Abstract
Amblyopia screening during childhood is critical for early detection and successful treatment. In the current study, we develop and evaluate a screening method that exploits the imbalanced interocular inhibition between amblyopic and fellow eyes. In nineteen subjects with anisometropic amblyopia and twenty-two age-matched subjects with myopia, we measured the area under the contrast sensitivity functions (AUCSFs) in eight monocular conditions defined by tested eye (left, right), patching of the untested eye (translucent, opaque), and refractive status (corrected, uncorrected). For each test eye, we defined the inhibition index as the ratio between AUCSF values obtained in the translucent and opaque patching conditions of the untested eye. To evaluate the screening potential of the inhibition index, we compared results from patients with amblyopia and myopia. With and without optical correction, the index was significantly lower in the amblyopic eye than in the fellow eye of the amblyopic subjects and both eyes of the myopic subjects. No significant difference was found among the two eyes of the myopic subjects and the fellow eyes of the amblyopic subjects. With the inhibition index as the predictor, a logistic regression model successfully discriminated amblyopic eyes from myopic eyes with 100% accuracy in the uncorrected condition. In the corrected condition, with the inhibition index and interocular visual acuity difference as predictors, amblyopic eyes were likewise discriminated from myopic eyes with 100% accuracy. This pattern of CSF changes, caused by the different patching modes of the untested eye, provides a potential CSF signature to discriminate anisometropic amblyopia from myopia.
Keywords: amblyopia screening, AUCSF, interocular inhibition, interocular difference
1. Introduction
Early detection is critical for successful treatment of amblyopia (“lazy eye”), the most common cause of monocular visual impairment in children and young adults that affects 2–5% of the population (Holmes and Clarke 2006). Earlier detection and correction of the underlying cause (strabismus, refractive error, and/or form deprivation) provides better long-term treatment outcomes (Atkinson, Braddick et al. 1996, Eibschitz-Tsimhoni, Friedman et al. 2000).
A typical amblyopia screening/diagnosis procedure includes assessments of visual acuity, stereo acuity, and presence of amblyopiogenic factors (e.g. strabismus and refractive errors) (Simons 2005, Holmes and Clarke 2006, Wu and Hunter 2006). With no single agreed-upon criterion, one common metric for diagnosing amblyopia is the interocular difference in visual acuity, after exclusion of potential organic defects, refractive error, and strabismus. Recent studies have proposed other potential metrics for diagnosing amblyopia which include interocular differences in contrast sensitivity, in particular the difference in high-cutoff spatial frequency between the eyes (Hess, Wang et al. 1999, Kiorpes, Tang et al. 1999, Hou, Huang et al. 2010). Over the past 20 years, David Hunter and colleagues (Hunter, Nassif et al. 2004, Loudon, Rook et al. 2011) have developed the Pediatric Vision Scanner (PVS), a portable device based on retinal birefringence scanning, to automatically detect strabismus, amblyopia, and other serious eye conditions in children as young as 2 years of age. A recent study showed that 97.3% of children who were given a “pass” result by PVS had normal eyes, while effectively 100% of children receiving a “refer” result had amblyopia, strabismus, or other vision loss that required medical attention (Loudon, Rook et al. 2011). Studies evaluating candidate screening methods for amblyopia have concluded that although tests may differ in terms of screening sensitivity and specificity, the estimated likelihood of amblyopia occurrence was comparable among the tests (Schmidt, Maguire et al. 2004, Group 2005), and combining different tests could improve diagnostic accuracy (Chou, Dana et al. 2011).
Although amblyopia screening can be performed in schools, by pediatricians or eye care specialists, automated photo-screening programs usually require specialized instruments and specific expertise (Ehrlich, Reinecke et al. 1983, Simons 2005). For example, the most common screening procedure assesses visual acuity differences in the potential amblyopic eye between conditions with and without (naked eye) refractive correction. The procedures mandate good compliance, proper eye alignment and refractive error measurements, and accurate prescription of eye glasses (Ingram 1977, Castanes 2002). The screening process can be expensive and inefficient, and is not always easily accessible (Sjöstrand and Abrahamsson 1996, Carlton, Karnon et al. 2008). There remains an important need for efficient, easy-to-perform, affordable, and reliable screening methods.
In the current study, we tested the efficacy of a simple and effective interocular inhibition procedure (IIP), for discriminating anisometropic amblyopia from myopia, which are commonly confused in visual examination without proper optical correction. Using the binocular combination paradigm (cite Ding and Sperling), we have demonstrated that deficient binocular vision in anisometropic amblyopia resulted from a combination of monocular signal attenuation and stronger inhibition from the fellow eye to the amblyopic eye (Huang, Zhou et al. 2011) (Huang, Zhou et al. 2010)(+our earlier paper). The IIP is based on the observation of the stronger inhibition of the fellow eye on the amblyopic eye in anisometropic amblyopia (Baker, Meese et al. 2008, Huang, Zhou et al. 2011) and the approximately balanced inhibition between the eyes in normal and myopic vision (Huang, Baker et al. 2012, Zhou, McNeal et al. 2014). We hypothesized that opaque and translucent patching over the fellow eye would result in different contrast sensitivities in the amblyopic eye, while different patching over the amblyopic eye would not change the contrast sensitivity of the fellow eye. For normal and myopic subjects, patching with opaque and translucent materials over one eye would not change the contrast sensitivity of the other eye. Specifically, if one eye is stronger than the other, patching the stronger eye with an opaque material will lead to better function in the weaker eye compared to patching it with a translucent material because patching with an opaque material can better release the weaker eye from inhibition exerted by the stronger eye.
Lai et al. (Lai, Alexander et al. 2011) evaluated interocular interaction in children with anisometropic amblyopia, anisometropia but no amblyopia, and normal vision. They measured and compared visual acuity, contrast sensitivity (6 c/deg), and alignment acuity (6 c/deg) of the non-dominant eye when the (untested) dominant eye was either fully occluded with an opaque patch or partially occluded with a center dark square (4.62 cd/m2, calculated based on their setup). The authors found that the difference between each measure in the two patching conditions was significantly greater for children with anisometropic amblyopia than the other two groups.
In this study, we focused on comparing contrast sensitivity of the tested eye when the untested eye was patched either with an opaque or a translucent material. The study differs from Lai et al. (2011) in three aspects: (1) we measured the full contrast sensitivity function with the quick CSF procedure (Lesmes, Lu et al. 2010) instead of contrast sensitivity at a single frequency, (2) we compared opaque and translucent patching conditions (0 vs 22.64 cd/m2) while Lai et al. compared opaque and dark patching conditions (0 vs 4.62 cd/m2), and (3) in addition to tests under full optical correction, we included a naked eye condition to test the procedure without optical correction. The procedure would be most valuable when optical correction is not available.
The quick CSF method (Lesmes, Lu et al. 2010) was used to measure contrast sensitivity functions (CSF) in the test eye while the untested eye was covered with opaque or translucent patching. For the test eye, the area under CSF (AUCSF) and cutoff spatial frequencies were obtained for both translucent and opaque patching conditions, and an inhibition index was calculated as the AUCSF ratio between patching conditions. The same procedure was repeated as the patching was switched between the previously tested and untested eyes. We report that changing the patching of the fellow eye drastically changed the CSFs in the amblyopic eye. The same manipulation had no significant impact on the CSFs of the fellow eyes of amblyopic subjects, nor on the CSF of the myopic subjects. The effect provides a simple and efficient method to discriminate anisometropic amblyopia from uncorrected myopia. The IIP may be further developed and tested for early amblyopia screening.
2. Methods
2.1 Subjects
Nineteen adult subjects (mean age: 22.6±0.7 years) with anisometropic amblyopia and twenty-two myopic adults (mean age: 23.1±0.8 years) participated in the study. Detailed characteristics of the subjects, including age, sex, optical correction, and their corrected and uncorrected acuity, are listed in Tables 1 and 2. The myopic subjects were recruited from the Institute of Psychology, Chinese Academy of Sciences, and nearby universities; the amblyopic subjects were referred from local ophthalmology/optometry clinics. All myopic subjects and the fellow eyes of the amblyopic subjects had corrected-to-normal vision. All participants were naive to psychophysical experiments. Written informed consent was obtained from each subject and their guardians/parents after explanation of the nature of the study. The experimental protocol was approved by the IRB of the Institute of Psychology, Chinese Academy of Sciences, and carried out in accordance with the Declaration of Helsinki.
Table 1.
Characteristics of amblyopic participants
| No. | Sex | Age(y) | AE |
FE |
||
|---|---|---|---|---|---|---|
| Correction | Acuity(LogMAR)* | Correction | Acuity(LogMAR)* | |||
| 1 | F | 24 | +0.50DS:+0.25DC×45 | 0.58/0.57 | −9.00DS | 0.66/0.09 |
| 2 | F | 6 | +2.50DS:+1.50DC×80 | 0.34/0.29 | +2.00DS:+0.50DC×83 | 0.18/0.07 |
| 3 | F | 32 | +2.50DS:+1.50DC×30 | Null/0.76 | +0.50DS | Null/−0.02 |
| 4 | F | 18 | −1.00DS:+1.25DC×107 | 0.28/0.28 | −2.75DS:−0.75×158 | 0.68/−0.02 |
| 5 | F | 26 | +3.50DS | 0.58/0.38 | −3.00DS | 0.27/−0.07 |
| 6 | M | 28 | +4.5DS | 0.58/0.48 | −2.75DS | 0.57/0.07 |
| 7 | F | 25 | +2.75DS | 0.07/0.07 | Plano | 0.06/0.06 |
| 8 | M | 31 | +1.00DS:+0.50DC×90 | 0.63/0.55 | −3.5DS:+0.50DC×10 | 0.36/−0.02 |
| 9 | F | 24 | +1.23DS:+1.25DC×90 | 0.38/0.25 | Plano | −0.13/−0.13 |
| 10 | M | 24 | +2.75DS:+1.50DC×105 | 0.73/0.48 | Plano | −0.02/−0.02 |
| 11 | M | 8 | +0.75DS:+1.50DC×70 | Null/0.38 | +1.75DS | Null/−0.13 |
| 12 | M | 17 | +4.37DS:−3.12DC×23 | 0.68/0.73 | +0.87DS:−0.37DC×170 | −0.17/−0.20 |
| 13 | F | 23 | +0.25DS | Null/1.30 | +0.50DC×180 | Null/−0.02 |
| 14 | F | 20 | +5.00DS | 0.38/0.29 | +1.00DS | −0.10/−0.25 |
| 15 | F | 11 | +2.25DS:+1.00DC×78 | 1.10/1.10 | Plano | −0.02/−0.02 |
| 16 | F | 13 | +2.50DS:+1.00DC×115 | 0.18/0.09 | −1.00DS | 0.18/−0.02 |
| 17 | M | 12 | −5.25DS:−0.50DC×44 | 1.05/0.18 | −2.00DS:−0.75DC×7 | 0.45/0.00 |
| 18 | M | 9 | +3.00DS:−5.00DC×175 | 0.07/−0.02 | +1.50DS:−4.00DC×175 | 0.07/−0.02 |
| 19 | F | 23 | +2.00DS:+0.50DC×79 | 0.78/0.28 | Plano | −0.02/−0.02 |
Acuities were arranged as ‘Naked condition/Full corrected condition’.
Table 2.
Characteristics of myopic participants
| No. | Sex | Age(y) | OD |
OS |
||
|---|---|---|---|---|---|---|
| Correction | Acuity(LogMAR) * | Acuity (LogMAR) * | ||||
| 1 | F | 25 | −6.50DS | 0.58/−0.07 | −6.50DS | 0.48/−0.13 |
| 2 | M | 23 | −4.0DS | 0.85/0.00 | −3.0DS | 0.85/−0.02 |
| 3 | M | 27 | −6.0DS:−1.0DC×180 | 0.68/−0.10 | −7.5DS:−1.0DC×180 | 0.78/−0.02 |
| 4 | M | 22 | −3.50DS:−0.75DC×7 | 0.38/−0.03 | −3.50DS:−1.00DC×176 | 0.26/−0.13 |
| 5 | M | 26 | −6.0DS | 0.88/0.00 | −4.75DS | 0.88/−0.11 |
| 6 | M | 35 | −6.50DS | 0.58/−0.02 | −5.50DS | 0.48/−0.02 |
| 7 | M | 24 | −3.5DS | 0.48/−0.07 | −3.5DS | 0.40/−0.03 |
| 8 | M | 25 | −3.00DS | 0.66/−0.15 | −2.00DS:−5.0DC×165 | 0.58/−0.11 |
| 9 | M | 23 | −1.75DS | 0.57/−0.02 | −1.5DS | 0.48/0.00 |
| 10 | M | 25 | −5.50DS | 0.58/0.09 | −7.0DS | 0.58/0.07 |
| 11 | M | 32 | −4.50DS | 0.68/−0.05 | −4.50DS | 0.68/−0.03 |
| 12 | F | 29 | −1.75DS | −0.11/−0.11 | −1.75DS | −0.13/−0.14 |
| 13 | F | 24 | −2.75DS | 0.40/−0.20 | −2.75DS | 0.34/−0.23 |
| 14 | M | 27 | −8.00DS | 0.88/0.28 | −5.00DS | 0.68/0.28 |
| 15 | M | 25 | −3.00DS | 0.76/0.09 | −2.00DS | 0.40/−0.01 |
| 16 | M | 23 | −3.5DS | 0.88/0.07 | −3.0DS | 0.78/−0.02 |
| 17 | M | 24 | −4.0DS | 0.73/−0.10 | −3.0DS | 0.27/0.00 |
| 18 | F | 19 | −4.0DS | 0.57/0.06 | −3.0DS | 0.29/−0.07 |
| 19 | F | 23 | −3.0DS | 0.85/−0.07 | −2.0DS | 0.85/−0.10 |
| 20 | M | 22 | −3.0DS | 0.47/−0.11 | −5.0DS | 0.66/−0.11 |
| 21 | F | 22 | −5.0DS | 0.76/−0.03 | −5.0DS | 0.73/−0.04 |
| 22 | M | 21 | −4.25DS | 0.88/−0.04 | −4.0DS | 0.68/−0.04 |
Acuities were arranged as ‘Naked condition/Full corrected condition’.
2.2 Stimuli and Apparatus
The stimuli were 2.5°×2.5° vertical sine wave gratings. To minimize edge effects, a half-Gaussian ramp (σ=0.25°) was added to the edges of the gratings to blend them into the background. Stimuli were generated using a computer running Matlab based on Psychtoolbox extensions (Brainard 1997, Pelli 1997) and presented on a gamma-corrected monitor with a spatial resolution of 1600×1200 pixels and a refresh rate of 85 Hz. A special circuit was used to produce 14-bit gray-level resolution (Li, Lu et al. 2003). The mean luminance of the display was 28.3 cd/m2. A chin rest was used to constrain head movements during the experiment. Subjects viewed the displays in fovea in a dimly light room at a distance of 1.14 m.
.3 qCSF Implementation
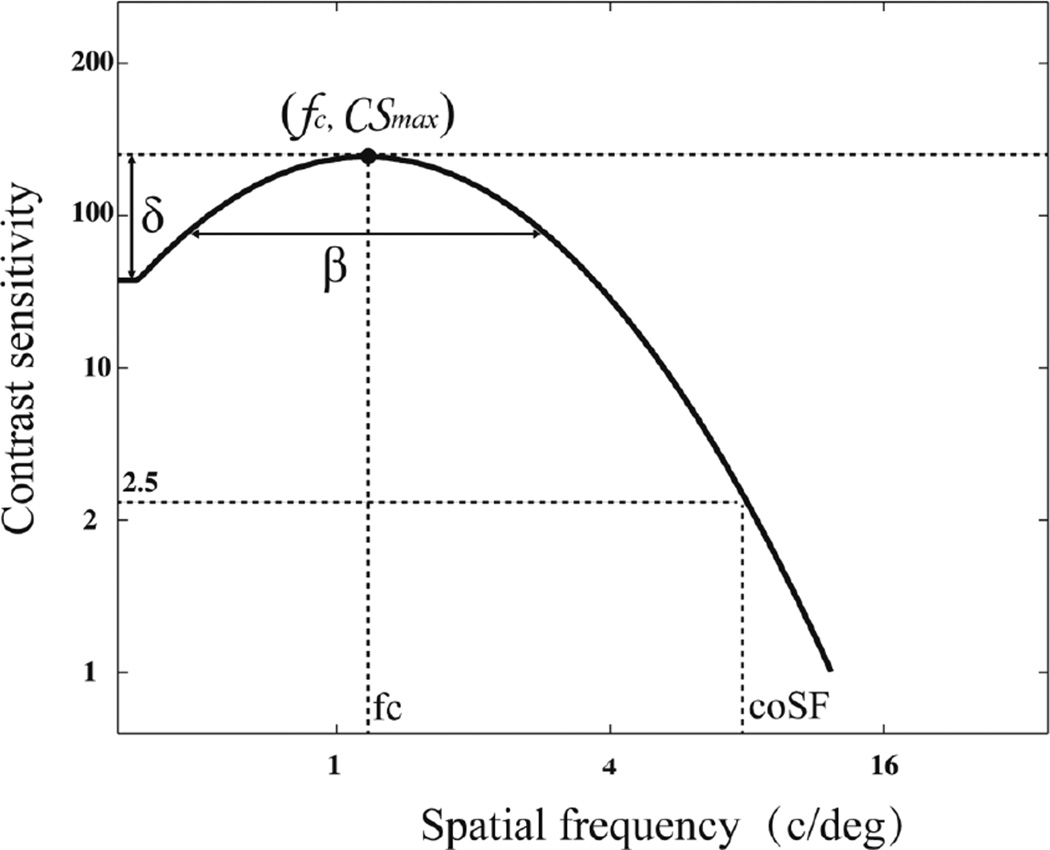
Recently, Lesmes et al (Lesmes, Lu et al. 2010) developed the quick CSF method (qCSF) to accurately estimate the contrast sensitivity function with greatly reduced testing times (e.g., 50 trials in a 2IFC task). As shown in Figure 1, the CSF is characterized by a four-parameter truncated log parabola (Watson and Ahumada 2005, Hou, Huang et al. 2010, Lesmes, Lu et al. 2010) with four parameters: (1) the peak gain (CSmax); (2) the peak spatial frequency (fc); (3) the truncated fall-off on the low-frequency side (δ); and (4) the bandwidth (fullwidth at half-maximum) (β). Different combinations of parameter values are assigned an initial probability, creating a four-dimensional probability density function (pdf). The pdf is updated using Bayes’ rule and subject’s response in detecting grating of a certain combination of spatial frequency and contrast level (Watson and Pelli 1983, King-Smith, Grigsby et al. 1994, Cobo-Lewis 1996, Kontsevich and Tyler 1999, Kujala and Lukka 2006, Lesmes, Jeon et al. 2006, Kim, Pitt et al. 2013). The spatial frequency and contrast of the stimulus in the next trial is chosen from all possible combinations of spatial frequency and contrast conditions such that the expected outcome will result in the largest reduction in the entropy (i.e. largest information gain) of the pdf. The method has been recently validated in applications studying amblyopia (Hou et al), peripheral vision (Rosén, Lundström et al. 2014) and second-order perception (Reynaud, Tang et al. 2014).
Figure 1.
CSF Parameterization. The spatial contrast sensitivity function, which describes reciprocal contrast threshold as a function of spatial frequency, can be described by four parameters: (1) the peak gain, max g (2) the peak frequency, max f (3) the bandwidth (fullwidth at half-maximum),b , and (4) the truncated fall-off on the low-frequency side. The qCSF method rapidly estimates the CSF by directly estimating these four parameters.
We implemented the qCSF procedure in exactly the same way as described in Lesmes, et al (Lesmes, Lu et al. 2010). Briefly, the stimulus space consisted of gratings with contrasts ranging from 0.1% to 99% in steps of 1.5 dBs and spatial frequencies from 0.5 to 16 cpd in steps of 3 dBs. The qCSF’s parameter space is a four-dimensional grid; the ranges of possible CSF parameters were: 1.2 to 1200 for peak gain, .25 to 24 cpd for peak frequency, .25 to 8 octaves for bandwidth, and .01 to 4 for truncation level. A diffused prior was used to initiate the procedure (Lesmes, et al, 2010)
The CSF curve was obtained after 50 qCSF trials. The area under contrast sensitivity function (AUCSF) was calculated by integrating over spatial frequency from 0.5 to 16 c/deg. The cutoff spatial frequency (coSF), defined as the spatial frequency that corresponds to a contrast sensitivity of 2.5, was computed from the CSF curve.
2.4 Procedure
A two-interval forced choice (2IFC) paradigm was used in the qCSF procedure. Each trial consisted of an initial 294-ms fixation in the center of the display and two153-ms stimulus intervals separated by an inter-stimulus interval (ISI) of 588-ms. A brief tone signaled the onset of each interval. The grating was only presented in one of the two intervals. Subjects were asked to indicate the interval that contained the grating using the computer keyboard. No feedback was provided.
2.5 Design
Visual acuity (VA) was measured for both eyes under optically corrected and uncorrected conditions using a Chinese Tumbling E chart (Mou 1966) with the untested eye covered by an opaque patch. To minimize effects of optical adaptation, visual acuity in the naked eye condition was measured at least 30 minutes after subjects took off their glasses.
The CSF was measured in eight test conditions: 2 test eyes (left/right eyes for myopic subjects or amblyopic/fellow eyes for amblyopic subjects) ×2 levels of optical correction (with/without glasses) ×2 patching conditions (the untested eye covered with opaque or translucent patching). While the opaque material blocked light completely, the translucent patch deprived form perception and lowered stimulus luminance by about 20%, i.e., the mean luminance in the non-tested eye was about 80% of that of the tested eye or 22.6 cd/m2.
Because we were interested in interocular inhibition index, subjects who did not complete tests in both patching conditions, mainly due to scheduling difficulties, were excluded from statistical analysis. We ended up with 19 effective measurements in the amblyopic eye with glasses condition, 7 in the amblyopic eye without glasses condition, 19 in the fellow eye with glasses condition, 6 in the fellow eye without glasses condition, 40 in the myopic eye with glasses condition (20 left and 20 right eyes), and 21 in the myopic eye without glasses condition (11 left and 10 right eyes). To guarantee that the CSF’s were measured at the same light adaption level, we asked subjects to step out of the test room into a regular indoor light room for about five minutes between tests. The four conditions with glasses and the other four without glasses were tested in separate blocks and counterbalanced across subjects.
2.6 Analysis
For each test eye, we defined the inhibition index for that eye to be the AUCSF ratio between the translucent and opaque patching conditions (Figures 2 and 3). An inhibition index significantly below 1.0 signifies that the tested eye is inhibited more by a light-patched untested eye than by a dark-patched untested eye. An index near 1.0 signifies that the tested eye is equally inhibited by the dark and light-patched untested eye. Inhibition indices in different conditions were compared with ANOVA and LSD multiple comparisons correction.
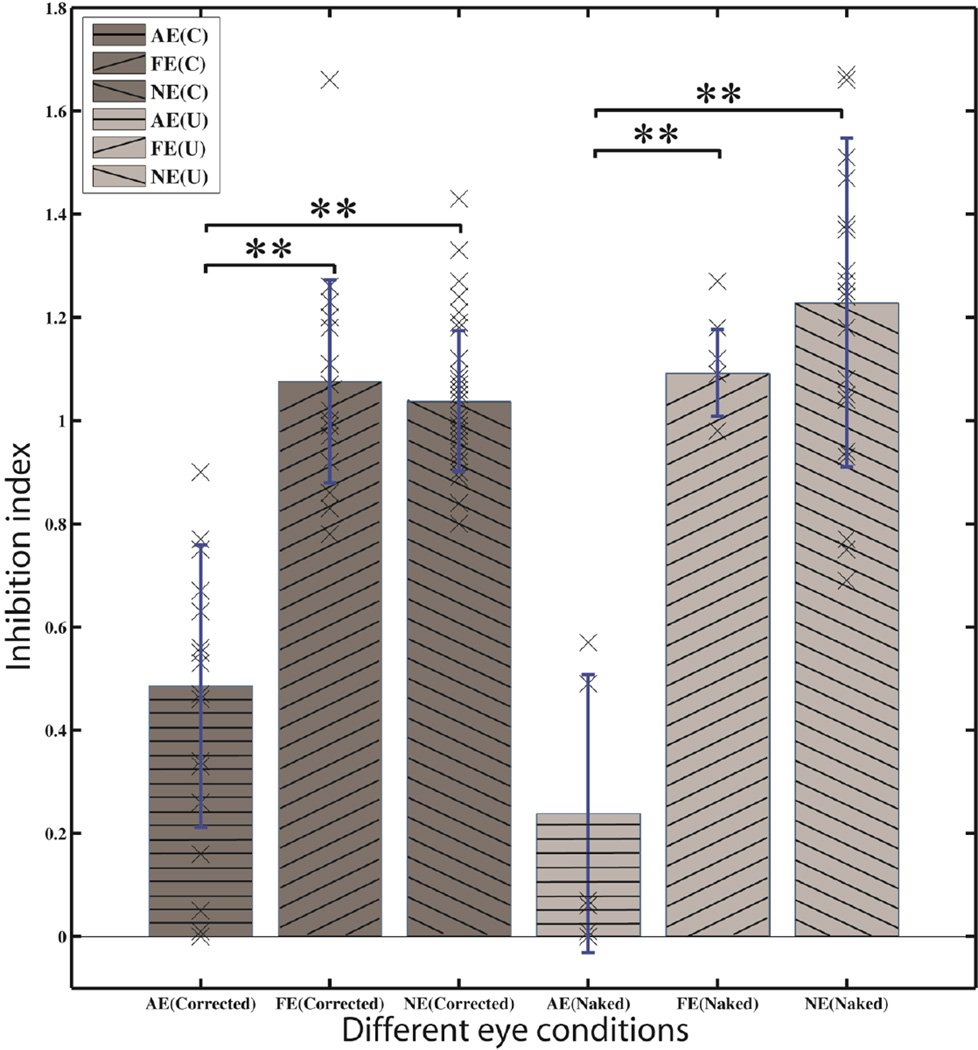
Figure 2.
Inhibition index in amblyopic and myopic vision. First three bars (dark gray) represent data from the amblyopic (horizontal line) and fellow eyes of the amblyopic subjects (left oriented line), and the average of the left and right eyes of the myopic subjects (right oriented) in the optically corrected condition. The last three bars (light gray) represent data in the naked eye condition. The crosses stand for individual data. Asterisks indicate significant difference between conditions (p<0.01).
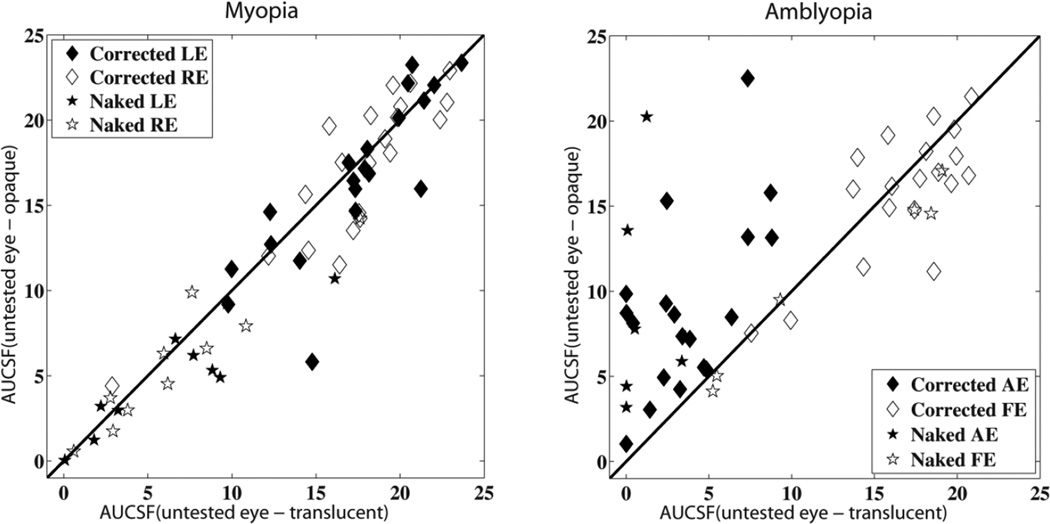
Figure 3.
AUCSF for myopic (left) and amblyopic (right) subjects. X-axis: AUCSF of the tested eye when the untested eye was covered with translucent materials; Y-axis: AUCSF of the tested eye when the untested eye was covered with opaque materials. The red diagonal line is the identity line with a slope of 1.0. Corrected AE/LE: Amblyopic/left eye tested with glasses; Corrected FE/RE: fellow/right eye tested with glasses; Corrected AE/LE (filled diamonds): Amblyopic/left eye tested with glasses; Corrected FE/RE (open diamonds): fellow/right eye tested with glasses; Naked AE/LE (filled stars): Amblyopic/left eye tested without glasses; Naked FE/RE (open stars): fellow/right eye tested without glasses;
The lower the inhibition index is, the greater the imbalance between the two eyes. Pearson’s correlations between the degree of amblyopia (e.g. visual acuity and AUCSF difference between amblyopic and fellow eyes) and the inhibition indices were calculated using SPSS for Windows (Version 19.0; SPSS, Inc., Chicago, IL, USA).
To determine effective predictors that discriminate anisometropic amblyopia from myopia, we applied a logistic regression model with amblyopia as the dichotomized outcome, inhibition index and interocular differences in visual acuity, AUCSF, and high-cutoff spatial frequency as independent predictors (Version 19.0; SPSS, Inc., Chicago, IL, USA):
| (1) |
where P(amb) is the probability of a subject being amblyopic, ratio represents inhibition index, area represents interocular AUCSF difference, VA represents interocular visual acuity difference, coSF represents interocular cutoff spatial frequency difference, i.e., the difference between the cutoff spatial frequencies in the two eyes, and βs are the coefficients.
A stepwise selection method was used to select effective predictors, in which the inclusion of a particular predictor is based on the significance of the score statistic, and the exclusion of a particular predictor is based on the probability of a likelihood-ratio statistic (Forward Selection-Conditional in SPSS 19.0). The logistic regression models in the corrected and uncorrected conditions were evaluated separately.
3. Results
We compared visual acuity in the two eyes of the amblyopic and myopic subjects, for both the optically corrected and uncorrected conditions. Under correction, there were significant differences (p<0.01) between acuities in the amblyopic (0.44±0.08, mean LogMAR±s.e.) and fellow eyes (−0.04±0.02) of the amblyopic subjects, and between acuity in the amblyopic eye and corrected-to-normal eyes of the myopic subjects (−0.04±0.02). Without optical correction, no significant visual acuity difference was found among the amblyopic, fellow, and myopic eyes (0.41±0.10, 0.29±0.10 and 0.52±0.10, respectively; p>0.10). Visual acuity was not a good metric for discriminating amblyopia from myopia without optical correction in these subjects.
From CSFs obtained in each condition, we derived the area under CSF (AUCSF) and the cutoff spatial frequencies. The AUCSF characterizes spatial vision over a wide range of spatial frequencies (Lesmes, Z-L et al. , van Gaalen, Jansonius et al. 2009). The cutoff spatial frequency characterizes the spatial resolution limit of the visual system (Campbell and Green 1965, Regan, Raymond et al. 1981, Zhou, Huang et al. 2006, Hou, Huang et al. 2010). Visual acuity and cutoff spatial frequency were highly correlated in all the test conditions (Pearson Correlation, R=−0.717, P<0.01).
Without optical correction, the AUCSF in the amblyopic eyes (8.92±2.27) was comparable to that in the fellow eyes (10.00±2.07) and myopic eyes (6.35±0.92 average across two eyes) in the opaque patching condition (p>0.10). However, the AUCSF in the amblyopic eyes (1.26±0.60) was significantly lower than that in the fellow eyes (10.71±2.88) and slightly lower than the myopic eyes (5.40±0.92) in the translucent patching condition (p<0.01 and p=0.58, respectively). With optical correction, the AUCSF in the amblyopic eye (9.04±1.18) was significantly lower than that in the fellow eyes (15.86±0.88) and myopic eyes (16.80±0.72) in the opaque patching condition (p<0.01). In addition, the AUCSF in the amblyopic eyes (3.71±0.66) was significantly lower than that in the fellow eyes (16.71±0.82) and myopic eyes (16.79±0.72) in the translucent patching condition (p<0.01).
With optical correction, the inhibition index was significantly lower (all p<0.01) in the amblyopic eyes (0.43±0.07) than that in the fellow eyes (1.08±0.05) of the amblyopic subjects, and in both eyes of the myopic subjects (1.10±0.08 and 1.03±0.04 for left and right eyes, respectively). Removing optical correction led to the same qualitative results: the inhibition index was 0.17±0.09 for the amblyopic eyes, and 1.15±0.05, 1.17±0.09 and 1.28±0.10 for the fellow eyes of the amblyopic subjects, and the left and right eyes of the myopic subjects, respectively. No significant difference was found among the left and right eyes of the myopic subjects and the fellow eyes of the amblyopic subjects in both optical correction conditions (all p>0.10). The inhibition index was highly correlated with interocular visual acuity difference in the optically corrected condition (R=0.639, p<0.01) but not in the uncorrected condition (R=0.003, p>0.1).
The logistic regression analysis revealed that the best discriminating factor(s) was the inhibition index in the uncorrected condition, but a combination of inhibition index and the interocular visual acuity difference in the corrected condition. With the inhibition index as the predictor, amblyopic and myopic eyes were discriminated with 100% accuracy in the naked eye condition (β0 =171.27, β1=−272.36; χ(1)=20.73, p<0.01). In the optically corrected condition, using the inhibition index as the single predictor discriminated amblyopic from myopic eyes with 82.9% accuracy; adding interocular visual acuity difference as a second predictor increased the accuracy to 100% (β0 =306.82, β1=−260.48;β4 =−12.30; χ(2)=56.62, p<0.01).
The predictive powers of the variables discriminating amblyopia from myopia, including interocular visual acuity difference, interocular cutoff spatial frequency difference, interocular AUCSF difference, and inhibition index, are listed in Table 3. In the uncorrected condition, only the inhibition index successfully identified amblyopia at 100% accuracy; in the optically corrected condition, interocular visual acuity difference predicted amblyopia with 95.1% accuracy; and adding inhibition index increased the accuracy to 100%.
Table 3.
The power of single metrics and the best discriminator(s) in identifying amblyopia.
| Factors | Amblyopia Identifying (%) | |
|---|---|---|
| Naked | Corrected | |
| Interocular Visual Acuity Difference (IVAD) | Null | 95.1 |
| Interocular AUCSF Difference (Translucent) | Null | 82.9 |
| Interocular AUCSF Difference (Opaque) | Null | 75.6 |
| Interocular Cutoff Difference (Translucent) | Null | 65.9 |
| Interocular Cutoff Difference(Opaque) | Null | Null |
| Inhibition Index (I2) | 100 | 82.9 |
| Best Combination of Metric(s) | 100(I2) | 100(I2 and IVAD) |
Null: the particular predictor didn’t significant improve the discriminating power relative to the case without any predictors (with only the constant).
4. Discussion
By manipulating the patching condition (translucent vs. opaque) in the untested eye and exploiting the asymmetrical interocular inhibition between the two eyes in amblyopia (Huang, Zhou et al. 2011, Lai, Alexander et al. 2012), we demonstrated that the qCSF procedure in combination with patching can be used to effectively screen anisometropic amblyopia without optical correction.
Our results are related to interocular suppression and/or dichoptic masking from the DC component of the stimulus presented to the non-tested eye. Yang & Stevenson (Yang and Stevenson 1999) applied an interocular luminance masking paradigm in normal subjects and found that grating contrast sensitivity of the tested eye was greatly decreased when the mean luminance of a steady uniform field presented to the non-tested eye increased. Lai et al., (2011) found that, with proper optical correction, visual acuity, contrast sensitivity and alignment sensitivity differences of the amblyopic eye between the fully and partially patched fellow eye conditions were significantly higher in anisometropic children with amblyopia, compared to those without amblyopia or normal controls. Freeman & Jolly (1994) found that visual acuity in one eye was not affected by occluding or increasing the luminance in the untested eye uniformly in normal subjects, but presenting a uniform lit field to the untested fellow eye significantly reduced visual acuity in the tested eye in strabismic subjects (Freeman and Jolly 1994). On the other hand, Wildsoet, et al. (Wildsoet, Wood et al. 1998) found that the test eye of normal subjects had the worst letter acuity and contrast sensitivity when the untested eye was occluded with a white opaque patch instead of a translucent (frosted) or a +1.50 D (frogging) lens. . Using a dichoptic masking paradigm, Hess and colleagues found that adding an opaque patch, a stationary or temporal modulating uniform filed (1, 2, and 3 Hz) to the untested eye yielded similar outputs of letter contrast sensitivity in both normal and amblyopic subjects (Huang, Baker et al. 2012, Zhou, McNeal et al. 2014). Several factors may account for the different results: (1) different patching materials: As suggested by Wildsoet et al. (1998), they used a white opaque patching material while most of the other studies used black opaque patching materials; (2) type of amblyopia: Hess and colleagues tested subjects with strabismic amblyopia while we studied anisometropic amblyopia (Huang, Baker et al. 2012, Zhou, McNeal et al. 2014); (3) task and stimulus: Wildsoet et al. (Wildsoet, Wood et al. 1998), (Huang, Baker et al. 2012) and Zhou, McNeal et al. 2014) measured either contrast acuity and/or contrast sensitivity with broad-band letters while we measured contrast sensitivity function with sine-wave gratings with relatively narrow bandwidth.
Most amblyopes are of anisometropic and strabismic types. In the current study, we only tested the efficacy of the interocular inhibition procedure to discriminate aisometropic amblyopia from myopia. Although Baker, Meese & Hess (Baker, Meese et al. 2008) concluded that interocular suppression and binocular combination are essentially intact in strabismic amblyopia based on results from interocular masking tests, stronger inhibition from the fellow fixating eye to the amblyopic eye has been long established as a mechanism for visual deficits in strabismic amblyopia (Sireteanu, Fronius et al. 1981, Harrad and Hess 1992, Harrad, Sengpiel et al. 1996). Application of bicuculline (GABA receptor blocker) has been found to be effective in reversing the binocular responsiveness of cortical cells in strabismic animals (Mower, Christen et al. 1984), suggesting that the input from the strabismic eye may be functionally suppressed in strabismic amblyopia (Sengpiel, Jirmann et al. 2006). Using a global motion identification paradigm, several studies have demonstrated that interocular suppression might play a primary role in both anisometropic and strabismic amblyopia (Black, Thompson et al. 2011, Li, Thompson et al. 2011, Zhou, Huang et al. 2013). Others have found that contrast interference threshold (the lower the threshold, the stronger the inhibition from the fellow eye to the amblyopic eye) was smaller in strabismic amblyopia than in anisometropic amblyopia (Narasimhan, Harrison et al. 2012), indicating interocular suppression may be even stronger in strabismic amblyopia. All these results suggest that the interocular inhibition procedure can be applied to screen strabismic amblyopia. Because strabismic amblyopia is mechanistically different from anisometropic amblyopia (Levi and Klein 1982, Song, Levi et al. 2014), it would be important to test the interocular inhibition procedure in strabismic amblyopia.
Early and accurate detection are two key components of amblyopia treatment. Early vision screening is strongly recommended by the American Academy of Pediatrics (AAP) to detect amblyopia to allow successful treatment (Castanes 2002, Wall, Marsh-Tootle et al. 2002). On the other hand, previous findings recommend using joint tests to screen with greater diagnostic accuracy (Chou, Dana et al. 2011). The test developed and evaluated in the current study can be applied without eye alignment, refractive error measurement, and prescription of eye glasses. The tests are efficient, easy-to-perform, and can be quite affordable. In this study, we have only tested adult subjects. Further tests of the procedure on children are necessary to evaluate its potential for early amblyopia screening.
Recently, the qCSF procedure has been implemented on an iPad (Dorr, Lesmes et al. 2013), making it possible to conduct our screening on a portable device and simultaneously on many subjects. It would be interesting to compare our method to other methods, e.g. letter acuity, stereo acuity, autorefractor, and photoscreeners, and evaluate the best screening combinations in a large population.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (NSFC 31230032), the Knowledge Innovation Program of the Chinese Academy of Sciences (Y3CX102003 to CBH), Institute of Psychology, CAS, and the National Eye Institute (EY021553 to ZLL).
Financial Disclosure:
LAL and Z-LL have an intellectual property interest in methods for measuring contrast sensitivity (US 7938538), and equity interest in a company (Adaptive Sensory Technology) commercializing the technology. Z-LL, C-BH, WLJ, JWZ, and LAL have an intellectual property interest in methods for screening amblyopia (U.S. Provisional Patent Application Serial No. 61/987,700). LAL holds employment in Adaptive Sensory Technology.
References
- Atkinson J, Braddick O, Bobier B, Anker S, Ehrlich D, King J, Watson P, Moore A. Two infant vision screening programmes: prediction and prevention of strabismus and amblyopia from photo-and videorefractive screening. Eye. 1996;10(2):189–198. doi: 10.1038/eye.1996.46. [DOI] [PubMed] [Google Scholar]
- Baker DH, Meese TS, Hess RF. Contrast masking in strabismic amblyopia: attenuation, noise, interocular suppression and binocular summation. Vision Res. 2008;48(15):1625–1640. doi: 10.1016/j.visres.2008.04.017. [DOI] [PubMed] [Google Scholar]
- Black JM, Thompson B, Maehara G, Hess RF. A compact clinical instrument for quantifying suppression. Optometry & Vision Science. 2011;88(2):E334–E343. doi: 10.1097/OPX.0b013e318205a162. [DOI] [PubMed] [Google Scholar]
- Brainard DH. The psychophysics toolbox. Spatial vision. 1997;10(4):433–436. [PubMed] [Google Scholar]
- Campbell F, Green D. Optical and retinal factors affecting visual resolution. The Journal of Physiology. 1965;181(3):576. doi: 10.1113/jphysiol.1965.sp007784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlton J, Karnon J, Czoski-Murray C, Smith K, Marr J. The clinical effectiveness and cost-effectiveness of screening programmes for amblyopia and strabismus in children up to the age of 4–5 years: a systematic review and economic evaluation. 2008 doi: 10.3310/hta12250. [DOI] [PubMed] [Google Scholar]
- Castanes M. Major review: The underutilization of vision screening (for amblyopia, optical anomalies and strabismus) among preschool age children. Binocular vision & strabismus quarterly. 2002;18(4):217–232. [PubMed] [Google Scholar]
- Chou R, Dana T, Bougatsos C. Screening for visual impairment in children ages 1–5 years: update for the USPSTF. Pediatrics: peds. 2011 doi: 10.1542/peds.2010-0462. 2010-0462. [DOI] [PubMed] [Google Scholar]
- Cobo-Lewis AB. 29th Annual Meeting of the Society for Mathematical Psychology. North Carolina: University of North Carolina, Chapel Hill; 1996. An Adaptive Method for Estimating Multiple Parameters of a Psychometric Function. [Google Scholar]
- Dorr M, Lesmes LA, Lu Z-L, Bex PJ. Rapid and reliable assessment of the contrast sensitivity function on an iPad. Investigative ophthalmology & visual science. 2013;54(12):7266–7273. doi: 10.1167/iovs.13-11743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ehrlich MI, Reinecke RD, Simons K. Preschool vision screening for amblyopia and strabismus. Programs, methods, guidelines, 1983. Survey of ophthalmology. 1983;28(3):145–163. doi: 10.1016/0039-6257(83)90092-9. [DOI] [PubMed] [Google Scholar]
- Eibschitz-Tsimhoni M, Friedman T, Naor J, Eibschitz N, Friedman Z. Early screening for amblyogenic risk factors lowers the prevalence and severity of amblyopia. Journal of American Association for Pediatric Ophthalmology and Strabismus. 2000;4(4):194–199. doi: 10.1067/mpa.2000.105274. [DOI] [PubMed] [Google Scholar]
- Freeman AW, Jolly N. Visual loss during interocular suppression in normal and strabismic subjects. Vision research. 1994;34(15):2043–2050. doi: 10.1016/0042-6989(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Group V. i. P. S. Sensitivity of screening tests for detecting vision in preschoolers-targeted vision disorders when specificity is 94% Optometry & Vision Science. 2005;82(5):432–438. doi: 10.1097/01.OPX.0000162660.14378.30. [DOI] [PubMed] [Google Scholar]
- Harrad R, Hess R. Binocular integration of contrast information in amblyopia. Vision research. 1992;32(11):2135–2150. doi: 10.1016/0042-6989(92)90075-t. [DOI] [PubMed] [Google Scholar]
- Harrad R, Sengpiel F, Blakemore C. Physiology of suppression in strabismic amblyopia. British Journal of Ophthalmology. 1996;80(4):373–377. doi: 10.1136/bjo.80.4.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess RF, Wang Y-Z, Demanins R, Wilkinson F, Wilson HR. A deficit in strabismic amblyopia for global shape detection. Vision research. 1999;39(5):901–914. doi: 10.1016/s0042-6989(98)00157-6. [DOI] [PubMed] [Google Scholar]
- Holmes JM, Clarke MP. Amblyopia. The Lancet. 2006;367(9519):1343–1351. doi: 10.1016/S0140-6736(06)68581-4. [DOI] [PubMed] [Google Scholar]
- Hou F, Huang CB, Lesmes L, Feng LX, Tao L, Zhou YF, Lu ZL. qCSF in clinical application: efficient characterization and classification of contrast sensitivity functions in amblyopia. Invest Ophthalmol Vis Sci. 2010;51(10):5365–5377. doi: 10.1167/iovs.10-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou F, Huang CB, Tao L, Feng L, Zhou Y, Lu ZL. qCSF in Clinical Application: Efficient Characterization and Classification of Contrast Sensitivity Functions in Amblyopia. Invest Ophthalmol Vis Sci. 2010 doi: 10.1167/iovs.10-5468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-B, Zhou J, Lu Z-L, Zhou Y. Deficient binocular combination reveals mechanisms of anisometropic amblyopia: Signal attenuation and interocular inhibition. Journal of vision. 2011;11(6):4. doi: 10.1167/11.6.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C-B, Zhou J, Zhou Y, Lu Z-L. Contrast and Phase Combination in Binocular Vision. PLoS ONE. 2010;Volume 5(Issue 12) doi: 10.1371/journal.pone.0015075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang PC, Baker DH, Hess RF. Interocular suppression in normal and amblyopic vision: spatio-temporal properties. J Vis. 2012;12(11) doi: 10.1167/12.11.29. [DOI] [PubMed] [Google Scholar]
- Hunter DG, Nassif DS, Winsor R, Gramatikov BI, Guyton DL, Piskun NV. Pediatric Vision Screener 1: instrument design and operation. Journal of biomedical optics. 2004;9(6):1363–1368. doi: 10.1117/1.1805560. [DOI] [PubMed] [Google Scholar]
- Ingram R. Refraction as a basis for screening children for squint and amblyopia. British Journal of Ophthalmology. 1977;61(1):8–15. doi: 10.1136/bjo.61.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim W, Pitt MA, Lu Z-L, Steyvers M, Myung JI. A Hierarchical Adaptive Approach to Optimal Experimental Design. 2013 doi: 10.1162/NECO_a_00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King-Smith PE, Grigsby SS, Vingrys AJ, Benes SC, Supowit A. Efficient and unbiased modifications of the QUEST threshold method: theory, simulations, experimental evaluation and practical implementation. Vision Res. 1994;34(7):885–912. doi: 10.1016/0042-6989(94)90039-6. [DOI] [PubMed] [Google Scholar]
- Kiorpes L, Tang C, Movshon JA. Factors limiting contrast sensitivity in experimentally amblyopic macaque monkeys. Vision research. 1999;39(25):4152–4160. doi: 10.1016/s0042-6989(99)00130-3. [DOI] [PubMed] [Google Scholar]
- Kontsevich LL, Tyler CW. Bayesian adaptive estimation of psychometric slope and threshold. Vision Res. 1999;39(16):2729–2737. doi: 10.1016/s0042-6989(98)00285-5. [DOI] [PubMed] [Google Scholar]
- Kujala JV, Lukka TJ. Bayesian adaptive estimation: The next dimension. Journal of Mathematical Psychology. 2006;50(4):369–389. [Google Scholar]
- Lai XJ, Alexander J, He M, Yang Z, Suttle C. Visual functions and interocular interactions in anisometropic children with and without amblyopia. Investigative ophthalmology & visual science. 2011;52(9):6849–6859. doi: 10.1167/iovs.10-6755. [DOI] [PubMed] [Google Scholar]
- Lai XJ, Alexander J, He MG, Yang ZK, Suttle C. A novel apparatus for interocular interaction evaluation in children with and without anisometropic amblyopia. Clin Exp Optom. 2012;95(4):410–420. doi: 10.1111/j.1444-0938.2012.00753.x. [DOI] [PubMed] [Google Scholar]
- Lesmes LA, Jeon ST, Lu ZL, Dosher BA. Bayesian adaptive estimation of threshold versus contrast external noise functions: the quick TvC method. Vision Res. 2006;46(19):3160–3176. doi: 10.1016/j.visres.2006.04.022. [DOI] [PubMed] [Google Scholar]
- Lesmes LA, Lu Z-L, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: The quick CSF method. Journal of Vision. 2010;10(3) doi: 10.1167/10.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesmes LA, Lu ZL, Baek J, Albright TD. Bayesian adaptive estimation of the contrast sensitivity function: the quick CSF method. J Vis. 2010;10(3):17. doi: 10.1167/10.3.17. 11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lesmes LA, Z-L L, J B, T A. Efficient Adaptive Estimation of the Contrast Sensitivity Function: the qCSF method. J Vis. doi: 10.1167/10.3.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levi DM, Klein S. Differences in vernier discrimination for grating between strabismic and anisometropic amblyopes. Investigative Ophthalmology & Visual Science. 1982;23(3):398–407. [PubMed] [Google Scholar]
- Li J, Thompson B, Lam CS, Deng D, Chan LY, Maehara G, Woo GC, Yu M, Hess RF. The role of suppression in amblyopia. Invest Ophthalmol Vis Sci. 2011;52(7):4169–4176. doi: 10.1167/iovs.11-7233. [DOI] [PubMed] [Google Scholar]
- Li X, Lu Z-L, Xu P, Jin J, Zhou Y. Generating high gray-level resolution monochrome displays with conventional computer graphics cards and color monitors. Journal of neuroscience methods. 2003;130(1):9–18. doi: 10.1016/s0165-0270(03)00174-2. [DOI] [PubMed] [Google Scholar]
- Loudon SE, Rook CA, Nassif DS, Piskun NV, Hunter DG. Rapid, high-accuracy detection of strabismus and amblyopia using the pediatric vision scanner. Investigative ophthalmology & visual science. 2011;52(8):5043–5048. doi: 10.1167/iovs.11-7503. [DOI] [PubMed] [Google Scholar]
- Mou T. Logarithmic visual acuity chart and five-score recording. Chinese Journal of Ophthalmology. 1966;13(1):96–106. [Google Scholar]
- Mower GD, Christen WG, Burchfiel JL, Duffy FH. Microiontophoretic bicuculline restores binocular responses to visual cortical neurons in strabismic cats. Brain research. 1984;309(1):168–172. doi: 10.1016/0006-8993(84)91024-2. [DOI] [PubMed] [Google Scholar]
- Narasimhan S, Harrison ER, Giaschi DE. Quantitative measurement of interocular suppression in children with amblyopia. Vision research. 2012;66:1–10. doi: 10.1016/j.visres.2012.06.007. [DOI] [PubMed] [Google Scholar]
- Pelli DG. The VideoToolbox software for visual psychophysics: Transforming numbers into movies. Spatial vision. 1997;10(4):437–442. [PubMed] [Google Scholar]
- Regan D, Raymond J, Ginsburg A, Murray T. Contrast sensitivity, visual acuity and the discrimination of Snellen letters in multiple sclerosis. Brain. 1981;104(2):333–350. doi: 10.1093/brain/104.2.333. [DOI] [PubMed] [Google Scholar]
- Reynaud A, Tang Y, Zhou Y, Hess RF. A normative framework for the study of second-order sensitivity in vision. Journal of vision. 2014;14(9):3. doi: 10.1167/14.9.3. [DOI] [PubMed] [Google Scholar]
- Rosén R, Lundström L, Venkataraman AP, Winter S, Unsbo P. Quick contrast sensitivity measurements in the periphery. Journal of vision. 2014;14(8):3. doi: 10.1167/14.8.3. [DOI] [PubMed] [Google Scholar]
- Schmidt P, Maguire M, Dobson V, Quinn G, Ciner E, Cyert L, Kulp MT, Moore B, Orel-Bixler D, Redford M. Comparison of preschool vision screening tests as administered by licensed eye care professionals in the Vision In Preschoolers Study. Ophthalmology. 2004;111(4):637–650. doi: 10.1016/j.ophtha.2004.01.022. [DOI] [PubMed] [Google Scholar]
- Sengpiel F, Jirmann K-U, Vorobyov V, Eysel UT. Strabismic suppression is mediated by inhibitory interactions in the primary visual cortex. Cerebral Cortex. 2006;16(12):1750–1758. doi: 10.1093/cercor/bhj110. [DOI] [PubMed] [Google Scholar]
- Simons K. Amblyopia characterization, treatment, and prophylaxis. Surv Ophthalmol. 2005;50(2):123–166. doi: 10.1016/j.survophthal.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Sireteanu R, Fronius M, Singer W. Binocular interaction in the peripheral visual field of humans with strabismic and anisometropic amblyopia. Vision research. 1981;21(7):1065–1074. doi: 10.1016/0042-6989(81)90011-0. [DOI] [PubMed] [Google Scholar]
- Sjöstrand J, Abrahamsson M. Prevention of amblyopia and the concept of cure. European journal of ophthalmology. 1996;7(2):121–129. doi: 10.1177/112067219700700201. [DOI] [PubMed] [Google Scholar]
- Song S, Levi DM, Pelli DG. A double dissociation of the acuity and crowding limits to letter identification, and the promise of improved visual screening. Journal of Vision. 2014;14(5):3. doi: 10.1167/14.5.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Gaalen KW, Jansonius NM, Koopmans SA, Terwee T, Kooijman AC. Relationship between contrast sensitivity and spherical aberration: comparison of 7 contrast sensitivity tests with natural and artificial pupils in healthy eyes. Journal of Cataract & Refractive Surgery. 2009;35(1):47–56. doi: 10.1016/j.jcrs.2008.09.016. [DOI] [PubMed] [Google Scholar]
- Wall TC, Marsh-Tootle W, Evans HH, Fargason CA, Jr, Ashworth CS, Hardin JM. Compliance with vision-screening guidelines among a national sample of pediatricians. Ambulatory Pediatrics. 2002;2(6):449–455. doi: 10.1367/1539-4409(2002)002<0449:cwvsga>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Watson AB, Ahumada AJ. A standard model for foveal detection of spatial contrast. Journal of Vision. 2005;5(9):717–740. doi: 10.1167/5.9.6. [DOI] [PubMed] [Google Scholar]
- Watson AB, Pelli DG. QUEST: a Bayesian adaptive psychometric method. Percept Psychophys. 1983;33(2):113–120. doi: 10.3758/bf03202828. [DOI] [PubMed] [Google Scholar]
- Wildsoet C, Wood J, Maag H, Sabdia S. The effect of different forms of monocular occlusion on measures of central visual function. Ophthalmic and Physiological Optics. 1998;18(3):263–268. [PubMed] [Google Scholar]
- Wu C, Hunter DG. Amblyopia: diagnostic and therapeutic options. American journal of ophthalmology. 2006;141(1):175–184. doi: 10.1016/j.ajo.2005.07.060. e172. [DOI] [PubMed] [Google Scholar]
- Yang J, Stevenson SB. Post-retinal processing of background luminance. Vision research. 1999;39(24):4045–4051. doi: 10.1016/s0042-6989(99)00116-9. [DOI] [PubMed] [Google Scholar]
- Zhou J, Huang PC, Hess RF. Interocular suppression in amblyopia for global orientation processing. J Vis. 2013;13(5):19. doi: 10.1167/13.5.19. [DOI] [PubMed] [Google Scholar]
- Zhou J, McNeal S, Babu RJ, Baker DH, Bobier WR, Hess RF. Time course of dichoptic masking in normals and suppression in amblyopes. Invest Ophthalmol Vis Sci. 2014;55(7):4098–4104. doi: 10.1167/iovs.14-13969. [DOI] [PubMed] [Google Scholar]
- Zhou Y, Huang C, Xu P, Tao L, Qiu Z, Li X, Lu ZL. Perceptual learning improves contrast sensitivity and visual acuity in adults with anisometropic amblyopia. Vision Res. 2006;46(5):739–750. doi: 10.1016/j.visres.2005.07.031. [DOI] [PubMed] [Google Scholar]