Highlights
-
•
Picornaviral RdRPs are responsible for the polyadenylation of viral RNA.
-
•
Reiterative transcription mechanisms occur during replication of poly(A) tails.
-
•
Conserved RdRP structures influence the size of poly(A) tails.
-
•
Common features of picornavirus RdRPs and telomerase reverse transcriptase.
-
•
Poly(A) tails are a telomere of picornavirus RNA genomes.
Keywords: Picornavirales, Picornaviridae, RNA-dependent RNA polymerase, 3Dpol, Reiterative transcription, Polyadenylation
Abstract
Poly(A) tails are functionally important features of all picornavirus RNA genomes. Some viruses have genomes with relatively short poly(A) tails (encephalomyocarditis virus) whereas others have genomes with longer poly(A) tails (polioviruses and rhinoviruses). Here we review the polyadenylation of picornavirus RNA as it relates to the structure and function of 3Dpol. Poliovirus 3Dpol uses template-dependent reiterative transcription mechanisms as it replicates the poly(A) tails of viral RNA (Steil et al., 2010). These mechanisms are analogous to those involved in the polyadenylation of vesicular stomatitis virus and influenza virus mRNAs. 3Dpol residues intimately associated with viral RNA templates and products regulate the size of poly(A) tails in viral RNA (Kempf et al., 2013). Consistent with their ancient evolutionary origins, picornavirus 3Dpol and telomerase reverse transcriptase (TERT) share structural and functional features. Structurally, both 3Dpol and TERT assume a “right-hand” conformation with thumb, palm and fingers domains encircling templates and products. Functionally, both 3Dpol and TERT use template-dependent reiterative transcription mechanisms to synthesize repetitive sequences: poly(A) tails in the case of picornavirus RNA genomes and DNA telomeres in the case of eukaryotic chromosomes. Thus, picornaviruses and their eukaryotic hosts (humans and animals) maintain the 3′ ends of their respective genomes via evolutionarily related mechanisms.
1. Introduction
Picornaviruses are ubiquitous and infect a diverse range of animals, insects and plants. The great variety of picornaviruses is consistent with their ancient origins (Koonin et al., 2008). Based on shared molecular features, picornaviruses are taxonomically organized by order, family, genus, species, and virus (Table 1 ) (Le Gall et al., 2008). The Picornavirales order includes five families: Picornaviridae, Dicistroviridae, Iflaviridae, Marnaviridae and Secoviridae (Le Gall et al., 2008). Hundreds of human and animal pathogens are distributed among 26 genera and 46 species groups in the Picornaviridae family (Knowles et al., 2012). Humans (CDC, 2010), apes and monkeys (Van Nguyen et al., 2014), pigs (Van Dung et al., 2014), cattle (Grubman and Baxt, 2004), mice (Denis et al., 2006), seals (Kapoor et al., 2008), shrimp (Aranguren et al., 2013), turtles (Farkas et al., 2014), birds (Boros et al., 2013) and bees (Chen et al., 2014) are but a few of the hosts frequently infected by these widespread viruses.
Table 1.
Picornaviruses (Picornavirales order).
| Family | Genus | Species groups | Type species |
|---|---|---|---|
| Picornaviridae | 26 genera: Aphtho-, Aquama-, Avihepato-, Avisi-, Cardio-, Cosa-, Dicipi-, Entero-, Erbo-, Galli-, Hepato-, Hunni-, Kobu-, Megri-, Mischi-, Mosa-, Osci-, Parecho-, Pasi-, Passeri, Rosa-, Sali-, Sapelo-, Seneca-, Tescho-, & Tremoviruses. | Aphthoviruses Cardioviruses Enteroviruses A-H Rhinoviruses A-C others |
Foot & mouth disease virus (FMDV) Encephalomyocarditis virus (EMCV) Polio, Coxsackie, Echo & Enteroviruses Rhinoviruses others |
| Dicistroviridae |
Cripavirus Apavirus |
Cripivirus Apavirus |
Cricket paralysis virus (CrPV) Acute bee paralysis virus |
| Iflaviridae | Iflavirus | Deformed wing virus Slow bee paralysis virus 7 others |
Deformed wing virus Slow bee paralysis virus |
| Marnaviridae | Marnavirus | Heterosigma akashiwo | Heterosigma akashiwo RNA virus |
| Secoviridae |
Como- Faba- and Nepoviruses Chera- Sadwa- Sequi- Torrado- & Waikaviruses |
Cowpea mosaic virus Parsnip yellow fleck virus |
|
Poly(A) tails are a characteristic feature of viral RNA genomes in the Picornavirales order, with one potential exception (Sequiviruses) (Le Gall et al., 2008). There are inconsistent reports regarding the presence or absence of a poly(A) tail in some sequiviruses in the Secoviridae family: parsnip yellow fleck virus, lettuce mottle virus and dandelion yellow mosaic virus (Jadao et al., 2007, Menzel and Vetten, 2008, Turnbull-Ross et al., 1992). Two groups failed to detect poly(A) tails in these sequiviruses (Jadao et al., 2007, Turnbull-Ross et al., 1992) whereas another group reports the presence of a 3′ terminal poly(A) tail in parsnip yellow fleck virus (Menzel and Vetten, 2008). Additional characterization of viruses in the Secoviridae family are warranted to confirm the presence or absence of a poly(A) tail in these viruses (Sanfacon et al., 2009). Here, we review the nature of picornavirus poly(A) tails and the manner in which they are maintained during viral replication.
2. Picornavirus RNA genomes and RNA replication
Picornavirus RNA genomes, like the enterovirus genome illustrated here (Fig. 1A), have a number of characteristic features, including a viral protein (VPg) at the 5′ end and a poly(A) tail of variable length at the 3′ end (Le Gall et al., 2008). One long open reading frame encodes capsid proteins (VP1-VP4) and non-structural proteins associated with host cell interactions and RNA replication (2Apro, 2B, 2CATPase, 3A, 3BVPg, 3Cpro and 3Dpol). A comparison of Picornaviridae RNA genomes reveals some notable differences between genera (Boros et al., 2014, Le Gall et al., 2008); however, common features suggest shared mechanisms of viral RNA replication. Shared genomic features important for RNA replication include phylogenetically related VPg proteins (Sun et al., 2014), cis-acting replication elements (CREs) involved in VPg uridylylation (Cordey et al., 2008, Steil and Barton, 2009a), an ATPase required for RNA replication (Sweeney et al., 2010), the viral RNA-dependent RNA polymerase (Lescar and Canard, 2009) and 3′ terminal poly(A) tails. While 3Dpol is consistently encoded at the 3′ end of picornaviral ORFs (Le Gall et al., 2008, Son et al., 2014, Van Dung et al., 2014), CREs are located at variable locations in different picornavirus RNA genomes (Cordey et al., 2008, Steil and Barton, 2009a). CREs have been predicted and/or experimentally defined for various genera and species groups in the Picornaviridae family including group A, B and C rhinoviruses (Cordey et al., 2008, McKnight and Lemon, 1998, Yang et al., 2002), group A, B, C and D enteroviruses (Goodfellow et al., 2000, Goodfellow et al., 2003, Paul et al., 2000, Shen et al., 2008, van Ooij et al., 2006b), aphthoviruses (Mason et al., 2002), hepatoviruses (Yang et al., 2008), cardioviruses (Lobert et al., 1999), parechoviruses (Al-Sunaidi et al., 2007) and sapeloviruses (Son et al., 2014). CREs have not been predicted or defined for viruses in the Dicistroviridae, Iflaviridae, Marnaviridae or Secoviridae families, so it remains to be determined whether all viruses in the Picornavirales order use template-dependent VPg uridylylation during viral RNA replication (Steil and Barton, 2009a). CRE-dependent VPg uridylylation and the initiation of picornavirus RNA synthesis are reviewed elsewhere in this issue by Paul and Wimmer (Paul and Wimmer, 2015). The diagram of RNA replication in Fig. 1C is simplified to emphasize common features of picornavirus replication which likely apply to other viruses throughout the Picornavirales order. In particular, we expect that the viral RNA-dependent RNA polymerases of viruses in the Picornaviridae, Dicistroviridae, Iflaviridae, Marnaviridae and Secoviridae families replicate the poly(A) tails of their RNA genomes (Fig. 1C). As discussed in some detail in this review, there is good evidence to indicate that 3Dpol replicates the poly(A) tail of poliovirus RNA (Kempf et al., 2013, Steil et al., 2010). Future experimental work will determine if other viruses in the Picornavirales order replicate the poly(A) tail of their respective genomes, as illustrated in Fig. 1C.
Fig. 1.
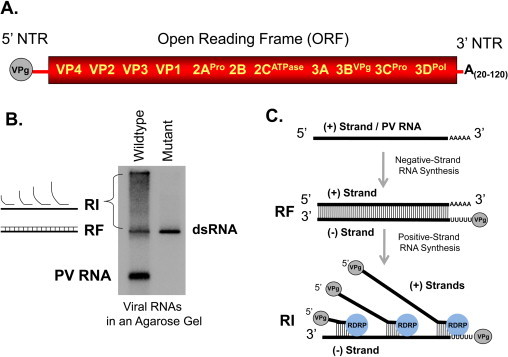
Enterovirus RNA genomes and viral RNA replication. (A) Picornaviruses have a poly(A) tail of variable length at the 3′ end of the viral RNA genome. (B) Cell-free replication of poliovirus RNA. Poliovirus RNA replicons [designated as DNVR27 and RNA2 in (Steil and Barton, 2008)] were incubated in HeLa cell-free translation reactions containing 2 mM guanidine HCl to form preinitiation RNA replication complexes (PIRCs). The PIRCs were incubated for 1 h at 37 °C in reaction mixtures containing [α-32P]CTP and nonradioactive ATP, GTP and UTP. Radiolabeled RNAs were fractionated by electrophoresis in a non-denaturing 1% agarose/Tris–Borate EDTA (TBE) gel and detected by phosphorimaging (Steil, 2008). The wildtype replicon (DNVR 27) has a wildtype 5′ terminus whereas the mutant replicon (RNA2) has two non-viral Gs at the 5′ end which inhibit positive-strand RNA synthesis (Steil, 2008, Steil and Barton, 2008). (C) Diagram of viral RNA replication. The viral RNA-dependent RNA polymerase (3Dpol) replicates the poly(A) tail, making VPg-linked poly(U) sequences at the 5′ end of negative-strand RNA. Then, during positive-strand RNA synthesis, 3Dpol uses the poly(U) sequences at the 5′ end of negative-strand RNA as the template for the polyadenylation of nascent positive-strands.
Picornavirus RNA genomes serve as both the viral mRNA required for viral protein synthesis and as a template for negative-strand synthesis during viral RNA replication. Following viral mRNA translation, non-structural proteins, in concert with cis-active RNA structures in the viral RNA templates, form membrane-anchored replication complexes in the cytoplasm of infected cells (reviewed in (Steil and Barton, 2009a)). Remarkably, all of the metabolic steps of viral replication (viral mRNA translation, polyprotein processing, RNA replication and virus assembly) are recapitulated in cell-free reactions containing cytoplasmic extracts from uninfected host cells (Molla et al., 1991). Cell-free virus replication, first achieved with poliovirus (Barton and Flanegan, 1993, Molla et al., 1991), was subsequently achieved with both encephalomyocarditis virus (EMCV) (Fata-Hartley and Palmenberg, 2005, Svitkin and Sonenberg, 2003) and rhinovirus RNAs (Todd et al., 1997). A cell-free replication system was also developed for positive-strand RNA plant viruses in the alpha-like and carmo-like virus supergroups (Komoda et al., 2004). The synchronous and sequential nature of viral mRNA translation (Kempf and Barton, 2008a, Kempf and Barton, 2008b), viral RNA replication (Barton and Flanegan, 1997) and virus assembly (Barton and Flanegan, 1993) within cell-free reactions has been exploited to better understand these individual steps of replication. Viral RNA replication is monitored in cell-free reactions by including radiolabled NTPs in the reactions (Fig. 1B). Radiolabel from NTPs is incorporated into negative- and positive-strand viral RNAs as they are synthesized by the viral RNA-dependent RNA polymerase 3Dpol. The viral RNAs radiolabled in cell-free reactions (Fig. 1B) are consistent with the expected intermediates of RNA replication (Fig. 1C).
Viral RNA replication occurs in sequential steps. First, positive-strand RNA templates are transcribed by 3Dpol, the picornavirus RNA-dependent RNA polymerase, into complementary negative-strand products (Fig. 1C, negative-strand RNA synthesis). VPg and its uridylylated derivatives prime the initiation of negative-strand RNA synthesis (Steil and Barton, 2008, Steil and Barton, 2009b) using 3′-terminal poly(A) sequences on the viral RNA templates (Sharma et al., 2005), resulting in VPg-linked poly(U) products at the 5′ end of negative-strand RNA intermediates (Steil et al., 2010). In turn, negative-strand RNA intermediates are used as templates for positive-strand RNA synthesis (Fig. 1C, positive-strand RNA synthesis). Uridylylated VPg (VPgpUpUOH) primes positive-strand RNA synthesis on complementary adenosine bases at the 3′ end of negative-strand templates (Sharma et al., 2005, Steil and Barton, 2008, Steil and Barton, 2009b). Multiple copies of positive-strand RNA are made simultaneously on each negative-strand RNA template, leading to the formation of replicative-intermediate (RI) RNA. Mutations that specifically disable positive-strand RNA synthesis lead to the accumulation of replicative form (RF) RNA (Fig. 1B and C) (Morasco et al., 2003, Murray and Barton, 2003, Steil and Barton, 2008, Steil and Barton, 2009b).
Notably, 3Dpol replicates the poly(A) tail of viral RNA, synthesizing VPg-linked poly(U) at the 5′ end of negative-strands (Fig. 1C) (Steil et al., 2010). Subsequently, VPg-linked poly(U) intermediates function as templates for the polyadenylation of nascent positive-strand RNA (Fig. 1C) (Steil et al., 2010).
3. Template-dependent reiterative transcription mechanisms
Viral RNA replication mechanisms need to ensure the faithful replication of viral RNA genomes. To maintain the integrity of picornavirus RNA genomes, it is imperative that poly(A) tails be regenerated on new viral RNAs during each round of viral replication. In theory, the poly(A) tails could be synthesized by host poly(A) polymerases (PAPs) (Laishram, 2014), by the viral RNA-dependent RNA polymerase, 3Dpol (as diagramed in Fig. 1C), or both. We found that 3Dpol is primarily responsible for the synthesis of poliovirus poly(A) tails (Kempf et al., 2013, Steil et al., 2010). Under normal conditions, 3Dpol replicates the poly(A) tail during viral RNA replication (Steil et al., 2010, Kempf et al., 2013). Cellular PAPs have been shown to restore viral poly(A) tails when they are deleted experimentally (Liu et al., 2008, Raju et al., 1999, Tacahashi and Uyeda, 1999; van Ooij et al., 2006); however, cellular PAPs do not appear to impact the overall size of poliovirus poly(A) tails, as 3Dpol alanine substitution mutations impact the overall size of poly(A) tails in viral RNA (Kempf et al., 2013). If cellular PAPs were primarily responsible for the size of poly(A) tails, then mutations in 3Dpol would not change the overall size of poly(A) tails to the extent that they do (Kempf et al., 2013). Based on these findings, poliovirus 3Dpol appears to use template-dependent reiterative transcription mechanisms analogous to those involved in the polyadenylation of Mononegavirales and Orthomyxovirus mRNAs (Table 2 ).
Table 2.
Reiterative transcription and polyadenylation of viral mRNAs.
| Virus | RdRP | RNA template | Citations |
|---|---|---|---|
| VSV Rhabdoviridae |
L protein | Intergenic U7 |
Schubert et al. (1980) Hunt et al. (1984) Barr and Wertz (2001) |
| Sendai Paramyxoviridae |
L protein | Intergenic U7 | Hausmann et al. (1999) |
| Influenza Orthomyxoviridae |
PB1 | U5 adjacent to panhandle |
Robertson et al. (1981) Poon et al. (1999) Zheng et al. (1999) |
| Poliovirus Picornaviridae | 3Dpol | Poly(A) tail & VPg-linked poly(U) | Steil et al. (2010),Kempf et al. (2013) |
Template-dependent reiterative transcription by the viral polymerase involves the production of RNA products that are longer than the corresponding RNA template. VSV and Sendai L proteins use intergenic U7 sequences to template the polyadenylation of viral mRNAs, where the poly(A) tail is substantially longer than the corresponding U7 template sequence (Barr and Wertz, 2001, Hausmann et al., 1999, Hunt et al., 1984, Schubert et al., 1980) (Table 2). In a similar manner, influenza virus PB1 uses U5 sequences for the polyadenylation of viral mRNAs (Poon et al., 1999, Pritlove et al., 1999, Robertson et al., 1981, Zheng et al., 1999) (Table 2). In the case of poliovirus poly(A) tails, the situation is more complicated due to the heterogeneous length of natural poly(A) tails, which range from 20 to 120 bases long (Fig. 2A, Polio). 3Dpol faithfully copies short poly(A) tails into short VPg-linked poly(U) intermediates (viral RNA with a poly A32 → VPg-linked poly U32 at the 5′ end of negative-strand intermediates) (Steil et al., 2010). Then, in turn, the VPg-linked poly U32 is used to make poly(A) tails from 20 to 120 bases long on new RNA genomes (Steil et al., 2010). In cases where the poly(A) tail is already long (greater than 52 bases in length), 3Dpol makes VPg-linked poly(U) products that are substantially longer (VPg-linked polyU(>120)) (Steil et al., 2010). In turn, VPg-linked poly U120 is used to make poly(A) tails 20–120 bases long (Steil et al., 2010). We do not yet appreciate the precise mechanisms used by 3Dpol to maintain poly(A) tails that range from 20 to 120 bases long; however, this is an active topic of investigation in our laboratory. Picornavirus poly(A) tails are highly diverse: EMCV maintains RNA genomes with relatively short poly(A) tails whereas poliovirus and rhinovirus maintain RNA genomes with longer poly(A) tails (Fig. 2A) (Ahlquist and Kaesberg, 1979). Features of poliovirus 3Dpol impact poly(A) tail lengths. Alanine substitution mutations in poliovirus 3Dpol change the size of poly(A) tails in virion RNA (Fig. 2B) (Kempf et al., 2013). Some mutations result in shorter poly(A) tails (R128A 3Dpol) whereas other mutations result in longer poly(A) tails (L419A 3Dpol). While there is comprehensive data regarding the size distribution of poly(A) tails in three picornavirus genomes (Fig. 2A, EMCV, PV and HRV), to the best of our knowledge there are no studies describing the size distribution of poly(A) tails in other picornaviruses. Nonetheless, as described below, we expect the data implicating poliovirus 3Dpol in the polyadenylation of viral RNA to be broadly applicable to other picornaviruses, and perhaps all polyadenylated positive-strand RNA viruses.
Fig. 2.
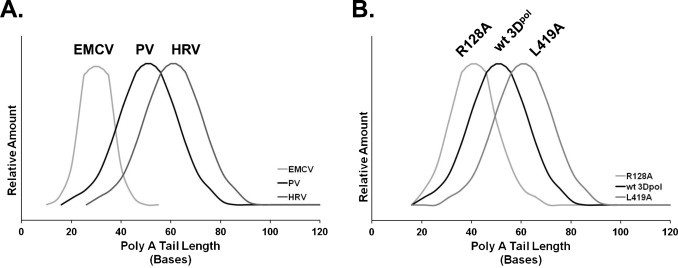
Size distribution of poly(A) tails in picornavirus RNA genomes. (A) Picornavirus RNA genomes have poly(A) tails of variable length, with notable differences in the size distribution when comparing genus and species groups (Ahlquist and Kaesberg, 1979). Encephalomyocarditis virus (EMCV) RNA has relatively short poly(A) tails [range: 10–58 bases long; mean: 28 bases long] whereas poliovirus (PV) [range: 20–120; mean: 53 bases long] (Kempf et al., 2013) and rhinovirus (HRV) RNAs have longer poly(A) tails (Ahlquist and Kaesberg, 1979). (B) Alanine substitution mutations in poliovirus 3Dpol lead to changes in the size of poly(A) tails in RNA genomes (Kempf et al., 2013). 3Dpol R128A mutation results in shorter poly(A) tails (mean: 44 bases long) whereas L419A mutation results in longer poly(A) tails (mean: 66 bases long). The graphs display the size distribution of poly(A) tails for EMCV, PV and HRV RNAs based on published data (Ahlquist and Kaesberg, 1979, Kempf et al., 2013).
4. 3Dpol structures implicated in the polyadenylation of viral RNA
Picornavirus 3Dpol is well studied and its roles in viral RNA replication are well established. It shares common amino acid motifs with RNA-dependent RNA polymerases from retro, positive-strand, negative-strand and dsRNA virus families (Te Velthuis, 2014). Atomic resolution structures of 3Dpol are known for poliovirus (Gong and Peersen, 2010, Thompson and Peersen, 2004), coxsackievirus (Campagnola et al., 2008, Gong et al., 2013), rhinovirus (Appleby et al., 2005, Gong et al., 2013, Love et al., 2004), enterovirus 71 (Chen et al., 2013), foot-and-mouth disease virus (Ferrer-Orta et al., 2004, Ferrer-Orta et al., 2009), and EMCV (Vives-Adrian et al., 2014) (Fig. 3 ). In addition to solving several apo structures (3Dpol structures without RNA templates or products), the Peersen lab successfully isolated and crystallized 3Dpol elongation complexes (Gong et al., 2013, Gong and Peersen, 2010, Sholders and Peersen, 2014). The atomic structure of 3Dpol elongation complexes provides significant insight into the manner in which 3Dpol interacts with viral RNA templates and products (Fig. 3A). Ongoing work is focused on the manner in which viral RNA templates and products translocate as the polymerase synthesizes RNA (Sholders and Peersen, 2014). Template-dependent reiterative transcription mechanisms, like those envisioned in the polyadenylation of viral RNA, would require substantial structural rearrangements of viral RNA templates and products within 3Dpol elongation complexes.
Fig. 3.
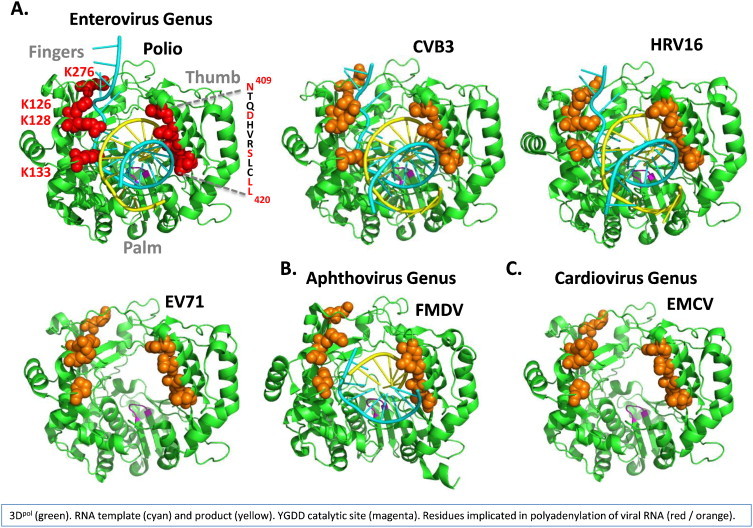
3Dpol structures implicated in the polyadenylation of viral RNA. (A) Enterovirus 3Dpol (Polio, CVB3, HRV16 and EV71) (B) Aphthovirus (FMDV) 3Dpol. (C) Cardiovirus (EMCV) 3Dpol. 3Dpols (green). RNA template (cyan) and product (yellow). YGDD catalytic site (magenta). Residues implicated in the polyadenylation of viral RNA (red-poliovirus, orange-corresponding residues in other viruses as noted in Table 3) (Kempf et al., 2013). Protein Data Bank files for polio (4K4T), CVB3 (4K4X), HRV16 (4K50), EV71 (4IKA), FMDV (2E9T) and EMCV (4NZ0).
Alanine substitution mutations implicate specific features of 3Dpol in the polyadenylation of viral RNA (Table 3 and Fig. 3A) (Kempf et al., 2013). Residues in the fingers and thumb domain of 3Dpol affect the size of poly(A) tails in poliovirion RNA (Table 3 and Fig. 3A). Charged amino acid residues in the fingers domain interact with viral RNA templates and products while a thumb domain α-helix fits within the minor groove of dsRNA products as they leave the active site (Fig. 4A). The structural orientation of these residues makes it clear that 3Dpol regulates the size of poly(A) tails, in part, through conserved elements that interact with viral RNA templates and products (Table 3 and Fig. 3). Therefore, based on the common mechanisms of picornaviral replication (Fig. 1) and the conserved features of poliovirus 3Dpol implicated in the polyadenylation of viral RNA (Table 3 and Fig. 3), we expect that 3Dpol is responsible for the polyadenylation of all picornavirus RNA genomes. Polymorphisms in 3Dpol among different picornaviruses likely affect the size of poly(A) tails in RNA genomes.
Table 3.
Conserved structures of 3Dpol implicated in the polyadenylation of picornaviral RNA*.
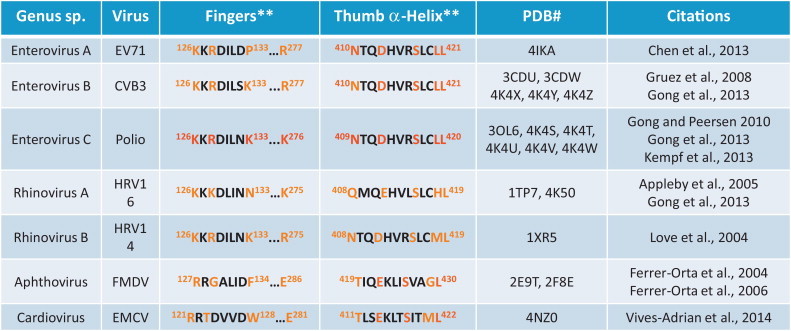
* Red: Poliovirus 3Dpol residues implicated in the polyadenylation of viral RNA (Kempf et al., 2013).
Orange: Residues at corresponding locations in other 3Dpol sequences and structures.
** 3Dpol amino acid alignments (Chen et al., 2013; Gruez et al., 2008; Ferrer-Orta et al., 2004).
Fig. 4.
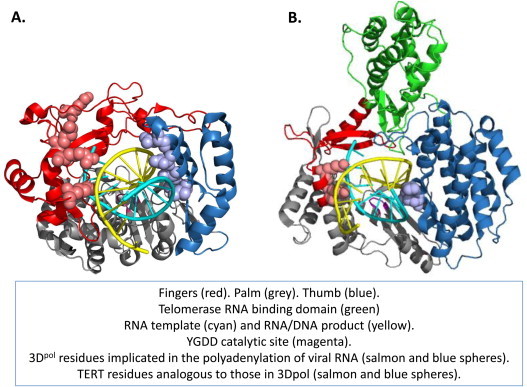
Structural and functional parallels between 3Dpol and telomerase reverse transcriptase (TERT). (A) Poliovirus 3Dpol elongation complex (PDB: 4K4T) (Gong and Peersen, 2010). (B) TERT structure including RNA template and DNA product (PDB: 3KYL) (Mitchell et al., 2010).
5. Unresolved questions
5.1. What is the biological impact of shorter or longer poly(A) tails?
At present, it is unclear why viruses like EMCV evolved to maintain relatively short poly(A) tails whereas others (polioviruses and rhinoviruses) evolved to maintain longer poly(A) tails (Fig. 2A). Considering circularized mRNPs, where eIF4G interacts with poly(A) binding protein (PABP) (Svitkin et al., 2001), it is possible that viral IRES elements and 2Apro activity impact the optimal size of poly(A) tails. In addition, the size of poly(A) tails may impact the manner in which viral mRNAs interact with mRNA turnover machinery in cells, as deadenylase and Xrn1 are recruited specifically to polyadenylated mRNAs (Doidge et al., 2012). Poliovirus 2Apro increases viral mRNA stability (Kempf and Barton, 2008a). Furthermore, 2Apro-dependent increases in viral mRNA stability are coincident in time with the cleavage of eIF4G (Kempf and Barton, 2008a). The cleavage of eIF4G by 2Apro liberates the NH-terminal portion of eIF4G from circularized viral mRNPs, and in so doing may also dissociate cellular mRNA turnover machinery from circularized viral mRNPs (Kempf and Barton, 2008a). Enteroviruses and rhinoviruses, which cleave eIF4G with 2Apro, have longer poly(A) tails than EMCV, which does not cleave eIF4G. Thus, enterovirus and rhinovirus mRNAs are translated in 2Apro-modified polysomes, perhaps to uncouple mRNA turnover machinery from viral mRNAs (Kempf and Barton, 2008a). Shorter poly(A) tails, like those in EMCV, may provide some relief from host mRNA turnover machinery, although this possibility has not been substantiated experimentally.
Stress granules are another consideration (White and Lloyd, 2012). Does the length of poly(A) tails impact the manner in which viral mRNAs interact with stress granules? Enterovirus and rhinovirus 3 Cpro cleave PABP and G3BP, stress granule proteins (White et al., 2007, White and Lloyd, 2011); however, the length of poly(A) tails as they relate to stress granule formation has not been examined experimentally.
5.2. Factors other than 3Dpol regulating the size of picornavirus poly(A) tails?
Our working model of reiterative transcription suggests that 3Dpol pauses during VPg-linked poly(U) and poly(A) synthesis, nascent dsRNA products melt, realign, reanneal and resume elongation, thereby making RNA products that are longer than the template (Steil et al., 2010). The 3′ NTR of viral RNAs might impact the manner in which 3Dpol pauses during VPg-linked poly(U) synthesis. Likewise, VPg at the 5′ end of negative-strand RNA templates could prevent 3Dpol from running off the end of RNA templates during positive-strand RNA synthesis, provoking reiterative transcription during the polyadenylation of nascent (+) strands, especially on RNA templates with relatively short VPg-linked poly(U) sequences (Steil et al., 2010). 3Dpol oligomers (Bentham et al., 2012, Lyle et al., 2002) or protein complexes (Shen et al., 2008) might impact the manner in which nascent dsRNA products melt, realign and reanneal; however, there is no direct evidence implicating 3Dpol oligomers or protein complexes in these events. 3′NTR mutations are reported to impact the size of poly(A) tails (van Ooij et al., 2006a). Furthermore, PABP could influence the replication of poly(A) tails, although its contribution appears to be dispensable (Svitkin et al., 2007).
5.3. How are poly(A) tails maintained on other positive-strand RNA virus genomes?
Cellular PAPs synthesize poly(A) tails in a template-independent manner, downstream of characteristic polyadenylation signals (AAUAAA and AUUAAA) (Laishram, 2014). Poly(A) tails on herpesvirus mRNAs (Majerciak et al., 2013) and cellular mRNAs (Ni et al., 2013) are synthesized by cellular PAPs (Laishram, 2014). DNA viruses and retroviruses have 3′ terminal polyadenylation signals that are used regularly by cellular PAPs (Schrom et al., 2013). Among polyadenylated positive-strand RNA viruses, only potexviruses have 3′ terminal polyadenylation signals that could be used regularly by cellular PAPs (Osman et al., 2014). We expect that most polyadenylated positive-strand RNA viruses, like picornaviruses (Kempf et al., 2013, Steil et al., 2010), replicate their poly(A) tails with their viral RNA-dependent RNA polymerases. The presence of long poly(U) sequences in alphavirus (Sawicki and Gomatos, 1976) and coronavirus (Wu et al., 2013) negative-strand RNA intermediates are consistent with these predictions. Nonetheless, some polyadenylated positive-strand RNA viruses have been shown to use cellular PAPs to repair defective genomes lacking poly(A) tails (Liu et al., 2008, Raju et al., 1999, Tacahashi and Uyeda, 1999, van Ooij et al., 2006a).
6. Structural and functional parallels between 3Dpol and telomerase reverse transcriptase (TERT)
Consistent with their ancient evolutionary origins (Nakamura and Cech, 1998), picornavirus 3Dpol and TERT share structural and functional features. Structurally, both 3Dpol and TERT assume a “right-hand” conformation with thumb, palm and fingers domains encircling templates and products (Fig. 4) (Gillis et al., 2008, Gong and Peersen, 2010, Mason et al., 2011, Mitchell et al., 2010). Functionally, both 3Dpol and TERT use template-dependent reiterative transcription mechanisms to synthesize repetitive sequences at the ends of chromosomes: poly(A) tails in the case of picornavirus RNA genomes and DNA telomeres in the case of eukaryotic chromosomes (Blackburn, 1999, Kempf et al., 2013, Steil et al., 2010). These two enzymes have diverged to such a great degree that there is little amino acid homology evident beyond the catalytic residues in the palm domain. Nonetheless, there are strikingly similar fingers domain and thumb domain residues gripping the templates and products as they exit the respective molecules (Fig. 4). Based on these features of 3Dpol and TERT, it is reasonable to think of 3Dpol reiterative transcription mechanisms as telomerase-like aspects of viral RNA replication. Likewise, it is reasonable to consider poly(A) tails to be a telomere of picornavirus RNA genomes.
The concept of telomeres in positive-strand RNA virus genomes is not new (Rao et al., 1989). A tRNA-like element at the 3′ end of brome mosaic virus RNA was ascribed telomere functions long ago (Rao et al., 1989). Telomeres have two characteristic features: (1) mechanisms to renew themselves, and (2) mechanisms to protect the remainder of the genome. Cellular CCA-adding enzyme can renew the integrity of the tRNA-like element at the 3′ end of brome mosaic virus RNA genomes (Rao et al., 1989). Aminoacylation reinforces the integrity of the tRNA-like element (Rao et al., 1989). Furthermore, the tRNA-like element of brome mosaic virus RNA protects the viral RNA genome from host cell mRNA turnover machinery. In the case of picornaviruses, reiterative transcription mechanisms of 3Dpol renew 3′ poly(A) tails on viral RNA genomes during viral RNA replication (Kempf et al., 2013, Steil et al., 2010). In turn, the poly(A) tail, via interactions with PABP and other factors, protects the viral RNA genome from mRNA turnover machinery (Kempf and Barton, 2008a, Kempf and Barton, 2008b). Thus, we think it is reasonable to consider both poly(A) tails and tRNA-like elements to be telomeres of positive-strand RNA virus genomes.
Acknowledgements
Supported by the National Institutes of Health (AI042189). We thank Ben Steil for the contribution of data and Daphne Cooper for critical evaluation of the manuscript.
References
- CDC Nonpolio enterovirus and human parechovirus surveillance – United States, 2006–2008. MMWR. Morb. Mortal. Wkly. Rep. 2010;59(48):1577–1580. [PubMed] [Google Scholar]
- Ahlquist P., Kaesberg P. Determination of the length distribution of poly(A) at the 3′ terminus of the virion RNAs of EMC virus, poliovirus, rhinovirus, RAV-61 and CPMV and of mouse globin mRNA. Nucleic Acids Res. 1979;7(5):1195–1204. doi: 10.1093/nar/7.5.1195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Sunaidi M., Williams C.H., Hughes P.J., Schnurr D.P., Stanway G. Analysis of a new human parechovirus allows the definition of parechovirus types and the identification of RNA structural domains. J. Virol. 2007;81(2):1013–1021. doi: 10.1128/JVI.00584-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appleby T.C., Luecke H., Shim J.H., Wu J.Z., Cheney I.W., Zhong W., Vogeley L., Hong Z., Yao N. Crystal structure of complete rhinovirus RNA polymerase suggests front loading of protein primer. J. Virol. 2005;79(1):277–288. doi: 10.1128/JVI.79.1.277-288.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranguren L.F., Salazar M., Tang K., Caraballo X., Lightner D. Characterization of a new strain of Taura syndrome virus (TSV) from Colombian shrimp farms and the implication in the selection of TSV resistant lines. J. Invertebr. Pathol. 2013;112(1):68–73. doi: 10.1016/j.jip.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Barr J.N., Wertz G.W. Polymerase slippage at vesicular stomatitis virus gene junctions to generate poly(A) is regulated by the upstream 3′-AUAC-5′ tetranucleotide: implications for the mechanism of transcription termination. J. Virol. 2001;75(15):6901–6913. doi: 10.1128/JVI.75.15.6901-6913.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D.J., Flanegan J.B. Coupled translation and replication of poliovirus RNA in vitro: synthesis of functional 3D polymerase and infectious virus. J. Virol. 1993;67(2):822–831. doi: 10.1128/jvi.67.2.822-831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barton D.J., Flanegan J.B. Synchronous replication of poliovirus RNA: initiation of negative-strand RNA synthesis requires the guanidine-inhibited activity of protein 2C. J. Virol. 1997;71(11):8482–8489. doi: 10.1128/jvi.71.11.8482-8489.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentham M., Holmes K., Forrest S., Rowlands D.J., Stonehouse N.J. Formation of higher-order foot-and-mouth disease virus 3D(pol) complexes is dependent on elongation activity. J. Virol. 2012;86(4):2371–2374. doi: 10.1128/JVI.05696-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackburn E.H. Telomerase. In: Gesteland R.F., Cech T.R., Atkins J.F., editors. The RNA World: The Nature of Modern RNA Suggests a Prebiotic RNA. 2nd ed. Cold Spring Harbor Laboratory Press; Cold Spring Harbor, NY: 1999. pp. 609–635. [Google Scholar]
- Boros A., Kiss T., Kiss O., Pankovics P., Kapusinszky B., Delwart E., Reuter G. Genetic characterization of a novel picornavirus distantly related to the marine mammal-infecting aquamaviruses in a long-distance migrant bird species, European roller (Coracias garrulus) J. Gen. Virol. 2013;94(Pt 9):2029–2035. doi: 10.1099/vir.0.054676-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boros A., Pankovics P., Reuter G. Avian picornaviruses: molecular evolution, genome diversity and unusual genome features of a rapidly expanding group of viruses in birds. Infect. Genet. Evol. 2014;28C:151–166. doi: 10.1016/j.meegid.2014.09.027. [DOI] [PubMed] [Google Scholar]
- Campagnola G., Weygandt M., Scoggin K., Peersen O. Crystal structure of coxsackievirus B3 3Dpol highlights the functional importance of residue 5 in picornavirus polymerases. J. Virol. 2008;82(19):9458–9464. doi: 10.1128/JVI.00647-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Wang Y., Shan C., Sun Y., Xu P., Zhou H., Yang C., Shi P.Y., Rao Z., Zhang B., Lou Z. Crystal structure of enterovirus 71 RNA-dependent RNA polymerase complexed with its protein primer VPg: implication for a trans mechanism of VPg uridylylation. J. Virol. 2013;87(10):5755–5768. doi: 10.1128/JVI.02733-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.P., Pettis J.S., Corona M., Chen W.P., Li C.J., Spivak M., Visscher P.K., DeGrandi-Hoffman G., Boncristiani H., Zhao Y., vanEngelsdorp D., Delaplane K., Solter L., Drummond F., Kramer M., Lipkin W.I., Palacios G., Hamilton M.C., Smith B., Huang S.K., Zheng H.Q., Li J.L., Zhang X., Zhou A.F., Wu L.Y., Zhou J.Z., Lee M.L., Teixeira E.W., Li Z.G., Evans J.D. Israeli acute paralysis virus: epidemiology, pathogenesis and implications for honey bee health. PLoS Pathog. 2014;10(7):e1004261. doi: 10.1371/journal.ppat.1004261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordey S., Gerlach D., Junier T., Zdobnov E.M., Kaiser L., Tapparel C. The cis-acting replication elements define human enterovirus and rhinovirus species. RNA. 2008;14(8):1568–1578. doi: 10.1261/rna.1031408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denis P., Liebig H.D., Nowotny N., Billinis C., Papadopoulos O., O’Hara R.S., Knowles N.J., Koenen F. Genetic variability of encephalomyocarditis virus (EMCV) isolates. Vet. Microbiol. 2006;113(1–2):1–12. doi: 10.1016/j.vetmic.2005.10.032. [DOI] [PubMed] [Google Scholar]
- Doidge R., Mittal S., Aslam A., Winkler G.S. Deadenylation of cytoplasmic mRNA by the mammalian Ccr4-Not complex. Biochem. Soc. Trans. 2012;40(4):896–901. doi: 10.1042/BST20120074. [DOI] [PubMed] [Google Scholar]
- Farkas S.L., Ihasz K., Feher E., Bartha D., Jakab F., Gal J., Banyai K., Marschang R.E. Sequencing and phylogenetic analysis identifies candidate members of a new picornavirus genus in terrestrial tortoise species. Arch Virol. 2014 doi: 10.1007/s00705-014-2292-z. [DOI] [PubMed] [Google Scholar]
- Fata-Hartley C.L., Palmenberg A.C. Dipyridamole reversibly inhibits mengovirus RNA replication. J. Virol. 2005;79(17):11062–11070. doi: 10.1128/JVI.79.17.11062-11070.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer-Orta C., Agudo R., Domingo E., Verdaguer N. Structural insights into replication initiation and elongation processes by the FMDV RNA-dependent RNA polymerase. Curr. Opin. Struct. Biol. 2009;19(6):752–758. doi: 10.1016/j.sbi.2009.10.016. [DOI] [PubMed] [Google Scholar]
- Ferrer-Orta C., Arias A., Perez-Luque R., Escarmis C., Domingo E., Verdaguer N. Structure of foot-and-mouth disease virus RNA-dependent RNA polymerase and its complex with a template-primer RNA. J. Biol. Chem. 2004;279(45):47212–47221. doi: 10.1074/jbc.M405465200. [DOI] [PubMed] [Google Scholar]
- Gillis A.J., Schuller A.P., Skordalakes E. Structure of the Tribolium castaneum telomerase catalytic subunit TERT. Nature. 2008;455(7213):633–637. doi: 10.1038/nature07283. [DOI] [PubMed] [Google Scholar]
- Gong P., Kortus M.G., Nix J.C., Davis R.E., Peersen O.B. Structures of coxsackievirus, rhinovirus, and poliovirus polymerase elongation complexes solved by engineering RNA mediated crystal contacts. PLoS one. 2013;8(5):e60272. doi: 10.1371/journal.pone.0060272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong P., Peersen O.B. Structural basis for active site closure by the poliovirus RNA-dependent RNA polymerase. Proc. Natl. Acad. Sci. U. S. A. 2010;107(52):22505–22510. doi: 10.1073/pnas.1007626107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow I., Chaudhry Y., Richardson A., Meredith J., Almond J.W., Barclay W., Evans D.J. Identification of a cis-acting replication element within the poliovirus coding region. J. Virol. 2000;74(10):4590–4600. doi: 10.1128/jvi.74.10.4590-4600.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodfellow I.G., Polacek C., Andino R., Evans D.J. The poliovirus 2C cis-acting replication element-mediated uridylylation of VPg is not required for synthesis of negative-sense genomes. J. Gen. Virol. 2003;84(Pt 9):2359–2363. doi: 10.1099/vir.0.19132-0. [DOI] [PubMed] [Google Scholar]
- Grubman M.J., Baxt B. Foot-and-mouth disease. Clin. Microbiol. Rev. 2004;17(2):465–493. doi: 10.1128/CMR.17.2.465-493.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gruez A., Selisko B., Roberts M., Bricogne G., Bussetta C., Jabafi I., Coutard B., De Palma A.M., Neyts J., Canard B. The crystal structure of coxsackievirus B3 RNA-dependent RNA polymerase in complex with its protein primer VPg confirms the existence of a second VPg binding site on Picornaviridae polymerases. J Virol, 2008;82(19):9577–9590. doi: 10.1128/JVI.00631-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hausmann S., Garcin D., Morel A.S., Kolakofsky D. Two nucleotides immediately upstream of the essential A6G3 slippery sequence modulate the pattern of G insertions during Sendai virus mRNA editing. J. Virol. 1999;73(1):343–351. doi: 10.1128/jvi.73.1.343-351.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt D.M., Smith E.F., Buckley D.W. Aberrant polyadenylation by a vesicular stomatitis virus mutant is due to an altered L protein. J. Virol. 1984;52(2):515–521. doi: 10.1128/jvi.52.2.515-521.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadao A.S., Krause-Sakate R., Liberti D., Pavan M.A., Echer M.M., Svanella-Dumas L., Zerbini F.M., Candresse T., Le Gall O. Further characterization of two sequiviruses infecting lettuce and development of specific RT-PCR primers. Arch. Virol. 2007;152(5):999–1007. doi: 10.1007/s00705-006-0895-8. [DOI] [PubMed] [Google Scholar]
- Kapoor A., Victoria J., Simmonds P., Wang C., Shafer R.W., Nims R., Nielsen O., Delwart E. A highly divergent picornavirus in a marine mammal. J. Virol. 2008;82(1):311–320. doi: 10.1128/JVI.01240-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf B.J., Barton D.J. Poliovirus 2A(Pro) increases viral mRNA and polysome stability coordinately in time with cleavage of eIF4G. J. Virol. 2008;82(12):5847–5859. doi: 10.1128/JVI.01514-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf B.J., Barton D.J. Poly(rC) binding proteins and the 5′ cloverleaf of uncapped poliovirus mRNA function during de novo assembly of polysomes. J. Virol. 2008;82(12):5835–5846. doi: 10.1128/JVI.01513-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kempf B.J., Kelly M.M., Springer C.L., Peersen O.B., Barton D.J. Structural features of a picornavirus polymerase involved in the polyadenylation of viral RNA. J. Virol. 2013;87(10):5629–5644. doi: 10.1128/JVI.02590-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knowles N.J., Hovi T., Hyypia T., King A.M.Q., Lindberg A.M., Pallanch M.A., Palmenberg A.C., Simmonds P., Skern T., Stanway G., Yamashita T., Zell R. Picornaviridae. In: King A.M., Adams M.J., Carstens E.B., Lefkowitz E.J., editors. Virus Taxonomy: Classification and Nomenclature of Viruses: Ninth Report of the International Committee on Taxonomy of Viruses. Elsevier; San Diego: 2012. pp. 855–880. [Google Scholar]
- Komoda K., Naito S., Ishikawa M. Replication of plant RNA virus genomes in a cell-free extract of evacuolated plant protoplasts. Proc. Natl. Acad. Sci. U. S. A. 2004;101(7):1863–1867. doi: 10.1073/pnas.0307131101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E.V., Wolf Y.I., Nagasaki K., Dolja V.V. The Big Bang of picorna-like virus evolution antedates the radiation of eukaryotic supergroups. Nat. Rev. Microbiol. 2008;6(12):925–939. doi: 10.1038/nrmicro2030. [DOI] [PubMed] [Google Scholar]
- Laishram R.S. Poly(A) polymerase (PAP) diversity in gene expression – star-PAP vs canonical PAP. FEBS Lett. 2014;588(14):2185–2197. doi: 10.1016/j.febslet.2014.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Gall O., Christian P., Fauquet C.M., King A.M., Knowles N.J., Nakashima N., Stanway G., Gorbalenya A.E. Picornavirales, a proposed order of positive-sense single-stranded RNA viruses with a pseudo-T = 3 virion architecture. Arch. Virol. 2008;153(4):715–727. doi: 10.1007/s00705-008-0041-x. [DOI] [PubMed] [Google Scholar]
- Lescar J., Canard B. RNA-dependent RNA polymerases from flaviviruses and Picornaviridae. Curr. Opin. Struct. Biol. 2009;19(6):759–767. doi: 10.1016/j.sbi.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Liu G.Q., Ni Z., Yun T., Yu B., Zhu J.M., Hua J.G., Chen J.P. Rabbit hemorrhagic disease virus poly(A) tail is not essential for the infectivity of the virus and can be restored in vivo. Arch. Virol. 2008;153(5):939–944. doi: 10.1007/s00705-008-0079-9. [DOI] [PubMed] [Google Scholar]
- Lobert P.E., Escriou N., Ruelle J., Michiels T. A coding RNA sequence acts as a replication signal in cardioviruses. Proc. Natl. Acad. Sci. U. S. A. 1999;96(20):11560–11565. doi: 10.1073/pnas.96.20.11560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Love R.A., Maegley K.A., Yu X., Ferre R.A., Lingardo L.K., Diehl W., Parge H.E., Dragovich P.S., Fuhrman S.A. The crystal structure of the RNA-dependent RNA polymerase from human rhinovirus: a dual function target for common cold antiviral therapy. Structure. 2004;12(8):1533–1544. doi: 10.1016/j.str.2004.05.024. [DOI] [PubMed] [Google Scholar]
- Lyle J.M., Bullitt E., Bienz K., Kirkegaard K. Visualization and functional analysis of RNA-dependent RNA polymerase lattices. Science. 2002;296(5576):2218–2222. doi: 10.1126/science.1070585. [DOI] [PubMed] [Google Scholar]
- Majerciak V., Ni T., Yang W., Meng B., Zhu J., Zheng Z.M. A viral genome landscape of RNA polyadenylation from KSHV latent to lytic infection. PLoS Pathog. 2013;9(11):e1003749. doi: 10.1371/journal.ppat.1003749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason M., Schuller A., Skordalakes E. Telomerase structure function. Curr. Opin. Struct. Biol. 2011;21(1):92–100. doi: 10.1016/j.sbi.2010.11.005. [DOI] [PubMed] [Google Scholar]
- Mason P.W., Bezborodova S.V., Henry T.M. Identification and characterization of a cis-acting replication element (cre) adjacent to the internal ribosome entry site of foot-and-mouth disease virus. J. Virol. 2002;76(19):9686–9694. doi: 10.1128/JVI.76.19.9686-9694.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight K.L., Lemon S.M. The rhinovirus type 14 genome contains an internally located RNA structure that is required for viral replication. RNA. 1998;4(12):1569–1584. doi: 10.1017/s1355838298981006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menzel W., Vetten H.J. Complete nucleotide sequence of an isolate of the Anthriscus strain of Parsnip yellow fleck virus. Arch. Virol. 2008;153(11):2173–2175. doi: 10.1007/s00705-008-0240-5. [DOI] [PubMed] [Google Scholar]
- Mitchell M., Gillis A., Futahashi M., Fujiwara H., Skordalakes E. Structural basis for telomerase catalytic subunit TERT binding to RNA template and telomeric DNA. Nat. Struct. Mol. Biol. 2010;17(4):513–518. doi: 10.1038/nsmb.1777. [DOI] [PubMed] [Google Scholar]
- Molla A., Paul A.V., Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991;254(5038):1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- Morasco B.J., Sharma N., Parilla J., Flanegan J.B. Poliovirus cre(2C)-dependent synthesis of VPgpUpU is required for positive- but not negative-strand RNA synthesis. J. Virol. 2003;77(9):5136–5144. doi: 10.1128/JVI.77.9.5136-5144.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray K.E., Barton D.J. Poliovirus CRE-dependent VPg uridylylation is required for positive-strand RNA synthesis but not for negative-strand RNA synthesis. J. Virol. 2003;77(8):4739–4750. doi: 10.1128/JVI.77.8.4739-4750.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura T.M., Cech T.R. Reversing time: origin of telomerase. Cell. 1998;92(5):587–590. doi: 10.1016/s0092-8674(00)81123-x. [DOI] [PubMed] [Google Scholar]
- Ni T., Yang Y., Hafez D., Yang W., Kiesewetter K., Wakabayashi Y., Ohler U., Peng W., Zhu J. Distinct polyadenylation landscapes of diverse human tissues revealed by a modified PA-seq strategy. BMC Genomics. 2013;14:615. doi: 10.1186/1471-2164-14-615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osman T.A., Olsthoorn R.C., Livieratos I.C. Role of the Pepino mosaic virus 3′-untranslated region elements in negative-strand RNA synthesis in vitro. Virus Res. 2014;190:110–117. doi: 10.1016/j.virusres.2014.06.018. [DOI] [PubMed] [Google Scholar]
- Paul A.V., Rieder E., Kim D.W., van Boom J.H., Wimmer E. Identification of an RNA hairpin in poliovirus RNA that serves as the primary template in the in vitro uridylylation of VPg. J. Virol. 2000;74(22):10359–10370. doi: 10.1128/jvi.74.22.10359-10370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul A.V., Wimmer E. Initiation of protein-primed picornavirus RNA synthesis. Virus Research. 2015;206:12–26. doi: 10.1016/j.virusres.2014.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon L.L., Pritlove D.C., Fodor E., Brownlee G.G. Direct evidence that the poly(A) tail of influenza A virus mRNA is synthesized by reiterative copying of a U track in the virion RNA template. J. Virol. 1999;73(4):3473–3476. doi: 10.1128/jvi.73.4.3473-3476.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritlove D.C., Poon L.L., Devenish L.J., Leahy M.B., Brownlee G.G. A hairpin loop at the 5′ end of influenza A virus virion RNA is required for synthesis of poly(A)+ mRNA in vitro. J. Virol. 1999;73(3):2109–2114. doi: 10.1128/jvi.73.3.2109-2114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raju R., Hajjou M., Hill K.R., Botta V., Botta S. In vivo addition of poly(A) tail and AU-rich sequences to the 3′ terminus of the Sindbis virus RNA genome: a novel 3′-end repair pathway. J. Virol. 1999;73(3):2410–2419. doi: 10.1128/jvi.73.3.2410-2419.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao A.L., Dreher T.W., Marsh L.E., Hall T.C. Telomeric function of the tRNA-like structure of brome mosaic virus RNA. Proc. Natl. Acad. Sci. U. S. A. 1989;86(14):5335–5339. doi: 10.1073/pnas.86.14.5335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson J.S., Schubert M., Lazzarini R.A. Polyadenylation sites for influenza virus mRNA. J. Virol. 1981;38(1):157–163. doi: 10.1128/jvi.38.1.157-163.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanfacon H., Wellink J., Le Gall O., Karasev A., van der Vlugt R., Wetzel T. Secoviridae: a proposed family of plant viruses within the order Picornavirales that combines the families Sequiviridae and Comoviridae, the unassigned genera Cheravirus and Sadwavirus, and the proposed genus Torradovirus. Arch. Virol. 2009;154(5):899–907. doi: 10.1007/s00705-009-0367-z. [DOI] [PubMed] [Google Scholar]
- Sawicki D.L., Gomatos P.J. Replication of semliki forest virus: polyadenylate in plus-strand RNA and polyuridylate in minus-strand RNA. J. Virol. 1976;20(2):446–464. doi: 10.1128/jvi.20.2.446-464.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrom E.M., Moschall R., Schuch A., Bodem J. Regulation of retroviral polyadenylation. Adv. Virus Res. 2013;85:1–24. doi: 10.1016/B978-0-12-408116-1.00001-X. [DOI] [PubMed] [Google Scholar]
- Schubert M., Keene J.D., Herman R.C., Lazzarini R.A. Site on the vesicular stomatitis virus genome specifying polyadenylation and the end of the L gene mRNA. J. Virol. 1980;34(2):550–559. doi: 10.1128/jvi.34.2.550-559.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma N., O’Donnell B.J., Flanegan J.B. 3′-Terminal sequence in poliovirus negative-strand templates is the primary cis-acting element required for VPgpUpU-primed positive-strand initiation. J. Virol. 2005;79(6):3565–3577. doi: 10.1128/JVI.79.6.3565-3577.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen M., Reitman Z.J., Zhao Y., Moustafa I., Wang Q., Arnold J.J., Pathak H.B., Cameron C.E. Picornavirus genome replication. Identification of the surface of the poliovirus (PV) 3C dimer that interacts with PV 3Dpol during VPg uridylylation and construction of a structural model for the PV 3C2-3Dpol complex. J. Biol. Chem. 2008;283(2):875–888. doi: 10.1074/jbc.M707907200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sholders A.J., Peersen O.B. Distinct conformations of a putative translocation element in poliovirus polymerase. J. Mol. Biol. 2014;426(7):1407–1419. doi: 10.1016/j.jmb.2013.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Son K.Y., Kim D.S., Kwon J., Choi J.S., Kang M.I., Belsham G.J., Cho K.O. Full-length genomic analysis of Korean porcine Sapelovirus strains. PLoS ONE. 2014;9(9):e107860. doi: 10.1371/journal.pone.0107860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil B.P. University of Colorado; 2008. Protein primers and a telomerase-like mechanism of polioviruis RNA replication maintain the 3′ end of the RNA genome. (thesis) [Google Scholar]
- Steil B.P., Barton D.J. Poliovirus cis-acting replication element-dependent VPg Uridylylation lowers the Km of the initiating nucleoside triphosphate for viral RNA replication. J. Virol. 2008;82(19):9400–9408. doi: 10.1128/JVI.00427-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil B.P., Barton D.J. Cis-active RNA elements (CREs) and picornavirus RNA replication. Virus Res. 2009;139(2):240–252. doi: 10.1016/j.virusres.2008.07.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil B.P., Barton D.J. Conversion of VPg into VPgpUpUOH before and during poliovirus negative-strand RNA synthesis. J. Virol. 2009;83(24):12660–12670. doi: 10.1128/JVI.01676-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steil B.P., Kempf B.J., Barton D.J. Poly(A) at the 3′ end of positive-strand RNA and VPg-linked poly(U) at the 5′ end of negative-strand RNA are reciprocal templates during replication of poliovirus RNA. J. Virol. 2010;84(6):2843–2858. doi: 10.1128/JVI.02620-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y., Guo Y., Lou Z. Formation and working mechanism of the picornavirus VPg uridylylation complex. Curr. Opin. Virol. 2014;9C:24–30. doi: 10.1016/j.coviro.2014.09.003. [DOI] [PubMed] [Google Scholar]
- Svitkin Y.V., Costa-Mattioli M., Herdy B., Perreault S., Sonenberg N. Stimulation of picornavirus replication by the poly(A) tail in a cell-free extract is largely independent of the poly(A) binding protein (PABP) RNA. 2007;13(12):2330–2340. doi: 10.1261/rna.606407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y.V., Imataka H., Khaleghpour K., Kahvejian A., Liebig H.D., Sonenberg N. Poly(A)-binding protein interaction with elF4G stimulates picornavirus IRES-dependent translation. RNA. 2001;7(12):1743–1752. [PMC free article] [PubMed] [Google Scholar]
- Svitkin Y.V., Sonenberg N. Cell-free synthesis of encephalomyocarditis virus. J. Virol. 2003;77(11):6551–6555. doi: 10.1128/JVI.77.11.6551-6555.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweeney T.R., Cisnetto V., Bose D., Bailey M., Wilson J.R., Zhang X., Belsham G.J., Curry S. Foot-and-mouth disease virus 2C is a hexameric AAA+ protein with a coordinated ATP hydrolysis mechanism. J. Biol. Chem. 2010;285(32):24347–24359. doi: 10.1074/jbc.M110.129940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacahashi Y., Uyeda I. Restoration of the 3′ end of potyvirus RNA derived from Poly(A)-deficient infectious cDNA clones. Virology. 1999;265(1):147–152. doi: 10.1006/viro.1999.0027. [DOI] [PubMed] [Google Scholar]
- Te Velthuis A.J. Common and unique features of viral RNA-dependent polymerases. Cell. Mol. Life Sci. 2014;71(22):4403–4420. doi: 10.1007/s00018-014-1695-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson A.A., Peersen O.B. Structural basis for proteolysis-dependent activation of the poliovirus RNA-dependent RNA polymerase. EMBO J. 2004;23(17):3462–3471. doi: 10.1038/sj.emboj.7600357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd S., Towner J.S., Semler B.L. Translation and replication properties of the human rhinovirus genome in vivo and in vitro. Virology. 1997;229(1):90–97. doi: 10.1006/viro.1996.8416. [DOI] [PubMed] [Google Scholar]
- Turnbull-Ross A.D., Reavy B., Mayo M.A., Murant A.F. The nucleotide sequence of parsnip yellow fleck virus: a plant picorna-like virus. J. Gen. Virol. 1992;73(Pt 12):3203–3211. doi: 10.1099/0022-1317-73-12-3203. [DOI] [PubMed] [Google Scholar]
- Van Dung N., Anh P.H., Van Cuong N., Hoa N.T., Carrique-Mas J., Hien V.B., Campbell J., Baker S., Farrar J., Woolhouse M.E., Bryant J.E., Simmonds P. Prevalence, genetic diversity and recombination of species G enteroviruses infecting pigs in Vietnam. J. Gen. Virol. 2014;95(Pt 3):549–556. doi: 10.1099/vir.0.061978-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Nguyen D., Harvala H., Ngole E.M., Delaporte E., Woolhouse M.E., Peeters M., Simmonds P. High rates of infection with novel enterovirus variants in wild populations of mandrills and other old world monkey species. J. Virol. 2014;88(11):5967–5976. doi: 10.1128/JVI.00088-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooij M.J., Polacek C., Glaudemans D.H., Kuijpers J., van Kuppeveld F.J., Andino R., Agol V.I., Melchers W.J. Polyadenylation of genomic RNA and initiation of antigenomic RNA in a positive-strand RNA virus are controlled by the same cis-element. Nucleic Acids Res. 2006;34(10):2953–2965. doi: 10.1093/nar/gkl349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ooij M.J., Vogt D.A., Paul A., Castro C., Kuijpers J., van Kuppeveld F.J., Cameron C.E., Wimmer E., Andino R., Melchers W.J. Structural and functional characterization of the coxsackievirus B3 CRE(2C): role of CRE(2C) in negative- and positive-strand RNA synthesis. J. Gen. Virol. 2006;87(Pt 1):103–113. doi: 10.1099/vir.0.81297-0. [DOI] [PubMed] [Google Scholar]
- Vives-Adrian L., Lujan C., Oliva B., van der Linden L., Selisko B., Coutard B., Canard B., van Kuppeveld F.J., Ferrer-Orta C., Verdaguer N. The crystal structure of a cardiovirus RNA-dependent RNA polymerase reveals an unusual conformation of the polymerase active site. J. Virol. 2014;88(10):5595–5607. doi: 10.1128/JVI.03502-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.P., Cardenas A.M., Marissen W.E., Lloyd R.E. Inhibition of cytoplasmic mRNA stress granule formation by a viral proteinase. Cell Host Microbe. 2007;2(5):295–305. doi: 10.1016/j.chom.2007.08.006. [DOI] [PubMed] [Google Scholar]
- White J.P., Lloyd R.E. Poliovirus unlinks TIA1 aggregation and mRNA stress granule formation. J. Virol. 2011;85(23):12442–12454. doi: 10.1128/JVI.05888-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White J.P., Lloyd R.E. Regulation of stress granules in virus systems. Trends Microbiol. 2012;20(4):175–183. doi: 10.1016/j.tim.2012.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.Y., Ke T.Y., Liao W.Y., Chang N.Y. Regulation of coronaviral poly(A) tail length during infection. PLoS one. 2013;8(7):e70548. doi: 10.1371/journal.pone.0070548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Rijnbrand R., McKnight K.L., Wimmer E., Paul A., Martin A., Lemon S.M. Sequence requirements for viral RNA replication and VPg uridylylation directed by the internal cis-acting replication element (cre) of human rhinovirus type 14. J. Virol. 2002;76(15):7485–7494. doi: 10.1128/JVI.76.15.7485-7494.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Yi M., Evans D.J., Simmonds P., Lemon S.M. Identification of a conserved RNA replication element (cre) within the 3Dpol-coding sequence of hepatoviruses. J. Virol. 2008;82(20):10118–10128. doi: 10.1128/JVI.00787-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng H., Lee H.A., Palese P., Garcia-Sastre A. Influenza A virus RNA polymerase has the ability to stutter at the polyadenylation site of a viral RNA template during RNA replication. J. Virol. 1999;73(6):5240–5243. doi: 10.1128/jvi.73.6.5240-5243.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]


