Abstract
Early and accurate identification of people at high risk of premature death may assist in the targeting of preventive therapies in order to improve overall health. To identify novel biomarkers for all-cause mortality, we performed untargeted metabolomics in the Atherosclerosis Risk in Communities (ARIC) Study. We included 1,887 eligible ARIC African Americans, and 671 deaths occurred during a median follow-up period of 22.5 years (1987–2011). Chromatography and mass spectroscopy identified and quantitated 204 serum metabolites, and Cox proportional hazards models were used to analyze the longitudinal associations with all-cause and cardiovascular mortality. Nine metabolites, including cotinine, mannose, glycocholate, pregnendiol disulfate, α-hydroxyisovalerate, N-acetylalanine, andro-steroid monosulfate 2, uridine, and γ-glutamyl-leucine, showed independent associations with all-cause mortality, with an average risk change of 18% per standard-deviation increase in metabolite level (P < 1.23 × 10−4). A metabolite risk score, created on the basis of the weighted levels of the identified metabolites, improved the predictive ability of all-cause mortality over traditional risk factors (bias-corrected Harrell's C statistic 0.752 vs. 0.730). Mannose and glycocholate were associated with cardiovascular mortality (P < 1.23 × 10−4), but predictive ability was not improved beyond the traditional risk factors. This metabolomic analysis revealed potential novel biomarkers for all-cause mortality beyond the traditional risk factors.
Keywords: all-cause mortality, cardiovascular mortality, metabolomics, risk factors
All-cause mortality is a traditional measure of overall disease burden in public health, and currently, cardiovascular disease (CVD) is the leading age-adjusted cause of death in the United States (1). Early and accurate identification of people at high risk of premature death, particularly CVD-related death, could assist in the targeting of preventive therapies in order to improve overall health. Previous studies have shown that levels of circulating biomarkers have the potential to predict all-cause mortality and CVD mortality (2–7). However, there remains considerable unexplained risk, and a systematic way to fully explore the effect of many circulating molecules is lacking. Metabolomics, which characterizes small-molecule metabolites produced by a variety of biochemical and cellular processes, takes us a step closer to this ideal and is one path toward new biomarker discovery. By using targeted metabolomic technology, Fischer et al. (8) recently identified 4 metabolites (out of 106) that are associated with all-cause and CVD mortality among the Estonian and Finnish populations, but the majority of the targeted metabolites (e.g., 85 of the 106) were lipids. Therefore, the predictive ability of the untargeted metabolome, consisting of many metabolites in other categories, remains unclear.
Untargeted metabolomic technologies capture numerous small molecules in a biosample representing multiple pathways and functional classes, and thus provide the opportunity to more fully assess relationships between diverse metabolites and mortality. African-American adults have higher rates of all-cause mortality and CVD mortality than other US racial/ethnic groups (9), but the exact mechanism underlying such disparity is not clear. To date, no study appears to have explored the metabolomic antecedents of all-cause and CVD mortality in a large African-American population. In a well-characterized, population-based sample of African Americans from the Atherosclerosis Risk in Communities (ARIC) Study, we explored the longitudinal association of the serum metabolome, quantified by means of untargeted metabolomic technology, with all-cause mortality and CVD mortality after a median of 22.5 years of follow-up.
METHODS
Study population
The ARIC Study is a prospective cohort study designed to investigate the etiology and predictors of CVD. The ARIC Study enrolled 15,792 adults from 4 US communities (Forsyth County, North Carolina; Jackson, Mississippi; selected suburbs of Minneapolis, Minnesota; and Washington County, Maryland) between 1987 and 1989. A detailed description of the ARIC Study's design and methods has been published elsewhere (10). Metabolomic profiles were measured in 1,977 African Americans from the Jackson field center, and among them, 1,887 participants with complete mortality follow-up information and covariates were included in this study.
Cause of death for study participants was ascertained through annual cohort follow-up, community-wide hospital surveillance, and linkage with local and national death registries. The date and cause of death were verified by death certificate review. All-cause mortality was defined as death from any cause, and CVD mortality was defined as any death in which the principal cause was cardiovascular in nature, using International Classification of Diseases, Ninth Revision, codes 390–459 or International Classification of Diseases, Tenth Revision, codes I00–I99. For this analysis, the vital status of study participants was followed from baseline through December 31, 2011.
For covariates, demographic information was obtained during an in-home interview. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or use of antihypertensive medication. Antihypertensive medication use was recorded by self-report and ascertained by inventory. Diabetes was defined as fasting glucose concentration ≥126 mg/dL, nonfasting glucose concentration ≥200 mg/dL, a self-report of a physician's diagnosis of diabetes or “sugar in the blood,” or use of oral diabetes medication or insulin. Prevalent CVD was defined by evidence of prior myocardial infarction, heart failure, or stroke, history of physician-diagnosed heart attack, or previous coronary reperfusion procedure. Smoking status was self-reported and was categorized as current smoker or non–current smoker. Plasma total cholesterol and high-density lipoprotein cholesterol concentrations were determined by standardized enzymatic methods. Estimated glomerular filtration rate was calculated using the Chronic Kidney Disease Epidemiology Collaboration equation (11).
Measurement of the metabolome
Metabolite profiling was measured using baseline fasting serum samples that had been stored at −80°C since collection. More than 600 metabolites were detected and quantified by Metabolon, Inc. (Durham, North Carolina) using an untargeted metabolomic quantification protocol based on gas chromatography–mass spectrometry and liquid chromatography–mass spectrometry (12, 13). Metabolites were excluded from this analysis if 1) more than 80% of the samples had values below the detection limit or 2) the reliability coefficient, based on a comparison of 2 samples from 60 individuals collected 4–6 weeks apart, was less than 0.60. After these exclusions, 204 metabolites, including 118 named compounds within 8 super-pathways (including amino acids, carbohydrates, vitamins, energy, lipids, nucleotides, peptides, and xenobiotics) and 86 unnamed compounds, were involved and analyzed in this study. The unnamed compounds were assigned tags beginning with “X” and followed by numbers (e.g., “X-12345”).
Statistical analysis
Out of 204 metabolites, 187 had values above the detection limit in at least 50% of the samples and were analyzed as continuous variables, where “below detection” values were imputed as the lowest detected value for that metabolite in all samples. After imputation, each of the 187 metabolites was centered by its mean and scaled by its standard deviation prior to the analysis. The other 17 metabolites with 50%–80% “below detection” values were analyzed as ordinal variables with 3 levels: 1) values below the detection limit, 2) detected values below the median of all “above detection” values, and 3) detected values at or above the median.
We assessed the longitudinal associations of metabolite levels with all-cause mortality and CVD mortality using Cox proportional hazards models, with adjustment for age, sex, and traditional risk factors, including body mass index, systolic blood pressure, antihypertensive medication use, diabetes status, prevalent CVD, current smoking status, estimated glomerular filtration rate, and total cholesterol and high-density lipoprotein cholesterol levels. The proportional hazards assumption was tested using the methods developed by Grambsch and Therneau (14). Statistical significance at this stage was defined as P < 1.23 × 10−4, accounting for testing of 204 metabolites and 2 outcomes using Bonferroni correction.
Because of the potential correlation between metabolites, we next evaluated the independent associations of the significant metabolites using a forward stepwise analysis of all-cause mortality and CVD mortality. The metabolite with the smallest P value identified in the Cox proportional hazards model was included as a predictor, adjusting for traditional risk factors as described above. Subsequently, the metabolite with the next-smallest P value in the Cox proportional hazards model was included as a predictor, adjusting for traditional risk factors and the first metabolite. The process was repeated until no additional metabolite was significant at P < 0.05.
A metabolite risk score was generated by summing the levels of independent mortality-related metabolites weighted by the regression coefficients observed in the Cox model for each individual metabolite. We divided the metabolite risk scores into quartiles to estimate the associations with each outcome using a Cox proportional hazards model adjusting for the same covariates as those described above. We investigated the ability of the model to predict risk using Harrell's C statistic (15, 16), for which the censoring distribution is considered when calculating the concordance probability. We performed 10-fold cross-validation to derive a bias-corrected Harrell's C statistic and to obtain a more precise assessment of model performance. In addition, we calculated the net reclassification index and the integrated discrimination index based on 20-year risk of death according to Pencina et al. (17). Net reclassification index was assessed as a continuous measure and as a categorical measure by assigning individuals to one of 3 risk categories (<6%, 6%–20%, and >20%). All statistical analyses were performed using R (R Development Core Team, R Foundation for Statistical Computing, Vienna, Austria (http://www.r-project.org)).
RESULTS
A total of 1,887 eligible ARIC African Americans were included in this study, with a median follow-up period of 22.5 years. During the follow-up time period, there were 671 deaths in this study sample, and 259 of the 671 deaths were from CVD. Baseline characteristics of the study sample by mortality status are shown in Table 1. Male sex, prevalent diabetes, hypertension, prevalent CVD, current smoking, and lower high-density lipoprotein cholesterol levels were individually associated with death events.
Table 1.
Baseline Characteristics of Participants by Mortality Status Among African Americans in the ARIC Study, 1987–2011
| Characteristic | Type of Death Event |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| None |
All-Cause Mortality |
CVD Deaths Only |
|||||||
| No. | % | Mean (SD) | No. | % | Mean (SD) | No. | % | Mean (SD) | |
| Total | 1,216 | 671 | 259 | ||||||
| Age, years | 51.5 (5.3) | 55.3 (5.7) | 55.6 (5.6) | ||||||
| Male sex | 376 | 30.9 | 304 | 45.3 | 119 | 45.9 | |||
| Diabetes | 132 | 10.9 | 176 | 26.2 | 87 | 33.6 | |||
| Prevalent CVD | 76 | 6.3 | 117 | 17.4 | 62 | 23.9 | |||
| Current smoking | 269 | 22.1 | 277 | 41.3 | 104 | 40.2 | |||
| Hypertension | 568 | 46.7 | 442 | 65.9 | 205 | 79.2 | |||
| Antihypertensive medication use | 416 | 34.2 | 308 | 45.9 | 148 | 57.1 | |||
| Body mass indexa | 29.7 (6.0) | 29.6 (6.2) | 30.5 (6.4) | ||||||
| HDL cholesterol, mg/dL | 56.7 (16.8) | 53.5 (17.7) | 51.0 (15.3) | ||||||
| Total cholesterol, mg/dL | 215.3 (45.0) | 214.0 (44.9) | 215.4 (45.8) | ||||||
| Serum-based eGFRCKD-EPI,b mL/minute/1.73 m2 | 106.1 (16.3) | 100.6 (21.0) | 98.8 (22.4) | ||||||
| Follow-up time, years | 23.2 (1.1) | 13.5 (6.6) | 13.0 (6.5) | ||||||
Abbreviations: ARIC, Atherosclerosis Risk in Communities; CKD-EPI, Chronic Kidney Disease Epidemiology Collaboration; CVD, cardiovascular disease; eGFR, estimated glomerular filtration rate; HDL, high-density lipoprotein; SD, standard deviation.
a Weight (kg)/height (m)2.
b eGFR was calculated using the CKD-EPI equation (11).
Metabolomic association with all-cause mortality
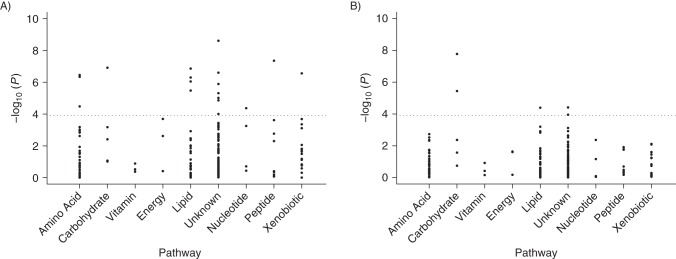
We identified 18 metabolites (11 named and 7 unnamed) among the 8 super-pathways that were significantly associated (P < 1.23 × 10−4) with incident all-cause mortality after adjustment for demographic and traditional risk factors (Figure 1A; full results are presented in Web Table 1, available at http://aje.oxfordjournals.org/). Higher levels were associated with increased risk of death for 14 out of 18 metabolites, and the other 4 metabolites appeared to have protective associations. Among the 11 named metabolites, the pairwise correlation ranged from −0.21 to 0.64, and thus the next step was to examine whether they had mutually independent associations with all-cause mortality. Nine metabolites showed independent associations, with an average risk change of 18% per standard-deviation increase in metabolite levels (Figure 2).
Figure 1.
Associations between 204 serum metabolites and incident all-cause mortality (A) and cardiovascular disease mortality (B), by metabolomic super-pathway, among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study, 1987–2011. The x-axis shows 8 metabolite super-pathways; the y-axis shows the common logarithm (log10) of the P value for each metabolite. The dotted gray line represents the statistical significance threshold (P < 1.23 × 10−4).
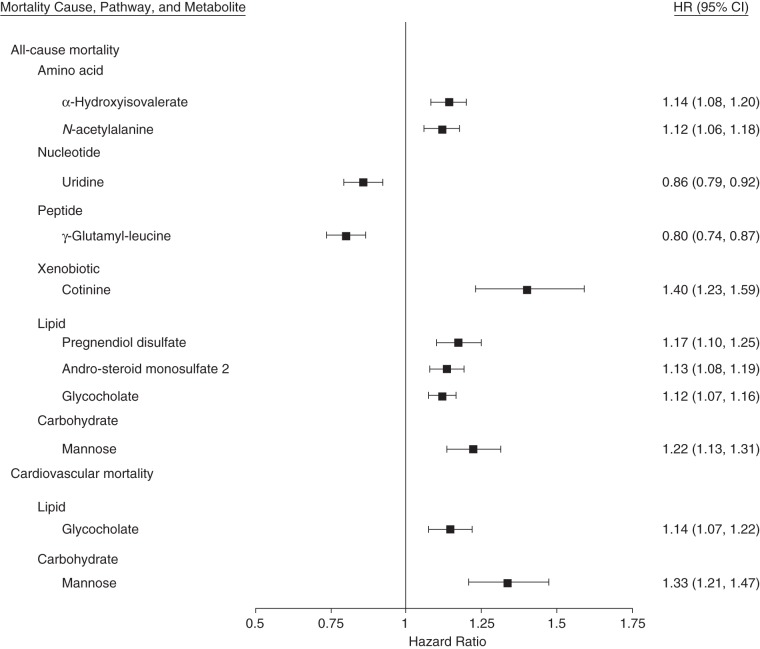
Figure 2.
Hazard ratios (HRs) and 95% confidence intervals (CIs) for named serum metabolites predicting the risk of all-cause and cardiovascular disease mortality among African Americans in the Atherosclerosis Risk in Communities (ARIC) Study, 1987–2011. HRs were estimated in Cox models adjusting for age, sex, body mass index, systolic blood pressure, antihypertensive medication use, diabetes status, current smoking status, prevalent cardiovascular disease status, plasma high-density lipoprotein cholesterol level, total cholesterol level, and estimated glomerular filtration rate.
Cotinine, an alkaloid in tobacco, was the strongest risk-increasing predictor of death (hazard ratio (HR) per standard-deviation (SD) increase = 1.40, 95% confidence interval (CI): 1.23, 1.59). Gamma-glutamyl-leucine, a dipeptide composed of γ-glutamate and leucine, was the most significant metabolite and the strongest risk-decreasing predictor (HR per SD =0.80, 95% CI: 0.74, 0.87). Uridine, one of the 5 standard nucleosides, also showed a modest protective association with death (HR per SD = 0.86, 95% CI: 0.79, 0.92). A metabolite risk score based on the weighted levels of the 9 metabolites showed a large effect size for the risk of death, where higher quartiles of the risk score were related to higher risk of death (P for trend < 0.001; Table 2). The concordance probability for the risk of death was also noticeably increased by adding the metabolite risk score to the traditional risk factors (bias-corrected Harrell's C statistic 0.752 vs. 0.730). When metabolite risk score was added to the traditional risk model, the net reclassification index based on the 20-year risk categories (<6%, 6%–20%, and >20%) was improved 7.1% (P < 0.001), whereas the continuous net reclassification index and the integrated discrimination index were 36.5% (P < 0.001) and 3.7% (P < 0.001), respectively.
Table 2.
Hazard Ratios for the Association of Metabolite Risk Score (Quartiles) With Incident All-Cause Mortality Among African Americans in the ARIC Study, 1987–2011
| Quartile of Metabolic Risk Scorea | Hazard Ratiob | 95% Confidence Interval | Mortality Rate (per 1,000 Person-Years) |
|---|---|---|---|
| 1 | 1.00 | Referent | 7.60 |
| 2 | 1.34 | 1.01, 1.79 | 12.00 |
| 3 | 2.02 | 1.53, 2.66 | 20.33 |
| 4 | 3.24 | 2.42, 4.33 | 36.62 |
Abbreviation: ARIC, Atherosclerosis Risk in Communities.
a Crude P for trend < 0.001 (calculated as a linear trend in quartile number).
b Hazard ratio for all-cause mortality generated from Cox proportional hazards regression. Covariates were: age, sex, body mass index, systolic blood pressure, antihypertensive medication use, diabetes status, current smoking status, prevalent cardiovascular disease status, plasma high-density lipoprotein cholesterol level, total cholesterol level, and estimated glomerular filtration rate from baseline measurement.
Metabolomic association with CVD mortality
Five metabolites (3 named and 2 unnamed) among 3 super-pathways were identified as being associated (P < 1.23 × 10−4) with incident CVD mortality using Cox models, after adjustment for demographic factors and traditional risk factors (Figure 1B; full results shown in Web Table 2), and all of them showed positive associations with CVD mortality. Two named metabolites, mannose (HR per SD = 1.33, 95% CI: 1.21, 1.47) and glycocholate (HR per SD = 1.14, 95% CI: 1.07, 1.22), were independently associated with CVD mortality. Both mannose and glycocholate were significantly related to all-cause mortality as well, with slightly larger magnitudes of effect sizes (Figure 2). Mannose, a C-2 epimer of glucose, had a high correlation with glucose levels (r = 0.86) but was persistently related to all-cause mortality and CVD mortality even after glucose levels were accounted for (data not shown). Similar to glucose (6), mannose showed a nonlinear relationship with mortality, where the third and fourth quartiles had a higher risk but the second quartile showed the lowest risk (Web Figure 1). No apparent improvement in the predictive ability for risk of CVD mortality was observed by adding mannose and glycocholate to the traditional risk factors (bias-corrected Harrell's C statistic 0.790 vs. 0.783).
DISCUSSION
We related the untargeted serum metabolome (n = 204 metabolites) to incident mortality (n = 671 cases) after 22.5 years of follow-up in a sample of 1,887 ARIC African-American study participants. We identified 9 metabolites that were associated with incident all-cause mortality, and 2 of the 9 metabolites were also associated with incident CVD mortality. These associations were independent of other risk factors, and a metabolite risk score, created by aggregating the information from 9 individual metabolites, significantly improved prediction of incident all-cause mortality.
In a recent targeted metabolomic study of 106 candidate biomarkers, Fischer et al. (8) reported that 4 biomarkers—α-1-acid glycoprotein, albumin, very-low-density lipoprotein particle size, and citrate—were predictive of all-cause mortality as well as CVD mortality. Three of these biomarkers—α-1-acid glycoprotein, albumin, and very-low-density lipoprotein particle size—are large molecules or molecular complexes, which were not measured by our methods. Citrate was measured in our study but was later excluded from the analysis due to high variability as measured 4–6 weeks apart (reliability coefficient = 0.375). We explored the association of citrate with all-cause mortality, and no significant association was observed among African Americans in ARIC (data not shown). A potential explanation may be the presence of population-specific effects on mortality or fluctuation of levels over time, as indicated by citrate's overall high variability.
In this study, 7 metabolites were shown to have positive associations with all-cause mortality. Cotinine, a metabolite of nicotine found in tobacco, was the strongest predictor of mortality. It is well-established that smoking increases risk of death (18, 19). In our study, we found that about 13% of non–current smokers had detectable cotinine levels, and in a stratified analysis, cotinine was strongly associated with high risk of death for both non–current and current smokers (data not shown). The findings presented here suggest a dose-response relationship between cotinine and death, in addition to overt smoking status. Glucose, a biomarker of diabetes, has been shown to have a J-shaped association with mortality (6, 20). Mannose is a sugar monomer, which can be formed from glucose. Here we report that mannose, similar to glucose, has a J-shaped association with all-cause mortality. Interestingly, its association with mortality persists after accounting for glucose levels. Mannose is also involved in protein glycosylation (21), suggesting a mechanistic influence on mortality outside of hyperglycemia, possibly through the changes in protein glycosylation that occur in response to inflammation (22–24). Zheng et al. (25) previously reported that a metabolomic pattern consisting of 16 metabolites derived from principal component analysis was associated with risk of developing hypertension, and elevated blood pressure levels are risk factors for all-cause mortality (26). In the present study, we observed that 2 metabolites, pregnendiol disulfate and α-hydroxyisovalerate, which are involved in the previously reported hypertension-related metabolomic pattern, increased the risk of all-cause mortality. Glycocholate is an indirect cholesterol-derived bile acid (27), and little is known about its biological function. Total cholesterol levels have an impact on all-cause mortality (28, 29); however, the correlation between glycocholate and cholesterol in our study was low (r = −0.09). N-acetylalanine is an amino acid and andro-steroid monosulfate 2 is an androgen metabolite in serum, while their biological functions are largely unknown. Here we report that high levels of N-acetylalanine and andro-steroid monosulfate 2 have modest associations with all-cause mortality in African Americans; however, their biological functions and health effects need further investigation.
In contrast to the 6 metabolites mentioned above, uridine and γ-glutamyl-leucine were inversely associated with all-cause mortality. Uridine is a pyrimidine nucleoside involved in glycolysis. Studies have demonstrated that uridine has a protective effect on neurological disorders by enhancing glycolytic energy production (30, 31), and it protects neurons against ischemic insult-induced neuronal death in animal models (32). Gamma-glutamyl-leucine, a dipeptide composed of γ-glutamate and leucine, is an incomplete breakdown product of protein digestion/catabolism. In animal studies, an increased level of γ-glutamyl-leucine is an indicator of an anti-obesogenic metabolism (33). Obesity has been associated with higher risk of all-cause mortality (34), and our findings suggested that an anti-obesogenic metabolism may be beneficial for overall health and longevity.
Studies have shown that inflammatory and hyperglycemia biomarkers, such as B-type natriuretic peptide, C-reactive protein, cardiac troponins, and glucose, are associated with the risk of all-cause mortality, as well as CVD mortality (3, 6, 35). CVD mortality is the largest component of age-adjusted mortality in the United States (1). In our study, all metabolites identified as being associated with all-cause mortality showed nominal significance with CVD mortality, while two of them, mannose and glycocholate, reached our stringent significance threshold. Mannose and glycocholate are involved in glucose and cholesterol metabolisms, 2 key factors that regulate the prognosis and progression of CVD (36).
To our knowledge, this is the first population-based untargeted metabolomics study to identify novel biomarkers for mortality in African Americans. However, potential limitations of this study deserve consideration. We limited the analyses to those metabolites with few missing values and high reliability coefficients; therefore, possible associations between the excluded metabolites and mortality need further investigation. The participants in the present study were African Americans; it is yet unknown whether our findings could be generalized to other ethnic groups. The focus of this study was all-cause mortality and CVD mortality; future studies on death from other causes, such as cancer, are warranted. Our study applied untargeted metabolomic technologies, which capture both named and unnamed metabolites. Besides named metabolites, we identified several unnamed metabolites associated with all-cause and CVD mortality. Little is known about these unnamed metabolites, and their chemical structures need further exploration in order to obtain critical insight into pathogenic mechanisms of mortality.
In summary, we found 9 metabolites that were associated with all-cause mortality, and 2 of them were associated with CVD mortality. In addition, the metabolite risk score, a collective measurement of 9 individual metabolites, prominently enhanced the prediction of all-cause mortality. Our results highlight the importance of evaluating metabolomic biomarkers in a prospective cohort study to help identify novel molecular mechanisms that influence human health.
Supplementary Material
ACKNOWLEDGMENTS
Author affiliations: Department of Epidemiology, Human Genetics and Environmental Sciences, School of Public Health, University of Texas, Houston, Texas (Bing Yu, Eric Boerwinkle); Department of Epidemiology, Gillings School of Global Public Health, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina (Gerardo Heiss); Metabolon, Inc., Durham, North Carolina (Danny Alexander); Division of Nephrology, School of Medicine, Johns Hopkins University, Baltimore, Maryland (Morgan E. Grams); Welch Center for Prevention, Epidemiology, and Clinical Research, Johns Hopkins Medical Institutions, Baltimore, Maryland (Morgan E. Grams); and Human Genome Sequencing Center, Baylor College of Medicine, Houston, Texas (Eric Boerwinkle).
The Atherosclerosis Risk in Communities (ARIC) Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C. The metabolomics measurements were sponsored by the National Human Genome Research Institute (grant 3U01HG004402-02S1).
The authors thank the staff of the ARIC Study for their important contributions.
Conflict of interest: none declared.
REFERENCES
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: Final Data for 2010. Atlanta, GA: National Center for Health Statistics; 2013. (National vital statistics reports, vol. 61, no. 4). [PubMed] [Google Scholar]
- 2.Steffen LM, Jacobs DR Jr, Stevens J et al. Associations of whole-grain, refined-grain, and fruit and vegetable consumption with risks of all-cause mortality and incident coronary artery disease and ischemic stroke: the Atherosclerosis Risk in Communities (ARIC) Study. Am J Clin Nutr. 2003;783:383–390. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Gona P, Larson MG et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;35525:2631–2639. [DOI] [PubMed] [Google Scholar]
- 4.Clarke R, Emberson JR, Breeze E et al. Biomarkers of inflammation predict both vascular and non-vascular mortality in older men. Eur Heart J. 2008;296:800–809. [DOI] [PubMed] [Google Scholar]
- 5.Kaptoge S, Di Angelantonio E, Lowe G et al. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;3759709:132–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seshasai SR, Kaptoge S, Thompson A et al. Diabetes mellitus, fasting glucose, and risk of cause-specific death. N Engl J Med. 2011;3649:829–841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hallengren E, Almgren P, Engström G et al. Fasting levels of high-sensitivity growth hormone predict cardiovascular morbidity and mortality: the Malmö Diet and Cancer Study. J Am Coll Cardiol. 2014;6414:1452–1460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fischer K, Kettunen J, Würtz P et al. Biomarker profiling by nuclear magnetic resonance spectroscopy for the prediction of all-cause mortality: an observational study of 17,345 persons. PLoS Med. 2014;112:e1001606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Go AS, Mozaffarian D, Roger VL et al. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. Circulation. 2014;1293:e28–e292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. Am J Epidemiol. 1989;1294:687–702. [PubMed] [Google Scholar]
- 11.Levey AS, Stevens LA, Schmid CH et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;1509:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Evans AM, DeHaven CD, Barrett T et al. Integrated, nontargeted ultrahigh performance liquid chromatography/electrospray ionization tandem mass spectrometry platform for the identification and relative quantification of the small-molecule complement of biological systems. Anal Chem. 2009;8116:6656–6667. [DOI] [PubMed] [Google Scholar]
- 13.Ohta T, Masutomi N, Tsutsui N et al. Untargeted metabolomic profiling as an evaluative tool of fenofibrate-induced toxicology in Fischer 344 male rats. Toxicol Pathol. 2009;374:521–535. [DOI] [PubMed] [Google Scholar]
- 14.Grambsch PM, Therneau TM. Proportional hazards tests and diagnostics based on weighted residuals. Biometrika. 1994;813:515–526. [Google Scholar]
- 15.Harrell FE Jr, Califf RM, Pryor DB et al. Evaluating the yield of medical tests. JAMA. 1982;24718:2543–2546. [PubMed] [Google Scholar]
- 16.Harrell FE Jr, Lee KL, Mark DB. Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med. 1996;154:361–387. [DOI] [PubMed] [Google Scholar]
- 17.Pencina MJ, D'Agostino RB Sr, Steyerberg EW. Extensions of net reclassification improvement calculations to measure usefulness of new biomarkers. Stat Med. 2011;301:11–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Qiao Q, Tervahauta M, Nissinen A et al. Mortality from all causes and from coronary heart disease related to smoking and changes in smoking during a 35-year follow-up of middle-aged Finnish men. Eur Heart J. 2000;2119:1621–1626. [DOI] [PubMed] [Google Scholar]
- 19.Gellert C, Schöttker B, Brenner H. Smoking and all-cause mortality in older people: systematic review and meta-analysis. Arch Intern Med. 2012;17211:837–844. [DOI] [PubMed] [Google Scholar]
- 20.Kowall B, Rathmann W, Heier M et al. Categories of glucose tolerance and continuous glycemic measures and mortality. Eur J Epidemiol. 2011;268:637–645. [DOI] [PubMed] [Google Scholar]
- 21.Durand G, Seta N. Protein glycosylation and diseases: blood and urinary oligosaccharides as markers for diagnosis and therapeutic monitoring. Clin Chem. 2000;466:795–805. [PubMed] [Google Scholar]
- 22.Malhotra R, Wormald MR, Rudd PM et al. Glycosylation changes of IgG associated with rheumatoid arthritis can activate complement via the mannose-binding protein. Nat Med. 1995;13:237–243. [DOI] [PubMed] [Google Scholar]
- 23.Novokmet M, Lukić E, Vučković F et al. Changes in IgG and total plasma protein glycomes in acute systemic inflammation. Sci Rep. 2014;4:4347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gkrania-Klotsas E, Langenberg C, Sharp SJ et al. Seropositivity and higher immunoglobulin g antibody levels against cytomegalovirus are associated with mortality in the population-based European Prospective Investigation of Cancer-Norfolk cohort. Clin Infect Dis. 2013;5610:1421–1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Zheng Y, Yu B, Alexander D et al. Metabolomics and incident hypertension among blacks: the Atherosclerosis Risk in Communities Study. Hypertension. 2013;622:398–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stamler J, Dyer AR, Shekelle RB et al. Relationship of baseline major risk factors to coronary and all-cause mortality, and to longevity: findings from long-term follow-up of Chicago cohorts. Cardiology. 1993;82(2-3):191–222. [DOI] [PubMed] [Google Scholar]
- 27.Buttar HS, Li T, Ravi N. Prevention of cardiovascular diseases: role of exercise, dietary interventions, obesity and smoking cessation. Exp Clin Cardiol. 2005;104:229–249. [PMC free article] [PubMed] [Google Scholar]
- 28.Smith GD, Shipley MJ, Marmot MG et al. Plasma cholesterol concentration and mortality. The Whitehall Study. JAMA. 1992;2671:70–76. [PubMed] [Google Scholar]
- 29.Stamler J, Daviglus ML, Garside DB et al. Relationship of baseline serum cholesterol levels in 3 large cohorts of younger men to long-term coronary, cardiovascular, and all-cause mortality and to longevity. JAMA. 2000;2843:311–318. [DOI] [PubMed] [Google Scholar]
- 30.Connolly GP, Duley JA. Uridine and its nucleotides: biological actions, therapeutic potentials. Trends Pharmacol Sci. 1999;205:218–225. [DOI] [PubMed] [Google Scholar]
- 31.Amante DJ, Kim J, Carreiro ST et al. Uridine ameliorates the pathological phenotype in transgenic G93A-ALS mice. Amyotroph Lateral Scler. 2010;116:520–530. [DOI] [PubMed] [Google Scholar]
- 32.Choi JW, Shin CY, Choi MS et al. Uridine protects cortical neurons from glucose deprivation-induced death: possible role of uridine phosphorylase. J Neurotrauma. 2008;256:695–707. [DOI] [PubMed] [Google Scholar]
- 33.Sansbury BE, Bhatnagar A, Hill BG. Impact of nutrient excess and endothelial nitric oxide synthase on the plasma metabolite profile in mice. Front Physiol. 2014;5:453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flegal KM, Kit BK, Orpana H et al. Association of all-cause mortality with overweight and obesity using standard body mass index categories: a systematic review and meta-analysis. JAMA. 2013;3091:71–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dallmeier D, Denkinger M, Peter R et al. Sex-specific associations of established and emerging cardiac biomarkers with all-cause mortality in older adults: the ActiFE Study. Clin Chem. 2015;612:389–399. [DOI] [PubMed] [Google Scholar]
- 36.Bemis CE, Gorlin R, Kemp HG et al. Progression of coronary artery disease. A clinical arteriographic study. Circulation. 1973;473:455–464. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.