Abstract
Aims
Cardiomyocyte (CM) proliferation increases from the inner trabecular to outer compact myocardium in fetal hearts. We determined if canonical Wnt signaling has directional and graded activity to maintain this CM proliferation gradient. Moreover, we investigated whether perturbation of Wnt signaling intensity could modulate CM proliferative activity.
Methods and results
With confocal microscopy and image analysis we found that the Wnt effector, β-catenin, formed a signaling gradient which positively correlated with CM proliferative activity across ventricular walls of wild type (WT) embryos at embryonic day (E) 13.5 and 17.5. Negative Wnt regulators, adenomatosis polyposis coli (APC), had a reverse distribution pattern. The activation of canonical Wnt/β-catenin signaling by deletion of Apc in CMs led to ventricular hyperplasia with no adverse effects on fetal survival or CM differentiation. In contrast, cardiac deletion of β-catenin resulted in ventricular hypoplasia and fetal demise by E14.5. We further revealed differential distribution and regulation of three cyclin Ds in fetal hearts. Cyclin D1 was mainly expressed in endothelial cells. Although both cyclin D2 and D3 were present in CMs, only cyclin D2 was regulated by Wnt signaling perturbation: downregulation by β-catenin deletion and upregulation by Apc knockout.
Conclusion
Canonical Wnt signaling is asymmetrical and graded across ventricular walls and positively regulates CM proliferation via cyclin D2.
Keywords: Adenomatosis polyposis coli (APC), β-catenin, heart, development, Wnt, proliferation
Graphical Abstract
1. Introduction
Many signaling pathways including canonical Wnt/β-catenin signaling have been implicated in early heart chamber formation 1, 2, but their roles in the later stages of cardiac development remain poorly defined. During early cardiogenesis, the canonical Wnt/β-catenin pathway regulates various cellular processes, from heart field determination to cardiomyocyte (CM) commitment and differentiation 3. After heart chambers have formed, CM proliferation increases from the inner trabecular to the outer compact myocardium and is largely responsible for the growth of normal fetal ventricles 4, 5. How this proliferation gradient is maintained remains poorly understood. It is well documented that canonical Wnt/β-catenin signaling has asymmetric activity during embryonic patterning 6. Whether this pathway has directional and graded activity in the heart has not been examined in details.
β-Catenin is an important signal transducer in the canonical Wnt/β-catenin pathway 7. Upon Wnt stimulation, β-catenin is stabilized, enters the nucleus, and binds to transcription factors, including T cell factor/lymphoid enhancer-binding factor (TCF/LEF) family members, which activate target gene transcription 8, 9. Although disruption of β-catenin in the heart during development is universally lethal, it is controversial and highly debatable whether this canonical regulation of Wnt signaling in β-catenin exists in the heart 3. Adenomatosis polyposis coli (APC) interacts with β-catenin to form the so called destruction complex including glycogen synthase kinase (GSK)-3β and axin1/2 3, 7. GSK-3β phosphorylates β-catenin on serines 33/37 and threonine 41, promoting its degradation through the ubiquitin/proteasome system. Global deletion of GSK-3β promotes cardiac hyperplasia, but seems to have no major effect on β-catenin stability 10. On the other hand, chemical inhibition of GSK-3β in cultured neonatal rat CMs enhances proliferation with concomitant β-catenin activation 11.
APC mutation or deletion prevents the formation of this complex and thereby has an essential role in inactivating canonical Wnt signaling 12. Genetic APC truncation mutations promote β-catenin-mediated signaling and cause colonic adenoma and adenocarcinoma 12, 13. Germline homozygous Apc mutation prevents gastrulation in mice 14 and interferes with cardiac loop morphogenesis in zebrafish 15 resulting in excessive endocardial cushions. How Apc loss-of-function affects mammalian heart development remains undetermined. Using Cre-Lox technology, we activated and inhibited Wnt signaling through cardiac specific deletion of Apc and β-catenin respectively. Our data demonstrate that β-catenin is required for CM proliferation. APC deletion activates β-catenin signaling and promotes CM proliferation through the the upregulation of cyclin D2.
2. Methods
2.1 Animals
Mice with two loxP sites inserted in introns 1 and 6 of the β-catenin gene were generously donated to the research community by Dr. Rolf Kemler (Max-Planck Institute of Immunology, Germany) (Brault et al, 2001) and maintained at The Jackson Laboratory (Bar Harbor, ME). Mice with two loxP sites flanking the exon 14 of Apc 12, 16 were generated by Dr. Tetsuo Noda at The Cancer Institute of Japanese Foundation for Cancer Research (Tokyo, Japan) and acquired from Dr. Bart O. Williams at the Van Andel Research lnstitute (Grand Rapids, MI, USA). Transgenic mice expressing Cre recombinase under the control of the α-myosin heavy chain promoter (αMHC-Cre) were created as previously described 17. Homozygous Apc or β-catenin loxP-floxed (Apcf/f or β-cateninf/f) mice were crossed with αMHC-Cre mice to generate heterozygous loxP-floxed mice negative for αMHC-Cre and positive for αMHC-Cre. Heterozygous loxP-floxed mice positive for αMHC-Cre were bred with homozygous loxP-floxed mice to produce homozygous loxP-floxed or deleted Apc and β-catenin mice. Pregnant dams were sacrificed via isoflurane inhalation, and then followed by cervical dislocation. Embryos were harvested from the pregnant dam through a hysterectomy at gestational ages between E13.5 to E17.5. Genomic DNA was isolated and purified with DNeasy Blood & Tissue Kit (Qiagen, Valencia, CA). αMHC-Cre transgene, loxP-floxed and wild type β-catenin genes were amplified by PCR as described previously (Qu J et al, 2007). Analysis for Apc alleles was done by multiplex PCR using Apc-P3 primer 5′-GTTCTGTATCATGGAAAGATAGGTGGTC-3′, Apc-P4 primer 5′-CACTCAAAACGCTTTTGAGGGTTGATTC-3′, Apc-P5 primer 5′-GAGTACGGGGTCTCTGTCTCAGTGAA-3′, targeted allele, deleted allele, and wild-type allele amplified as 314-bp (P3 and P4), 258-bp (P3 and P5), and 226-bp (P3 and P4) PCR products, respectively 12, 16. All procedures were performed in accordance with the Guide for the Care and Use of Laboratory Animals (US Department of Health, Education, and Welfare, Department of Health and Human Services, NIH Publication 85-23), and approved by the University of Rochester Animal Care and Use Committee.
2.2 RNA isolation and RT-PCR
Total RNA was extracted from isolated ventricles of E13.5 and 17.5 embryos using the Trizol reagent from Invitrogen (Life Technologies Corporation, Grand Island, NY) following the manufacturer’s instructions. For TaqMan® Assays, real-time RT-PCR was performed with Applied Biosystems 7900HT Sequence Detection System in the Functional Genomics Center of University of Rochester on six pooled ventricles in duplicate with primers for Axin 2 ordered from Applied Biosystems (Fig. S3C). Data were normalized and standardized with SDS2.2 software provided by Applied System. For custom designed primers (Table S3), real time RT-PCR was performed on at least three different animals in triplicate with MyiQ™ Real-Time PCR Detection System. Results were normalized with Gapdh and analyzed with the comparative Ct method. RT-PCR for detecting Apc deletion was carried out with the DreamTaq Green Buffer (Life Technologies Corporation, Grand Island, NY) and visualized with 3% agarose gels using F546 primer 5′-TGAGGAATTTGTCTTGGCGAG-3′ and R721 primer 5′-GCACTTCCCATGGCAATCATT-3′.
2.3 Histology and immunohistochemistry
Murine hearts were fixed in 4% paraformaldehyde at room temperature for 30 min and embedded in paraffin. Four μm sections were used for hematoxylin and eosin or immunohistochemical staining as previously described18. Antigen retrieval was performed in EDTA buffer (pH: 9.0) by heating to 99°C for 20 minutes with a PT Link system from Dako (Carpinteria, CA). Endogenous peroxidase activity was quenched with 3% hydrogen peroxide and non-specific binding was blocked with 10% non-immune goat serum (Invitrogen, Carlsbad, CA). Primary antibodies (Table S5) were incubated overnight in a moisturized chamber at 4°C. The signal was amplified with the Histostain-SP Kit (Life Technologies Corporation, Grand Island, NY) according to manufacturer’s instruction.
2.4 Immunofluorescent labeling and confocal microscopy
Paraformaldehyde-fixed hearts were washed with PBS and immersed in 30% sucrose/PBS for 30 min to 1 hour and then embedded with O.C.T compound. Five μm sections were washed with 0.1 mol/L glycine for 25 min in order to reduce non-specific crosslinking activity of residual paraformaldehyde, immersed in 10% normal goat serum for 60 min to block non-specific binding, and then incubated overnight at 4°C with primary antibodies (Table S5). After washing 6 times with PBS, secondary antibody labeled with DyLight-555 or 488 (Life Technologies Corporation, Grand Island, NY) was added. Nuclear counterstain was performed with 4′, 6-Diamidino-2-Phenylindole (DAPI, Sigma-Aldrich, St. Louis, MO). Confocal images were collected with Olympus FV1000 confocal microscope (Olympus America Inc., Melville, NY) under uniform settings. For negative controls, primary antibodies were omitted or substituted with rabbit serum under the same conditions.
2.5 Quantification of fluorescent intensity and measurement of ventricular wall thickness
Five μm frozen sections were labeled with β-catenin, APC and costained with sarcomeric α-actinin (Actinin) and DAPI. Ventricles were scanned at 40× and 0.55 μm z-intervals with an Olympus FV1000 confocal microscope. Five sequential images along the z-axis were stacked to generate a merged image for further intensity quantification by ImageJ (Fiji). The intensity of β-catenin and APC was measured in all Actinin positive regions of 40× magnification images, and presented as the mean fluorescence intensity in pixels. The mean nuclear pixel intensity of β-catenin was measured in DAPI stained CM nuclei of all Actinin positive regions at 40× (Fig. 1G).
Figure 1.
Representative confocal images and quantitation of Wnt signaling and CM proliferation gradients in the ventricular wall of normal E13.5 embryos. A and B, β-catenin is stronger at the compact (Com) than trabecular (Tra) layer and quantitation of average β-catenin intensity (B, bar graph) in Actinin positive CMs that contain membranous and nuclear β-catenin (B). C and D, Ki67 gradient with low power view (C) and double labeling with sarcomeric α-actinin (Actinin, D) and quantitation of percentage of Ki67 (bar graph) positive CMs in Com and Tra layers. E and F, APC decorates Actinin positive CMs, but not Actinin negative endothelial cells (triangles) and quantitation shows higher APC intensity in Tra than in Com layer. G, An illustration (left) shows how nuclear β-catenin intensity was measured for entire images as well as bar graphs demonstrate average nuclear β-catenin intensity in Com and Tra layers. Regions of interest (ROIs) are semi-automatically outlined by image J based on all Actinin positive areas. H, A diagram illustrates that β-catenin is expressed at the highest levels in the rapidly proliferating compact myocardium, forming a decreasing intensity gradient from the epicardium to the endocardium. In contrast, APC shows the strongest expression at the slowly proliferating trabecular layer form an increasing intensity gradient from the epicardium to the endocardium. Dotted lines show visceral pericardium and bars=50μm. Data represent mean ± SD. N=3-4 independent experiments.
Compact layer thickness was perpendicularly measured from the epicardium to the junction of compact and trabecular layers in the Actinin positive region at five random locations from three sections per heart.
2.6 SDS gel electrophoresis and Western blotting
Isolated ventricles from E17.5 hearts were collected and extracted with RIPA buffer (Life Technologies Corporation, Grand Island, NY). An equal amount of total protein, determined with the Bio-Rad Coomassie Protein Assay (Bio-Rad Laboratories, Hercules, CA) from each group, was loaded and separated by 4-12% NuPAGE® Bis-Tris Pre-Cast gels (Life Technologies Corporation, Grand Island, NY). Target proteins were detected with specific antibodies using SuperSignal™ West Pico Chemiluminescent Substrate (Thermo, Rockford, IL). Protein band intensity was quantified with NIH ImageJ software (http://rsb.info.nih.gov/ij/) using relative densitometric values on the duplicates of at least three independent experiments from each group.
2.7 Statistics
Fluorescent intensity and real time RT-PCR data were presented as mean ± standard deviation (SD) of the mean. Means among groups were analyzed with SPSS 13.0 software (IBM Corporation, Armonk, NY) using Independent-Sample T Test for two groups and One-Way ANOVA for more than two groups. Post hoc multiple comparisons were then performed to determine if a statistical significance was detected between groups. Fisher’s least significant difference (LSD) was used to compare group means when equal variances were present. If equal variances were not assumed, differences between group means were calculated by Tamhane’s T2 test.
3. Results
3.1 Canonical Wnt signaling gradient correlates with CM proliferative activity
CMs show a proliferation gradient across the ventricular wall with the highest proliferation rate at the epicardial compact layer of embryonic hearts 4. A recent report revealed that proliferating CMs that are often seen at the compact layer are positive for active nuclear β-catenin, indicating Wnt signaling intensity may control cardiac proliferation 19. In the intestines, Wnt signaling intensity correlates with the proliferative activity from the base of crypt to the surface epithelial cells 20, 21. We wondered whether canonical Wnt/β-catenin signaling also formed similar signaling gradient across the ventricular wall to regulate CM proliferation. We found that Wnt signaling components did indeed show graded and directional distribution in the ventricular wall of wild type (WT) embryos at embryonic day (E) 13.5 (Fig. 1). The Wnt effector β-catenin had an intensity gradient with the highest level at the epicardial compact layer (Fig. 1A) corresponding to CM proliferation activity reveled by Ki67 (Fig. 1C and D). Although β-catenin is predominantly membranous (Fig 1B), its nuclear signals were clearly detected at higher magnification (Fig. S1A), with a similar intensity gradient (Fig. 1G, bar graph) to that of total β-catenin (Fig. 1B, bar graph) from the outer to inner layer across the ventricular wall. Reversely, the Wnt suppressor APC (Fig. 1E and F) demonstrated the strongest intensity at the innermost trabecular layer.
3.2 Cardiac β-catenin deletion inhibited CM proliferation
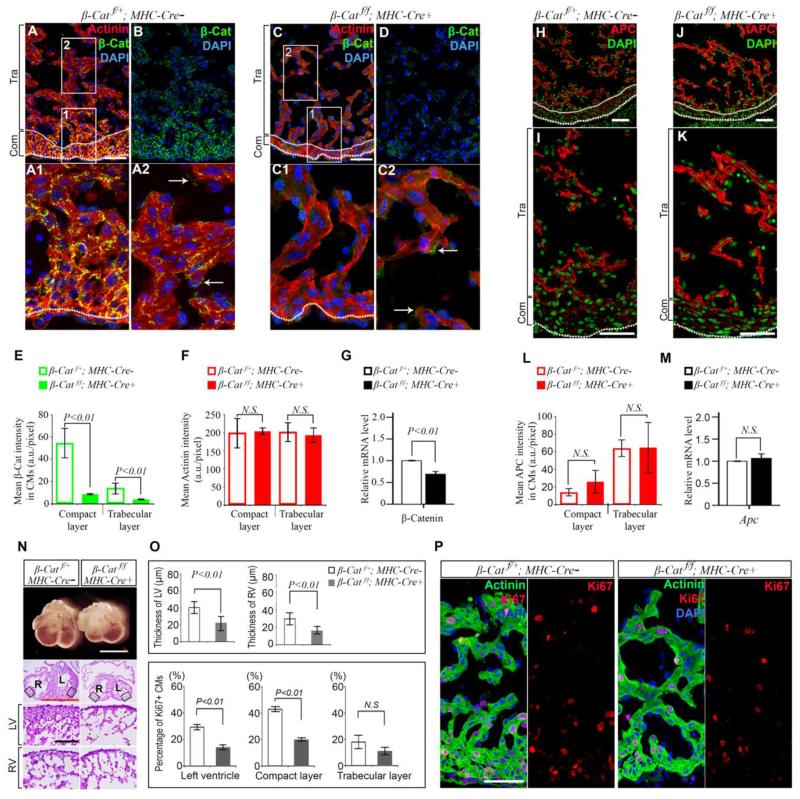
The α-myosin heavy chain (αMHC) is transiently expressed in differentiating CMs during cardiac chamber formation from E10.5 to 14.5 22 and αMHC promoter driven Cre recombinase (αMHC-Cre) can be used to delete loxP floxed genes, specifically in fetal CMs 17. To elucidate the cell-autonomous role of β-catenin in CMs, we introduced αMHC-Cre into mice with loxP sites inserted in introns 1 and 6 of the β-catenin gene (β-Catf)23 to specifically delete β-catenin in CMs. Compared to WT (β-Catf/+;αMHC-Cre−) or heterozygous β-catenin deletion (β-Catf/+;αMHC-Cre+) siblings (Fig. 2A and 2B), β-catenin was significantly reduced in homozygous β-catenin conditional knockout (β-Catf/f;αMHC-Cre+=β-Cat cKO) mice in protein (Fig. 2C and 2D) and mRNA levels (Fig. 2G) by E13.5. RosamT/mG reporter mice for Cre activity revealed efficient conversion of red to green membrane-targeted signals by αMHC-Cre in both compact and trabecular layers at E13.5 (Fig. S1B). As reported previously, heterozygous loss of β-catenin in CMs had no detectable effects on mouse development and no homozygous β-catenin deleted mice were detected at birth 24. No gross or histologic abnormality was observed at E10.5 and E11.5 in β-Cat cKO mice. Embryos with β-Cat cKO were also grossly unremarkable at E12.5, but had slight expansion of the pericardial space, indicating pericardial edema. At 13.5, all genotypes were present at the expected numbers (Table S1). A small percentage of β-Cat cKO embryos (4 out of 18) were grossly pale and microscopically necrotic, consistent with autolysis. The remaining littermates (14 out of 18) were grossly normal (Fig. S2A), but shown signs of congestive heart failure with pericardial edema (Fig. S2B and S2C). Hearts were smaller with thinner left and right ventricular walls in β-Cat cKO embryos compared to that of β-Catf/+;αMHC-Cre− WT controls (Fig. 2N and Fig. 2O, upper panel). β-Catenin was significantly reduced in CMs at both compact and trabecular layers, resulting in the loss of normal β-catenin signaling gradient along the ventricular wall (Fig 2C and 2D). Consistent with our hypothesis, Ki67 positive CMs decreased more than 50% in the compact layer from 43.0% in WT to 19.7% in β-Cat cKO mice at E13.5. The trabecular layer had low proliferative activity and less β-catenin than the compact layer in normal embryos (Fig. 2O, lower panel and Fig. 2P). Thus, β-catenin deletion had less significant impact on Ki67 labeling in the trabecular layer. pH3S10 (Fig. S3A and S3B) were also reduced in CMs of β-Cat cKO compared to WT embryos. On the other hand, APC intensity gradient remained intact in β-Cat cKO ventricles (Fig. 2J and K) similar to WT controls (Fig. 2 H and I). There was a slight increase in APC at the compact layer, but no statistical significance was detected by quantitative confocal microscopy (Fig. 2L). Real-time RT-PCR also did not reveal statistical significance in Apc mRNA levels between cKO and WT groups (Fig. 2M). No increase in apoptosis was detected by TUNEL labeling and anti-cleaved active caspase 3 staining in the heart of β-Cat cKO embryos at E13.5 (Fig. S2D). At E14.5, β-Cat cKO embryos were reduced in numbers (Table S1) and demonstrated autolysis and absorption. These findings suggest that β-catenin is required for normal cardiac development and necessary to maintain higher proliferation rate in the compact layer.
Figure 2.
Cardiac deletion of β-catenin results in ventricular hypoplasia at E13.5. A and B, Double labeling of β-catenin and sarcomeric α-actinin (Actinin) in WT (A and B) and β-catenin (C and D) deleted hearts (β-Cat cKO) shows effective deletion of β-catenin in Actinin positive CMs and thinning of ventricular walls in β-Cat cKO hearts; Arrows indicated β-catenin signals from endothelial cells. E, Average β-catenin intensity is downregulated in Actinin positive CMs of β-Cat cKO hearts. F, Average Actinin intensity shows no difference between compact and trabecular layers or among WT and β-Cat cKO hearts. G, Real time RT-PCR reveals downregulation of β-catenin mRNA levels in β-Cat cKO heart. H to K shows APC expression by confocal microscopy (H and J, lower power; I and K, higher power), average intensity (L) or real time RT-PCR (M). APC is mildly upregulated in β-Cat cKO hearts, but this change is not statistically significant. N, Cardiac morphology shows that β-Cat cKO mice have smaller hearts with thinner left (L) and right (R) ventricular walls compared to WT controls; Boxed regions are zoomed in adjacent images; Scale bars, 1 mm (white and red) and 100 μm (black). O, Ventricular wall thickness (upper panel) in β-Cat cKO and WT mice and percentage of Ki67 positive CMs (lower panel) in ventricular walls of β-Cat cKO and WT mice. P, Double labeling of Ki67 with Actinin. Data represent mean ± SD. N=3-4 independent experiments.
3.3 Cardiac Apc deletion promotes CM proliferation
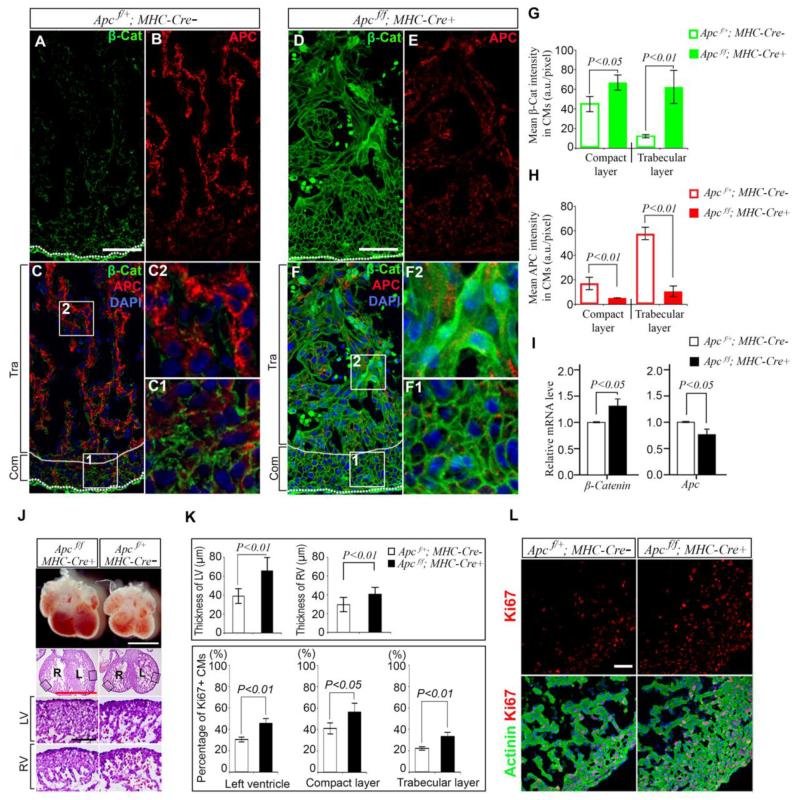
Adenomatosis polyposis coli (APC) suppresses canonical Wnt signaling by promoting β-catenin degradation and its germline homozygous Apc mutation impairs cardiac loop morphogenesis in zebrafish 15 resulting in excessive endocardial cushions. How Apc loss-of-function affects mammalian heart development remains undetermined. We employed αMHC-Cre to delete Apc in fetal CMs with a well-characterized mouse model containing LoxP sites flanked at the exon 14 of the Apc gene (Apcf) 12, 16. Genomic PCR and RT-PCR demonstrated that αMHC-Cre mediated cardiac-specific excision of exon 14 (Fig. S4). All genotypes were present at expected numbers (Table S2). Embryos with homozygous Apc deletion (Apcf/f;αMHC-Cre+= Apc cKO) showed normal morphology from E13.5 to E17.5 compared with Apcf/+; αMHC-Cre− (Apc WT) control embryos. Hearts from Apc cKO embryos were larger and had thicker ventricular walls at E13.5 (Fig. 3J and 3K, upper panel) and at E17.5 (Fig 4A) compared to their WT siblings. As expected, APC gradient was disrupted and APC expression was dramatically reduced in the heart in Apc cKO embryos at E13.5 and E17.5 (Fig.3E and Fig.S5B). Consequently, β-catenin was increased in the heart and accumulated in the nucleus of CMs in Apc cKO mice E13.5 (Fig. 3F2) and E17.5 (Fig. S5B1 and S5B2). Western blots demonstrated the reduction of APC and accumulation of β-catenin in Apc cKO hearts (Fig. S6). Proliferation activity of CMs as indicated by Ki67 increased 36% in compact layer and 50% in trabecular layers over WT littermates at E13.5 respectively (Fig. 3K, lower panel and 3L). This increase continued to E17.5 by 42% in the compact layer and 52% in the trabecular layer (Fig. 4B and 4C, lower panel) in Apc cKO embryos over their WT siblings. pH3S10 also revealed an increase in in Apc cKO CMs at E13.5 (Fig. S3A and S3B) and E17.5 (Fig. 4B and 4C, upper panel). These results indicate that APC with higher expression level in the inner trabecular layer is an important repressor of canonical Wnt/β-catenin signaling in the heart to inhibit CM proliferation.
Figure 3.
Wnt activation by cardiac APC deletion promotes CM proliferation at E13.5. A to G, Double labeling of APC with β-catenin in WT (A-C) and Apc (D-F) deleted hearts (cKO) shows effective deletion of Apc and upregulation of β-catenin. G, Average β-catenin intensity is increased in Apc cKO hearts. H, Average APC intensity is decreased in Apc cKO hearts. I, Real time RT-PCR reveals downregulation of Apc and upregulation of β-catenin mRNA levels in Apc cKO heart. J, Cardiac morphology shows that Apc cKO mice have bigger hearts with thicker left (L) and right (R) ventricular walls compared to WT controls; Boxed regions are zoomed in adjacent images; Scale bars, 1 mm (white and, red) and 100 μm (black). K, Ventricular wall thickness (upper panel) in Apc cKO and WT mice and percentage of Ki67 positive CMs (lower panel) in ventricular wall of cKO and WT mice. L, Double labeling of Ki67 with Actinin. Data represent mean ± SD. N=3 independent experiments.
Figure 4.
Cardiac morphology and proliferation activity in compound Apc and β-catenin (β-Cat) deletion hearts at E17.5. A, Gross and H&E morphology of individual hearts with left ventricular walls zoomed in the bottom row. Scale bars, 1 mm (white and red) and 100 μm (black). B, pH3S10 or Ki67 co-stained with Actinin and DAPI. C, Percentage of pH3S10 and Ki67 positive CMs in β-Cat and Apc compound cKO mice at E17.5. Data are presented as mean ± SD from 3 to 4 embryos in each genotype. * P<0.05 and ** P<0.01 indicate statistically significant difference between the groups (ANOVA).
3.4 Genetic interaction of β-catenin with Apc
To dissect the genetic interaction of APC with β-catenin in CMs during later stage of heart development, we generated Apc and β-catenin compound deletion mice. The loss of one copy of β-catenin dampened the effect of Apc cKO on CM proliferation (Fig 4C) and β-catenin signaling (Fig. S5C) at E17.5. This result indicates that β-catenin is a downstream effector regulated by APC. On the other hand, one or two copy loss of Apc could only partially extend the survival of β-Cat cKO embryos from E14.5 to E17.5 (Fig. S4E). By this age, Apc deletion could not prevent the loss of β-catenin signaling across the ventricle (Fig. S5D and S5E) in β-Cat cKO embryos. These findings are consistent with the role of APC in β-catenin stability 13. Consequently, β-catenin degrades slower and has a longer half-life in β-catenin/Apc double than in β-catenin single cKO mutants. Therefore, β-catenin/Apc double mutants live slightly longer than β-catenin single mutants.
3.5 Wnt target and cell cycle gene expression by β-catenin and Apc deletion
Axin2 is ubiquitously expressed in many tissues and is considered a universal target of canonical Wnt pathway 25. By real-time RT PCR, Axin2 transcripts were increased by 4.5-fold in Apc cKO mice and decreased by 70% in β-Cat cKO mice at E13.5 (Fig. S3C), indicating canonical Wnt signaling is regulated by APC and β-catenin in the heart. D-type cyclins and c-myc are among the earliest identified canonical Wnt targets 26-29. Upregulation of c-myc was identified in Apc cKO mice, but its downregulation was not detected in β-Cat cKO mice at E13.5 (Fig. 6A and Table S4). Additionally, no significant increase of c-myc was observed in Apc cKO mice at E17.5 (Fig. 6B). Although Cyclin D1 is a clear target in Apc mutant tumor cells 27, 28, only Cyclin D2 was immediately induced by Apc loss-of-function in colon 29. Our data demonstrated that the expression levels of Cyclin D1 and Cyclin D3 were not changed in either Apc or β-catenin deleted hearts at E13.5 (Fig. 5A and 5E) or E17.5 (Fig. 5B and 5F). In contrast, Cyclin D2 mRNA levels were significantly increased in Apc cKO hearts at E13.5 (Fig. 5E) and E17.5 (Fig. 5F), and decreased in β-Cat cKO mice at E17.5 (Fig. 6B). Similarly, only cyclin D2, but not D1 and D3 proteins were elevated in at E17.5 (Fig. S6). However, cyclin D2 mRNA was only slightly, but not statistically significantly decreased in β-Cat cKO mice at E13.5 (Fig. 5E) partially due to its high expression in endothelial cells. Interestingly, cyclin D1 was mainly expressed in endothelial cells while cyclin D2 and D3 were detected in both CMs and endothelial cells (Fig. 5A-D). Again, only cyclin D2 was increased in CMs of Apc cKO mice and decreased (Fig. 5C) in β-Cat cKO mice by immunofluorescent staining. In parallel, protein levels of proliferation markers: Ki67 and pH3S10 were elevated in Apc cKO at E17.5 (Fig. S6).
Figure 6.
Gene expression by real time RT-PCR in cardiac β-catenin (β-Cat) and Apc deleted hearts at E13.5 and E17.5. A, A heat map shows log2-fold changes of selective genes in β-Cat cKO and Apc cKO mice compared to their WT siblings. B, A heat map (upper) and bar graphs (bottom) reveal log2-fold changes of selective genes in compound Apc and β-Cat deletion hearts at E17.5. Post hoc multiple comparisons were performed in these target genes. Targets with statistical significance among groups are marked in red. N=3-4 for each genotype. Each sample contains 3 technical replicates.
Figure 5.
Expression of D-type cyclins in E13.5 and E17.5 embryos. A and B, Cyclin D1 and cyclin D3 expression is not affected by either β-catenin (β-Cat, A, E13.5) or Apc (B, E17.5) deletion. Interesting, cyclin D1 is mainly expressed in endothelial cells while cyclin D3 is detected in both CMs and endothelial cells. C, Cyclin D2 is expressed in both CMs and endothelial cells and its expression increases in CMs of Apc cKO mice, but decreases in CMs of β-Cat cKO mice at E13.5. D, Cyclin D2 continues to increase in both compact and trabecular CMs that are deleted of Apc and contain membrane-targeted green instead of red fluorescent protein at E17.5. E and F, Expression of cyclin D1, D2 and D3 by real time RT-PCR in cardiac β-Cat and Apc deleted hearts at E13.5 (E) and E17.5 (F). Scale bars= 50 μm. N=3-4 independent experiments.
3.6 Wnt signaling perturbation on cardiac gene expression
In many cell types, the proliferation rate is tightly correlated with differentiation status. In zebrafish, global loss of APC function has been shown to promote CM proliferation, but inhibit maturation15. We examined the expression of Gata4, Hand1, Hand2, Myh6, Myh7, Myl2, Myl7, Nppa (ANF), Nkx2.5, Pdgfra, SMA, Tbx2, Tbx3, Tbx5, Tbx18, Tbx20 and WT1 by real time RT-PCR (Fig. 6A and Table S4). Hand1, Hand2, Myh7, Myl7, Nppa, SMA, Tbx3 and Tbx5 were upregulated while Myh6, Tbx2 and Tbx18 were downregulated by cardiac Apc deletion. However, these changes were less than 40% among different genotypes except Nppa and Myl7. These results suggest that Wnt signaling mainly regulate cardiac proliferation rather than differentiation after E13.5.
4. Discussion
The role of canonical Wnt signaling in cardiac development remains controversial and needs to be further defined with genetic manipulation of key signaling molecules in a cell and developmental stage specific manner. In Drosophila and mice, canonical Wnt/β-catenin signaling is required for cardiac field specification and morphogenesis 30. On the other hand, cardiogenesis in gastrulating amphibians and chicks requires canonical Wnt/β-catenin signaling inhibition 31, 32. A biphasic role, stimulatory before and inhibitory during and after gastrulation, has therefore been stipulated for the canonical Wnt pathway in cardiogenesis 33. However, this proposed inhibitory effect after gastrulation is contradicted by the fact that β-catenin deletion in cardiac progenitor cells and their differentiated descendants through Cre driven by several cardiac promoters such as Isl1, Mef2c, MesP1, Nkx2.5, or SM22α impairs cardiac development and fetal survival 34-37. The activity of these promoters in migrating cardiac committed cells as well as their offspring settling in the heart proper makes it difficult to distinguish the roles of canonical Wnt/β-catenin signaling in cell migration, differentiation, and proliferation of cardiac lineage cells. Additionally, the embryonic lethality of these models prevented exploring how β-catenin-mediated canonical Wnt signaling regulates cardiac chamber maturation and CM expansion after cardiac looping morphogenesis. Through genetic deletion of Apc/β-catenin by αMHC-Cre, we have shown that APC controls asymmetrical Wnt signaling across ventricular walls and suppresses β-catenin activity in the trabecular layer. Conversely, high β-catenin levels are essential for CM proliferation and normal ventricular maturation.
4.1 Canonical Wnt signaling gradient and cell proliferation
After cardiac chamber morphogenesis and initial four chamber formation, CMs proliferate rapidly for ventricular compaction and septation 4. Closer to the birth, CM cell cycle activity decreases significantly 5. A second wave of DNA replication occurs during first 2 weeks after birth, resulting in CM binucleation, but not cell division 5. Global and regional deficiency in CM proliferation can lead to ventricular septal defect and non-compaction. Therefore, CM cell cycle regulation is essential to understand normal ventricular maturation. We have confirmed that CM proliferation forms a decreasing gradient from the outer to the inner myocardium 4. More importantly, we have revealed that negative Wnt regulator APC is mainly expressed at the inner trabecular layer while Wnt nuclear β-catenin effector has the highest level at the outer compact myocardium. Similarly, Buikema et al have reported that there are more nuclear active positive CMs in the compact than trabecular layer 19. These findings indicate that canonical Wnt/β-catenin has directional and graded activity across the ventricular wall to regulate CM proliferation. It is well known that canonical Wnt/β-catenin signaling has asymmetric activity and directional signaling during development 6. Wnt signaling intensity has also been reported to correlate with the proliferative activity from the base of the crypt to the surface epithelial cells of the intestines 20, 21.
4.2 Canonical Wnt signaling promotes and is required for CM proliferation after chamber formation
Our results have shown that CM cannot proliferate sufficiently to build normal ventricles without β-catenin. On the other hand, Wnt activation by cardiac Apc deletion promotes CM proliferation without significant effect on CM differentiation in late embryonic development. Similarly, Wnt signaling activation and inhibition in CMs expressing myosin light chain (MIC) 2v which is a ventricular form of MLC2 38 demonstrate that β-catenin stabilization by deleting its exon 3 enhances CM cell cycle activity 19. Global and cardiac specific deletion of GSK-3β which negatively regulates stability also promotes CM proliferation in neonatal and adult hearts 10, 39. These results indicate that canonical Wnt signaling can promote CM proliferation and is required for cardiac chamber maturation during embryonic development.
4.3 Wnt signaling regulates cell cycle gene expression
All three type of cyclin Ds are expressed in the heart 5. However, cyclin D1 is not detected by Western blotting in CMs isolated from neonatal hearts 40. Our localization study confirms that cyclin D1 is expressed in endothelial cells, but not CMs at E13.5 and E17.5. In addition, cyclin D1 expression is not affected by cardiac Wnt activation and inhibition. Cyclin D3 is equally expressed in CMs and endothelial cells, and shows no significant change upon Wnt activation and inhibition. In WT embryos, more cyclin D2 is present in endothelial cells than CMs. Cardiac Wnt activation by Apc deletion increases cyclin D2 in CMs, but not endothelial cells. Conversely, cardiac β-catenin deletion decreases cyclin D2 expression in CMs. Stabilizing β-catenin in CMs before E13.5 has been previously shown to enhance cyclin D2 expression 41, 42. Moreover, cyclin D2 overexpression is more effective to promote CM proliferation than cyclin D1 and D3 overexpression after cardiac injury 43. All these findings indicate that cyclin D2 is the most significantly affected one of all 3 cyclin Ds by cardiac Wnt signaling manipulation and may have important roles in CM cell cycle regulation during all stages of heart development and in cardiac repair after damage.
4.4 Cell autonomous vs cell non-autonomous cell cycle control
The roles of canonical Wnt/β-catenin signaling in cardiogenesis are dependent on the developmental stage and cellular context 3. Before gastrulation, canonical Wnt/β-catenin signaling promotes cardiac commitment and differentiation in many species. In contrast, differential effects of this pathway on CM expansion have been reported among mice, zebrafish, amphibians, and chicks after gastrulation. Conditional deletion and transgenic overexpression of Wnt signaling component in this study and previous investigations 19, 42 have demonstrated that this pathway enhances and is necessary for CM expansion in mice after gastrulation. On the other hand, several studies have indicated that cardiogenesis in zebrafish, amphibians and chicks requires Wnt signaling inhibition after gastrulation 31, 32, 44. In the chick, posterior lateral plate mesoderm normally forms blood, but Wnt inhibition induces cardiac specific gene expression from this tissue 31. Similarly, Wnt suppression can induce cardiogenesis in explants from noncardiogenic ventral marginal zone mesoderm in Xenopus laevis 32. Global Wnt activation also inhibits heart specification after gastrulation in Zebrafish 44. Nevertheless, these experiments with Wnt manipulation in intact animal, organ culture, or tissue implants could not separate cell autonomous vs non-cell autonomous effect. This cell vs non-cell autonomous difference in CM proliferation has also been observed in retinoid acid signaling 45. Global deficiency in retinoid acid receptor RXRα causes ventricular hypoplasia while cardiac specific deletion of RXRα has not significant impact on normal cardiac development. With cardiac specific Wnt activation and inhibition, we have shown that the cell autonomous role of canonical Wnt/beta-catenin signaling in CMs is to promote rather than inhibit CM expansion after looping morphogenesis. Therefore, Wnt stimulation could be explored to strengthen ventricular development in patients with ventricular hypoplasia, promote cardiac regeneration in vivo, and expand differentiated CMs in vitro.
Supplementary Material
Highlights.
-
1)
β-cat played a critical role in maintaining CM proliferation gradient.
-
2)
APC formed an intensity gradient opposite to β-cat and CM proliferative activity.
-
3)
With Apc/β-cat double cKO, we found that Wnt signaling regulated cyclin D2.
Acknowledgements
We thank Drs. Rolf Kemler, Tetsuo Noda, Michael D. Schneider, and Bart O. Williams for their generosity to share mouse models with the scientific community.
Sources of Funding
The project described was supported by the National Institute of Health (NIH) grant R01 HL111480 (F Li) and a Grant-in-Aid award 10GRNT4460014 (F Li) from the American Heart Association Greater River Affiliate and the Lawrence J. and Florence A. DeGeorge Charitable Trust. Dr. Haodong Xu is supported by the NIH grant K08 HL088127.
Abbreviations
- APC
adenomatosis polyposis coli
- cKO
conditional knockout
- CM
cardiomyocyte
- E
embryonic day
- WT
wild type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author contributions
Bo Ye and Faqian Li designed the experiments. Bo Ye and Ning Hou collected data. Faqian Li, Haodong Xu, Bo Ye, Ning Hou, Lu Xiao, Yifan, Xu and James Boyer analyzed data and prepared the manuscript.
Conflict of Interest
None
References
- [1].Hurlstone AFL, Haramis A-PG, Wienholds E, Begthel H, Korving J, van Eeden F, et al. The Wnt/[beta]-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–7. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- [2].Olson EN. Gene Regulatory Networks in the Evolution and Development of the Heart. Science. 2006;313:1922–7. doi: 10.1126/science.1132292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–98. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- [4].Sedmera D, Reckova M, DeAlmeida A, Coppen SR, Kubalak SW, Gourdie RG, et al. Spatiotemporal pattern of commitment to slowed proliferation in the embryonic mouse heart indicates progressive differentiation of the cardiac conduction system. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2003;274A:773–7. doi: 10.1002/ar.a.10085. [DOI] [PubMed] [Google Scholar]
- [5].Soonpaa MH, Kim KK, Pajak L, Franklin M, Field LJ. Cardiomyocyte DNA synthesis and binucleation during murine development. American Journal of Physiology - Heart and Circulatory Physiology. 1996;271:H2183–H9. doi: 10.1152/ajpheart.1996.271.5.H2183. [DOI] [PubMed] [Google Scholar]
- [6].Larabell CA, Torres M, Rowning BA, Yost C, Miller JR, Wu M, et al. Establishment of the dorso-ventral axis in Xenopus embryos is presaged by early asymmetries in beta-catenin that are modulated by the Wnt signaling pathway. J Cell Biol. 1997;136:1123–36. doi: 10.1083/jcb.136.5.1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Miller JR, Moon RT. Signal transduction through beta-catenin and specification of cell fate during embryogenesis. Genes Dev. 1996;10:2527–39. doi: 10.1101/gad.10.20.2527. [DOI] [PubMed] [Google Scholar]
- [8].Behrens J, von Kries JP, Kuhl M, Bruhn L, Wedlich D, Grosschedl R, et al. Functional interaction of beta-catenin with the transcription factor LEF-1. Nature. 1996;382:638–42. doi: 10.1038/382638a0. [DOI] [PubMed] [Google Scholar]
- [9].Grigoryan T, Wend P, Klaus A, Birchmeier W. Deciphering the function of canonical Wnt signals in development and disease: conditional loss- and gain-of-function mutations of {beta}-catenin in mice. Genes Dev. 2008;22:2308–41. doi: 10.1101/gad.1686208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Kerkela R, Kockeritz L, Macaulay K, Zhou J, Doble BW, Beahm C, et al. Deletion of GSK-3beta in mice leads to hypertrophic cardiomyopathy secondary to cardiomyoblast hyperproliferation. J Clin Invest. 2008;118:3609–18. doi: 10.1172/JCI36245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Tseng A-S, Engel FB, Keating MT. The GSK-3 Inhibitor BIO Promotes Proliferation in Mammalian Cardiomyocytes. Chemistry & Biology. 2006;13:957–63. doi: 10.1016/j.chembiol.2006.08.004. [DOI] [PubMed] [Google Scholar]
- [12].Shibata H, Toyama K, Shioya H, Ito M, Hirota M, Hasegawa S, et al. Rapid colorectal adenoma formation initiated by conditional targeting of the Apc gene. Science. 1997;278:120–3. doi: 10.1126/science.278.5335.120. [DOI] [PubMed] [Google Scholar]
- [13].Su LK, Vogelstein B, Kinzler KW. Association of the APC tumor suppressor protein with catenins. Science. 1993;262:1734–7. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- [14].Fodde R, Edelmann W, Yang K, van Leeuwen C, Carlson C, Renault B, et al. A targeted chain-termination mutation in the mouse Apc gene results in multiple intestinal tumors. Proceedings of the National Academy of Sciences of the United States of America. 1994;91:8969–73. doi: 10.1073/pnas.91.19.8969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Hurlstone AF, Haramis AP, Wienholds E, Begthel H, Korving J, Van Eeden F, et al. The Wnt/beta-catenin pathway regulates cardiac valve formation. Nature. 2003;425:633–7. doi: 10.1038/nature02028. [DOI] [PubMed] [Google Scholar]
- [16].Cole AM, Myant K, Reed KR, Ridgway RA, Athineos D, Van den Brink GR, et al. Cyclin D2-cyclin-dependent kinase 4/6 is required for efficient proliferation and tumorigenesis following Apc loss. Cancer Res. 2010;70:8149–58. doi: 10.1158/0008-5472.CAN-10-0315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Agah R, Frenkel PA, French BA, Michael LH, Overbeek PA, Schneider MD. Gene recombination in postmitotic cells. Targeted expression of Cre recombinase provokes cardiac-restricted, site-specific rearrangement in adult ventricular muscle in vivo. J Clin Invest. 1997;100:169–79. doi: 10.1172/JCI119509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Yi XP, Gerdes AM, Li F. Myocyte redistribution of GRK2 and GRK5 in hypertensive, heart-failure-prone rats. Hypertension. 2002;39:1058–63. doi: 10.1161/01.hyp.0000019130.09167.3b. [DOI] [PubMed] [Google Scholar]
- [19].Buikema JW, Mady AS, Mittal NV, Atmanli A, Caron L, Doevendans PA, et al. Wnt/β-catenin signaling directs the regional expansion of first and second heart field-derived ventricular cardiomyocytes. Development. 2013;140:4165–76. doi: 10.1242/dev.099325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Gregorieff A, Pinto D, Begthel H, Destrée O, Kielman M, Clevers H. Expression Pattern of Wnt Signaling Components in the Adult Intestine. Gastroenterology. 2005;129:626–38. doi: 10.1016/j.gastro.2005.06.007. [DOI] [PubMed] [Google Scholar]
- [21].Angus-Hill ML, Elbert KM, Hidalgo J, Capecchi MR. T-cell factor 4 functions as a tumor suppressor whose disruption modulates colon cell proliferation and tumorigenesis. Proceedings of the National Academy of Sciences. 2011;108:4914–9. doi: 10.1073/pnas.1102300108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Zammit PS, Kelly RG, Franco D, Brown N, Moorman AF, Buckingham ME. Suppression of atrial myosin gene expression occurs independently in the left and right ventricles of the developing mouse heart. Dev Dyn. 2000;217:75–85. doi: 10.1002/(SICI)1097-0177(200001)217:1<75::AID-DVDY7>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- [23].Brault V, Moore R, Kutsch S, Ishibashi M, Rowitch DH, McMahon AP, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–64. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- [24].Qu J, Zhou J, Ping Yi X, Dong B, Zheng H, Miller LM, et al. Cardiac-specific haploinsufficiency of β-catenin attenuates cardiac hypertrophy but enhances fetal gene expression in response to aortic constriction. Journal of Molecular and Cellular Cardiology. 2007;43:319–26. doi: 10.1016/j.yjmcc.2007.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Lustig B, Jerchow B, Sachs M, Weiler S, Pietsch T, Karsten U, et al. Negative Feedback Loop of Wnt Signaling through Upregulation of Conductin/Axin2 in Colorectal and Liver Tumors. Mol Cell Biol. 2002;22:1184–93. doi: 10.1128/MCB.22.4.1184-1193.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].He TC, Sparks AB, Rago C, Hermeking H, Zawel L, da Costa LT, et al. Identification of c-MYC as a target of the APC pathway [see comments] Science. 1998;281:1509–12. doi: 10.1126/science.281.5382.1509. [DOI] [PubMed] [Google Scholar]
- [27].Shtutman M, Zhurinsky J, Simcha I, Albanese C, D’Amico M, Pestell R, et al. The cyclin D1 gene is a target of the beta-catenin/LEF-1 pathway. Proc Natl Acad Sci U S A. 1999;96:5522–7. doi: 10.1073/pnas.96.10.5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Tetsu O, McCormick F. [beta]-Catenin regulates expression of cyclin D1 in colon carcinoma cells. Nature. 1999;398:422–6. doi: 10.1038/18884. [DOI] [PubMed] [Google Scholar]
- [29].Sansom OJ, Reed KR, Hayes AJ, Ireland H, Brinkmann H, Newton IP, et al. Loss of Apc in vivo immediately perturbs Wnt signaling, differentiation, and migration. Genes Dev. 2004;18:1385–90. doi: 10.1101/gad.287404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Cohen ED, Tian Y, Morrisey EE. Wnt signaling: an essential regulator of cardiovascular differentiation, morphogenesis and progenitor self-renewal. Development. 2008;135:789–98. doi: 10.1242/dev.016865. [DOI] [PubMed] [Google Scholar]
- [31].Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–27. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–15. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc Natl Acad Sci U S A. 2007;104:9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [34].Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proc Natl Acad Sci U S A. 2007;104:10894–9. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Ai D, Fu X, Wang J, Lu MF, Chen L, Baldini A, et al. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. Proc Natl Acad Sci U S A. 2007;104:9319–24. doi: 10.1073/pnas.0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Klaus A, Saga Y, Taketo MM, Tzahor E, Birchmeier W. Distinct roles of Wnt/beta-catenin and Bmp signaling during early cardiogenesis. Proc Natl Acad Sci U S A. 2007;104:18531–6. doi: 10.1073/pnas.0703113104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Cohen ED, Wang Z, Lepore JJ, Lu MM, Taketo MM, Epstein DJ, et al. Wnt/beta-catenin signaling promotes expansion of Isl-1-positive cardiac progenitor cells through regulation of FGF signaling. J Clin Invest. 2007;117:1794–804. doi: 10.1172/JCI31731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Chen J, Kubalak SW, Minamisawa S, Price RL, Becker KD, Hickey R, et al. Selective Requirement of Myosin Light Chain 2v in Embryonic Heart Function. Journal of Biological Chemistry. 1998;273:1252–6. doi: 10.1074/jbc.273.2.1252. [DOI] [PubMed] [Google Scholar]
- [39].Woulfe KC, Gao E, Lal H, Harris D, Fan Q, Vagnozzi R, et al. Glycogen Synthase Kinase-3{beta} Regulates Post-Myocardial Infarction Remodeling and Stress-Induced Cardiomyocyte Proliferation In Vivo. Circ Res. 2010;106:1635–45. doi: 10.1161/CIRCRESAHA.109.211482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Poolman RA, Brooks G. Expressions and Activities of Cell Cycle Regulatory Molecules During the Transition from Myocyte Hyperplasia to Hypertrophy. Journal of Molecular and Cellular Cardiology. 1998;30:2121–35. doi: 10.1006/jmcc.1998.0808. [DOI] [PubMed] [Google Scholar]
- [41].Ai D, Fu X, Wang J, Lu M-F, Chen L, Baldini A, et al. Canonical Wnt signaling functions in second heart field to promote right ventricular growth. PNAS. 2007;104:9319–24. doi: 10.1073/pnas.0701212104. %R 101073/pnas0701212104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [42].Kwon C, Arnold J, Hsiao EC, Taketo MM, Conklin BR, Srivastava D. Canonical Wnt signaling is a positive regulator of mammalian cardiac progenitors. Proceedings of the National Academy of Sciences. 2007;104:10894–9. doi: 10.1073/pnas.0704044104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Pasumarthi KBS, Nakajima H, Nakajima HO, Soonpaa MH, Field LJ. Targeted Expression of Cyclin D2 Results in Cardiomyocyte DNA Synthesis and Infarct Regression in Transgenic Mice. Circ Res. 2005;96:110–8. doi: 10.1161/01.RES.0000152326.91223.4F. [DOI] [PubMed] [Google Scholar]
- [44].Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, et al. From the Cover: Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proceedings of the National Academy of Sciences. 2007;104:9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Chen J, Kubalak SW, Chien KR. Ventricular muscle-restricted targeting of the RXRalpha gene reveals a non-cell-autonomous requirement in cardiac chamber morphogenesis. Development. 1998;125:1943–9. doi: 10.1242/dev.125.10.1943. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.