Abstract
Although heart rate and temperature are continuously monitored in patients during recovery following surgery, measures that extract direct manifestations of neural regulation of autonomic circuits from the beat-to-beat heart rate may be more sensitive to outcome. We explore the relationship between features of autonomic regulation and survival in the prairie vole, a small mammal, with features of vagal regulation of the heart similar to humans. Cardiac vagal regulation is manifested in the beat-to-beat heart rate variability (HRV) pattern and can be quantified by extracting measures of the amplitude of periodic oscillations associated with spontaneous breathing. Thus, monitoring beat-to-beat heart rate patterns post-surgery in the prairie vole may provide an opportunity to dynamically assess autonomic adjustments during recovery. Surgeries to implant telemetry devices to monitor body temperature and continuous ECG in prairie voles are routinely performed in our laboratory. Ten of these implanted prairie voles died within 48 h post-surgery. To compare the post-surgery autonomic trajectories with typical surviving prairie voles, the post-surgery data from 17 surviving prairie voles were randomly selected. The data are reported hourly for 27 prairie voles between 6 and 14 h (1 h before the demise of the first subject) post-surgery. Receiver operator curves were calculated hourly for each variable to evaluate sensitivity in discriminating survival. The data illustrate that measures of HRV are the most sensitive indicators. These findings provide a foundation for investigating further neural mechanisms of cardiovascular function.
Keywords: Autonomic nervous system, Surgery recovery, Anesthesia, Heart rate variability, Animal model
1. Introduction
Monitoring heart rate and thermoregulatory functions during recovery are standard practices in clinical medicine following surgical procedures requiring general anesthesia. Although these are robust indicators of an irreversible multisystem shutdown that precedes death, other variables that reflect dynamically changing neural tone may provide an earlier indicator of demise. Deschamps and Denault (2008) argue for inclusion of real-time monitoring of heart rate variability (HRV) and autonomically-targeted reactive drug management during anesthesia and post-operative recovery. Underlying this suggestion is a large literature illustrating that the dynamically changing amplitude of periodic beat-to-beat heart rate patterns reflect brainstem modulation of the heart (Porges, 2007). However, current clinical electrocardiogram (ECG) technology undersamples the signal, limiting the sensitivity of beat-to-beat changes in rate over time. Further, real-time measurement of heart rate variability is in its infancy. As we move closer to the wide-spread availability of continuous monitoring of beat-to-beat blood pressure and heart rate in clinical settings, the possibility of applying dynamic measures of neural regulation of the heart is moving closer to feasibility. This study was conducted to provide a foundation for developing a valid and reliable animal model system to eventually study neural control of the autonomic nervous system more directly, with translational potential to human conditions.
The rhythmic changes in beat-to-beat heart rate pattern reflect the modulation of neural influences to the sino-atrial node. A high frequency component in HRV, which manifests a respiratory rhythm, is commonly known as respiratory sinus arrhythmia (RSA). In mammals, RSA is mediated primarily through the myelinated vagal efferent pathways originating in a medullary nucleus, the nucleus ambiguus (Porges, 1995). Due to its strong neurophysiological basis, RSA has been a primary focus of research applying HRV to clinical conditions (e.g., obesity, (Tonhajzerova et al., 2008), sudden death, (Groh et al., 2008), irritable bowel syndrome, (Spetalen et al., 2008)). Lower frequency (LF) HRV components have also been studied. Often these studies investigate oscillations in humans with a period of approximately 12 to 20 s, a frequency shared with other cardiovascular variables including blood pressure and vasomotor activity. The neural mechanisms mediating LF in heart rate are poorly understood and have been assumed to represent mixed vagal and sympathetic influences (Axelrod et al., 1981), however this assumption has been questioned since the rhythm can be completely blocked in normal subjects with atropine (cholinergic receptor blockage) and is not affected by beta-adrenergic receptor blockade (Jokkel et al., 1995; Eckberg, 2003; Porges, 2007). Similarly in the vole, HRV is virtually eliminated by atropine and not depressed by beta-adrenergic receptor blockade (Grippo et al., 2007).
Greater HRV, usually measured by an increase in the amplitude of RSA, is generally reported to be associated with positive cardiovascular health (Routledge et al., 2002). In post-hoc analyses, depressed HRV has been linked to several autonomic disorders including sudden death (Mustonen et al., 1997) (Cygankiewicz et al., 2008), hypertension and diabetes (Masi et al., 2007). Depressed HRV is also associated with psychiatric disorders including depression (Licht et al., 2008) and anxiety (Friedman, 2007). RSA is profoundly depressed during anesthesia (Donchin et al., 1985) (Ibrahim et al., 2001) (Pomfrett et al., 1993); post-surgical recovery of RSA parallels a return to consciousness (Donchin et al., 1985), and pre-surgical levels of RSA are reported to be predictive of neurosurgical outcome (Donchin et al., 1992). Hence, the study of HRV in an animal model is useful for understanding in a controlled environment the relationships of relevant systems as they pertain to outcome and the development of potential autonomically-targeted interventions.
The prairie vole (Microtus ochrogaster) is a rodent species that displays several social behaviors similar to humans (Carter et al., 1995). Also, relative to many small mammals, the prairie vole has elevated parasympathetic (vagal) cardiac tone and a pattern of autonomic regulation and reactivity similar to humans (Grippo et al., 2007). Given these features, investigation of autonomic recovery following surgery in prairie voles may provide a translational model generating insights leading to a better understanding of the relation between recovery and autonomic state in human clinical settings.
The present study was designed to investigate autonomic function in prairie voles following a minor surgical procedure, to provide a foundation for developing a useful animal model for the investigation of mechanisms underlying autonomic function and health. In the current study, physiologic parameters (i.e, RSA, LF, temperature, and heart period) were monitored in prairie voles following surgical implantation of electrodes and transmitters to determine if the indices of autonomic regulation would provide lead indicators of survival or demise. Based on the literature evaluating heart rate patterns in humans with multi-factorial trauma (Mowery et al., 2008), we hypothesized that measures of brainstem regulation of the heart manifested in the indices of HRV (i.e., RSA, LF) would be the strongest predictors of survival. Further, we expected, as brain regulation of these systems diminished, that heart rate would decouple (i.e. become less correlated) from the HRV measures.
2. Methods
2.1. Animals
The twenty-seven female prairie voles studied were descendants of a wild stock caught near Champaign, Illinois. Female prairie voles do not have spontaneous puberty or an estrous cycle. The ovaries of the adult female prairie vole remain inactive until the female has physical contact with a male, hence allowing for the use of reproductively intact animals without the need for controlling the estrous cycle (Carter et al., 1987). Offspring were removed from the breeding pairs at 21 days of age and housed in same-sex sibling pairs until the commencement of the experimental procedures. Prior to and following surgery, the prairie voles were maintained on a 14/10 h light/dark cycle (lights on at 0630 h), with a temperature of 25±1 °C and relative humidity of 21±4 g/m3. Animals were allowed food (Purina rabbit chow) and water ad libitum. All procedures were conducted according to National Institutes of Health’s Guide for the Care and Use of Laboratory Animals and approved by the University of Illinois Institutional Animal Care and Use Committee.
2.2. Group assignment
Animals that died between 14 and 48 h post-surgery (n=10) were categorized as the “demise” group. Survival of 14 h was determined by the earliest animal to die from the selection pool. Animals that survived at least twelve days post-surgery (n=17) were categorized as the “survival” group. None of the animals in the “survival” group died at any point during the study. Animals that died during surgery were excluded from the study.
2.3. General surgery procedures, sample notes, and details
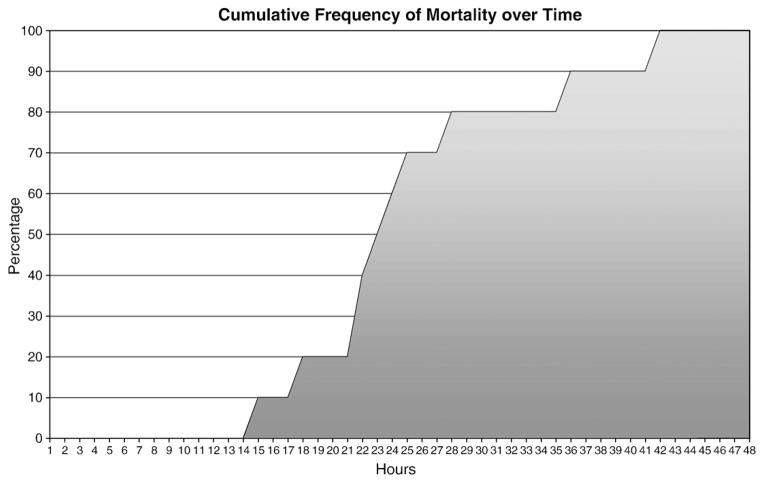
The animals for this project (“survival” and “demise” groups) were both obtained from a larger dataset designed to investigate autonomic responses and social behavior (see Grippo et al., 2007). During the data collection, it was noted that a few of the animals died within two days of surgery (see Fig. 1, graph of mortality vs hours post-surgery). All surgeries were performed by a comparative neuroscientist, with exactly the same instruments, procedures, and pre- and postoperative care with effort to hold all variables constant. All dead and surviving animals were chosen from studies when the surgeon was experienced with the specific procedures (approx. 1 year or more). Dead animals and surviving animals were chosen from the same groups of animals. This is a short, minimally-invasive procedure in the context of rodent surgeries.
Fig. 1.
Mortality vs hours post-surgery.
A total of 81 surgeries were performed over a 3 year period. There were 10 deaths (12.4% of animals) within two days of surgery. Seventeen animals were chosen from the survivors as a comparison group pseudorandomly (by ID number, without availability of clinical information). There were no significant differences between survive and demise groups on either weight or age using independent samples t-tests: age: t(29)=0.03, p>0.05, weight: t(29)=0.18, p>0.05 (see Table 1). All surgeries were performed by a well-trained investigator and were performed at approximately the same time of day (~8 am–12 pm). Surgery time was 30–45 min per animal. The anesthesia used was ketamine/xyaline (5:1 ratio; 67 mg/kg katamine and 13.33 mg/kg xyaline, sc). Ketamine has a half-life of 2.5 h. If a supplemental anesthetic injection was necessary during surgery, it was administered at a volume of 0.01 ml/animal (5:1 ratio). All environmental variables (including room temperature, humidity level, and number of personnel), pre-operative procedures (including preparation of surgical area, preparation of instruments, and preparation of surgeon), and post-operative procedures and monitoring (including monitoring of animals, and availability of food and water) were identical in both groups.
Table 1.
Age and weight between groups.
| Age (days, mean, SD) | Weight (grams, mean, SD) | p-value | |
|---|---|---|---|
| Survive | 73+−15.0 | 48+−8.6 | >.05 |
| Demise | 73+−16.8 | 47+−7.2 | >.05 |
2.4. Telemetric transmitter implantation
Wireless radio transmitters [Data Sciences International (DSI), St. Paul, MN; model TA10ETA-F20] were implanted intraperitoneally under aseptic conditions, during the light period, for long-term ECG recordings. Animals were anesthetized with ketamine and xylazine (67 mg/kg and 13.33 mg/kg, respectively, sc; NLS Animal Health, Owings Mills, MD). Transmitter implantation was similar to procedures reported in the literature (Sgoifo et al., 1996; Grippo et al., 2007) Immediately following recovery from surgery, animals were housed in custom-designed divided cages (Grippo et al., 2007) for five days, to permit adequate healing of suture wounds, and were then returned to the home cage (with the same-sex sibling).
2.5. Radiotelemetric recordings
ECG signals were recorded with a radiotelemetry receiver (DSI, St. Paul, MN; sampling rate 5 kHz, 12-bit precision digitizing). Activity level and body temperature were monitored via the receiver (sampling rate 256 Hz). All the parameters were recorded for at least 2 min every hour starting 5 h after surgery.
2.6. Quantification of radiotelemetric recordings
2.6.1. Quantification of telemetric variables
Quantification of telemetric variables was similar to those previously described (Grippo et al., 2007). Multiple segments of 1–5 min of stable, continuous data were used to evaluate heart period, HRV, and temperature. Preliminary spectral analyses identified two frequency components: a frequency at the spontaneous breathing rate between approximately 1.0 and 4.0 Hz that defined RSA oscillations, and a frequency slower than breathing in the 0.2 to 1.0 Hz range that defined LF oscillations in the prairie vole. Data segments were matched across subjects and time points, and were used to calculate all physiological variables.
Beat-to-beat heart period, temperature, and activity were quantified using vendor software (DSI, St. Paul, MN). R-wave detections were verified with custom-designed software. The ECG signal was exported into a data file and synchronized with the sequential beat-to-beat heart periods. Data were visually scanned for outliers due to missed or faulty R-wave detections. Missed or faulty detections were corrected using the synchronized ECG pattern as a guide, with custom-designed software (Cardio-Edit; Brain–Body Center, UIC). All data were collected using Dataquest ART, version 4.1. Acquisition software, and data were prepared for analysis using Dataquest ART, version 4.1 Analysis software (Data Sciences International, St. Paul, MN).
RSA was assessed with time-frequency procedures (Porges, 1985; Porges and Bohrer, 1990) that have been validated with humans (Porges, 2007) and modified for the prairie vole (Grippo et al., 2007). The amplitude of RSA represents the functional vagal impact on the sino-atrial node of myelinated vagal efferent pathways originating in the nucleus ambiguus. To deal with the possibility that violating the assumption of stationarity can distort time series analyses of RSA, the following procedures were implemented: 1) the R–R intervals (heart period) were time-sampled into equal time intervals with a sampling rate of 20 Hz; 2) the time series were detrended with a moving polynomial filter (Porges and Byrne, 1992) that removed variance in the series below 1 Hz for RSA (i.e., 21-point cubic polynomial) and below 0.2 Hz for LF (i.e., 51-point cubic polynomial), 3) the spectral analyses identified the peak amplitude of RSA and LF from the detrended data. RSA was operationally defined as the natural log of the sum of the power within the respiratory bandwidth of 1.0–4.0 Hz and LF was operationally defined as the natural log of the sum of the power within the 0.2–1.0 Hz band.
3. Statistical methods
For both groups, the data were visually scanned over all hours and across all measures. Both groups generally appeared approximately normal on visual inspection across all metrics. There was no evidence of bimodal distribution. On some hours, on some metrics, there were violations of kurtosis and skewness.
To determine group differences, repeated measures analyses of variance were calculated on each physiological variable using survival as a group variable and hour following surgery as the repeated measure. Due to the potential influence of violations of normality, confirmatory nonparametric analyses of variance were also calculated. To evaluate the covariance among variables and to determine if group classification influenced the covariation among the variables, correlations were calculated across and within each group. Receiver operator curves (ROC) were calculated each hour to determine the sensitivity of each variable in distinguishing group classifications. Logistic stepwise multivariate regressions were used to determine the best predictive model of demise among the four physiological variables by hour. The Statistical Package for Social Sciences (SPSS) version 17 was used to perform all statistical analyses. A probability of p<.05 was considered to be statistically significant.
4. Results
4.1. Analyses of variance
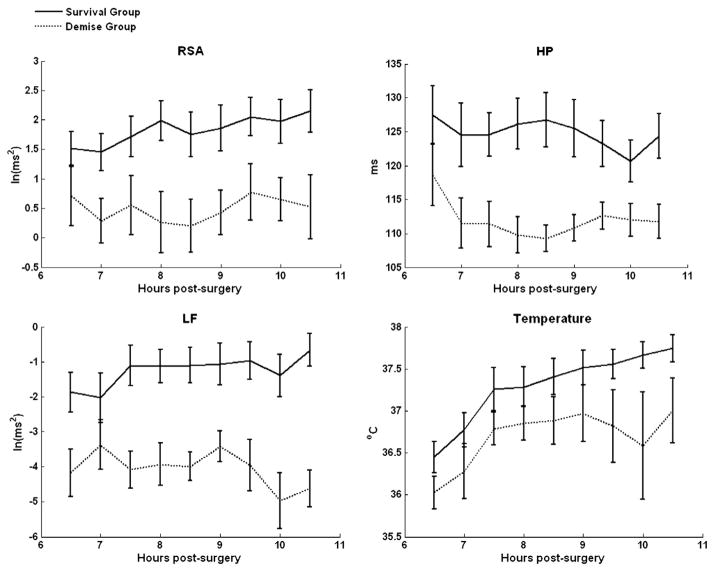
Analyses on group differences identified significant group differences in RSA, F(1, 24),=7.849, p<.05 (survive mean=1.85, SD=1.43; demise mean=0.48 SD=1.44), LF, F(1, 24)=13.569, p<.05 (survive mean=1.25, SD=2.31; demise mean=3.52 SD=1.83), and heart period, F(1, 24)=6.667, p<.05) (survive mean=124.78, SD=15.49; demise mean=112.46 SD=8.97), but not in temperature. Temperature, collapsed across groups, significantly increased across the repeated hour-by-hour assessments, F(8, 176)=23.780, p<.05) (hour 6 mean temp=36.32, SD=.73; hour 14 mean temp=37.53, SD=1.02). There were no significant within group interactions. Since some of the distributions deviated from normal on some hours, nonparametric analyses were also conducted to verify findings. The findings from the nonparametric analyses were consistent with the ANOVA results. As illustrated in Fig. 2, the significant group differences for RSA, LF, and heart period were observable early and were maintained across the repeated assessments.
Fig. 2.
Survival and demise trajectory comparisons across physiologic indicators. RSA = respiratory sinus arrhythmia, HP = heart period, LF = low frequency heart rate variability. Note: Error bars represent ± 1 Standard Error of the Mean. All points with error bar separation are significantly different.
4.2. Correlations
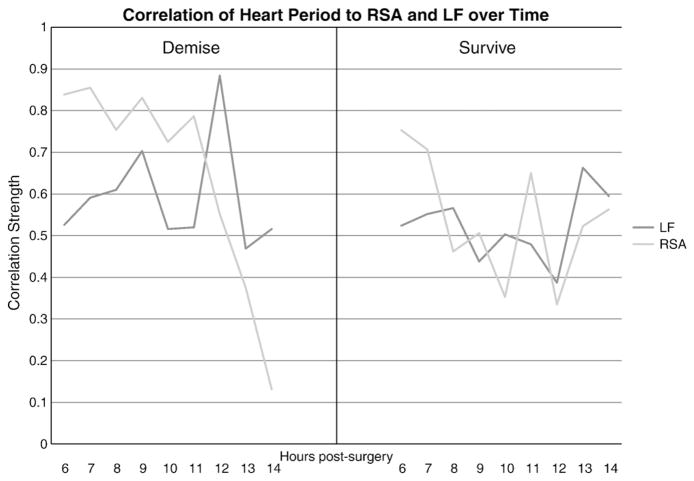
Correlations among the physiological variables within each group were calculated at each hour. In the survival group, correlations between RSA, LF, and HP were moderate to high over time and consistently significantly related. Temperature was the least strongly correlated with the other variables (data not shown). In contrast, in the demise group, the four physiological variables were not consistently correlated and were decoupled by hour 14 (i.e., no significant correlations) (see Fig. 3 for decoupling of heart period from RSA and LF). RSA and heart period decoupling appears most pronounced in downward trend in the demise group.
Fig. 3.
Correlation of heart period to RSA and LF over time in demise (depicted at left) and survival (depicted at right) groups. Note decoupling of RSA from heart period. RSA = respiratory sinus arrhythmia, LF = low frequency heart rate variability.
4.3. Efficacy of physiological variables in group classification
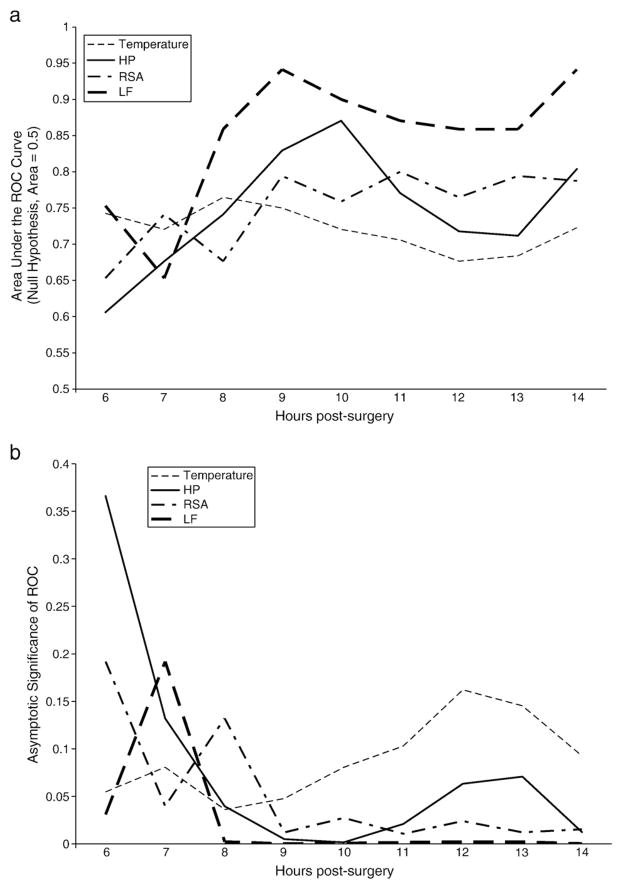
To evaluate the relative sensitivity of the physiological variables in classifying demise and survival groups, receiver operating characteristic (ROC) curves were calculated for each variable, hour by hour. The ROC is a combined measure of sensitivity and specificity, and significance is tested against an assignment of members to each group based on chance alone. Fig. 3 illustrates the hour-by-hour efficacy of each variable. Fig. 3a illustrates the area under the curve (AUC) for each physiological variable during each hour; a variable with no correlation to group membership would have an AUC of 0.5. The AUC reflects the efficacy of the variable in accurately identifying a prairie vole as being in the “demise” group, with larger AUC indicating increased efficacy. The statistical significances for the AUC data are illustrated in Fig. 3b. Table 2 shows the false positives and false negatives over time across the four physiological indicators. The ROC curves illustrate that the HRV measures (RSA, LF) and heart period performed well, providing an effective and reliable index of group membership by hour 9 (p<.05) (Fig 4a,b).
Table 2.
False positives and false negatives per hour across physiological indicators.
| Hours | Temperature
|
RSA
|
LF
|
HP
|
||||
|---|---|---|---|---|---|---|---|---|
| FP | FN | FP | FN | FP | FN | FP | FN | |
| 6 | 6 | 2 | 13 | 6 | 8 | 4 | 10 | 7 |
| 7 | 6 | 3 | 8 | 4 | 10 | 6 | 10 | 5 |
| 8 | 6 | 3 | 9 | 6 | 3 | 1 | 9 | 4 |
| 9 | 8 | 2 | 5 | 4 | 1 | 0 | 6 | 3 |
| 10 | 10 | 3 | 8 | 5 | 2 | 0 | 5 | 3 |
| 11 | 10 | 3 | 5 | 3 | 3 | 0 | 7 | 3 |
| 12 | 13 | 3 | 6 | 4 | 1 | 1 | 8 | 4 |
| 13 | 13 | 3 | 5 | 3 | 4 | 2 | 9 | 4 |
| 14 | 8 | 4 | 4 | 4 | 1 | 0 | 3 | 2 |
RSA = Respiratory sinus arrhythmia, HP = heart period, LF = low frequency heart rate variability. FP = false positive, FN = false negative.
Fig. 4.
a) Receiver operating characteristic (area under the curve) indicating relative sensitivity of physiologic indicators over time to survival RSA = respiratory sinus arrhythmia, HP = heart period, LF = low frequency heart rate variability. b) Receiver operating characteristic curves (probability of significant effect) indicating relative sensitivity of physiologic indicators over time to survival RSA = respiratory sinus arrhythmia, HP = heart period, LF = low frequency heart rate variability.
4.4. Multiple regressions
All four variables (RSA, LF, heart period, and temperature) were entered into a multivariate stepwise logistic regression to determine which variables would provide the earliest and strongest prediction of survival and whether combinations of the variables would improve the prediction model. The analysis indicated that at hour six, RSA was the earliest and strongest predictor of survival (OR 4.49, 95% CI=2.07–9.76). The overall model accuracy for hour 6 was 88.2% for survival, and 50% for demise. At hour 7, both RSA and LF contribute independent variance to survival (RSA, OR 4.80, 95% CI=1.49–9.10; LF, OR 3.05, 95% CI=1.034–8.98). The overall model accuracy for hour 7 was 88.2% for survival and 75.0% for demise. From hour 8 through 14, LF was the strongest predictor of survival and the only predictor remaining in the final model. By hour 14 (LF OR 3.96, 95% CI=1.86–8.42) the overall predictive accuracy of the model containing only LF was 93.8% for survival and 71.4% for demise.
5. Discussion
The present study was designed to provide a foundation for developing an animal model to investigate specifically the neural mechanisms of autonomic and cardiovascular function. Prairie voles offer a good potential analog to human autonomic performance due to their high tonic levels of vagal influence on the heart compared to other small mammals such as rats and mice. While the present study did not aim to present a model of a specific type of surgery (e.g., pacemaker implantation) or medication, it does provide a groundwork for the future development of specific models. This study is the first to describe autonomic function following a surgical intervention in prairie voles.
The primary finding from this study is that measures of HRV (i.e., RSA, LF) indicate demise earlier and more consistently than the traditional measures of heart rate (i.e., heart period) and body temperature. The earliest predictor of demise is RSA at hour six. However, consistency is not achieved until hour 8, at which point LF remains a strong, and strengthening, predictor of demise through the end of recording. ROC analyses indicate consistent significant sensitivity and specificity for LF and RSA at hour 9. In contrast heart period significantly discriminates at hours 8–11 and 14, while temperature significantly discriminates only during hour 8. The decrease in heart period of the demise group is likely attributable to changes in pacemaker function due to an interaction between reduced vagal regulation and local neurochemical changes in the sino-atrial node.
The most striking difference between the groups was the consistent depression of both HRV measures in the demise group. It is unclear why LF was a stronger predictor than RSA over time. LF is thought to represent different autonomic influences than RSA. It has been proposed that LF either reflects the combined contributions of the sympathetic and parasympathetic inputs to the heart (Axelrod et al., 1981) or possibly represents the influence of the unmyelinated vagus originating in the dorsal motor nucleus of the vagus (Porges, 2007). Consistent with the dorsal motor nucleus hypothesis, loss of LF might indicate a more global shutdown of brainstem regulation of the autonomic nervous system and loss of the adaptive transitory functions, including baroreceptor reflexes, that this circuit generally mediates. Thus, depressed LF might index a loss of the neural protection from episodes of syncope, bradycardia, and apnea. Consistent with this hypothesis, a recent study reported that decreased LF was a predictive factor for acute coronary events (Perkiomaki et al., 2008).
Further, there is evidence of decoupling of heart period from HRV components over time in the demise group. Decoupling suggests a lack of neural input into cardiac cycle control. In a recent study, Morris et al (Morris et al., 2006a), in a sample of 1425 trauma patients (human), report approximately 55.9% of their sample had decoupling of HRV from heart rate. Mowery et al. (2008) report cardiac decoupling in 145 trauma patients correlates with intracranial hypertension and mortality. However, it should be noted that both Mowery et al (2008) and Morris et al. (2006b) defined cardiac uncoupling solely on the basis of reduced HRV. While reduced HRV may indicate cardiac uncoupling, directly assessing it via examination of the relationships of autonomic components may offer additional critical information. Exploring these phenomena in prairie voles and potential interventions may prove fruitful.
Polyvagal Theory (Porges, 2007) suggests phylogenetically differentiated afferent and efferent systems involved in autonomic control that serve different adaptive purposes. LF may modulate mechanisms, via pathways originating in the dorsal motor nucleus of the vagus, involved in immobilization, response to inflammation, and states of healing. Decrease in LF in the demise group, potentially, suggests failure of central nervous system management of reparative resources subsequent to the surgical trauma.
Because this is the first study to investigate autonomic function using continuous electrocardiographic recordings in prairie voles following a surgical intervention, there are some limitations. One limitation of the study is a lack of assessment of cause of death. Because of the nature of the research question, we are unable to control the specific cause or time of death as we are studying the natural course of death or survival following surgery, which makes interpretation of the data more difficult (no random assignment of groups). However, this allows for a better representation of autonomic characteristics that may be relevant in human patients. Moreover, technical limitations of the study design prevented data collection from the animals prior to the 5th hour after surgery. In future studies, continuous monitoring immediately after surgery will provide a framework from which to understand the progression of HRV through post-operative recovery.
Since prairie voles have, similar to humans, high vagal regulation of the heart, the prairie vole may provide a valuable comparative model to explore the impact of trauma (e.g., surgery) on outcomes via monitoring the neural regulation of the heart via beat-to-beat heart rate patterns. In a clinical setting it would be very powerful to have a predictor of survival that is useful regardless of exact cause of death. Indeed, if we determine the exact cause of death for each human that dies in the clinical setting, we still only have this information post-mortem. But if we can create a model that can teach us more about neural control of autonomic function, we can potentially translate this information to the clinical setting, and develop an index survival vulnerability using measures of autonomic function as predictors. However, to further develop a comparative model with translational value, future research should include post-mortem investigation in order to parse specific autonomic correlates and ensure that the potential model reflects our knowledge of human autonomic signatures to various etiologies. This study provides a foundation upon which additional investigation of the neural mechanisms of autonomic and cardiovascular function can be conducted, with translational potential to human clinical conditions. Future research may evaluate interventions designed to improve neural regulation of the heart after surgery (e.g., pharmaceutical, mechanical, or environmental) using the prairie vole model. In addition to mechanistic research with animal models, investigations of human case study trials in which beat-to-beat heart rate were monitored would provide an important step in expanding our preliminary findings to the clinical arena.
Acknowledgments
This study is conducted and supported by NIA, NIH and NIMH. John Williamson is a recipient of NIA (F32 AG027648-01A1), PI: JBW (2006–2008). Stephen Porges is a recipient of NIMH (R21 MH0674467), PI: SWP. Angela Grippo is a recipient of NIMH (F32 MH73233), PI: AJG and NIMH (R21 MH77581), PI: AJG. Sue Carter is a recipient of NIMH (R01 MH72935), PI: CSC. David Nyenhuis and Philip Gorelick are recipients of NIA (R01 AG17934), PI: PBG (2000–2003) and DLN (2003–2006).
References
- Axelrod S, Gordon D, Ubel FA, Shannon DC, Berger AC, Cohen RJ. Power spectrum analysis of heart rate fluctuation: a quantitative probe of beat-to-beat cardiovascular control. Science. 1981;213:220–222. doi: 10.1126/science.6166045. [DOI] [PubMed] [Google Scholar]
- Carter CS, DeVries AC, Getz LL. Physiological substrates of mammalian monogamy: the Prairie vole model. Neurosci Biobehav Rev. 1995;19:303–314. doi: 10.1016/0149-7634(94)00070-h. [DOI] [PubMed] [Google Scholar]
- Carter CS, Witt DM, Schneider J, Harris ZL, Volkening D. Male stimuli are necessary for female sexual behavior and uterine growth in prairie voles (Microtus ochrogaster) Horm Behav. 1987;21:74–82. doi: 10.1016/0018-506x(87)90032-8. [DOI] [PubMed] [Google Scholar]
- Cygankiewicz I, Zareba W, de Luna AB. Prognostic value of Holter monitoring in congestive heart failure. Cardiol J. 2008;15:313–323. [PubMed] [Google Scholar]
- Donchin Y, Feld JM, Porges SW. Respiratory sinus arrhythmia during recovery from isoflurane-nitrous oxide anesthesia. Anesth Analg. 1985;64:811–815. [PubMed] [Google Scholar]
- Donchin Y, Constantini S, Szold A, Byrne EA, Porges SW. Cardiac vagal tone predicts outcome in neurosurgical patients. Crit Care Med. 1992;20:941–949. doi: 10.1097/00003246-199207000-00008. [DOI] [PubMed] [Google Scholar]
- Deschamps A, Denault A. Analysis of heart rate variability: a useful tool to evaluate autonomic tone in the anesthetized patient? Can J Anesth. 2008;55:208–213. doi: 10.1007/BF03021504. [DOI] [PubMed] [Google Scholar]
- Eckberg DL. The human respiratory gate. J Physiol. 2003;548:339–352. doi: 10.1113/jphysiol.2003.037192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman BH. An autonomic flexibility — neurovisceral integration model of anxiety and cardiac vagal tone. Biol Psychol. 2007;74:185–199. doi: 10.1016/j.biopsycho.2005.08.009. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Lamb DG, Carter CS, Porges SW. Cardiac regulation in the socially monogamous prairie vole. Physiol Behav. 2007;90:386–393. doi: 10.1016/j.physbeh.2006.09.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groh WJ, Groh MR, Saha C, Kincaid JC, Simmons Z, Ciafaloni E, Pourmand E, Pourmand R, Otten RF, Bhakta D, Nair GV, Marashdeh MM, Zipes DP, Pascuzzi RM. Electrocardiographic abnormalities and sudden death in myotonic dystrophy type 1. N Engl J Med. 2008;358:2688–2697. doi: 10.1056/NEJMoa062800. [DOI] [PubMed] [Google Scholar]
- Ibrahim A, Taraday J, Kharasch E. Bispectral index monitoring during sedation with sevoflurance, midazolam, and propofol. Anesthesiology. 2001;95:1151–1159. doi: 10.1097/00000542-200111000-00019. [DOI] [PubMed] [Google Scholar]
- Jokkel G, Bonyhay I, Kollai M. Heart rate variability after complete autonomic blockade in man. J Auton Nerv Sys. 1995;51:85–89. doi: 10.1016/0165-1838(95)80010-8. [DOI] [PubMed] [Google Scholar]
- Licht CM, de Geus EJ, Zitman FG, Hoogenijk WJ, van Dyck R, Penninx BW. Association between major depressive disorder and heart rate variability in the Netherlands Study of Depression and Anxiety (NESDA) Arch Gen Psychiatry. 2008;65:1358–1367. doi: 10.1001/archpsyc.65.12.1358. [DOI] [PubMed] [Google Scholar]
- Masi CM, Hawkley LC, Rickett EM, Cacioppo JT. Respiratory sinus arrhythmia and diseases of aging: obesity, diabetes mellitus, and hypertension. Biol Psychol. 2007;74:212–223. doi: 10.1016/j.biopsycho.2006.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris JA, Jr, Norris PR, Ozdas A, Waitman LR, Harrell FE, Jr, Williams AE, Cau H, Jenkins JM. Reduced heart rate variability: an indicator of cardiac uncoupling and diminished physiologic reserve in 1425 trauma patients. J Trauma. 2006a;60:1165–1173. doi: 10.1097/01.ta.0000220384.04978.3b. [DOI] [PubMed] [Google Scholar]
- Mowery NT, Norris PR, Riordan W, Jenkins JM, Williams AE, Morris JA., Jr Cardiac uncoupling and heart rate variability are associated with intracranial hypertension and morality: a study of 145 trauma patients with continuous monitoring. J Trauma. 2008:621–627. doi: 10.1097/TA.0b013e3181837980. [DOI] [PubMed] [Google Scholar]
- Mustonen J, Uusitupa M, Mantysaari M, Lansimies E, Pyorala K, Laasko M. Changes in autonomic nervous function during the 4-year follow-up in middle-aged diabetic and nondiabetic subjects initially free of coronary heart disease. J Intern Med. 1997;241:227–235. doi: 10.1046/j.1365-2796.1997.116126000.x. [DOI] [PubMed] [Google Scholar]
- Perkiomaki JS, Jokinen V, Tapanainen J, Airaksinen KE, Huikuri HV. Autonomic — markers as predictors of nonfatal acute coronary events after myocardial infarction. Ann Noninvasive Electrocardiol. 2008;13:120–129. doi: 10.1111/j.1542-474X.2008.00211.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pomfrett CJ, Barrie JR, Healy TE. Respiratory sinus arrhythmia: an index of light anaesthesia. Br J Anesth. 1993;71:212–217. doi: 10.1093/bja/71.2.212. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jr, Norris PR, Ozdas A, Waitman LR, Harrell FE, Jr, Williams AE, Cau H, Jenkins JM. Reduced heart rate variability: an indicator of cardiac uncoupling and diminished physiologic reserve in 1425 trauma patients. J Trauma. 2006b;60:1165–1173. doi: 10.1097/01.ta.0000220384.04978.3b. heritage. A polyvagal theory. Psychophysiology. 32, 301–318. [DOI] [PubMed] [Google Scholar]
- Porges SW. Method and apparatus for evaluating rhythmic oscillations in aperiodic physiological response systems. 4,510,944 United States Patent number. 1995
- Porges SW, Bohrer RE. Analyses of periodic processes in psychophysiological research. In: Cacioppo JT, Tassinary LG, editors. Principles of Psychophysiology: Physical, Social, and Inferential Elements. Cambridge University Press; New York: 1990. pp. 708–753. [Google Scholar]
- Porges SW, Byrne EA. Research methods for measurement of heart rate and respiration. Biol Psychol. 1992;324:93–130. doi: 10.1016/0301-0511(92)90012-j. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal perspective. Biol Psychol. 2007;74:116–143. doi: 10.1016/j.biopsycho.2006.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Routledge HC, Chowdhary S, Townend JN. Heart rate variability — a therapeutic target? J Clin Pharm Ther. 2002;27:85–92. doi: 10.1046/j.1365-2710.2002.00404.x. [DOI] [PubMed] [Google Scholar]
- Sgoifo A, Stilli D, Medici D, Gallo P, Aimi B, Musso E. Electrode positioning for reliable telemetry ECG recordings during social stress in unrestrained rats. Physiol Behav. 1996;60:1397–1401. doi: 10.1016/s0031-9384(96)00228-4. [DOI] [PubMed] [Google Scholar]
- Spetalen S, Sandvik L, Blomhoff S, Jacobsen MB. Autonomic function at rest and in response to emotional and rectal stimuli in women with irritable bowel syndrome. Dig Dis Sci. 2008;53:1652–1659. doi: 10.1007/s10620-007-0066-0. [DOI] [PubMed] [Google Scholar]
- Tonhajzerova I, Javorka M, Trunkvalterova Z, Chroma O, Javorkova J, Lazarova Z, Ciljakova M, Javorka K. Cardio-respiratory interaction and autonomic dysfunction in obesity. J Physiol Pharmacol. 2008;59:709–718. [PubMed] [Google Scholar]