Abstract
Background
This study aimed to analyze the potential function of lncRNA CCAT2 in cervical cancer cell proliferation and apoptosis.
Material/Methods
Expression level of CCAT2 in cervical cancer cell lines (HeLa, CaSki, and SiHa) was detected by quantitative real-time PCR. CCAT2 knockdown was established by transfecting siRNA into human cervical cancer cells. Its effects on cell proliferation were studied using cell-counting kit-8 assay. The effect of CCAT2 on cervical cancer cells cycle and apoptosis was assessed by flow cytometry assay.
Results
CCK8 assay showed that CCAT2 knockdown inhibited cell proliferation in HeLa, CaSki, and SiHa cells. The flow cytometry confirmed the results that knockdown of CCAT2 could induce cervical cancer cells cycle G1 phase arrestment and trigger the cells apoptosis.
Conclusions
LncRNA CCAT2 promoted the proliferation and survival of cervical cancer cells.
MeSH Keywords: Apoptosis; Cell Proliferation; RNA, Long Noncoding; Uterine Cervical Neoplasms
Background
World-wide, cervical cancer is one of the most common malignancies and is the second most frequent cause of cancer-related deaths among women [1]. Although the prognosis of cervical cancer has been improved remarkably in recent years, the pathophysiological mechanisms contributing to cervical cancer are still largely unknown [2]. To detect cervical cancer in the early stage and reduce its mortality, a more comprehensive understanding of the molecular pathogenesis of cervical cancer is of paramount importance.
Increasing evidence has indicated that the long non-coding RNAs (lncRNAs) are not only involved in tumorigenesis but also may act as prognostic indicators [3–5]. LncRNAs are defined as non-coding RNA molecules with over 200 nucleotides; they may play vital functional roles in chromatin modifying, regulation of transcription genes, and post-transcriptional management [6,7].
Colon cancer-associated transcript 2 (CCAT2), a 1752-bp lncRNA that maps to chromosome 8q24.21, was originally detected as being highly expressed in colorectal cancer, and it promotes tumor process metastasis during carcinogenesis [8]. Previous studies suggest that CCAT2 is upregulated in gastric, breast, esophageal, and lung carcinoma tissues compared with adjacent normal tissues [9–12]. Recently, Chen et al. reported that CCAT2 is correlated with cervical cancer metastasis and serves as a marker of poor prognosis in cervical cancer patients [13]. However, the effects of CCAT2 in cervical cancer cells are still unclear; therefore, we performed this study to explore its role in cervical cancer. Cell proliferation and cell apoptosis, which play essential roles in tumor development and progression, are the main research orientation of CCAT2 in cervical cancer in the current study.
Material and Methods
Cell culture
The research protocol was approved by the Ethics Committee of the First Affiliated Hospital of Wenzhou Medical University. Three human cervical cancer cell lines – CaSki, HeLa, and SiHa – were cultured in RPMI-1640 medium containing 10% fetal bovine serum. In a humidified incubator, cells were grown under 37°C in the presence of 5% CO2.
Total RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
According to manufacturer’s instructions, the TRIzol reagent (Invitrogen) was used to extract the total RNA from the cervical cancer cell line. The total RNA was reverse transcribed into cDNA using the one-step RT-PCR kit (TaKaRa). qRT-PCR analysis of lncRNA CCAT2 was performed using CCAT2-specific primers: (forward, 5′-CCCTGGTCAAATTGCTTAACCT-3′, reverse, 5′-TTATTCGTCCCTCTGTTTTATGGAT-3′) and the SYBR® Pre-mix Dimmer Eraser kit (TaKaRa) in an ABI Prism 7500 (Applied Biosystems). GAPDH served as the endogenous control. The relative mRNA expression change was calculated using 2−ΔΔCt method.
Cell transfection
We purchased CCAT2 siRNA and its negative control siRNA from Shanghai GenePharma Company. The CCAT2 siRNA sequences were as follows: 5′UUAACCUCUUCCUAUCUCATT3′ (sense) and 5′UGAG AUAGGAAGAGGUUAATT3′ (antisense). According to the manufacturer’s instructions, we transfected the cervical cancer cells siRNA by using Lipofectamine 2000 (Invitrogen) to knock down their expression of CCAT2. Cells were harvested 48 h after transfection and used in the qRT-PCR, cell proliferation, and flow cytometry assays.
Cell proliferation assay
Proliferation assays were performed using CCK8 (Dojindo). Cells were plated in 96-well plates in triplicate at approximately 1000 cells per well and cultured in the growth medium. Cells were then treated with the indicated reagent and the numbers of cells per well were measured by the absorbance (450 nm) of reduced water-soluble tetrazolium salt (WST) at the indicated time points.
Flow cytometric analysis
Cells were transfected with CCAT2 siRNA or its respective control and harvested at 48 h after transfection. After the propidium iodide or AnnexinV/PI double-staining, the cells were assessed for cell cycle or apoptosis by using a flow cytometer (BD Biosciences). All assays were repeated at least 3 times.
Statistical analyses
IBM SPSS 19.0 statistical software was used to analyze the data. The inter-group expression differences between cell lines, the expression changes after transfection, cell cycle, and cell apoptosis assays were analyzed using an independent samples t test. All data are showed as the mean ±SD. All of the p values were 2-sided and a p value of less than 0.05 was considered to indicate a statistically significant difference.
Results
Cervical cancer cells proliferation was inhibited by CCAT2 knockdown
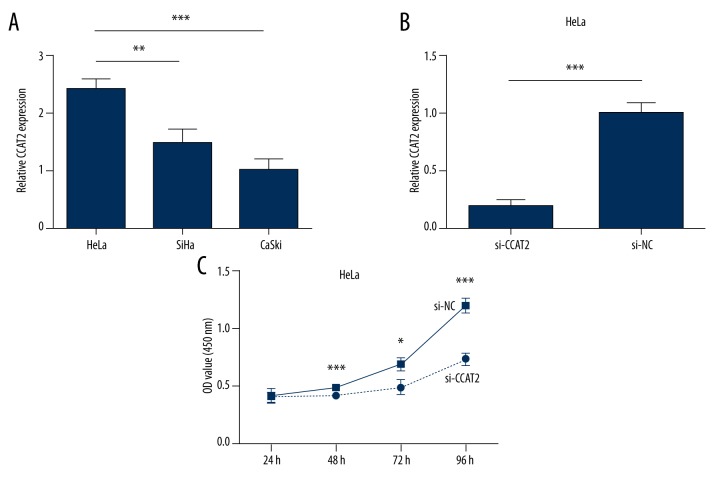
As a previous study demonstrated, CCAT2 expression is significantly higher in cervical cancer tissue than in adjacent normal tissues [13]. We assessed the expression of CCAT2 in 3 human cervical cancer cell lines by qRT-PCR. As shown by Figure 1A, CCAT2 was most highly expressed in the HeLa cell line. Then, we selected cervical cancer cell line HeLa for CCAT2 knockdown by siRNA. qRT-PCR assay confirmed that CCAT2 was significantly downregulated by siRNA compared with negative control (Figure 1B).
Figure 1.
Relative expression of CCAT2 in 3 cervical cancer cell lines was carried out by qRT-PCR (A). Knockdown effects of siRNA CCAT2 in HeLa cell line (B). CCK8 assay to test the function of lncRNA CCAT2. Downregulation of CCAT2 inhibited HeLa cells growth (C). Statistical analyses were performed with the independent samples t test. All data are shown as the mean ±SD.
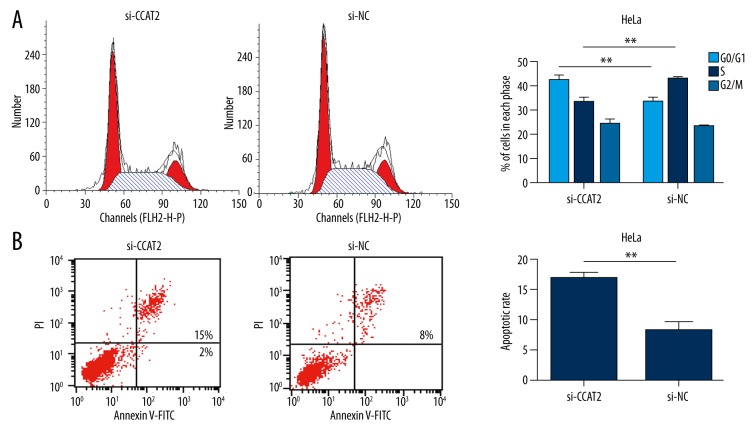
As indicated in the CCK8 assay (Figure 1C), HeLa cells transfected with CCAT2 siRNA showed significant cell proliferation inhibition (Figure 1C). In addition, the percentage of cells in the G0/G1 phase increased from 34% to 43%, and S phase decreased from 44% to 33% in HeLa cells transfected with CCAT2 siRNA (Figure 2A). Taken together, these findings suggest that CCAT2 can promote cervical cancer cell proliferation in vitro.
Figure 2.
Flow cytometric analysis to test the role of CCAT2 on HeLa cells cycle and apoptosis. Downregulation of CCAT2 promoted cell cycle arrest at G1 phase (A) and triggered cell apoptosis (B). Statistical analyses were performed with the independent samples t test. All data are showed as the mean ±SD.
Cervical cancer cells apoptosis was promoted by CCAT2 knockdown
We next performed flow cytometry assay to elucidate the function of CCAT2 in regulation of cell apoptosis in the HeLa cell line. Compared with the control group, the apoptotic rate of HeLa cell line was obviously increased after transfecting them with CCAT2 siRNA (8.3±1.0% vs. 17.0±1.5%, P<0.01, Figure 2B). This result showed that knockdown of CCAT2 promoted cervical cancer cells apoptosis, which may contribute to inhibition of cervical cancer.
Discussion
Recently, lncRNAs were discovered to be dysregulated in a variety of diseases, especially carcinomas [14,15]. LncRNAs have a special role in regulating cellular activities, including proliferation, apoptosis, and differentiation [16–18]. As a novel lncRNA in a non-coding RNA world, CCAT2 was initially reported for its overexpression in primary colorectal cancer, wherein CCAT2 promotes cancer invasion, proliferation, and metastasis [8]. In recent years, the dysregulated CCAT2 expression in other cancer tissues and cell lines has been reported; therefore, we investigated whether CCAT2 plays a functional role in cervical cancer process.
MiRNAs can regulate the expression of massive target genes that encode proteins, which may lead to the change of biological function [19,20]. A growing body of literature has proposed that miRNAs/lncRNAs can target lncRNAs/miRNAs [21,22]. Previous studies found that CCAT2 up-regulated MYC, miR-17-5p, and miR-20a through TCF7L2-mediated transcriptional regulation and the physical interaction between CCAT2 and TCF7L2, resulting in activation of the WNT signaling pathway, and CCAT2 itself is a WNT downstream target [8]. Furthermore, it is also related to cell growth regulation in several cancers, such as breast cancer, gastric cancer, and lung carcinoma, by regulating multiple target genes, including PI3K, Akt, and Crk [12,23]. Chen et al. recently suggested that over-expression of CCAT2 is related to the prognosis of cervical cancer and may serve as a new prognostic biomarker [13].
In the present study we confirmed that the proliferation and survival of cervical cancer cells were both significantly inhibited when the expression level of CCAT2 was knocked down by siRNA transfection. Furthermore, CCK8 assay and cell cycle assay both suggested that CCAT2 can induce the proliferation of cervical cancer cells. Apoptosis assay indicated that CCAT2 inhibited apoptosis in cervical cancer cells. Chen et al. reported that CCAT2 is correlated with cervical cancer metastasis and is as a predictor of poor prognosis in cervical cancer patients [13]. These findings indicate that CCAT2 may be a potential therapeutic target for cervical cancer intervention.
Conclusions
LncRNA CCAT2 promoted the proliferation and survival of cervical cancer cells. Although the underlying mechanism is not yet fully revealed, our work provides further knowledge in this area.
Footnotes
Source of support: Departmental sources
References
- 1.Jemal A, Bray F, Center MM, et al. Global cancer statistics. Cancer J Clin. 2011;61:69–90. doi: 10.3322/caac.20107. [DOI] [PubMed] [Google Scholar]
- 2.Tewari KS, Sill MW, Long HJ, et al. Improved survival with bevacizumab in advanced cervical cancer. N Engl J Med. 2014;370:734–43. doi: 10.1056/NEJMoa1309748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ying L, Huang Y, Chen H, et al. Downregulated MEG3 activates autophagy and increases cell proliferation in bladder cancer. Mol Biosyst. 2013;9:407–11. doi: 10.1039/c2mb25386k. [DOI] [PubMed] [Google Scholar]
- 4.Zhao Y, Guo Q, Chen J, et al. Role of long non-coding RNA HULC in cell proliferation, apoptosis and tumor metastasis of gastric cancer: A clinical and in vitro investigation. Oncol Rep. 2014;31:358–64. doi: 10.3892/or.2013.2850. [DOI] [PubMed] [Google Scholar]
- 5.Huang JF, Guo YJ, Zhao CX, et al. Hepatitis B virus X protein (HBx)-related long noncoding RNA (lncRNA) down-regulated expression by HBx (Dreh) inhibits hepatocellular carcinoma metastasis by targeting the intermediate filament protein vimentin. Hepatology. 2013;57:1882–92. doi: 10.1002/hep.26195. [DOI] [PubMed] [Google Scholar]
- 6.Mercer TR, Dinger ME, Mattick JS. Long non-coding RNAs: insights into functions. Nature reviews Genetics. 2009;10:155–159. doi: 10.1038/nrg2521. [DOI] [PubMed] [Google Scholar]
- 7.Fachel AA, Tahira AC, Vilella-Arias SA, et al. Expression analysis and in silico characterization of intronic long noncoding RNAs in renal cell carcinoma: Emerging functional associations. Mol Cancer. 2013;12:140. doi: 10.1186/1476-4598-12-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ling H, Spizzo R, Atlasi Y, et al. CCAT2, a novel noncoding RNA mapping to 8q24, underlies metastatic progression and chromosomal instability in colon cancer. Genome Res. 2013;23:1446–61. doi: 10.1101/gr.152942.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qiu M, Xu Y, Yang X, et al. CCAT2 is a lung adenocarcinoma-specific long non-coding RNA and promotes invasion of non-small cell lung cancer. Tumour Biol. 2014;35:5375–80. doi: 10.1007/s13277-014-1700-z. [DOI] [PubMed] [Google Scholar]
- 10.Wang CY, Hua L, Yao KH, et al. Long non-coding RNA CCAT2 is up-regulated in gastric cancer and associated with poor prognosis. Int J Clin Exp Pathol. 2015;8:779–85. [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang X, Xu Y, He C, et al. Elevated expression of CCAT2 is associated with poor prognosis in esophageal squamous cell carcinoma. J Surg Oncol. 2015;111:834–39. doi: 10.1002/jso.23888. [DOI] [PubMed] [Google Scholar]
- 12.Redis RS, Sieuwerts AM, Look MP, et al. CCAT2, a novel long non-coding RNA in breast cancer: Expression study and clinical correlations. Oncotarget. 2013;4:1748–62. doi: 10.18632/oncotarget.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Liu L, Zhu W. Up-regulation of long non-coding RNA CCAT2 correlates with tumor metastasis and poor prognosis in cervical squamous cell cancer patients. Int J Clin Exp Pathol. 2015;8:13261–66. [PMC free article] [PubMed] [Google Scholar]
- 14.Yang F, Huo XS, Yuan SX, et al. Repression of the long noncoding RNA-LET by histone deacetylase 3 contributes to hypoxia-mediated metastasis. Mol Cell. 2013;49:1083–96. doi: 10.1016/j.molcel.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 15.Gupta RA, Shah N, Wang KC, et al. Long non-coding RNA HOTAIR reprograms chromatin state to promote cancer metastasis. Nature. 2010;464:1071–76. doi: 10.1038/nature08975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ma MZ, Li CX, Zhang Y, et al. Long non-coding RNA HOTAIR, a c-Myc activated driver of malignancy, negatively regulates miRNA-130a in gallbladder cancer. Mol Cancer. 2014;13:156. doi: 10.1186/1476-4598-13-156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wu XS, Wang XA, Wu WG, et al. MALAT1 promotes the proliferation and metastasis of gallbladder cancer cells by activating the ERK/MAPK pathway. Cancer Biol Ther. 2014;15:806–14. doi: 10.4161/cbt.28584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen CL, Tseng YW, Wu JC, et al. Suppression of hepatocellular carcinoma by baculovirus-mediated expression of long non-coding RNA PTENP1 and MicroRNA regulation. Biomaterials. 2015;44:71–81. doi: 10.1016/j.biomaterials.2014.12.023. [DOI] [PubMed] [Google Scholar]
- 19.Han F, Wu Y, Jiang W. MicroRNA-18a decreases choroidal endothelial cell proliferation and migration by inhibiting HIF1A expression. Med Sci Monit. 2015;21:1642–47. doi: 10.12659/MSM.893068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yan G, Li B, Xin X, et al. MicroRNA-34a promotes hepatic stellate cell activation via targeting ACSL1. Med Sci Monit. 2015;21:3008–15. doi: 10.12659/MSM.894000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nie W, Ge HJ, Yang XQ, et al. LncRNA-UCA1 exerts oncogenic functions in non-small cell lung cancer by targeting miR-193a-3p. Cancer Lett. 2016;371:99–106. doi: 10.1016/j.canlet.2015.11.024. [DOI] [PubMed] [Google Scholar]
- 22.Kang Y, Song J, Kim D, et al. PCGEM1 stimulates proliferation of osteoarthritic synoviocytes by acting as a sponge for miR-770. J Orthop Res. 2016;34(3):412–18. doi: 10.1002/jor.23046. [DOI] [PubMed] [Google Scholar]
- 23.Cai Y, He J, Zhang D. Long noncoding RNA CCAT2 promotes breast tumor growth by regulating the Wnt signaling pathway. Onco Targets Ther. 2015;8:2657–64. doi: 10.2147/OTT.S90485. [DOI] [PMC free article] [PubMed] [Google Scholar]