Abstract
Objective
To systematically review and assess the efficacy, different treatment protocols (formulation, dosage, and duration), and safety of nystatin for treating oral candidiasis.
Methods
Four electronic databases were searched for trials published in English till July 1, 2015. Randomized controlled trials comparing nystatin with other antifungal therapies or a placebo were included. Clinical and/or mycological cure was the outcome evaluation. A meta-analysis or descriptive study on the efficacy, treatment protocols, and safety of nystatin was conducted.
Results
The meta-analysis showed that nystatin pastille was significantly superior to placebo in treating denture stomatitis. Nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. The descriptive investigations showed that administration of nystatin suspension and pastilles in combination for 2 weeks might achieve a higher clinical and mycological cure rate, and using the nystatin pastilles alone might have a higher mycological cure rate, when compared with using nystatin suspensions alone. Nystatin pastilles at a dose of 400,000 IU resulted in a significantly higher mycological cure rate than that administrated at a dose of 200,000 IU. Furthermore, treatment with nystatin pastilles for 4 weeks seemed to have better clinical efficacy than treatment for 2 weeks. Descriptive safety assessment showed that poor taste and gastrointestinal adverse reaction are the most common adverse effects of nystatin.
Conclusion
Nystatin pastille was significantly superior to placebo in treating denture stomatitis, while nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. Indirect evidence from a descriptive study demonstrated that administration of nystatin pastille alone or pastille and suspension in combination is more effective than that of suspension alone; prolonged treatment duration for up to 4 weeks can increase the efficacy of nystatin. More well designed and high quality randomized control studies are needed to confirm these findings.
Keywords: nystatin, oral candidiasis, systematic review, meta-analysis, safety, dosage forms, treatment duration
Introduction
Oral candidiasis, which is the most common human fungal infection, is characterized by an overgrowth of Candida species in the superficial epithelium of the oral mucosa.1,2 It has been associated with multiple host risk factors, including impaired salivary gland function, denture wearing, oral mucosa disruption, drug use (long-term administration of broad-spectrum antibiotics, corticosteroids, antidepressants, antineoplastic, drugs, and immunosuppressant), age (common in neonates and the elderly), endocrine alterations (diabetes mellitus, pregnancy, renal failure, and hyperthyroidism), dietary factors (high-carbohydrate diet and iron-deficiency anemia), cancer, and HIV infection.3–5 Elimination of the predisposing factors is an important strategy in treating oral candidiasis.
Various topical and systemic agents are currently available for the treatment of oral candidiasis.3 Systemic antifungal agents, including triazoles, fluconazole, and itraconazole, are appropriate for patients who do not respond to or are intolerant to topical treatment and those at high risk of developing systemic infections.3,6 However, numerous drug interactions and decreased susceptibility of species other than Candida albicans toward azoles limit the application of systemic antifungal agents.7,8 Topical antifungal agents, such as nystatin, amphotericin B, miconazole, and clotrimazole, are recommended typically as the first-line treatment for uncomplicated cases of oral candidiasis.2,9,10
Nystatin is a membrane-active polyene macrolide produced by Streptomyces noursei strains and is available in various forms, such as oral suspension, topical cream, and oral pastille.11–15 Nystatin is not absorbed from gastrointestinal tract when orally administered.5 Therefore, the topical use of nystatin is considered the most common route of administration in dentistry, as systemic exposure is minimal. Further, nystatin also plays an important role in the prophylaxis of oral and systemic candidiasis in full-term and premature newborns, infants, and immunocompromised patients (eg, AIDS patients, cancer patients, and organ transplant recipients), as it is associated with a low incidence of drug interactions and acceptable costs, especially in developing countries.16–19 The common recommended dose for topical use of nystatin is 200,000–600,000 IU qid for children and adults, and 100,000–200,000 IU qid for newborns and infants.18,20 Treatment duration can vary from 1 or 2 to 4 weeks.5,21–23 Up to now, there is no consensus on the formulation, dosage, or treatment duration of nystatin in the treatment of oral candidiasis. The aim of this study was to summarize and assess the efficacy, different treatment protocols (formulation, dosage, and duration), and safety of nystatin in different patient populations with oral candidiasis by a meta-analysis and systematic review.
Materials and methods
This review was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines.24
Inclusion criteria
Randomized controlled trials that compared nystatin (at any dosage and in any form) with other antifungal therapies or a placebo were included in this review. The diagnosis of oral candidiasis was based on clinical diagnosis with or without confirmation by mycological tests. There were no restrictions on patients’ age, sex, or race. The primary outcome was the clinical cure rate: the patient was considered to be cured if the oral lesion and symptoms had completely resolved. The secondary outcome was the mycological cure rate: the patient was considered to be cured if the smear or culture test showed negative results.
Database and search strategies
Four electronic databases were searched by two independent reviewing authors (XL, CZ): the Cochran Central Register of Controlled Trials (CENTRAL) (The Cochrane Library 2015, Issue 6), PubMed (July 1, 2015), EMBASE (July 1, 2015), and Science Citation Index (July 1, 2015). The following terms were searched in combination: (“oral candidiasis” OR “oral candidiases” OR “thrush” OR “oral moniliases” OR “oral moniliasis” OR “oropharyngeal candidiasis” OR “candidal stomatitis” OR “muguet” OR “prosthetic stomatitis” OR “angular cheilitis” OR “rhomboid glossitis”) AND (“nystatin” OR “fungicidin”) AND (“randomized controlled trial” OR “randomized controlled study” OR “RCT”). Manual searches were also conducted as a supplement.
Data extraction and quality assessment
The two review authors (XL, CZ) were independently responsible for scanning titles and abstracts, selecting studies, reading full reports, extracting data, and assessing the quality of studies; these steps were performed in duplicate by each of these authors. All the relevant data of each included study, including author, year of publication, region, risk factors, characteristics of the patients, detailed interventions, outcomes, and adverse effects, were extracted and summarized in a table format. The quality of the included studies was assessed using the Cochrane Handbook for Systematic Review of Interventions and the Rev Man 5.2.0 software. The following assessment criteria were used to assess the quality of the studies: 1) random sequence generation (if the study did not use this method, it was considered to have a selection bias), 2) allocation concealment (selection bias), 3) blinding of participants and personnel (performance bias), 4) blinding of outcome assessment (detection bias), 5) incomplete outcome data (attrition bias), 6) selective reporting (reporting bias), and 7) other biases. The Kappa coefficient was used to calculate inter-rater agreement with regard to study inclusion and quality assessment. A third reviewer (HH or ZMY) was invited to make an assessment if the two review authors could not reach a consensus.
Data synthesis and analysis
The efficacy of nystatin versus placebo and nystatin versus fluconazole was evaluated using the Stata 12.0 (StataCorp LP, College Station, TX, USA) software. Results were expressed as odds ratio (OR) together with the 95% confidence interval (CI), and plotted on a forest plot. The inconsistency index I2 was calculated to assess the variation caused by heterogeneity. When P was >0.10 and I2 was <25%, the fixed-effect model was used, which assumes the same homogeneity of effect size across all studies. When P was <0.10 and I2 was >25%, inter-study heterogeneity was deemed statistically significant, and a random-effects model was employed.25 A descriptive study was conducted on studies evaluating the efficacy of nystatin versus other antifungal treatments due to the limited number of studies or marked heterogeneity in many aspects of the study characteristics.
Results
Results of the search
A total of 379 abstracts were extracted from the four databases. Finally, only eleven trials with a total of 1,148 patients were included in the present analysis (Figure 1). On the basis of the inclusion criteria, 153 duplicate publications, 182 irrelevant studies, four reviews, four nonclinical studies, four noncontrolled studies, one retrospective study, 12 studies with an unmatched study design, five studies with unavailable full texts, and three publications that were not in English were excluded. The Kappa value of inter-reviewer agreement for study inclusion was 0.83. No studies that met the requirements were obtained by the manual search.
Figure 1.
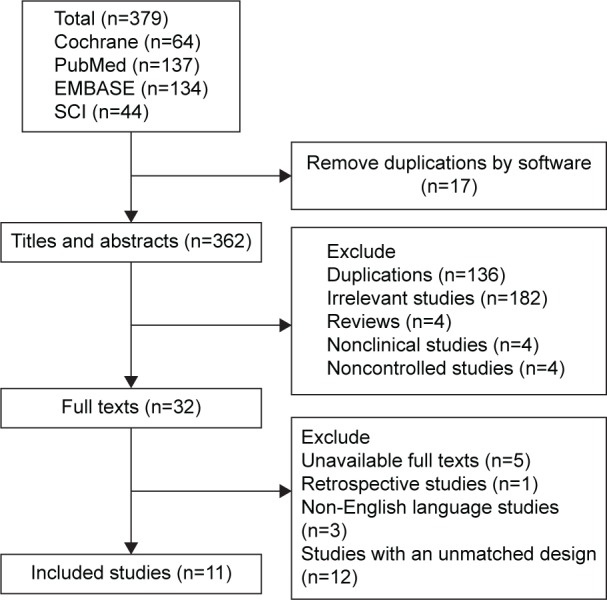
Trial flow and study selection.
Abbreviation: SCI, Science Citation Index.
Characteristics of the included studies
Three trials were performed in patients with denture stomatitis;26–28 three trials were conducted on infants or children;20,29,30 three trials included HIV or AIDS patients;31–33 one trial was on hospitalized cancer patients;34 and one trial was performed in several groups of patients, including those with xerostomia, HIV, immunosuppression in conjunction with organ transplantation, and wearing of dentures.35 Nystatin was used in the suspension and pastille forms; the dosage ranged from 100,000 to 1,100,000 IU three to five times a day; and the treatment duration was 10 to 30 days (Table 1).
Table 1.
Characteristics of the included studies
| Author | Year | Region | Risk factor | Nystatin group
|
Control group
|
Nystatin group
|
Control group
|
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age (mean) | Sex ratio (F:M) | N | Age (mean) | Sex ratio (F:M) | N | Formulation | Dose | Frequency (times/day) | Duration (days) | Medication | Formulation | Duration | ||||
| Mima et al26 | 2012 | Brazil | Denture | 62.45 y | 3:1 | 20 | 61.25 y | 13:7 | 20 | Suspension | 100,000 IU | 4 | 15 | Photodynamic therapy | – | 15 d |
| Johnson et al27 | 1989 | USA | Denture | – | – | 8 8 |
– | – | 8 | Pastille | 200,000 IU 400,000 IU |
5 | 14 | Placebo | Pastille | 14 d |
| Nairn28 | 1975 | England | Denture | – | – | 13 | – | – | 18 15 |
Pastille | 500,000 IU | 4 | 30 | Amphotericin B placebo | Tablet | 1 month |
| Goins et al29 | 2002 | USA | Infants | 1–12 mo | – | 28 | 1–12 mo | – | 17 | Suspension | 100,000 IU | 4 | 10 | Fluconazole | Suspension | 7 d |
| Hoppe20 | 1997 | Germany | Infants | 130.8 d | 57.6:42.4 | 107 | 132.0 d | 48:52 | 105 | Suspension | 100,000 IU | 4 | 12 | Miconazole | Gel | 12 d |
| Flynn et al30 | 1995 | USA | Infants Children | 6 mo to 13 y | – | 88 | 6 mo to 13 y | – | 94 | Suspension | 400,000 IU | 4 | 14 | Fluconazole | Suspension | 14 d |
| Moshi et al31 | 1998 | Tanzania | AIDS | 15–59 y | – | 91 | 15–59 y | – | 91 | Suspension | 100,000 IU | 3 | 14 | Sodium benzoate | Solution | 14 d |
| Pons et al32 | 1997 | USA | HIV AIDS | 38 y | 1:7 | 84 | 38 y | 1:7 | 83 | Suspension | 500,000 IU | 4 | 14 | Fluconazole | Suspension | 14 d |
| Nyst et al33 | 1992 | Zairian | AIDS | 35.4 y | 25:22 | 47 | 34.5 y | 27:22 23:22 | 49 45 |
Suspension | 200,000 IU | 4 | 14 | Gentian violet ketoconazole | Suspension troche | 14 d |
| Meunier et al34 | 1990 | Belgium | Cancer patients | – | – | 24 | – | – | 18 | Suspension + pastille | 1,000,000 IU 100,000 IU |
3 | 10–12 | Ketoconazole | Tablet | 10–12 d |
| Blomgren et al35 | 1998 | Sweden | Multigroup patients | 60.7 y | 16:14 | 33 | 58.4 y | 18:12 | 34 | Solution | 100,000 IU | 4 | 21 | Fluconazole | Capsule | 7 d |
Abbreviations: d, days; mo, months; y, years; F, female, M, male.
Risk of bias and quality of the included studies
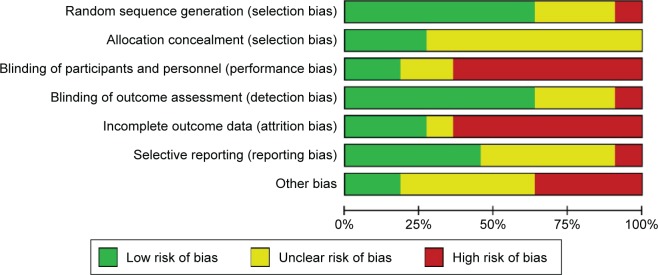
None of the included studies met all the seven assessment criteria (Table S1). Most studies were found to have a high risk of a performance and attrition bias and a moderate risk of other biases. The overall risk of each bias is presented in Figure 2, and the risk of each bias in each of the studies separately is presented in Figure 3. A 100% agreement was achieved on study quality among the reviewers.
Figure 2.
Risk of bias graph: the overall risk of each bias is presented as a percentage representing the risk in all the included studies.
Figure 3.
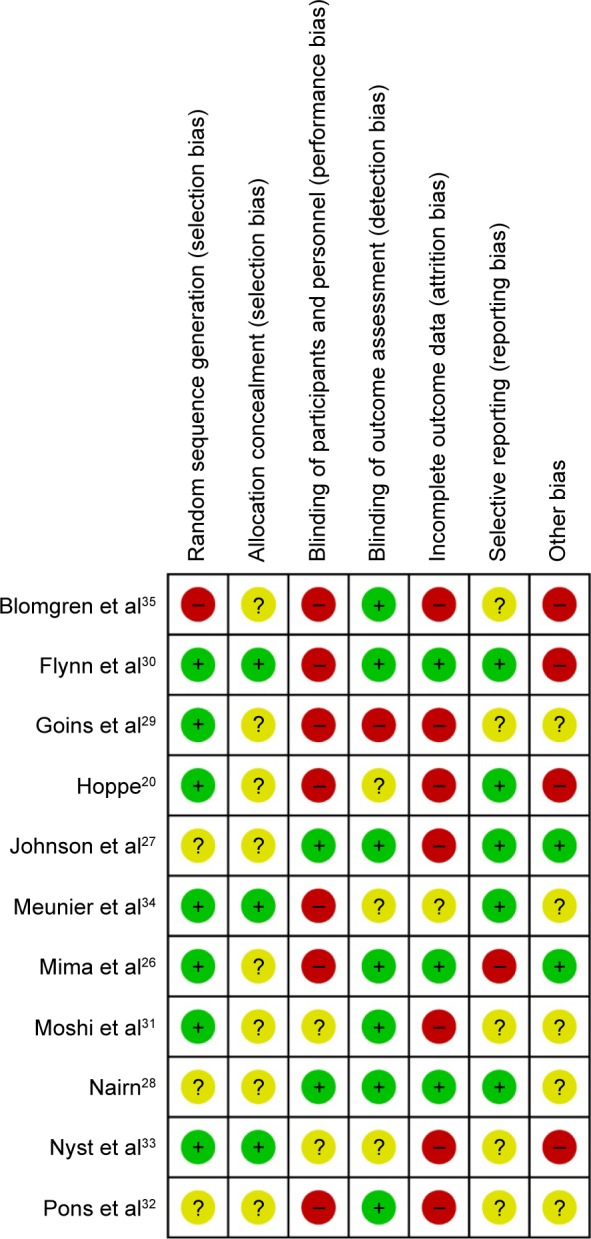
Risk of bias summary: the risk of each bias in each of the included studies is shown separately.
Note: +, ?, − indicate the low bias, uncertain, and high bias, respectively.
Efficacy assessment
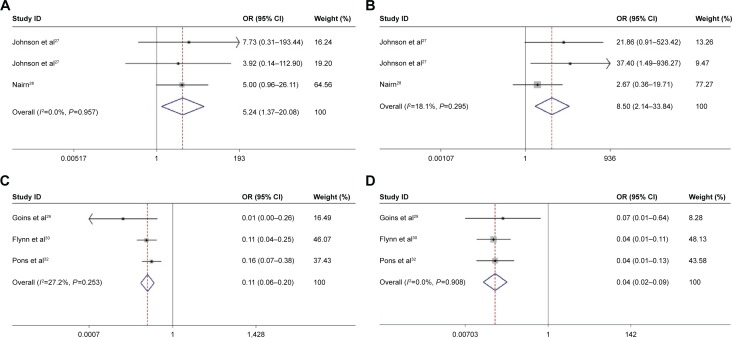
The clinical and mycological cure rates associated with nystatin and the control treatments are summarized in Table 2. Two studies comparing the efficacy of nystatin pastilles to the placebo in treating denture stomatitis were analyzed by meta-analysis.27,28 The results showed that the efficacy of nystatin pastilles was significantly superior to that of the placebo treatment (clinical OR =5.24, 95% CI =1.37–20.08, P=0.957; mycological OR =8.50, 95% CI =2.14–33.84, P=0.295; Figure 4A and B; Table 3). Three studies evaluating the efficacy of nystatin suspension and fluconazole for treating oral or oropharyngeal candidiasis in infants, children (1 month to 13 years old), and HIV/AIDS patients were also studied by meta-analysis.29,30,32 The results of these three studies demonstrated that the efficacy of the nystatin suspension was significantly inferior to that of fluconazole (clinical OR =0.11, 95% CI =0.06–0.20, P=0.253; mycological OR =0.04, 95% CI =0.02–0.09, P=0.908; Figure 4C and D; Table 3).
Table 2.
Clinical and mycological efficacy of nystatin and the control treatments
| Author | Risk factor | Clinical cure rate
|
Mycological cure rate
|
||||
|---|---|---|---|---|---|---|---|
| Nystatin (%) | Control (%) | OR (95% CI) | Nystatin (%) | Control (%) | OR (95% CI) | ||
| Mima et al26 | Denture | 53 | 45 | 1.22 (0.35–4.24) | – | – | – |
| Johnson et al27 | Denture | 28.6 | 0 | 7.73 (0.31–193.44) | 57.1 | 0 | 21.86 (0.91–523.42) |
| 14.3 | 0 | 3.92 (0.14–112.90) | 71.4 | 0 | 37.40 (1.49–936.27) | ||
| Nairn28 | Denture | 76.9 | 88.8 | 0.42 (0.06–2.95) | 40 | 6.25 | 10.00 (0.92–108.82) |
| 76.9 | 40 | 5.00 (0.96–26.11) | 40 | 20 | 2.67 (0.36–19.71) | ||
| Goins et al29 | Infants | 28.6 | 100 | 0.01 (0.00–0.26) | 5.6 | 73.3 | 0.07 (0.01–0.64) |
| Hoppe20 | Infants | 54.1 | 99 | 0.01 (0.00–0.09) | 8.2 | 54.1 | 0.08 (0.03–0.18) |
| Flynn et al30 | Infants and children | 51 | 91 | 0.11 (0.04–0.25) | 11 | 76 | 0.04 (0.01–0.11) |
| Moshi et al31 | AIDS | 63.5 | 55.6 | 1.24 (0.65–2.37) | – | – | – |
| Pons et al32 | HIV/AIDS | 52 | 87 | 0.16 (0.07–0.38) | 6 | 60 | 0.04 (0.01–0.13) |
| Nyst et al33 | AIDS | 9 | 42 | 0.13 (0.03–0.67) | 13 | 62 | 0.09 (0.02–0.40) |
| 9 | 43 | 0.12 (0.02–0.66) | 13 | 57 | 0.12 (0.03–0.50) | ||
| Meunier et al34 | Cancer patients | 87.5 | 72.2 | 2.69 (0.55–13.20) | 66 | 61 | 1.27 (0.36–4.54) |
| Blomgren et al35 | Multigroup patients | 16.7 | 30 | 0.47 (0.14–1.61) | – | – | – |
Abbreviations: CI, confidence interval; OR, odds ratio.
Figure 4.
Forest plots evaluating the efficacy of nystatin (fixed-effect model).
Notes: (A) The overall clinical efficacy of nystatin was significantly superior to the placebo in treating denture stomatitis; (B) the overall mycological efficacy of nystatin was significantly superior to the placebo in treating denture stomatitis; (C) the overall clinical efficacy of nystatin was significantly inferior to fluconazole for treating oral or oropharyngeal candidiasis in infants, children, and HIV/AIDS patients; and (D) the overall mycological efficacy of nystatin was significantly inferior to fluconazole for treating oral or oropharyngeal candidiasis in infants, children, and HIV/AIDS patients.
Abbreviations: CI, confidence interval; OR, odds ratio.
Table 3.
Meta-analysis of the efficacy of nystatin compared with that of the placebo and fluconazole
| Risk factor | Control | Study number | Patient number | Clinical cure rate
|
Mycological cure rate
|
||||
|---|---|---|---|---|---|---|---|---|---|
| OR (95% CI) | P-value | I2 (%) | OR (95% CI) | P-value | I2 (%) | ||||
| Denture | Placebo | 3* | 52 | 5.24 (1.37–20.08) | 0.957 | 0.0 | 8.50 (2.14–33.84) | 0.295 | 18.1 |
| Infants, children HIV/AIDS | Fluconazole | 3 | 394 | 0.11 (0.06–0.20) | 0.253 | 27.2 | 0.04 (0.02–0.09) | 0.908 | 0.0 |
Note:
One study was divided into two investigations and included in the meta-analysis.
Abbreviations: CI, confidence interval; OR, odds ratio.
A descriptive investigation was conducted on the other seven studies due to the limited number of studies or marked heterogeneity in many aspects of the study characteristics.20,26,28,31,33–35 The efficacy of nystatin pastilles was compared to that of other drugs in two studies.28,34 The results showed that nystatin pastilles had similar clinical efficacy (79.6%–87.5%) to amphotericin B (88.8%)28 and ketoconazole (72.2%).34 The efficacy of nystatin suspension was compared to that of other treatment strategies in four studies.20,26,31,33 These studies showed that the clinical and mycological efficacy of nystatin suspensions (9%–54.1% and 5.6%–13%, respectively) was significantly inferior to that of miconazole (99% and 54.1%, respectively),20 gentian violet (42% and 62%, respectively), and ketoconazole (43% and 57%, respectively)33 in treating oral candidiasis in infants, children, and HIV/AIDS patients, while nystatin suspensions had similar clinical efficacy (53%–63.5%) to photodynamic therapy (45%)26 and sodium benzoate (55.6%).31 These results showed that nystatin pastilles had a relatively high clinical efficacy (79.6%–87.5%) in treating oral candidiasis. However, nystatin suspension seemed to be inferior to miconazole, gentian violet, and ketoconazole in treating oral candidiasis in infants, children, and HIV/AIDS patients (for details, Tables 1 and 2).
Formulation, dosage, and duration of nystatin treatment
Due to obvious diversity in study design, the analysis of different treatment protocols (formulation, dosage, and duration) of nystatin was not qualified for meta-analysis; only descriptive investigations were conducted. In the included eleven studies, there are three formulations of nystatin, including suspension form, pastille form, and a combination of the suspension and pastille forms. For patients with denture stomatitis, the clinical cure rate with the suspension form was 53%,26 and the clinical and mycological cure rates with the pastille form only were 14.3%–76.9% and 40%–71.4%, respectively.27,28 Researches on infants, children, and HIV/AIDS patients with oral candidiasis showed that the clinical cure rate was 9%–63.5% and the mycological cure rate was 5.6%–13% with the use of the suspension form.20,29–33 Meunier et al34 found that clinical and mycological cures were achieved in 87.5% and 66% of cancer patients, respectively, with oral candidiasis by using the suspension and pastille forms in combination. The results of these studies indicated that combined administration of nystatin in the suspension and pastille form for 2 weeks might achieve a higher clinical and mycological cure rate than administration of the nystatin suspension alone in the treatment of oral candidiasis. Moreover, use of the pastille alone for 2 weeks might result in a higher mycological cure rate than use of the suspension alone (Table 4).
Table 4.
Summary of the usage and efficacy of nystatin
| Risk factor | Formulation | Dose | Frequency (times/day) |
Duration (days) |
Clinical cure rate (%) |
Mycological cure rate (%) |
|---|---|---|---|---|---|---|
| Denture | Suspension | 100,000 IU | 4 | 15 | 53 | – |
| Pastille | 200,000/400,000 IU | 5 | 14 | 28.6/14.3 | 57.1/71.4 | |
| Pastille | 500,000 IU | 4 | 30 | 76.9 | 40 | |
| Young age (in infants and children) | Suspension | 100,000–400,000 IU | 4 | 10–14 | 28.6–54.1 | 5.6–11 |
| HIV/AIDS | Suspension | 100,000–500,000 IU | 3–4 | 14 | 9–63.5 | 6–13 |
| Cancer | Suspension + pastille | 1,000,000+100,000 IU | 3 | 10–12 | 87.5 | 66 |
| Multiple | Suspension | 100,000 IU | 4 | 21 | 16.7 | – |
Johnson et al27 compared the efficacy of the nystatin pastilles administered in two dosages (200,000 and 400,000 IU) for treating denture stomatitis. No significant difference was found in clinical efficacy (28.6% and 14.3%, OR =5.67, 95% CI =0.56–56.96) between the two dosages, while the mycological efficacy of the 400,000 IU nystatin pastilles was significantly higher than that of the 200,000 IU pastilles (71.4% and 57.1%, OR =28.33, 95% CI =2.95–271.81).
The treatment duration of nystatin was 2 to 4 weeks. Researches on denture stomatitis and oral candidiasis in infants, children, and HIV/AIDS patients showed that the clinical and mycological cure rates were 9%–63.5% and 6%–13%, respectively, with the use of the suspension form for 2 weeks.30–33 Blomgren et al35 found that a 16.7% clinical cure rate was achieved when the suspension form was used for 3 weeks. Studies on denture stomatitis showed that using nystatin pastilles for 2 weeks could achieve a 14.3%–28.6% clinical cure rate and a 57.1%–71.4% mycological cure rate27 and that using the pastille form for 4 weeks could achieve a 76.9% clinical cure rate and a 40% mycological cure rate.28 These findings indicated that nystatin administration of 4 weeks has a better clinical efficacy than 2 weeks in the treatment of denture stomatitis (Table 4).
Safety assessment
Eight out of the eleven studies reported the adverse effects of nystatin.20,28–33,35 Two of the eight studies reported that nystatin had no adverse effects.29,33 One study did not show the details of the adverse effects.31 Poor taste (the incidence was 61.5% in one study)28 and gastrointestinal adverse reactions, including vomiting, nausea, diarrhea, anorexia, and abdominal pain (the incidence was 0.01%–0.06% in four studies), were the most common adverse effects reported (Table 5).20,30,32,35
Table 5.
Adverse effects of nystatin and the control treatments
| Author | Risk factor | Adverse effects of nystatin | Adverse effects of the control treatment |
|---|---|---|---|
| Mima et al26 | Denture | – | – |
| Johnson et al27 | Denture | – | – |
| Nairn28 | Denture | Unpleasant taste in eight patients | Unpleasant taste in five patients |
| Goins et al29 | Infants | None | None |
| Hoppe20 | Infants | Ten adverse events in six patients (vomiting, diarrhea) | Seven events in six patients (vomiting, diarrhea) |
| Flynn et al30 | Young age (infants and children) | Three patients (vomiting, nausea, diarrhea, anorexia, abdominal pain), one patient (rash, headache) | Six patients (vomiting, nausea, diarrhea, anorexia, abdominal pain), one patient (rash, headache) |
| Moshi et al31 | AIDS | Ten patients | 14 patients |
| Pons et al32 | HIV | Vomiting in one patient | Nausea in one patient, and elevated liver enzyme concentrations in two patients |
| Nyst et al33 | AIDS | None | Irritation and small superficial oral ulcers in two patients |
| Meunier et al34 | Cancer | – | – |
| Blomgren et al35 | Multiple | Nausea in one patient | None |
Discussion
Oral candidiasis is an opportunistic infection of oral cavity. It is common among the elderly and infants, particularly in those elderly patients who wear dentures. It can also be a mark of some systemic disease, such as diabetes mellitus, cancer, and immunodeficiency diseases. The prevalence and incidence of all forms of oral candidiasis have increased in recent decades. Approximately 54% of people who wear removable dentures suffer from oral candidiasis.36 Thrush occurs in ~1% to 37% of systematically healthy infants.29 In all, 15%–60% of cancer patients will develop oral candidiasis because of immunosuppression.2 More than 90% of patients with AIDS may suffer from oral candidiasis at some time during their illness.37 Although the appearance and development of azoles and echinocandins antifungal agents, which had better tastes and less gastrointestinal adverse reactions, provided more clinical options, topical therapy, such as nystatin, is still one of the main recommended treatments for oral candidiasis due to its high efficacy, low cost, and less side effects, especially in developing countries.11,18 One study showed that nystatin was the most commonly prescribed antifungal agent for the treatment of oral candidiasis in Jordan (78.2%).38
In 2009, the Infectious Diseases Society of America updated its clinical practice guidelines for the management of candidiasis. In this guideline, nystatin suspension at a concentration of 100,000 U/mL and a dosage of 4–6 mL qid, or one to two nystatin pastilles (200,000 U each) administered qid for 7–14 days, is recommended for mild oropharyngeal candidiasis.39 In addition, the World Health Organization recommended that topical therapy with nystatin suspension or pastilles can be an alternative to oral fluconazole for treating oropharyngeal candidiasis in HIV-positive children and adults.40 Even so, few evidences were found on the efficacy of nystatin for oral candidiasis in clinical practice. Furthermore, the applications of nystatin were varied among different patient populations and countries. So, ensuring the detailed indications of nystatin for different types of oral candidiasis is of great importance.
In the present review, the meta-analysis of the limited studies showed that the efficacy of nystatin pastilles was significantly superior to placebo in treating denture stomatitis, while nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. Further, the descriptive investigations showed that administration of nystatin suspension and pastilles in combination for 2 weeks might achieve a higher clinical and mycological cure rates (87.5% and 66%) than if nystatin suspension alone is used. Nystatin pastilles at a dose of 400,000 IU resulted in a significantly higher mycological cure rate than the dose of 200,000 IU. With regard to treatment duration, administration of nystatin pastilles for 4 weeks showed better clinical efficacy (76.9%) than its administration for 2 weeks. Poor taste and gastrointestinal adverse reaction were the most common adverse effects of nystatin.
Polyene antibiotics exert their antifungal effects by interacting with ergosterol in the fungal cell membrane, creating pores, and subsequently increasing the inflow and outflow of many materials.41 However, the treatment is effective only if these antibiotics are administered over a sufficient period of time. Nystatin suspension was not a good choice for infants, children, and HIV/AIDS patients with oral candidiasis, probably because of its short-term action on the oral mucosa.5 Moreover, exposure to nystatin at a concentration 0.25 to 1 times the minimum inhibitory concentration value for 30 minutes resulted in a postantifungal effect with an average duration range of 3.1 to 6.3 hours in several Candida isolates.16 Nystatin shows a remarkable postantifungal effect, which is defined as the delay in fungal regrowth that persists after a brief exposure to an antifungal agent.16 Therefore, nystatin in the topical pastille form seems to be more effective in treating oral candidiasis than oral nystatin suspension.
Candida species colonize in the oral mucosa via adhesion to buccal epithelial cells, germ tube formation, and relative cell surface hydrophobicity.42–45 Therefore, topical drugs that get absorbed into the oral epithelium are necessary for killing yeast hyphae growing within the tissue.23 In previous studies, the treatment duration of nystatin varied from 1 to 6 week(s). Richardson and Jones8 proposed that nystatin solutions need to be used for at least 1 week after resolution of symptoms, usually for 4 weeks for the primary treatment of oral candidiasis. Moreover, in recurrent cases, the duration of treatment should be at least 4 to 6 weeks.27 In agreement with these findings, in this review, our descriptive investigation showed that 4 weeks of nystatin administration seemed to have better clinical efficacy than 2 weeks of nystatin usage.
There are two limitations to this analysis. First, very few clinical trials with heterogeneity were available. Second, several studies were considered to be at a high risk of performance and attrition bias, and at a moderate risk of other biases. Moreover, 72.7% of the studies did not provide enough information about allocation concealment. The inconsistent quality of the included studies would impact the credibility of the results. Therefore, clinicians need to view the results of this research with caution. All these deficiencies indicate that well designed and high quality randomized controlled trial a study are needed in the future.
Conclusion
Nystatin pastille was significantly superior to placebo in treating denture stomatitis, while nystatin suspension was not superior to fluconazole in treating oral candidiasis in infants, children, or HIV/AIDS patients. Indirect evidence from a descriptive study demonstrated that administration of nystatin pastille alone or pastille and suspension in combination is more effective than that of suspension alone; prolonged treatment duration for up to 4 weeks can increase the efficacy of nystatin. More well designed and high quality randomized control studies are needed to confirm these findings.
Supplementary material
Table S1.
Risk of bias and quality assessment of the included studies
| Author | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Mima et al26 | Low bias | Uncertain | High bias | Low bias | Low bias | High bias | Low bias |
| Johnson et al27 | Uncertain | Uncertain | Low bias | Low bias | High bias | Low bias | Low bias |
| Nairn28 | Uncertain | Uncertain | Low bias | Low bias | Low bias | Low bias | Uncertain |
| Goins et al29 | Low bias | Uncertain | High bias | High bias | High bias | Uncertain | Uncertain |
| Hoppe20 | Low bias | Uncertain | High bias | Uncertain | High bias | Low bias | High bias |
| Flynn et al30 | Low bias | Low bias | High bias | Low bias | Low bias | Low bias | High bias |
| Moshi et al31 | Low bias | Uncertain | Uncertain | Low bias | High bias | Uncertain | Uncertain |
| Pons et al32 | Uncertain | Uncertain | High bias | Low bias | High bias | Uncertain | Uncertain |
| Nyst et al33 | Low bias | Low bias | Uncertain | Uncertain | High bias | Uncertain | High bias |
| Meunier et al34 | Low bias | Low bias | High bias | Uncertain | Uncertain | Low bias | Uncertain |
| Blomgren et al35 | High bias | Uncertain | High bias | Low bias | High bias | Uncertain | High bias |
Acknowledgments
This work was supported by the Program for New Clinical Techniques and Therapies of Peking University School and Hospital of Stomatology (PKUSSNCT-14A01) and Natural Science Foundation of China (81000441, 81570985).
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Melkoumov A, Goupil M, Louhichi F, Raymond M, de Repentigny L, Leclair G. Nystatin nanosizing enhances in vitro and in vivo antifungal activity against Candida albicans. J Antimicrob Chemother. 2013;68(9):2099–2105. doi: 10.1093/jac/dkt137. [DOI] [PubMed] [Google Scholar]
- 2.Akpan A, Morgan R. Oral candidiasis. Postgrad Med J. 2002;78(922):455–459. doi: 10.1136/pmj.78.922.455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Epstein JB, Polsky B. Oropharyngeal candidiasis: a review of its clinical spectrum and current therapies. Clin Ther. 1998;20(1):40–57. doi: 10.1016/s0149-2918(98)80033-7. [DOI] [PubMed] [Google Scholar]
- 4.Coronado-Castellote L, Jimenez-Soriano Y. Clinical and microbiological diagnosis of oral candidiasis. J Clin Exp Dent. 2013;5(5):e279–e286. doi: 10.4317/jced.51242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Samaranayake LP, Keung Leung W, Jin L. Oral mucosal fungal infections. Periodontol 2000. 2009;49:39–59. doi: 10.1111/j.1600-0757.2008.00291.x. [DOI] [PubMed] [Google Scholar]
- 6.Hoepelman IM, Dupont B. Oral candidiasis: the clinical challenge of resistance and management. Int J Antimicrob Agents. 1996;6(3):155–159. doi: 10.1016/0924-8579(95)00050-x. [DOI] [PubMed] [Google Scholar]
- 7.Richardson MD, Warnock DW. Fungal Infection: Diagnosis and Management. Oxford: Blackwell Publishing; 2003. [Google Scholar]
- 8.Richardson MD, Jones BL. Therapeutic Guidelines in Systemic Fungal Infections. 3rd ed. London: Remedica Publishing; 2007. [Google Scholar]
- 9.Antifungal chemotherapy in patients with acquired immunodeficiency syndrome British Society for Antimicrobial Chemotherapy Working Party. Lancet. 1992;340(8820):648–651. [PubMed] [Google Scholar]
- 10.Como JA, Dismukes WE. Oral azole drugs as systemic antifungal therapy. N Engl J Med. 1994;330(4):263–272. doi: 10.1056/NEJM199401273300407. [DOI] [PubMed] [Google Scholar]
- 11.Kaur IP, Kakkar S. Topical delivery of antifungal agents. Expert Opin Drug Deliv. 2010;7(11):1303–1327. doi: 10.1517/17425247.2010.525230. [DOI] [PubMed] [Google Scholar]
- 12.Epstein JB. Antifungal therapy in oropharyngeal mycotic infections. Oral Surg Oral Med Oral Pathol. 1990;69(1):32–41. doi: 10.1016/0030-4220(90)90265-t. [DOI] [PubMed] [Google Scholar]
- 13.Guida RA. Candidiasis of the oropharynx and esophagus. Ear Nose Throat J. 1988;67(11):832, 834–836, 838–840. [PubMed] [Google Scholar]
- 14.Greenspan D. Treatment of oropharyngeal candidiasis in HIV-positive patients. J Am Acad Dermatol. 1994;31(3 Pt 2):S51–S55. doi: 10.1016/s0190-9622(08)81268-6. [DOI] [PubMed] [Google Scholar]
- 15.Wong SSW, Samaranayake LP, Seneviratne CJ. In pursuit of the ideal antifungal agent for Candida infections: high-throughput screening of small molecules. Drug Discov Today. 2014;19(11):1721–1730. doi: 10.1016/j.drudis.2014.06.009. [DOI] [PubMed] [Google Scholar]
- 16.Fernandez Campos F, Calpena Campmany AC, Rodriguez Delgado G, Lopez Serrano O, Clares Naveros B. Development and characterization of a novel nystatin-loaded nanoemulsion for the buccal treatment of candidosis: ultrastructural effects and release studies. J Pharm Sci. 2012;101(10):3739–3752. doi: 10.1002/jps.23249. [DOI] [PubMed] [Google Scholar]
- 17.Howell A, Isaacs D, Halliday R, Australasian Study Group For Neonatal I Oral nystatin prophylaxis and neonatal fungal infections. Arch Dis Child Fetal Neonatal Ed. 2009;94(6):F429–F433. doi: 10.1136/adc.2008.157123. [DOI] [PubMed] [Google Scholar]
- 18.Sklenar Z, Scigel V, Horackova K, Slanar O. Compounded preparations with nystatin for oral and oromucosal administration. Acta Pol Pharm. 2013;70(4):759–762. [PubMed] [Google Scholar]
- 19.Gotzsche PC, Johansen HK. Nystatin prophylaxis and treatment in severely immunodepressed patients. Cochrane Database Syst Rev. 2014;9:CD002033. doi: 10.1002/14651858.CD002033.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hoppe JE. Treatment of oropharyngeal candidiasis and candidal diaper dermatitis in neonates and infants: review and reappraisal. Pediatr Infect Dis J. 1997;16(9):885–894. doi: 10.1097/00006454-199709000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Niimi M, Firth NA, Cannon RD. Antifungal drug resistance of oral fungi. Odontology. 2010;98(1):15–25. doi: 10.1007/s10266-009-0118-3. [DOI] [PubMed] [Google Scholar]
- 22.Williams DW, Kuriyama T, Silva S, Malic S, Lewis MA. Candida biofilms and oral candidosis: treatment and prevention. Periodontol 2000. 2011;55(1):250–265. doi: 10.1111/j.1600-0757.2009.00338.x. [DOI] [PubMed] [Google Scholar]
- 23.Rautemaa R, Ramage G. Oral candidosis – clinical challenges of a biofilm disease. Crit Rev Microbiol. 2011;37(4):328–336. doi: 10.3109/1040841X.2011.585606. [DOI] [PubMed] [Google Scholar]
- 24.Shamseer L, Moher D, Clarke M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015;349:g7647. doi: 10.1136/bmj.g7647. [DOI] [PubMed] [Google Scholar]
- 25.Xie S, Xu H, Shan X, Liu B, Wang K, Cai Z. Clinicopathological and prognostic significance of survivin expression in patients with oral squamous cell carcinoma: evidence from a meta-analysis. PLoS One. 2015;10(2):e0116517. doi: 10.1371/journal.pone.0116517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mima EG, Vergani CE, Machado AL, et al. Comparison of photodynamic therapy versus conventional antifungal therapy for the treatment of denture stomatitis: a randomized clinical trial. Clin Microbiol Infect. 2012;18(10):E380–E388. doi: 10.1111/j.1469-0691.2012.03933.x. [DOI] [PubMed] [Google Scholar]
- 27.Johnson GH, Taylor TD, Heid DW. Clinical evaluation of a nystatin pastille for treatment of denture-related oral candidiasis. J Prosthetic Dent. 1989;61(6):699–703. doi: 10.1016/s0022-3913(89)80045-9. [DOI] [PubMed] [Google Scholar]
- 28.Nairn RI. Nystatin and amphotericin B in the treatment of denture-related candidiasis. Oral Surg Oral Med Oral Pathol. 1975;40(1):68–75. doi: 10.1016/0030-4220(75)90348-5. [DOI] [PubMed] [Google Scholar]
- 29.Goins RA, Ascher D, Waecker N, Arnold J, Moorefield E. Comparison of fluconazole and nystatin oral suspensions for treatment of oral candidiasis in infants. Pediatr Infect Dis J. 2002;21(12):1165–1167. doi: 10.1097/00006454-200212000-00017. [DOI] [PubMed] [Google Scholar]
- 30.Flynn PM, Cunningham CK, Kerkering T, et al. Oropharyngeal candidiasis in immunocompromised children: a randomized, multicenter study of orally administered fluconazole suspension versus nystatin. The Multicenter Fluconazole Study Group. J Pediatr. 1995;127(2):322–328. doi: 10.1016/s0022-3476(95)70321-7. [DOI] [PubMed] [Google Scholar]
- 31.Moshi AH, Jorgensen AF, Pallangyo K. Treatment of oral candidiasis: a study to determine the clinical response of sodium benzoate compared with nystatin suspension. AIDS. 1998;12(16):2237–2238. [PubMed] [Google Scholar]
- 32.Pons V, Greenspan D, Lozada-Nur F, et al. Oropharyngeal candidiasis in patients with AIDS: randomized comparison of fluconazole versus nystatin oral suspensions. Clin Infect Dis. 1997;24(6):1204–1207. doi: 10.1086/513664. [DOI] [PubMed] [Google Scholar]
- 33.Nyst MJ, Perriens JH, Kimputu L, Lumbila M, Nelson AM, Piot P. Gentian violet, ketoconazole and nystatin in oropharyngeal and esophageal candidiasis in Zairian AIDS patients. Ann Soc Belg Med Trop. 1992;72(1):45–52. [PubMed] [Google Scholar]
- 34.Meunier F, Gérain J, Snoeck R. Oral treatment of oropharyngeal candidiasis with nystatin versus ketoconazole in cancer patients. Drug Invest. 1990;2(2):71–75. [Google Scholar]
- 35.Blomgren J, Berggren U, Jontell M. Fluconazole versus nystatin in the treatment of oral candidosis. Acta Odontol Scand. 1998;56(4):202–205. doi: 10.1080/00016359850142790. [DOI] [PubMed] [Google Scholar]
- 36.Cumming CG, Wight C, Blackwell CL, Wray D. Denture stomatitis in the elderly. Oral Microbiol Immunol. 1990;5(2):82–85. doi: 10.1111/j.1399-302x.1990.tb00232.x. [DOI] [PubMed] [Google Scholar]
- 37.Feigal DW, Katz MH, Greenspan D, et al. The prevalence of oral lesions in HIV-infected homosexual and bisexual men: three San Francisco epidemiological cohorts. AIDS. 1991;5(5):519–525. doi: 10.1097/00002030-199105000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Al-Shayyab MH, Abu-Hammad OA, Al-Omiri MK, Dar-Odeh NS. Antifungal prescribing pattern and attitude towards the treatment of oral candidiasis among dentists in Jordan. Int Dent J. 2015;65(4):216–226. doi: 10.1111/idj.12173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Pappas PG, Kauffman CA, Andes D, et al. Clinical practice guidelines for the management of candidiasis: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis. 2009;48(5):503–535. doi: 10.1086/596757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.WHO Guidelines Approved by the Guidelines Review Committee . Guidelines on the Treatment of Skin and Oral HIV-Associated Conditions in Children and Adults. Geneva: World Health Organization; 2014. Copyright (c) World Health Organization 2014. [PubMed] [Google Scholar]
- 41.Kinoshita H, Yoshioka M, Ihara F, Nihira T. Cryptic antifungal compounds active by synergism with polyene antibiotics. J Biosci Bioeng. 2015 Aug 29; doi: 10.1016/j.jbiosc.2015.08.003. Epub. [DOI] [PubMed] [Google Scholar]
- 42.Ellepola AN, Joseph BK, Chandy R, Khan ZU. The postantifungal effect of nystatin and its impact on adhesion attributes of oral Candida dubliniensis isolates. Mycoses. 2014;57(1):56–63. doi: 10.1111/myc.12102. [DOI] [PubMed] [Google Scholar]
- 43.Jayatilake JA, Samaranayake LP. Experimental superficial candidiasis on tissue models. Mycoses. 2010;53(4):285–295. doi: 10.1111/j.1439-0507.2010.01879.x. [DOI] [PubMed] [Google Scholar]
- 44.Sitheeque MA, Samaranayake LP. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia) Crit Rev Oral Biol Med. 2003;14(4):253–267. doi: 10.1177/154411130301400403. [DOI] [PubMed] [Google Scholar]
- 45.Samaranayake LP, Fidel PL, Naglik JR, et al. Fungal infections associated with HIV infection. Oral Dis. 2002;8(Suppl 2):151–160. doi: 10.1034/j.1601-0825.8.s2.6.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1.
Risk of bias and quality assessment of the included studies
| Author | Random sequence generation | Allocation concealment | Blinding of participants and personnel | Blinding of outcome assessment | Incomplete outcome data | Selective reporting | Other bias |
|---|---|---|---|---|---|---|---|
| Mima et al26 | Low bias | Uncertain | High bias | Low bias | Low bias | High bias | Low bias |
| Johnson et al27 | Uncertain | Uncertain | Low bias | Low bias | High bias | Low bias | Low bias |
| Nairn28 | Uncertain | Uncertain | Low bias | Low bias | Low bias | Low bias | Uncertain |
| Goins et al29 | Low bias | Uncertain | High bias | High bias | High bias | Uncertain | Uncertain |
| Hoppe20 | Low bias | Uncertain | High bias | Uncertain | High bias | Low bias | High bias |
| Flynn et al30 | Low bias | Low bias | High bias | Low bias | Low bias | Low bias | High bias |
| Moshi et al31 | Low bias | Uncertain | Uncertain | Low bias | High bias | Uncertain | Uncertain |
| Pons et al32 | Uncertain | Uncertain | High bias | Low bias | High bias | Uncertain | Uncertain |
| Nyst et al33 | Low bias | Low bias | Uncertain | Uncertain | High bias | Uncertain | High bias |
| Meunier et al34 | Low bias | Low bias | High bias | Uncertain | Uncertain | Low bias | Uncertain |
| Blomgren et al35 | High bias | Uncertain | High bias | Low bias | High bias | Uncertain | High bias |