Abstract
Caffeine inhibits the G2 checkpoint activated by DNA damage and enhances the toxicity of DNA-damaging agents towards p53-defective cancer cells. The relationship between structure and G2 checkpoint inhibition was determined for 56 caffeine analogs. Replacement of the methyl group at position 3 or 7 resulted in loss of activity, while replacement at position 1 by ethyl or propyl increased activity slightly. 8-Substituted caffeines retained activity, but were relatively insoluble. The structure-activity profile did not resemble those for other known pharmacological activities of caffeine. The active analogs also potentiated the killing of p53-defective cells by ionizing radiation, but none was as effective as caffeine.
Keywords: caffeine, cell cycle checkpoint, DNA damage, radio-sensitization, structure-activity
Introduction
Caffeine is a well-known inhibitor of the G2 checkpoint (1–3). The G2 checkpoint is a biochemical mechanism activated by DNA damage during the G2 phase of the cell cycle. It temporarily delays entry into mitosis to allow time for DNA repair and prevent the propagation of genetic abnormalities (4,5). Caffeine can prevent cells from arresting in G2 and it can override G2 arrest (1). A DNA damage checkpoint also operates in the G1 phase of the cell cycle. The G1 checkpoint delays entry into S phase to permit DNA repair and prevent replication of damaged DNA (6). Over 60% of human tumors are defective for the tumor suppressor protein p53 (7), a protein essential for the G1 checkpoint. Unlike normal cells, p53 cells are unable to arrest in G1 after DNA damage. However, their G2 checkpoint, although impaired (8–10), still provides them with an opportunity to repair DNA damage and to continue dividing.
DNA-damaging agents constitute the major class of treatment for cancer. Considerable interest has arisen in the possible use of G2 checkpoint inhibitors as a means of enhancing the sensitivity of p53− tumor cells to DNA-damaging treatments (11–14). The inhibitors should have little effect on the survival of normal cells, since such cells can activate the G1 checkpoint and the G2 checkpoint is strong. However, the inhibitors should increase the killing of p53− cancer cells, since they will now proceed into mitosis with excessive DNA damage, a lethal event.
The potential of this approach has been tested on paired cell lines differing only in their p53 status, using caffeine or the caffeine analog pentoxifylline in combination with either ionizing radiation or cisplatin. In all cases, there was enhanced killing of p53− cells over p53+ cells (15–19). Caffeine, however, is not suitable for clinical use because it has many other pharmacological activities, including toxicity at the concentration required to inhibit the checkpoint (20).
As part of a search for more suitable G2 checkpoint inhibitors, we have examined the activity of 56 analogs of caffeine. We discuss the relationship between the structure of the analogs, activity as G2 checkpoint inhibitors, other pharmacological activities, and ability to selectively potentiate killing of p53− cells by ionizing radiation.
Materials and methods
Caffeine derivatives
8-Cyclopentyl-l-propylxanthine (21) was alkylated at the 3- and 7-positions using methyl iodide and potassium carbonate in dimethylformamide at room temperature to afford 8-cyclopentyl caffeine in 86% yield after purification by preparative thin-layer chromatography. The mass spectrum, proton nuclear magnetic resonance spectrum and analytical data confirmed the structure. 1-Allyl-3,7-dimethyl-8-phenylxanthine (22) was hydrogenated over 10% Pd-C in a mixture of dimethylformamide:ethanol (1:2). The reaction was complete in 20 min to provide 3,7-dimethyl-8-phenyl-l-propylxanthine in 89% yield. The mass spectrum, proton nuclear magnetic resonance spectrum and analytical data confirmed the structure. The remaining caffeine derivatives, listed in Tables I–III, were synthesised as described previously (21–26) or were from standard commercial sources.
Table I.
Effect of single structural alterations of caffeine on G2 checkpoint inhibition, solubility and cytotoxicity.
| Compound number |
Structural alteration | G2 checkpoint inhibition (mM)a |
Solubilityb | Cytotoxicity IC50 (mM)c |
|---|---|---|---|---|
| 1 | 1-CH3 (caffeine) | 0.6 | ++ | >2 |
| 2 | 1-H (theobromine) | Inactive | + | 2 |
| 3 | 1-CH2CH3 | 0.4 | ++ | >2 |
| 4 | 1-CH2CH2CH3 | 0.2 | ++ | >2 |
| 5 | 1-CH2CH2CH2CH2C(O)CH3(pentoxifylline) | 0.8 | ++ | >2 |
| 6 | 1-CH2OCH3 | 2 | ++ | >2 |
| 7 | 1-CH2CH=CH2 | >2 | ++ | >2 |
| 8 | 1-CH2C≡CH | Inactive | ++ | >2 |
| 9 | 1-CH2CN | Inactive | ++ | >2 |
| 10 | 1-CH2C(O)OCH2CH3 | Inactive | ++ | >2 |
| 11 | 1-Benzyl | Inactive | ++ | >2 |
| 12 | 3-H (paraxanthine) | Inactive | ++ | >2 |
| 13 | 3-CH2C≡CH | Inactive | ++ | >2 |
| 14 | 3-CH2CH2CH3 | Inactive | ++ | >2 |
| 15 | 7-H (theophylline) | >2 | ++ | >2 |
| 16 | 7-CH2CH=CH2 | Inactive | − | >2 |
| 17 | 7-CH2C≡CH | Inactive | + | >2 |
| 18 | 7-CH2CH2CH3 | >2 | ++ | >2 |
| 19 | 7-CH2CH2Cl | Inactive | ++ | >2 |
| 20 | 7-CH2CH2OH | Inactive | ++ | >2 |
| 21 | 7-CH2CN | Inactive | ++ | >2 |
| 22 | 7-Benzyl | Inactive | + | 0.4 |
| 23 | 8-Phenyl | 0.7 | − | >2 |
| 24 | 8-Cyclohexyl | 0.2 | − | 0.7 |
| 25 | 8-CH3 | 0.7 | ++ | 2 |
| 26 | 8-CF3 | Inactive | − | >2 |
| 27 | 8-Cyclobutyl | 0.7 | + | 0.5 |
| 28 | 8-Br | 0.2 | + | 1 |
| 29 | 8-Chlorostyryl | Inactive | − | 0.05 |
Concentration required to achieve 50% of the maximal G2 checkpoint inhibition elicited by caffeine.
++, no precipitates observed at all on concentrations tested; +, precipitates observed at concentrations above 0.5 mM; −, precipitates observed at concentrations below 0.5 mM.
Determined by MTT assay as described in Materials and methods.
Table III.
Effect of multiple structural alterations of caffeine on G2 checkpoint inhibition, solubility and cytotoxicity.
| Compound number |
Structural alterations | G2 checkpoint inhibition (mM)a |
Solubilityb | Cytotoxicity IC50 (mM)c |
|||
|---|---|---|---|---|---|---|---|
| 1 | 1-CH3 | 3-CH3 | 7-CH3 (caffeine) | 0.6 | ++ | >2 | |
| 46 | 1-H | 3-CH2CH2CH3 | 7-H (enprofylline) | Inactive | ++ | >2 | |
| 47 | 1-CH2CH2CH3 | 3-H | 7-H | + | 1 | ||
| 48 | 1-CH2CH2CH3 | 3-CH2CH2CH3 | 7-H | Inactive | + | 0.5 | |
| 49 | 1-CH2CH2CH3 | 3-CH2CH2CH3 | 7-CH2CH=CH2 | Inactive | ++ | 0.5 | |
| 50 | 1-H | 3-CH2CH=CH2 | 7-CH2CH=CH2 | Inactive | + | 1 | |
| 51 | 1-CH2C≡CH | 3-CH2C≡CH | 7-CH2C≡CH | Inactive | − | 2 | |
| 52 | 1-CH2CHCH(CH3)2 | 3-CH2CH(CH3)2 | 7-H | Inactive | − | >2 | |
| 53 | 1-CH2CH2CH3 | 3-CH2CH2CH3 | 8-cyclopentyl | Inactive | − | 0.05 | |
| 54 | 1-CH2CH2CH3 | 3-CH2CH2CH3 | 8-cyclohexyl | Inactive | − | 0.2 | |
| 55 | 1-CH2CH2CH3 | 3-H | 7-H | 8-cyclopentyl | Inactive | − | >2 |
| 56 | 1-CH2CH2CH3 | 3-CH2CH2CH3 | 7-H | 8-cyclohexyl | Inactive | − | 0.02 |
| 57 | 1-CH2CH2CH3 | 3-CH2CH2CH3 | 7-H | 8-cyclopentyl | Inactive | − | 0.02 |
Concentration required to achieve 50% of the maximal G2 checkpoint inhibition elicited by caffeine.
++, no precipitates observed at all concentrations tested; +, precipitates observed at concentrations above 0.5 mM; −, precipitates observed at concentrations below 0.5 mM.
Determined by MTT assay as described in Materials and methods.
G2 checkpoint inhibition assay
G2 checkpoint inhibition was determined using a cell-based assay described in detail in ref. 14. Briefly, human mammary carcinoma MCF-7 p53− cells plated in 96-well tissue culture plates were irradiated with 6.5 Gy from a 60Co source and were treated with caffeine analogs 16 h after irradiation for 8 h in the presence of 100 ng/ml nocodazole, and G2 checkpoint inhibition was determined by ELISA using the TG3 antibody, which measures mitotic cells-Results obtained by ELISA were confirmed periodically by counting mitotic cells using fluorescence microscopy (27).
Cytotoxicity assay
Cells were seeded at 500 per well in 96-well plates, grown overnight, and then irradiated with 6 Gy. Other cells were not irradiated and served as controls. Caffeine analogs were added immediately after irradiation and the cells were incubated for 20 h. The analogs were removed and the cells were allowed to grow in fresh medium until cells not treated with caffeine analogs had approached confluence. This was usually after three days for unirradiated cells and five days for cells irradiated with 6 Gy. Cell survival was measured using a soluble tetrazolium salt assay (Cell-Titer96™; Promega).
Results
We recently developed a cell-based assay for G2 checkpoint inhibition using human breast carcinoma MCF-7 cells lacking p53 function (14). Exposure of cells to ionizing radiation causes them to arrest in G2. Subsequent treatment with a G2 checkpoint inhibitor causes the cells to escape G2 arrest, and addition of nocodazole traps them in mitosis. Mitotic cells are then measured by ELISA using the TG-3 antibody, which recognizes a phosphorylated form of nucleolin present only in mitotic cells.
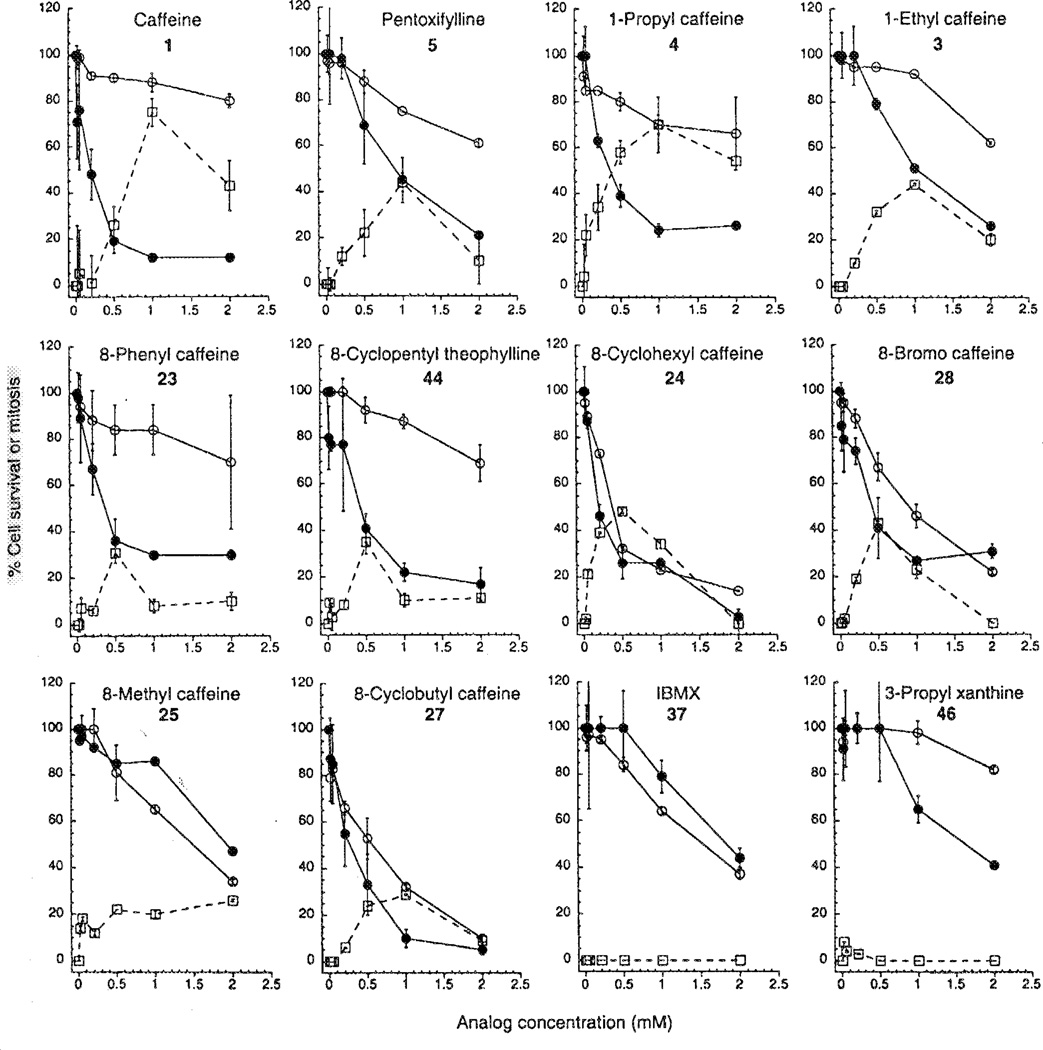
Caffeine (Fig. 1) showed dose-dependent G2 checkpoint inhibition with half-maximal activity (IC50) at 0.6 mM (Table I) and maximal activity at 1 mM (Fig. 2). 1 mM caffeine caused 75% of cells to overcome G2 arrest and enter mitosis (Fig. 2). At higher concentrations caffeine showed less activity (Fig. 2). This narrow active range is often seen with G2 checkpoint inhibitors (14) and is thought to be due to high concentrations inhibiting kinases or phosphatases that are required for entry into mitosis.
Figure 1.
Structural formula of caffeine.
Figure 2.
The G2 checkpoint inhibition and potentiation of γ-irradiation-induced cytotoxicity by caffeine and eleven analogs. The G2 checkpoint inhibition (□) is expressed as the % G2-arrested cells induced to enter mitosis, as described in Materials and methods. The cytotoxicity of the compounds without γ-irradiation (○) is expressed as % cell survival compared with untreated controls. The cytotoxicity with γ-irradiation (●) is expressed as % cell survival compared with cells treated with 6 Gy irradiation alone. The 6 Gy irradiation induced about 80% cell death.
Caffeine alone showed little toxicity towards MCF-7 p53− cells, as shown by the fairly flat curve of % survival versus caffeine concentration (Fig. 2). However, caffeine strongly enhanced cell killing by γ-irradiation, as shown by the greatly increased slope of the curve in cells exposed to 6 Gy of irradiation (Fig. 2). This phenomenon has been described previously (15–19).
Fifty-six analogs of caffeine were tested in the G2 checkpoint inhibition assay. Those showing more activity than caffeine were then tested for their cytotoxicity and for their ability to enhance cell killing by γ-irradiation.
Single structural alterations of caffeine
The activity of the caffeine analogs with only one structural modification, namely replacement of the methyl at position 1, 3 or 7 or substitution at the 8-position are presented in Table I. Pentoxifylline (5) is a caffeine analog with a 1-hexanonyl substituent, previously shown to display G2 checkpoint inhibition (15,17). Pentoxifylline showed half-maximal activity at 0.8 mM and maximal activity at 1 mM (Fig. 2, Table I). However, it was less efficacious than caffeine (Fig. 2). causing only 45% of cells to enter mitosis at its optimal concentration. Pentoxifylline was slightly more toxic than caffeine on its own (Fig. 2). It also potentiated killing of p53− MCF-7 cells, but more weakly than caffeine (Fig. 2, Table I).
The activities of analogs with other substituents at position 1 are shown in Table I. Replacement of the 1-methyl group of caffeine with H, as in theobromine (2), resulted in complete loss of activity. A small increase in the size of the 1-substituent resulted in a slight increase in activity, with IC50 of 0.4 mM for 1-ethyl (3) and 0.2 mM for 1-propyl (4). Increasing the size or polarity of the 1-substituent further caused a decrease in activity, with IC50 of 0.8 mM for pentoxifylline (5) and 2 mM for 1-methoxymethyl (6) or eliminated it completely as with 1-allyl (7), 1-propargyl (8), 1-methylcyano (9), 1-ethylcarboxymethyl (10) or 1-benzyl (11). However, lost, of activity may simply result from insolubility of the compound in cell culture medium. Therefore, the solubility of the caffeine analogs in culture medium was also determined by monitoring the presence of precipitates during incubation, using a microscope with 40-fold magnification. All the 1-substituted caffeine analogs were completely soluble at concentrations ≤2 mM except for theobromine, which precipitated at concentrations >0.5 mM (Table I).
The results indicate that an alkyl substituent is required at the 1-position to inhibit the G2 checkpoint, with caffeine being slightly less potent than the 1-ethyl (3) and 1-propyl (4) analogs (IC50 of 0.6 mM, 0.4 mM and 0.3 mM respectively), but more efficaceous, producing 75% mitotic cells compared with 70% (3) and 42% (4) (Fig. 2). The cytotoxicity of the 1-ethyl (3) and 1-propyl (4) analogs was determined. When used alone, they were more cytotoxic than caffeine and comparable to pentoxifylline (Fig. 2). In combination with ionizing radiation, the 1-ethyl analog showed more enhancement of killing than the 1-propyl analog (Fig. 2). However, neither enhanced killing as much as caffeine.
Replacement of the methyl group at the 3-position of caffeine with H (paraxanthine, 12), 3-propyl (14) or 3-propargyl (13) resulted in inactive compounds (Table I). Similarly, replacement of the methyl group at the 7-position of caffeine with other moieties either resulted in an inactive compound, or in the case of replacement with H (theophylline, 15) or 7-propyl (18), in reduced activity.
Replacement of H at position 8 of caffeine with 8-methyl (25), 8-phenyl (23), or 8-cyclobutyI (27) had no significant effect on activity relative to caffeine (Table I). Replacement with 8-bromine (28) or 8-cyclohexyl (24) slightly increased activity, whereas replacement with 8-trifluoromethyl (26) or 8-chlorostyryl (29) caused loss of activity (Table I). The 8-phenyl analog (23) showed activity at 0.5 mM, but not at higher concentrations (Fig. 2), probably because of very poor solubility. Similarly, 8-substituted analogs 24, 27 and 28 showed reduced activity at high concentrations (Fig. 2). The 8-methyl analog (25) was soluble, but showed only moderate G2 checkpoint inhibition (Fig. 2). The 8-phenyl analog (23) showed moderate cytotoxicity alone and significant enhancement of killing by ionizing radiation (Fig. 2). Analogs 24, 25, 27 and 28 showed significant toxicity alone and little or no enhancement of killing by ionizing radiation, as shown by the similarity of the curves (Fig. 2).
Collectively, the results indicate that the methyl groups at positions 1, 3 and 7 are important for G2 checkpoint inhibition by caffeine, but that slightly bulkier groups can be accommodated at position 1, but not at positions 3 and 7. Addition of a variety of groups at position 8 is tolerated and may even enhance activity. However, poor solubility was a problem for nearly all 8-substituted caffeines.
Multiple structural alterations of caffeine
We next determined the combined effects of two structural alterations on the activity of caffeine. All analogs except one were inactive as G2 checkpoint inhibitors (Table II). The sole exception was analog 44 in which the 7-methyl of caffeine was replaced with H and the 8-H with cyclopentyl. This analog 44 had G2 checkpoint activity, low intrinsic cytotoxicity, and caused significant enhancement of killing by ionizing radiation (Fig. 2). However, the compound was poorly soluble and showed weak G2 checkpoint inhibition at high concentrations, probably as a result of poor solubility (Fig. 2, Table II). Analog 37, 3-isobutyl-l-methylxanthine (IBMX), showed no enhancement of cytotoxicity by ionizing radiation (Fig. 2), but was cytotoxic alone (Table II).
Table II.
Effect of two structural alterations of caffeine on G2 checkpoint inhibition, solubility and cytotoxicity.
| Compound number |
Structural alterations | G2 checkpoint inhibition (mM)a |
Solubilityb | Cytotoxicity IC50 (mM)c |
|
|---|---|---|---|---|---|
| 1 | 1-CH3 | 3-CH3 (caffeine) | 0.6 | ++ | >2 |
| 30 | 1-CH2CH3 | 3-CH2CH3 | Inactive | ++ | 2 |
| 31 | 1-CH2C≡CH | 3-H | Inactive | ++ | >2 |
| 32 | 1-H | 3-H | Inactive | + | >2 |
| 33 | 1-CH2CH=CH2 | 3-CH2CH=CH2 | Inactive | ++ | >2 |
| 34 | 1-CH2C≡CH | 7-CH2C≡CH | Inactive | − | 1 |
| 35 | 1-CH2CH2CH3 | 8-phenyl | Inactive | − | 0.2 |
| 36 | 1-CH2CH2CH3 | 8-cyclopentyl | Inactive | + | 0.2 |
| 37 | 3-CH2CH(CH3)2 | 7-H (IBMX) | Inactive | ++ | 1 |
| 38 | 3-CH2CH(CH3)2 | 7-CH2C≡CH | Inactive | ++ | 2 |
| 39 | 3-CH2CH2CH3 | 7-CH2CH2CH3 | Inactive | ++ | 1 |
| 40 | 3-CH2C≡CH | 7-CH2C≡CH | Inactive | + | 2 |
| 41 | 3-H | 7-H | Inactive | + | >2 |
| 42 | 3-CH2CHCH3 | 7-H | Inactive | ++ | >2 |
| 43 | 7-H | 8-CF3 | Inactive | − | >2 |
| 44 | 7-H | 8-cyclopentyl | 0.6 | − | 2 |
| 45 | 7-H | 9-CH3 (isocaffeine) | Inactive | ++ | >2 |
Concentration required to achieve 50% of the maximal G2 checkpoint inhibition elicited by caffeine.
++, no precipitates observed at all on concentrations tested; +, precipitates observed at concentrations above 0.5 mM; −, precipitates observed at concentrations below 0.5 mM.
Determined by MTT assay as described in Materials and methods.
None of the caffeine analogs with multiple structural alterations showed any activity in the assay (Table III). Most showed low solubility and high intrinsic cytotoxicity. Analog 46 (enprofylline) showed some enhancement of cytotoxicity by ionizing radiation (Fig. 2).
Discussion
Caffeine was recognized as a G2 checkpoint inhibitor as early as 1974 (1). The ability of caffeine to induce damaged cells to undergo mitosis before properly repairing lesions in their DNA was proposed as the basis for its well-documented ability to potentiate the lethality of DNA-damaging agents (28). Subsequently two caffeine analogs have also been found to be significant G2 checkpoint inhibitors: pentoxifylline (3,7-dimethyl-l-(5-oxohexyl)xanthine) (29) and its hydroxy metabolite lisofylline (3,7-dimethyl-l-(5-R-hydroxyhexyl)xanthine) (17). We have carried out an extensive comparison of 56 caffeine analogs for their ability to inhibit the G2 checkpoint and to enhance the toxicity of DNA damage in p53− MCF-7 cells. Nine caffeine analogs showed G2 checkpoint inhibition with IC50 values less than 2 mM. Three of the active compounds were altered in the 1-position of caffeine, namely the 1-ethyl (3), the 1-propyl (4) and the 1-hexanonyl (pentoxifylline, 5) analogs. The 1-propyl analog (4) showed half maximal activity at 0.5 mM compared with caffeine at 0.6 mM and showed maximal activity at 1 mM, as did caffeine. Both caffeine and 4 caused 70–75% of the cells to escape G2 arrest. The other six active compounds were 8-substituted caffeines (23, 24, 25, 27 and 28) and an 8-substituted theophylline (44). All six of these 8-substituted analogs had poor solubility. None of the other active analogs caused as much escape at the optimal concentration as did caffeine and the 1-propyl analog (4).
The analogs showing significant G2 checkpoint inhibition and low intrinsic cytotoxicity, namely caffeine, the 1-ethyl- (3), 1-propyl (4) and 1-hexanonyl (pentoxyfylline, 5), 8-phenyl-caffeine (23) and 8-cyclopentyltheophylline (44) all potentiated killing of cells by ionizing radiation. Other analogs (24, 25, 27 and 28) that showed G2 checkpoint activity either showed little or no potentiation with ionizing radiation, possibly because of their high intrinsic cytotoxicity. One analog (46) that had no G2 checkpoint activity did not potentiate killing by ionizing radiation, while another analog (37, IBMX) with no activity did show some enhancement. The relatively good correlation between G2 checkpoint inhibition and potentiation of killing by ionizing radiation is consistent with checkpoint inhibition playing a role in the enhancement.
Caffeine and caffeine analogs have many pharmacological activities, including competitive antagonism at adenosine receptors (22–24), inhibition of phosphodiesterases (30), release of calcium from intracellular stores (31,32), tracheal relaxation (33) and inhibition of the cystic fibrosis chloride channels (34). It is clear that the present structure-activity profile for G2 checkpoint inhibition by caffeine analogs does not correlate with their profile of activity at any of these other targets. For example, replacement of methyl with larger substituents at positions 1, 3 and 7 increases potency as adenosine receptor antagonists (24) but eliminates G2 checkpoint inhibition. Compound 37 is a potent phosphodiesterase inhibitor (30) and compound 51 is a strong tracheal relaxant (33) but both are inactive as checkpoint inhibitors. Analog 8 is inactive as a calcium release agent (31) but it is more potent than caffeine as a checkpoint inhibitor. The results indicate that caffeine overcomes DNA damage-induced G2 arrest by a mechanism distinct from any yet described for alkylxanthines.
Entry into mitosis is controlled by Cdc2 kinase, which is composed of the p34cdc2 catalytic subunit and a cyclin regulatory subunit. Cdc2 is maintained in an inactive state during G2 phase by inhibitory phosphorylation of the catalytic subunit and is activated at the onset of mitosis by dephosphorylation by Cdc25 phosphatase (6). The activity of Cdc25 is controlled in part by the Chk2 kinase, which is itself phosphorylated and activated after DNA damage in an ATM kinase-dependent manner (35,36). Caffeine treatment inhibits the radiation-induced activation of Chk2 (37), and causes activation of Cdc25 and Cdc2, but does not affect them directly. It does however inhibit the kinase activity of immunoprecipitated ATM, suggesting it inhibits ATM directly (37,38).
It is remarkable that inhibition of the G2 checkpoint by caffeine shows such severe structural constraints, with only alterations in the substituent at the 1-position and substitution at the 8-position being tolerated. Furthermore, combined alteration at both the 1- and 8-position does not appear to be tolerated. 8-Phenylcaffeine (23) and 8-cyclohexylcaffeine (24) retain activity but the corresponding 8-phenyl-l-propyl analog (35) and the 8-cyclopentyl-l-propyl analog (36) are inactive. It would be interesting to determine whether the structure-activity requirement for ATM kinase inhibition is similar to that for G2 checkpoint inhibition.
We show a generally good correlation between G2 checkpoint inhibition by caffeine analogs and their synergistic cytotoxic effects with ionizing radiation on p53− cells. However, at least one caffeine analog, compound 46, enhanced the cytotoxicity without inhibiting the checkpoint, raising the possibility that enhancement of cytotoxicity by caffeine may result from more than just G2 checkpoint inhibition. Indeed, it has been proposed that the effects of caffeine may also be due to inhibition of DNA repair (39). With regard to therapeutic potential, none of the analogs tested in the present study, showed a marked increase in activity. Solubility was a problem for many, and the concentrations required would seem to preclude in vivo use because of undesirable side effects. However, the observation that relatively large substitutions at position 1 are tolerated suggests that further alterations at that position may yield more selective or potent G2 checkpoint inhibitors.
Acknowledgments
This research was supported by a grant from the Canadian Breast Cancer Research Initiative to M.R.
References
- 1.Walters RA, Gurley LR, Tobey RA. Effects of caffeine on radiation-induced phenomena associated with cell cycle traverse of mammalian cells. Biophys J. 1974;14:99–118. doi: 10.1016/S0006-3495(74)70002-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rowley R, Zorch M, Leeper DB. Effect of caffeine on radiation-induced mitotic delay: delayed expression of G2 arrest. Radiat Res. 1984;97:178–185. [PubMed] [Google Scholar]
- 3.Zampetti-Bosseler F, Scott D. The effect of caffeine on X-ray induced mitotic delay in normal and ataxia-telangiectasia fibroblasts. Mutat Res. 1985;143:251–256. doi: 10.1016/0165-7992(85)90089-2. [DOI] [PubMed] [Google Scholar]
- 4.Hartwell L, Weinert T, Kadyk L, Garvik B. Cell cycle checkpoints, genomic integrity, and cancer. Cold Spring Harbor Symp Quant Biol. 1994;59:259–263. doi: 10.1101/sqb.1994.059.01.030. [DOI] [PubMed] [Google Scholar]
- 5.Kaufmann WK, Paules RS. DNA damage and cell cycle checkpoints. FASEB J. 1996;10:238–247. doi: 10.1096/fasebj.10.2.8641557. [DOI] [PubMed] [Google Scholar]
- 6.Elledge SJ. Cell cycle checkpoints: preventing an identity crisis. Science. 1996;274:1664–1672. doi: 10.1126/science.274.5293.1664. [DOI] [PubMed] [Google Scholar]
- 7.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Mutations in the p53 tumor suppressor gene: clues to cancer etiology and molecular pathogenesis. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 8.Bunz F, Dutriaux A, Lengauer C, Waldman T, Zhou S, Brown JP, Sedivy JM, Kinzler KW, Vogelstein B. Requirement for p53 and p21 to sustain G2 arrest after DNA damage. Science. 1998;282:1497–1501. doi: 10.1126/science.282.5393.1497. [DOI] [PubMed] [Google Scholar]
- 9.Innocente SA, Abrahamson JL, Cogswell JP, Lee JM. p53 regulates a G2 checkpoint through cyclin B1. Proc Natl Acad Sci USA. 1999;96:2147–2152. doi: 10.1073/pnas.96.5.2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schwartz D, Almog N, Peled A, Goldfinger N, Rotter V. Role of wild type p53 in the G2 phase: regulation of the gamma-irradiation-induced delay and DNA repair. Oncogene. 1997;15:2597–2607. doi: 10.1038/sj.onc.1201436. [DOI] [PubMed] [Google Scholar]
- 11.Murray AW. Creative blocks: cell-cycle checkpoints and feedback controls. Nature. 1992;359:599–604. doi: 10.1038/359599a0. [DOI] [PubMed] [Google Scholar]
- 12.Weinert T, Lydall D. Cell cycle checkpoints, genetic instability and cancer. Semin Cancer Biol. 1993;4:129–140. [PubMed] [Google Scholar]
- 13.Nurse P. Checkpoint pathways come of age. Cell. 1997;91:865–867. doi: 10.1016/s0092-8674(00)80476-6. [DOI] [PubMed] [Google Scholar]
- 14.Roberge M, Berlinck RGS, Xu L, Anderson HJ, Lim LY, Curman D, Stringer CM, Friend SH, Davies P, Haggarty SJ, Kelly MT, Britton R, Piers E, Andersen RJ. High-throughput assay for G2 checkpoint inhibitors and identification of the structurally novel compound isogranulatimide. Cancer Res. 1998;58:5701–5706. [PubMed] [Google Scholar]
- 15.Fan S, Smith ML, Rivet DJ, II, Duba D, Zhan Q, Kohn KW, Fornace AJ, Jr, O'Connor PM. Disruption of p53 function sensitizes breast cancer MCF-7 cells to cisplatin and pentoxifylline. Cancer Res. 1995;55:1649–1654. [PubMed] [Google Scholar]
- 16.Powell SN, De Frank JS, Connell P, Eogan M, Preffer F, Dombkowski D, Tang W, Friend S. Differential sensitivity of p53− and p53+ cells to caffeine-induced radiosensitization and override of G2 delay. Cancer Res. 1995;55:1643–1648. [PubMed] [Google Scholar]
- 17.Russell KJ, Wiens LW, Demers GW, Galloway DA, Le T, Rice GC, Bianco JA, Singer JW, Groudine M. Preferential radiosensitization of G1 checkpoint-deficient cells by methylxanthines. Int J Radiat Oncol Biol Phys. 1996;36:1099–1106. doi: 10.1016/s0360-3016(96)00432-4. [DOI] [PubMed] [Google Scholar]
- 18.Yao S-L, Akhtar AJ, McKenna KA, Bedi GC, Sidransky D, Mabry M, Ravi SJ, Collector MI, Jones RJ, Sharkis SJ, Fuchs EJ, Bedi A. Selective radiosensitization of p53-deficient cells by caffeine-mediated activation of p34cdc2 kinase. Nat Med. 1996;2:1140–1143. doi: 10.1038/nm1096-1140. [DOI] [PubMed] [Google Scholar]
- 19.Bracey TS, Williams AC, Paraskeva C. Inhibition of radiation-induced G2 delay potentiates cell death by apoptosis and/or the induction of giant cells in colorectal tumor cells with disrupted p53 function. Clin Cancer Res. 1997;3:1371–1381. [PubMed] [Google Scholar]
- 20.Arnaud MJ. The pharmacology of caffeine. Prog Drug Res. 1987;31:273–313. doi: 10.1007/978-3-0348-9289-6_9. [DOI] [PubMed] [Google Scholar]
- 21.Muller CE, Shi D, Manning M, Jr, Daly J. Synthesis of paraxanthine analogs (1,7-disubstituted xanthines) and other xanthines unsubstituted at the 3-position: structure-activity relationships at adenosine receptors. J Med Chem. 1993;36:3341–3349. doi: 10.1021/jm00074a015. [DOI] [PubMed] [Google Scholar]
- 22.Daly JW, Padgett WL, Shamim MT. Analogs of caffeine and theophylline: effect of structural alterations on affinity at adenosine receptors. J Med Chem. 1986;29:1305–1308. doi: 10.1021/jm00157a035. [DOI] [PubMed] [Google Scholar]
- 23.Shamim MT, Ukena D, Padgett WL, Daly JW. Effects of 8-phenyl and 8-cycloalkyl substituents on the activity of mono-, di-, and trisubstituted alkylxanthines with substitution at the 1-, 3-, and 7-positions. J Med Chem. 1989;32:1231–1237. doi: 10.1021/jm00126a014. [DOI] [PubMed] [Google Scholar]
- 24.Daly JW, Hide I, Muller CE, Shamim M. Caffeine analogs: structure-activity relationships at adenosine receptors. Pharmacology. 1991;42:309–321. doi: 10.1159/000138813. [DOI] [PubMed] [Google Scholar]
- 25.Jacobson KA, Gallo-Rodriguez C, Melman N, Fischer B, Maillard M, van Bergen A, van Galen PJM, Karton Y. Structure-activity relationships of 8-styrylxanthines as A2-seleclive adenosine antagonists. J Med Chem. 1993;36:1332–1342. doi: 10.1021/jm00062a005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobson KA, Shi D, Gallo-Rodriguez C, Manning M, Muller C, Daly JW, Neumeyer JL, Kiriasis L, Pfleiderer W. Effect of trifluoromethyl - and other substituents on activity of xanthines at adenosine receptors. J Med Chem. 1993;36:2639–2644. doi: 10.1021/jm00070a007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo XW, Th'ng JP, Swank RA, Anderson HJ, Tudan C, Bradbury EM, Roberge M. Chromosome condensation induced by fostriecin does not require p34cdc2 kinase activity and histone H1 hyperphosphorylation, but is associated with enhanced histone H2A and H3 phosphorylation. EMBO J. 1995;14:976–985. doi: 10.1002/j.1460-2075.1995.tb07078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lau CC, Pardee AB. Mechanism by which caffeine potentiates the lethality of nitrogen mustard. Proc Natl Acad Sci USA. 1982;79:2942–2946. doi: 10.1073/pnas.79.9.2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fingert HJ, Chang JD, Pardee AB. Cytotoxic, cell cycle, and chromosomal effects of methylxanthines in human tumor cells treated with alkylating agents. Cancer Res. 1986;46:2463–2467. [PubMed] [Google Scholar]
- 30.Choi OH, Shamim MT, Padgett WL, Daly JW. Caffeine and theophylline analogues: correlation of behavioral effects with activity as adenosine receptor antagonists and as phosphodiesterase inhibitors. Life Sci. 1988;43:387–398. doi: 10.1016/0024-3205(88)90517-6. [DOI] [PubMed] [Google Scholar]
- 31.Muller CE, Daly JW. Stimulation of calcium release by caffeine analogs in pheochromocytoma cells. Biochem Pharmacol. 1993;46:1825–1829. doi: 10.1016/0006-2952(93)90589-o. [DOI] [PubMed] [Google Scholar]
- 32.Liu W, Mcissner G. Structure-activity relationship of xanthines and skeletal muscle ryanodine receptor/Ca2+ release channels. Pharmacology. 1997;54:135–143. doi: 10.1159/000139480. [DOI] [PubMed] [Google Scholar]
- 33.Brackett LE, Shamim MT, Daly JW. Activities of caffeine, theophylline, and enprofylline analogs as tracheal relaxants. Biochem Pharmacol. 1990;39:1897–1904. doi: 10.1016/0006-2952(90)90607-m. [DOI] [PubMed] [Google Scholar]
- 34.Chappe V, Mettey Y, Vierfond JM, Hanrahan JW, Gola M, Verrier B, Becq F. Structural basis for specificity and potency of xanthine derivatives as activators of the CFTR chloride channel. Br J Pharmacol. 1998;123:683–693. doi: 10.1038/sj.bjp.0701648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Matsuoka S, Huang M, Elledge SJ. Linkage of ATM to cell cycle regulation by the Chk2 protein kinase. Science. 1998;282:1893–1897. doi: 10.1126/science.282.5395.1893. [DOI] [PubMed] [Google Scholar]
- 36.Chaturvedi P, Eng WK, Zhu Y, Mattern MR, Mishra R, Hurle MR, Zhang X, Annan RS, Lu Q, Faucette LF, Scott GF, Li X, Carr SA, Johnson RK, Winkler JD, Zhou B-BS. Mammalian Chk2 is a downstream effector of the ATM-dependent DNA damage checkpoint pathway. Oncogene. 1999;18:4047–4054. doi: 10.1038/sj.onc.1202925. [DOI] [PubMed] [Google Scholar]
- 37.Blasina A, Price BD, Turennc GA, McGowan CH. Caffeine inhibits the checkpoint kinase ATM. Curr Biol. 1999;9:1135–1138. doi: 10.1016/s0960-9822(99)80486-2. [DOI] [PubMed] [Google Scholar]
- 38.Sarkaria JN, Busby EC, Tibbetts RS, Roos P, Taya Y, Karnitz LM, Abraham RT. Inhibition of ATM and ATR kinase activities by the radiosensitizing agent, caffeine. Cancer Res. 1999;59:4375–4382. [PubMed] [Google Scholar]
- 39.Musk SRR, Steel GC. Override of the radiation-induced mitotic block in human tumour cells by methylxanthines and its relationship to the potentiation of cytotoxicity. Int J Radiat Biol. 1990;57:1105–1112. doi: 10.1080/09553009014551221. [DOI] [PubMed] [Google Scholar]