Abstract
Asymmetries in upper limb position sense have been explained in the context of a left limb advantage derived from differences in hemispheric specialization in the processing of kinesthetic information. However, it is not clearly understood how the comparison of perceptual information associated with passive limb displacement and the corresponding matching movement resulting from the execution of a motor command contributes to these differences. In the present study, upper limb position sense was investigated in 12 right-hand-dominant young adults performing wrist position matching tasks which varied in terms of interhemispheric transfer, memory retrieval and whether the reference position was provided by the same or opposite limb. Right and left hand absolute matching errors were similar when the reference and matching positions were produced by the same hand but were 36% greater when matching the reference position with the opposite hand. When examining the constant errors generated from matching movements made with the same hand that provided the reference, the right and left hand matching errors (≈3°) were similar. However, when matching with the opposite limb, a large overshoot (P <0.05) characterized the error when the right hand matched the left hand reference while a large undershoot (P <0.05) characterized the error when the left hand matched the right hand reference. The overshoot and undershoot were of similar magnitude (≈4°). Although asymmetries in the central processing of proprioceptive information such as inter-hemispheric transfer may exist, the present study suggests that asymmetries in position sense predominantly result from a difference in the “gain of the respective proprioceptive sensory-motor loops”. This new hypothesis is strongly supported by a dual-linear model representing the right and left hand sensory-motor systems as well as morphological and physiological data.
Keywords: Proprioception, Perception, Position sense, Human, Wrist
Introduction
Asymmetries in upper limb position sense have been investigated using matching paradigms requiring memory retrieval and/or interhemispheric transfer of proprioceptive information, whereby one limb is passively positioned and then this reference position is actively reproduced with the same or contralateral limb. In some studies the absolute matching errors were less for the left than right limb when the same limb provided the reference and matching movements. This result was interpreted as a right hemisphere/left limb advantage in detecting and processing proprioceptive position information (Roy and MacKenzie 1978; Carnahan and Elliott 1987; Nishizawa and Saslow 1987; Riolo-Quinn 1991). However, other studies showed no significant differences in matching performance between the limbs (Wrisberg and Winter 1985; Carson et al. 1990a, b; Imanaka et al. 1995). These contrasting results may stem from differences in muscle innervations that may be primarily unilateral for distal musculature and bilateral for proximal musculature (see Imanaka et al. 1995 for review). In addition, differences in matching performance may also be dependent upon the context in which kinesthetic perception is tested. Indeed, methodological paradigms vary largely between studies, which may include simultaneous tasks, sequential reproduction with or without delays or with or without vision, planar versus spatial movements.
In contralateral matching tasks, where the reference and matching limbs are opposite, the observed asymmetries were found to be dependent upon whether the dominant or non-dominant limb produced the reference or performed the match (Worringham and Stelmach 1985; Rodier et al. 1991; Goble et al. 2006; Goble and Brown 2007; Adamo et al. 2008), unilateral muscle fatigue (Walsh et al. 2004; Allen and Khattab 2006; Walsh et al. 2006; Allen et al. 2007) or unilateral muscle tendon vibration (Goodwin et al. 1972a). In addition, contralateral matching tasks have illustrated that the direction of the constant error, quantified as an overshoot or undershoot, is limb/hemisphere specific (Roll 1981; Worringham and Stelmach 1985; Rodier et al. 1991; Clark et al. 1995; Yamauchi et al. 2004). Yamauchi et al. (2004) proposed that the directional transfer of spatial information from the left limb/right hemisphere to the right limb/left hemisphere accounted for greater right limb overshoots, as the right limb was required to match a movement based on a poorer representation of the left limb reference position. In contrast, left limb matching showed less of an overshoot, suggesting that when matching left limb movements from a right limb reference position, the transfer of information related to movement amplitude was more efficient. Although other studies (Roll 1981; Worringham and Stelmach 1985; Clark et al. 1995) reported asymmetries in upper limb matching movements, a clear interpretation was not provided. Nevertheless, Rodier et al. (1991) noted that in the context of their experiment a strong relationship existed between the magnitudes of the errors produced by each hand, as a larger right limb overshoot was consistently opposed by a larger left limb undershoot or smaller overshoot.
Perhaps the most remarkable evidence that the perception of limb-specific differences contributes to kinesthetic and kinematic asymmetries may be found in studies that examined the effects of muscle tendon vibration. As early as 1972 Goodwin et al. assumed that applying vibration to the tendon of the muscle antagonist of the displaced reference limb induced an increase in the firing frequency of the Ia afferents that led to an overshoot when reproducing the movement with the opposite limb, while applying a similar vibration to the matching limb produced an undershoot. Thus, a difference in the perception between the two limbs contributed to the direction of the matching error. Microneurographic recordings of Ia afferents confirmed Goodwin’s hypothesis (Burke et al. 1976; Roll and Vedel 1982).
However, most matching studies using contralateral matching tasks were based on the implicit assumption that both limb/hemisphere systems are intrinsically identical in terms of perception. The goal of the present work was to test the hypothesis that a difference in the gains of the respective right and left proprioceptive sensory-motor systems contributes significantly to asymmetries in movement reproduction. Wrist matching movements were investigated in three experimental conditions to determine the influences of the side (left/right) producing the reference and hand dominance (right) on position sense asymmetry.
Methods
Participants
Twelve young (6 females; 6 males, mean age 22.1 ± 2 years) right-hand-dominant individuals participated as paid volunteers in the study. All participants were free from any upper limb neurological and musculoskeletal conditions that might impair task performance and demonstrated full upper limb range of motion. All participants signed an informed consent form approved by the institutional review board of the University of Michigan.
Experimental setup
Participants were blindfolded and seated with the upper arms positioned in 70–80° abduction and 20° of horizontal shoulder flexion while the elbows remained in a fixed posture of 120° extension. The forearms were supported in a neutral position by fixed horizontal levers. The axis of wrist joint rotation corresponding to flexion and extension movements was aligned with the pivot point of each manipulandum. The hands were stabilized on the moving lever of the manipulanda by lightly wrapping them in a fine elastic mesh. Two servo-motors (Smartmotor™) were programmed to passively displace the moving levers of the manipulanda at a speed of 20°/s. Hence, all reference movements as well as all returns to the initial wrist joint positions were passively imposed, while only matching movements were produced actively.
Experimental conditions
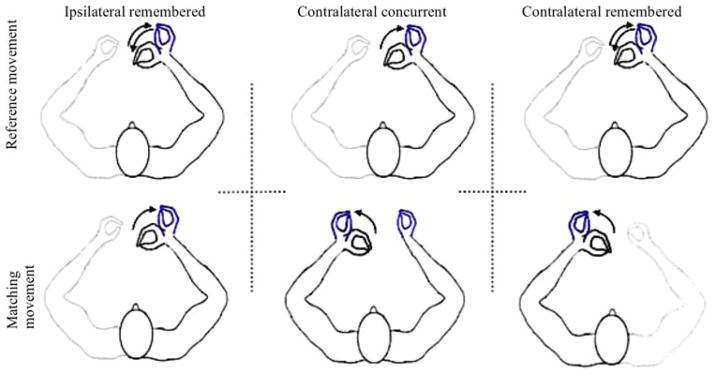
Three wrist position-matching conditions, which varied in terms of memory requirements and the need for inter-hemispheric transfer, were randomly presented to each subject. Using the right (dominant) or left (non-dominant) hand, the reference position was generated from a start position of 15° wrist flexion and passively extended 40°. The participants were instructed to accurately reproduce the passive displacements at the speed imposed by the motor. A schematic of the matching conditions is shown in Fig. 1.
Fig. 1.
Schematic of the three wrist position matching conditions as viewed from above. Top panel shows the reference movement in the three conditions. Bottom panel shows the matching movement when reproduced with the same (ipsilateral) or opposite (contralateral concurrent and contralateral remembered) hand. Arrows indicate direction of the movements. Both the right and left hand provided the reference. Right hand reference positions are shown here
In ipsilateral remembered (IR) matching, the reference and matching movements were performed with the same hand. Memory of perception associated with the reference was used to produce the matching movement. In contralateral concurrent (CC) matching, the reference hand was held in the reference position while the movement was reproduced with the opposite hand. In this condition, feedback about the reference and matching hand positions was available simultaneously and information from each hemisphere had to be compared. A transfer from one hemisphere to the other is required to produce the matching movement. In contralateral remembered (CR) matching, the reference hand was displaced to the reference position, returned to the initial start position and then the reference was matched with the opposite hand. In this condition, memory of the perception of movement elicited by the reference position was compared with the matching information and interhemispheric transfer is required to perform the match. The reference position was maintained for 2 s before the return in the IR and CR conditions. The three matching conditions were performed with the right (dominant) and left (non-dominant) hands providing the reference position. For all participants, three test trials were preceded by two practice trials.
Data acquisition and processing
Wrist joint rotation was recorded as the voltage output from precision potentiometers mounted beneath the pivot of each manipulandum. The analog signals were digitized at 100 Hz, low pass filtered (fourth order Butterworth, zero phase lag, 6 Hz cut off frequency) and converted to calibrated angular displacement values using custom designed software (LabVIEW™ National Instruments). Absolute matching error, constant error, movement time and movement smoothness associated with the matching movement were calculated. Absolute error was calculated as the absolute angular difference between the reference and matching limb position. Constant error was calculated as the direction of the matching error relative to the reference position. An overshoot described a matching movement amplitude larger than the reference position whereas an undershoot described a movement amplitude smaller than the reference position. Movement time was calculated utilizing the respective times of onset and offset values 2 SD greater than the averaged value of a 200 ms pre-movement baseline signal obtained from the differentiated position record. Movement smoothness was evaluated by calculating the jerk score between the end point of the movement (displacement angle) normalized for movement distance and duration as follows:
where j is the third time derivative of the position signal, d is the movement duration, and l is the movement amplitude (Teulings et al. 1997; Seidler et al. 2001). For each matching condition (3) and hand (2) combination, three matching trials were averaged, constituting a total of 18 measures for each subject for each dependent variable.
Statistical analysis
A two-way analysis of variance was conducted to determine the main effects of condition (ipsilateral remembered, contralateral concurrent, contralateral remembered) and hand (right, left) as well as interactions for each dependent variable. Significance was set at P ≤ 0.05. To determine which factors influenced the main and interaction effects, post hoc tests (Tukey Honestly Significant Differences—HSD—for multiple comparisons) were conducted to compare conditions. Paired t tests were used to distinguish differences between hands.
Results
Absolute error
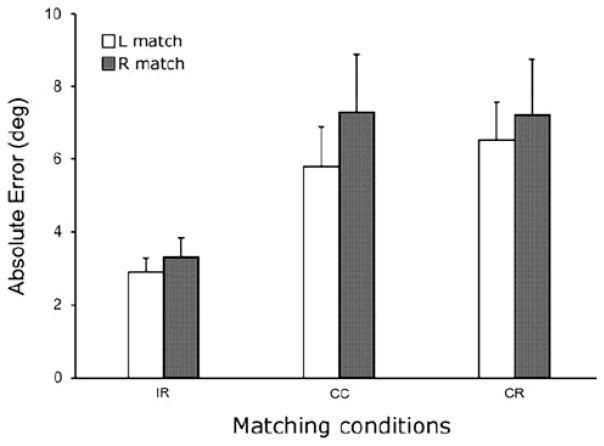
The absolute errors corresponding to each condition and hand are illustrated in Fig. 2. Matching errors were 36% greater in the contralateral than ipsilateral conditions. The analysis of variance showed a significant main effect for condition only (F(2,66) = 6.8 P <0.01). Post-hoc analyses showed that when compared to the ipsilateral condition, matching errors were greater in the CC (t(46) = 3.3, P = 0.001) and CR (t(46) = 3.8, P <0.001) conditions, respectively. However, matching errors were not significantly different between the contralateral conditions and were not influenced by which hand (P = 0.35) performed the matching.
Fig. 2.
Mean (+1 SE) absolute errors for different matching conditions: (IR ipsilateral remembered, CC contralateral concurrent, CR contralateral remembered) when matching with the right (filled bars) and left (open bars) hands
Constant error
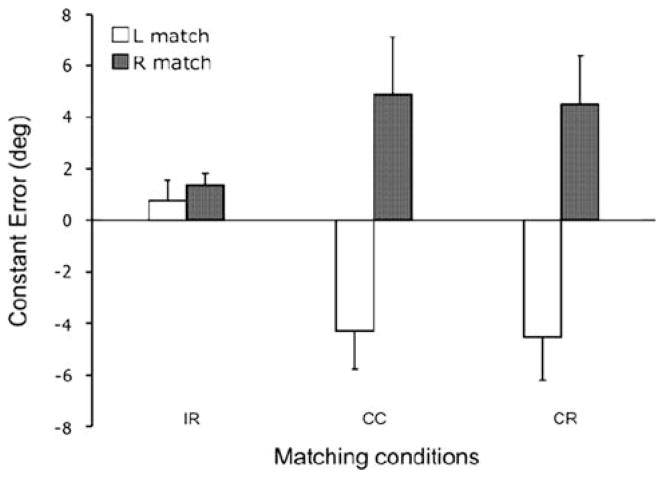
The ANOVA showed a significant main effect for hand (F(1,66) = 23.3, P <0.001) and a hand × condition interaction effect (F(2,66) = 4.7, P = 0.012) for the constant error. The constant error corresponded to a large overshoot when the right hand matched the left hand reference position and a large undershoot when the left hand matched the right hand reference position in the CC (t(22) = 3.5, P = 0.003) and CR (t(22) = 3.4, P = 0.002) conditions, as shown in Fig. 3. The right and left hand matching errors were similar in both contralateral conditions. In the ipsilateral condition the constant error was similar for both hands.
Fig. 3.
Mean (+1 SE) constant error for each matching conditions: (IR ipsilateral remembered, CC contralateral concurrent, CR contralateral remembered) when matching with the right (filled bars) and left (open bars) hands
Movement time
The ANOVA showed a significant main effect for condition (F(2,66) = 3.9, P = 0.02). The post-hoc analysis indicated a significant difference in movement time between the contralateral concurrent and contralateral remembered conditions only (t(46) = 2.5, P = 0.02). Matching time (Table 1) was not influenced by which hand performed the match (P = 0.96). However the movement amplitude was 20% smaller (about 8°) for the left than right hand (see Fig. 3), which shows that the left hand was significantly slower than the right hand. In other words, both hands took about the same amount of time to produce the match even though the left hand reproduced a smaller movement.
Table 1.
Matching movement time
| Condition | IR | CC | CR |
|---|---|---|---|
| Right hand | 1.60 ± 0.22 | 1.59 ± 0.37 | 1.78 ± 0.33 |
| Left hand | 1.65 ± 0.24 | 1.52 ± 0.21 | 1.83 ± 0.39 |
Time = s ± SD
Movement smoothness
Although movements tended to be less smooth (mean ± SD) for the left (movement smoothness = 152 ± 67) than the right (movement smoothness = 134 ± 66) hand, these differences were not statistically significant (P = 0.25) under any condition (P = 0.09).
Discussion
Greater absolute matching errors in the contralateral than ipsilateral conditions indicate that utilization of proprioceptive information from the opposite limb contributes to differences in matching performance. In the context of contralateral matching, the overshoot and undershoot expressed by the constant errors, reveals an asymmetry between right and left hand matching. This result strongly suggests that differences in perception and/or reproduction may exist between the two sensory-motor systems.
Asymmetries in motor performance
The dynamic dominance theory (Sainburg 2002) proposes that in right handed individuals the dominant right limb/left hemisphere system is specialized for controlling limb dynamics and the non-dominant left limb/right hemisphere system is specialized for controlling static limb position. Support for hemispheric specialization of movement and position control has been found in studies investigating movement kinematics in populations affected by neurological disorders. Individuals affected by left hemisphere damage presented deficits associated with movement trajectory control, while those with right hemisphere damage presented deficits associated with position control (Haaland and Harrington 1989a, b; Haaland et al. 2004). In the present study, right wrist movements were on average faster and smoother than left wrist movements, which tend to be in agreement with a specific mode of control for each limb/hemisphere system. However, the systematic difference in the direction of the constant error between the two hands in contralateral matching conditions is most likely independent of this specialization.
Asymmetries in position sense
Memory-transfer effects
Asymmetries in position sense have been investigated using ipsilateral (Roy and MacKenzie 1978; Nishizawa and Saslow 1987; Riolo-Quinn 1991; Carnahan and Elliott 1987), contralateral (Worringham and Stelmach 1985; Rodier et al. 1991) or a combination of ipsilateral and contralateral matching paradigms (Goble et al. 2006; Goble and Brown 2007; Adamo et al. 2007; Adamo et al. 2008). In these studies, the magnitude of limb differences in absolute matching errors was dependent upon whether the limb tested served as the reference or generated the match, the displacement amplitude of the reference position, memory and transfer requirements, as well as the age of the participants. In the context of contralateral limb matching, asymmetry has been explained in terms of a directional advantage in the transfer of information or a limb specific advantage. Following an earlier model proposed by Haude et al. (1987) and Yamauchi et al. (2004) proposed that the directional transfer of spatial information was more efficient from the left to right hemisphere than in the opposite direction. This interpretation is in agreement with asymmetries observed in the transfer of movement dynamics in reaching movements (Criscimagna-Hemminger et al. 2003) and manual performance in visuomotor tasks (Parlow and Kinsbourne 1989). In another interpretation, the difference in limb matching accuracy has been associated with a non-preferred left limb/right hemisphere advantage for processing proprioceptive feedback whereby matching left limb elbow positions from right limb reference positions resulted in less absolute matching errors than in the reverse situation (Goble et al. 2006; Goble and Brown 2007). A directional advantage in favor of the left/non-preferred limb is in agreement with hypothesis of a predominant use of feedback control mode utilization by that limb (Wang and Sainburg 2004). Although several position sense studies have reported an advantage of one limb over the other in terms of matching accuracy, this asymmetry is not systematic and appears to be strongly related to the context in which the perception of limb displacement is tested (see Carson et al. 1990b; Imanaka et al. 1995; for review). For example, the simultaneous performance with both thumbs of ipsilateral matching tasks (Roy and MacKenzie 1978; Riolo-Quinn 1991) is adding an attentional constraint contributing to the observed asymmetry, as predicted by attentional models (see Imanaka et al. 1995). Furthermore, most studies only report the absolute error, which masks the direction of the error (overshoot/undershoot).
Limb—specific directional position error
The direction of the matching error based on an overshoot or undershoot of the reference position provides important information about the extent to which limb specific information contributes to movement reproduction. In the absence of learning, overshooting with the right limb when matching a left limb reference (Roll 1981; Worringham and Stelmach 1985; Clark et al. 1995; Yamauchi et al. 2004) and less overshooting or undershooting with the left limb when matching a right limb reference (Worringham and Stelmach 1985) have been observed in various conditions. However, these studies have neglected to consider the extent to which position information associated with the perception of movement amplitude from the opposite limb may have contributed to these differences. Taken together, the perception of limb displacement and the requirement of the matching tasks, suggest that the expression of a perceptive effect is strongly dependent on the context in which it occurs, as illustrated by other studies showing the importance of the perceptual context in the determination of motor responses (Roll et al. 1980; Feldman and Latash 1982; Lackner and Taublieb 1983; Carson et al. 1990b; Martin et al. 1990; Lackner et al. 2000).
Limb specific differences in perception
In the present study, the non-significant right/left limb differences in matching errors observed in the ipsilateral conditions suggests that a hemisphere/limb specific specialization for processing proprioceptive information may neither adequately explain this absence of difference, nor the right/left directional differences observed in contra-lateral matching tasks. In the contralateral concurrent and contralateral remembered conditions, all participants showed a predominant overshooting of the left hand reference position when matching with the right hand. In contrast, left hand matches in the same conditions exhibited undershooting of the right hand reference position. Similar results, including overshooting with the right limb when matching a left limb reference (Roll 1981; Worringham and Stelmach 1985; Clark et al. 1995; Yamauchi et al. 2004) and less overshooting or undershooting with the left limb when matching a right limb reference (Soechting 1982; Worringham and Stelmach 1985) have been observed in various conditions. More specifically, in this study, the magnitude of directional error represented by the average difference between overshoot and undershoot is greater than 8°. This rather large value may require an explanation extending beyond the utilization of a specific feedback control mode or directional transfer benefit. Indeed, the interpretation of the asymmetry should also include the influence of the perception associated with the kinesthetic feedback to reflect the evidence that the proprioceptive information giving rise to the perception of the position to be reproduced comes from the opposite limb.
Asymmetries in limb position matching may be explained by understanding the influence of limb perception on the directional error of matching. Upper limb position matching experiments that introduce a perturbation, such as localized fatigue or tendon vibration have illustrated how a difference in perception influences matching performance. For example, when matching with a non-fatigued limb a reference position established by flexion of a fatigued limb, the movement ended in a more extended position (participants undershot the target); while in the reverse condition, participants overshot the target (Walsh et al. 2004; Allen and Khattab 2006; Walsh et al. 2006; Allen et al. 2007). The effect of muscle tendon vibration was initially illustrated by Goodwin et al. (1972b). A large matching error resulted from a difference in the perception of position between the limbs as the perceived magnitude of elongation of the vibrated reference arm muscle was artificially increased by the vibration-induced increase in muscle spindle activity (Burke et al. 1976; Roll and Vedel 1982). Hence, when matching the perception of the reference limb, the subject overshot the target. Overall, these studies demonstrates that a difference in the perception of the respective limb positions contribute to an asymmetry in limb movement reproductions regardless of the directional transfer of limb position information, suggesting that the transfer advantage (Haude et al. 1987; Yamauchi et al. 2004) is not sufficient to explain asymmetry in position sense.
Proprioceptive sensory-motor gain hypothesis and model
A proprioceptive sensory-motor gain hypothesis based on a difference in the relationship between passive limb displacement and the corresponding perception of the displacement for each limb/hemisphere system is proposed. In the present study, overshooting with the right hand and undershooting with the left hand indicates that the gain is higher for the left than right hand. It must be kept in mind that contralateral matching of the right hand corresponded to the perception of position established by the left limb. If the perceptual gain is higher for the left than right limb, the right limb will produce a larger displacement to match the perception associated with the reference position established by the left limb. Therefore, the right limb will produce an overshoot when matching the left limb reference. In the reverse condition, the left limb will produce a smaller displacement than the right limb reference to evoke a perception equivalent to that associated with the right reference. Thus the left limb will undershoot the reference position provided by the right limb.
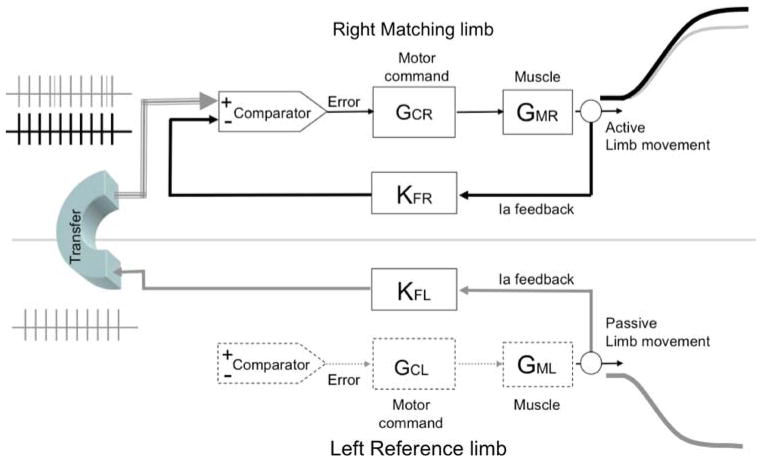
A simplified model of a closed loop system can be used to demonstrate and validate this hypothesis. The sensory-motor feedback loops corresponding to each hand can be represented by their components as described in Fig. 4. The diagram represents only the case in which the right hand matches a reference provided by the left hand. In the Laplace domain (see for example Jagacinski and Flach 2003) the transfer function of each element can be characterized by a gain and the relationship between the input and output be expressed by the following equation:
| (1) |
where Y represents the output or movement amplitude, I the input corresponding to the desired movement amplitude based on proprioceptive feedback, Gc the gain of the controller or motor command, Gm the gain of the muscle and Kf the gain of the proprioceptive feedback. In the case illustrated in Fig. 4, the matching movement output can be represented by:
| (2) |
where r and l denote the right and left sides, since the inputs to the comparator correspond respectively to the feedbacks provided by the left (Kl · Yl) and right (Kr · Yr) hand. Equation (2) can be rewritten under the form
Fig. 4.
Dual linear model representing the sensory-motor feedback loops of each limb in the Laplace domain. For the sake of clarity, the model represents only the case right hand matching the left hand reference, including only the muscle spindles proprioceptive feedback (Ia feedback). KF, GC and GM correspond to the gains of the respective transfer functions representing the feedback, motor command and muscle components. R and L denote right and left limb. KF represents the gain of the proprioceptive perceptual path while GC and GM represent the gain of the motor path. This model assumes that the closed loop gain is greater for the left non-dominant than the right dominant hand system as shown in text
| (3) |
Conversely when the left hand matches a reference provided by the right hand
| (4) |
it can be noted that the fraction terms in Eqs. (3) and (4) represent the closed loop gains of the right and left sensory-motor systems considered, respectively, as indicated in Eq. (1). Hence as the experimental results show that Yr >Yl when the amplitude of the reference and matching movements are perceived equal (Kr · Yr = Kl · Yl) then
| (5) |
and
| (6) |
where ε represent the difference between the right and left hand movement amplitudes.
Equation (5) can be rearranged Hr/Hl = Yr/Yl = Yl + ε/Yl = 1 + ε/Yl to show that Hl = Hr(1 + ε/Yl) and thus Hl >Hr, which shows that the proprioceptive sensory-motor gain or closed loop gain is greater for the let than the right hand, as proposed.
This result and the proprioceptive sensory-motor gain hypothesis are compatible with the absence of a limb/hemisphere specific gain effect in the ipsilateral remembered condition. When matching in the IR condition the perception of movement displacement is congruent with the intended movement in the matching limb, as matching does not rely on the non-equivalent perception provided by another limb.
Asymmetries in the monosynaptic reflex further support this hypothesis. Aimonetti et al. (1999) demonstrated that in right hand dominant individuals the gain of the mono-synaptic reflex was larger for the right than left wrist. These authors assumed that asymmetrical effectiveness of proprioceptive assistance might result from preferential use of the right hand in skilled movements. Hence, this study demonstrated that sensory-motor responses might be a function of the utilization of the limb. A similar interpretation may apply to a supraspinal loop. Since the right dominant hand benefits from larger cortical representation than the left non-dominant hand in humans (Kim et al. 1993; Classen et al. 1998; Baraldi et al. 1999) and animals (Nudo et al. 1992, 1996) as a result of cortical plasticity, it may be presumed that cortical representation corresponding to right hand use translates into a higher sensory-motor resolution, and as such, the right hand/left hemisphere system may not need a high proprioceptive sensory-motor gain. Therefore, the proposed hypothesis is also in agreement with anatomical differences between the right and left sensory-motor systems.
Overall, in that perspective, the constant error does not indicate that the left hand would be more accurate than the right hand but rather suggests that the difference in gain between the two limbs, and in some instances an offset difference, create a bias and eventually an apparent reduction in error for the left hand. This hypothesis is not in contradiction with a control mode advantage of the right hemisphere/left limb or directional transfer of information advantage but rather indicates that the interpretation of an advantage, particularly when accuracy is the outcome measure and when one limb is required to reproduce the reference provided by the other should be approached with caution. Hence, in the context of contralateral matching, any advantage or disadvantage of one limb may be primarily related to the other limb due to a difference in the perception elicited by each limb/hemisphere system.
Acknowledgments
A National Institute on Aging T32 training grant AG000114 to DA, and a National Institute on Aging R03 grant AG 025120-01 to Dr. S. Brown supported this work, performed in the Division of Kinesiology at the University of Michigan. The authors are grateful to J. Foulke and E. Claxton for designing the motorization of the manipulanda and providing technical assistance for the development of the control interface. We also thank C. Waechter for her help in data collection.
Contributor Information
Diane E. Adamo, Email: dadamo@wayne.edu, Institute of Gerontology, Wayne State University, 226 Knapp Building, 87 East Ferry Street, Detroit, MI 48202, USA
Bernard J. Martin, Email: martinbj@umich.edu, Department of Industrial and Operations Engineering, The University of Michigan, 1205 Beal Avenue, Ann Arbor, MI 48109-2117, USA
References
- Adamo DE, Alexander NB, Brown SH. The influence of age and physical activity on upper limb proprioceptive ability. J Aging Phys Act. 2008 doi: 10.1123/japa.17.3.272. (In press) [DOI] [PubMed] [Google Scholar]
- Adamo DE, Martin BJ, Brown SH. Age-related differences in upper limb proprioceptive acuity. Percept Mot Skills. 2007;104:1297–1309. doi: 10.2466/pms.104.4.1297-1309. [DOI] [PubMed] [Google Scholar]
- Aimonetti JM, Morin D, Schmied A, Vedel JP, Pagni S. Proprioceptive control of wrist extensor motor units in humans: dependence on handedness. Somatosens Mot Res. 1999;16:11–29. doi: 10.1080/08990229970618. [DOI] [PubMed] [Google Scholar]
- Allen SC, Khattab A. The tendency to altered perception of airflow resistance in aged subjects might be due mainly to a reduction in diaphragmatic proprioception. Med Hypotheses. 2006;67:1406–1410. doi: 10.1016/j.mehy.2006.05.046. [DOI] [PubMed] [Google Scholar]
- Allen TJ, Ansems GE, Proske U. Effects of muscle conditioning on position sense at the human forearm during loading or fatigue of elbow flexors and the role of the sense of effort. J Physiol. 2007;580:423–434. doi: 10.1113/jphysiol.2006.125161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baraldi P, Porro CA, Serafini M, Pagnoni G, Murari C, Corazza R, Nichelli P. Bilateral representation of sequential finger movements in human cortical areas. Neurosci Lett. 1999;269:95–98. doi: 10.1016/s0304-3940(99)00433-4. [DOI] [PubMed] [Google Scholar]
- Burke D, Hagbarth KE, Lofstedt L, Wallin BG. The responses of human muscle spindle endings to vibration during isometric contraction. J Physiol. 1976;261:695–711. doi: 10.1113/jphysiol.1976.sp011581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carnahan H, Elliott D. Pedal asymmetry in the reproduction of spatial locations. Cortex. 1987;23:157–159. doi: 10.1016/s0010-9452(87)80028-x. [DOI] [PubMed] [Google Scholar]
- Carson RG, Chua R, Elliott D, Goodman D. The contribution of vision to asymmetries in manual aiming. Neuropsychologia. 1990a;28:1215–1220. doi: 10.1016/0028-3932(90)90056-t. [DOI] [PubMed] [Google Scholar]
- Carson RG, Elliott D, Goodman D, Dickinson J. Manual asymmetries in the reproduction of a 3-dimensional spatial location. Neuropsychologia. 1990b;28:99–103. doi: 10.1016/0028-3932(90)90090-b. [DOI] [PubMed] [Google Scholar]
- Clark FJ, Larwood KJ, Davis ME, Deffenbacher KA. A metric for assessing acuity in positioning joints and limbs. Exp Brain Res. 1995;107:73–79. doi: 10.1007/BF00228018. [DOI] [PubMed] [Google Scholar]
- Classen J, Liepert J, Wise SP, Hallett M, Cohen LG. Rapid plasticity of human cortical movement representation induced by practice. J Neurophysiol. 1998;79:1117–1123. doi: 10.1152/jn.1998.79.2.1117. [DOI] [PubMed] [Google Scholar]
- Criscimagna-Hemminger SE, Donchin O, Gazzaniga MS, Shadmehr R. Learned dynamics of reaching movements generalize from dominant to nondominant arm. J Neurophysiol. 2003;89:168–176. doi: 10.1152/jn.00622.2002. [DOI] [PubMed] [Google Scholar]
- Feldman AG, Latash ML. Inversions of vibration-induced sensomotor events caused by supraspinal influences in man. Neurosci Lett. 1982;31:147–151. doi: 10.1016/0304-3940(82)90107-0. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Task-dependent asymmetries in the utilization of proprioceptive feedback for goal-directed movement. Exp Brain Res. 2007;180:693–704. doi: 10.1007/s00221-007-0890-7. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Brown SH. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res. 2006;168:307–311. doi: 10.1007/s00221-005-0280-y. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. A systematic distortion of position sense produced by muscle vibration. J Physiol. 1972a;221:8P–9P. [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. Proprioceptive illusions induced by muscle vibration: contribution by muscle spindles to perception? Science. 1972b;175:1382–1384. doi: 10.1126/science.175.4028.1382. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Elsinger CL, Mayer AR, Durgerian S, Rao SM. Motor sequence complexity and performing hand produce differential patterns of hemispheric lateralization. J Cogn Neurosci. 2004;16:621–636. doi: 10.1162/089892904323057344. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington D. The role of the hemispheres in closed loop movements. Brain Cogn. 1989a;9:158–180. doi: 10.1016/0278-2626(89)90027-4. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL. Hemispheric control of the initial and corrective components of aiming movements. Neuropsychologia. 1989b;27:961–969. doi: 10.1016/0028-3932(89)90071-7. [DOI] [PubMed] [Google Scholar]
- Haaland KY, Harrington DL, Grice JW. Effects of aging on planning and implementing arm movements. Psychol Aging. 1993;8:617–632. doi: 10.1037//0882-7974.8.4.617. [DOI] [PubMed] [Google Scholar]
- Haude RH, Morrow-Tlucak M, Fox DM, Pickard KB. Differential visual field-interhemispheric transfer: can it explain sex and handedness differences in lateralization? Percept Mot Skills. 1987;65:423–429. doi: 10.2466/pms.1987.65.2.423. [DOI] [PubMed] [Google Scholar]
- Imanaka K, Abernethy B, Yamauchi M, Funase K, Nishihira Y. Hemispace asymmetries and laterality effects in arm positioning. Brain Cogn. 1995;29:232–253. doi: 10.1006/brcg.1995.1280. [DOI] [PubMed] [Google Scholar]
- Jagacinski R, Flach J. Control theory for humans: quantitative approaches to modeling performance. Lawrence Erlbaum Associates; Mahwah: 2003. [Google Scholar]
- Kim SG, Ashe J, Hendrich K, Ellermann JM, Merkle H, Ugurbil K, Georgopoulos AP. Functional magnetic resonance imaging of motor cortex: hemispheric asymmetry and handedness. Science. 1993;261:615–617. doi: 10.1126/science.8342027. [DOI] [PubMed] [Google Scholar]
- Lackner JR, Taublieb AB. Reciprocal interactions between the position sense representations of the two forearms. J Neurosci. 1983;3:2280–2285. doi: 10.1523/JNEUROSCI.03-11-02280.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lackner JR, Rabin E, DiZio P. Fingertip contact suppresses the destabilizing influence of leg muscle vibration. J Neurophysiol. 2000;84:2217–2224. doi: 10.1152/jn.2000.84.5.2217. [DOI] [PubMed] [Google Scholar]
- Martin BJ, Roll JP, Hugon M. Modulation of cutaneous flexor responses induced in man by vibration-elicited proprioceptive or exteroceptive inputs. Aviat Space Environ Med. 1990;61:921–928. [PubMed] [Google Scholar]
- Nishizawa S, Saslow CA. Lateralization of kinesthetically guided spatial perception. Cortex. 1987;23:485–494. doi: 10.1016/s0010-9452(87)80009-6. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Jenkins WM, Merzenich MM, Prejean T, Grenda R. Neurophysiological correlates of hand preference in primary motor cortex of adult squirrel monkeys. J Neurosci. 1992;12:2918–2947. doi: 10.1523/JNEUROSCI.12-08-02918.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM. Use-dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci. 1996;16:785–807. doi: 10.1523/JNEUROSCI.16-02-00785.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlow SE, Kinsbourne M. Asymmetrical transfer of training between hands: implications for interhemispheric communication in normal brain. Brain Cogn. 1989;11:98–113. doi: 10.1016/0278-2626(89)90008-0. [DOI] [PubMed] [Google Scholar]
- Riolo-Quinn L. Relationship of hand preference to accuracy on a thumb-positioning task. Percept Mot Skills. 1991;73:267–273. [PubMed] [Google Scholar]
- Rodier S, Euzet JP, Gahery Y, Paillard J. Crossmodal versus intramodal evaluation of the knee joint angle : A normative study in a population of young adults. Hum Mov Sci. 1991;10:689–712. [Google Scholar]
- Roll JP. Thèse de doctorat Es Sciences. Université Aix-Marseille I; 1981. Contribution de la proprioception musculaire a la perception et au contrôle du momvement chez l’homme. [Google Scholar]
- Roll JP, Vedel JP. Kinaesthetic role of muscle afferents in man, studied by tendon vibration and microneurography. Exp Brain Res. 1982;47:177–190. doi: 10.1007/BF00239377. [DOI] [PubMed] [Google Scholar]
- Roll JP, Gilhodes JC, Tardy-Gervet MF. Effects of vision on tonic vibration response of a muscle or its antagonists in normal man (author’s transl) Experientia. 1980;36:70–72. doi: 10.1007/BF02003980. [DOI] [PubMed] [Google Scholar]
- Roy EA, MacKenzie C. Handedness effects in kinesthetic spatial location judgements. Cortex. 1978;14:250–258. doi: 10.1016/s0010-9452(78)80051-3. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res. 2002;142:241–258. doi: 10.1007/s00221-001-0913-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidler RD, Alberts JL, Stelmach GE. Multijoint movement control in Parkinson’s disease. Exp Brain Res. 2001;140:335–344. doi: 10.1007/s002210100829. [DOI] [PubMed] [Google Scholar]
- Soechting JF. Does position sense at the elbow reflect a sense of elbow joint angle or one of limb orientation? Brain Res. 1982;248:392–395. doi: 10.1016/0006-8993(82)90601-1. [DOI] [PubMed] [Google Scholar]
- Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp Neurol. 1997;146:159–170. doi: 10.1006/exnr.1997.6507. [DOI] [PubMed] [Google Scholar]
- Walsh LD, Hesse CW, Morgan DL, Proske U. Human forearm position sense after fatigue of elbow flexor muscles. J Physiol. 2004;558:705–715. doi: 10.1113/jphysiol.2004.062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh LD, Allen TJ, Gandevia SC, Proske U. Effect of eccentric exercise on position sense at the human forearm in different postures. J Appl Physiol. 2006;100:1109–1116. doi: 10.1152/japplphysiol.01303.2005. [DOI] [PubMed] [Google Scholar]
- Wang J, Sainburg RL. Interlimb transfer of novel inertial dynamics is asymmetrical. J Neurophysiol. 2004;92:349–360. doi: 10.1152/jn.00960.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worringham CJ, Stelmach GE. The contribution of gravitational torques to limb position sense. Exp Brain Res. 1985;61:38–42. doi: 10.1007/BF00235618. [DOI] [PubMed] [Google Scholar]
- Wrisberg CA, Winter TP. Reproducing the end location of a positioning movement: the long and short of it. J Mot Behav. 1985;17:242–254. doi: 10.1080/00222895.1985.10735347. [DOI] [PubMed] [Google Scholar]
- Yamauchi M, Imanaka K, Nakayama M, Nishizawa S. Lateral difference and interhemispheric transfer on arm-positioning movement between right and left handers. Percept Mot Skills. 2004;98:1199–1209. doi: 10.2466/pms.98.3c.1199-1209. [DOI] [PubMed] [Google Scholar]