Abstract
OBJECTIVES
Perioperative blood pressure management by targeting individualized optimal blood pressure, determined by cerebral blood flow autoregulation monitoring, may ensure sufficient renal perfusion. The purpose of this study was to evaluate changes in the optimal blood pressure for individual patients, determined during cardiopulmonary bypass (CPB) and during early postoperative period in intensive care unit (ICU). A secondary aim was to examine if excursions below optimal blood pressure in the ICU are associated with risk of cardiac surgery-associated acute kidney injury (CSA-AKI).
METHODS
One hundred and ten patients undergoing cardiac surgery had cerebral blood flow monitored with a novel technology using ultrasound tagged near infrared spectroscopy (UT-NIRS) during CPB and in the first 3 h after surgery in the ICU. The correlation flow index (CFx) was calculated as a moving, linear correlation coefficient between cerebral flow index measured using UT-NIRS and mean arterial pressure (MAP). Optimal blood pressure was defined as the MAP with the lowest CFx. Changes in optimal blood pressure in the perioperative period were observed and the association of blood pressure excursions (magnitude and duration) below the optimal blood pressure [area under the curve (AUC) < OptMAP mmHgxh] with incidence of CSA-AKI (defined using Kidney Disease: Improving Global Outcomes criteria) was examined.
RESULTS
Optimal blood pressure during early ICU stay and CPB was correlated (r = 0.46, P < 0.0001), but was significantly higher in the ICU compared with during CPB (75 ± 8.7 vs 71 ± 10.3 mmHg, P = 0.0002). Thirty patients (27.3%) developed CSA-AKI within 48 h after the surgery. AUC < OptMAP was associated with CSA-AKI during CPB [median, 13.27 mmHgxh, interquartile range (IQR), 4.63–20.14 vs median, 6.05 mmHgxh, IQR 3.03–12.40, P = 0.008], and in the ICU (13.72 mmHgxh, IQR 5.09–25.54 vs 5.65 mmHgxh, IQR 1.71–13.07, P = 0.022).
CONCLUSIONS
Optimal blood pressure during CPB and in the ICU was correlated. Excursions below optimal blood pressure (AUC < OptMAP mmHgXh) during perioperative period are associated with CSA-AKI. Individualized blood pressure management based on cerebral autoregulation monitoring during the perioperative period may help improve CSA-AKI-related outcomes.
Keywords: Cerebral autoregulation, Acute kidney injury, Haemodynamic management, Perioperative care
INTRODUCTION
Prior work from our group supports the contention that real-time monitoring of cerebral blood flow (CBF) autoregulation to guide intraoperative blood pressure management may help ensure better organ perfusion for patients undergoing cardiac surgery than the current standard of care, based on empiric blood pressure targets [1–5]. We have found that the lower limits of CBF autoregulation are broad (i.e. 40–90 mmHg) and it is difficult to predict based on preoperative demographic and blood pressure data [6]. Furthermore, our prior publications show that intraoperatively for patients undergoing cardiac surgery, the magnitude and duration that mean arterial blood pressure (MAP) is outside the limits of autoregulation is associated with postoperative complications such as kidney injury (CSA-AKI), major organ morbidity and mortality, including stroke, and delirium [1–3, 5].
Monitoring of cerebral autoregulation in our investigations thus far has been based on transcranial Doppler measurement of middle cerebral artery CBF velocity [6–9]. Transcranial Doppler has many limitations that limit its use to mainly research including the need for special equipment and technical training, susceptibility to motion and electrical artefact and inability to find trans-temporal insonating windows in some patients. Clinical translation of cerebral autoregulation monitoring to diverse practice settings will require the availability of devices that provide reliable, continuous measurement of CBF or its surrogates but with minimal care-giver intervention. We have recently demonstrated that a non-invasive, continuous monitor of CBF using an innovative ultrasound tagged near infrared spectroscopy (UT-NIRS) method compared favourably with transcranial Doppler for cerebral autoregulation monitoring of patients during cardiopulmonary bypass (CPB) [10].
Haemodynamic fluctuations do not stop with completion of surgery and occur frequently after cardiac surgery in patients after they have been admitted to the intensive care unit (ICU). We have found that postoperative blood pressure excursions below the optimal blood pressure, determined intraoperatively with cerebral autoregulation monitoring, are associated with compromised brain perfusion [4]. Monitoring of cerebral autoregulation for guiding blood pressure targets in the ICU, thus, might have similar benefits for patient outcomes as we have found during CPB. It is currently unknown, however, whether optimal MAP determined with cerebral autoregulation monitoring during CPB remains unchanged postoperatively in the ICU when the patients have pulsatile blood flow and whether blood pressure aberrations are associated with compromised perfusion to other organs than just the brain. The purpose of this study was to assess whether optimal MAP determined by cerebral autoregulation monitoring in the ICU after surgery is similar to that measured during CPB. We further sought to examine if excursions below optimal blood pressure in the ICU are associated with risk of cardiac surgery-associated acute kidney injury (CSA-AKI). For this study, cerebral autoregulation monitoring was performed with a novel UT-NIRS method.
METHODS
Between July 2013 and June 2015, 110 patients undergoing cardiac surgery with CPB were enrolled in a prospective observational study (clinical trial registration no. NCT02084394). All patients being admitted for cardiac surgery fulfilling the criteria were approached for participation in the study. The inclusion criteria included patients 50 years or older undergoing CPB; patient who can speak and understand English; and patients who understand the nature of the study and are willing to sign the consent form. The exculsion criteria included emergency surgery patients; patients with neurocognitive disorders; minimental state examination score less than 21; patients taking antipsychotic drugs; patients with implant in the brain underneath CerOx probes; and women who are pregnant. The study was approved by the Institutional Review Board of The Johns Hopkins Medical Institutions and all patients provided written, informed consent before participation.
The methodology detailed below along with its rationale has already been published by our group [10].
Patient care
Perioperative care, standard for routine cardiac surgery cases, was provided to the patients including monitoring of direct radial artery blood pressure and anaesthesia with midazolam, fentanyl and isoflurane. CPB was performed with a target flow between 2.0 and 2.4 l/min/m2 and α-stat pH management. Arterial blood gases were measured 10 min after initiation of CPB, and then hourly. Normocarbia was maintained by adjusting gas flow on the heart–lung machine. This was based on continuous in-line blood gas monitoring.
Monitoring of cerebral blood flow autoregulation
Ultrasound tagged-NIRS monitoring was performed using a CerOx™ (Ornim, Inc., Kfar Saba, Israel) monitor during CPB and in the ICU for 3 h after surgery [11–13]. Three hours post-surgery monitoring was chosen as patients were still in sleep and the readings from the CerOx monitoring were stable excluding any potential confounding variables associated with body movements. Adhesive pads were attached on both sides of the forehead, and probes were attached to the adhesive pads using the manufacturer's guidelines. A circumferential elastic band was then placed around the head to secure the probes in direct contact with the scalp. Analogue arterial pressure data from the operating room haemodynamic monitor and CerOx™ signals were sampled with an analogue-to-digital converter at 60 Hz and then processed with ICM + software version 6.1 (University of Cambridge, Cambridge, UK) as previously described [8, 9]. To limit the analysis to the frequency of slow vasogenic waves (0.05–0.003 Hz), relevant to cerebral autoregulation, the signals were time-integrated as non-overlapping 10-s mean values, which is equivalent to applying a moving average filter with a 10-s time window and re-sampling at 0.1 Hz. The latter was performed to eliminate high-frequency noise from the respiratory and pulse frequencies, while allowing detection of oscillations and transients that occur below 0.05 Hz [8, 9]. The signals were further high pass filtered with a DC cut-off set at 0.003 Hz to remove slow drifts associated with haemodilution at the onset of bypass, blood transfusions, cooling and rewarming. A continuous, moving Pearson's correlation coefficient was calculated between the changes in MAP and CerOx™ data, generating the variables correlation flow index (CFx) [10]. Consecutive paired 10-s averaged values from 300-s duration were used for each calculation, incorporating 30 data points for each index [8, 9]. CFx approaches zero or is negative when cerebral autoregulation is functional whereas CFx value approaches 1 when reaching limits of cerebral autoregulation.
Patient outcomes
The development of CSA-AKI was defined based on the Kidney Disease: Improving Global Outcomes (KDIGO) classification system: Stage 1: 1.5–1.9 times baseline or >0.3 mg/dl increase in serum creatinine; Stage 2: an increase in plasma creatinine × 2.0–2.9 from baseline; and Stage 3: an increase in plasma creatinine × 3 or increase in serum creatinine to >4.0 mg/dl or initiation of renal replacement therapy [14, 15]. The last serum creatinine measured before surgery was used as the preoperative creatinine. Serum creatinine levels were measured in the Clinical Chemistry Laboratory of Johns Hopkins Hospital (Roche Diagnostics, Indianapolis, IN, USA). The estimated glomerular filtration rate (eGFR) was calculated by using Chronic Kidney Disease Epidemiology Collaboration creatinine equation [16]. Patients meeting any of the KDIGO criteria were considered to have CSA-AKI.
Data analysis
The normality of the distribution of the data was assessed by the Kolmogorov–Smirnov test. Normally distributed continuous variables were analysed by the Student's t-test and paired t-test was used for paired analysis. Data that remained non-normally distributed after logarithmic transformation were analysed by Mann–Whitney or Wilcoxon test for paired comparison. Categorical variables were analysed by Fisher's exact test. Test for correlation was performed by Pearson's correlation and Spearman's correlation for data that were not normally distributed.
To compare optimal MAP determined during CPB and in the ICU, CFx were categorized and averaged in 5-mmHg MAP bins for each patient [9]. Optimal MAP was defined as the MAP with the best autoregulation or that MAP where CFx was lowest or least correlated with changes in blood pressure. The right- and left-sided optimal MAP values were averaged to define the individual optimal MAP value.
The patients were dichotomized into two groups based on whether or not they developed CSA-AKI. Blood pressure excursions below the optimal blood pressure were defined as the sum of the product of the difference in MAP from the optimal blood pressure and the time spent at each mean arterial pressure (MAP) below optimal MAP (AUC < OptMAP). This variable is equivalent to area under the curve (AUC): ∑ΔMAP in mmHg below the optimal MAP × time on CPB in h at each MAP, with units of mmHgxHr.
Multivariate logistic regression for incidence of AKI was evaluated including the primary variable, blood pressure excursions below the optimal blood pressure and other possible confounding factors: total CPB time; total cross-clamp time; preoperative eGFR; surgical procedures other than isolated CABG [17, 18]. P values less than 0.05 were considered statistically significant. All analyses were performed using Stata (Version 13.1, Stata Corp, College Station, TX, USA).
RESULTS
Demographic and clinical data for the 110 patients included in the study are listed in Table 1. The mean age of the patients was 65 ± 8.8 years and the duration of CPB was 129 ± 64 min. Physiological data during CPB and for the first 3 h in the ICU are listed in Table 2. There was a significant decrease in average CFx in the ICU compared with that measured during CPB (ICU: 0.12 ± 0.10 vs CPB: 0.33 ± 0.17, P < 0.0001), indicating better preserved average autoregulation after surgery with return of pulsatile flow. MAP was higher in the ICU than during CPB (75.0 ± 8.5 vs 70.0 ± 7.5 mmHg, P < 0.0001). There were other differences between arterial blood gas results and haemoglobin levels between the CPB and ICU study periods (Table 2).
Table 1:
Demographic and operative characteristics of the studied patients
| Entire population (n = 110) | |
|---|---|
| Age (years)a | 65 ± 8.8 |
| Male (%) | 67 (60.9%) |
| Baseline estimated glomerular filtration rate (ml × min−1 × 1.73 m−2)a | 69.1 ± 19.8 |
| Chronic obstructive pulmonary disease (%) | 12 (10.9%) |
| Hypertension (%) | 79 (71.8%) |
| Diabetes (%) | 33 (30.0%) |
| Coronary artery disease (%) | 73 (66.4%) |
| Previous percutaneous coronary intervention (%) | 18 (16.4%) |
| Valve disease (%) | 46 (41.8%) |
| Previous smoker (%) | 54 (49.1%) |
| Current smoker (%) | 18 (16.4%) |
| Aspirin (%) | 77 (70.0%) |
| Angiotensin-converting enzyme inhibitors-I (%) | 30 (27.3%) |
| Calcium channel blocker (%) | 27 (24.5%) |
| β-Adrenergic receptor blocker (%) | 61 (55.5%) |
| Statin (%) | 65 (59.1%) |
| Diuretics (%) | 38 (34.5%) |
| Peripheral vascular disease (%) | 8 (7.3%) |
| Prior carotid endarterectomy (%) | 1 (0.9%) |
| Prior cerebral vascular accident (%) | 6 (5.5%) |
| Prior TIA (%) | 3 (2.7%) |
| Surgical procedure | |
| CABG | 58 (52.8%) |
| CABG + AVR | 16 (14.5%) |
| AVR/MVR | 34 (30.9%) |
| Others | 2 (1.8%) |
| Duration of cardiopulmonary bypass (min)a | 129 ± 64.2 |
| Total cross-clamp time (min)a | 81 ± 29.0 |
| Postoperative outcome | |
| Acute kidney injury (%) | 30 (27.3%) |
| Low cardiac output syndrome (%) | 23 (20.9%) |
| Major morbidity and mortality (%) | 32 (29.1%) |
| Operative death (%) | 3 (2.7%) |
LCOS was defined as the usage of inotropes for 24 h or new intra-aortic balloon pump insertion.
LCOS: low cardiac output syndrome;
AVR: aortic valve replacement; CABG: coronary artery bypass graft; LVAD: left ventricular assisting device placement; MVR: mitral valve replacement or repair; Statin: HMG-CoA reductase inhibitors; TIA: transient ischaemic attack.
aData are listed as mean ± SD.
Table 2:
Physiological data during CPB and the first 3 h in the ICU
| CPB | ICU | P-value | |
|---|---|---|---|
| Correlation flow indexa | 0.33 ± 0.17 | 0.12 ± 0.10 | <0.0001 |
| Mean arterial pressure (mmHg)a | 70 ± 7.5 | 75 ± 8.5 | <0.0001 |
| pHa | 7.38 ± 0.03 | 7.35 ± 0.05 | <0.0001 |
| PO2 (mmHg)a | 252.2 ± 37.9 | 129.1 ± 44.9 | <0.0001 |
| PCO2 (mmHg)a | 41.1 ± 1.9 | 38.6 ± 4.6 | <0.0001 |
| HCO3 (mmol/l)a | 24.5 ± 1.5 | 20.9 ± 2.1 | <0.0001 |
| Mean haemoglobin (g/dl)a | 9.3 ± 1.2 | 10.4 ± 1.4 | <0.0001 |
CPB: cardiopulmonary bypass; ICU: intensive care unit; SD: standard deviation.
aData are listed as mean ± SD.
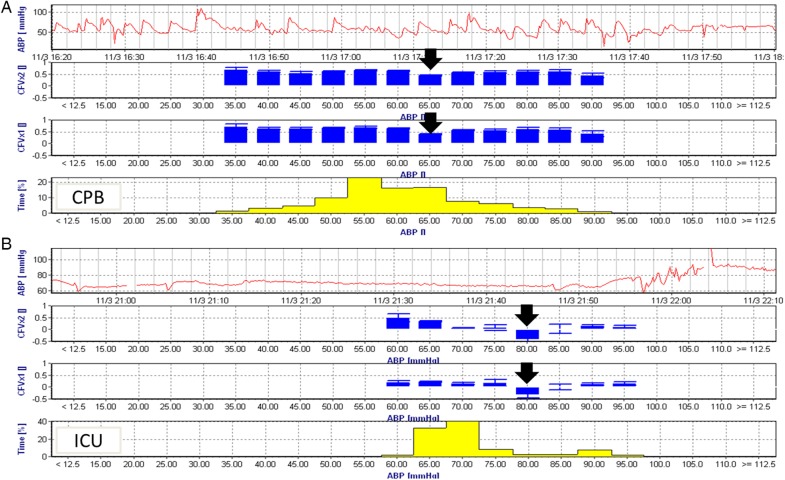
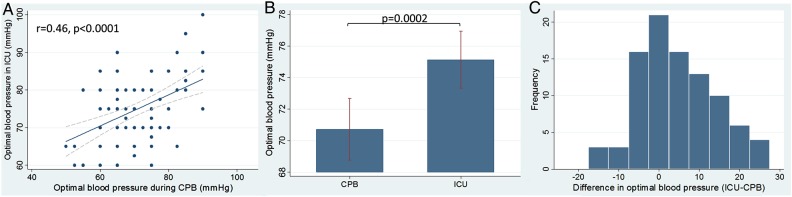
In the ICU, optimal MAP could not be identified in 18 (16.4%) patients due to limited variability in blood pressure. A representative CBF autoregulation monitoring recording is shown in Fig. 1. Optimal MAP measured during CPB was correlated with optimal MAP measured in the ICU (r = 0.46, 95% CI, 0.28–0.61, P < 0.0001) as shown in Fig. 2A. Optimal MAP measured in the ICU was higher than that measured during CPB (75 ± 8.7 vs 71 ± 10.3 mmHg, P = 0.0002) as shown in Fig. 2B. The distribution of optimal MAP in the ICU compared with that measured during CPB is shown in Fig. 2C. Optimal MAP in the ICU was higher in 49 (53.3%) patients, the same in 13 (14.1%) patients and lower in 30 (32.6%) patients compared with that measured during CPB.
Figure 1:
A representative cerebral autoregulation monitoring recording from a single patient obtained during CPB (A) and after ICU admission (B). The top graph in each recording is the time series of ABP while the next two graphs represent the CFx from the left and right brain hemispheres. The latter represent the average CFx values placed in 5 mmHg blood pressure bins. When cerebral blood flow is in the autoregulated range, CFx is close to zero but when blood pressure is below or above the autoregulation range CFx approaches 1. The bottom graph (B) represents the percentage of time during the recording where blood pressure was in each 5 mmHg bins. Optimal mean arterial pressure is defined as the pressure with the lowest CFx (black arrow) or that blood pressure with the best autoregulation. Note, that during CPB (A), the optimal blood pressure is 65 mmHg. During the ICU period (B) the optimal blood pressure was 80 mmHg. The time scales between the two periods are different but note that blood pressure variability was more pronounced during CPB than in the ICU. Blood pressure increased around 21:25 h in the ICU likely coinciding with emergence from sedation and weaning from mechanical ventilation. CPB: cardiopulmonary bypass; ICU: intensive care unit; ABP: arterial pressure; CFx: correlation flow index.
Figure 2:
The relationship between optimal MAP determined with cerebral autoregulation monitoring measured during CPB and in the ICU in the immediate postoperative period. (A) Optimal MAP blood pressure measured during CPB and in the ICU is correlated r = 0.46, P < 0.0001. (B) Average optimal MAP is higher in the ICU compared with during CPB (P = 0.0002). (C) Distribution of the differences between optimal MAP in the ICU and during CPB where zero is the same optimal MAP between the two study periods. Optimal MAP was higher in the ICU than during CPB in 49 (53.3%) patients, while it was lower in 30 (32.6%) patients. MAP: mean arterial pressure; CPB: cardiopulmonary bypass; ICU: intensive care unit.
Thirty patients (27.3%) developed CSA-AKI within 48 h after CPB. Among those who developed AKI, 24 patients (21.8%) were Stage 1, 5 patients (4.6%) were Stage 2 and 1 patient (0.9%) was Stage 3. Demographic and operative data for patients who either developed or did not develop CSA-AKI is given in Table 3. There was a trend towards longer duration of CPB (147 ± 53.8 vs 122 ± 66.9 min, P = 0.085) and significantly longer duration of aortic cross-clamping (92 ± 30.9 vs 77 ± 27.4 min, P = 0.014) for patients who developed CSA-AKI. There were no significant differences in other preoperative variables (Table 2).
Table 3:
Demographic data for patients with and without AKI
| No AKI (n = 80) | AKI (n = 30) | P-value | |
|---|---|---|---|
| Age (years)a | 65 ± 8.7 | 65 ± 9.2 | 0.87 |
| Male (%) | 48 (60.0%) | 19 (63.3%) | 0.83 |
| LVEFb | 55 (49–60) | 58 (50–60) | 0.16 |
| Baseline estimated glomerular filtration rate (ml × min−1 × 1.73 m−2)a | 70.3 ± 19.1 | 66.0 ± 21.8 | 0.32 |
| Baseline creatinine (mg/dl)a | 1.1 ± 0.8 | 1.2 ± 0.5 | 0.71 |
| Creatinine at 48 h after surgery (mg/dl)a | 1.1 ± 0.6 | 1.9 ± 0.6 | <0.0001 |
| Chronic obstructive pulmonary disease (%) | 8 (10.0%) | 4 (13.3%) | 0.73 |
| Hypertension (%) | 56 (70.0%) | 23 (76.7%) | 0.64 |
| Diabetes (%) | 25 (31.3%) | 8 (26.7%) | 0.82 |
| Coronary artery disease (%) | 55 (68.8%) | 18 (60.0%) | 0.5 |
| Previous percutaneous coronary intervention (%) | 13 (16.3%) | 5 (16.7%) | 1 |
| Valve disease (%) | 32 (40.0%) | 14 (46.7%) | 0.67 |
| Previous smoker (%) | 37 (46.3%) | 17 (56.7%) | 0.39 |
| Current smoker (%) | 11 (13.8%) | 7 (23.3%) | 0.25 |
| Aspirin (%) | 57 (71.3%) | 20 (66.7%) | 0.65 |
| Angiotensin-converting enzyme inhibitors-I (%) | 24 (30.0%) | 6 (20.0%) | 0.35 |
| Calcium channel blocker (%) | 17 (21.3%) | 10 (33.3%) | 0.22 |
| β-Adrenergic receptor blocker (%) | 46 (57.5%) | 15 (50.0%) | 0.67 |
| Statin (%) | 48 (60.0%) | 17 (56.7%) | 0.83 |
| Diuretics (%) | 28 (35.0%) | 10 (33.3%) | 1 |
| Peripheral vascular disease (%) | 4 (5.0%) | 4 (13.3%) | 0.21 |
| Prior carotid endarterectomy (%) | 1 (1.3%) | 0 (0.0%) | 1 |
| Prior cerebral vascular accident (%) | 5 (6.3%) | 1 (3.3%) | 1 |
| Prior TIA (%) | 2 (2.5%) | 1 (3.3%) | 1 |
| Surgical procedure | |||
| CABG | 47 (58.8%) | 11 (36.7%) | 0.113 |
| CABG + AVR | 9 (11.3%) | 7 (23.3%) | |
| AVR/MVR | 23 (28.8%) | 11 (36.7%) | |
| Others | 1 (1.3%) | 1 (3.3%) | |
| Duration of cardiopulmonary bypass (min)a | 122 ± 66.9 | 147 ± 53.8 | 0.08 |
| Total cross-clamp time (min)a | 77 ± 27.4 | 92 ± 30.9 | 0.014 |
| Postoperative hospital stay (days)b | 6 (5–8) | 9 (7–11) | 0.0002 |
AVR: aortic valve replacement; AKI: acute kidney injury; CABG: coronary artery bypass graft; LVAD: left ventricular assisting device placement; MVR: mitral valve replacement or repair; Statin: HMG-CoA reductase inhibitors; TIA: transient ischaemic attack; LVEF: left ventricular ejection fraction; SD: standard deviation.
aData are listed as mean ± SD for continuous variables that were normally distributed.
bData are listed as median (interquartile range) for continuous variables that were non-normally distributed.
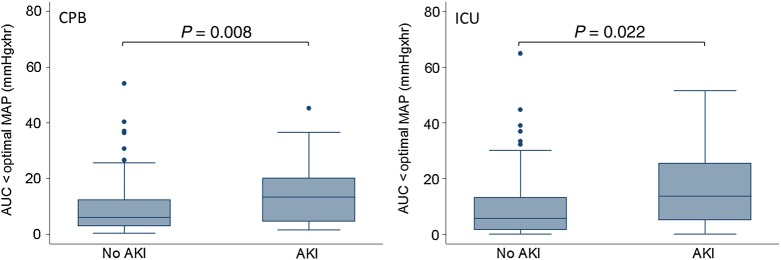
The association between AUC < OptMAP during CPB and in the ICU was evaluated for their relationship with risk for CSA-AKI (Fig. 3). There were significant differences in AUC < OptMAP during CPB [AKI, median, 13.27 mmHgxh, interquartile range (IQR), 4.63–20.14 vs no AKI, median, 6.05 mmHgxh, IQR 3.03–12.40, P = 0.008] and in the ICU (AKI, median, 13.72 mmHgxh, IQR 5.09–25.54 vs no AKI, median, 5.65 mmHgxh, IQR 1.71–13.07, P = 0.022) for patients with and without CSA-AKI. However, there were no significant differences in blood pressure excursion below specific MAP (i.e. 60, 70, 80 mmHg) during CPB and in the ICU (Table 4).
Figure 3:
Box and whisker plot showing blood pressure excursion below the optimal blood pressure during CPB and in the ICU for patients with and without CSA-AKI. The horizontal line in the shaded box represents the median value, and the shaded box represents the interquartile range. The error bars below and above the shaded area represents ±1.5 × the interquartile range; points beyond the error bar are outliers. CPB: cardiopulmonary bypass; ICU: intensive care unit; CSA-AKI: cardiac surgery-associated acute kidney injury.
Table 4:
The effect of blood pressure excursion below specific MAP during CPB or during the first 3 h after admission to the ICU for the entire cohort and for those with and without AKI
| Entire | No AKI | AKI | P-value | |
|---|---|---|---|---|
| CPB | ||||
| AUC <60 mmHg (mmHgxhr) | 3.93 (1.82–7.36) | 3.73 (1.75–6.21) | 4.54 (1.91–9.98) | 0.31 |
| AUC <70 mmHg (mmHgxhr) | 11.85 (6.87–20.88) | 11.47 (6.78–16.91) | 15.95 (8.45–26.34) | 0.16 |
| AUC <80 mmHg (mmHgxhr) | 26.58 (17.67–39.88) | 24.31 (17.50–34.23) | 34.80 (20.83–49.72) | 0.06 |
| ICU | ||||
| AUC <60 mmHg (mmHgxhr) | 0.73 (0.14–3.64) | 0.59 (0.14–2.90) | 1.15 (0.07–5.74) | 0.48 |
| AUC <70 mmHg (mmHgxhr) | 8.08 (2.32–20.94) | 7.58 (2.46–18.92) | 9.61 (2.20–24.23) | 0.56 |
| AUC <80 mmHg (mmHgxhr) | 28.92 (15.13–47.88) | 28.25 (15.90–46.71) | 32.18 (14.03–52.93) | 0.7 |
There were no significant difference in blood pressure excursion below specific cut-offs (i.e. 60, 70, 80 mmHg) and incidence of AKI. However, blood pressure excursions below the optimal blood pressure measured by cerebral autoregulation monitoring were associated with incidence of AKI in both during CPB and in the ICU. The data are listed as median (interquartile range).
AUC: area under the curve; MAP: mean arterial pressure; CPB: cardiopulmonary bypass; ICU: intensive care unit; AKI: acute kidney injury.
Multivariate logistic regression for CSA-AKI showed that the blood pressure excursion below the optimal blood pressure during CPB and in the ICU was independently associated with CSA-AKI after adjusting for possible confounding variables including total CPB time, total cross-clamp time, preoperative eGFR and surgery other than isolated CABG (Table 5).
Table 5:
Logistic regression model for cardiac surgery-associated acute kidney injury
| Odds ratio | Standard error | 95% CI | P-value | |
|---|---|---|---|---|
| Total bypass time | 1.00 | 0.004 | 0.99–1.01 | 0.20 |
| Total cross-clamp time | 1.01 | 0.012 | 0.99–1.03 | 0.45 |
| Surgery other than isolated CABG | 0.95 | 0.456 | 0.36–2.43 | 0.91 |
| Preoperative eGFR | 0.99 | 0.012 | 0.96–1.01 | 0.30 |
| AUC < optimal blood pressure | 1.03 | 0.011 | 1.01–1.05 | 0.017 |
Total bypass time, total cross-clamp time, surgery other than isolated CABG, preoperative eGFR and the sum of blood pressure excursions below the optimal blood pressure during CPB and in the ICU were included in the model.
AUC: area under the curve; CABG: coronary artery bypass graft; CI: confidence interval; eGFR: estimated glomerular filtration rate; ICU: intensive care unit.
DISCUSSION
In this study, we found that, for the most patients, the optimal MAP in the ICU was higher than during CPB but there was interindividual variability. There was significant correlation between optimal MAP determined during CPB and in the ICU. Further, we found that excursions of blood pressure below the optimal MAP (AUC < OptMAP) for cerebral autoregulation occurring both during CPB and in the ICU were associated with incidence of CSA-AKI.
CBF measurement for cerebral autoregulation monitoring in this study was with a novel UT-NIRS device that we have previously validated against transcranial Doppler [10]. In that study, mean velocity index (Mx) was compared with CFx during CPB. Similar to CFx, Mx is a moving linear correlation coefficient between transcranial Doppler measured CBF velocity and MAP [7, 9]. When MAP is outside the autoregulation range, both Mx and CFx are more positive as CBF is dependent on changes in blood pressure (i.e. ‘pressure passive’). We found that the optimal MAP based on Mx and CFx was correlated (P < 0.001) and was in agreement (bias −2.85 ± 8.54, 95% limits of agreement −19.60 to 13.89). Coherence between ipsilateral middle CBF velocity and cerebral flow index values averaged 0.61 ± 0.07 (95% CI, 0.59–0.63). This novel UT-NIRS device overcomes many of the technical limitations of the transcranial Doppler monitoring including ease of use without the need for specialized training and the lack of dependence on obtaining a trans-temporal window to measure CBF velocity. This device, therefore, could be applied to diverse clinical conditions for monitoring of CBF.
The development of CSA-AKI is well established to be associated with poor patient outcomes including higher morbidity and mortality during hospitalization and after hospital discharge [19–21]. While the aetiology of CSA-AKI is complex, our prior work suggests that renal hypoperfusion during CPB is a risk factor. In those studies, we found that the product of the magnitude and duration that MAP was below the lower limit of autoregulation was associated with CSA-AKI [2]. In the current study, we show for the first time that not only are blood pressure excursions below optimal MAP based on cerebral autoregulation monitoring during CPB were associated with CSA-AKI, but also that excursions after surgery in the ICU are also linked to risk for this complication. Thus, individualizing blood pressure management decisions based on cerebral autoregulation monitoring may better ensure renal perfusion during and immediately after cardiac surgery.
The findings of the current study complement our prior studies where we found a relationship between MAP in the ICU after cardiac surgery and evidence of brain cellular injury [4]. In that study, average MAP (mean ± standard deviation) in the ICU after surgery was 74 ± 7.3 mmHg; whereas optimal MAP during CPB was 78 ± 12.8 mmHg (P = 0.008). The incidence of hypotension varied from 22 to 37% based on standard definitions that are based on decrements of preoperative blood pressure measurements (i.e. 20%, 30% below baseline etc.). In contrast, hypotension based on cerebral autoregulation determined optimal MAP occurred in 54% of patients (P < 0.001 vs standard definitions). There was no relationship between standard definitions of hypotension and plasma levels of the brain-specific protein glial fibrillary acidic protein (GFAP) levels, but MAP < optimal was positively related with POD1 GFAP levels (Coef, 1.77; 95% CI, 1.27–2.48; P = 0.001). Those results suggest that blood pressure management after surgery based on cerebral autoregulation determined optimal MAP may better ensure brain perfusion than basing blood pressure targets on empiric definitions. The results of the present study appear to extend those findings to the kidney whereby optimizing MAP in the ICU based on cerebral autoregulation monitoring results offers the potential to ensure better renal perfusion.
This study has several limitations. Cerebral autoregulation monitoring in the ICU was only performed for the first 3 h after surgery. This time period was chosen as patient movement and dislodgement of the monitoring sensors was common during weaning from mechanical ventilation and extubation. Blood pressure ranges observed in this time period were also not enough for 16.4% of the patients who were not able to define their optimal blood pressure. However, optimal blood pressure was observed in all patients during CPB. Further studies with clinical end-points will be needed to see if assuming the value obtained on CPB can be a good option for blood pressure management after surgery. There is also a possibility that a longer observation period during ICU with CerOx monitoring may provide more data points to obtain cerebral autoregulation curve. While we found a relationship between blood pressure below optimal MAP and CSA-AKI, we did not incorporate preoperative cardiac output in the analysis as it was not available for all patients. However, the result of our study showed that low cardiac output syndrome after surgery was not associated with incidence of CSA-AKI at 48 h (P = 0.19). Only future studies will help establish whether raising blood pressure with volume or pharmacologically targeting specific MAP values and/or adjustment of cardiac output in the optimal autoregulation range improve renal outcomes. Our analysis also does not include the possibility of other haemodynamic insult after 3 h of monitoring in the ICU which may have an impact on incidence of AKI.
In conclusion, optimal blood pressure based on cerebral autoregulation monitoring determined during CPB is correlated with that measured after surgery in the ICU. Blood pressure excursions below the optimal blood pressure measured during CPB and in the first 3 h after surgery in the ICU were associated with CSA-AKI. The novel UT-NIRS technology used in this study may provide a clinically feasible method for monitoring of CBF in patients during and after cardiac surgery.
Funding
This work was supported by Ornim, Inc., Kfar Saba, Israel (to Kaushik Mandal, KM); Japan Heart Association/Bayer Yakuhin Grant for Abroad (to Daijiro Hori) and the National Institutes of Health (RO1 092259, Charles Hogue, principal investigator).
Conflict of interest: Charles Hogue serves as an advisor to Ornim Medical, Inc. and has stock buying options. Kaushik Mandal was the principal investigator and was in part funded by grant from Ornim, Inc., Kfar Saba, Israel.
REFERENCES
- 1.Ono M, Brady K, Easley RB, Brown C, Kraut M, Gottesman RF, et al. Duration and magnitude of blood pressure below cerebral autoregulation threshold during cardiopulmonary bypass is associated with major morbidity and operative mortality. J Thorac Cardiovasc Surg 2014;147:483–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ono M, Arnaoutakis GJ, Fine DM, Brady K, Easley RB, Zheng Y, et al. Blood pressure excursions below the cerebral autoregulation threshold during cardiac surgery are associated with acute kidney injury. Crit Care Med 2013;41:464–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hori D, Brown C, Ono M, Rappold T, Sieber F, Gottschalk A, et al. Arterial pressure above the upper cerebral autoregulation limit during cardiopulmonary bypass is associated with postoperative delirium. Br J Anaesth 2014;113:1009–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hori D, Ono M, Rappold TE, Conte JV, Shah AS, Cameron DE, et al. Hypotension after cardiac operations based on autoregulation monitoring leads to brain cellular injury. Ann Thorac Surg 2015;100:487–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ono M, Joshi B, Brady K, Easley RB, Zheng Y, Brown C, et al. Risks for impaired cerebral autoregulation during cardiopulmonary bypass and postoperative stroke. Br J Anaesth 2012;109:391–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Joshi B, Ono M, Brown C, Brady K, Easley RB, Yenokyan G, et al. Predicting the limits of cerebral autoregulation during cardiopulmonary bypass. Anesth Analg 2012;114:503–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Joshi B, Brady K, Lee J, Easley B, Panigrahi R, Smielewski P, et al. Impaired autoregulation of cerebral blood flow during rewarming from hypothermic cardiopulmonary bypass and its potential association with stroke. Anesth Analg 2010;110:321–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brady KM, Mytar JO, Lee JK, Cameron DE, Vricella LA, Thompson WR, et al. Monitoring cerebral blood flow pressure autoregulation in pediatric patients during cardiac surgery. Stroke 2010;41:1957–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brady K, Joshi B, Zweifel C, Smielewski P, Czosnyka M, Easley RB, et al. Real-time continuous monitoring of cerebral blood flow autoregulation using near-infrared spectroscopy in patients undergoing cardiopulmonary bypass. Stroke 2010;41:1951–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hori D, Hogue CW, Jr, Shah A, Brown C, Neufeld KJ, Conte JV, et al. Cerebral autoregulation monitoring with ultrasound tagged near infrared spectroscopy in cardiac surgery patients. Anesth Analg 2015;121:1187–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schytz HW, Guo S, Jensen LT, Kamar M, Nini A, Gress DR, et al. A new technology for detecting cerebral blood flow: a comparative study of ultrasound tagged NIRS and 133Xe-SPECT. Neurocrit Care 2012;17:139–45. [DOI] [PubMed] [Google Scholar]
- 12.Ron A, Raceheli N, Breskin I, Metzger Y, Silman Z, Kamar M, et al. Measuring tissue blood flow using ultrasound modulated diffused light. Proc. SPIE 8223, photons plus Ultrasound: Imaging and Sensing 2012. 82232J; doi:10.1117/12.9906349.
- 13.Mahan GD, Engler WE, Tiemann JJ, Uzgiris E. Ultrasonic tagging of light: theory. Proc Natl Acad Sci USA 1998;95:14015–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roy AK, Mc Gorrian C, Treacy C, Kavanaugh E, Brennan A, Mahon NG, et al. A comparison of traditional and novel definitions (RIFLE, AKIN, and KDIGO) of acute kidney injury for the prediction of outcomes in acute decompensated heart failure. Cardiorenal Med 2013;3:26–37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kellum JA, Lameire N. KDIGO AKI Guideline Work group. Diagnosis, evaluation and management of acute kidney injury: KDIGO clinical practice Guideline for Acute Kidney Injury (section 2). Kidney Int Suppl 2012;2:19–36. [Google Scholar]
- 16.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, et al. Expressing the modification of diet in renal disease study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 2007;53:766–72. [DOI] [PubMed] [Google Scholar]
- 17.Parolari A, Pesce LL, Pacini D, Mazzanti V, Salis S, Sciacovelli C, et al. Risk factors for perioperative acute kidney injury after adult cardiac surgery: role of perioperative management. Ann Thorac Surg 2012;93:584–91. [DOI] [PubMed] [Google Scholar]
- 18.Rodrigues AJ, Evora PR, Bassetto S, Alves Junior L, Scorzoni Filho A, Araujo WF, et al. Risk factors for acute renal failure after heart surgery. Rev Bras Cir Cardiovasc 2009;24:441–6. [DOI] [PubMed] [Google Scholar]
- 19.Dasta JF, Kane-Gill SL, Durtschi AJ, Pathak DS, Kellum JA. Costs and outcomes of acute kidney injury (AKI) following cardiac surgery. Nephrol Dial Transplant 2008;23:1970–4. [DOI] [PubMed] [Google Scholar]
- 20.Chertow GM, Levy EM, Hammermeister KE, Grover F, Daley J. Independent association between acute renal failure and mortality following cardiac surgery. Am J Med 1998;104:343–8. [DOI] [PubMed] [Google Scholar]
- 21.Chertow GM, Burdick E, Honour M, Bonventre JV, Bates DW. Acute kidney injury, mortality, length of stay, and costs in hospitalized patients. J Am Soc Nephrol 2005;16:3365–70. [DOI] [PubMed] [Google Scholar]