Abstract
Bufei Jianpi formula (BJF) has long been used as a therapeutic agent in the treatment of COPD. Systems pharmacology identified 145 active compounds and 175 potential targets of BJF in a previous study. Additionally, BJF was previously shown to effectively prevent COPD and its comorbidities, such as ventricular hypertrophy, by inhibition of inflammatory cytokine production, matrix metalloproteinases expression, and other cytokine production, in vivo. However, the system-level mechanism of BJF for the treatment of COPD is still unclear. The aim of this study was to gain insight into its system-level mechanisms by integrating transcriptomics, proteomics, and metabolomics together with systems pharmacology datasets. Using molecular function, pathway, and network analyses, the genes and proteins regulated in COPD rats and BJF-treated rats could be mainly attributed to oxidoreductase activity, antioxidant activity, focal adhesion, tight junction, or adherens junction. Furthermore, a comprehensive analysis of systems pharmacology, transcript, protein, and metabolite datasets is performed. The results showed that a number of genes, proteins, metabolites regulated in BJF-treated rats and potential target proteins of BJF were involved in lipid metabolism, cell junction, oxidative stress, and inflammatory response, which might be the system-level therapeutic mechanism of BJF treatment.
Keywords: Bufei Jianpi formula, system-level therapeutic mechanism, transcriptomic, proteomic, metabolomic
Introduction
COPD is a serious health problem characterized primarily by irreversible airflow limitation and systemic inflammation, which represents a substantial economic burden in the world.1,2 Despite great progress in the treatment of COPD, drug therapies have not changed significantly.3 Therefore, the development of novel therapeutic drugs and strategies to improve the efficacy in treating this deadly disease is still urgently needed.
Bufei Jianpi formula (BJF), a traditional Chinese medicine (TCM), has provided effective relief of symptoms in patients with COPD. Our clinical study demonstrated that BJF had extensive pharmacological effects on patients with COPD, such as alleviating the clinical symptoms of stable COPD, reducing the exacerbation frequency, delaying acute exacerbation, and improving pulmonary function and exercise capacity.4
Increasing evidences demonstrate that, in treating complex illnesses, including COPD, treatment formula containing multiple drugs with distinct but related mechanisms can usually exert synergistic therapeutic effects and amplify the therapeutic efficacies of each agent.5,6 In the previous study, we constructed a systems pharmacological model by combining active compound prediction, target prediction, and network pharmacology to clarify the active compounds and therapeutic mechanism of BJF. Systems pharmacology successfully identified 145 bioactive ingredients from BJF and predicted 175 potential targets of the active components contained in BJF. Moreover, we experimentally demonstrated that BJF was effective for the treatment of COPD and ventricular hypertrophy due to its inhibitory effect on the inflammatory cytokine, and hypertrophic factors expression, protease–antiprotease imbalance, and collagen deposition, in vivo.7 Systems pharmacology, as an understanding of the function of the biological system, provided a system-wide view of the therapeutic mechanism of BJF in treating COPD rats. However, we should provide more experimental evidences to validate the systems pharmacology predictions at system level.
Transcriptomics–proteomics–metabolomics-profiling techniques provide holistic approaches to reveal the complex biological processes and function of herbal compound recipe.8–10 Transcriptomics has been used in many in vivo studies to find the whole genome, which, however, cannot provide the details of the alternative splicing or even protein expression itself. Proteomics is a promising approach to directly study the expressed proteins and protein functions in a cellular context. Moreover, metabolomics provides the metabolic information, that is, the consequence of the transcriptome and proteome.11 Thus, comprehensive analyses of the transcriptomics, proteomics, and metabolomics datasets have the potential to provide a system view of the complex biological processes and function of herbal compounds.12
We previously analyzed metabolite changes in lung tissues of COPD rats, and identified 30 strongly regulated metabolites resulting from intervention by BJF treatment.13 In this study, we further analyzed the molecular effect of BJF on COPD rats using the datasets of transcripts and proteins derived from lung tissues. Finally, the aim of this study was to integrate systems pharmacology, transcriptomics, proteomics, and metabolomics data streams, which provided a system picture of molecular mechanisms of BJF in treating COPD rats.
Materials and methods
Chemicals and animals
Klebsiella pneumoniae (strain ID: 46114) was purchased from the National Center for Medical Culture Collection (CMCC, Beijing, People’s Republic of China). Tobacco (Hongqi Canal® Filter tip cigarette; tobacco type, tar: 10 mg; nicotine content: 1.0 mg; carbon monoxide: 12 mg) was obtained from China Tobacco Henan Industrial Co., Ltd (Zhengzhou, People’s Republic of China). Thirty-two Sprague Dawley rats (16 male and 16 female; 200±20 g) were purchased from the Experimental Animal Center of Henan Province (Zhengzhou, People’s Republic of China). The animals were reared in cages with free access to food and water under standard laboratory conditions. All animals were handled with humane care throughout the experiment.
COPD model, drug administration, and lung samples
COPD rat model was prepared as described earlier. Briefly, 22 rats were placed in a closed box exposed to tobacco and repeated K. pneumoniae infections.14
On week 9, COPD rats were randomly divided into two groups with ten rats in each group. COPD rats were intragastrically treated with normal saline (2 mL) and BJF at the dose of 4.84 g/kg per day. The healthy control rats were also administered intragastrically normal saline (2 mL) for the same amount of time. All the rats were sacrificed at week 20. The lung tissues were shock-frozen in liquid nitrogen and stored at −80°C before use.
The components of BJF were as follows: Astragali Radix 15 g, Polygonati Rhizoma 15 g, Codonopsis Radix 15 g, Atractylodis Macrocephalae Rhizoma 12 g, Poria 12 g, Fritillariae Thunbergii Bulbus 9 g, Magnoliae Officinalis Cortex 9 g, Citri Reticulatae Pericarpium 9 g, Asteris Tatarici Radix 9 g, Pheretima 12 g, Ardisiae Japonicae Herba 15 g, and Epimedii Herba 6 g. The herbs were identified and prepared in fluid extract according to the standard operating procedure. The experimental protocol was approved by the Experimental Animal Care and Ethics Committee of the First Affiliated Hospital, Henan University of Traditional Chinese Medicine. The animal experiments were conducted in accordance with guidelines of the Committee on the Care and Use of Laboratory Animals of the First Affiliated Hospital, Henan University of Traditional Chinese Medicine, People’s Republic of China.
Gene expression analyses with microarrays
For microarray analysis, six rats were used for each group for microarray study. Total RNA was isolated from lung tissues of six rats from each of the three experimental groups by TRIzol reagent (Thermo Fisher Scientific, Waltham, MA, USA) and purified using a Qiagen RNeasy Micro Kit (Qiagen NV, Venlo, the Netherlands). RNA integrity was checked using standard agarose gel electrophoresis and ethidium bromide staining.
Purified RNA was polymerase chain reaction amplified using a First Strand cDNA Synthesis Kit (Hoffman-La Roche Ltd., Basel, Switzerland) and labeled using an Agilent Quick Amp Kit (Agilent Technologies, Santa Clara, CA, USA). Then, RNA was hybridized with Agilent Whole Rat Genome Oligo Microarray (4×44 K) and washed. Finally, the slides were analyzed using an Agilent DNA microarray scanner (part number G2505B).
The raw microarray data were further analyzed using Agilent GeneSpring GX software Version 11.0. Processed data were subsequently filtered for significant detection (Student’s t-test screening, P<0.05) and differential expression versus COPD model rats (fold change, |log ratio| >1).
Protein expression analysis
Lung tissue protein was isolated from the three experimental groups. In each group, six rats were taken out for the analysis of protein expression. In brief, the lung tissues were lysed using the lysis buffer containing 4% sodium dodecyl sulfate, 0.1 M dithiothreitol, and 0.1 M Tris pH 8.0 and homogenized in a mechanical homogenizer (Retsch Technology, Haan, Germany). The lysates were clarified by centrifugation and then stored at −80°C.
For proteolytic digestion, trypsin (Hoffman-La Roche Ltd.) solution (protein/trypsin ratio 1:30) was added to the protein solution and incubated for 24 hours at 37°C. To label tryptic peptides with 8-plex iTRAQ labeling reagents (ABSciex, Darmstadt, Germany), each of the samples was reconstituted with isopropanol individually, and the labeling procedure was performed according to the manufacturer’s protocol.
For strong cation exchange fractionation, buffers A (10 mM KH2PO4 in 25% acetonitrile [ACN] at pH 3) and B (10 mM KH2PO4 and 2 M KCl in 25% ACN at pH 3) were used as the mobile phase. The peptide mixtures were dissolved in buffer A and then loaded onto a PolySULFOETHYL A column. Peptides were fractionated using an 86-minute gradient from 0% to 100% of buffer B and monitored at optical density 214 nm. Fractions were collected every minute from the retention times of 8–58 minutes. Then, the fractions were dried, dissolved in 0.1% formic acid (FA), and used for liquid chromatography–mass spectrometry (LC-MS) analysis.
LC-MS/MS analysis was performed on a nano liquid chromatograph (Shimadzu, Kyoto, Japan) coupled online to a quadrupole time-of-flight mass spectrometer (Bruker Daltonics, Bremen, Germany). For the LC-MS/MS analysis, water with 0.1% FA and ACN with 0.1% FA were used as the mobile phase. The peptide sample was injected into a pulled tip column (15 cm ×100 μm inner diameter) containing C18 Reprosil, 5 μm particles (Nikkyo Technos, Tokyo, Japan), and separated online using increasing amounts of acetonitrile containing 0.1% FA (mobile phase B) at 300 nL/min. Gradient conditions were as follows: 5%–34% B (0–25 minutes), 34%–60% B (25–30 minutes), 80% B held for 4 minutes, and 5%–80% B (1 minute).
The identification results were obtained from Mascot. The criteria used for protein quantitation were as follows: two unique peptide hits, two quantitative peptides, and disabling of outlier removal. The reporter ion ratio for each identified peptide was determined by Mascot. The loess and global median normalization were used to process the proteomics data. Data were log2 transformed and analyzed on both peptide and protein level. Statistical significance of observed fold-change ratios was determined by one sample t-test. P-values <0.05 were considered statistically significant for proteins. Fold change higher than 1.0 is for upregulation or lower than 1.0 is for downregulation.
Gene/protein set enrichment, network, and pathway analyses
The molecular function of transcripts and proteins was explored by BiNGO, a Cytoscape v3.1.1 plugin.15 Pathway enrichment analysis of transcripts and proteins was performed using the Database for Annotation, Visualization and Integrated Discovery and Kyoto Encyclopedia of Genes and Genomes database. We considered regulated pathways only as statistically significant, if the P-value was ≤0.05. We applied Metscape to analyze the integrated pathway of gene, protein, and metabolomics data.16 We also applied ClueGO, a Cytoscape plugin, to explore the molecular function of the proteins.17 In the MetaboAnalyst 3.0, the pathway analysis module combines the results from powerful pathway enrichment analysis with the pathway topology analysis to help us identify the most relevant pathways involved in the conditions under this study.18 Using STRING 10, we generated interaction networks based on the overlapping proteins between the targets of BJF and proteomic measurements of BJF-treated rats.19
Results and discussion
Transcriptomics analysis results of BJF-treated COPD rats
In the previous study, systems pharmacology successfully identified the bioactive ingredients and revealed the potential targets of the multiple components contained in BJF. Subsequently, we validated that BJF was effective for the treatment of COPD and ventricular hypertrophy due to its inhibitory effect on the inflammatory cytokine, and hypertrophic factors expression, protease–antiprotease imbalance and collagen deposition, in vivo.7 In this study, to investigate the system-wide mechanism of BJF in treating COPD rats, we further examined the effect of BJF on transcriptional profiles of lung tissues.
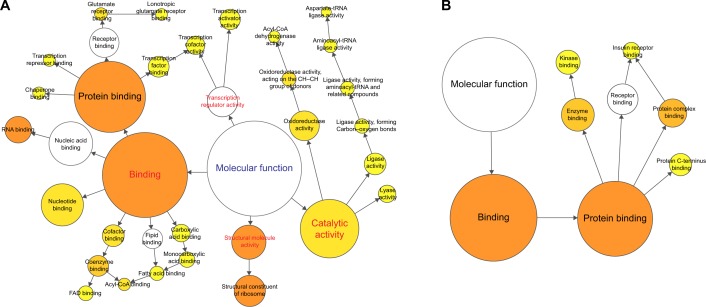
Using microarray-based RNA expression analysis, we detected ~41,000 expressed genes in lung tissues. There were 2,463 and 1,012 genes differently expressed at COPD model (versus control) and BJF treatment (versus COPD model) rats, respectively (Tables S1 and S2). These transcripts regulated in lung tissues of COPD model rats could be mapped to various molecular functions such as regulation of oxidoreductase activity, nucleic acid binding, channel activity, fatty acid binding, glucose, or fatty acid metabolism (Figure 1A). However, transcripts regulated only in BJF-treated rats were mapped to several molecular functions, such as kinase binding, protein complex binding, and insulin receptor binding (Figure 1B).
Figure 1.
Molecular functions of regulated genes in lung tissues of rats with COPD and BJF-treated rats.
Notes: BiNGO mapped the predominant functional themes of the tested gene set on the GO hierarchy and produced an intuitive and a customizable visual representation of the results. The area of a node was proportional to the number of genes in the test set annotated to the corresponding GO category. (A) Representative molecular function of regulated genes in lung tissues of rats with COPD. (B) Representative molecular function of regulated genes in lung tissues of BJF-treated rats.
Abbreviations: BJF, Bufei Jianpi formula; GO, gene ontology; FAD, flavin adenine dinucleotide.
In addition, in the COPD model group, the regulated genes were mapped to many different pathways, such as fatty acid metabolism, valine, leucine and isoleucine degradation, and propanoate metabolism (Table 1). The genes regulated in the BJF-treated group could be mapped to adherens junction, focal adhesion, endocytosis, wnt signaling pathway, etc. (Table 2).
Table 1.
Analyzed pathways of transcriptomics data regulated in lung tissues of rats with COPD
| Term | Count | % | P-value |
|---|---|---|---|
| Valine, leucine, and isoleucine degradation | 15 | 0.1128 | 1.43E-06 |
| Propanoate metabolism | 12 | 0.0902 | 7.33E-06 |
| Ribosome | 19 | 0.1429 | 1.02E-05 |
| Endocytosis | 30 | 0.2256 | 1.06E-04 |
| Fatty acid metabolism | 11 | 0.0827 | 4.45E-04 |
| Graft-versus-host disease | 12 | 0.0902 | 5.80E-04 |
| Viral myocarditis | 16 | 0.1203 | 8.03E-04 |
| Antigen processing and presentation | 16 | 0.1203 | 9.08E-04 |
| Type I diabetes mellitus | 12 | 0.0902 | 0.002375 |
| Allograft rejection | 11 | 0.0827 | 0.002977 |
| Aminoacyl-tRNA biosynthesis | 9 | 0.0677 | 0.005296 |
| Butanoate metabolism | 8 | 0.0602 | 0.006394 |
| Autoimmune thyroid disease | 11 | 0.0827 | 0.008398 |
| Cell cycle | 17 | 0.1278 | 0.012759 |
| Beta-alanine metabolism | 6 | 0.0451 | 0.015285 |
| Other glycan degradation | 5 | 0.0376 | 0.021132 |
| Limonene and pinene degradation | 4 | 0.0301 | 0.035787 |
| p53 signaling pathway | 10 | 0.0752 | 0.036731 |
| Spliceosome | 15 | 0.1128 | 0.038974 |
| Pyruvate metabolism | 7 | 0.0526 | 0.049506 |
Table 2.
Analyzed pathways of transcriptomics data regulated in lung tissues of Bufei-Jianpi-formula-treated rats
| Term | Count | % | P-value |
|---|---|---|---|
| Renal cell carcinoma | 8 | 1.5625 | 0.002333 |
| Endocytosis | 14 | 2.7344 | 0.002719 |
| Adherens junction | 8 | 1.5625 | 0.003481 |
| Focal adhesion | 13 | 2.5391 | 0.00587 |
| Pathways in cancer | 17 | 3.3203 | 0.01044 |
| Hedgehog signaling pathway | 6 | 1.1719 | 0.012268 |
| Huntington’s disease | 12 | 2.3438 | 0.012719 |
| Protein export | 3 | 0.5859 | 0.022752 |
| Wnt signaling pathway | 9 | 1.7578 | 0.040431 |
| Type II diabetes mellitus | 5 | 0.9766 | 0.041517 |
Proteomics analysis results of BJF-treated COPD rats
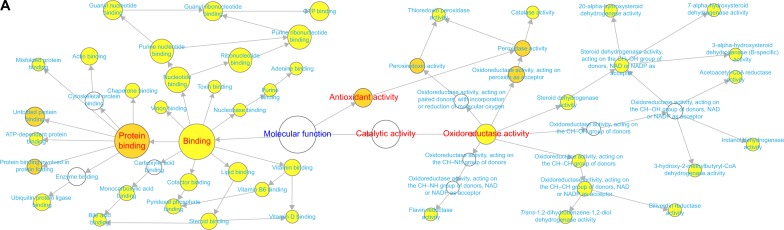
To characterize the protein expression profiles of rats, expression levels of proteins were detected using LC-MS-based proteomics. Comparing to those in healthy control rats, 192 proteins were identified in the COPD model rats (Table S3). After the treatment with BJF, comparing to those in the COPD model rats, 191 proteins were detected in BJF-treated rats (Table S4). The COPD rats (192 proteins) shared 100 common proteins with the BJF-treated rats (191 proteins). Out of the 100 proteins, expression changes of 42 proteins in the COPD model were inhibited by the BJF treatment (Table S5). These 42 proteins could be attributed to various molecular functions, such as oxidoreductase activity and antioxidant activity (peroxidase activity, catalase activity, thioredoxin peroxidase activity; Figure 2A), which might be involved in the therapeutic mechanism of BJF in treating COPD.
Figure 2.
Molecular functions of regulated proteins in lung tissues of COPD rats and BJF-treated rats.
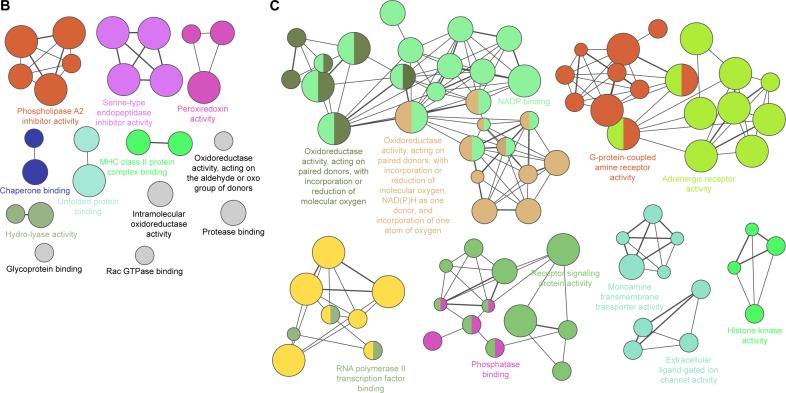
Notes: BiNGO mapped the predominant functional themes of the 42 overlapping proteins between COPD rats and BJF-treated rats and produced an intuitive and a customizable visual representation of the results (A). The molecular functions of 192 regulated proteins in COPD rats (B), and 191 regulated proteins in BJF-treated rats (C) were analyzed by ClueGO: functionally grouped network with terms as nodes linked, and functionally related groups partially overlap; the node size represents the term enrichment significance.
Abbreviation: BJF, Bufei Jianpi formula.
In addition, the 192 proteins regulated in COPD rats could be attributed to multiple molecular functions, including phospholipase A2 inhibitor activity, peroxiredoxin activity, and oxidoreductase activity, acting on the aldehyde or oxo group of donors, etc. (Figure 2B). In the BJF-treated group, 191 regulated proteins were mainly related to oxidoreductase activity, adrenergic receptor activity, receptor signaling protein activity, and extracellular ligand-gated ion channel activity (Figure 2C).
In addition, the proteins regulated in COPD rats and BJF-treated rats were mapped to various pathways, such as focal adhesion, tight junction, adherens junction, and leukocyte trans-endothelial migration (Tables 3 and 4).
Table 3.
Analyzed pathways of proteomics data regulated in lung tissues of rats with COPD
| Term | Count | % | P-value |
|---|---|---|---|
| Glycolysis/gluconeogenesis | 10 | 0.4179 | 6.64E-06 |
| HCM | 9 | 0.3761 | 5.29E-05 |
| Dilated cardiomyopathy | 9 | 0.3761 | 8.70E-05 |
| Pyruvate metabolism | 6 | 0.2507 | 3.62E-04 |
| Glyoxylate and dicarboxylate metabolism | 4 | 0.1672 | 7.99E-04 |
| Tight junction | 9 | 0.3761 | 0.001079 |
| Citrate cycle (TCA cycle) | 5 | 0.2089 | 0.00125 |
| Leukocyte transendothelial migration | 8 | 0.3343 | 0.002374 |
| Focal adhesion | 10 | 0.4179 | 0.003903 |
| Tryptophan metabolism | 5 | 0.2089 | 0.004803 |
| Valine, leucine, and isoleucine degradation | 5 | 0.2089 | 0.006122 |
| Adherens junction | 6 | 0.2507 | 0.00651 |
| Cardiac muscle contraction | 6 | 0.2507 | 0.008115 |
| Propanoate metabolism | 4 | 0.1672 | 0.015539 |
| Prion diseases | 4 | 0.1672 | 0.018217 |
| Fatty acid metabolism | 4 | 0.1672 | 0.029489 |
| ARVC | 5 | 0.2089 | 0.030762 |
| Purine metabolism | 7 | 0.2925 | 0.03707 |
Abbreviations: ARVC, arrhythmogenic right ventricular cardiomyopathy; HCM, hypertrophic cardiomyopathy; TCA, tricarboxylic acid.
Table 4.
Analyzed pathways of proteomics data regulated in lung tissues of Bufei-Jianpi-formula-treated rats
| Term | Count | % | P-value |
|---|---|---|---|
| Focal adhesion | 16 | 9.0395 | 5.73E-07 |
| Tight junction | 13 | 7.3446 | 1.26E-06 |
| Adherens junction | 9 | 5.0847 | 2.66E-05 |
| Regulation of actin cytoskeleton | 14 | 7.9096 | 3.54E-05 |
| Leukocyte transendothelial migration | 10 | 5.6497 | 1.09E-04 |
| HCM | 8 | 4.5198 | 4.49E-04 |
| Dilated cardiomyopathy | 8 | 4.5198 | 6.83E-04 |
| ECM–receptor interaction | 7 | 3.9548 | 0.002147 |
| Prion diseases | 5 | 2.8249 | 0.002534 |
| Glycolysis/gluconeogenesis | 7 | 3.9548 | 0.002584 |
| ARVC | 6 | 3.3898 | 0.007467 |
| Antigen processing and presentation | 6 | 3.3898 | 0.01515 |
| Cardiac muscle contraction | 5 | 2.8249 | 0.040267 |
| Valine, leucine, and isoleucine degradation | 4 | 2.2599 | 0.040457 |
Abbreviations: ARVC, arrhythmogenic right ventricular cardiomyopathy; ECM, extracellular matrix; HCM, hypertrophic cardiomyopathy.
COPD is known to be accompanied by pulmonary inflammation, oxidative stress, and muscle fiber dysfunction.20,21 These results suggested that BJF treatment could improve these COPD symptoms probably by regulating multiple molecular functions and pathways, such as oxidoreductase and antioxidant activity, focal adhesion, tight junction, adherens junction, and leukocyte trans-endothelial migration pathway.
Holistic views on gene, protein, and metabolite data
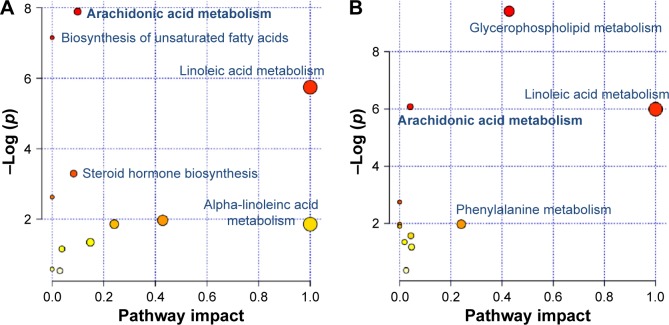
In the previous study, we detected 49 and 30 metabolites regulated in the COPD model (versus control) and BJF-treated (versus COPD model) groups, respectively.13 These metabolites were primarily involved in lipid metabolism (Figure 3A and B). In this study, a number of genes and proteins were identified, which deserve further attention to their system functions. Therefore, we further explored the system biological interpretation of the genes, proteins, and metabolites by integrating transcriptomics, proteomics, and metabolomics data.
Figure 3.
Pathway analysis of the metabolites regulated in lung tissues of COPD rats and BJF-treated rats.
Notes: Global metabolic disorders of the most relevant pathways were revealed using the MetaboAnalyst. A Google-map style interactive visualization system was implemented to facilitate data exploration and generate pathway views. (A) Representative pathway analysis of the metabolites in lung tissues of COPD rats. (B) Representative pathway analysis of the metabolites in lung tissues of BJF-treated rats.
Abbreviation: BJF, Bufei Jianpi formula.
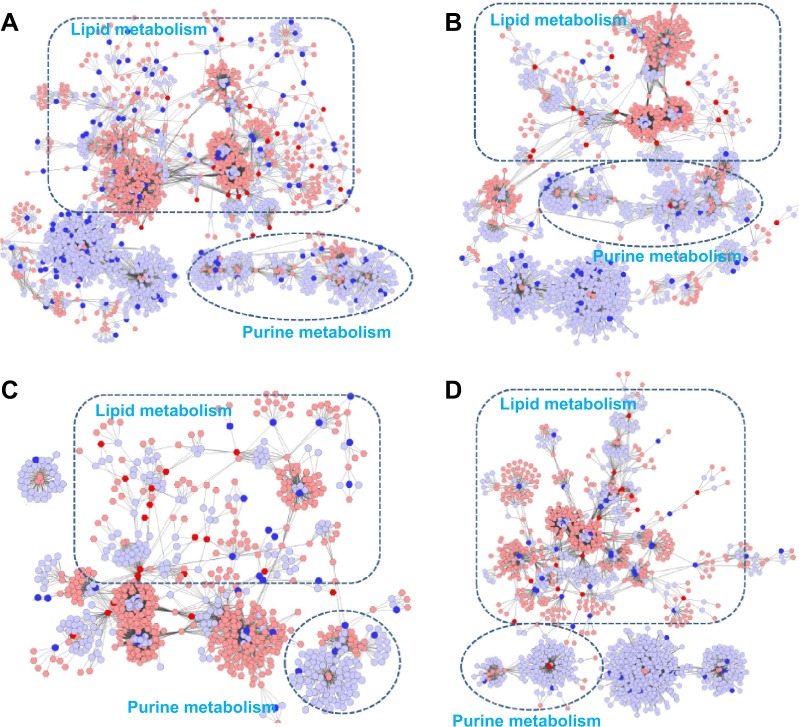
Using the Metscape software, we investigated the latent relationships of the metabolite, gene, and protein measurements by constructing the correlation network diagram. As shown in Figure 4A and B, according to the transcriptomics and metabolomics data of COPD rats and BJF-treated rats, we constructed two gene–metabolite networks, which were mainly related to lipid metabolism and purine metabolism. Interestingly, a number of genes and most of the metabolites were involved in lipid metabolism. Subsequently, we constructed two protein–metabolite networks based on the metabolomics and proteomics data of COPD rats and BJF-treated rats. As shown in Figure 4C and D, we observed that the protein–metabolite networks could be divided into two primary groups: lipid metabolism and purine metabolism, and more than half of proteins and almost all of metabolites were involved in lipid metabolism.
Figure 4.
Correlation networks of the metabolites, genes, and proteins regulated in lung tissues of COPD rats and BJF-treated rats.
Notes: The compound reaction network with compounds (the pink and red hexagons) and metabolic enzymes (the gray and blue rounds) as nodes and reactions as edges, was constructed using Metscape. Inputted compounds are shown in red, inputted genes and proteins are shown in blue. Existing compounds that populate the Metscape system were shown in pink. Existing genes and proteins were shown in gray. (A) Representative metabolites–gene network of COPD model group. (B) Representative metabolite–gene network of the BJF-treated group. (C) Representative metabolites–protein network of the COPD model group. (D) Representative metabolite–protein network of the BJF-treated group.
Abbreviation: BJF, Bufei Jianpi formula.
All of the abovementioned results indicated that lipid metabolism disorders might play a critical role in the COPD development and BJF intervention in a preclinical setting.
To explore the system relationships of the transcript and protein measurements, we compared the significant pathways derived from transcriptomics and proteomics data of COPD rats, and identified three common pathways including fatty acid metabolism, propanoate metabolism, and valine, leucine, and isoleucine degradation (Tables 1 and 3). In addition, the transcripts and proteins regulated in BJF-treated rats were mapped into three common pathways: adherens junction, tight junction, regulation of actin cytoskeleton, and dilated cardiomyopathy (Tables 2 and 4).
Integrating systems pharmacology, transcriptomics, proteomics, and metabolomics data
In the previous study, systems pharmacology successfully identified 175 targets of the active compounds contained in BJF.7 To investigate the system mechanism of BJF in treating COPD, we integrated transcriptomics, proteomics, and metabolomics data streams with systems pharmacology to provide a globe view of therapeutic mechanism of BJF treatment.
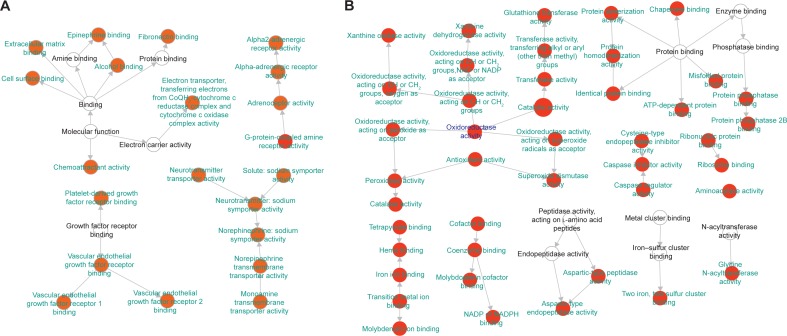
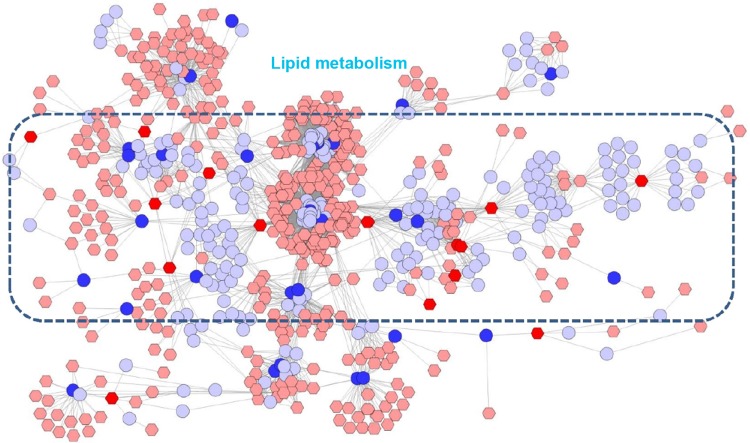
In this article, we first analyzed the overlapping proteins between the targets of BJF and transcript measurements of BJF-treated rats. As shown in Figure 5A, there were seven overlapping proteins (VEGFA, SLC6A2, ADRA2B, gyrB, CYCS, ampC, and katA) between them, which were associated with various biological functions, such as vascular endothelial growth factor receptor 1 binding, chemoattractant activity, and G-protein-coupled amine receptor activity. Subsequently, we identified eleven overlapping proteins (GSTM1, GSTP1, CTSD, MPO, HSPA5, CAT, ampC, kata, XDH, SOD1, and gyrB) between the targets of BJF and proteomic measurements of BJF-treated rats. Interestingly, the molecular functions of eleven overlapping proteins were mainly related to oxidoreductase and antioxidant activity (Figure 5B). However, no overlapping pathways were detected between the potential targets and transcript/protein measurements of BJF-treated rats. Finally, we constructed a correlation network diagram to investigate the latent relationships of the potential targets and metabolomics measurements of BJF-treated rats. In Figure 6, the results show that the network mainly consisted of lipid metabolism pathways.
Figure 5.
Molecular functions of the overlapping proteins.
Notes: BiNGO mapped the predominant functional themes of the tested protein set on the GO hierarchy and produced an intuitive and customizable visual representation of the results. The area of a node was proportional to the number of proteins in the test set annotated to the corresponding GO category. (A) Representative molecular function of overlapping proteins between the potential targets and transcript measurements in lung tissues of BJF-treated rats. (B) Representative molecular function of overlapping proteins between the potential targets and proteome measurements in lung tissues of BJF-treated rats.
Abbreviation: BJF, Bufei Jianpi formula.
Figure 6.
Correlation networks of the metabolites regulated in BJF-treated rats and target proteins.
Notes: The compound reaction network with compounds (the pink and red hexagons) and metabolic enzymes (the gray and blue rounds) as nodes and reactions as edges, was constructed using Metscape. Inputted compounds are shown in red, and inputted target proteins are shown in blue. Existing compounds that populate the Metscape system were shown in pink. Existing proteins were shown in gray.
Abbreviation: BJF, Bufei Jianpi formula.
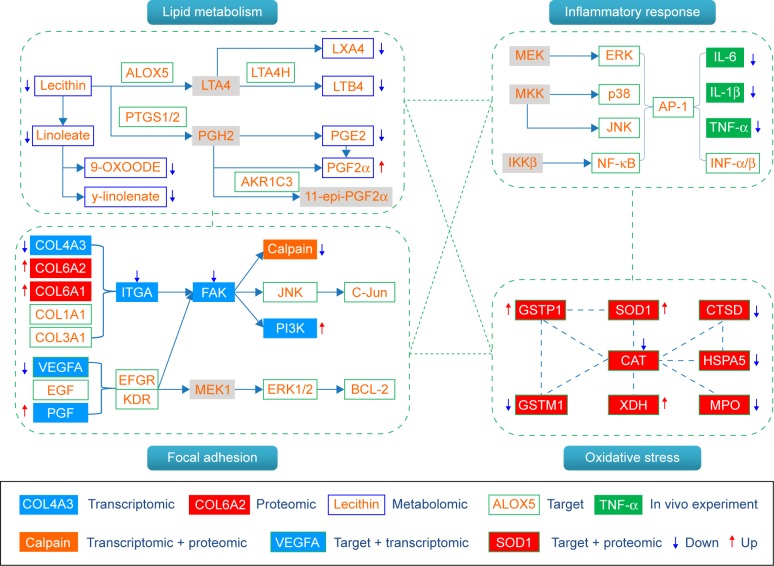
As discussed earlier, we constructed a system-level picture of the therapeutic mechanism of BJF by integrating systems pharmacology, transcript, protein, and metabolite datasets. As shown in Figure 7, the systems pharmacology targets and three-omics data were integrated, and the comprehensive picture was mainly consisted of four groups: lipid metabolism, inflammatory response, cell junction, and oxidative stress. In the previous study, we found that the expression levels of interleukin (IL)-1β, IL-6, and tumor necrosis factor-α were significantly decreased by BJF treatment. In addition, ERK, p38, JNK, and NF-κB were the potential targets of BJF.7 These results implied that BJF could achieve its ameliorative effect on COPD rats by suppressing inflammatory responses. In this study, upon BJF treatment, arachidonic acid metabolism and linoleic acid metabolism were the significant deregulated pathways in lung tissues. In these pathways, the level of lecithin, linoleate, 9-OXOODE, γ-linolenate, LXA4, LTB4, and PGE2 were suppressed by BJF treatment. Moreover, these metabolites, such as LXA4, LTB4, PGE2, and PGF2α, were involved in inflammatory processes in the airways of patients with COPD.22–25 In addition, according to the systems pharmacology predictions, ALOX5 PTGS1/2, LTA4H, and AKR1C3, the important enzymes involved in these deregulated pathways were the potential targets of BJF. Therefore, the results indicated that BJF achieved its anti-inflammatory activity by suppressing the lipid metabolism and the production of pre-inflammatory cytokines.
Figure 7.
Comprehensive analysis of the potential targets of BJF, transcriptomics, proteomics, and metabolomics regulations in lung tissues of BJF-treated rats.
Notes: The potential targets, transcriptomics, proteomics, and metabolomics data are presented as rectangles with different colors. Red arrow stands for upregulated, blue arrow for downregulated, and gray rectangle for unregulated.
Abbreviation: BJF, Bufei Jianpi formula.
The inflammatory responses associated with COPD are believed to be triggered by oxidative stress.26,27 In this study, we detected eleven overlapping proteins between the targets of BJF and proteomic measurements of BJF-treated rats, which were mainly involved in oxidative stress.28 For instance, the antioxidant proteins such as SOD1 and GSTP1 were involved in the pathogenesis of COPD,29–31 which were upregulated by BJF treatment. Additionally, increased myeloperoxidase levels are associated with rapid lung function decline.32,33 In proteomic study, we found that the expression levels of myeloperoxidase were decreased by BJF treatment. These results suggested that BJF achieved its anti-inflammatory activity by regulating the oxidative stress status.
Moreover, focal adhesion, the overlapping pathway among transcriptomic measurements, proteomic measurements, and the potential targets of BJF were significantly regulated by BJF treatment (Table 5). For instance, FAK, PI3K, JNK, c-Jun, and ERK1/2, involved in the pathogenesis of COPD progression,34–36 were the potential targets of BJF.
Table 5.
Analyzed pathways of the potential target
| Term | Count | % | P-value |
|---|---|---|---|
| Neuroactive ligand–receptor interaction | 28 | 0.7718 | 2.33E-11 |
| Pathways in cancer | 26 | 0.7166 | 1.25E-07 |
| Bladder cancer | 10 | 0.2756 | 4.18E-07 |
| Calcium signaling pathway | 18 | 0.4961 | 6.48E-07 |
| Prostate cancer | 12 | 0.3308 | 6.68E-06 |
| ALS | 9 | 0.2481 | 2.95E-05 |
| Non-small-cell lung cancer | 9 | 0.2481 | 3.39E-05 |
| Glioma | 9 | 0.2481 | 1.06E-04 |
| Small-cell lung cancer | 10 | 0.2756 | 1.49E-04 |
| Colorectal cancer | 10 | 0.2756 | 1.49E-04 |
| Gap junction | 10 | 0.2756 | 2.33E-04 |
| Pancreatic cancer | 9 | 0.2481 | 2.73E-04 |
| VEGF signaling pathway | 9 | 0.2481 | 3.63E-04 |
| Metabolism of xenobiotics by cytochrome P450 | 8 | 0.2205 | 4.93E-04 |
| Drug metabolism | 8 | 0.2205 | 6.03E-04 |
| Progesterone-mediated oocyte maturation | 9 | 0.2481 | 9.17E-04 |
| T-cell receptor signaling pathway | 10 | 0.2756 | 9.80E-04 |
| Insulin signaling pathway | 11 | 0.3032 | 0.001285 |
| Prion diseases | 6 | 0.1654 | 0.001287 |
| Melanoma | 8 | 0.2205 | 0.001367 |
| GnRH signaling pathway | 9 | 0.2481 | 0.002145 |
| Focal adhesion | 13 | 0.3583 | 0.002765 |
| NOD-like receptor signaling pathway | 7 | 0.1929 | 0.003321 |
| ErbB signaling pathway | 8 | 0.2205 | 0.004394 |
| Thyroid cancer | 5 | 0.1378 | 0.004572 |
| Type II diabetes mellitus | 6 | 0.1654 | 0.004846 |
| Alzheimer’s disease | 11 | 0.3032 | 0.005136 |
| Endometrial cancer | 6 | 0.1654 | 0.007473 |
| Arginine and proline metabolism | 6 | 0.1654 | 0.008098 |
| Neurotrophin signaling pathway | 9 | 0.2481 | 0.008989 |
| Toll-like receptor signaling pathway | 8 | 0.2205 | 0.009847 |
| Arachidonic acid metabolism | 6 | 0.1654 | 0.010189 |
| Graft-versus-host disease | 5 | 0.1378 | 0.013197 |
| Oocyte meiosis | 8 | 0.2205 | 0.015298 |
| Vascular smooth muscle contraction | 8 | 0.2205 | 0.016754 |
| Apoptosis | 7 | 0.1929 | 0.016888 |
| p53 signaling pathway | 6 | 0.1654 | 0.022214 |
| Renal cell carcinoma | 6 | 0.1654 | 0.024849 |
| Cell cycle | 8 | 0.2205 | 0.028683 |
| Glutathione metabolism | 5 | 0.1378 | 0.030289 |
| Fc epsilon RI signaling pathway | 6 | 0.1654 | 0.037359 |
| MAPK signaling pathway | 12 | 0.3308 | 0.0418 |
Abbreviations: ALS, amyotrophic lateral sclerosis; NOD, nucleotide oligomerization domain; VEGF, vascular endothelial growth factor; MAPK, mitogen-activated protein kinase.
Overall, we demonstrated that BJF provided protective and therapeutic benefits against COPD by regulating multiple biological functions, such as lipid metabolism, oxidative stress, inflammatory response, and focal adhesion pathway.
Conclusion
Three-omics technologies have emerged as the promising tools to find the complex mechanism of TCM in the form of systems biology.37,38 Systems pharmacology is applied to conduct a comprehensive analysis of the dynamic interactions between the components of TCM and a biological system. Combining systems pharmacology, transcriptomics, proteomics, and metabolomics data is becoming more important to detect the system-level therapeutic mechanism of TCM. However, attempts thus far to integrate system biology data streams have been met with limited success.
In this study, we first characterized the transcriptomic and proteomic profiles of the lung tissues derived from COPD rats and BJF-treated rats. The regulated transcripts and proteins were attributed to multiple functions, such as oxidoreductase activity and antioxidant activity. The metabolomics profiles of the lung tissues were previously characterized, and the regulated metabolites were mainly involved in lipid metabolism. Finally, we sought to obtain a comprehensive picture of systems pharmacology, transcriptomics, proteomics, and metabolomics data. The potential targets derived from systems pharmacology and three-omics data were integrated, and the system-level results indicated that BJF achieved its ameliorative effect over COPD via mechanisms that might be dependent on its system-wide effects on lipid metabolism, inflammatory response, cell junction pathways, and oxidative stress.
In summary, integrative application of systems pharmacology, transcriptome, proteome, and metabolome has potential implications toward understanding the system mechanism of action of TCM.
Acknowledgments
The research is supported by the National Natural Science Fund of China (influence and long-term effects of three Tiao-Bu Fei-Shen therapies in rats with COPD on regulation of multidimensional molecular network, No 81130062).
Footnotes
Authors contributions
JSL and SYL designed the outline of the study. PZ performed experiments, conceived the study, wrote the draft of the manuscript, and revised the manuscript. LPY, YGT, and YL were involved in performing experiments, acquisition of data, and statistical analysis. All authors contributed toward data analysis, drafting and critically revising the paper and agree to be accountable for all aspects of the work. All the authors read and approved the final version of the manuscript.
Disclosure
The authors report no conflicts of interest in this study.
References
- 1.Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease GOLD executive summary. Am J Respir Crit Care Med. 2013;187:347–365. doi: 10.1164/rccm.201204-0596PP. [DOI] [PubMed] [Google Scholar]
- 2.Bafadhel M, McCormick M, Saha S, et al. Profiling of sputum inflammatory mediators in asthma and chronic obstructive pulmonary disease. Respiration. 2012;83:36–44. doi: 10.1159/000330667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Woodruff PG, Agusti A, Roche N, Singh D, Martinez FJ. Current concepts in targeting chronic obstructive pulmonary disease pharmacotherapy: making progress towards personalised management. Lancet. 2015;385:1789–1798. doi: 10.1016/S0140-6736(15)60693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li SY, Li JS, Wang MH, et al. Effects of comprehensive therapy based on traditional Chinese medicine patterns in stable chronic obstructive pulmonary disease: a four-center, open-label, randomized, controlled study. BMC Complement Altern Med. 2012;12:197. doi: 10.1186/1472-6882-12-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li S. Network systems underlying traditional Chinese medicine syndrome and herb formula. Curr Bioinform. 2009;4:188–196. [Google Scholar]
- 6.Jia W, Gao WY, Yan YQ, et al. The rediscovery of ancient Chinese herbal formulas. Phytother Res. 2004;18:681–686. doi: 10.1002/ptr.1506. [DOI] [PubMed] [Google Scholar]
- 7.Zhao P, Li J, Li Y, Tian Y, Wang Y, Zheng C. Systems pharmacology-based approach for dissecting the active ingredients and potential targets of the Chinese herbal Bufei Jianpi formula for the treatment of chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:2633–2656. doi: 10.2147/COPD.S94043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang Q, Tan YX, Yin PY, et al. Metabolic characterization of hepatocellular carcinoma using nontargeted tissue metabolomics. Cancer Res. 2013;73:4992–5002. doi: 10.1158/0008-5472.CAN-13-0308. [DOI] [PubMed] [Google Scholar]
- 9.Heijne WH, Kienhuis AS, van Ommen B, Stierum RH, Groten JP. Systems toxicology: applications of toxicogenomics, transcriptomics, proteomics and metabolomics in toxicology. Expert Rev Proteomics. 2005;2:767–780. doi: 10.1586/14789450.2.5.767. [DOI] [PubMed] [Google Scholar]
- 10.Meierhofer D, Weidner C, Sauer S. Integrative analysis of transcriptomics, proteomics, and metabolomics data of white adipose and liver tissue of high-fat diet and rosiglitazone-treated insulin-resistant mice identified pathway alterations and molecular hubs. J Proteome Res. 2014;13:5592–5602. doi: 10.1021/pr5005828. [DOI] [PubMed] [Google Scholar]
- 11.Su G, Burant CF, Beecher CW, Athey BD, Meng F. Integrated metabolome and transcriptome analysis of the NCI60 dataset. BMC Bioinformatics. 2011;12(suppl 1):S36. doi: 10.1186/1471-2105-12-S1-S36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gehlenborg N, O’Donoghue SI, Baliga NS, et al. Visualization of omics data for systems biology. Nat Methods. 2010;7:S56–S68. doi: 10.1038/nmeth.1436. [DOI] [PubMed] [Google Scholar]
- 13.Li JS, Yang LP, Li Y, et al. Metabolomics study on model rats of chronic obstructive pulmonary disease treated with Bu-Fei Jian-Pi. Mol Med Rep. 2015;11:1324–1333. doi: 10.3892/mmr.2014.2843. [DOI] [PubMed] [Google Scholar]
- 14.Li Y, Li SY, Li JS, et al. A rat model for stable chronic obstructive pulmonary disease induced by cigarette smoke inhalation and repetitive bacterial infection. Biol Pharm Bull. 2012;35:1752–1760. doi: 10.1248/bpb.b12-00407. [DOI] [PubMed] [Google Scholar]
- 15.Maere S, Heymans K, Kuiper M. BiNGO: a cytoscape plugin to assess overrepresentation of gene ontology categories in biological networks. Bioinformatics. 2005;21:3448–3449. doi: 10.1093/bioinformatics/bti551. [DOI] [PubMed] [Google Scholar]
- 16.Karnovsky A, Weymouth T, Hull T, et al. Metscape 2 bioinformatics tool for the analysis and visualization of metabolomics and gene expression data. Bioinformatics. 2012;28:373–380. doi: 10.1093/bioinformatics/btr661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bindea G, Mlecnik B, Hackl H, et al. ClueGO: a cytoscape plug-in to decipher functionally grouped gene ontology and pathway annotation networks. Bioinformatics. 2009;25:1091–1093. doi: 10.1093/bioinformatics/btp101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xia JG, Sinelnikov IV, Han B, Wishart DS. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015;43:W251–W257. doi: 10.1093/nar/gkv380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Szklarczyk D, Franceschini A, Wyder S, et al. STRING v10: protein-protein interaction networks, integrated over the tree of life. Nucleic Acids Res. 2015;43:D447–D452. doi: 10.1093/nar/gku1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.MacNee W. Pathogenesis of chronic obstructive pulmonary disease. Clin Chest Med. 2007;28:479–513. doi: 10.1016/j.ccm.2007.06.008. [DOI] [PubMed] [Google Scholar]
- 21.Barnes PJ, Stockley RA. COPD: current therapeutic interventions and future approaches. Eur Respir J. 2005;25:1084–1106. doi: 10.1183/09031936.05.00139104. [DOI] [PubMed] [Google Scholar]
- 22.Tufvesson E, Bjermer L, Ekberg M. Patients with chronic obstructive pulmonary disease and chronically colonized with Haemophilus influenzae during stable disease phase have increased airway inflammation. Int J Chron Obstruct Pulmon Dis. 2015;10:881–889. doi: 10.2147/COPD.S78748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tulah AS, Parker SG, Moffatt MF, Wardlaw AJ, Connolly MJ, Sayers I. The role of ALOX5AP, LTA4H and LTB4R polymorphisms in determining baseline lung function and COPD susceptibility in UK smokers. BMC Med Genet. 2011;12:173. doi: 10.1186/1471-2350-12-173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang R, Li M, Zhou S, et al. Effect of a single nucleotide polymorphism in miR-146a on COX-2 protein expression and lung function in smokers with chronic obstructive pulmonary disease. Int J Chron Obstruct Pulmon Dis. 2015;10:463–473. doi: 10.2147/COPD.S74345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hoffmeyer F, Harth V, Bunger J, Bruning T, Raulf-Heimsoth M. Leukotriene B4, 8-iso-prostaglandin F2 alpha, and pH in exhaled breath condensate from asymptomatic smokers. J Physiol Pharmacol. 2009;60(suppl 5):57–60. [PubMed] [Google Scholar]
- 26.Rahman I, Adcock IM. Oxidative stress and redox regulation of lung inflammation in COPD. Eur Respir J. 2006;28:219–242. doi: 10.1183/09031936.06.00053805. [DOI] [PubMed] [Google Scholar]
- 27.Rahman I, MacNee W. Antioxidant pharmacological therapies for COPD. Curr Opin Pharmacol. 2012;12:256–265. doi: 10.1016/j.coph.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inonu H, Doruk S, Sahin S, et al. Oxidative stress levels in exhaled breath condensate associated with COPD and smoking. Respir Care. 2012;57:413–419. doi: 10.4187/respcare.01302. [DOI] [PubMed] [Google Scholar]
- 29.Harju T, Kaarteenaho-Wiik R, Sirvio R, et al. Manganese superoxide dismutase is increased in the airways of smokers’ lungs. Eur Respir J. 2004;24:765–771. doi: 10.1183/09031936.04.00121203. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka K, Sato K, Aoshiba K, Azuma A, Mizushima T. Superiority of PC-SOD to other anti-COPD drugs for elastase-induced emphysema and alteration in lung mechanics and respiratory function in mice. Am J Physiol Lung Cell Mol Physiol. 2012;302:L1250–L1261. doi: 10.1152/ajplung.00019.2012. [DOI] [PubMed] [Google Scholar]
- 31.Lakhdar R, Denden S, Mouhamed MH, et al. Correlation of EPHX1, GSTP1, GSTM1, and GSTT1 genetic polymorphisms with antioxidative stress markers in chronic obstructive pulmonary disease. Exp Lung Res. 2011;37:195–204. doi: 10.3109/01902148.2010.535093. [DOI] [PubMed] [Google Scholar]
- 32.Park HY, Man SF, Tashkin D, et al. The relation of serum myeloperoxidase to disease progression and mortality in patients with chronic obstructive pulmonary disease (COPD) PLoS One. 2013;8:e61315. doi: 10.1371/journal.pone.0061315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Churg A, Marshall CV, Sin DD, et al. Late intervention with a myeloperoxidase inhibitor stops progression of experimental chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2012;185:34–43. doi: 10.1164/rccm.201103-0468OC. [DOI] [PubMed] [Google Scholar]
- 34.Hsu AC, Starkey MR, Hanish I, et al. Targeting PI3K-p110alpha suppresses influenza virus infection in chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;191:1012–1023. doi: 10.1164/rccm.201501-0188OC. [DOI] [PubMed] [Google Scholar]
- 35.Huang Y, Meng XM, Jiang GL, et al. Studies on mitogen-activated protein kinase signaling pathway in the alveolar macrophages of chronic bronchitis rats. Mol Cell Biochem. 2015;400:97–105. doi: 10.1007/s11010-014-2266-1. [DOI] [PubMed] [Google Scholar]
- 36.Mitani A, Ito K, Vuppusetty C, Barnes PJ, Mercado N. Restoration of corticosteroid sensitivity in chronic obstructive pulmonary disease by inhibition of mammalian target of rapamycin. Am J Respir Crit Care Med. 2016;193:143–153. doi: 10.1164/rccm.201503-0593OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Quintana-Murci L, Chaix R, Wells RS, et al. Where west meets east: the complex mtDNA landscape of the southwest and Central Asian corridor. Am J Hum Genet. 2004;74:827–845. doi: 10.1086/383236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang A, Sun H, Wang Z, Sun W, Wang P, Wang X. Metabolomics: towards understanding traditional Chinese medicine. Planta Med. 2010;76:2026–2035. doi: 10.1055/s-0030-1250542. [DOI] [PubMed] [Google Scholar]