offspring44,45.
Abnormalities of the PCOS maternal-fetal environment
To date, however, the link between androgen excess in utero and the maternal environment remains unclear. Maternal serum androgen levels in midgestation are greater in PCOS than normal women46, but are unlikely to directly program PCOS in offspring if placental aromatization is normal26, even though reduced aromatase activity in term placenta from PCOS women likely contributes to elevated maternal androgen production25. Rather, an increased risk of developing maternal glucose intolerance in PCOS women may induce intrauterine hyperglycemia, which may stimulate fetal insulin release as a secretegogue for ovarian androgen production and/or folliculogenesis in the female fetus47–50. In support of this, prenatal testosterone administration to female rhesus monkeys impairs maternal-fetal glucose-insulin homeostasis and stimulates fetal insulin release51, consistent with several animal models establishing links between gestational hyperglycemia, fetal androgen excess and various adult PCOS-like phenotypes52.
Such maternal-fetal environment dysfunction may underlie several endocrine antecedents of PCOS previously reported in girls born to PCOS mothers. For example, infant girls born to PCOS mothers exhibit anti-mullerian hormone (AMH) overproduction as a marker of growing follicles, which persists in prepubertal life (along with exaggerated ovarian responsiveness to leuprolide administration) and improves when PCOS mothers receive metformin in pregnancy, beginning at or before conception53,54. In addition, serum leptin levels in newborns of PCOS women positively correlate with birth weight and maternal BMI at midgestation55. During puberty, enlarged ovaries and hyperinsulinemia in female children of PCOS women coexist with LH hypersecretion and androgen excess56–58.
In addition to glucose intolerance, PCOS women in pregnancy also are at increased risk of developing diabetes, pregnancy-induced hypertension, pre-eclampsia, preterm birth, impaired endovascular trophoblast invasion and abnormal placentation17,47–50,59, all of which may alter developmental programming of the infant. Successful fetal adaptation to maternal nutrient overabundance favors the development of large for gestational age infants, contributing to the positive association between maternal BMI at term and infant birth weight24,60,61. On the other hand, impaired fetal nutrient availability from placental insufficiency likely accompanies low infant birth weight associated with maternal diabetes61, PCOS pregnancies in Chilean and Iranian women23,62 and precocious puberty accompanying PCOS in northern Spanish women63. Therefore, different pathophysiological mechanisms, based upon the endocrine-metabolic status of the maternal-fetal environment, likely influence the birth weight of infants born to PCOS women.
Such pathophysiological mechanisms also may exert subtle developmental programming effects on the fetus after birth despite normal infant birth weight24,50,64–67. For example, exposure of female rhesus monkeys to prenatal testosterone excess impairs fetal glucose-insulin homeostasis without affecting infant birth weight and alters the trajectory of neonatal growth after birth51. As adults, female rhesus monkeys exposed to prenatal testosterone show disrupted development of subcutaneous abdominal adipocytes68, mimicking androgen inhibition of human adipose stem cell commitment to predipocyte formation69 and possible effects on a more primitive population of human adipose stem cells with pluripotent stem characteristics70.
Genetic and epigenetic mechanisms
The inherited nature of PCOS has been well established by family and twin studies. The prevalence of PCOS in female first-degree relatives of affected women is 20–40%, substantially higher than the general population prevalence71,72. Twin studies comparing the correlation of PCOS diagnosis between monozygotic and dizygotic twins have estimated the heritability of PCOS as 70%, suggesting that most susceptibility arises from genetic factors73. Heritability is commonly assumed to reflect the effects of inherited genomic variation; however, it may also reflect the effects from shared disease-predisposing environments. The latter is particularly relevant when considering that adverse intrauterine environments may contribute to disease risk, e.g. daughters of a woman with PCOS would be exposed to the same intrauterine environment. Thus, fetal androgen excess and consequent reprogramming may contribute to some portion of the 70% heritability.
Despite little progress in candidate gene studies of small sample sizes, genome-wide association studies (GWAS) in large Chinese cohorts with robust replication recently have identified variants in 11 genomic regions (loci) as risk factors for PCOS74,75. Assuming the gene nearest to each variant is responsible for the risk-altering effect, the GWAS-discoveries include DENND1A, LHCGR, THADA, FSHR, C9orf3, YAP1, RAB5B/SUOX, HMGA2, TOX3, INSR, and SUMO1P1. Only LHCGR, FSHR, and INSR, which encode receptors for LH/hCG, FSH and insulin, respectively, have clear functional relevance to the pathophysiology of PCOS. How the remaining genes might influence PCOS remains unknown.
Nevertheless, variants in some of these established PCOS susceptibility genes might influence fetal reprogramming of PCOS by altering 1) fetal or maternal androgen production, 2) fetal responsiveness to androgen exposure, 3) placental steroid production or clearance, or 4) placenta-related (e.g. placental insufficiency, abnormal placentation) as well as pregnancy-related (e.g. pre-eclampsia, pregnancy-induced hypertension, gestational diabetes) complications. For example, perhaps fetal ovaries with inherited PCOS-predisposing variants in LHCGR, unlike normal fetal ovaries37, express functional receptors, promoting excessive LH-stimulated androgen production at midgestation. Alternatively, INSR variation may increase fetal ovarian responsiveness to insulin, thereby promoting ovarian androgen production in the fetus in response to its own hyperinsulinemia from maternal hyperglycemia. That several PCOS genes (THADA, HMGA2, SUOX) have also been implicated in diabetes76–78 raises the possibility that fetal reprogramming is related to disturbed maternal-fetal glucose homeostasis. Equally important, several of these loci (LHCGR/FSHR region, INSR, TOX3) are associated with anthropometric measures (BMI, waist circumference, height)79,80, which could affect fetal reprogramming through fetal growth; while C9orf3 codes for aminopeptidase O, a testicular and placental protease generating angiotensin IV from angiotensin III81, which might promote placental dysfunction, pregnancy-induced hypertension, and fetal androgen excess.
Although the molecular mechanism of reprogramming by intrauterine events is unknown, epigenetic changes induced by an altered in utero environment is a likely mechanism82. Epigenetics refers to modifications of genomic DNA that can be passed to subsequent generations (such as DNA methylation and histone modification), allowing the environment to have permanent changes on gene expression. Rodents83,84 and rhesus monkeys85 experimentally exposed to androgen excess in utero have been found to exhibit alterations in DNA methylation at specific genes. Of interest, one of these genes is LHCGR83, one of the susceptibility genes discovered by GWAS in humans75, which demethylated would likely be overexpressed and capable of promoting fetal androgen excess and enhancing LH stimulation of adipogenesis86. Such altered LH signaling in visceral fat of LH hypersecreting infant and adult monkeys, exposed to androgen excess in utero, has been linked with differential DNA methylation of specific gene promoter sites in this fat depot85, which may be constrained by testosterone in its capacity to safely store fat68,69.
Future directions
Future studies need to examine how alterations in the maternal-fetal environment program adult PCOS by epigenetic modifications of fetal genetic susceptibility to PCOS after birth. If metabolic disorders of pregnancy, such as PCOS, obesity and diabetes mellitus, induce androgen overproduction by the midgestational human fetal ovary, then advanced fetal surveillance technologies and postnatal assessment of intrauterine androgen exposure will be required to understand whether adult PCOS can be reprogramed in susceptible female offspring. Equally important, abnormal placentation may simultaneously affect fetal adaptation to maternal nutrient availability, with altered placental DNA methylation87 or other epigenetic and metabolic abnormalities influencing fetal growth, infant birth weight and long-term physiology of the offspring43,88,89. As the number of robust susceptibility loci for PCOS emerges from ongoing GWAS data, additional studies will be needed to interrogate the possible role of these genes in fetal reprogramming of PCOS.
Finally, experimental constraints on using human fetal tissue for biomedical research limit our knowledge of the relationships between the human fetus and its maternal environment. Therefore new knowledge of how developmental programming affects human health requires animal models to explore the probable fetal origins of adult disease. Such animal studies need to examine how developmentally relevant endocrine/paracrine factors and genes interact to govern human fetal development, including the role of ovarian steroidogenesis in the developmental programming of target tissues. With such information, new clinical strategies promise to improve the endocrine-metabolic status of PCOS women in pregnancy, optimize long-term health of their offspring, and minimize susceptibility to acquiring PCOS and its metabolic derangements in future generations.
Acknowledgments
This study was funded by a grant from The Eunice Kennedy Shriver National Institute of Child Health & Human Development (NICHD), NIH through cooperative agreement U54 HD071836.
References
- 1.Zawadzki J, Dunaif A. Diagnostic criteria for polycystic ovary syndrome: towards a rational approach. In: Dunaif A, Givens JR, Haseltine FP, Merriam GR, editors. Polycystic Ovary Syndrome. Boston, MA: Blackwell Scientific; 1992. pp. 377–384. [Google Scholar]
- 2.Azziz R, Marin C, Hoq L, Badamgarav E, Song P. Health care-related economic burden of the polycystic ovary syndrome during the reproductive life span. J Clin Endocrinol Metab. 2005;90:4650–4658. doi: 10.1210/jc.2005-0628. [DOI] [PubMed] [Google Scholar]
- 3.The Rotterdam ESHRE/ASRM-sponsored PCOS consensus workshop group: Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome (PCOS) Hum Reprod. 2004;19:41–47. doi: 10.1093/humrep/deh098. [DOI] [PubMed] [Google Scholar]
- 4.Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO. The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89:2745–2749. doi: 10.1210/jc.2003-032046. [DOI] [PubMed] [Google Scholar]
- 5.Fauser BCJM, Tarlatzis B, Rebar R, et al. Consensus on women’s health aspects of polycystic ovary syndrome. The Amsterdam ESHRE/ASRM-sponsored 3rd PCOS consensus workshop group. Fertil Steril. 2012;97:28–38. doi: 10.1016/j.fertnstert.2011.09.024. [DOI] [PubMed] [Google Scholar]
- 6.March WA, Moore VM, Willson KJ, Phillips DIW, Norman RJ, Davies MJ. The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod. 2010;25:544–551. doi: 10.1093/humrep/dep399. [DOI] [PubMed] [Google Scholar]
- 7.Carmina E, Chu MC, Longo RA, Rini GB, Lobo RA. Phenotypic variation in hyperandrogenic women influences the findings of abnormal metabolic and cardiovascular risk parameters. J Clin Endocrinol Metab. 2005;90:2545–2549. doi: 10.1210/jc.2004-2279. [DOI] [PubMed] [Google Scholar]
- 8.Welt CK, Gudmundsson JA, Arason G, et al. Characterizing discrete subsets of polycystic ovary syndrome as defined by the Rotterdam criteria: the impact of weight on phenotype and metabolic features. J Clin Endocrinol Metab. 2006;91:4842–4848. doi: 10.1210/jc.2006-1327. [DOI] [PubMed] [Google Scholar]
- 9.Azziz R, Carmina E, Dewailly D, et al. POSITION STATEMENT: Criteria for defining polycystic ovary syndrome as a predominantly hyperandrogenic syndrome: an Androgen Excess Society guideline. J Clin Endocrinol Metab. 2006;91:4237–4245. doi: 10.1210/jc.2006-0178. [DOI] [PubMed] [Google Scholar]
- 10.Barnes RB, Rosenfield RL, Ehrmann DA, et al. Ovarian hyperandrogynism as a result of congenital adrenal virilizing disorders: evidence for perinatal masculinization of neuroendocrine function in women. J Clin Endocrinol Metab. 1994;79:1328–1333. doi: 10.1210/jcem.79.5.7962325. [DOI] [PubMed] [Google Scholar]
- 11.Merke DP, Cutler GB., Jr New ideas for medical treatment of congenital adrenal hyperplasia. Endocrinol Metab Clin North Am. 2001;30:121–135. doi: 10.1016/s0889-8529(08)70022-7. [DOI] [PubMed] [Google Scholar]
- 12.Phocas I, Chryssikopoulos A, Sarandakou A, Rizos D, Trakakis E. A contribution to the classification of cases of non-classic 21-hydroxylase-deficient congenital adrenal hyperplasia. Gynecol Endocrinol. 1995;9:229–238. doi: 10.3109/09513599509160451. [DOI] [PubMed] [Google Scholar]
- 13.Stikkelbroeck NM, Hermus AR, Braat DD, Otten BJ. Fertility in women with congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Obstet Gynecol Surv. 2003;58:275–284. doi: 10.1097/01.OGX.0000062966.93819.5B. [DOI] [PubMed] [Google Scholar]
- 14.Dumesic DA, Abbott DH, Padmanabhan V. PCOS and its Developmental Origins. Rev Endocr Metab Disord. 2007;8:127–141. doi: 10.1007/s11154-007-9046-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kuijper EAM, Vink JM, Lambalk CB, Boomsma DI. Prevalence of polycystic ovary syndrome in women from opposite-sex twin pairs. J Clin Endocrinol Metab. 2009;94:1987–1990. doi: 10.1210/jc.2009-0191. [DOI] [PubMed] [Google Scholar]
- 16.Cattrall FR, Vollenhoven BJ, Weston GC. Anatomical evidence for in utero androgen exposure in women with polycystic ovary syndrome. Fertil Steril. 2005;84:1689–1692. doi: 10.1016/j.fertnstert.2005.05.061. [DOI] [PubMed] [Google Scholar]
- 17.Palomba S, Marotta A, Cello AD, et al. Pervasive developmental disorders in children of hyperandrogenic women with polycystic ovary syndrome: a longitudinal case-control study. Clin Endocrinol. 2012;77:898–904. doi: 10.1111/j.1365-2265.2012.04443.x. [DOI] [PubMed] [Google Scholar]
- 18.Lujan M, Bloski T, Chizen D, Lehotay D, Pierson R. Digit ratios do not serve as anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Hum Reprod. 2010;25:204–211. doi: 10.1093/humrep/dep363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lujan ME, Podolski AJ, Chizen DR, Lehotay DC, Pierson RA. Digit ratios by computer-assisted analysis confirm lack of anatomical evidence of prenatal androgen exposure in clinical phenotypes of polycystic ovary syndrome. Reprod Biol Endocrinol. 2010;8:156–163. doi: 10.1186/1477-7827-8-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Abbott AD, Colman RJ, Tiefenthaler R, Dumesic DA, Abbott DH. Early-to-mid gestation fetal testosterone increases right hand 2D:4D finger length ratio in polycystic ovary syndrome-like monkeys. PLoS ONE. 2012;7:e42372. doi: 10.1371/journal.pone.0042372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Barry JA, Kay AR, Navaratnarajah R, et al. Umbilical vein testosterone in female infants born to mothers with polycystic ovary syndrome is elevated to male levels. J Obstet Gynaecol. 2010;30:444–446. doi: 10.3109/01443615.2010.485254. [DOI] [PubMed] [Google Scholar]
- 23.Mehrabian F, Kelishadi R. Comparison of the metabolic parameters and androgen level of umbilical cord blood in newborns of mothers with polycystic ovary syndrome and controls. J Res Med Sci. 2012;17:207–211. [PMC free article] [PubMed] [Google Scholar]
- 24.Anderson H, Fogel N, Grebe SK, Singh RJ, Taylor RL. Dunaif A Infants of women with polycystic ovary syndrome have lower cord blood androstenedione and estradiol levels. J Clin Endocrinol Metab. 2010;95:2180–2186. doi: 10.1210/jc.2009-2651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maliqueo M, Lara HE, Sánchez F, Echiburú B, Crisosto N, Sir-Petermann T. Placental steroidogenesis in pregnant women with polycystic ovary syndrome. Eur J Obstet Gynecol Reprod Biol. 2013;166:151–155. doi: 10.1016/j.ejogrb.2012.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Hickey M, Sloboda DM, Atkinson HC, et al. The relationship between maternal and umbilical cord androgen levels and polycystic ovary syndrome in adolescence: a prospective cohort study. J Clin Endocrinol Metab. 2009;94:3714–3720. doi: 10.1210/jc.2009-0544. [DOI] [PubMed] [Google Scholar]
- 27.Cole B, Hensinger K, Maciel GAR, Chang RJ, Erickson GF. Human fetal ovary development involves the spatiotemporal expression of P450c17 protein. J Clin Endocrinol Metab. 2006;91:3654–3661. doi: 10.1210/jc.2006-0641. [DOI] [PubMed] [Google Scholar]
- 28.Mesiano S. The endocrinology of human pregnancy and fetoplacental neuroendocrine development. In: Strauss JF III, Barbieri RL, editors. Yen and Jaffe’s Reproductive Endocrinology: Physiology, Pathophysiology, and Clinical Management. Sixth. Philadelphia, PA: Saunders Elsevier; 2009. pp. 248–281. [Google Scholar]
- 29.Fowler PA, Anderson RA, Saunders PT, et al. Development of Steroid Signaling Pathways during Primordial Follicle Formation in the Human Fetal Ovary. J Clin Endocrinol Metab. 2011;96:1754–1762. doi: 10.1210/jc.2010-2618. [DOI] [PubMed] [Google Scholar]
- 30.van de Beek C, Thijssen JHH, Cohen-Kettenis PT, van Goozen SHM, Buitelaar JK. Relationships between sex hormones assessed in amniotic fluid, and maternal and umbilical cord serum: what is the best source of information to investigate the effects of fetal hormonal exposure? Horm Behav. 2004;46:663–669. doi: 10.1016/j.yhbeh.2004.06.010. [DOI] [PubMed] [Google Scholar]
- 31.Goodarzi M, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 32.Adashi EY. The ovarian follicular apparatus. In: Adashi EY, Rock JA, Rosenwaks Z, editors. Reproductive Endocrinology, Surgery, and Technology. Philadelphia, PA: Lippincott-Raven; 1996. pp. 18–40. [Google Scholar]
- 33.Hatzirodos N, Bayne RA, Irving-Rodgers HF, et al. Linkage of regulators of TGF-β activity in the fetal ovary to polycystic ovary syndrome. FASEB J. 2011;25:2256–2265. doi: 10.1096/fj.11-181099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voutilainen R, Miller WL. Developmental expression of genes for the stereoidogenic enzymes P450scc (20,22-desmolase), P450c17 (17-hydroxy- lase/17,20-lyase), and P450c21 (21-hydroxylase) in the human fetus. J Clin Endocrinol Metab. 1986;63:1145–1150. doi: 10.1210/jcem-63-5-1145. [DOI] [PubMed] [Google Scholar]
- 35.Shifren JL, Osathanondh R, Yeh J. Human fetal ovaries and uteri: developmental expression of genes encoding the insulin, insulin-like growth factor I, insulin-like growth factor II receptors. Fertil Steril. 1993;59:1036–1040. doi: 10.1016/s0015-0282(16)55924-x. [DOI] [PubMed] [Google Scholar]
- 36.Payne AH, Jaffe RB. Androgen formation from pregnenolone sulfate by the human fetal ovary. J Clin Endocrinol Metab. 1974;39:300–304. doi: 10.1210/jcem-39-2-300. [DOI] [PubMed] [Google Scholar]
- 37.Wilson EA, Jawad MJ. The effect of trophic agents on fetal ovarian steroidogenesis in organ culture. Fertil Steril. 1979;32:73–79. doi: 10.1016/s0015-0282(16)44119-1. [DOI] [PubMed] [Google Scholar]
- 38.George FW, Wilson JD. Conversion of androgen to estrogen by the human fetal ovary. J Clin Endocrinol Metab. 1978;47:550–555. doi: 10.1210/jcem-47-3-550. [DOI] [PubMed] [Google Scholar]
- 39.Pepe GJ, Billiar RB, Albrecht ED. Regulation of baboon fetal ovarian folliculogenesis by estrogen. Mol Cell Endocrinol. 2006;247:41–46. doi: 10.1016/j.mce.2005.11.045. [DOI] [PubMed] [Google Scholar]
- 40.Albrecht ED, Pepe GJ. Estrogen regulation of placental angiogenesis and fetal ovarian development during primate pregnancy. Int J Dev Biol. 2010;54:397–408. doi: 10.1387/ijdb.082758ea. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Reyes FI, Winter JS, Faiman C. Studies on human sexual development. I. Fetal gonadal and adrenal sex steroids. J Clin Endocrinol Metab. 1973;37:74–78. doi: 10.1210/jcem-37-1-74. [DOI] [PubMed] [Google Scholar]
- 42.Beck Peccoz P, Padmanabhan V, Baggiani AM, et al. Maturation of hypothalamic-pituitary-gonadal function in normal human fetuses: circulating levels of gonadotropins, their common alpha-subunit and free testosterone, and discrepancy between immunological and biological activities of circulating follicle-stimulating hormone. J Clin Endocrinol Metab. 1991;73:525–532. doi: 10.1210/jcem-73-3-525. [DOI] [PubMed] [Google Scholar]
- 43.Barbieri RL, Saltzman DH, Torday JS, Randall RW, Frigoletto FD, Ryan KJ. Elevated concentrations of the β-subunit of human chorionic gonadotropin and testosterone in the amniotic fluid of gestations of diabetic mothers. Am J Obstet Gynecol. 1986;154:1039–1043. doi: 10.1016/0002-9378(86)90746-5. [DOI] [PubMed] [Google Scholar]
- 44.Driscoll SG, Benirschke K, Curtis GW. Neonatal deaths among infants of diabetic mothers. Postmortem findings in ninety-five infants. Am J Dis Child. 1960;100:818–835. doi: 10.1001/archpedi.1960.04020040820004. [DOI] [PubMed] [Google Scholar]
- 45.Hultquist GT, Olding LB. Endocrine pathology of infants of diabetic mothers. A quantitative morphological analysis including a comparison with infants of iso-immunized and of non-diabetic mothers. Acta Endocrinol (Copenh) 1981;241(Suppl):1–202. [PubMed] [Google Scholar]
- 46.Sir-Petermann, Maliqueo TM, Angel B, Lara HE, Pérez-Bravo F, Recabarren SE. Maternal serum androgens in pregnant women with polycystic ovarian syndrome: possible implications in prenatal androgenization. Hum Reprod. 2002;17:2573–2579. doi: 10.1093/humrep/17.10.2573. [DOI] [PubMed] [Google Scholar]
- 47.Boomsma CM, Eijkemans MJC, Hughes EG, Visser GHA, Fauser BCJM, Macklon NS. A meta-analysis of pregnancy outcomes in women with polycystic ovary syndrome. Hum Reprod Update. 2006;12:673–683. doi: 10.1093/humupd/dml036. [DOI] [PubMed] [Google Scholar]
- 48.Kjerulff LE, Sanchez-Ramos L, Duffy D. Pregnancy outcomes in women with polycystic ovary syndrome: a metaanalysis. Am J Obstet Gynecol. 2011;204:558.e1–558.e6. doi: 10.1016/j.ajog.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 49.Altieri P, Gambineri A, Prontera O, Cionci G, Franchina M, Pasquali R. Maternal polycystic ovary syndrome may be associated with adverse pregnancy outcomes. Eur J Obstet Gynecol Reprod Biol. 2010;149:31–36. doi: 10.1016/j.ejogrb.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 50.Bjercke S, Dale PO, Tanbo T, Storeng R, Ertzeid G, Abyholm T. Impact of insulin resistance on pregnancy complications and outcome in women with polycystic ovary syndrome. Gynecol Obstet Invest. 2002;54:94–98. doi: 10.1159/000067719. [DOI] [PubMed] [Google Scholar]
- 51.Abbott DH, Bruns CR, Barnett DK, et al. Experimentally-induced gestational androgen excess disrupts glucoregulation and stimulates growth in fetal and neonatal female rhesus monkeys. Am J Physiol Endocrinol Metab. 2010;299:E741–E751. doi: 10.1152/ajpendo.00058.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Abbott Semin Reprod Med. Present Edition, reference pending. 2013 [Google Scholar]
- 53.Sir-Petermann T, Codner E, Maliqueo M, et al. Increased anti-Mullerian hormone serum concentrations in prepubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2006;91:3105–3109. doi: 10.1210/jc.2005-2693. [DOI] [PubMed] [Google Scholar]
- 54.Crisosto N, Echiburú B, Maliqueo M, et al. Improvement of hyperandrogenism and hyperinsulinemia during pregnancy in women with polycystic ovary syndrome: possible effect in the ovarian follicular mass of their daughters. Fertil Steril. 2012;97:218–224. doi: 10.1016/j.fertnstert.2011.10.002. [DOI] [PubMed] [Google Scholar]
- 55.Maliqueo M, Echiburu B, Crisosto N, et al. Metabolic parameters in cord blood of newborns of women with polycystic ovary syndrome. Fertil Steril. 2009;92:277–282. doi: 10.1016/j.fertnstert.2008.04.022. [DOI] [PubMed] [Google Scholar]
- 56.Crisosto N, Codner E, Maliqueo M, et al. Anti-Mullerian hormone levels in peripubertal daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2007;92:2739–2743. doi: 10.1210/jc.2007-0267. [DOI] [PubMed] [Google Scholar]
- 57.Kent SC, Gnatuk CL, Kunselman AR, Demers LM, Lee PA, Legro RS. Hyperandrogenism and hyperinsulinism in children of women with polycystic ovary syndrome: a controlled study. J Clin Endocrinol Metab. 2008;93:1662–1669. doi: 10.1210/jc.2007-1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sir-Petermann T, Codner E, Pérez V, et al. Metabolic and reproductive features before and during puberty in daughters of women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2009;94:1923–1930. doi: 10.1210/jc.2008-2836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yamamoto M, Feigenbaum SL, Crites Y, et al. Risk of preterm delivery in non-diabetic women with polycystic ovarian syndrome. J Perinatol. 2012;32:770–776. doi: 10.1038/jp.2011.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cresswell JL, Barker DJ, Osmond C, Egger P, Phillips DI, Fraser RB. Fetal growth, length of gestation, and polycystic ovaries in adult life. Lancet. 1997;350:1131–1135. doi: 10.1016/s0140-6736(97)06062-5. [DOI] [PubMed] [Google Scholar]
- 61.Mumm H, Kamper-Jørgensen M, Nybo Andersen AM, Glintborg D, Andersen M. Birth weight and polycystic ovary syndrome in adult life: a register-based study on 523,757 Danish women born 1973–1991. Fertil Steril. 2013;99:777–782. doi: 10.1016/j.fertnstert.2012.11.004. [DOI] [PubMed] [Google Scholar]
- 62.Sir-Petermann T, Hitchsfeld C, Maliqueo M, et al. Birth weight in offspring of mothers with polycystic ovarian syndrome. Hum Reprod. 2005;20:2122–2126. doi: 10.1093/humrep/dei009. [DOI] [PubMed] [Google Scholar]
- 63.Ibanez L, Potau N, Francois I, de Zegher F. Precocious Pubarche, Hyperinsulinism, and Ovarian Hyperandrogenism in Girls: Relation to Reduced Fetal Growth. J Clin Endocrinol Metab. 1998;83:3558–3562. doi: 10.1210/jcem.83.10.5205. [DOI] [PubMed] [Google Scholar]
- 64.Legro RS, Roller RL, Dodson WC, Stetter CM, Kunselman AR, Dunaif A. Associations of birthweight and gestational age with reproductive and metabolic phenotypes in women with polycystic ovarian syndrome and their first-degree relatives. J Clin Endocrinol Metab. 2010;95:789–799. doi: 10.1210/jc.2009-1849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Laitinen J, Taponen S, Martikainen H, et al. Body size from birth to adulthood as a predictor of self-reported polycystic ovary syndrome symptoms. Int J Obes Relat Metab Disord. 2003;27:710–715. doi: 10.1038/sj.ijo.0802301. [DOI] [PubMed] [Google Scholar]
- 66.Haakova L, Cibula D, Rezabek K, Hill M, Fanta M, Zivny J. Pregnancy outcome in women with PCOS and in controls matched by age and weight. Hum Reprod. 2003;18:1438–1441. doi: 10.1093/humrep/deg289. [DOI] [PubMed] [Google Scholar]
- 67.Mikola M, Hiilesmaa V, Halttunen M, Suhonen L, Tiitinen A. Obstetric outcome in women with polycystic ovarian syndrome. Hum Reprod. 2001;16:226–229. doi: 10.1093/humrep/16.2.226. [DOI] [PubMed] [Google Scholar]
- 68.Chazenbalk GD, Aguilera P, Keller E, Dumesic DA, Abbott DH. Altered transition of adipose stem cell commitment to early preadipocyte differentiation in subcutaneous abdominal adipose tissue of adult PCOS-like female rehsus monkeys. Paper presented at: 95th Annual Meeting of the Endocrine Society; June 15–18, 2013; San Francisco, CA. Poster Mon-555. [Google Scholar]
- 69.Chazenbalk G, Singh P, Irge D, Shah A, Abbott DH, Dumesic DA. Androgens inhibit adipogenesis during human adipose stem cell commitment to predipocyte formation. Steroids. 2013;78:920–926. doi: 10.1016/j.steroids.2013.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Heneidi S, Simerman AA, Keller E, et al. Awakened by Cellular Stress: Isolation and Characterization of a Novel Population of Pluripotent Stem Cells Derived from Human Adipose Tissue. PlosOne. doi: 10.1371/journal.pone.0064752. 10.1371/journal.pone.0064752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Legro RS, Driscoll D, Strauss JF, 3rd, Fox J, Dunaif A. Evidence for a genetic basis for hyperandrogenemia in polycystic ovary syndrome. Proc Natl Acad Sci U S A. 1998;95:14956–14960. doi: 10.1073/pnas.95.25.14956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kahsar-Miller MD, Nixon C, Boots LR, Go RC, Azziz R. Prevalence of polycystic ovary syndrome (PCOS) in first-degree relatives of patients with PCOS. Fertil Steril. 2001;75:53–58. doi: 10.1016/s0015-0282(00)01662-9. [DOI] [PubMed] [Google Scholar]
- 73.Vink JM, Sadrzadeh S, Lambalk CB, Boomsma DI. Heritability of polycystic ovary syndrome in a Dutch twin-family study. J Clin Endocrinol Metab. 2006;91:2100–2104. doi: 10.1210/jc.2005-1494. [DOI] [PubMed] [Google Scholar]
- 74.Chen ZJ, Zhao H, He L, et al. Genome-wide association study identifies susceptibility loci for polycystic ovary syndrome on chromosome 2p16.3, 2p21 and 9q33.3. Nat Genet. 2011;43:55–59. doi: 10.1038/ng.732. [DOI] [PubMed] [Google Scholar]
- 75.Shi Y, Zhao H, Shi Y, et al. Genome-wide association study identifies eight new risk loci for polycystic ovary syndrome. Nat Genet. 2012;44:1020–1025. doi: 10.1038/ng.2384. [DOI] [PubMed] [Google Scholar]
- 76.Zeggini E, Scott LJ, Saxena R, et al. Meta-analysis of genome-wide association data and large-scale replication identifies additional susceptibility loci for type 2 diabetes. Nat Genet. 2008;40:638–645. doi: 10.1038/ng.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Voight BF, Scott LJ, Steinthorsdottir V, et al. Twelve type 2 diabetes susceptibility loci identified through large-scale association analysis. Nat Genet. 2010;42:579–589. doi: 10.1038/ng.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hakonarson H, Qu HQ, Bradfield JP, et al. A novel susceptibility locus for type 1 diabetes on Chr12q13 identified by a genome-wide association study. Diabetes. 2008;57:1143–1146. doi: 10.2337/db07-1305. [DOI] [PubMed] [Google Scholar]
- 79.Lango Allen H, Estrada K, Lettre G, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Fox CS, Heard-Costa N, Cupples LA, Dupuis J, Vasan RS, Atwood LD. Genome-wide association to body mass index and waist circumference: the Framingham Heart Study 100K project. BMC Med Genet. 2007;8(Suppl 1):S18. doi: 10.1186/1471-2350-8-S1-S18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Díaz-Perales A, Quesada V, Sánchez LM, et al. Identification of human aminopeptidase O, a novel metalloprotease with structural similarity to aminopeptidase B and leukotriene A4 hydrolase. J Biol Chem. 2005;280:14310–14317. doi: 10.1074/jbc.M413222200. [DOI] [PubMed] [Google Scholar]
- 82.Li Z, Huang H. Epigenetic abnormality: a possible mechanism underlying the fetal origin of polycystic ovary syndrome. Med Hypotheses. 2008;70:638–642. doi: 10.1016/j.mehy.2006.09.076. [DOI] [PubMed] [Google Scholar]
- 83.Zhu JQ, Zhu L, Liang XW, Xing FQ, Schatten H, Sun QY. Demethylation of LHR in dehydroepiandrosterone-induced mouse model of polycystic ovary syndrome. Mol Hum Reprod. 2010;16:260–266. doi: 10.1093/molehr/gap089. [DOI] [PubMed] [Google Scholar]
- 84.Qu F, Wang FF, Yin R, et al. A molecular mechanism underlying ovarian dysfunction of polycystic ovary syndrome: hyperandrogenism induces epigenetic alterations in the granulosa cells. J Mol Med (Berl) 2012;90:911–923. doi: 10.1007/s00109-012-0881-4. [DOI] [PubMed] [Google Scholar]
- 85.Xu N, Kwon S, Abbott DH, et al. Epigenetic mechanism underlying the development of polycystic ovary syndrome (PCOS)-like phenotypes in prenatally androgenized rhesus monkeys. PLoS One. 2011;6:e27286. doi: 10.1371/journal.pone.0027286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Dos Santos E, Dieudonné MN, Leneveu MC, Pecquery R, Serazin V, Giudicelli Y. In vitro effects of chorionic gonadotropin hormone on human adipose development. J Endocrinol. 2007;194:313–325. doi: 10.1677/JOE-06-0101. [DOI] [PubMed] [Google Scholar]
- 87.Park BH, Kim YJ, Park JS, et al. Folate and homocysteine levels during pregnancy affect DNA methylation in human placenta. J Prev Med Pub Health. 2005;38:437–442. [PubMed] [Google Scholar]
- 88.Rees WD, Wilson FA, Maloney CA. Sulfur amino acid metabolism in pregnancy: the impact of methionine in the maternal diet. J Nutr. 2006;136(Suppl 6):1701S–1705S. doi: 10.1093/jn/136.6.1701S. [DOI] [PubMed] [Google Scholar]
- 89.Kwong WY, Miller DJ, Wilkins AP, et al. Maternal low protein diet restricted to the preimplantation period induces a gender-specific change on hepatic gene expression in rat fetuses. Mol Reprod Dev. 2007;74:48–56. doi: 10.1002/mrd.20606. [DOI] [PubMed] [Google Scholar]